Asymmetric Firing Rate from Crayfish Left and Right Caudal Photoreceptors Due to Blue and Green Monochromatic Light Pulses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Extracellular Recordings
2.3. Statistical Analysis
3. Results
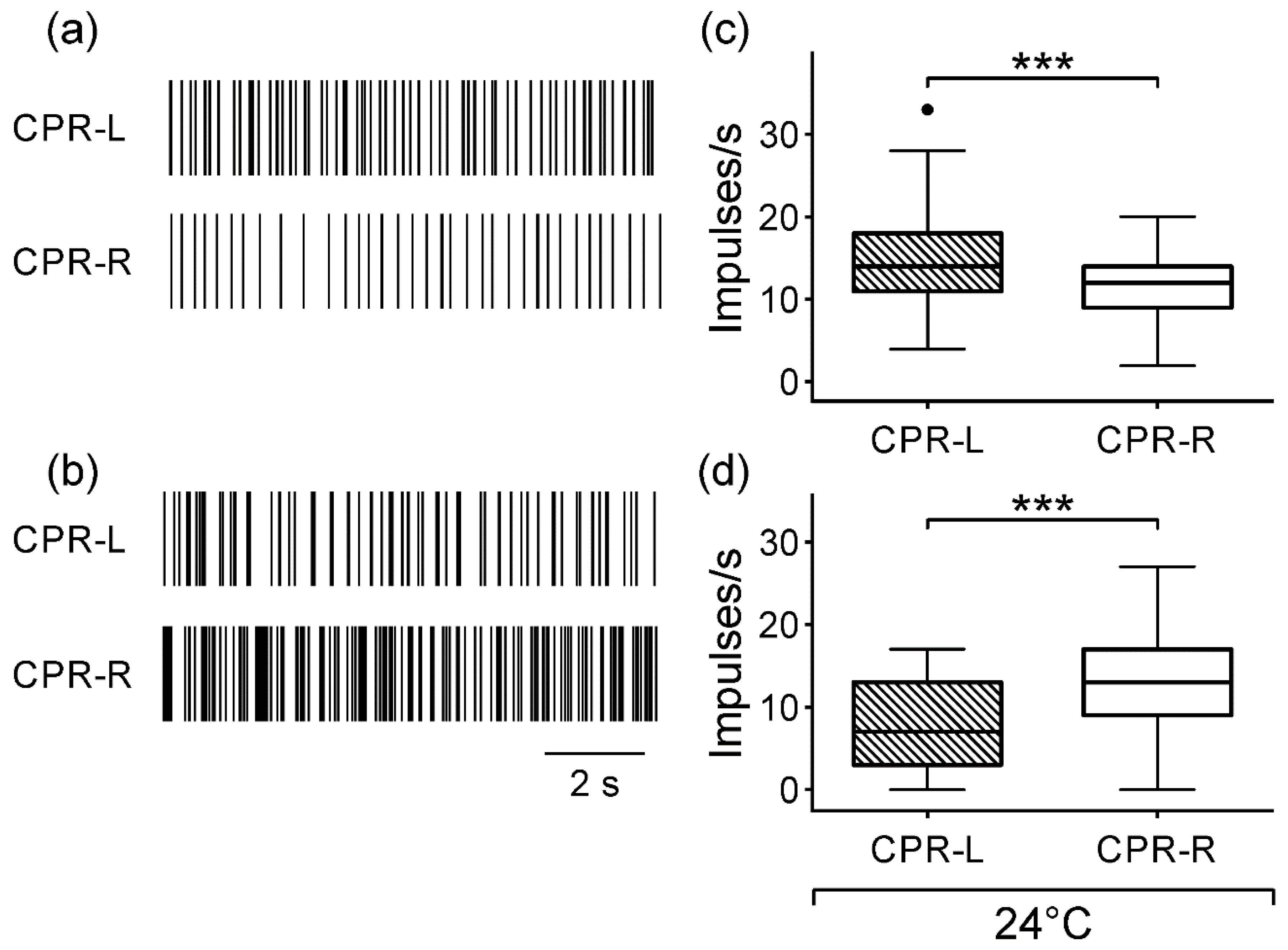
3.1. Spontaneous CPR Activity in Darkness
3.2. Spontaneous Activity of CPRs at Lower Temperature
3.3. Response from CPRs to Monochromatic Blue Light at Room Temperature
3.4. Response from CPRs to Monochromatic Blue Light at Lower Temperature
3.5. Response of CPRs to Monochromatic Green Light at Room Temperature
3.6. Response to Green Monochromatic Light Pulses from CPRs at Lower Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Sosa, L.; Calderón-Rosete, G.; Flores, G. Circadian and ultradian rhythms in the crayfish caudal photoreceptor. Synapse 2008, 62, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sosa, L.; Calderón-Rosete, G.; Anaya, V.; Flores, G. The caudal photoreceptor in crayfish: An overview. In Photoreceptors: Physiology, Types and Abnormalities; Akutagawa, E., Ozaki, K., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 59–78. ISBN 978-1-61942-619-1. [Google Scholar]
- Nilsson, D.E. The evolution of eyes and visually guided behaviour. Philos. Trans. R. Soc. B 2009, 364, 2833–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommen, Q.; Pfeuty, B.; Schatt, P.; Bijoux, A.; Bouget, F.Y.; Lefranc, M. Probing entrainment of Ostreococcus tauri circadian clock by green and blue light through a mathematical modeling approach. Front. Genet. 2015, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Genco, M.C.; Marlow, E.D.; Benton, J.L.; Beltz, B.S.; Sandeman, D.C. Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol. Int. 2009, 26, 1136–1168. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sosa, L.; Calderón-Rosete, G.; Ortega-Cambranis, A.; De-Miguel, F.F. Octopamine cyclic release and its modulation of visual sensitivity in crayfish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 203, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Dircksen, H. Circadian clocks in crustaceans: Identified neuronal and cellular systems. Front. Biosci. 2010, 15, 1040–1074. [Google Scholar] [CrossRef]
- Kennedy, D. Physiology of photoreceptor neurons in the abdominal nerve cord of the crayfish. J. Gen. Physiol. 1963, 46, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, L.A. The crayfish caudal photoreceptor: Advances and questions after the first half century. J. Comp. Physiol. 1988, 91, 61–68. [Google Scholar] [CrossRef]
- Simon, T.W.; Edwards, D.H. Light-evoked walking in crayfish: Behavioral and neuronal responses triggered by the caudal photoreceptor. J. Comp. Physiol. A 1990, 166, 745–755. [Google Scholar] [CrossRef]
- Wilkens, L.A.; Larimer, J.L. Photosensitivity in the 6th abdominal ganglion of decapod crustaceans: A comparative study. J. Comp. Physiol. A 1976, 106, 69–75. [Google Scholar] [CrossRef]
- Larimer, J.L. The interneurons of the abdominal positioning system of the crayfish. Brain Behav. Evol. 2000, 55, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, L.A.; Larimer, J.L. The CNS photoreceptor of crayfish: Morphology and synaptic activity. J. Comp. Physiol. 1972, 80, 389–407. [Google Scholar] [CrossRef]
- Kruszewska, B.; Larimer, J.L. Specific second messengers activate the caudal photoreceptor of crayfish. Brain Res. 1993, 618, 32–40. [Google Scholar] [CrossRef]
- Gotow, T.; Nishi, T. A new photosensory function for simple photoreceptors, the intrinsically photoresponsive neurons of the sea slug. Onchidium. Front. Cell. Neurosci. 2009, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Hermann, H.T.; Olsen, R.E. Afferent stochastic modulation of crayfish caudal photoreceptor units. J. Gen. Physiol. 1968, 51, 534–551. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.H. Crayfish extraretinal photoreception. I. Behavioral and motorneuronal responses to abdominal illumination. J. Exp. Biol. 1984, 109, 291–306. [Google Scholar] [PubMed]
- Hermann, H.T.; Olsen, R.E. Dynamic statistics of crayfish caudal photoreceptors. Biophys. J. 1967, 7, 279–296. [Google Scholar] [CrossRef]
- Nesbit, S.C.; Van Hoof, A.G.; Le, C.C.; Dearworth, J.R. Extracellular recording of light responses from optic nerve fibers and the caudal photoreceptor in the crayfish. J. Undergrad. Neurosci. Educ. 2015, 14, A29–A38. [Google Scholar] [PubMed]
- Kingston, A.C.N.; Cronin, T.W. Short- and long-wavelength-sensitive opsins are involved in photoreception both in the retina and throughout the central nervous system of crayfish. J. Comp. Physiol. A 2015, 201, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.C.N.; Cronin, T.W. Diverse distributions of extraocular opsins in crustaceans, Cephalopods, and Fish. Integr. Comp. Biol. 2016, 56, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.J.; Dannenhauer, K.; Kohler, M.; Labhart, T.; Gesemann, M. Opsin evolution and expression in arthropod compound eyes and ocelli: Insights from the cricket Gryllus bimaculatus. BMC Evol. Biol. 2012, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Terakita, A.; Nagata, T. Functional properties of opsins and their contribution to light-sensing physiology. Zool. Sci. 2014, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Preface. In Brain Asymmetry of Structure and/or Function, 1st ed.; Roger, L.J., Ed.; MDPI: Basel, Switzerland, 2017; p. vii. ISBN 978-3-03842-551-9. Available online: https://www.mdpi.com/journal/symmetry/special_issues/brain_asymmetry (accessed on 18 August 2018).
- Levin, M.; Klar, A.J.S.; Ramsdell, A.F. Introduction to provocative questions in left–right asymmetry. Philos. Trans. R. Soc. B 2016, 371, 20150399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobert, O. Development of left/right asymmetry in the Caenorhabditis elegans nervous system: From zygote to postmitotic neuron. Genesis 2014, 52, 528–543. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.R. What determines direction of asymmetry: Genes, environment or chance? Philos. Trans. R. Soc. B 2016, 371, 20150417. [Google Scholar] [CrossRef] [PubMed]
- Tobo, S.; Takeuchi, Y.; Hori, M. Morphological asymmetry and behavioral laterality in the crayfish, Procambarus clarkii. Ecol. Res. 2012, 27, 53. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Tobo, S.; Hori, M. Morphological asymmetry of the abdomen and behavioral laterality in atyid shrimps. Zool. Sci. 2008, 25, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angilletta, J.M.; Wilson, R.S. Cryptic asymmetry: Unreliable signals mask asymmetric performance of crayfish weapons. Biol. Lett. 2012, 8, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Hisada, M. Neuroanatomy of the terminal (sixth abdominal) ganglion of the crayfish, Procambarus clarkii (Girard). Cell Tissue Res. 1986, 243, 273–288. [Google Scholar] [CrossRef]
- Mulloney, B.; Tschuluun, N.; Hall, W.M. Architectonics of crayfish ganglia. Microsc. Res. Tech. 2003, 60, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Hermann, H.T. Analysis of the properties of the crayfish caudal photoreceptor (PRU-photoreceptor unit). In Experiments in Physiology and Biochemistry; Kerkut, G.A., Ed.; Academic Press: London, UK, 1972; Volume 3, pp. 155–192. [Google Scholar]
- Pacheco-Ortiz, J.A.; Sánchez-Hernández, J.C.; Rodríguez-Sosa, L.; Calderón-Rosete, G.; Villagran-Vargas, E. Left-right asymmetry in firing rate of extra-retinal photosensitive neurons in the crayfish. Gen. Physiol. Biophys. 2018, 37, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Van Harreveld, A. A physiological solution for fresh water crustaceans. Proc. Soc. Exp. Biol. Med. 1936, 34, 428–432. [Google Scholar] [CrossRef]
- Gold, C.; Henze, D.A.; Koch, C.; Buzsáki, G. On the origin of the extracellular action potential waveform: A modeling study. J. Neurophysiol. 2006, 95, 3113–3128. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.B.; Pearson, K.G. Predicted amplitude and form of action potentials recorded from unmyelinated nerve fibers. J. Theor. Biol. 1971, 32, 539–558. [Google Scholar] [CrossRef]
- Pettersen, K.H.; Einevoll, G.T. Amplitude variability and extracellular low-pass filtering of neuronal spikes. Biophys. J. 2008, 94, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Villagran-Vargas, E.; Ludu, A.; Hustert, R.; Jackson, A.; Heimburg, T. Periodic solutions and refractory period in the soliton theory for nerves and the locust femoral nerve. Biophys. Chem. 2011, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D. Responses from the crayfish caudal photoreceptor. Am. J. Ophthalmol. 1958, 46, 19–26. [Google Scholar] [CrossRef]
- Feudel, U.; Neiman, A.; Pei, X.; Wojtenek, W.; Braun, H.; Huber, M.; Moss, F. Homoclinic bifurcation in a Hodgkin-Huxley model of thermally sensitive neurons. Chaos 2000, 10, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.U.; Alaburda, A.; Hounsgaard, J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 2007, 315, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Broccard, F.D.; Garcia-Perez, E.; Bonifazi, P.; Ruaro, M.E.; Torre, V. On the dynamics of the spontaneous activity in neuronal networks. PLoS ONE. 2007, 2, e439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelle, B.A. Simple eyes, extraocular photoreceptors and opsins in the American horseshoe crab. Integr. Comp. Biol. 2016, 56, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Belanger, J.H. Temperature acclimation of the caudal photoreceptor response in the crayfish Orconectes rusticus (Girard). Can. J. Zool. 1988, 66, 1168–1171. [Google Scholar] [CrossRef]
- Masser, M.; Rouse, D. Australian Red Claw Crayfish; Southern Regional Aquaculture Center of the United States: Stoneville, MS, USA, 1997; Publication No. 244; 8p. [Google Scholar]
- Caplan, J.S.; Williams, A.H.; Marder, E. Many parameter sets in a multicompartment model oscillator are robust to temperature perturbations. J. Neurosci. 2014, 34, 4963–4975. [Google Scholar] [CrossRef] [PubMed]
- Marder, E.; Haddad, S.A.; Goeritz, M.L.; Rosenbaum, P.; Kispersky, T. How can motor systems retain performance over a wide temperature range? Lessons from the crustacean stomatogastric nervous system. J. Comp. Physiol. A 2015, 201, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Serpico, D.; Frasnelli, E. Where the standard approach in comparative neuroscience fails and where it works: General intelligence and brain asymmetries. Comp. Cognit. Behav. Rev. 2018, 13, 95–98. [Google Scholar] [CrossRef]
- Lira-Oliver, A. Modulation of the intensity of the spectral components of polychromatic light within certain regions in space by passive methods by strategically using material optical properties and texture. Technologies 2018, 6, 11. [Google Scholar] [CrossRef]
- Van Diepen, H.C.; Foster, R.G.; Meijer, J.H. A Colourful Clock. PLoS Biol. 2015, 13, e1002160. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 2016, 22, 61–72. Available online: http://www.molvis.org/molvis/v22/61 (accessed on 21 May 2018). [PubMed]
- Renart, A.; Machens, C.K. Variability in neural activity and behavior. Curr. Opin. Neurobiol. 2014, 25, 211–220. [Google Scholar] [CrossRef] [PubMed]








| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Test Statistics Z (c) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.59 (0.03) | 30 | 0.68282 | 9×10−7 | 6.58921 | p < 0.001 | |
| CPR-R | 0.42 (0.03) | 30 | 0.93946 | 0.08795 | ||||
| Time to peak (ms) | CPR-L | 0.43 (0.09) | 30 | 0.95206 | 0.19189 | 3.00895 | p < 0.01 | |
| CPR-R | 0.35 (0.11) | 30 | 0.9511 | 0.18091 | ||||
| Duration (ms) | CPR-L | 1.98 (0.2) | 30 | 0.984 | 0.91894 | −3.17161 | p < 0.01 | |
| CPR-R | 2.17 (0.37) | 30 | 0.85515 | 8×10−4 |
| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Test Statistics Z (c) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.32 (0.01) | 30 | 0.94446 | 0.11998 | 6.54584 | p < 0.001 | |
| CPR-R | 0.28 (0.02) | 30 | 0.77587 | 2×10−5 | ||||
| Time to peak (ms) | CPR-L | 0.46 (0.10) | 30 | 0.95222 | 0.19377 | −4.03904 | p < 0.001 | |
| CPR-R | 0.58 (0.12) | 30 | 0.97715 | 0.74575 | ||||
| Duration (ms) | CPR-L | 3.03 (0.37) | 30 | 0.98494 | 0.93615 | −0.25296 | p > 0.05 | |
| CPR-R | 3.06 (0.51) | 30 | 0.96227 | 0.35367 |
| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Test Statistics Z (c) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.47 (0.03) | 15 | 0.94433 | 0.43991 | 38.76548 | p < 0.001 | |
| CPR-R | 0.14 (0.02) | 15 | 0.91513 | 0.16231 | ||||
| Time to peak (ms) | CPR-L | 0.49 (0.13) | 15 | 0.92547 | 0.23327 | 2.57623 | p < 0.01 | |
| CPR-R | 0.36 (0.18) | 15 | 0.8421 | 0.01345 | ||||
| Duration (ms) | CPR-L | 2.07 (0.29) | 15 | 0.9157 | 0.1656 | 7.17465 | p < 0.001 | |
| CPR-R | 1.28 (0.31) | 15 | 0.95422 | 0.59316 |
| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.68 (0.03) | 15 | 0.97872 | 0.96003 | 3.91696 | p < 0.001 |
| CPR-R | 0.65 (0.02) | 15 | 0.94619 | 0.46662 | |||
| Time to peak (ms) | CPR-L | 0.5 (0.16) | 15 | 0.94079 | 0.39247 | 0.08234 | p > 0.05 |
| CPR-R | 0.5 (0.15) | 15 | 0.94734 | 0.48351 | |||
| Duration (ms) | CPR-L | 2 (0.24) | 15 | 0.98085 | 0.97496 | 1.56449 | p > 0.05 |
| CPR-R | 1.88 (0.19) | 15 | 0.93889 | 0.36857 |
| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Test Statistics Z (c) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.46 (0.07) | 15 | 0.79677 | 0.00334 | 4.64968 | p < 0.001 | |
| CPR-R | 0.14 (0.01) | 15 | 0.90523 | 0.11443 | ||||
| Time to peak (ms) | CPR-L | 0.42 (0.11) | 15 | 0.91022 | 0.13645 | 2.65413 | p < 0.05 | |
| CPR-R | 0.33 (0.09) | 15 | 0.94658 | 0.47229 | ||||
| Duration (ms) | CPR-L | 1.89 (0.27) | 15 | 0.98379 | 0.98896 | 5.30763 | p < 0.001 | |
| CPR-R | 1.25 (0.38) | 15 | 0.89033 | 0.06783 |
| Spikes | Mean (SD) | n | Normality Test (a) | p-Value | t Statistic (b) | Test Statistics Z (c) | Pairwise Comparison | |
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | CPR-L | 0.59 (0.05) | 15 | 0.57364 | 1.47341 × 10−5 | 4.65747 | p < 0.001 | |
| CPR-R | 0.42 (0.02) | 15 | 0.93599 | 0.33457 | ||||
| Time to peak (ms) | CPR-L | 0.43 (0.07) | 15 | 0.92184 | 0.20549 | 1.46147 | p > 0.05 | |
| CPR-R | 0.39 (0.09) | 15 | 0.94504 | 0.44991 | ||||
| Duration (ms) | CPR-L | 1.90 (0.14) | 15 | 0.94479 | 0.44638 | −3.88483 | p < 0.001 | |
| CPR-R | 2.16 (0.21) | 15 | 0.95637 | 0.62964 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, J.C.; Pacheco-Ortiz, J.A.; Rodríguez-Sosa, L.; Calderón-Rosete, G.; Villagran-Vargas, E. Asymmetric Firing Rate from Crayfish Left and Right Caudal Photoreceptors Due to Blue and Green Monochromatic Light Pulses. Symmetry 2018, 10, 389. https://doi.org/10.3390/sym10090389
Sánchez-Hernández JC, Pacheco-Ortiz JA, Rodríguez-Sosa L, Calderón-Rosete G, Villagran-Vargas E. Asymmetric Firing Rate from Crayfish Left and Right Caudal Photoreceptors Due to Blue and Green Monochromatic Light Pulses. Symmetry. 2018; 10(9):389. https://doi.org/10.3390/sym10090389
Chicago/Turabian StyleSánchez-Hernández, Juan C., José Agustín Pacheco-Ortiz, Leonardo Rodríguez-Sosa, Gabina Calderón-Rosete, and Edgar Villagran-Vargas. 2018. "Asymmetric Firing Rate from Crayfish Left and Right Caudal Photoreceptors Due to Blue and Green Monochromatic Light Pulses" Symmetry 10, no. 9: 389. https://doi.org/10.3390/sym10090389
APA StyleSánchez-Hernández, J. C., Pacheco-Ortiz, J. A., Rodríguez-Sosa, L., Calderón-Rosete, G., & Villagran-Vargas, E. (2018). Asymmetric Firing Rate from Crayfish Left and Right Caudal Photoreceptors Due to Blue and Green Monochromatic Light Pulses. Symmetry, 10(9), 389. https://doi.org/10.3390/sym10090389





