Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy?
Abstract
1. Introduction
2. The Effect of Pregnancy and the Burden of Viral Respiratory Infections
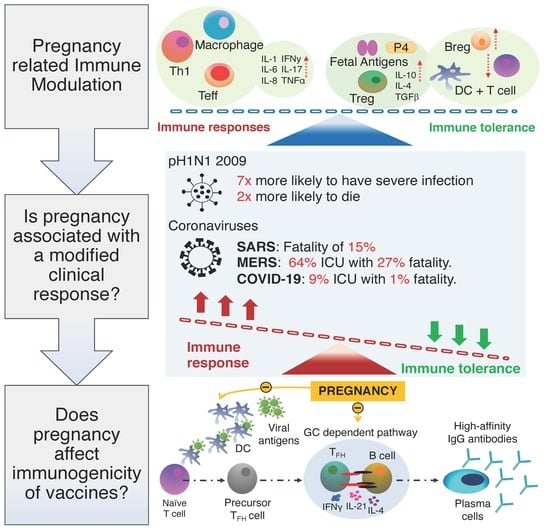
2.1. Pregnancy Related Immune Modulation
2.2. Is Pregnancy Associated with a Modified Clinical Response to Respiratory Virus
2.3. ACE-R and Pregnancy
3. What Is the Current Knowledge on Antibody Producing B Cells and Where is More Research Required?
4. Benefits of Maternal Immunisation and the Immunogenicity of Vaccines in Pregnancy?
4.1. Routinely Administered Vaccines in Pregnancy
4.1.1. Tetanus, Diphtheria, and Acellular Pertussis (Tdap)
4.1.2. Influenza Vaccine
4.2. Vaccines Available during Pregnancy if Medically Necessary
4.3. Vaccines Avoided during Pregnancy
4.4. Maternal Vaccines being Developed
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knight, M.; Kenyon, S.; Brocklehurst, P.; Neilson, J.; Shakespeare, J.; Lives, K.J.S. (Eds.) Improving Mothers’ Care-Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012; National Perinatal Epidemiology Unit, University of Oxford: Oxford, UK, 2014. [Google Scholar]
- Beigi, R.H.; Venkataramanan, R.; Caritis, S.N. Oseltamivir for influenza in pregnancy. Semin. Perinatol. 2014, 38, 503–507. [Google Scholar] [CrossRef]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Di Pietrantonj, C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018, 2018, CD001269. [Google Scholar] [CrossRef]
- Shah, N.M.; Imami, N.; Kelleher, P.; Barclay, W.S.; Johnson, M.R. Pregnancy-related immune suppression leads to altered influenza vaccine recall responses. Clin. Immunol. 2019, 208, 108254. [Google Scholar] [CrossRef]
- Naidu, M.A.; Muljadi, R.; Davies-Tuck, M.; Wallace, E.M.; Giles, M.L. The optimal gestation for pertussis vaccination during pregnancy: A prospective cohort study. Am. J. Obstet. Gynecol. 2016, 215, 237.e1–237.e6. [Google Scholar] [CrossRef]
- Rice, T.F.; Diavatopoulos, D.A.; Smits, G.P.; Van Gageldonk, P.G.M.; Berbers, G.A.M.; Van Der Klis, F.R.; Vamvakas, G.; Donaldson, B.; Bouqueau, M.; Holder, B.; et al. Antibody responses to Bordetella pertussis and other childhood vaccines in infants born to mothers who received pertussis vaccine in pregnancy-a prospective, observational cohort study from the United Kingdom. Clin. Exp. Immunol. 2019, 197, 1–10. [Google Scholar] [CrossRef]
- Baxter, R.; Bartlett, J.; Fireman, B.; Lewis, E.; Klein, N.P. Effectiveness of Vaccination During Pregnancy to Prevent Infant Pertussis. Pediatrics 2017, 139, e20164091. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Respiratory physiology of pregnancy. Breathe 2015, 11, 297–301. [Google Scholar] [CrossRef]
- Zollner, J.; Howe, L.G.; Edey, L.F.; O’Dea, K.P.; Takata, M.; Gordon, F.; Leiper, J.; Johnson, M. The response of the innate immune and cardiovascular systems to LPS in pregnant and nonpregnant mice†. Biol. Reprod. 2017, 97, 258–272. [Google Scholar] [CrossRef]
- Cerbulo-Vázquez, A.; Figueroa-Damian, R.; Arriaga-Pizano, L.; Hernandez-Andrade, E.; Mancilla-Herrera, I.; Mejia, L.A.F.; Arteaga-Troncoso, G.; Lopez-Macias, C.I.R.; Isibasi, A.; Mancilla-Ramírez, J. Pregnant Women Infected with Pandemic H1N1pdm2009 Influenza Virus Displayed Overproduction of Peripheral Blood CD69+ Lymphocytes and Increased Levels of Serum Cytokines. PLoS ONE 2014, 9, e107900. [Google Scholar] [CrossRef]
- Court, O.; Kumar, A.; Parrillo, J.E. Clinical review: Myocardial depression in sepsis and septic shock. Crit. Care 2002, 6, 500. [Google Scholar] [CrossRef] [PubMed]
- Razazi, K.; Boissier, F.; Surenaud, M.; Bedet, A.; Seemann, A.; Carteaux, G.; De Prost, N.; Brun-Buisson, C.; Hue, S.; Dessap, A.M. A multiplex analysis of sepsis mediators during human septic shock: A preliminary study on myocardial depression and organ failures. Ann. Intensiv. Care 2019, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Klouche, K.; Pommet, S.; Amigues, L.; Bargnoux, A.S.; Dupuy, A.M.; Machado, S.; Serveaux-Delous, M.; Morena, M.; Jonquet, O.; Cristol, J.P. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: Relationships with systolic myocardial dysfunction and intensive care unit mortality. J. Intensive Care Med. 2014, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Nasu, M. A review of sepsis-induced cardiomyopathy. J. Intensiv. Care 2015, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.P.; Mccord, J.; Otero, R.; Jacobsen, G.; Loomba, M. Clinical Utility of B-Type Natriuretic Peptide in Early Severe Sepsis and Septic Shock. J. Intensiv. Care Med. 2007, 22, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Bonney, E.A. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet. Gynecol. Clin. N. Am. 2016, 43, 679–698. [Google Scholar] [CrossRef]
- Vanders, R.L.; Murphy, V.E.; Gibson, P.; Hansbro, P.M.; Wark, P.A. CD8 T cells and dendritic cells: Key players in the attenuated maternal immune response to influenza infection. J. Reprod. Immunol. 2015, 107, 1–9. [Google Scholar] [CrossRef]
- Gluhovschi, C.; Gluhovschi, G.; Petrica, L.; Velciov, S.; Gluhovschi, A. Pregnancy Associated with Systemic Lupus Erythematosus: Immune Tolerance in Pregnancy and Its Deficiency in Systemic Lupus Erythematosus—An Immunological Dilemma. J. Immunol. Res. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. REVIEW ARTICLE: Inflammation and Implantation. Am. J. Reprod. Immunol. 2009, 63, 17–21. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Krishnan, L.; Guilbert, L.J.; Russell, A.S.; Wegmann, T.G.; Mosmann, T.R.; Belosevic, M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J. Immunol. 1996, 156, 644–652. [Google Scholar]
- Piccinni, M.-P. T cells in normal pregnancy and recurrent pregnancy loss. Reprod. Biomed. Online 2006, 13, 840–844. [Google Scholar] [CrossRef]
- Kwiatek, M.; Gęca, T.; Krzyzanowski, A.; Malec, A.; Kwaśniewska, A. Peripheral Dendritic Cells and CD4+CD25+Foxp3+ Regulatory T Cells in the First Trimester of Normal Pregnancy and in Women with Recurrent Miscarriage. PLoS ONE 2015, 10, e0124747. [Google Scholar] [CrossRef]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004, 10, 347–353. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Sun, G.-Q.; Hu, X.-H.; Kwak-Kim, J.; Liao, A.-H. The transdifferentiation of regulatory T and Th17 cells in autoimmune/inflammatory diseases and its potential implications in pregnancy complications. Am. J. Reprod. Immunol. 2017, 78, e12657. [Google Scholar] [CrossRef]
- Schober, L.; Radnai, D.; Schmitt, E.; Mahnke, K.; Sohn, C.; Steinborn, A. Term and preterm labor: Decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol. Cell Boil. 2012, 90, 935–944. [Google Scholar] [CrossRef]
- Shah, N.M.; Edey, L.F.; Imami, N.; Johnson, M.R. Human labour is associated with altered regulatory T cell function and maternal immune activation. Clin. Exp. Immunol. 2019, 199, 182–200. [Google Scholar] [CrossRef]
- Della Bella, S.; Giannelli, S.; Cozzi, V.; Signorelli, V.; Cappelletti, M.; Cetin, I.; Villa, M.L. Incomplete activation of peripheral blood dendritic cells during healthy human pregnancy. Clin. Exp. Immunol. 2011, 164, 180–192. [Google Scholar] [CrossRef]
- Vanders, R.L.; Gibson, P.G.; Murphy, V.E.; Wark, P.A.B. Plasmacytoid Dendritic Cells and CD8 T Cells from Pregnant Women Show Altered Phenotype and Function Following H1N1/09 Infection. J. Infect. Dis. 2013, 208, 1062–1070. [Google Scholar] [CrossRef]
- Engels, G.; Hierweger, A.M.; Hoffmann, J.; Thieme, R.; Thiele, S.; Bertram, S.; Dreier, C.; Infante, P.R.; Jacobsen, H.; Thiele, K.; et al. Pregnancy-Related Immune Adaptation Promotes the Emergence of Highly Virulent H1N1 Influenza Virus Strains in Allogenically Pregnant Mice. Cell Host Microbe 2017, 21, 321–333. [Google Scholar] [CrossRef]
- Gounder, A.P.; Boon, A.C.M. Influenza Pathogenesis: The Effect of Host Factors on Severity of Disease. J. Immunol. 2019, 202, 341–350. [Google Scholar] [CrossRef]
- Tesfaye, D.Y.; Gudjonsson, A.; Bogen, B.; Fossum, E. Targeting Conventional Dendritic Cells to Fine-Tune Antibody Responses. Front. Immunol. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Weinberg, E.D. Pregnancy-associated immune suppression: Risks and mechanisms. Microb. Pathog. 1987, 3, 393–397. [Google Scholar] [CrossRef]
- Tan, I.J.; Peeva, E.; Zandman-Goddard, G. Hormonal modulation of the immune system—A spotlight on the role of progestogens. Autoimmun. Rev. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Polikarpova, A.; Levina, I.; Sigai, N.; Zavarzin, I.; Morozov, I.; Rubtsov, P.; Guseva, A.; Smirnova, O.; Shchelkunova, T. Immunomodulatory effects of progesterone and selective ligands of membrane progesterone receptors. Steroids 2019, 145, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Lissauer, D.; Eldershaw, S.A.; Inman, C.; Coomarasamy, A.; Moss, P.A.H.; Kilby, M.D. Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 2015, 45, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ulrich, B.; Cho, J.; Park, J.; Kim, C.H. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J. Immunol. 2011, 187, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zang, S.; Bai, Y.; Yao, X.; Zhang, L. Effect of early pregnancy on the expression of progesterone receptor and progesterone-induced blocking factor in ovine lymph node. Theriogenology 2017, 93, 78–83. [Google Scholar] [CrossRef]
- Arck, P.; Hansen, P.J.; Jericevic, B.M.; Piccinni, M.-P.; Szekeres-Bartho, J. Progesterone During Pregnancy: Endocrine? Immune Cross Talk in Mammalian Species and the Role of Stress. Am. J. Reprod. Immunol. 2007, 58, 268–279. [Google Scholar] [CrossRef]
- Yudin, M.H. Risk management of seasonal influenza during pregnancy: Current perspectives. Int. J. Women’s Heal. 2014, 6, 681–689. [Google Scholar] [CrossRef]
- Honigsbaum, M. Revisiting the 1957 and 1968 influenza pandemics. Lancet 2020, 395, 1824–1826. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Bresee, J.S. Pandemic Influenza and Pregnant Women. Emerg. Infect. Dis. 2008, 14, 95–100. [Google Scholar] [CrossRef]
- Elliott, E. Pregnancy and Pandemic Flu. Clin. Infect. Dis. 2010, 50, 691–692. [Google Scholar] [CrossRef]
- Freeman, D.W.; Barno, A. Deaths from Asian Influenza Associated with Pregnancy. Am. J. Obstet. Gynecol. 1959, 78, 1172–1175. [Google Scholar] [CrossRef]
- Buchy, P.; Badur, S.; Kassianos, G.; Preiss, S.; Tam, J.S. Vaccinating pregnant women against influenza needs to be a priority for all countries: An expert commentary. Int. J. Infect. Dis. 2020, 92, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gould, V.M.W.; Francis, J.N.; Anderson, K.J.; Georges, B.; Cope, A.V.; Tregoning, J.S. Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Plaat, F.; Arrandale, L. Hypoxia in Pregnancy. Fetal Matern. Med. Rev. 2012, 23, 71–96. [Google Scholar] [CrossRef]
- Bhatia, P.; Bhatia, K. Pregnancy and the lungs. Postgrad. Med. J. 2000, 76, 683–689. [Google Scholar] [CrossRef]
- Joseph, J.; Sinha, A.; Paech, M.; Walters, B.N.J. Sepsis in pregnancy and early goal-directed therapy. Obstet. Med. 2009, 2, 93–99. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Catherine, N.-P.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Blackburn, S.T. Maternal, Fetal, & Neonatal Physiology; Elsevier Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Mullins, E.; Evans, D.; Viner, R.M.; O’Brien, P.; Morris, E. Coronavirus in pregnancy and delivery: Rapid review. Ultrasound Obstet. Gynecol. 2020, 55, 586–592. [Google Scholar] [CrossRef]
- Stawiski, E.W.; Diwanji, D.; Suryamohan, K.; Gupta, R.; Fellouse, F.A.; Sathirapongsasuti, F.; Liu, J.; Jiang, Y.-P.; Ratan, A.; Mis, M.; et al. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. BioRxiv 2020. [Google Scholar] [CrossRef]
- Buekens, P.; Alger, J.; Bréart, G.; Cafferata, M.L.; Harville, E.; Tomasso, G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob. Heal. 2020, 8, e877–e878. [Google Scholar] [CrossRef]
- (RCM) RCoOaGRaRCoM. Guidance for Healthcare Professionals on Coronavirus (COVID-19) Infection in Pregnancy; RCOG, Royal College of Midwives, Royal College of Paediatrics and Child Health, Public Health England and Public Health Scotland: London, UK, 2020. [Google Scholar]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.T.; Navis, G.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wooster, L.; Nicholson, C.J.; Sigurslid, H.H.; Cardenas, C.L.L.; Malhotra, R. Polymorphisms in the ACE2 Locus Associate with Severity of COVID-19 Infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Zhao, Y.; Xi, H.; Liu, C.; Qu, F.; Feng, X. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1209–1220. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45–47. [Google Scholar] [CrossRef]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529. [Google Scholar] [CrossRef]
- Li, G.; He, X.; Zhang, L.; Ran, Q.; Wang, J.; Xiong, A.; Wu, D.; Chen, F.; Sun, J.; Chang, C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020, 2020, 102463. [Google Scholar] [CrossRef]
- Bhattacharya, M. Understanding B Lymphocyte Development: A Long Way to Go; IntechOpen: London, UK, 2019. [Google Scholar]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Wen, B.; Xie, T.; Tang, P.; Hu, Y.; Huang, L.; Jin, K.; Zhang, P.; Liu, Z.; et al. Circulating CXCR3+ Tfh cells positively correlate with neutralizing antibody responses in HCV-infected patients. Sci. Rep. 2019, 9, 10090. [Google Scholar] [CrossRef]
- Bentebibel, S.-E.; Khurana, S.; Schmitt, N.; Kurup, P.; Mueller, C.; Obermoser, G.; Palucka, A.K.; Albrecht, R.; García-Sastre, A.; Golding, H.; et al. ICOS+PD-1+CXCR3+ T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci. Rep. 2016, 6, 26494. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Adelsberger, J.W.; Kemp, T.J.; Baseler, M.W.; Ledgerwood, J.E.; Pinto, L.A. Circulating CXCR5+CD4+ T Follicular-Like Helper Cell and Memory B Cell Responses to Human Papillomavirus Vaccines. PLoS ONE 2015, 10, e0137195. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Davydov, A.N.; Ladell, K.; McLaren, J.E.; Bonaiuti, P.; Metsger, M.; Ramsden, J.D.; Gilbert, S.C.; Lambe, T.; Price, D.A.; et al. CD4+ T Follicular Helper Cells in Human Tonsils and Blood Are Clonally Convergent but Divergent from Non-Tfh CD4+ Cells. Cell Rep. 2020, 30, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Herati, R.S.; Reuter, M.A.; Dolfi, D.V.; Mansfield, K.D.; Aung, H.; Badwan, O.Z.; Kurupati, R.K.; Kannan, S.; Ertl, H.; Schmader, K.E.; et al. Circulating CXCR5+PD-1+ Response Predicts Influenza Vaccine Antibody Responses in Young Adults but not Elderly Adults. J. Immunol. 2014, 193, 3528–3537. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K. The circulating life of a memory T-follicular helper cell. Clin. Transl. Immunol. 2017, 6, e141. [Google Scholar] [CrossRef]
- Ueno, H.; Banchereau, J.; Vinuesa, C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 2015, 16, 142–152. [Google Scholar] [CrossRef]
- Schmitt, N.; Bentebibel, S.-E.; Ueno, H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014, 35, 436–442. [Google Scholar] [CrossRef]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.S.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human Circulating PD-1+CXCR3−CXCR5+ Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 2013, 39, 758–769. [Google Scholar] [CrossRef]
- Cárdeno, A.; Magnusson, M.K.; Quiding-Jarbrink, M.; Lundgren, A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Sci. Rep. 2018, 8, 2729. [Google Scholar] [CrossRef]
- Bentebibel, S.-E.; Lopez, S.; Obermoser, G.; Schmitt, N.; Mueller, C.; Harrod, C.; Flano, E.; Mejias, A.; Albrecht, R.; Blankenship, D.; et al. Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination. Sci. Transl. Med. 2013, 5, 176ra32. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Kasahara, T.M.; Castro, J.R.; Sacramento, P.M.; Hygino, J.; Centurião, N.; Cassano, T.; Lopes, L.M.F.; Leite, S.; Silva, V.G.; et al. Pregnancy favors the expansion of circulating functional follicular helper T Cells. J. Reprod. Immunol. 2017, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Palacio, L.P.Q.; Fernández, E.R.; Hernández-Vásquez, Y.; Petray, P.B.; Postan, M. Circulating T Follicular Helper Cell Abnormalities Associated to Different Clinical Forms of Chronic Chagas Disease. Front. Microbiol. 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A. How T follicular helper cells and the germinal centre response change with age. Immunol. Cell Boil. 2013, 92, 72–79. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, R.; Gan, H.; Liang, Z.; Li, F.; Lin, T.; Luo, Y.; Cai, X.; He, F.; Shen, E. The effect of aging on the frequency, phenotype and cytokine production of human blood CD4 + CXCR5 + T follicular helper cells: Comparison of aged and young subjects. Immun. Ageing 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.O.; Zenclussen, A.C.; Jensen, F. The Role of B Cells in Pregnancy: The Good and the Bad. Am. J. Reprod. Immunol. 2013, 69, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Martins, C.; Leandro, M.J.; Nunes, G.; Sousa, M.-J.; Branco, J.C.; Borrego, L.-M. Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: A prospective observational study. BMC Pregnancy Childbirth 2016, 16, 139. [Google Scholar] [CrossRef]
- Guzman-Genuino, R.M.; Diener, K.R. Regulatory B Cells in Pregnancy: Lessons from Autoimmunity, Graft Tolerance, and Cancer. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- Cagigi, A.; Nilsson, A.; Pensieroso, S.; Chiodi, F. Dysfunctional B-cell responses during HIV-1 infection: Implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect. Dis. 2010, 10, 499–503. [Google Scholar] [CrossRef]
- Klopper, A. Pregnancy Proteins and Hormones in the Immune Response of Pregnancy; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1989; pp. 91–113. [Google Scholar]
- Zimmermann, P.; Perrett, K.P.; Messina, N.L.; Donath, S.; Ritz, N.; Van Der Klis, F.R.; Curtis, N. The Effect of Maternal Immunisation During Pregnancy on Infant Vaccine Responses. EClinicalMedicine 2019, 13, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Psarris, A.; Sindos, M.; Daskalakis, G.; Chondrogianni, M.E.; Panayiotou, S.; Antsaklis, P.; Loutradis, D. Immunizations during pregnancy: How, when and why. Eur. J. Obstet. Gynecol. Reprod. Boil. 2019, 240, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.K.; Heine, R.P. Vaccinations for Pregnant Women. Obstet. Gynecol. 2015, 125, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Wang, Y. Recent advances in the production of recombinant subunit vaccines inPichia pastoris. Bioengineered 2016, 7, 155–165. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaître, B.; Boukrid, M.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; De Tejada, B.M.; Siegrist, C.-A. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clin. Infect. Dis. 2016, 62, 829–836. [Google Scholar] [CrossRef]
- Tiwari, T.S.P.; Baughman, A.L.; Clark, T.A. First Pertussis Vaccine Dose and Prevention of Infant Mortality. Pediatrics 2015, 135, 990–999. [Google Scholar] [CrossRef]
- Vojtek, I.; Dieussaert, I.; Doherty, M.; Franck, V.; Hanssens, L.; Miller, J.; Bekkat-Berkani, R.; Kandeil, W.; Prado-Cohrs, D.; Vyse, A. Maternal immunization: Where are we now and how to move forward? Ann. Med. 2018, 50, 193–208. [Google Scholar] [CrossRef]
- Marshall, H.S.; McMillan, M.; Andrews, R.M.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccines Immunother. 2016, 12, 848–856. [Google Scholar] [CrossRef]
- Donegan, K.; King, B.; Bryan, P. Safety of pertussis vaccination in pregnant women in UK: Observational study. BMJ 2014, 349, g4219. [Google Scholar] [CrossRef]
- McMillan, M.; Clarke, M.; Parrella, A.; Fell, D.B.; Amirthalingam, G.; Marshall, H.S. Safety of Tetanus, Diphtheria, and Pertussis Vaccination During Pregnancy. Obstet. Gynecol. 2017, 129, 560–573. [Google Scholar] [CrossRef]
- WHO (World Health Organisation). Influenza Fact Sheets. Available online: https://wwwwhoint/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 28 May 2020).
- Costantino, C.; Vitale, F. Influenza vaccination in high-risk groups: A revision of existing guidelines and rationale for an evidence-based preventive strategy. J. Prev. Med. Hyg. 2016, 57, E13–E18. [Google Scholar] [PubMed]
- Louie, J.K.; Acosta, M.; Jamieson, D.J.; Honein, M.A. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N. Engl. J. Med. 2010, 362, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, Y.M.; Song, B.M.; Kim, H.S.; Seo, S.H. The 2009 Pandemic H1N1 Influenza Virus is More Pathogenic in Pregnant Mice Than Seasonal H1N1 Influenza Virus. Viral Immunol. 2012, 25, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Aldridge, J.R.; Duan, S.; Ghoneim, H.E.; Rehg, J.; Marjuki, H.; Boon, A.C.M.; McCullers, J.A.; Webby, R.J. Fatal Outcome of Pandemic H1N1 2009 Influenza Virus Infection Is Associated with Immunopathology and Impaired Lung Repair, Not Enhanced Viral Burden, in Pregnant Mice. J. Virol. 2011, 85, 11208–11219. [Google Scholar] [CrossRef]
- Kumar, A.; Meldgaard, T.S.; Bertholet, S. Novel Platforms for the Development of a Universal Influenza Vaccine. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Galvão, T.F.; Silva, M.T.; Zimmermann, I.R.; Lopes, L.A.B.; Bernardo, E.F.; Pereira, M.G. Influenza Vaccination in Pregnant Women: A Systematic Review. ISRN Prev. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Meier, S.; Bel, M.; Combescure, C.; Othenin-Girard, V.; Swali, R.A.; de Tejada, B.M.; Siegrist, C.A. Influenza Vaccination Given at Least 2 Weeks Before Delivery to Pregnant Women Facilitates Transmission of Seroprotective Influenza-specific Antibodies to the Newborn. Pediatric Infect. Dis. J. 2013, 32, 1374–1380. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Cox, R.J.; Brokstad, K.A.; Ogra, P. Influenza Virus: Immunity and Vaccination Strategies. Comparison of the Immune Response to Inactivated and Live, Attenuated Influenza Vaccines. Scand. J. Immunol. 2004, 59, 1–15. [Google Scholar] [CrossRef]
- Toback, S.L.; Beigi, R.; Tennis, P.; Sifakis, F.; Calingaert, B.; Ambrose, C.S. Maternal outcomes among pregnant women receiving live attenuated influenza vaccine. Influ. Other Respir. Viruses 2011, 6, 44–51. [Google Scholar] [CrossRef]
- Raj, R.S.; Bonney, E.A.; Phillippe, M. Influenza, Immune System, and Pregnancy. Reprod. Sci. 2014, 21, 1434–1451. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Weiner, J.; Cizmeci, D.; Hake, D.; Maertzdorf, J.; Kaufmann, S.H.E.; Leroux-Roels, G.; Maes, C.; Aerssens, A.; Calvert, A.; et al. Pregnancy has a minimal impact on the acute transcriptional signature to vaccination. npj Vaccines 2020, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.W.; Bayless, N.L.; Fukuyama, J.; Aziz, N.; Dekker, C.L.; Mackey, S.; Swan, G.E.; Davis, M.M.; Blish, C.A. Pregnancy Does Not Attenuate the Antibody or Plasmablast Response to Inactivated Influenza Vaccine. J. Infect. Dis. 2015, 212, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Schmader, K.E.; Kossenkov, A.V.; Doyle, S.; Kurupati, R.; Ertl, H.C.J. Immune response to influenza vaccination in the elderly is altered by chronic medication use. Immun. Ageing 2018, 15, 19. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines and vaccination against yellow fever: WHO Position Paper, June 2013—Recommendations. Vaccine 2015, 33, 76–77. [Google Scholar] [CrossRef]
- Kaaijk, P.; Luytjes, W.; Rots, N.Y. Vaccination against RSV. Hum. Vaccines Immunother. 2013, 9, 1263–1267. [Google Scholar] [CrossRef]
- Muñoz, F. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003, 21, 3465–3467. [Google Scholar] [CrossRef]
- Fortner, K.B.; Nieuwoudt, C.; Reeder, C.F.; Swamy, G.K. Infections in Pregnancy and the Role of Vaccines. Obstet. Gynecol. Clin. North Am. 2018, 45, 369–388. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Eberhardt, C. Review of maternal immunisation during pregnancy: Focus on pertussis and influenza. Swiss Med Wkly. 2017, 147. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, Z.; Greer, O.; Shah, N.M. Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy? Antibodies 2020, 9, 38. https://doi.org/10.3390/antib9030038
Saeed Z, Greer O, Shah NM. Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy? Antibodies. 2020; 9(3):38. https://doi.org/10.3390/antib9030038
Chicago/Turabian StyleSaeed, Zainab, Orene Greer, and Nishel Mohan Shah. 2020. "Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy?" Antibodies 9, no. 3: 38. https://doi.org/10.3390/antib9030038
APA StyleSaeed, Z., Greer, O., & Shah, N. M. (2020). Is the Host Viral Response and the Immunogenicity of Vaccines Altered in Pregnancy? Antibodies, 9(3), 38. https://doi.org/10.3390/antib9030038