The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review
Abstract
1. Introduction
2. Materials and Methods
3. Type of Monoclonal Antibodies
4. Mechanism of Action of Monoclonal Antibodies
5. Molecular Mechanisms Underlying HPV-Positive and HPV-Negative Carcinomas
6. Monoclonal Antibodies in HPV-Related Head/Neck Tumors: Advantages
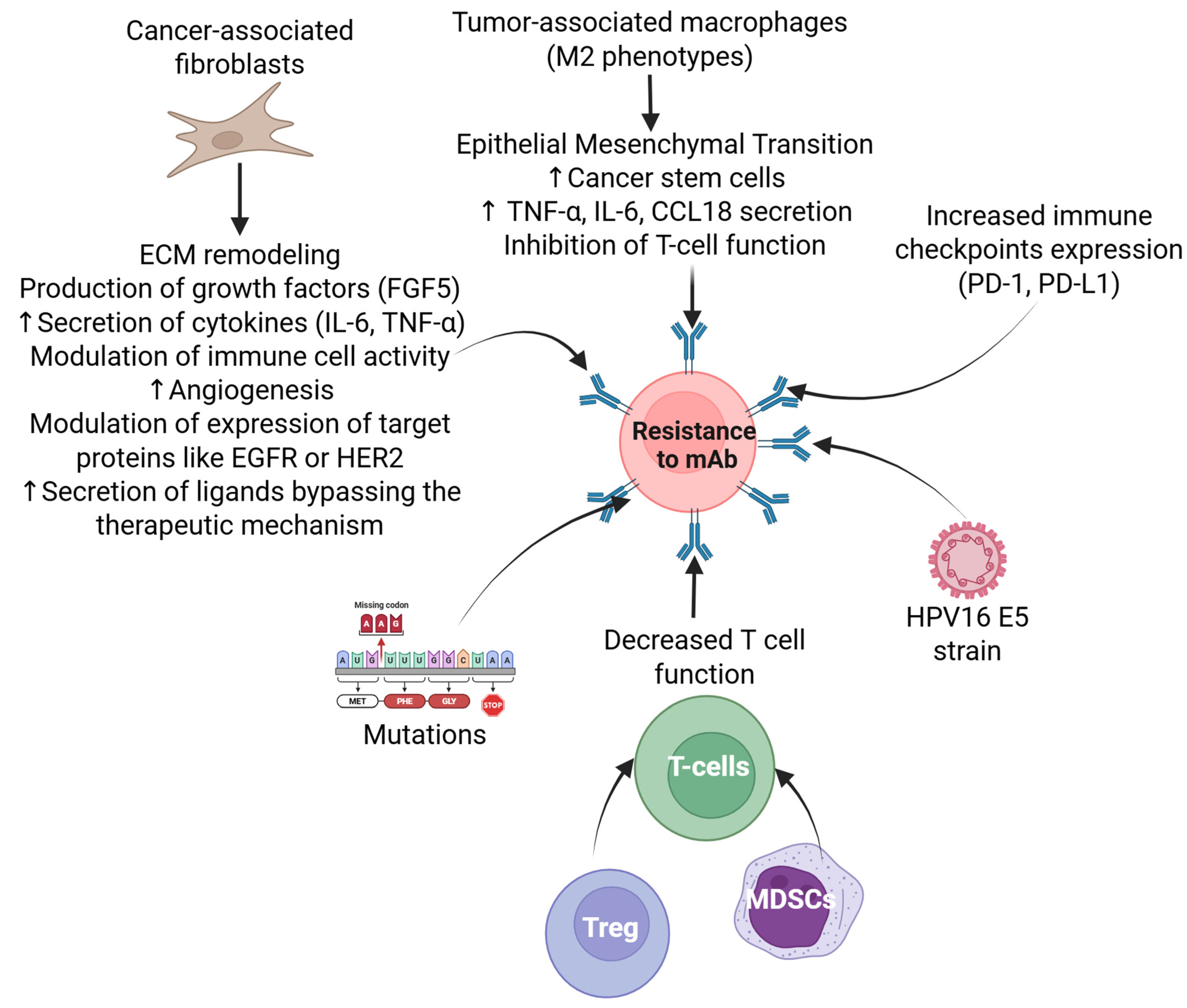
7. Resistance to Immunotherapy in HPV-Related Head and Neck Cancer
8. Challenges and Future Directions

9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4 |
| CAFs | Cancer-associated fibroblasts |
| EGFR | Epidermal growth factor receptor |
| HPV | Human papillomavirus |
| ICI | Immune checkpoint inhibitor |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| mAbs | Monoclonal antibodies |
| MHC | Major Histocompatibility Complex |
| MDSC | Myeloid-derived suppressor cells |
| NK cells | Natural killer cells |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| PD-L1 | Programmed death ligand 1 |
| PTEN | Phosphatase and tensin homolog |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor necrosis factor alpha |
| TAMs | Tumor-associated macrophages |
References
- Michaelraj, M.J.; Kuttiappan, K.; Ramasamy, S.; Rodrigues, F.A.E.; Govindaraj, S. Demographic profile and risk factors of head-and-neck squamous cell carcinoma in west Tamil Nadu: A cross-sectional observational study. Cancer Res. Stat. Treat. 2023, 6, 215–223. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.S.; Shuman, A.G.; Corner, G.W.; Shuk, E.; Sherman, E.J.; Elkin, E.B.; Hay, J.L.; Pfister, D.G. Sharing a diagnosis of HPV-related head and neck cancer: The emotions, the confusion, and what patients want to know. Head Neck 2013, 35, 1534–1541. [Google Scholar] [CrossRef][Green Version]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Lechner, M.; Jones, O.S.; Breeze, C.E.; Gilson, R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 2019, 19, 131–132. [Google Scholar] [CrossRef]
- Lim, Y.X.; D’Silva, N.J. HPV-associated oropharyngeal cancer: In search of surrogate biomarkers for early lesions. Oncogene 2024, 43, 543–554. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Felsher, M.; Waterboer, T.; Mirghani, H.; Mehanna, H.; Roberts, C.; Chen, Y.T.; Lynam, M.; Pedros, M.; Sanchez, E.; et al. Oral Human Papillomavirus Prevalence and Genotyping Among a Healthy Adult Population in the US. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 783–795. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Xiao, C.C.; Murphy, B.; Moore, M.; Fakhry, C.; Day, T.A. Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral. Oncol. 2015, 51, 727–730. [Google Scholar] [CrossRef]
- Galati, L.; Chiocca, S.; Duca, D.; Tagliabue, M.; Simoens, C.; Gheit, T.; Arbyn, M.; Tommasino, M. HPV and head and neck cancers: Towards early diagnosis and prevention. Tumour Virus Res. 2022, 14, 200245. [Google Scholar] [CrossRef]
- Bratman, S.V.; Bruce, J.P.; O’Sullivan, B.; Pugh, T.J.; Xu, W.; Yip, K.W.; Liu, F.F. Human Papillomavirus Genotype Association With Survival in Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016, 2, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012, 6 (Suppl. S1), S16–S24. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Mooren, J.J.; Lehnen, M.; Claessen, S.M.; Stenner, M.; Huebbers, C.U.; Weissenborn, S.J.; Wedemeyer, I.; Preuss, S.F.; Straetmans, J.M.; et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin. Cancer Res. 2009, 15, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar]
- Sher, D.J.; Adelstein, D.J.; Bajaj, G.K.; Brizel, D.M.; Cohen, E.E.W.; Halthore, A.; Harrison, L.B.; Lu, C.; Moeller, B.J.; Quon, H.; et al. Radiation therapy for oropharyngeal squamous cell carcinoma: Executive summary of an ASTRO Evidence-Based Clinical Practice Guideline. Pract. Radiat. Oncol. 2017, 7, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927. [Google Scholar] [CrossRef]
- Holzlohner, P.; Hanack, K. Generation of Murine Monoclonal Antibodies by Hybridoma Technology. J. Vis. Exp. 2017, 119, 54832. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef]
- Chackalamannil, S.; Rotella, D.; Ward, S. Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Nelson, A.L.; Dhimolea, E.; Reichert, J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 2010, 9, 767–774. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.; Boyer, M.; Jafri, Z.; Makeham, T.; Pham, T.; Khachigian, L.M.; Floros, P.; Dowling, E.; Fedder, K.; Shonka, D., Jr.; et al. Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2798. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Pol, J.; Bloy, N.; Eggermont, A.; Cremer, I.; Fridman, W.H.; Galon, J.; Marabelle, A.; Kohrt, H.; Zitvogel, L.; et al. Trial watch: Tumor-targeting monoclonal antibodies for oncological indications. Oncoimmunology 2015, 4, e985940. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Rodriguez-Nava, C.; Ortuno-Pineda, C.; Illades-Aguiar, B.; Flores-Alfaro, E.; Leyva-Vazquez, M.A.; Parra-Rojas, I.; Del Moral-Hernandez, O.; Vences-Velazquez, A.; Cortes-Sarabia, K.; Alarcon-Romero, L.D.C. Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer. Biomedicines 2023, 11, 1610. [Google Scholar] [CrossRef]
- Manso, T.; Kushwaha, A.; Abdollahi, N.; Duroux, P.; Giudicelli, V.; Kossida, S. Mechanisms of action of monoclonal antibodies in oncology integrated in IMGT/mAb-DB. Front. Immunol. 2023, 14, 1129323. [Google Scholar] [CrossRef] [PubMed]
- Geppert, T.D.; Lipsky, P.E. Activation of T lymphocytes by immobilized monoclonal antibodies to CD3. Regulatory influences of monoclonal antibodies to additional T cell surface determinants. J. Clin. Investig. 1988, 81, 1497–1505. [Google Scholar] [CrossRef]
- Han, X.; Vesely, M.D. Stimulating T Cells Against Cancer With Agonist Immunostimulatory Monoclonal Antibodies. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2019; Volume 342, pp. 1–25. [Google Scholar] [CrossRef]
- Bauman, J.E.; Ferris, R.L. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer 2014, 120, 624–632. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK cell-based cancer immunotherapies through receptor engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef]
- Kuzniewska, A.; Majeranowski, A.; Henry, S.; Kowalska, D.; Stasilojc, G.; Urban, A.; Zaucha, J.M.; Okroj, M. The Acquisition of Complement-Dependent Cytotoxicity by the Type II Anti-CD20 Therapeutic Antibody Obinutuzumab. Cancers 2023, 16, 49. [Google Scholar] [CrossRef]
- Janeway, C.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease; Garland Pub: New York, NY, USA, 2001; Volume 2. [Google Scholar]
- Peng, H.Y.; Hsiao, J.R.; Chou, S.T.; Hsu, Y.M.; Wu, G.H.; Shieh, Y.S.; Shiah, S.G. MiR-944/CISH mediated inflammation via STAT3 is involved in oral cancer malignance by cigarette smoking. Neoplasia 2020, 22, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Johnson, N.; Cooke, L.; Johnson, B.; Chen, Y.; Pandey, M.; Chandler, J.; Mahadevan, D. TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients. Cancers 2021, 13, 597. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef]
- Papillomaviruses, H. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2011. [Google Scholar]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, Y.; Chen, T.; Zhu, C. An E7-retinoblastoma protein pathway mechanism may account for the higher carcinogenic ability of HPV16 over HPV58 in cervical cancer. Transl. Cancer Res. 2024, 13, 1876–1886. [Google Scholar] [CrossRef]
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Bastian, S.; Sponagel, J.; Kuryshev, V.; Sultmann, H.; Scheffner, M.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015, 11, e1004712. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Kolesnik, M.; Stepien, E.; Polz-Dacewicz, M. The role of microRNA (miRNA) as a biomarker in HPV and EBV-related cancers. J. Pre-Clin. Clin. Res. 2021, 15, 104–110. [Google Scholar] [CrossRef]
- Elmusrati, A.; Wang, J.; Wang, C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- De Nola, R.; Loizzi, V.; Cicinelli, E.; Cormio, G. Dynamic crosstalk within the tumor microenvironment of uterine cervical carcinoma: Baseline network, iatrogenic alterations, and translational implications. Crit. Rev. Oncol. Hematol. 2021, 162, 103343. [Google Scholar] [CrossRef]
- Piwocka, O.; Piotrowski, I.; Suchorska, W.M.; Kulcenty, K. Dynamic interactions in the tumor niche: How the cross-talk between CAFs and the tumor microenvironment impacts resistance to therapy. Front. Mol. Biosci. 2024, 11, 1343523. [Google Scholar] [CrossRef] [PubMed]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017, 231, 21–33. [Google Scholar] [CrossRef]
- Evans, A.M.; Salnikov, M.; Gameiro, S.F.; Maleki Vareki, S.; Mymryk, J.S. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. J. Clin. Med. 2022, 11, 4825. [Google Scholar] [CrossRef] [PubMed]
- Shiri Aghbash, P.; Rasizadeh, R.; Sadri Nahand, J.; Bannazadeh Baghi, H. The role of immune cells and inflammasomes in Modulating cytokine responses in HPV-Related cervical cancer. Int. Immunopharmacol. 2025, 145, 113625. [Google Scholar] [CrossRef]
- Mehanna, H.; Evans, M.; Beasley, M.; Chatterjee, S.; Dilkes, M.; Homer, J.; O’Hara, J.; Robinson, M.; Shaw, R.; Sloan, P. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S90–S96. [Google Scholar] [CrossRef]
- Blanco, R.G.; Fakhry, C.; Ha, P.K.; Ryniak, K.; Messing, B.; Califano, J.A.; Saunders, J.R. Transoral robotic surgery experience in 44 cases. J. Laparoendosc. Adv. Surg. Tech. Part A 2013, 23, 900–907. [Google Scholar] [CrossRef]
- Pipkorn, P.; Sinha, P.; Kallogjeri, D.; Adkins, D.; Thorstad, W.T.; Rich, J.T.; Jackson, R.S. Outcomes of relapsed human papillomavirus-related oropharyngeal squamous cell carcinoma treated with curative intent. Head Neck 2019, 41, 1312–1319. [Google Scholar] [CrossRef]
- Takes, R.P.; Strojan, P.; Silver, C.E.; Bradley, P.J.; Haigentz, M., Jr.; Wolf, G.T.; Shaha, A.R.; Hartl, D.M.; Olofsson, J.; Langendijk, J.A. Current trends in initial management of hypopharyngeal cancer: The declining use of open surgery. Head neck 2012, 34, 270–281. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, R.; Yoshida, K.; Miyawaki, D.; Okamoto, Y.; Kiyota, N.; Saito, M.; Otsuki, N.; Nibu, K. Radiotherapy for stage I or II hypopharyngeal carcinoma. J. Radiat. Res. 2012, 53, 892–899. [Google Scholar] [CrossRef]
- Dohopolski, M.J.; Diao, K.; Hutcheson, K.A.; Akhave, N.S.; Goepfert, R.P.; He, W.; Lei, X.J.; Peterson, S.K.; Shen, Y.; Sumer, B.D.; et al. Long-term Patient-Reported Outcomes in a Population-Based Cohort Following Radiotherapy vs Surgery for Oropharyngeal Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.F.; Griffiths, R. Did the addition of concomitant chemotherapy to radiotherapy improve outcomes in hypopharyngeal cancer? A population-based study. Curr. Oncol. 2016, 23, 266–272. [Google Scholar] [CrossRef]
- Ravi, P.; Babu, S. Emerging immune checkpoint inhibitors for the treatment of oropharyngeal squamous cell carcinoma. Oral Oncol. Rep. 2024, 12, 100650. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Zhu, Z.; McGray, A.J.R.; Jiang, W.; Lu, B.; Kalinski, P.; Guo, Z.S. Improving cancer immunotherapy by rationally combining oncolytic virus with modulators targeting key signaling pathways. Mol. Cancer 2022, 21, 196. [Google Scholar] [CrossRef]
- Huang, Y.; Lan, Y.; Zhang, Z.; Xiao, X.; Huang, T. An Update on the Immunotherapy for Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 800315. [Google Scholar] [CrossRef]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef]
- Harrington, K.J.; Burtness, B.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Brana, I.; Baste, N.; Neupane, P.; et al. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. 2023, 41, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M. The role of immune checkpoint inhibitors in cancer therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Dostarlimab (Jemperli): CADTH Reimbursement Recommendation: Indication: Dostarlimab in Combination with Carboplatin and Paclitaxel for the Treatment of Adult Patients with Primary Advanced or Recurrent Mismatch Repair Deficient (dMMR)/Microsatellite Instability-High (MSI-H) Endometrial Cancer Who Are Candidates for Systemic Therapy; CADTH Reimbursement Reviews and Recommendations: Ottawa, ON, Canada, 2024.
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Yadav, R.; Mathur, I.; Haokip, H.R.; Pandey, A.K.; Kumar, V.; Jain, N. Dostarlimab: Review on success story and clinical trials. Crit. Rev. Oncol. Hematol. 2024, 198, 104374. [Google Scholar] [CrossRef]
- Rendon, A.; Rayi, A. Nivolumab. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Raedler, L.A. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 96–100. [Google Scholar]
- Flynn, J.P.; Gerriets, V. Pembrolizumab; StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Taberna, M.; Oliva, M.; Mesia, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 383. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Samuels, M.; Falkenius, J.; Bar-Ad, V.; Dunst, J.; van Triest, B.; Yachnin, J.; Rodriguez-Gutierrez, A.; Kuipers, M.; You, X.; Sarholz, B.; et al. A Phase 1 Study of the DNA-PK Inhibitor Peposertib in Combination With Radiation Therapy With or Without Cisplatin in Patients With Advanced Head and Neck Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 743–756. [Google Scholar] [CrossRef]
- Xi, M.; Zhu, J.; Zhang, F.; Shen, H.; Chen, J.; Xiao, Z.; Huangfu, Y.; Wu, C.; Sun, H.; Xia, G. Antibody-drug conjugates for targeted cancer therapy: Recent advances in potential payloads. Eur. J. Med. Chem. 2024, 276, 116709. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Luan, S.; Zheng, M.; Wang, Z.; Chen, Y.; Chen, X.; Tong, A.; Yang, H. Novel bispecific antibody-drug conjugate targeting PD-L1 and B7-H3 enhances antitumor efficacy and promotes immune-mediated antitumor responses. J. Immunother. Cancer 2024, 12, e009710. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shen, S.; Gong, C.; Wang, X.; Luo, F.; Luo, F.; Lei, Y.; Wang, Z.; Xu, S.; Ni, Q.; et al. Bispecific Antibody PD-L1 x CD3 Boosts the Anti-Tumor Potency of the Expanded Vgamma2Vdelta2 T Cells. Front. Immunol. 2021, 12, 654080. [Google Scholar] [CrossRef]
- Lancman, G.; Richter, J.; Chari, A. Bispecifics, trispecifics, and other novel immune treatments in myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 264–271. [Google Scholar] [CrossRef]
- Miyauchi, S.; Sanders, P.D.; Guram, K.; Kim, S.S.; Paolini, F.; Venuti, A.; Cohen, E.E.W.; Gutkind, J.S.; Califano, J.A.; Sharabi, A.B. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. 2020, 80, 732–746. [Google Scholar] [CrossRef]
- Park, R.; Chung, C.H. Advanced Human Papillomavirus-Negative Head and Neck Squamous Cell Carcinoma: Unmet Need and Emerging Therapies. Mol. Cancer Ther. 2024, 23, 1717–1730. [Google Scholar] [CrossRef]
- Saba, N.F.; Pamulapati, S.; Patel, B.; Mody, M.; Strojan, P.; Takes, R.; Makitie, A.A.; Cohen, O.; Pace-Asciak, P.; Vermorken, J.B.; et al. Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer. Cancers 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Meci, A.; Goyal, N.; Slonimsky, G. Mechanisms of Resistance and Therapeutic Perspectives in Immunotherapy for Advanced Head and Neck Cancers. Cancers 2024, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Babu, S.; Kumar, M.V.; Subbarayan, R. Emergence of Advanced Immunotherapy: New Horizons for HPV-Negative Head and Neck Squamous Cell Carcinoma. Oral Oncol. Rep. 2024, 12, 100670. [Google Scholar] [CrossRef]
- Picon, H.; Guddati, A.K. Mechanisms of resistance in head and neck cancer. Am. J. Cancer Res. 2020, 10, 2742–2751. [Google Scholar]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Tumor associated macrophage in HPV(+) tumors: Between immunosuppression and inflammation. Semin. Immunol. 2023, 65, 101671. [Google Scholar] [CrossRef]
- Lin, Z.; Li, G.; Jiang, K.; Li, Z.; Liu, T. Cancer therapy resistance mediated by cancer-associated fibroblast-derived extracellular vesicles: Biological mechanisms to clinical significance and implications. Mol. Cancer 2024, 23, 191. [Google Scholar] [CrossRef]
- Li, X.; Gonzalez-Maroto, C.; Tavassoli, M. Crosstalk between CAFs and tumour cells in head and neck cancer. Cell Death Discov. 2024, 10, 303. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Wu, C.; Huang, Q. Heterogeneity of cancer-associated fibroblasts in head and neck squamous cell carcinoma: Opportunities and challenges. Cell Death Discov. 2023, 9, 124. [Google Scholar] [CrossRef]
- Raudenska, M.; Balvan, J.; Hanelova, K.; Bugajova, M.; Masarik, M. Cancer-associated fibroblasts: Mediators of head and neck tumor microenvironment remodeling. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188940. [Google Scholar] [CrossRef]
- Adhim, Z.; Otsuki, N.; Kitamoto, J.; Morishita, N.; Kawabata, M.; Shirakawa, T.; Nibu, K. Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention against head and neck cancer-containing HPV16 cell lines. Acta Otolaryngol. 2013, 133, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Abboodi, F.; Buckhaults, P.; Altomare, D.; Liu, C.; Hosseinipour, M.; Banister, C.E.; Creek, K.E.; Pirisi, L. HPV-inactive cell populations arise from HPV16-transformed human keratinocytes after p53 knockout. Virology 2021, 554, 9–16. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef] [PubMed]
- Ladwa, R.; Chandra, J.; Woo, W.-P.; Finlayson, N.; Liu, H.; McGrath, M.; See, A.; Hughes, B.G.; Cooper, C.L.; Jackson, J.E. A phase Ib study to assess the safety of the human papillomavirus DNA vaccine (AMV002) in combination with durvalumab for HPV-associated oropharyngeal squamous cell carcinoma. Front. Oncol. 2024, 14, 1419258. [Google Scholar] [CrossRef]
- Jhawar, S.R.; Wang, S.J.; Thandoni, A.; Bommareddy, P.K.; Newman, J.H.; Marzo, A.L.; Kuzel, T.M.; Gupta, V.; Reiser, J.; Daniels, P.; et al. Combination oncolytic virus, radiation therapy, and immune checkpoint inhibitor treatment in anti-PD-1-refractory cancer. J. Immunother. Cancer 2023, 11, e006780. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, M.; Yang, C.; Wei, W.; He, X.; Cheng, G.; Wang, S. Combination therapy with oncolytic viruses and immune checkpoint inhibitors in head and neck squamous cell carcinomas: An approach of complementary advantages. Cancer Cell Int. 2023, 23, 1. [Google Scholar] [CrossRef]
- Nenclares, P.; Rullan, A.; Tam, K.; Dunn, L.A.; St John, M.; Harrington, K.J. Introducing Checkpoint Inhibitors Into the Curative Setting of Head and Neck Cancers: Lessons Learned, Future Considerations. In American Society of Clinical Oncology Educational Book. Annual Meeting; American Society of Clinical Oncology: Alexandria, VA, USA, 2022; pp. 1–16. [Google Scholar]
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Raty, J.K.; Yla-Herttuala, S. Challenges in monoclonal antibody-based therapies. Ann. Med. 2009, 41, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Mitchell, A.P.; Kesselheim, A.S.; Rome, B.N. Trends in prices of checkpoint inhibitors in the US, 2016–2023. J. Clin. Oncol. 2024, 42, 16. [Google Scholar] [CrossRef]
- Sifniotis, V.; Cruz, E.; Eroglu, B.; Kayser, V. Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies 2019, 8, 36. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Huang, S.; Kruser, T.J.; Nechrebecki, M.M.; Armstrong, E.A.; Benavente, S.; Gondi, V.; Hsu, K.T.; Harari, P.M. Mechanisms of acquired resistance to cetuximab: Role of HER (ErbB) family members. Oncogene 2008, 27, 3944–3956. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Markus, H.S.; Leslie, R.D.; Topol, E.J. Personalized medicine: Risk prediction, targeted therapies and mobile health technology. BMC Med. 2014, 12, 37. [Google Scholar] [CrossRef]
- Kothari, M.; Wanjari, A.; Acharya, S.; Karwa, V.; Chavhan, R.; Kumar, S.; Kadu, A.; Patil, R. A Comprehensive Review of Monoclonal Antibodies in Modern Medicine: Tracing the Evolution of a Revolutionary Therapeutic Approach. Cureus 2024, 16, e61983. [Google Scholar] [CrossRef]
- Olateju, O.A.; Zeng, Z.; Thornton, J.D.; Mgbere, O.; Essien, E.J. Management of metastatic melanoma in Texas: Disparities in the utilization of immunotherapy following the regulatory approval of immune checkpoint inhibitors. BMC Cancer 2023, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Omeogu, C.; Islam, J.Y.; Joshi, A.; Zhang, D.; Braithwaite, D.; Karanth, S.D.; Tailor, T.D.; Clarke, J.M.; Akinyemiju, T. Socioeconomic disparities in immunotherapy use among advanced-stage non-small cell lung cancer patients: Analysis of the National Cancer Database. Sci. Rep. 2023, 13, 8190. [Google Scholar] [CrossRef]
- Gnjatic, S.; Bronte, V.; Brunet, L.R.; Butler, M.O.; Disis, M.L.; Galon, J.; Hakansson, L.G.; Hanks, B.A.; Karanikas, V.; Khleif, S.N.; et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J. Immunother. Cancer 2017, 5, 44. [Google Scholar] [CrossRef]
- Pharaon, R.R.; Xing, Y.; Agulnik, M.; Villaflor, V.M. The Role of Immunotherapy to Overcome Resistance in Viral-Associated Head and Neck Cancer. Front. Oncol. 2021, 11, 649963. [Google Scholar] [CrossRef] [PubMed]
- Goli, V.A.R.; Butreddy, A. Biosimilar monoclonal antibodies: Challenges and approaches towards formulation. Chem. Biol. Interact. 2022, 366, 110116. [Google Scholar] [CrossRef]
- Galvao, T.F.; Livinalli, A.; Lopes, L.C.; Zimmermann, I.R.; Silva, M.T. Biosimilar monoclonal antibodies for cancer treatment in adults. Cochrane Database Syst. Rev. 2024, 11, CD013539. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Boersma, P.; Black, L.I. Human Papillomavirus Vaccination Among Adults Aged 18–26, 2013–2018. NCHS Data Brief. 2020, 354, 1–8. Available online: https://pubmed.ncbi.nlm.nih.gov/32487295/ (accessed on 22 March 2025).
- Liang, C.; Marsit, C.J.; McClean, M.D.; Nelson, H.H.; Christensen, B.C.; Haddad, R.I.; Clark, J.R.; Wein, R.O.; Grillone, G.A.; Houseman, E.A.; et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012, 72, 5004–5013. [Google Scholar] [CrossRef]
- Krsek, A.; Baticic, L.; Sotosek, V.; Braut, T. The Role of Biomarkers in HPV-Positive Head and Neck Squamous Cell Carcinoma: Towards Precision Medicine. Diagnostics 2024, 14, 1448. [Google Scholar] [CrossRef]
- Ramesh, P.S.; Devegowda, D.; Singh, A.; Thimmulappa, R.K. NRF2, p53, and p16: Predictive biomarkers to stratify human papillomavirus associated head and neck cancer patients for de-escalation of cancer therapy. Crit. Rev. Oncol. Hematol. 2020, 148, 102885. [Google Scholar] [CrossRef]
- Miniuk, M.; Reszec-Gielazyn, J.; Bortnik, P.; Borsukiewicz, A.; Mroczek, A. Novel Predictive Biomarkers in the Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Med. 2024, 13, 5876. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Qiu, S.; Wang, R. Therapeutic strategies of different HPV status in Head and Neck Squamous Cell Carcinoma. Int. J. Biol. Sci. 2021, 17, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Zaidi, M.; Fakhry, C.; Gaykalova, D.A. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017, 19, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wuerdemann, N.; Gultekin, S.E.; Putz, K.; Wittekindt, C.; Huebbers, C.U.; Sharma, S.J.; Eckel, H.; Schubotz, A.B.; Gattenlohner, S.; Buttner, R.; et al. PD-L1 Expression and a High Tumor Infiltrate of CD8+ Lymphocytes Predict Outcome in Patients with Oropharyngeal Squamous Cells Carcinoma. Int. J. Mol. Sci. 2020, 21, 5228. [Google Scholar] [CrossRef]
- Sanchez-Canteli, M.; Granda-Diaz, R.; Del Rio-Ibisate, N.; Allonca, E.; Lopez-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; Garcia-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.M.; Medeiros, F.; Azimpouran, M.; Venturina, M.; Balzer, B. PD-L1 Expression in HPV-associated Versus HPV-independent Invasive Vulvar Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2024, 43, 405–413. [Google Scholar] [CrossRef]
- Hongo, T.; Yamamoto, H.; Jiromaru, R.; Yasumatsu, R.; Kuga, R.; Nozaki, Y.; Hashimoto, K.; Matsuo, M.; Wakasaki, T.; Tamae, A.; et al. PD-L1 expression, tumor-infiltrating lymphocytes, mismatch repair deficiency, EGFR alteration and HPV infection in sinonasal squamous cell carcinoma. Mod. Pathol. 2021, 34, 1966–1978. [Google Scholar] [CrossRef]
- Cui, J.W.; Li, Y.; Yang, Y.; Yang, H.K.; Dong, J.M.; Xiao, Z.H.; He, X.; Guo, J.H.; Wang, R.Q.; Dai, B.; et al. Tumor immunotherapy resistance: Revealing the mechanism of PD-1/PD-L1-mediated tumor immune escape. Biomed. Pharmacother. 2024, 171, 116203. [Google Scholar] [CrossRef]
- Sakatani, T.; Kita, Y.; Fujimoto, M.; Sano, T.; Hamada, A.; Nakamura, K.; Takada, H.; Goto, T.; Sawada, A.; Akamatsu, S.; et al. IFN-Gamma Expression in the Tumor Microenvironment and CD8-Positive Tumor-Infiltrating Lymphocytes as Prognostic Markers in Urothelial Cancer Patients Receiving Pembrolizumab. Cancers 2022, 14, 263. [Google Scholar] [CrossRef]
- Baras, A.S.; Drake, C.; Liu, J.J.; Gandhi, N.; Kates, M.; Hoque, M.O.; Meeker, A.; Hahn, N.; Taube, J.M.; Schoenberg, M.P.; et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016, 5, e1134412. [Google Scholar] [CrossRef]

| mAb | Overall Response Rate | Progression-Free Survival | Overall Survival |
|---|---|---|---|
| Pembrolizumab | 35.6% combination vs. 36.3% standard care | No difference | 12.3 months vs. 10.3 months |
| Nivolumab | 13.3% in the nivolumab group vs. 5.8% in the standard therapy group | 19.7% vs. 9.9% at 6 months | 7.5 months vs. 5.1 months |
| Cetuximab | 22.9% in cetuximab + Irinotecan vs. 10.8% with monotherapy | 66% vs. 58% at 6 months | 8.6 months vs. 6.9 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalin, M.; Patel, S.; Coggins, C.; Rai, V. The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review. Antibodies 2025, 14, 37. https://doi.org/10.3390/antib14020037
Zalin M, Patel S, Coggins C, Rai V. The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review. Antibodies. 2025; 14(2):37. https://doi.org/10.3390/antib14020037
Chicago/Turabian StyleZalin, Michael, Shaan Patel, Carter Coggins, and Vikrant Rai. 2025. "The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review" Antibodies 14, no. 2: 37. https://doi.org/10.3390/antib14020037
APA StyleZalin, M., Patel, S., Coggins, C., & Rai, V. (2025). The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review. Antibodies, 14(2), 37. https://doi.org/10.3390/antib14020037








