Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
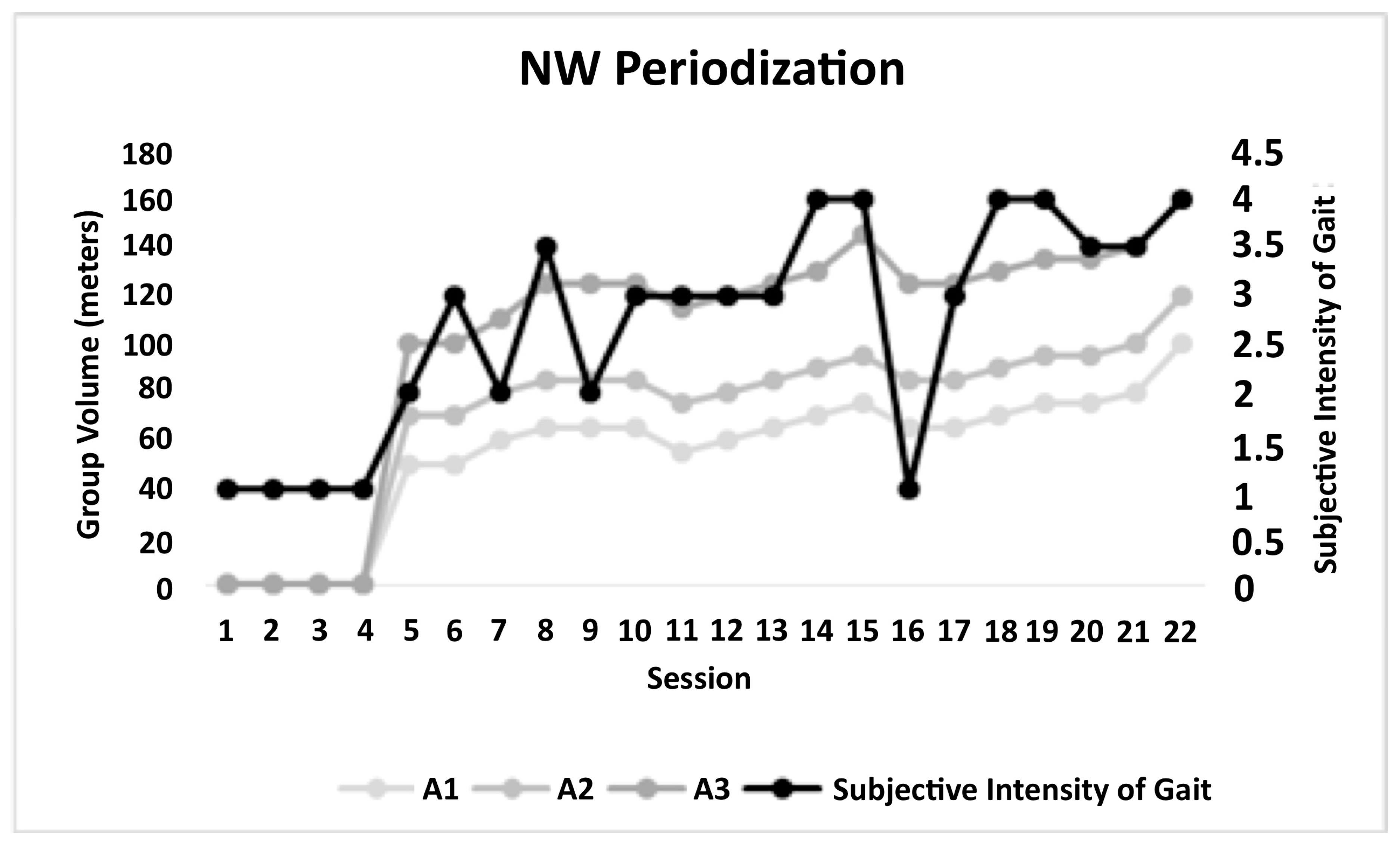
2.3. Assessment and Intervention Procedures
(m) = 493 + (2.2 × height) − (0.93 × weight) − (5.3 × age) + 17 m
(m) = 493 + (2.2 × height) − (0.93 × weight) − (5.3 × age) m
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Frazzitta, G.; Pezzoli, G.; Bertotti, G.; Maestri, R. Asymmetry and freezing of gait in parkinsonian patients. J. Neurol. 2013, 260, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Peterson, D.S.; Earhart, G.M. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture 2013, 38, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.; Villagra, F.; Castellote, J.M.; Pastor, M.A. Kinematic and Kinetic Patterns Related to Free-Walking in Parkinson’s Disease. Sensors 2018, 18, 4224. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, T.A.; van der Kooij, H.; Munneke, M.; Bloem, B.R. Gait disorders and balance disturbances in Parkinson’s disease: Clinical update and pathophysiology. Curr. Opin. Neurol. 2008, 21, 461–471. [Google Scholar] [CrossRef]
- Saunders, J.B.D.M.; Inman, V.T.; Eberhart, H.D. The major determinants in normal and pathological gait. J. Bone Jt. Surg. Am. 1953, 35, 543–558. [Google Scholar] [CrossRef]
- Cavagna, A.; Thys, H.; Zamboni, A. The sources of external work in level walking and running. J. Physiol. 1976, 262, 639–657. [Google Scholar] [CrossRef]
- Bianchi, L.; Angelini, D.; Orani, G.P.; Lacquaniti, F. Kinematic coordination in human gait: Relation to mechanical energy cost. J. Neurophysiol. 1998, 79, 2155–2170. [Google Scholar] [CrossRef]
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2008, 23, 631–640. [Google Scholar] [CrossRef]
- Shu, H.F.; Yang, T.; Yu, S.X.; Huang, H.D.; Jiang, L.L.; Gu, J.W.; Kuang, Y.Q. Aerobic exercise for Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e100503. [Google Scholar] [CrossRef] [Green Version]
- Hubble, R.P.; Naughton, G.; Silburn, P.A.; Cole, M.H. Trunk Exercises Improve Gait Symmetry in Parkinson Disease: A Blind Phase II Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2018, 97, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Manca, A.; Dragone, D.; Deriu, F.; Solla, P.; Secci, C.; Monticone, M.; Mercuro, G. Nordic Walking for the Management of People With Parkinson Disease: A Systematic Review. PM&R 2017, 9, 1157–1166. [Google Scholar] [CrossRef]
- Monteiro, E.P.; Franzoni, L.T.; Cubillos, D.M.; de Oliveira Fagundes, A.; Carvalho, A.R.; Oliveira, H.B.; Pantoja, P.D.; Schuch, F.B.; Rieder, C.R.; Martinez, F.G.; et al. Effects of Nordic walking training on functional parameters in Parkinson’s disease: A randomized controlled clinical trial. Scand. J. Med. Sci. Sports 2017, 27, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, L.T.; Monteiro, E.P.; Oliveira, H.B.; da Rosa, R.G.; Costa, R.R.; Rieder, C.; Martinez, F.G.; Peyré-Tartaruga, L.A. A 9-week Nordic and free walking improve postural balance in Parkinson’s disease. Sports Med. Int. Open 2018, 2, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomeñuka, N.A.; Oliveira, H.B.; Silva, E.S.; Costa, R.R.; Kanitz, A.C.; Liedtke, G.V.; Schuch, F.B.; Peyré-Tartaruga, L.A. Effects of Nordic walking training on quality of life, balance and functional mobility in elderly: A randomized clinical trial. PLoS ONE 2019, 14, e0211472. [Google Scholar] [CrossRef] [PubMed]
- Arcila, D.M.C.; Monteiro, E.P.; Gomeñuka, N.A.; Peyré-Tartaruga, L.A. Metodologia e Didática Pedagógica aplicada ao ensino da Caminhada Nórdica e Livre para pessoas com Doença de Parkinson I [Methodology and pedagogical didactics applied to the education of nordic walking and free walking for people with parkinson’s disease I]. Cad. RBCE 2018, 8, 72–83. [Google Scholar]
- Pellegrini, B.; Peyré-Tartaruga, L.A.; Zoppirolli, C.; Bortolan, L.; Savoldelli, A.; Minetti, A.E.; Schena, F. Mechanical energy patterns in Nordic walking: Comparisons with conventional walking. Gait Posture 2017, 51, 234–238. [Google Scholar] [CrossRef]
- Boccia, G.; Zoppirolli, C.; Bortolan, L.; Schena, F.; Pellegrini, B. Shared and task-specific muscle synergies of Nordic walking and conventional walking. Scand. J. Med. Sci. Sports 2018, 28, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, B.; Peyré-Tartaruga, L.A.; Zoppirolli, C.; Bortolan, L.; Bacchi, E.; Figard-Fabre, H.; Schena, F. Exploring muscle activation during Nordic walking: A comparison between conventional and uphill walking. PLoS ONE 2015, 10, e0138906. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.A.; Thaut, M.H.; McIntosh, G.C.; Rice, R.R. Components of EMG symmetry and variability in parkinsonian and healthy elderly gait. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 1–7. [Google Scholar] [CrossRef]
- Morris, M.E.; Huxham, F.; McGinley, J.; Dodd, K.; Iansek, R. The biomechanics and motor control of gait in Parkinson disease. Clin. Biomech. 2001, 16, 459–470. [Google Scholar] [CrossRef]
- Delval, A.; Salleron, J.; Bourriez, J.L.; Bleuse, S.; Moreau, C.; Krystkowiak, P.; Defebvre, L.; Devos, P.; Duhamel, A. Kinematic angular parameters in PD: Reliability of joint angle curves and comparison with healthy subjects. Gait Posture 2008, 28, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Mehrholz, J.; Kugler, J.; Storch, A.; Pohl, M.; Hirsch, K.; Elsner, B. Treadmill training for patients with Parkinson Disease. An abridged version of a Cochrane Review. Eur. J. Phys. Rehabil. Med. 2016, 52, 704–713. [Google Scholar] [PubMed]
- Tumas, V.; Borges, V.; Ballalai-Ferraz, H.; Zabetian, C.P.; Mata, I.F.; Brito, M.M.C.; Foss, M.P.; Novaretti, N.; Santos-Lobato, B.L. Some aspects of the validity of the Montreal Cognitive Assessment (MoCA) for evaluating cognitive impairment in Brazilian patients with Parkinson’s disease. Dement. Neuropsychol. 2016, 10, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.S.; de Sousa, A.C.; de Lucena, L.C.; Santiago, L.M.M.; Lindquist, A.R.R. Does dual task walking affect gait symmetry in individuals with Parkinson’s disease? Eur. J. Physiother. 2018, 21, 8–14. [Google Scholar] [CrossRef]
- O’neal, H.A.; Blair, S.N. Enhancing Adherence in Clinical Exercise Trials. Quest 2001, 53, 310–317. [Google Scholar] [CrossRef]
- Raciti, L.; Nicoletti, A.; Mostile, G.; Bonomo, R.; Contrafatto, D.; Dibilio, V.; Luca, A.; Sciacca, G.; Cicero, C.E.; Vasta, R.; et al. Validation of the UPDRS section IV for detection of motor fluctuations in Parkinson’s disease. Park. Relat. Disord. 2016, 27, 98–101. [Google Scholar] [CrossRef]
- Enright, P.L.; McBurnie, M.A.; Bittner, V.; Tracy, R.P.; McNamara, R.; Arnold, A.; Newman, A.B. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003, 123, 387–398. [Google Scholar] [CrossRef]
- Rosenthal, J.A. Qualitative Descriptors of Strength of Association and Effect Size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Espirito-santo, H.; Daniel, F. Calcular e apresentar tamanhos do efeito em trabalhos científicos (1): As limitações do p < 0.05 na análise de diferenças de médias de dois grupos. Rev. Port. Invest. Comp. Soc. 2015, 1, 3–6. [Google Scholar]
- Plotnik, M.; Giladi, N.; Balash, Y.; Peretz, C.; Hausdorff, J.M. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann. Neurol. 2005, 57, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Sen, S.; Eslinger, P.J.; Wagner, D.; Kong, L.; Lewis, M.M.; Du, G.; Huang, X. Side of motor onset is associated with hemisphere-specific memory decline and lateralized gray matter loss in Parkinson’s disease. Park. Relat Disord 2015, 21, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djaldetti, R.; Ziv, I.; Melamed, E. The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol. 2006, 5, 796–802. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Yogev, G.; Plotnik, M.; Peretz, C.; Giladi, N.; Hausdorff, J.M. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: When does the bilateral coordination of gait require attention? Exp. Brain Res. 2007, 177, 336–346. [Google Scholar] [CrossRef]
- Schenkman, M.L.; Clark, K.; Xie, T.; Kuchibhatla, M.; Shinberg, M.; Ray, L. Spinal movement and performance of a standing reach task in participants with and without Parkinson disease. Phys. Ther. 2001, 81, 1400–1411. [Google Scholar] [CrossRef]
- Djurić-Jovičić, M.; Belić, M.; Stanković, I.; Radovanović, S.; Kostić, V.S. Selection of gait parameters for differential diagnostics of patients with de novo Parkinson’s disease. Neurol. Res. 2017, 39, 853–861. [Google Scholar] [CrossRef]
- Meyer, C.; Killeen, T.; Easthope, C.S.; Curt, A.; Bolliger, M.; Linnebank, M.; Zörner, B.; Filli, L. Familiarization with treadmill walking: How much is enough? Sci. Rep. 2019, 9, 5232. [Google Scholar] [CrossRef]
- Ni, M.; Hazzard, J.B.; Signorile, J.F.; Luca, C. Exercise Guidelines for Gait Function in Parkinson’s Disease: A Systematic Review and Meta-analysis. Neurorehabil. Neural Repair 2018, 32, 872–886. [Google Scholar] [CrossRef]
- Zhou, L.; Gougeon, M.A.; Nantel, J. Nordic Walking Improves Gait Power Profiles at the Knee Joint in Parkinson’s Disease. J. Aging Phys. Act. 2018, 26, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Luna, N.M.S.; Lucareli, P.R.G.; Sales, V.C.; Speciali, D.; Alonso, A.C.; Peterson, M.D.; Rodrigues, R.B.M.; Fonoffc, E.T.; Barbosac, E.R.; Teixeira, M.J.; et al. Treadmill training in Parkinson’s patients after deep brain stimulation: Effects on gait kinematic. Neurorehabilitation 2018, 42, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, D.; Canepa, M.; Fernandez, A.M. The effect of treadmill and overground walking on preferred walking speed and gait kinematics in healthy, physically active older adults. Eur. J. Appl. Physiol. 2017, 117, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Seminati, E.; Nardello, F.; Zamparo, P.; Ardigó, L.P.; Faccioli, N.; Minetti, A.E. Anatomically Asymmetrical Runners Move More Asymmetrically at the Same Metabolic Cost. PLoS ONE 2013, 8, e74134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziuba, A.K.; Żurek, G.; Garrard, I.; Wierzbicka-Damska, I. Parameters in lower limbs during natural walking and Nordic walking at different speeds. Acta Bioeng. Biomech. 2015, 17, 95–101. [Google Scholar] [PubMed]

| Variable | Mean (Standard Deviation) |
|---|---|
| Total subjects (male/female) | 14 (7/7) |
| Gender (female/male) | 7/7 |
| Age (years) | 66.8 (±9.6) |
| Disease duration (years) | 7.2 (±5.4) |
| UPDRS (points) | 12.2 (±6.1) |
| H & Y | 1.5 (1–3) |
| MoCA | 26.6 (2.2) |
| Lower limb length (m) | 0.89 (0.05) |
| Body mass (kg) | 64.5 (±23.5) |
| Height (m) | 1.7 (±.86) |
| PRE | POST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| More Affected | Less Affected | More Affected | Less Affected | p-Value | ||||||
| Speed (m·s−1) | Mean (max; min) | Mean (max; min) | Mean (max; min) | Mean (max; min) | T | C | T*C | ES More Affected | ES Less Affected | |
| Flexion | - | - | - | - | - | - | - | - | - | - |
| Knee (degree) | 0.28 | 49.9 (45.7; 54.1) | 42.3 (35.7; 49.0) | 50.8 (46.2; 55.4) | 52.3 (47.3; 57.3) | 0.012 * | 0.236 | 0.007 * | 0.10 | 0.82 |
| Abduction | ||||||||||
| Hip (degree) | 0.28 | 1.1 (−1.9; 4.1) | 7.6 (4.7; 10.5) | 4.00 (2.04; 5.96) | 6.7 (4.7; 8.6) | 0.329 | 0.007 * | 0.040 * | 0.56 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanardi, A.P.J.; Martinez, F.G.; da Silva, E.S.; Casal, M.Z.; Martins, V.F.; Passos-Monteiro, E.; Haas, A.N.; Peyré-Tartaruga, L.A. Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease. Symmetry 2019, 11, 1481. https://doi.org/10.3390/sym11121481
Zanardi APJ, Martinez FG, da Silva ES, Casal MZ, Martins VF, Passos-Monteiro E, Haas AN, Peyré-Tartaruga LA. Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease. Symmetry. 2019; 11(12):1481. https://doi.org/10.3390/sym11121481
Chicago/Turabian StyleZanardi, Ana Paula J., Flávia G. Martinez, Edson S. da Silva, Marcela Z. Casal, Valéria F. Martins, Elren Passos-Monteiro, Aline N. Haas, and Leonardo A. Peyré-Tartaruga. 2019. "Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease" Symmetry 11, no. 12: 1481. https://doi.org/10.3390/sym11121481
APA StyleZanardi, A. P. J., Martinez, F. G., da Silva, E. S., Casal, M. Z., Martins, V. F., Passos-Monteiro, E., Haas, A. N., & Peyré-Tartaruga, L. A. (2019). Effects of Nordic Walking on Gait Symmetry in Mild Parkinson’s Disease. Symmetry, 11(12), 1481. https://doi.org/10.3390/sym11121481








