CD47 as a Potential Target to Therapy for Infectious Diseases
Abstract
:1. Introduction
2. CD47 Expression and Infection
3. Role of CD47 in Immune Response
3.1. CD47 and Innate Immunity
3.2. CD47 and Adaptive Immunity
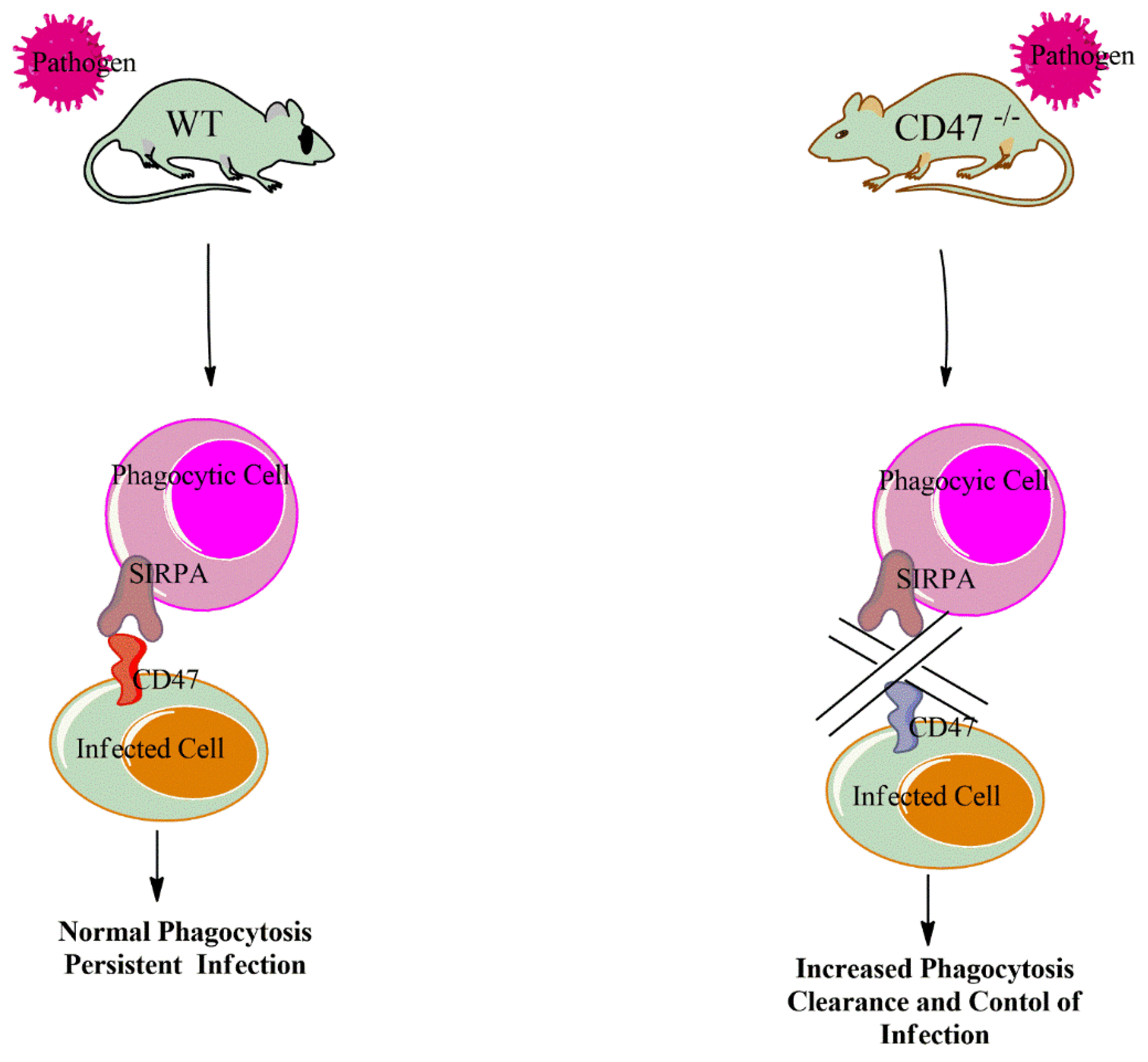
4. CD47 Genetic Inactivation and Infection
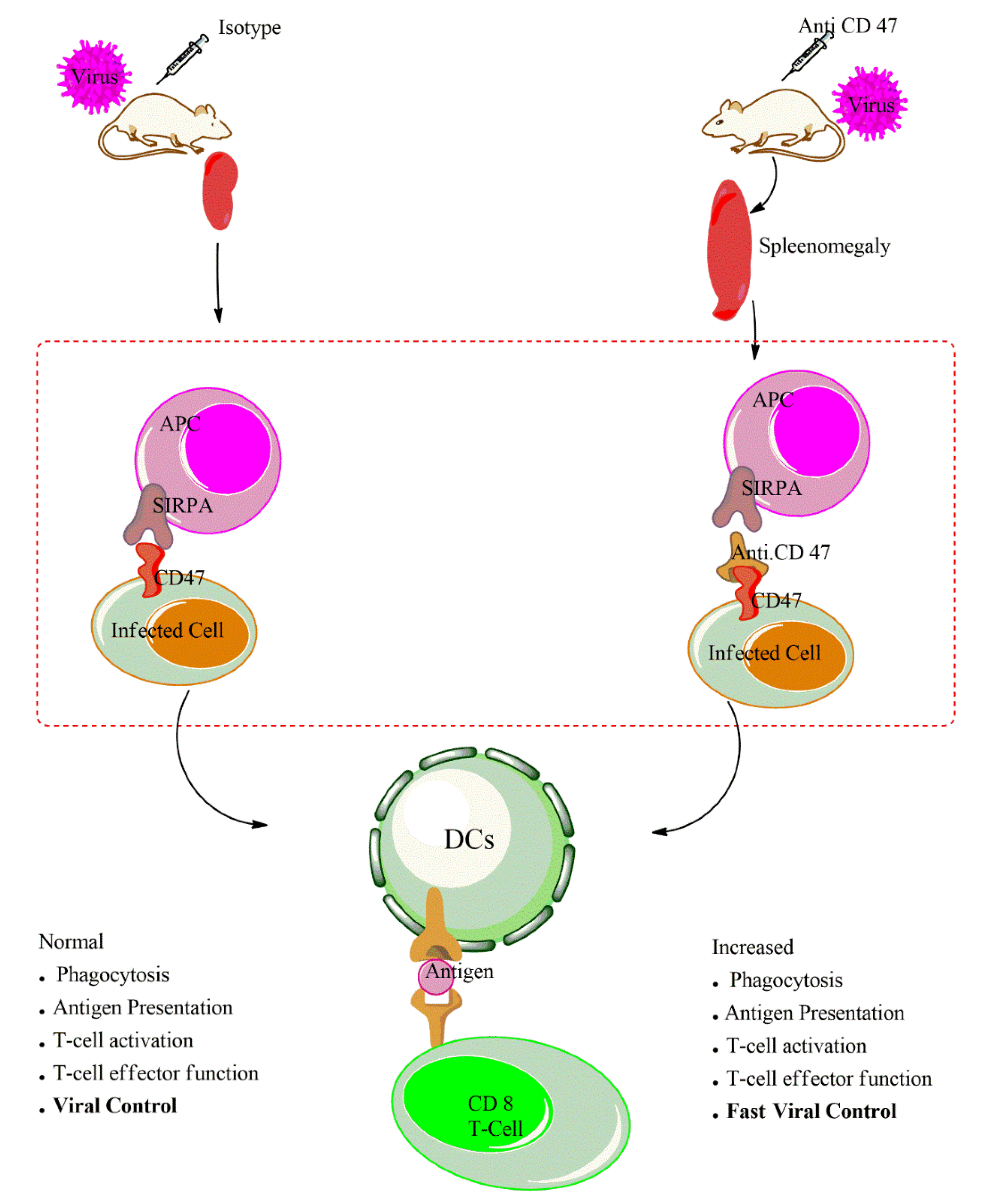
5. CD47-Blocking Antibodies
6. CD47 Blockade and APCs Activation During Infection
7. CD47 Blockade and T Cell Function During Infection
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, T.; Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Zhang, Y.; Song, Y.; Hu, J.; He, X.; et al. Tumor-intrinsic CD47 signal regulates glycolysis and promotes colorectal cancer cell growth and metastasis. Theranostics 2020, 10, 4056–4072. [Google Scholar] [CrossRef]
- Oldenborg, P.-A. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013, 2013, 614619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jin, S.; Guo, X.; Qian, W. Targeting the CD47-SIRPalpha signaling axis: Current studies on B-cell lymphoma immunotherapy. J. Int. Med. Res. 2018, 46, 4418–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; Van Rooijen, N.; Weissman, I.L.; Park, C.Y. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, L.; Yang, L.; Li, H.; Li, R.; Yu, J.; Yang, L.; Wei, F.; Yan, C.; Sun, Q.; et al. Anti-CD47 Antibody As a Targeted Therapeutic Agent for Human Lung Cancer and Cancer Stem Cells. Front. Immunol. 2017, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Anniss, A.M.; Sparrow, R.L. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus. Apher. Sci. 2002, 27, 233–238. [Google Scholar] [CrossRef]
- Burger, P.; Hilarius-Stokman, P.; De Korte, D.; Van Den Berg, T.K.; Van Bruggen, R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012, 119, 5512–5521. [Google Scholar] [CrossRef] [Green Version]
- Lian, S.; Xie, X.; Lu, Y.; Jia, L.; Lee, J. Checkpoint CD47 Function On Tumor Metastasis And Immune Therapy. OncoTargets Ther. 2019, 12, 9105–9114. [Google Scholar] [CrossRef] [Green Version]
- Folkes, A.S.; Feng, M.; Zain, J.M.; Abdulla, F.; Rosen, S.T.; Querfeld, C. Targeting CD47 as a cancer therapeutic strategy—The cutaneous T-cell lymphoma experience. Curr. Opin. Oncol. 2018, 30, 332–337. [Google Scholar] [CrossRef]
- Yuan, J.; He, H.; Chen, C.; Wu, J.; Rao, J.; Yan, H. Combined high expression of CD47 and CD68 is a novel prognostic factor for breast cancer patients. Cancer Cell Int. 2019, 19, 238. [Google Scholar] [CrossRef] [PubMed]
- Cham, L.B.; Dulgeroff, L.B.T.; Tal, M.C.; Adomati, T.; Li, F.; Bhat, H.; Huang, A.; Lang, P.A.; Moreno, M.E.; Rivera, J.M.; et al. Immunotherapeutic Blockade of CD47 Inhibitory Signaling Enhances Innate and Adaptive Immune Responses to Viral Infection. Cell Rep. 2020, 31, 107494. [Google Scholar] [CrossRef]
- Cameron, C.M.; Barrett, J.W.; Mann, M.; Lucas, A.; McFadden, G. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology 2005, 337, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré, D.; Martínez-Vicente, P.; Engel, P.; Angulo, A. Immunoglobulin superfamily members encoded by viruses and their multiple roles in immune evasion. Eur. J. Immunol. 2017, 47, 780–796. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Shao, R.; Huang, H.; Wang, X.; Rong, Z.; Lin, Y. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPa axis. Cancer Med. 2019, 8, 4245–4253. [Google Scholar] [PubMed] [Green Version]
- Lin, Y.; Zhao, J.-L.; Zheng, Q.-J.; Jiang, X.; Tian, J.; Liang, S.-Q.; Guo, H.-W.; Qin, H.-Y.; Liang, Y.-M.; Han, H. Notch Signaling Modulates Macrophage Polarization and Phagocytosis Through Direct Suppression of Signal Regulatory Protein α Expression. Front. Immunol. 2018, 9, 1744. [Google Scholar] [CrossRef]
- Bian, Z.; Shi, L.; Guo, Y.L.; Lv, Z.; Tang, C.; Niu, S.; Tremblay, A.; Venkataramani, M.; Culpepper, C.; Li, L.; et al. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5434–E5443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, N.B.; Schneider, K.M.; Massa, P.T. SHP-1-dependent macrophage differentiation exacerbates virus-induced myositis. J. Immunol. 2015, 194, 2796–2809. [Google Scholar] [CrossRef] [Green Version]
- Maile, L.A.; DeMambro, V.E.; Wai, C.; Lotinun, S.; Aday, A.W.; Capps, B.E.; Beamer, W.G.; Rosen, C.J.; Clemmons, D.R. An essential role for the association of CD47 to SHPS-1 in skeletal remodeling. J. Bone Miner. Res. 2011, 26, 2068–2081. [Google Scholar] [CrossRef]
- Iwamura, H.; Saito, Y.; Sato-Hashimoto, M.; Ohnishi, H.; Murata, Y.; Okazawa, H.; Kanazawa, Y.; Kaneko, T.; Kusakari, S.; Kotani, T.; et al. Essential roles of SIRPalpha in homeostatic regulation of skin dendritic cells. Immunol. Lett. 2011, 135, 100–107. [Google Scholar] [CrossRef]
- Florian, S.; Ghannadan, M.; Mayerhofer, M.; Aichberger, K.J.; Hauswirth, A.W.; Schernthaner, G.H.; Printz, D.; Fritsch, G.; Bohm, A.; Sonneck, K.; et al. Evaluation of normal and neoplastic human mast cells for expression of CD172a (SIRPalpha), CD47, and SHP-1. J. Leukoc. Biol. 2005, 77, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Viant, C.; Fenis, A.; Chicanne, G.; Payrastre, B.; Ugolini, S.; Vivier, E. SHP-1-mediated inhibitory signals promote responsiveness and anti-tumour functions of natural killer cells. Nat. Commun. 2014, 5, 6108. [Google Scholar] [CrossRef] [PubMed]
- Carmi, Y.; Prestwood, T.R.; Spitzer, M.H.; Linde, I.L.; Chabon, J.; Reticker-Flynn, N.E.; Bhattacharya, N.; Zhang, H.; Zhang, X.; Basto, P.; et al. Akt and SHP-1 are DC-intrinsic checkpoints for tumor immunity. JCI Insight 2016, 1, 89020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Piccione, E.C.; Juarez, S.; Liu, J.; Tseng, S.; Ryan, C.E.; Narayanan, C.; Wang, L.; Weiskopf, K.; Majeti, R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs 2015, 7, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Kojima, Y.; Volkmer, J.-P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; DiRenzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Ni, T.; Wang, J.; Liu, Y.; Fan, Q.; Wang, Y.; Huang, T.; Chu, Y.; Sun, X.; Wang, Y. CD47 Blockade Inhibits Tumor Progression through Promoting Phagocytosis of Tumor Cells by M2 Polarized Macrophages in Endometrial Cancer. J. Immunol. Res. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Métayer, L.E.; Vilalta, A.; Burke, G.A.; Brown, G.C. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by phagoptosis. Oncotarget 2017, 8, 60892–60903. [Google Scholar] [CrossRef] [Green Version]
- Ayi, K.; Lu, Z.; Serghides, L.; Ho, J.M.; Finney, C.A.; Wang, J.C.Y.; Liles, W.C.; Kain, K. CD47-SIRPα Interactions Regulate Macrophage Uptake of Plasmodium falciparum-Infected Erythrocytes and Clearance of MalariaIn Vivo. Infect. Immun. 2016, 84, 2002–2011. [Google Scholar] [CrossRef] [Green Version]
- Mittal, R.; Gonzalez-Gomez, I.; Prasadarao, N.V. Escherichia coliK1 Promotes the Ligation of CD47 with Thrombospondin-1 To Prevent the Maturation of Dendritic Cells in the Pathogenesis of Neonatal Meningitis. J. Immunol. 2010, 185, 2998–3006. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Khandelwal, S.; Kozakai, Y.; Sahu, B.; Kumar, S. CD47 regulates the phagocytic clearance and replication of the Plasmodium yoelii malaria parasite. Proc. Natl. Acad. Sci. USA 2015, 112, 3062–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Lee, Y.N.; Bian, Z.; Liu, Y.; Kang, S.M. CD47 Plays a Role as a Negative Regulator in Inducing Protective Immune Responses to Vaccination against Influenza Virus. J. Virol. 2016, 90, 6746–6758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Dee, Z.; Pidcock, K.; Gutierrez, L.S. Thrombospondin-1: Multiple Paths to Inflammation. Mediat. Inflamm. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daubon, T.; Léon, C.; Clarke, K.; Andrique, L.; Salabert, L.; Darbo, E.; Pineau, R.; Guérit, S.; Maitre, M.; Dedieu, S.; et al. Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nat. Commun. 2019, 10, 1146. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Soto-Pantoja, D.R.; Stein, E.V.; Liu, C.; Elkahloun, A.G.; Pendrak, M.L.; Nicolae, A.; Singh, S.P.; Nie, Z.; Levens, D.; et al. Thrombospondin-1 Signaling through CD47 Inhibits Self-renewal by Regulating c-Myc and Other Stem Cell Transcription Factors. Sci. Rep. 2013, 3, 1673. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Chen, K.; Gao, L.; Zheng, Y.; Yang, Y.-G. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016, 7, e2368. [Google Scholar] [CrossRef]
- Kamijo, H.; Miyagaki, T.; Takahashi-Shishido, N.; Nakajima, R.; Oka, T.; Suga, H.; Sugaya, M.; Sato, S. Thrombospondin-1 promotes tumor progression in cutaneous T-cell lymphoma via CD47. Leukemia 2020, 34, 845–856. [Google Scholar] [CrossRef]
- Khandelwal, S.; Van Rooijen, N.; Saxena, R.K. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 2007, 47, 1725–1732. [Google Scholar] [CrossRef]
- Peluso, M.O.; Adam, A.; Armet, C.M.; Zhang, L.; O’Connor, R.W.; Lee, B.H.; Lake, A.C.; Normant, E.; Chappel, S.C.; Hill, J.A.; et al. The Fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J. Immunother. Cancer 2020, 8, e000413. [Google Scholar] [CrossRef]
- Hayes, B.H.; Tsai, R.K.; Dooling, L.J.; Kadu, S.; Lee, J.Y.; Pantano, D.; Rodriguez, P.L.; Subramanian, S.; Shin, J.W.; Discher, D.E. Macrophages show higher levels of engulfment after disruption of cis interactions between CD47 and the checkpoint receptor SIRPalpha. J. Cell. Sci. 2020, 133, jcs237800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puro, R.J.; Bouchlaka, M.N.; Hiebsch, R.R.; Capoccia, B.J.; Donio, M.J.; Manning, P.T.; Frazier, W.A.; Karr, R.W.; Pereira, D.S. Development of AO-176, a next-generation humanized anti-cd47 antibody with novel anticancer properties and negligible red blood cell binding. Mol. Cancer Ther. 2020, 19, 835–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyong, D.A.; Loughland, J.R.; SheelaNair, A.; Andrew, D.; Rivera, F.D.L.; Piera, K.A.; William, T.; Grigg, M.J.; Barber, B.E.; Haque, A.; et al. Loss of complement regulatory proteins on red blood cells in mild malarial anaemia and in Plasmodium falciparum induced blood-stage infection. Malar. J. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, N.A.; El Fetouh, R.M.A.; Hafez, A.A.; Gad, A.; Kamal, M.M. The expression of CD47 and its association with 2,3-DPG levels in stored leuco-reduced blood units. Transfus. Clin. Biol. 2019, 26, 279–283. [Google Scholar] [CrossRef]
- Arias, C.F.; Arias, C.F. How do red blood cells know when to die? R. Soc. Open Sci. 2017, 4, 160850. [Google Scholar] [CrossRef] [Green Version]
- Wiewióra, M.; Piecuch, J.; Sędek, L.; Mazur, B.; Sosada, K. The effects of obesity on CD47 expression in erythrocytes. Cytom. Part B Clin. Cytom. 2015, 92, 485–491. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, X.; Chen, C.; He, H.; Liu, L.; Wu, J.; Yan, H. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol. Lett. 2019, 18, 3249–3255. [Google Scholar] [CrossRef] [Green Version]
- Betancur, P.A.; Abraham, B.J.; Yiu, Y.Y.; Willingham, S.B.; Khameneh, F.; Zarnegar, M.; Kuo, A.H.; McKenna, K.; Kojima, Y.; Leeper, N.J.; et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 2017, 8, 14802. [Google Scholar] [CrossRef]
- Nath, P.R.; Gangaplara, A.; Pal-Nath, D.; Mandal, A.; Maric, D.; Sipes, J.M.; Cam, M.; Shevach, E.M.; Roberts, D.D. CD47 Expression in Natural Killer Cells Regulates Homeostasis and Modulates Immune Response to Lymphocytic Choriomeningitis Virus. Front. Immunol. 2018, 9, 2985. [Google Scholar] [CrossRef] [Green Version]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gutgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matozaki, T.; Murata, Y.; Okazawa, H.; Ohnishi, H. Functions and molecular mechanisms of the CD47–SIRPα signalling pathway. Trends Cell Biol. 2009, 19, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Respatika, D.; Komori, S.; Washio, K.; Nishimura, T.; Kotani, T.; Murata, Y.; Okazawa, H.; Ohnishi, H.; Kaneko, Y.; et al. SIRPalpha(+) dendritic cells regulate homeostasis of fibroblastic reticular cells via TNF receptor ligands in the adult spleen. Proc. Natl. Acad. Sci. USA 2017, 114, E10151–E10160. [Google Scholar] [CrossRef] [Green Version]
- Meijles, D.N.; Sahoo, S.; Ghouleh, I.A.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.; Nouraie, M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, eaaj1784. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, J.; Zhang, X.; Li, J.; Zhao, P.; Fei, P. TSP1 ameliorates age-related macular degeneration by regulating the STAT3-iNOS signaling pathway. Exp. Cell Res. 2020, 388, 111811. [Google Scholar] [CrossRef]
- Sick, E.; Jeanne, A.; Schneider, C.; Dedieu, S.; Takeda, K.; Martiny, L. CD47 update: A multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br. J. Pharmacol. 2012, 167, 1415–1430. [Google Scholar] [CrossRef] [Green Version]
- Hagnerud, S.; Manna, P.P.; Cella, M.; Stenberg, A.; Frazier, W.A.; Colonna, M.; Oldenborg, P.-A. Deficit of CD47 Results in a Defect of Marginal Zone Dendritic Cells, Blunted Immune Response to Particulate Antigen and Impairment of Skin Dendritic Cell Migration. J. Immunol. 2006, 176, 5772–5778. [Google Scholar] [CrossRef]
- Van, V.Q.; Lesage, S.; Bouguermouh, S.; Gautier, P.; Rubio, M.; Levesque, M.; Nguyen, S.; Galibert, L.; Sarfati, M. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J. 2006, 25, 5560–5568. [Google Scholar] [CrossRef]
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 Is an Autocrine Negative Regulator of Human Dendritic Cell Activation. J. Exp. Med. 2003, 198, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, L.; Wilson, K.E.; Roberts, D.D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 2001, 166, 2427–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azcutia, V.; Routledge, M.; Williams, M.R.; Newton, G.; Frazier, W.A.; Manica, A.; Croce, K.J.; Parkos, C.A.; Schmider, A.B.; Turman, M.V.; et al. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol. Biol. Cell 2013, 24, 3358–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.-X.; Xu, M.M. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Tomiyama, Y.; Oritani, K.; Murayama, Y.; Ishikawa, J.; Kato, H.; Miyagawa, J.-I.; Honma, N.; Nishiura, T.; Matsuzawa, Y. Interaction Between Src Homology 2 Domain Bearing Protein Tyrosine Phosphatase Substrate-1 and CD47 Mediates the Adhesion of Human B Lymphocytes to Nonactivated Endothelial Cells. J. Immunol. 2002, 168, 3213–3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motegi, S.-I.; Okazawa, H.; Ohnishi, H.; Sato, R.; Kaneko, Y.; Kobayashi, H.; Tomizawa, K.; Ito, T.; Honma, N.; Bühring, H.; et al. Role of the CD47–SHPS-1 system in regulation of cell migration. EMBO J. 2003, 22, 2634–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Johansen, M.; Looney, M.; Brown, E.J.; Matthay, M.A. CD47 Deficiency Protects Mice from Lipopolysaccharide-Induced Acute Lung Injury and Escherichia coli Pneumonia. J. Immunol. 2008, 180, 6947–6953. [Google Scholar] [CrossRef] [Green Version]
- Navarathna, D.H.; Stein, E.V.; Lessey-Morillon, E.C.; Nayak, D.; Martin-Manso, G.; Roberts, D.D. CD47 Promotes Protective Innate and Adaptive Immunity in a Mouse Model of Disseminated Candidiasis. PLoS ONE 2015, 10, e0128220. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef] [Green Version]
- Li, W. Eat-me signals: Keys to molecular phagocyte biology and “Appetite” control. J. Cell. Physiol. 2012, 227, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Caberoy, N.B.; Zhou, Y.; Li, W. Can Phage Display Be Used as a Tool to Functionally Identify Endogenous Eat-Me Signals in Phagocytosis? J. Biomol. Screen. 2009, 14, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Sun, Q.; Chen, A.; Fan, J.; Yang, X.; Xu, L.; Du, P.; Qiu, W.; Zhang, W.; Wang, S.; et al. A fully human anti-CD47 blocking antibody with therapeutic potential for cancer. Oncotarget 2016, 7, 83040–83050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPalpha Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, C.; Chao, M.; Gibbs, C.; McCamish, M.; Liu, J.; Chen, J.; Majeti, R.; Weissman, I. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann. Oncol. 2019, 30, 486–489. [Google Scholar] [CrossRef]
- Pietsch, E.C.; Dong, J.; Cardoso, R.; Zhang, X.; Chin, D.; Hawkins, R.; Dinh, T.; Zhou, M.; Strake, B.; Feng, P.-H.; et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017, 7, e536. [Google Scholar] [CrossRef]
- Kauder, S.E.; Kuo, T.C.; Harrabi, O.; Chen, A.; Sangalang, E.; Doyle, L.; Rocha, S.S.; Bollini, S.; Han, B.; Sim, J.; et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 2018, 13, e0201832. [Google Scholar] [CrossRef] [Green Version]
- Engelbertsen, D.; Autio, A.; Verwilligen, R.; Depuydt, M.A.C.; Newton, G.; Rattik, S.; Levinsohn, E.; Saggu, G.; Jarolim, P.; Wang, H.; et al. Increased lymphocyte activation and atherosclerosis in CD47-deficient mice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wen, W.; Tang, L.; Qin, C.-J.; Lin, Y.; Zhang, H.-L.; Wu, H.; Ashton, C.; Wu, H.-P.; Ding, J.; et al. Inhibition of SIRPα in dendritic cells potentiates potent antitumor immunity. OncoImmunology 2016, 5, e1183850. [Google Scholar] [CrossRef] [Green Version]
- Veillette, A.; Chen, J. SIRPalpha-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Barrera, L.; Montes-Servín, E.; Hernandez-Martinez, J.-M.; García-Vicente, M.D.L.; Montes-Servín, E.; Herrera-Martínez, M.; Crispín, J.C.; Borbolla-Escoboza, J.R.; Arrieta, O. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br. J. Cancer 2017, 117, 385–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.M.; Pu, Y.; Han, D.; Shi, Y.; Cao, X.; Liang, H.; Chen, X.; Li, X.-D.; Deng, L.; Chen, Z.J.; et al. Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein α Signaling. Immunity 2017, 47, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Bian, Z.; Shi, L.; Niu, S.; Ha, B.; Tremblay, A.; Li, L.; Zhang, X.; Paluszynski, J.; Liu, M.; et al. Loss of Cell Surface CD47 Clustering Formation and Binding Avidity to SIRPalpha Facilitate Apoptotic Cell Clearance by Macrophages. J. Immunol. 2015, 195, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeClair, P.; Liu, C.-C.; Monajemi, M.; Reid, G.; Sly, L.M.; Lim, C.J. CD47-ligation induced cell death in T-acute lymphoblastic leukemia. Cell Death Dis. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeure, C.E.; Tanaka, H.; Mateo, V.; Rubio, M.; Delespesse, G.; Sarfati, M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 2000, 164, 2193–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Veer, M.J.; Holko, M.; Frevel, M.; Walker, E.; Der, S.; Paranjape, J.M.; Silverman, R.H.; Williams, B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001, 69, 912–920. [Google Scholar]
- Gulzar, N.; Copeland, K.F.T. CD8+ T-cells: Function and response to HIV infection. Curr. HIV Res. 2004, 2, 23–37. [Google Scholar] [CrossRef]
- Myers, L.M.; Tal, M.C.; Dulgeroff, L.B.T.; Carmody, A.B.; Messer, R.J.; Gulati, G.; Yiu, Y.Y.; Staron, M.M.; Angel, C.L.; Sinha, R.; et al. A functional subset of CD8(+) T cells during chronic exhaustion is defined by SIRPalpha expression. Nat. Commun. 2019, 10, 794. [Google Scholar] [CrossRef] [Green Version]
- Duhan, V.; Hamdan, T.A.; Xu, H.C.; Shinde, P.; Bhat, H.; Li, F.; Al-Matary, Y.; Haussinger, D.; Bexgovsek, J.; Friedrich, S.K.; et al. NK cell-intrinsic FcepsilonRIgamma limits CD8+ T-cell expansion and thereby turns an acute into a chronic viral infection. PLoS Pathog. 2019, 15, e1007797. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yu, G.-T.; Deng, W.-W.; Mao, L.; Yang, L.-L.; Ma, S.-R.; Bu, L.-L.; Kulkarni, A.B.; Zhang, W.-F.; Zhang, L.; et al. Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. OncoImmunology 2018, 7, e1397248. [Google Scholar] [CrossRef]
- Tal, M.C.; Dulgeroff, L.B.T.; Myers, L.; Cham, L.B.; Mayer-Barber, K.D.; Bohrer, A.C.; Castro, E.; Yiu, Y.Y.; Angel, C.L.; Pham, E.; et al. Upregulation of CD47 Is a Host Checkpoint Response to Pathogen Recognition. Mbio 2020, 11, e01293-20. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cham, L.B.; Adomati, T.; Li, F.; Ali, M.; Lang, K.S. CD47 as a Potential Target to Therapy for Infectious Diseases. Antibodies 2020, 9, 44. https://doi.org/10.3390/antib9030044
Cham LB, Adomati T, Li F, Ali M, Lang KS. CD47 as a Potential Target to Therapy for Infectious Diseases. Antibodies. 2020; 9(3):44. https://doi.org/10.3390/antib9030044
Chicago/Turabian StyleCham, Lamin B., Tom Adomati, Fanghui Li, Murtaza Ali, and Karl S. Lang. 2020. "CD47 as a Potential Target to Therapy for Infectious Diseases" Antibodies 9, no. 3: 44. https://doi.org/10.3390/antib9030044
APA StyleCham, L. B., Adomati, T., Li, F., Ali, M., & Lang, K. S. (2020). CD47 as a Potential Target to Therapy for Infectious Diseases. Antibodies, 9(3), 44. https://doi.org/10.3390/antib9030044






