Exploring and Exploiting the Symmetry-Breaking Effect of Cyclodextrins in Mechanomolecules
Abstract
1. Introduction
2. The Stereochemistry of CD-Based Mechanomolecules
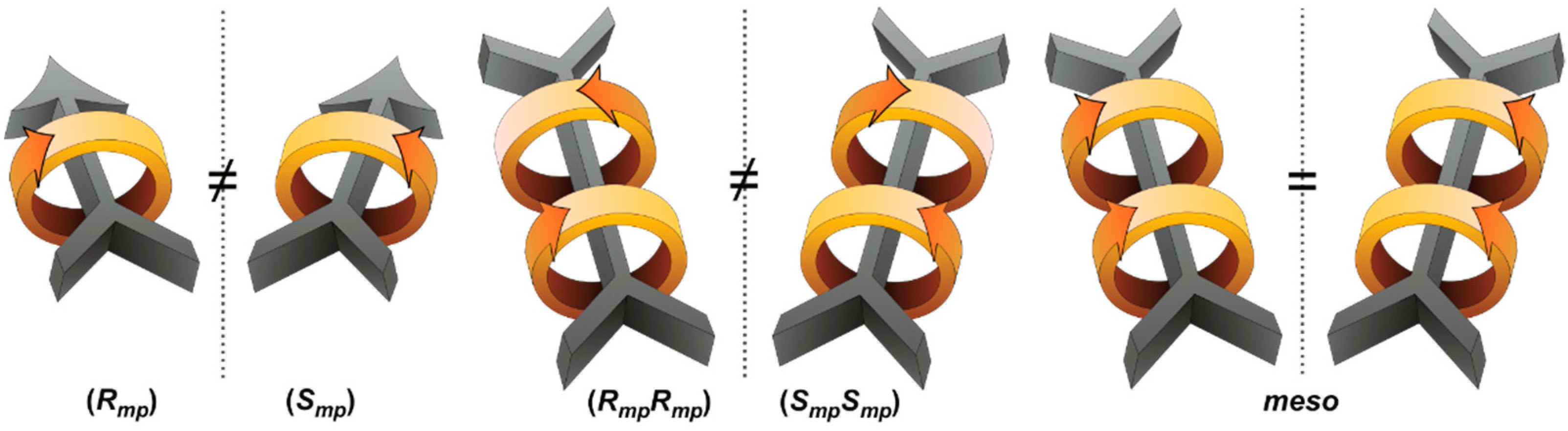
2.1. Orientational Mechanostereoisomers
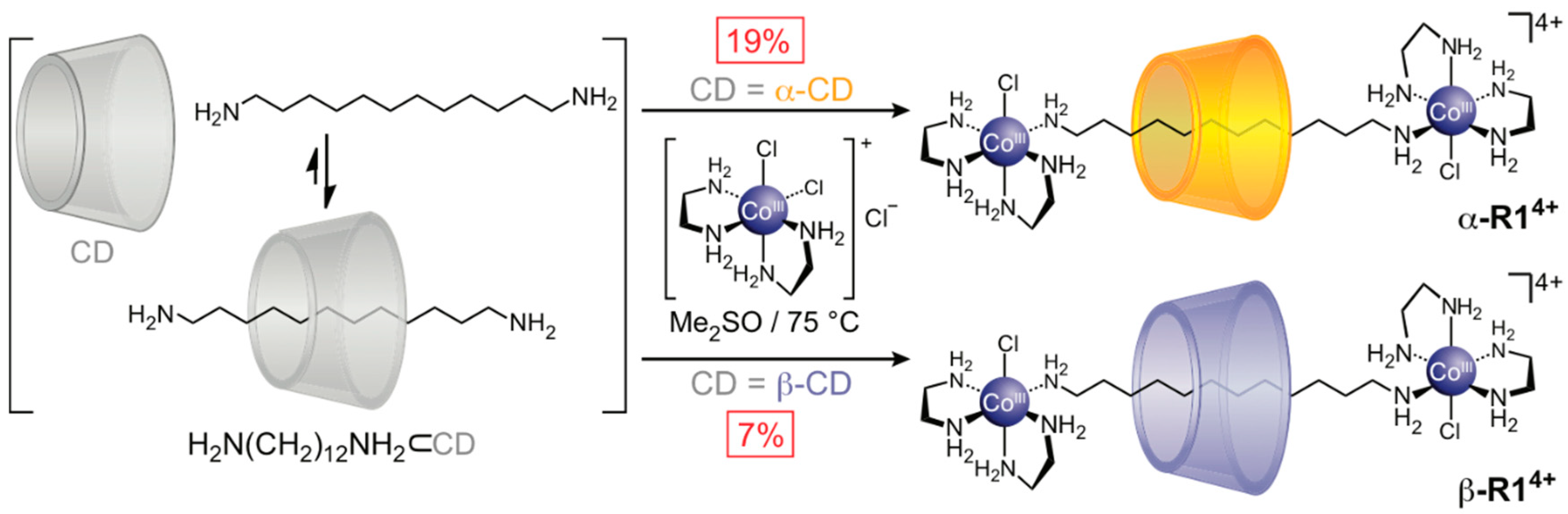
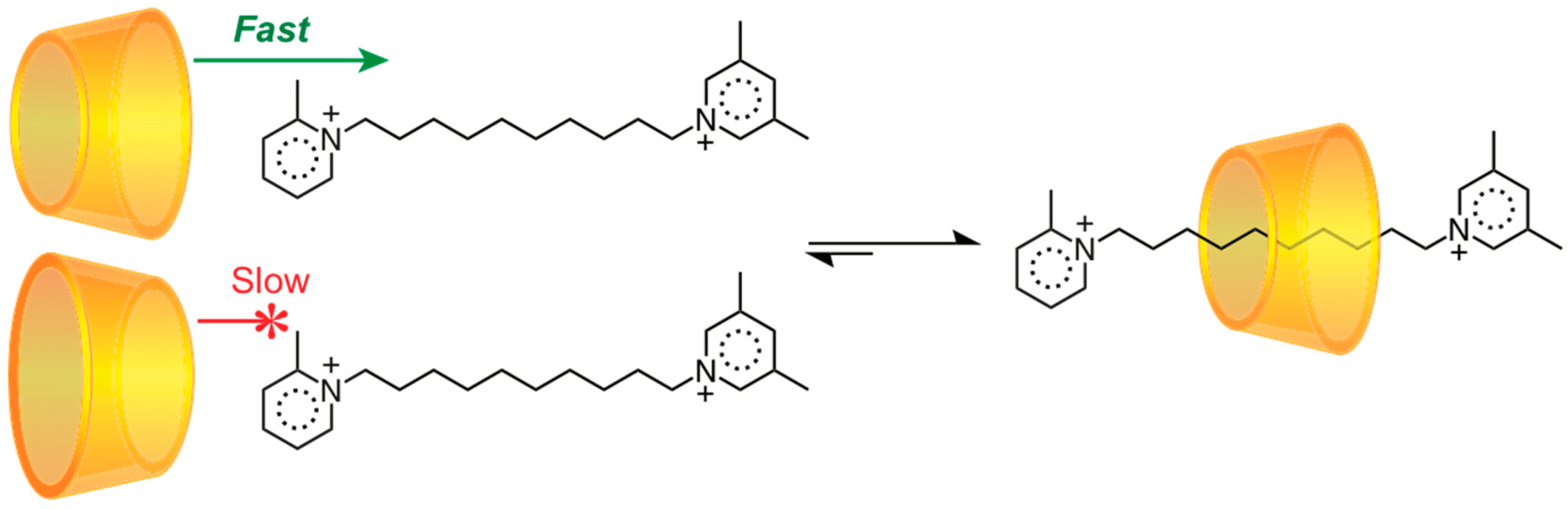
2.1.1. [2]Rotaxane Orientational Isomers
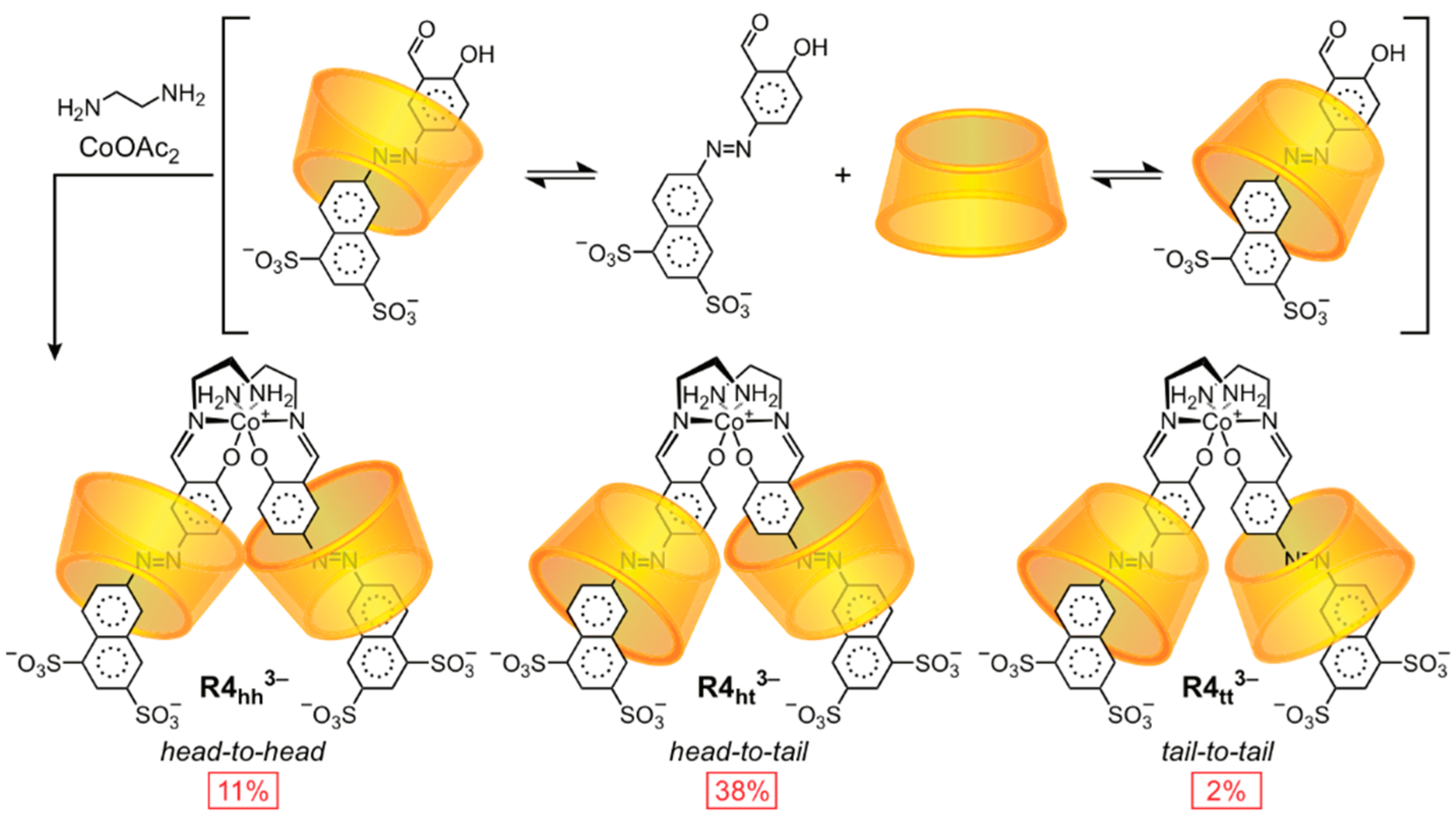
2.1.2. [3]Rotaxane Orientational Isomers
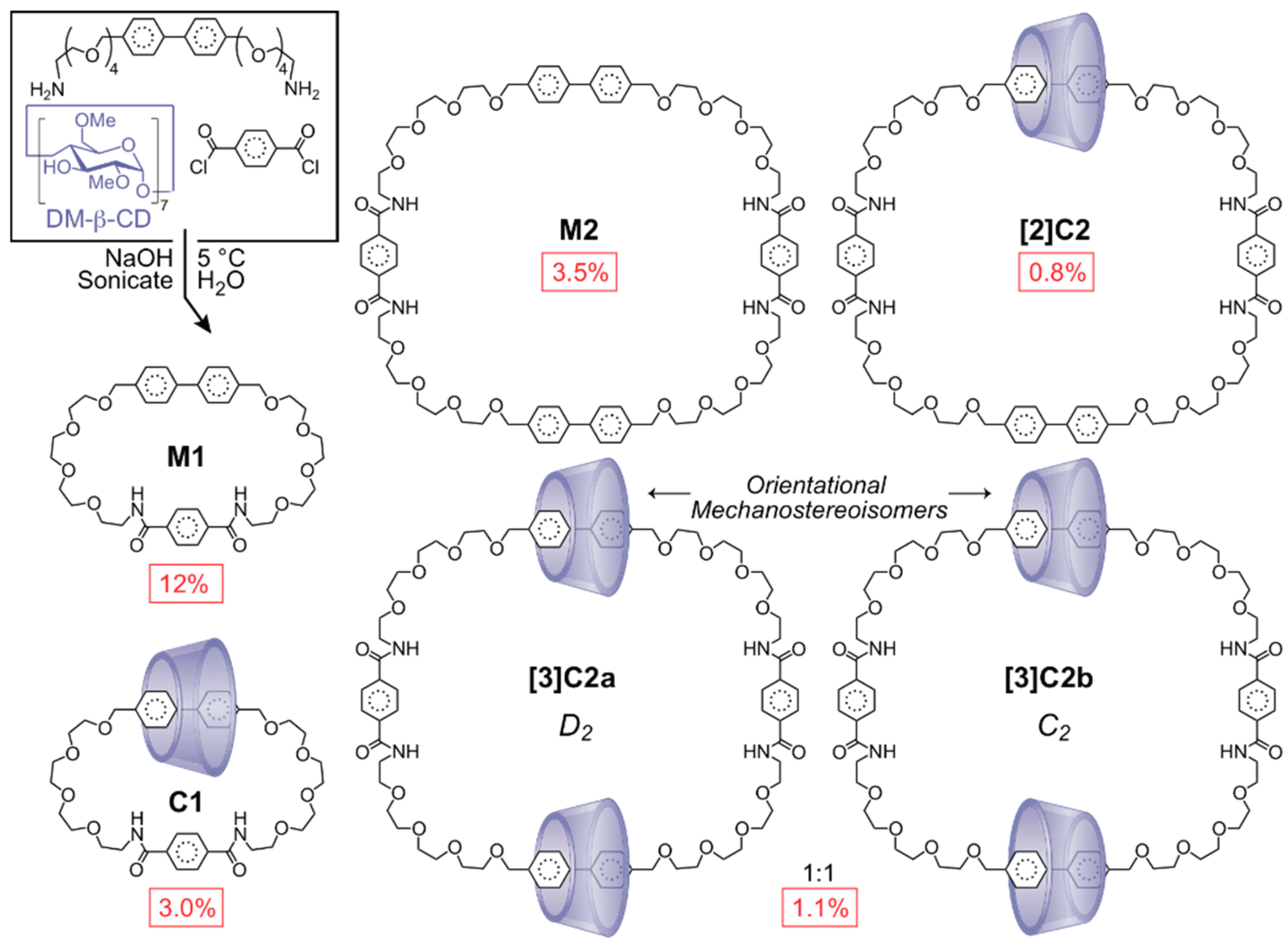
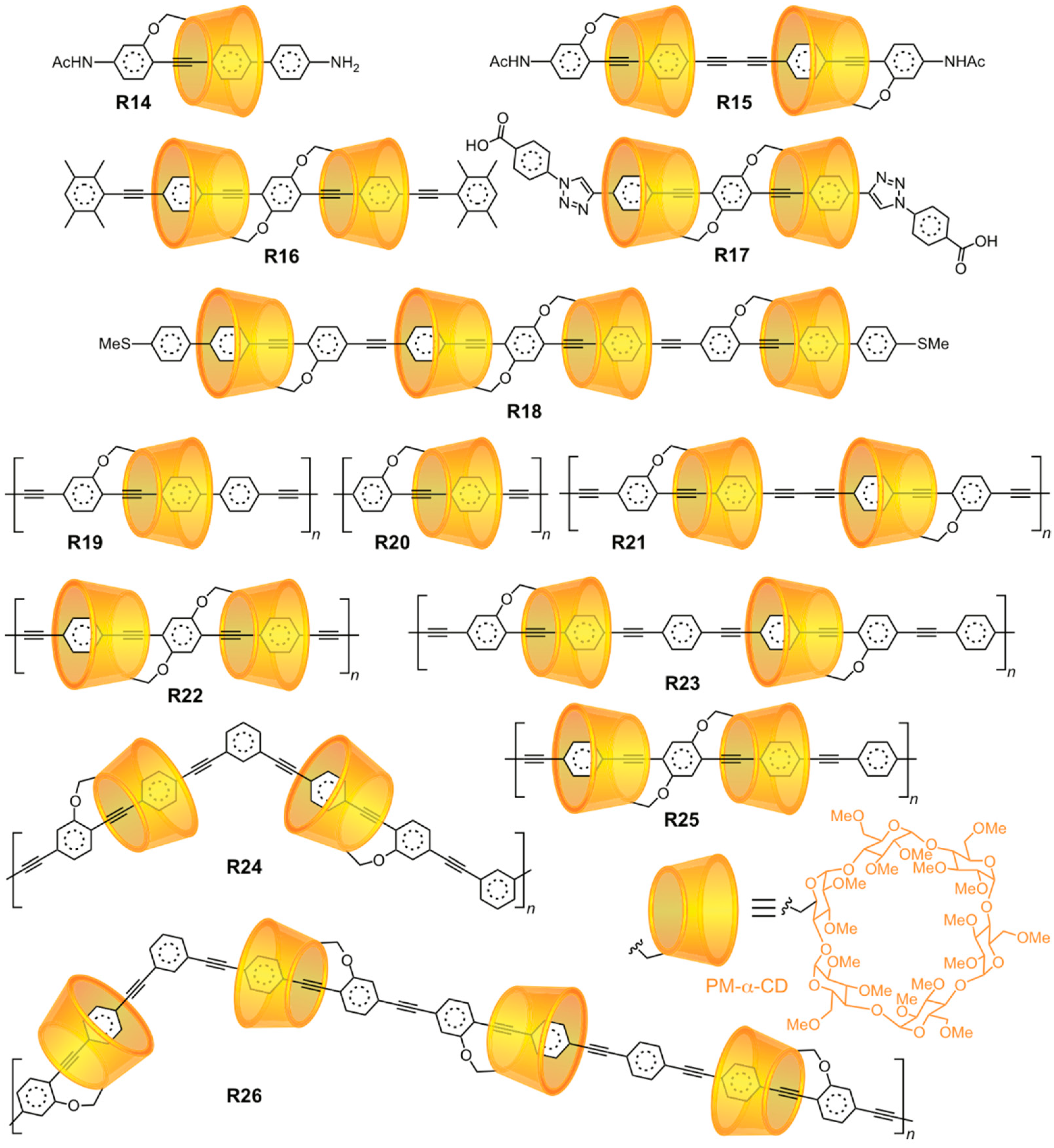
2.1.3. [n]Rotaxane Orientational Isomers
2.1.4. [3]Catenane Orientational Isomers
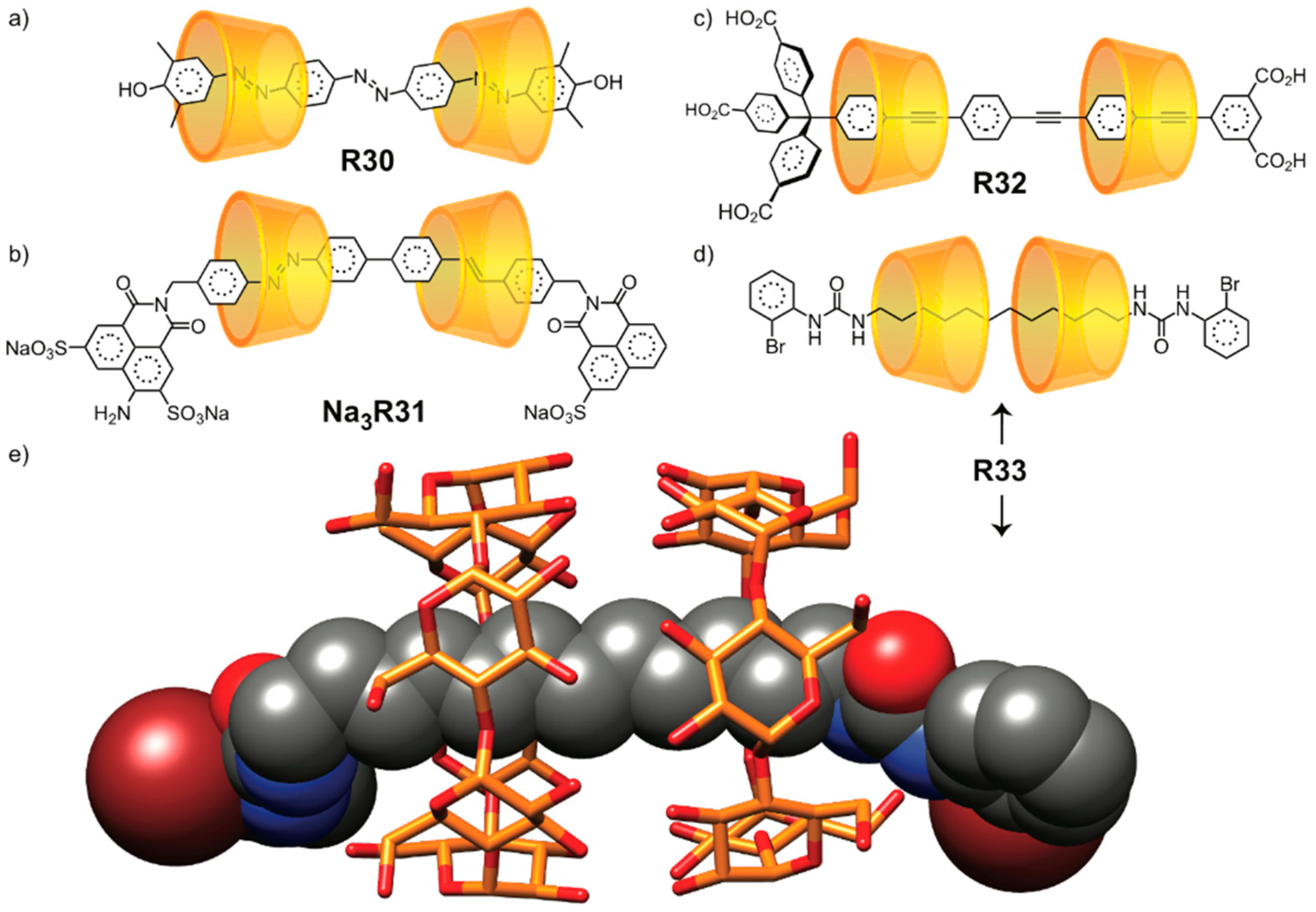
2.1.5. [n]Catenane Orientational Isomers
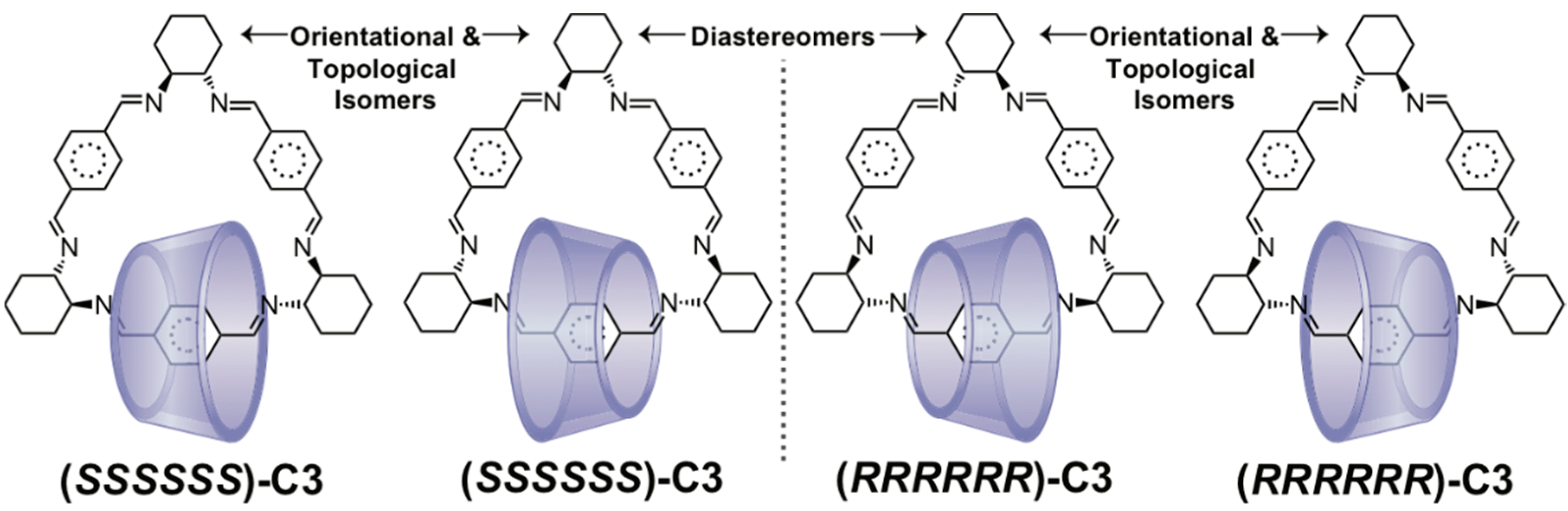
2.2. Mechanically Planar Chirality
2.3. Topological Chirality
3. Applications of CD Symmetry Breaking in Mechanomolecules
3.1. Stereoselectivc Synthesis
3.1.1. Regioselectivity
3.1.2. Orientational Selectivity
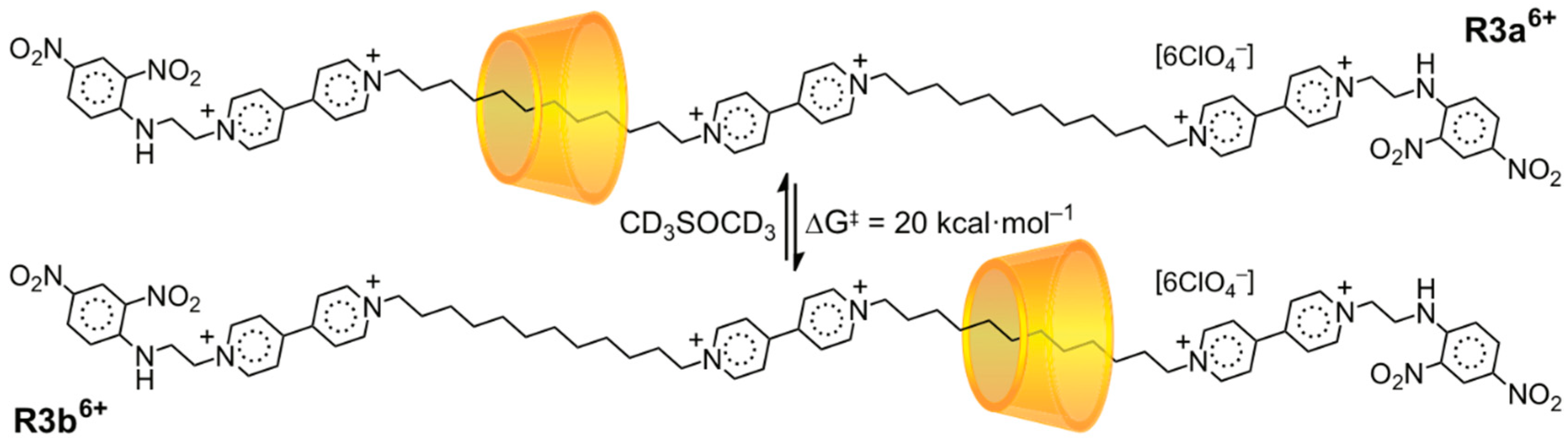
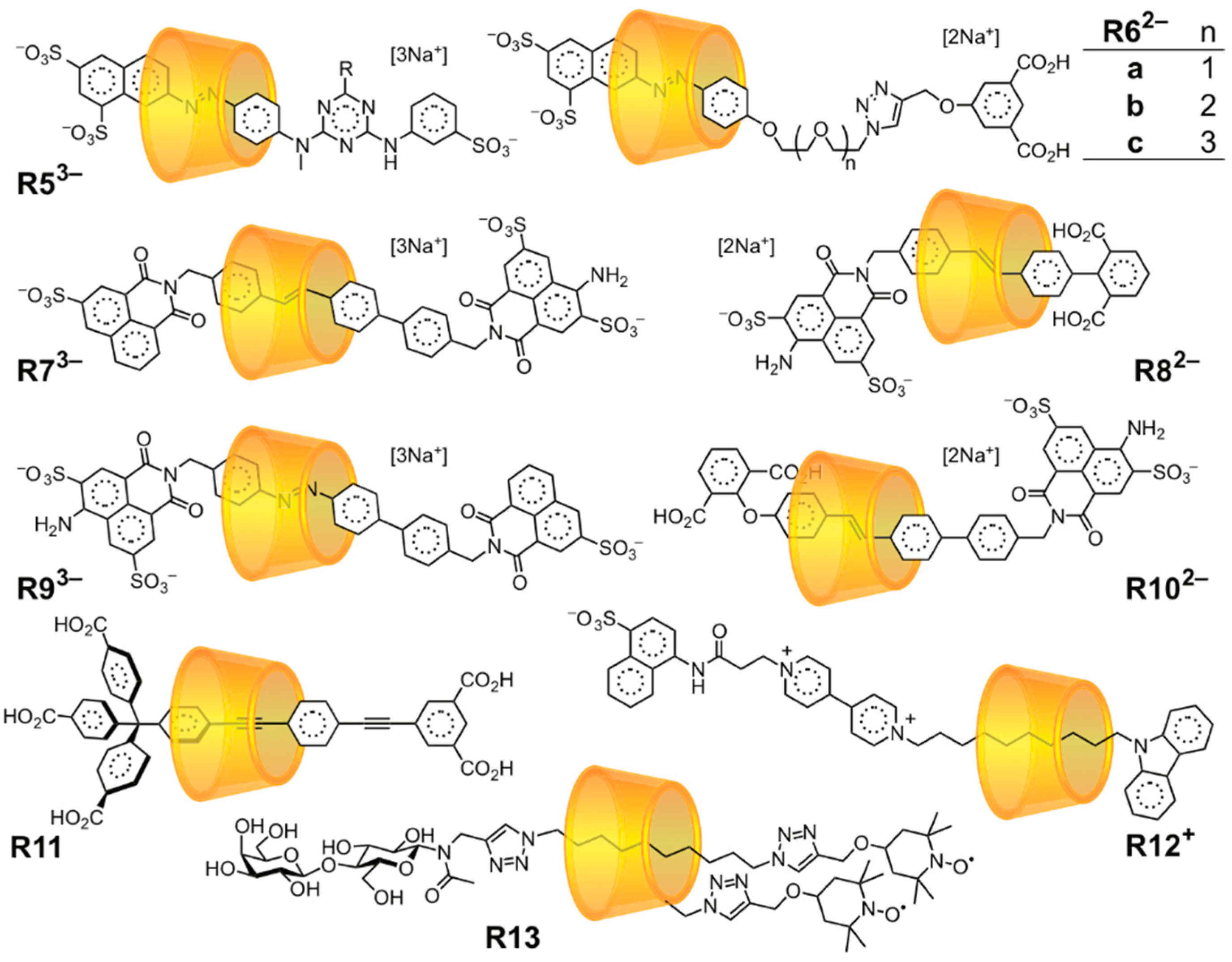
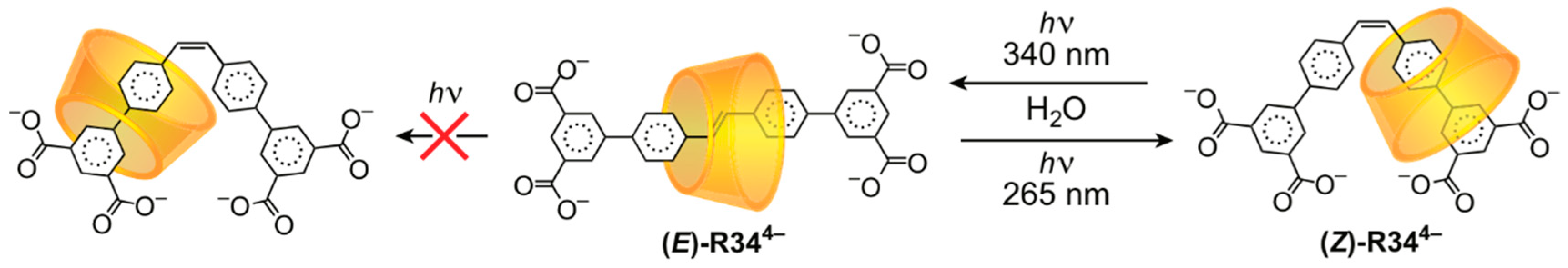
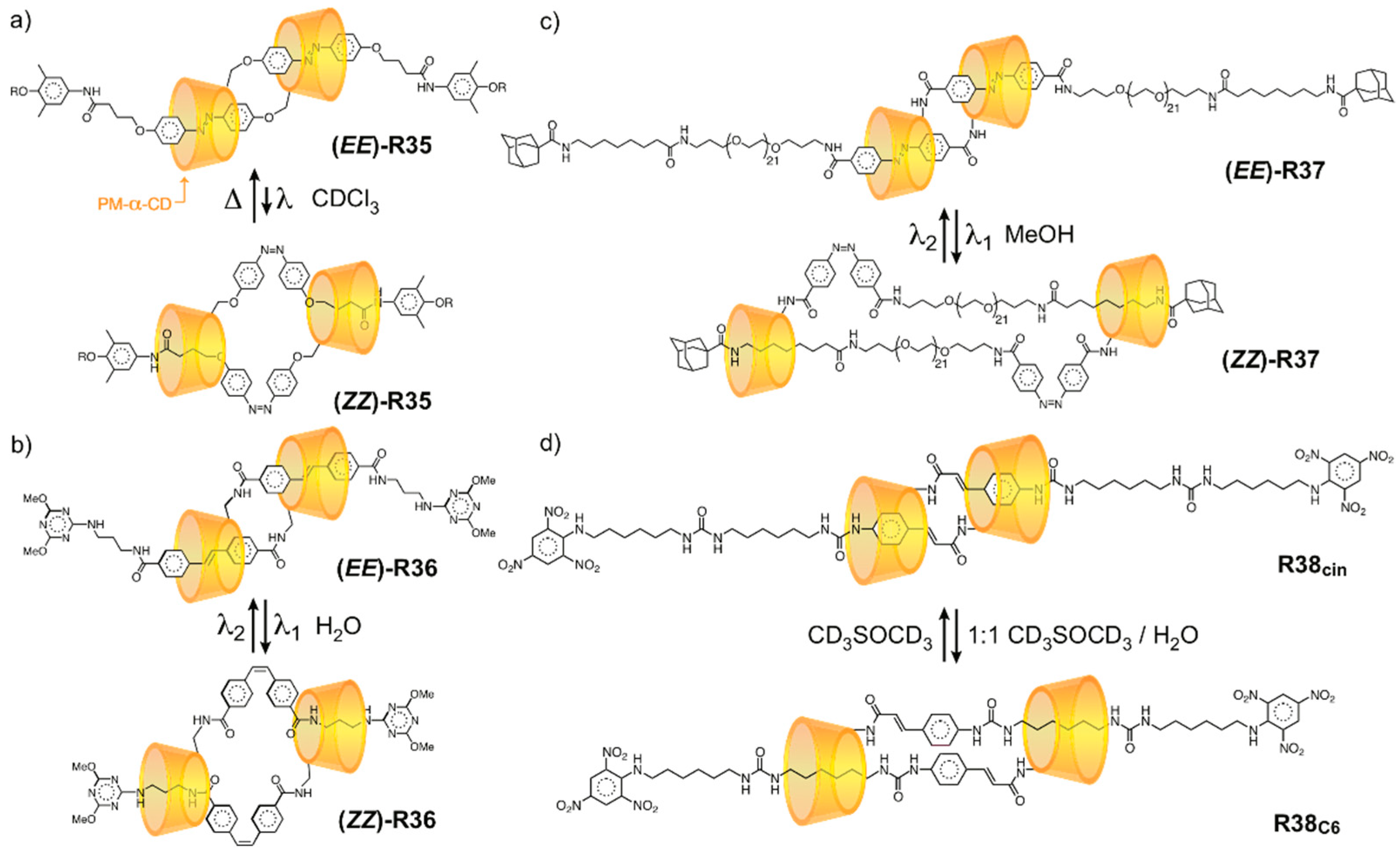
3.2. Mechanostereoselectivity—Biased Directional Motion
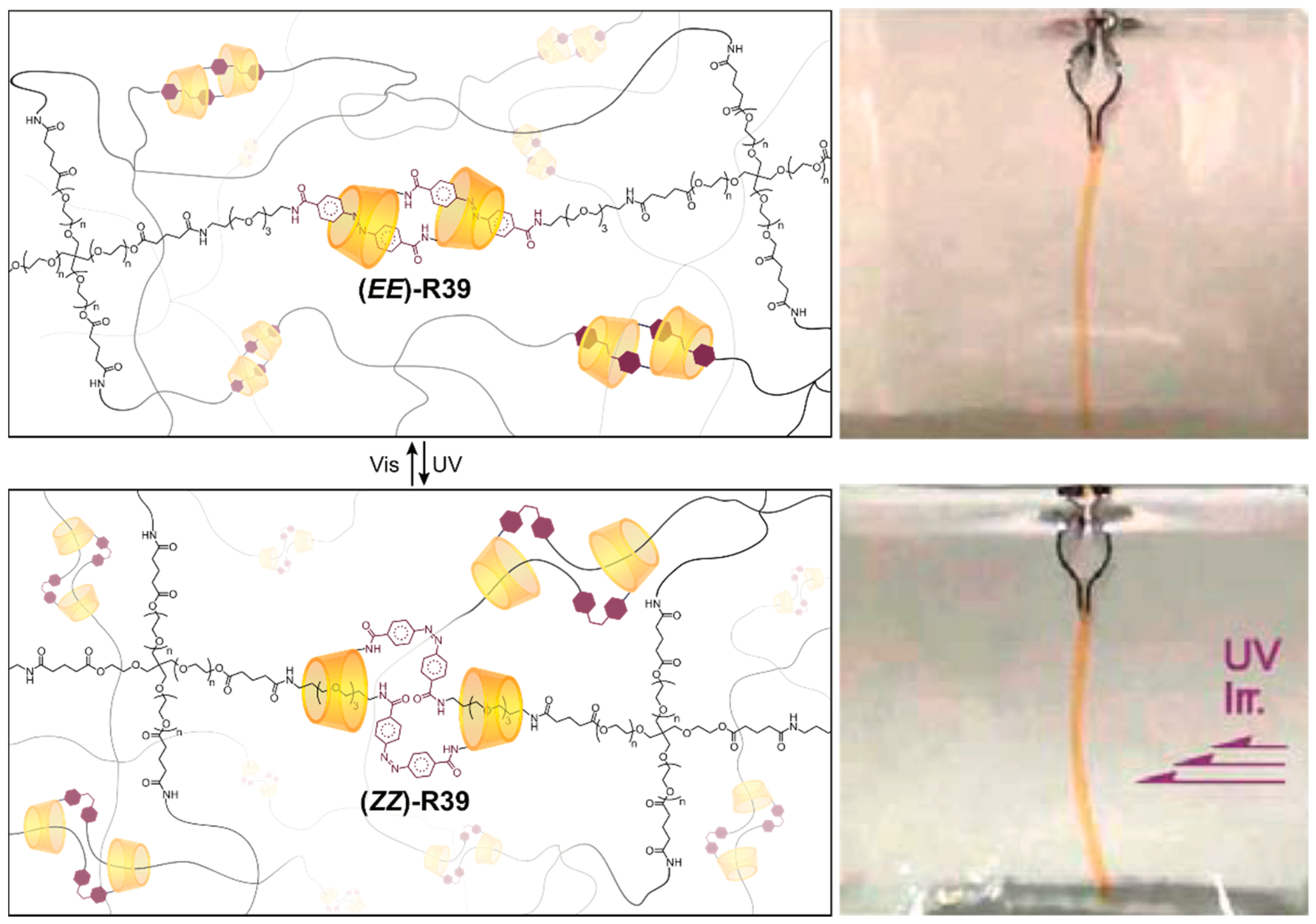
3.3. Networked “Slide-Ring” Materials
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Villiers, A. Sur La Transformation De La Fécule en Dextrine Par Le Ferment Butyrique. Compt. Rend. Fr. Acad. Sci. 1891, 112, 435–438. [Google Scholar]
- French, D.; Pulley, A.O.; Effenberger, J.A.; Rougvie, M.A.; Abdullah, M. Studies on the Schardinger Dextrins. XII. the Molecular Size and Structure of the Delta-, Epsilon-, Zeta-, and Eta-Dextrins. Arch. Biochem. Biophys. 1965, 111, 153–160. [Google Scholar] [CrossRef]
- Gessler, K.; Usón, I.; Takaha, T.; Krauss, N.; Smith, S.M.; Okada, S.; Sheldrick, G.M.; Saenger, W. V-Amylose at Atomic Resolution: X-Ray Structure of α-Cycloamylose with 26 Glucose Residues (Cyclomaltohexaicosaose). Proc. Natl. Acad. Sci. USA 1999, 96, 4246–4251. [Google Scholar] [CrossRef] [PubMed]
- French, D. The Schardinger Dextrins. Adv. Carbohydr. Chem. 1957, 12, 189–260. [Google Scholar] [PubMed]
- Freudenberg, K.; Cramer, F. Structure of the Schardinger Dextrins Alpha, Beta, and Gamma. Zeitschrift für Naturforschung 1948, 3b, 464. [Google Scholar] [CrossRef]
- Pringsheim, H. Chemistry of the Polysaccharides. Angew. Chem. Int. Ed. 1922, 35, 345–349. [Google Scholar] [CrossRef]
- Cramer, F. Uber Einschlubverbindungen, I. Mitteil.: Additionsverbuindungen Der Cycloamylosen. Chem. Ber. 1951, 84, 851–854. [Google Scholar] [CrossRef]
- Cramer, F. Über Einschlußverbindungen, II. Mitteil. Die Blauen Jodadditionsverbindungen Organischer Moleküle. Chem. Ber. 1951, 84, 855–859. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Cram, D.J. The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, R.L.; Sebastian, J.F.; Clowes, G.A.; Bender, M.L. Acceleration of Phenyl Ester Cleavage by Cycloamyloses. A Model for Enzymic Specificity. J. Am. Chem. Soc. 1967, 89, 3242–3253. [Google Scholar] [CrossRef]
- VanEtten, R.L.; Clowes, G.A.; Sebastian, J.F.; Bender, M.L. The Mechanism of the Cycloamylose-Accelerated Cleavage of Phenyl Esters. J. Am. Chem. Soc. 1967, 89, 3253–3262. [Google Scholar] [CrossRef]
- Breslow, R. Biomimetic Chemistry and Artificial Enzymes: Catalysis by Design. Acc. Chem. Res. 1995, 28, 146–153. [Google Scholar] [CrossRef]
- Li, S.; Purdy, W.C. Cyclodextrins and Their Applications in Analytical Chemistry. Chem. Rev. 1992, 92, 1457–1470. [Google Scholar] [CrossRef]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A Versatile Tool in Separation Science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef]
- Ke, C.; Smaldone, R.A.; Kikuchi, T.; Li, H.; Davis, A.P.; Stoddart, J.F. Quantitative Emergence of Hetero [4]Rotaxanes by Template-Directed Click Chemistry. Angew. Chem. Int. Ed. 2012, 52, 381–387. [Google Scholar] [CrossRef]
- Szejtli, J. Medicinal Applications of Cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as Food Ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F.; Nepogodiev, S.A. Cyclodextrin-Based Catenanes and Rotaxanes. Chem. Rev. 1998, 98, 1959–1976. [Google Scholar]
- Wenz, G.; Han, B.-H.; Müller, A. Cyclodextrin Rotaxanes and Polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. π-Conjugated Molecules Covered by Permethylated Cyclodextrins. Chem. Rec. 2011, 11, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Girek, T. Cyclodextrin-Based Rotaxanes. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 1–21. [Google Scholar] [CrossRef]
- Hashidzume, A.; Yamaguchi, H.; Harada, A. Cyclodextrin-Based Rotaxanes: From Rotaxanes to Polyrotaxanes and Further to Functional Materials. Eur. J. Org. Chem. 2019, 2019, 3344–3357. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Weinheim, Germany, 2016. [Google Scholar]
- Saito, H.; Yonemura, H.; Nakamura, H.; Matsuo, T. Stability and Exchange Properties of Through-Ring Cyclodextrin Complexes. Effects of Chain Length in Polymethylene Bis(1-pyridinium) as Guest Molecules. Chem. Lett. 1990, 19, 535–538. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakamura, H.; Matsuo, T. Formation of Through-Ring α-Cyclodextrin Complexes with α, Ω-Alkanedicarboxylate Anion. Effects of the Aliphatic Chain Length and Electrostatic Factors on the Complexation Behavior. Bull. Chem. Soc. Jpn. 1992, 65, 164–169. [Google Scholar] [CrossRef]
- Ogino, H. Relatively High-Yield Synthesis of Rotaxanes. Synthesis and Properties of Compounds Consisting of Cyclodextrins Threaded by α, Ω -Diaminoalkanes Coordinated to Cobalt(III) Complexes. J. Am. Chem. Soc. 1981, 103, 1303–1304. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Olson, M.A.; Botros, Y.Y.; Stoddart, J.F. Mechanostereochemistry. Pure Appl. Chem. 2010, 82, 1569–1574. [Google Scholar] [CrossRef]
- Isnin, R.; Kaifer, A.E. Novel Class of Asymmetric Zwitterionic Rotaxanes Based on α-Cyclodextrin. J. Am. Chem. Soc. 1991, 113, 8188–8190. [Google Scholar] [CrossRef]
- Isnin, R.; Kaifer, A.E. A New Approach to Cyclodextrin-Based Rotaxanes. Pure Appl. Chem. 1993, 65, 495–498. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Harada, A. A Cyclodextrin-Based Molecular Shuttle Containing Energetically Favored and Disfavored Portions in Its Dumbbell Component. Org. Lett. 2000, 2, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Buston, J.E.H.; Young, J.R.; Anderson, H.L. Rotaxane-Encapsulated Cyanine Dyes: Enhanced Fluorescence Efficiency and Photostability. Chem. Commun. 2000, 11, 905–906. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Anderson, H.L.; Marken, F. Enhanced Chemical Reversibility of Redox Processes in Cyanine Dye Rotaxanes. Chem. Commun. 2001, 11, 1046–1047. [Google Scholar] [CrossRef]
- Saudan, C.; Dunand, F.A.; Abou-Hamdan, A.; Bugnon, P.; Lye, P.G.; Lincoln, S.F.; Merbach, A.E. A Model for Sequential Threading of α-Cyclodextrin Onto a Guest: A Complete Thermodynamic and Kinetic Study in Water. J. Am. Chem. Soc. 2001, 123, 10290–10298. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, H.J. Isomeric [2]Rotaxanes and Unidirectional [2]Pseudorotaxane Composed of α-Cyclodextrin and Aliphatic Chain-Linked Carbazole-Viologen Compounds. Org. Lett. 2004, 6, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.J.; Macartney, D.H. Orientational Isomers of α-Cyclodextrin [2]Semi-Rotaxanes with Asymmetric Dicationic Threads. Org. Biomol. Chem. 2005, 3, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Takashima, Y.; Yamaguchi, H.; Harada, A. Kinetic Control of Threading of Cyclodextrins onto Axle Molecules. J. Am. Chem. Soc. 2005, 127, 12186–12187. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.J.; Macartney, D.H. Orientational Isomers of Cyclodextrin Semirotaxanes and Rotaxanes with Organic and Transition Metal Complex Stoppers. Supramol. Chem. 2007, 19, 537–546. [Google Scholar] [CrossRef]
- Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. A Molecular Reel: Shuttling of a Rotor by Tumbling of a Macrocycle. J. Org. Chem. 2010, 75, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Claridge, T.D.W.; Anderson, H.L. Metal-Driven Ligand Assembly in the Synthesis of Cyclodextrin [2] and [3]Rotaxanes. Org. Biomol. Chem. 2007, 5, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.W.; Klotz, E.J.F.; Odell, B.; Claridge, T.D.W.; Anderson, H.L. Solid-Phase Synthesis of Oligo (Phenylene Ethynylene) Rotaxanes. Angew. Chem. Int. Ed. 2007, 46, 6845–6848. [Google Scholar] [CrossRef]
- Craig, M.R.; Claridge, T.D.W.; Anderson, H.L.; Hutchings, M.G. Synthesis of a Cyclodextrin Azo Dye [3]Rotaxane as a Single Isomer. Chem. Commun. 1999, 16, 1537–1538. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Ma, X.; Tian, H. A [3]Rotaxane with Three Stable States That Responds to Multiple-Inputs and Displays Dual Fluorescence Addresses. Chem. Eur. J. 2005, 11, 5929–5937. [Google Scholar] [CrossRef]
- Akae, Y.; Koyama, Y.; Kuwata, S.; Takata, T. Cyclodextrin-Based Size-Complementary [3]Rotaxanes: Selective Synthesis and Specific Dissociation. Chem. Eur. J. 2014, 20, 17132–17136. [Google Scholar] [CrossRef]
- Akae, Y.; Sogawa, H.; Takata, T. Cyclodextrin-Based [3]Rotaxane-Crosslinked Fluorescent Polymer: Synthesis and De-Crosslinking Using Size Complementarity. Angew. Chem. Int. Ed. 2018, 57, 14832–14836. [Google Scholar] [CrossRef]
- Akae, Y.; Sogawa, H.; Takata, T. Synthesis of a Structure-Definite α-Cyclodextrin-Based Macromolecular [3]Rotaxane Using a Size-Complementary Method. Angew. Chem. Int. Ed. 2018, 57, 11742–11746. [Google Scholar] [CrossRef] [PubMed]
- Akae, Y.; Sogawa, H.; Takata, T. Effective Synthesis and Modification of α-Cyclodextrin-Based [3]Rotaxanes Enabling Versatile Molecular Design. Eur. J. Org. Chem. 2019, 22, 3605–3613. [Google Scholar] [CrossRef]
- Sakamoto, K.; Takashima, Y.; Yamaguchi, H.; Harada, A. Preparation and Properties of Rotaxanes Formed by Dimethyl-β-Cyclodextrin and Oligo(Thiophene)s with β-Cyclodextrin Stoppers. J. Org. Chem. 2007, 72, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, M.; Auzély, R.; Fort, S. Synthesis and Biological Evaluation of Multivalent Carbohydrate Ligands Obtained by Click Assembly of Pseudo-Rotaxanes. Org. Biomol. Chem. 2009, 7, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Suzaki, Y.; Osakada, K. [5]Rotaxanes Composed of α-Cyclodextrin and Pd or Pt Complexes with Alkylbipyridinium Ligands. Chem. Lett. 2008, 37, 182–183. [Google Scholar] [CrossRef]
- Taira, T.; Suzaki, Y.; Osakada, K. Pd-II and Pt-III Complexes with Amphiphilic Ligands: Formation of Micelles and [5]Rotaxanes with α-Cyclodextrin in Aqueous Solution. Chem. Asian J. 2008, 3, 895–902. [Google Scholar] [CrossRef]
- Taira, T.; Suzaki, Y.; Osakada, K. Metallohydrogel Formed From Amphiphilic Pd Complex and α-Cyclodextrin: Control of Its Sol-Gel Transition. Chem. Lett. 2013, 42, 1062–1064. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The Molecular Necklace: A Rotaxane Containing Many Threaded α-Cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Liu, P.; Chipot, C.; Shao, X.; Cai, W. How Do α-Cyclodextrins Self-Organize on a Polymer Chain? J. Phys. Chem. C 2012, 116, 17913–17918. [Google Scholar] [CrossRef]
- Lüttringhaus, A.; Cramer, F.; Prinzbach, H.; Henglein, F.M. Cyclisationen Von Langkettigen Dithiolen. Versuche Zur Darstellung Sich Umfassender Ringe Mit Hilfe Von Einschlußverbindungen. Liebigs Ann. Chem. 1958, 613, 185–198. [Google Scholar] [CrossRef]
- Armspach, D.; Ashton, P.R.; Moore, C.P.; Spencer, N.; Stoddart, J.F.; Wear, T.J.; Williams, D.J. Self-Assembly of Catenanes with Cyclodextrin Units. Angew. Chem. Int. Ed. Engl. 1993, 32, 854–858. [Google Scholar] [CrossRef]
- Armspach, D.; Ashton, P.R.; Ballardini, R.; Balzani, V.; Godi, A.; Moore, C.; Prodi, L.; Spencer, N.; Stoddart, J.F.; Tolley, M.S.; et al. Catenated Cyclodextrins. Chem. Eur. J. 1995, 1, 33–55. [Google Scholar] [CrossRef]
- Li, J.; Nowak, P.; Fanlo-Virgós, H.; Otto, S. Catenanes from Catenanes: Quantitative Assessment of Cooperativity in Dynamic Combinatorial Catenation. Chem. Sci. 2014, 5, 4968–4974. [Google Scholar] [CrossRef]
- Okada, M.; Harada, A. Poly(Polyrotaxane): Photoreactions of 9-Anthracene-Capped Polyrotaxane. Macromolecules 2003, 36, 9701–9703. [Google Scholar] [CrossRef]
- Higashi, T.; Morita, K.; Song, X.; Zhu, J.; Tamura, A.; Yui, N.; Motoyama, K.; Arima, H.; Li, J. One-Pot Synthesis of Cyclodextrin-Based Radial Poly [n]Catenanes. Commun. Chem. 2019, 2, 78. [Google Scholar] [CrossRef]
- Prelog, V.; Gerlach, H. Cycloenantiomerie Und Cyclodiastereomerie. 1. Mitteilung. Helv. Chim. Acta 1964, 47, 2288–2294. [Google Scholar] [CrossRef]
- Schill, G. Catenanes, Rotaxanes, and Knots; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Jäger, R.; Händel, M.; Harren, J.; Rissanen, K.; Vögtle, F. Chemistry with Rotaxanes: Intra-and Intermolecularly Covalently Linked Rotaxanes. Liebigs Ann. 1996, 1996, 1201–1207. [Google Scholar] [CrossRef]
- Yamamoto, C.; Okamoto, Y.; Schmidt, T.; Vögtle, F. Enantiomeric Resolution of Cycloenantiomeric Rotaxane, Topologically Chiral Catenane, and Pretzel-Shaped Molecules: Observation of Pronounced Circular Dichroism. J. Am. Chem. Soc. 1997, 119, 10547–10548. [Google Scholar] [CrossRef]
- Schmieder, R.; Hubner, G.; Seel, C.; Vögtle, F. The First Cyclodiasteromeric [3]Rotaxane. Angew. Chem. Int. Ed. 1999, 38, 3528–3530. [Google Scholar] [CrossRef]
- Reuter, C.; Schmieder, R.; Vögtle, F. From Rotaxanes to Knots. Templating, Hydrogen Bond Patterns, and Cyclochirality. Pure Appl. Chem. 2000, 72, 2233–2241. [Google Scholar] [CrossRef]
- Reuter, C.; Seel, C.; Nieger, M.; Vögtle, F. Chiral [1]Rotaxanes: X-Ray Structures and Chiroptical Properties. Helv. Chim. Acta 2000, 83, 630–640. [Google Scholar] [CrossRef]
- Eliel, E.; Wilen, S.; Mander, L. Stereochemistry of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Makita, Y.; Kihara, N.; Nakakoji, N.; Takata, T.; Inagaki, S.; Yamamoto, C.; Okamoto, Y. Catalytic Asymmetric Synthesis and Optical Resolution of Planar Chiral Rotaxane. Chem. Lett. 2007, 36, 162–163. [Google Scholar] [CrossRef]
- Bordoli, R.J.; Goldup, S.M. An Efficient Approach to Mechanically Planar Chiral Rotaxanes. J. Am. Chem. Soc. 2014, 136, 4817–4820. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Craig, M.R.; Hutchings, M.G.; Claridge, T.D.W.; Anderson, H.L. Rotaxane-Encapsulation Enhances the Stability of an Azo Dye, in Solution and When Bonded to Cellulose. Angew. Chem. Int. Ed. 2001, 40, 1071–1074. [Google Scholar] [CrossRef]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional Mesoporous Silica Nanoparticles for Photothermal-Controlled Drug Delivery in Vivo. Angew. Chem. Int. Ed. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhu, L.; Li, X.; Kwok, A.; Pan, X.; Zhao, Y. A Photoswitchable [2]Rotaxane Array on Graphene Oxide. Asian J. Org. Chem. 2012, 1, 314–318. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, L.; Li, X.; Kwok, A.; Li, X.; Ågren, H.; Zhao, Y. Photothermal-Responsive [2]Rotaxanes. RSC Adv. 2013, 3, 2341. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Qu, D.-H.; Ren, J.; Chen, K.; Tian, H. A Lockable Light-Driven Molecular Shuttle with a Fluorescent Signal. Angew. Chem. Int. Ed. 2004, 43, 2661–2665. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Ren, J.; Tian, H. A Light-Driven Rotaxane Molecular Shuttle with Dual Fluorescence Addresses. Org. Lett. 2004, 6, 2085–2088. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Ma, X.; Qu, D.-H.; Tian, H. Unidirectional Threading Synthesis of Isomer-Free [2]Rotaxanes. Chem. Eur. J. 2006, 12, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Song, H.J.; Chang, H.-J. Unidirectional α-Cyclodextrin-Based [2]Rotaxanes Bearing Viologen Unit on Axle. Tetrahedron Lett. 2006, 47, 3831–3834. [Google Scholar] [CrossRef]
- Casati, C.; Franchi, P.; Pievo, R.; Mezzina, E.; Lucarini, M. Unraveling Unidirectional Threading of α-Cyclodextrin in a [2]Rotaxane Through Spin Labeling Approach. J. Am. Chem. Soc. 2012, 134, 19108–19117. [Google Scholar] [CrossRef] [PubMed]
- Frisch, H.L.; Wasserman, E. Chemical Topology. J. Am. Chem. Soc. 1961, 83, 3789–3795. [Google Scholar] [CrossRef]
- Prelog, V. Problems in Chemical Topology. Chemistry Britain 1968, 4, 382. [Google Scholar]
- Sokolov, V.I. Topological Ideas in Stereochemistry. Russian Chem. Rev. 1973, 42, 452–463. [Google Scholar] [CrossRef]
- Walba, D.M. Topological Stereochemistry. Tetrahedron 1985, 41, 3161–3212. [Google Scholar] [CrossRef]
- Walba, D.M. A Topological Hierarchy of Molecular Chirality and Other Tidbits in Topological Stereochemistry. In New Developments in Molecular Chirality; Mezey, P.G., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1991; Volume 5, pp. 119–129. [Google Scholar]
- Chambron, J.-C.; Dietrich-Buchecker, C.; Dietrich-Buchecker, C.; Sauvage, J.-P. From Classical Chirality to Topologically Chiral Catenands and Knots. Top. Curr. Chem. 1993, 165, 131–162. [Google Scholar]
- Liang, C.; Mislow, K. Topological Chirality and Achirality of Links. J. Math. Chem. 1995, 18, 1–24. [Google Scholar] [CrossRef]
- Breault, G.A.; Hunter, C.A.; Mayers, P.C. Supramolecular Topology. Tetrahedron 1999, 55, 5265–5293. [Google Scholar] [CrossRef]
- Siegel, J.S. Chemical Topology and Interlocking Molecules. Science 2004, 304, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Fenlon, E.E. Open Problems in Chemical Topology. Eur. J. Org. Chem. 2008, 30, 5023–5035. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Pérez-García, L. Topology in Molecules Inspired, Seen and Represented. Chem. Soc. Rev. 2009, 38, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Forgan, R.S.; Sauvage, J.-P.; Stoddart, J.F. Chemical Topology: Complex Molecular Knots, Links, and Entanglements. Chem. Rev. 2011, 111, 5434–5464. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Sakamoto, S.; Yamaguchi, K.; Hong, J.-I. Versatile Formation of [2]Catenane and [2]Pseudorotaxane Structures; Threading and Noncovalent Stoppering by a Self-Assembled Macrocycle. Org. Lett. 2004, 6, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nie, Y.; Saha, M.L.; He, Z.; Jiang, L.; Zhou, Z.; Stang, P.J. Photoreversible [2] Catenane via the Host–Guest Interactions Between a Palladium Metallacycle and β-Cyclodextrin. Inorg. Chem. 2015, 54, 11807–11812. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, H.Y.; Yee, C.-C.; Hung Ng, A.W.; Hu, K. Strategies to Assemble Catenanes with Multiple Interlocked Macrocycles. Inorg. Chem. 2017, 57, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Tang, B. Synthesis of Diastereomeric Trianglamine-β-Cyclodextrin-[2]-Catenanes. Tetrahedron Lett. 2006, 47, 2985–2988. [Google Scholar] [CrossRef]
- Gawroński, J.; Kołbon, H.; Kwit, M.; Katrusiak, A. Designing Large Triangular Chiral Macrocycles: Efficient [3+3] Diamine−Dialdehyde Condensations Based on Conformational Bias. J. Org. Chem. 2000, 65, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Forgan, R.S.; Blackburn, A.K.; Boyle, M.M.; Schneebeli, S.T.; Stoddart, J.F. The Topological and Chemical Implications of Introducing Oriented Rings to [3]Catenanes. Supramol. Chem. 2014, 26, 192–201. [Google Scholar] [CrossRef]
- Meyer, C.D.; Forgan, R.S.; Chichak, K.; Peters, A.J.; Tangchaivang, N.; Cave, G.W.V.; Khan, S.I.; Cantrill, S.J.; Stoddart, J.F. The Dynamic Chemistry of Molecular Borromean Rings and Solomon Knots. Chem. Eur. J. 2010, 16, 12570–12581. [Google Scholar] [CrossRef] [PubMed]
- Escandar, G.M.; Muñoz de la Peña, A. Room-Temperature Phosphorescence of Acenaphthene in Aerated Solutions in the Presence of Bromoalcohols and γ-Cyclodextrin. Anal. Chim. Acta 1998, 370, 199–205. [Google Scholar] [CrossRef]
- Klotz, E.J.F.; Claridge, T.D.W.; Anderson, H.L. Homo- and Hetero-[3]Rotaxanes with Two Pi-Systems Clasped in a Single Macrocycle. J. Am. Chem. Soc. 2006, 128, 15374–15375. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, G.; Fujimoto, T.; Kaneda, T. Synthesis and Characterization of the First Pair of an Unlocked and a Locked Self-Inclusion Complex From a Permethylated α-Cyclodextrin Derivative. Chem. Lett. 2003, 32, 536–537. [Google Scholar] [CrossRef]
- Onagi, H.; Blake, C.J.; Easton, C.J.; Lincoln, S.F. Installation of a Ratchet Tooth and Pawl to Restrict Rotation in a Cyclodextrin Rotaxane. Chem. Eur. J. 2003, 9, 5978–5988. [Google Scholar] [CrossRef]
- Ma, X.; Qu, D.; Ji, F.; Wang, Q.; Zhu, L.; Xu, Y.; Tian, H. A Light-Driven [1]Rotaxane via Self-Complementary and Suzuki-Coupling Capping. Chem. Commun. 2007, 14, 1409–1411. [Google Scholar] [CrossRef]
- Terao, J. Permethylated Cyclodextrin-Based Insulated Molecular Wires. Polym. Chem. 2011, 2, 2444–2452. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Kambe, N. Synthesis of an Organic-Soluble π-Conjugated [1]Rotaxane. Chem. Lett. 2009, 38, 76–77. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Tsurui, K.; Kambe, N. Synthesis of a Linked [1]-[1] Rotaxane. Chem. Lett. 2009, 38, 190–191. [Google Scholar] [CrossRef]
- Tsuda, S.; Terao, J.; Tanaka, Y.; Maekawa, T.; Kambe, N. Synthesis of Linked Symmetrical [3] and [5]Rotaxanes Having an Oligomeric Phenylene Ethynylene (OPE) Core Skeleton as a π-Conjugated Guest via Double Intramolecular Self-Inclusion. Tetrahedron Lett. 2009, 50, 1146–1150. [Google Scholar] [CrossRef]
- Terao, J.; Ikai, K.; Kambe, N.; Seki, S.; Saeki, A.; Ohkoshi, K.; Fujihara, T.; Tsuji, Y. Synthesis of a Head-to-Tail-Type Cyclodextrin-Based Insulated Molecular Wire. Chem. Commun. 2011, 47, 6816–6818. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tsuda, S.; Tanaka, Y.; Okoshi, K.; Fujihara, T.; Tsuji, Y.; Kambe, N. Synthesis of Organic-Soluble Conjugated Polyrotaxanes by Polymerization of Linked Rotaxanes. J. Am. Chem. Soc. 2009, 131, 16004–16005. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tanaka, Y.; Tsuda, S.; Kambe, N.; Taniguchi, M.; Kawai, T.; Saeki, A.; Seki, S. Insulated Molecular Wire with Highly Conductive π-Conjugated Polymer Core. J. Am. Chem. Soc. 2009, 131, 18046–18047. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Tsuda, S.; Tsurui, K.; Kambe, N. Synthesis of Highly Insulated Molecular Wires by Polymerization of Organic-Soluble Symmetrical Linked Inclusion Complex Monomers. Macromol. Symp. 2010, 297, 54–60. [Google Scholar] [CrossRef]
- Terao, J.; Wadahama, A.; Matono, A.; Tada, T.; Watanabe, S.; Seki, S.; Fujihara, T.; Tsuji, Y. Design Principle for Increasing Charge Mobility of π-Conjugated Polymers Using Regularly Localized Molecular Orbitals. Nat. Commun. 2013, 4, 1691. [Google Scholar] [CrossRef]
- Terao, J.; Hosomi, T.; Masai, H.; Matsuda, W.; Seki, S.; Fujihara, T.; Tsuji, Y. Synthesis and Redox Response of Insulated Molecular Wire Elongated Through Iron–Terpyridine Coordination Bonds. Chem. Lett. 2014, 43, 1289–1291. [Google Scholar] [CrossRef]
- Frampton, M.J.; Anderson, H.L. Insulated Molecular Wires. Angew. Chem. Int. Ed. 2007, 46, 1028–1064. [Google Scholar] [CrossRef]
- Baxter, I.; Ashton, P.R.; Cantrill, S.J.; Fyfe, M.C.T.; Glink, P.T.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Supramolecular Daisy Chains. Angew. Chem. Int. Ed. 1998, 37, 1294–1297. [Google Scholar]
- Rotzler, J.; Mayor, M. Molecular Daisy Chains. Chem. Soc. Rev. 2013, 42, 44–62. [Google Scholar] [CrossRef]
- Fujimoto, T.; Sakata, Y.; Kaneda, T. The First Janus [2]Rotaxane. Chem. Commun. 2000, 21, 2143–2144. [Google Scholar] [CrossRef]
- Hoshino, T.; Miyauchi, M.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Daisy Chain Necklace: Tri [2]rotaxane Containing Cyclodextrins. J. Am. Chem. Soc. 2000, 122, 9876–9877. [Google Scholar] [CrossRef]
- Miyawaki, A.; Miyauchi, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Formation of Supramolecular Isomers; Poly [2]rotaxane and Supramolecular Assembly. Chem. Commun. 2008, 4, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Onagi, H.; Easton, C.J.; Lincoln, S.F. An Hermaphrodite [2]Rotaxane: Preparation and Analysis of Structure. Org. Lett. 2001, 3, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.E.; Lincoln, S.F.; Easton, C.J. The Foundation of a Light Driven Molecular Muscle Based on Stilbene and α-Cyclodextrin. Chem. Commun. 2008, 34, 3980–3982. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Aso, Y.; Kaneda, T. Linear Oligomers Composed of a Photochromically Contractible and Extendable Janus [2]Rotaxane. Chem. Commun. 2006, 29, 3072–3074. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, S.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Contraction of Supramolecular Double-Threaded Dimer Formed by α-Cyclodextrin with a Long Alkyl Chain. Org. Lett. 2007, 9, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Taura, D.; Hashidzume, A.; Harada, A. Light-Switchable Janus [2]Rotaxanes Based on α-Cyclodextrin Derivatives Bearing Two Recognition Sites Linked with Oligo (Ethylene Glycol). Chem. Asian J. 2010, 5, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, A.C.; Zhu, Z.; Cao, D.; Liu, W.-G.; Li, H.; Dey, S.K.; Basu, S.; Trabolsi, A.; Botros, Y.Y.; Goddard, W.A., III; et al. Radically Enhanced Molecular Switches. J. Am. Chem. Soc. 2012, 134, 16275–16288. [Google Scholar] [CrossRef]
- Fahrenbach, A.C.; Bruns, C.J.; Li, H.; Trabolsi, A.; Coskun, A.; Stoddart, J.F. Ground-State Kinetics of Bistable Redox-Active Donor–Acceptor Mechanically Interlocked Molecules. Acc. Chem. Res. 2014, 47, 482–493. [Google Scholar] [CrossRef]
- Stanier, C.A.; Alderman, S.J.; Claridge, T.D.; Anderson, H.L. Unidirectional Photoinduced Shuttling in a Rotaxane with a Symmetric Stilbene Dumbbell. Angew. Chem. Int. Ed. 2002, 41, 1769–1772. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-Based Molecular Muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Oshikiri, T.; Yamaguchi, H.; Takashima, Y.; Harada, A. Face Selective Translation of a Cyclodextrin Ring Along an Axle. Chem. Commun. 2009, 37, 5515–5517. [Google Scholar] [CrossRef] [PubMed]
- Hashidzume, A.; Kuse, A.; Oshikiri, T.; Adachi, S.; Yamaguchi, H.; Harada, A. A Pseudo-Rotaxane of α-Cyclodextrin and a Two-Station Axis Molecule Consisting of Pyridinium and Decamethylene Moieties, and its Deuteration in Deuterium Oxide. Tetrahedron 2017, 73, 4988–4993. [Google Scholar] [CrossRef]
- Hashidzume, A.; Kuse, A.; Oshikiri, T.; Adachi, S.; Okumura, M.; Yamaguchi, H.; Harada, A. Toward a Translational Molecular Ratchet: Face-Selective Translation Coincident with Deuteration in a Pseudo-Rotaxane. Sci. Rep. 2018, 8, 8950. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.R.; Bělohradský, M.; Philp, D.; Stoddart, J.F. Slippage—An Alternative Method for Assembling [2]Rotaxanes. J. Chem. Soc. Chem. Commun. 1993, 16, 1269–1274. [Google Scholar] [CrossRef]
- Raymo, F.M.; Stoddart, J.F. Slippage—A Simple and Efficient Way to Self-Assemble [n]Rotaxanes. Pure Appl. Chem. 1997, 69, 1987–1997. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Oshikiri, T.; Harada, A. Rotaxanes with Unidirectional Cyclodextrin Array. J. Phys. Condens. Matter 2006, 18, S1809–S1816. [Google Scholar] [CrossRef]
- Ito, K. Novel Cross-Linking Concept of Polymer Network: Synthesis, Structure, and Properties of Slide-Ring Gels with Freely Movable Junctions. Polymer 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Mayumi, K.; Ito, K. Structure and Dynamics of Polyrotaxane and Slide-Ring Materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef]
- Kato, K.; Ito, K. Polymer Networks Characterized by Slidable Crosslinks and the Asynchronous Dynamics of Interlocked Components. React. Funct. Polym. 2013, 73, 405–412. [Google Scholar] [CrossRef]
- Noda, Y.; Hayashi, Y.; Ito, K. From Topological Gels to Slide-Ring Materials. J. Appl. Polym. Sci. 2014, 131, 40509. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-Links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Araki, J.; Zhao, C.; Ito, K. Efficient Production of Polyrotaxanes From α-Cyclodextrin and Poly (Ethylene Glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. From High Molecular Weight Precursor Polyrotaxanes to Supramolecular Sliding Networks. the “Sliding Gels”. Polymer 2005, 46, 8494–8501. [Google Scholar] [CrossRef]
- Sakai, T.; Murayama, H.; Nagano, S.; Takeoka, Y.; Kidowaki, M.; Ito, K.; Seki, T. Photoresponsive Slide-Ring Gel. Adv. Mater. 2007, 19, 2023–2025. [Google Scholar] [CrossRef]
- Murayama, H.; Imran, A.B.; Nagano, S.; Seki, T.; Kidowaki, M.; Ito, K.; Takeoka, Y. Chromic Slide-Ring Gel Based on Reflection from Photonic Bandgap. Macromolecules 2008, 41, 1808–1814. [Google Scholar] [CrossRef]
- Araki, J. Polyrotaxane Derivatives. II. Preparation and Characterization of Ionic Polyrotaxanes and Ionic Slide-Ring Gels. J. Polym. Sci. Pol. Chem. 2011, 49, 2199–2209. [Google Scholar] [CrossRef]
- Murakami, T.; Schmidt, B.V.K.J.; Brown, H.R.; Hawker, C.J. One-Pot “Click” Fabrication of Slide-Ring Gels. Macromolecules 2015, 48, 7774–7781. [Google Scholar] [CrossRef]
- Kato, K.; Okabe, Y.; Okazumi, Y.; Ito, K. A Significant Impact of Host-Guest Stoichiometry on the Extensibility of Polyrotaxane Gels. Chem. Commun. 2015, 51, 16180–16183. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Sainou, N. Amino Acid-Derivatized Slide-Ring Gels: Chemical Crosslinking of Polyrotaxane Conjugates with Different Amino Acid Pendant Groups. Polymer 2015, 74, 133–143. [Google Scholar] [CrossRef]
- Kali, G.; Eisenbarth, H.; Wenz, G. One Pot Synthesis of a Polyisoprene Polyrotaxane and Conversion to a Slide-Ring Gel. Macromol. Rapid Commun. 2015, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Inoue, K.; Kidowaki, M.; Ito, K. Organic−Inorganic Hybrid Slide-Ring Gels: Polyrotaxanes Consisting of Poly (Dimethylsiloxane) and γ-Cyclodextrin and Subsequent Topological Cross-Linking. Macromolecules 2009, 42, 7129–7136. [Google Scholar] [CrossRef]
- Karino, T.; Okumura, Y.; Zhao, C.; Kataoka, T.; Ito, K.; Shibayama, M. SANS Studies on Deformation Mechanism of Slide-Ring Gel. Macromolecules 2005, 38, 6161–6167. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. Unveiling the Sliding Motion in Topological Networks: Influence of the Swelling Solvent on the Relaxation Dynamics. Adv. Mater. 2006, 18, 2847–2851. [Google Scholar] [CrossRef]
- Murata, N.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Anomaly in Stretching-Induced Swelling of Slide-Ring Gels with Movable Cross-Links. Macromolecules 2009, 42, 8485–8491. [Google Scholar] [CrossRef]
- Mayumi, K.; Tezuka, M.; Bando, A.; Ito, K. Mechanics of Slide-Ring Gels: Novel Entropic Elasticity of a Topological Network Formed by Ring and String. Soft Matter 2012, 8, 8179–8183. [Google Scholar] [CrossRef]
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-Responsive Polymer Membranes of Slide-Ring Gels with Movable Cross-Links. Adv. Mater. 2013, 25, 4636–4640. [Google Scholar] [CrossRef]
- Kato, K.; Yasuda, T.; Ito, K. Viscoelastic Properties of Slide-Ring Gels Reflecting Sliding Dynamics of Partial Chains and Entropy of Ring Components. Macromolecules 2013, 46, 310–316. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely Stretchable Thermosensitive Hydrogels by Introducing Slide-Ring Polyrotaxane Cross-Linkers and Ionic Groups into the Polymer Network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Zheng, Z.; Ding, X.; Peng, Y. Periodic Auto-Active Gels with Topologically “Polyrotaxane-Interlocked” Structures. Chem. Commun. 2014, 50, 6372–6374. [Google Scholar] [CrossRef] [PubMed]
- Karino, T.; Shibayama, M.; Okumura, Y.; Ito, K. SANS Study on Pulley Effect of Slide-Ring Gel. Phys. B Condens. Matter 2006, 385, 807–809. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Travelet, C.; Lapp, A.; Lindner, P.; Hadziioannou, G. Topological Polymer Networks with Sliding Cross-Link Points: The “Sliding Gels”. Relationship Between Their Molecular Structure and the Viscoelastic as Well as the Swelling Properties. Macromolecules 2007, 40, 535–543. [Google Scholar] [CrossRef]
- Kato, K.; Karube, K.; Nakamura, N.; Ito, K. The Effect of Ring Size on the Mechanical Relaxation Dynamics of Polyrotaxane Gels. Polym. Chem. 2015, 6, 2241–2248. [Google Scholar] [CrossRef]
- Iwaso, K.; Takashima, Y.; Harada, A. Fast Response Dry-Type Supramolecular Artificial Muscles with [c2]Daisy Chains. Nat. Chem. 2016, 8, 625–632. [Google Scholar] [CrossRef]
- Ikejiri, S.; Takashima, Y.; Osaki, M.; Yamaguchi, H.; Harada, A. Solvent-Free Photoresponsive Artificial Muscles Rapidly Driven by Molecular Machines. J. Am. Chem. Soc. 2018, 140, 17308–17315. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Pierro, T.; Neri, P. Sequence Stereoisomerism in Calixarene-Based Pseudo [3]Rotaxanes. Org. Lett. 2011, 13, 2098–2101. [Google Scholar] [CrossRef]
- Pierro, T.; Gaeta, C.; Talotta, C.; Casapullo, A.; Neri, P. Fixed or Invertible Calixarene-Based Directional Shuttles. Org. Lett. 2011, 13, 2650–2653. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Neri, P. Stereoprogrammed Direct Synthesis of Calixarene-Based [3]Rotaxanes. Org. Lett. 2012, 14, 3104–3107. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Mirra, S.; Margarucci, L.; Casapullo, A.; Neri, P. Catenation of Calixarene Annulus. Org. Lett. 2013, 15, 116–119. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Qi, Z.; Schalley, C.A.; Neri, P. Pseudorotaxanes with Self-Sorted Sequence and Stereochemical Orientation. Angew. Chem. Int. Ed. 2013, 52, 7437–7441. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Talotta, C.; Neri, P. Pseudorotaxane Orientational Stereoisomerism Driven by π-Electron Density. Chem. Commun. 2014, 50, 9917–9920. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Ciesa, F.; Fragassi, M.; Pochini, A.; Secchi, A. Selective Synthesis of Two Constitutionally Isomeric Oriented Calix [6]Arene-Based Rotaxanes. Angew. Chem. Int. Ed. 2005, 44, 278–281. [Google Scholar] [CrossRef]
- Arduini, A.; Calzavacca, F.; Pochini, A.; Secchi, A. Unidirectional Threading of Triphenylureidocalix [6]arene-Based Wheels: Oriented Pseudorotaxane Synthesis. Chem. Eur. J. 2003, 9, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Meng, Z.; Xiang, J.-F.; Xia, Y.-X.; Sun, Y.; Hu, S.-Z.; Chen, H.; Yao, J.; Chen, C.-F. Guest-Dependent Directional Complexation Based on Triptycene Derived Oxacalixarene: Formation of Oriented Rotaxanes. Chem. Sci. 2016, 7, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Z.; Chen, C.-F. Triptycene-Derived Oxacalixarene with Expanded Cavity: Synthesis, Structure and Its Complexation with Fullerenes C60 and C70. Chem. Commun. 2010, 46, 4199–4201. [Google Scholar] [CrossRef]
- Hu, S.-Z.; Chen, C.-F. Triptycene-Derived Oxacalixarenes as New Wheels for the Synthesis of [2]Rotaxanes: Acid-Base- and Metal-Ion-Switchable Complexation Processes. Chem. Eur. J. 2011, 17, 5424–5431. [Google Scholar] [CrossRef]
- Strutt, N.L.; Zhang, H.; Schneebeli, S.T.; Stoddart, J.F. Functionalizing Pillar[n]arenes. Acc. Chem. Res. 2014, 47, 2631–2642. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Aoki, T.; Yamagishi, T.-A. Thermally Responsive Shuttling Behavior of a Pillar[6]arene-Based [2]Rotaxane. Chem. Commun. 2012, 48, 6842–6844. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Aoki, T.; Kitajima, K.; Yamagishi, T.-A.; Hayashi, Y.; Kawauchi, S. High-Yield Diastereoselective Synthesis of Planar Chiral [2]- and [3]Rotaxanes Constructed From Per-ethylated Pillar[5]Arene and Pyridinium Derivatives. Chem. Eur. J. 2012, 18, 7493–7500. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamafuji, D.; Yamagishi, T.-A.; Brouwer, A.M. Förster Resonance Energy Transfer by Formation of a Mechanically Interlocked [2]Rotaxane. Chem. Commun. 2013, 49, 5468–5470. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, W.; Hou, D.; Meng, Q.; Zheng, R.; Li, C. Novel Binding Regioselectivity in the Interpenetration of a Non-Symmetric Axle Into a Non-Symmetric Pillar[5]arene Wheel. Chem. Commun. 2014, 50, 4820–4823. [Google Scholar] [CrossRef] [PubMed]
- Aav, R.; Mishra, K. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef]
- Lee, S.; Chen, C.-H.; Flood, A.H. A Pentagonal Cyanostar Macrocycle with Cyanostilbene CH Donors Binds Anions and Forms Dialkylphosphate [3]Rotaxanes. Nat. Chem. 2013, 5, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, J.; Saghatelian, A.; Cheley, S.; Bayley, H.; Ghadiri, M.R. Single DNA Rotaxanes of a Transmembrane Pore Protein. Angew. Chem. Int. Ed. 2004, 43, 3063–3067. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruns, C.J. Exploring and Exploiting the Symmetry-Breaking Effect of Cyclodextrins in Mechanomolecules. Symmetry 2019, 11, 1249. https://doi.org/10.3390/sym11101249
Bruns CJ. Exploring and Exploiting the Symmetry-Breaking Effect of Cyclodextrins in Mechanomolecules. Symmetry. 2019; 11(10):1249. https://doi.org/10.3390/sym11101249
Chicago/Turabian StyleBruns, Carson J. 2019. "Exploring and Exploiting the Symmetry-Breaking Effect of Cyclodextrins in Mechanomolecules" Symmetry 11, no. 10: 1249. https://doi.org/10.3390/sym11101249
APA StyleBruns, C. J. (2019). Exploring and Exploiting the Symmetry-Breaking Effect of Cyclodextrins in Mechanomolecules. Symmetry, 11(10), 1249. https://doi.org/10.3390/sym11101249