Characterization of Hidden Chirality: Two-Fold Helicity in β-Strands
Abstract
:1. Introduction
2. Materials and Methods
3. Results
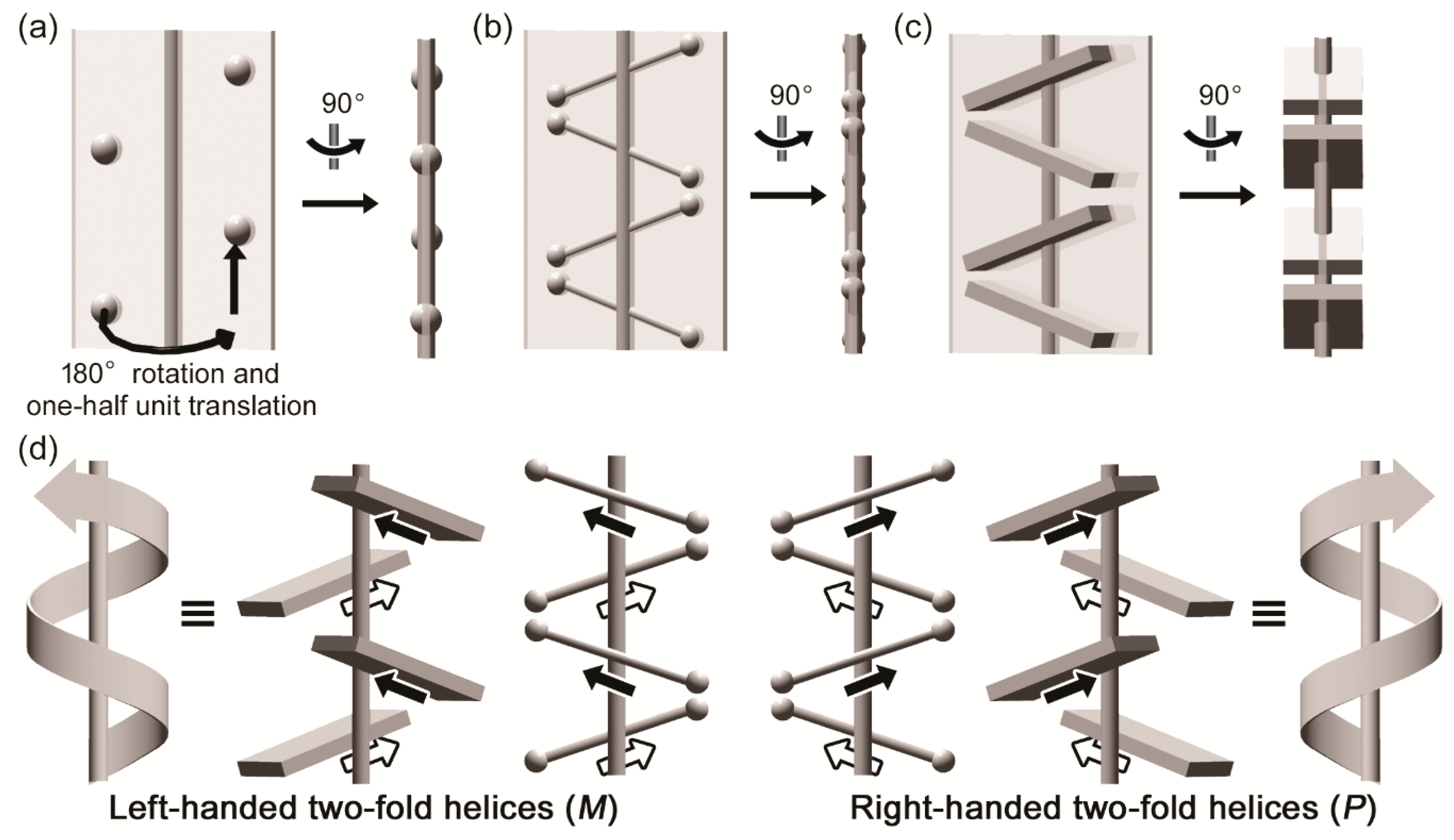
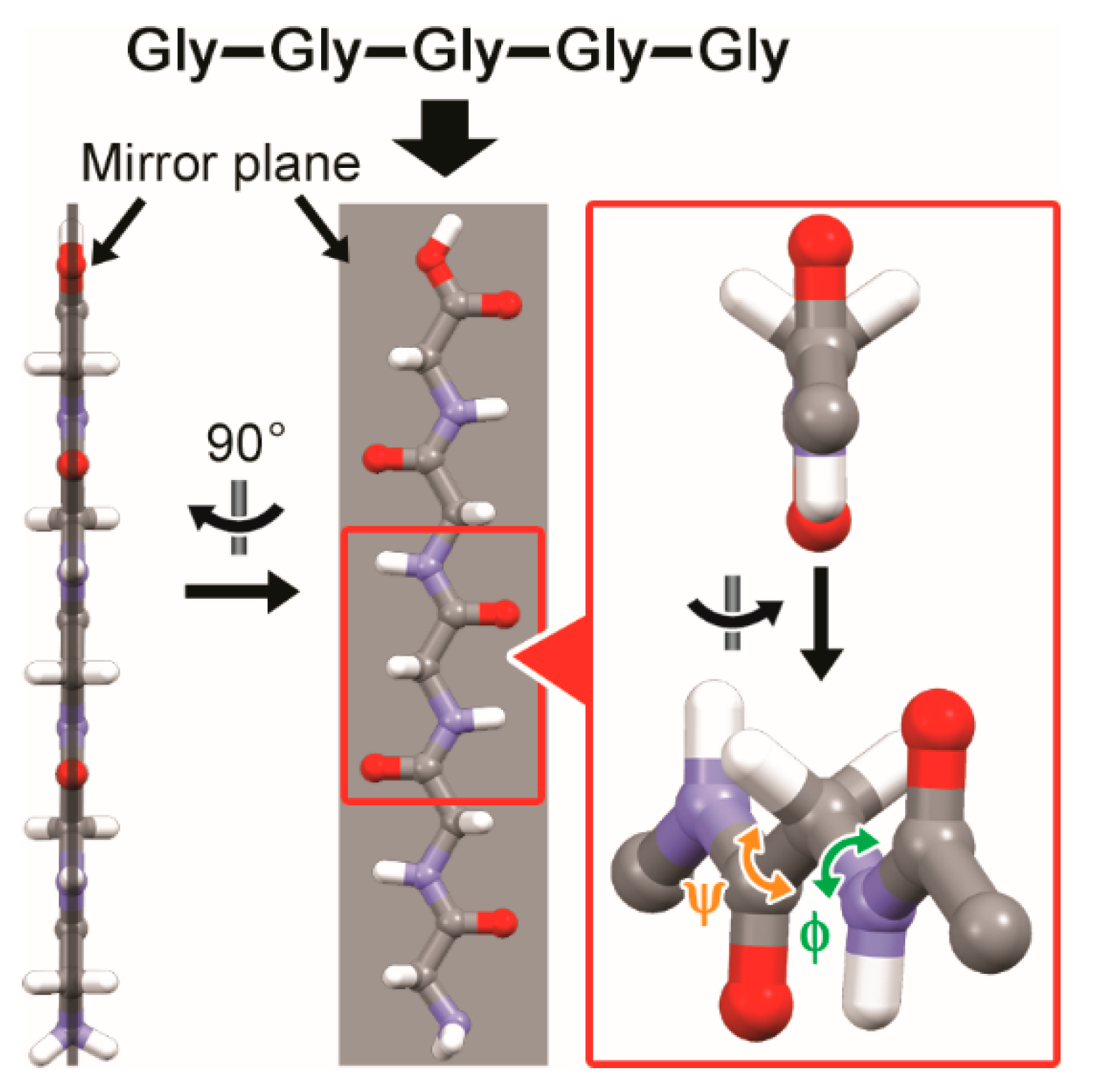
3.1. Two-Fold Helicity of Extended Linear Pentaglycine
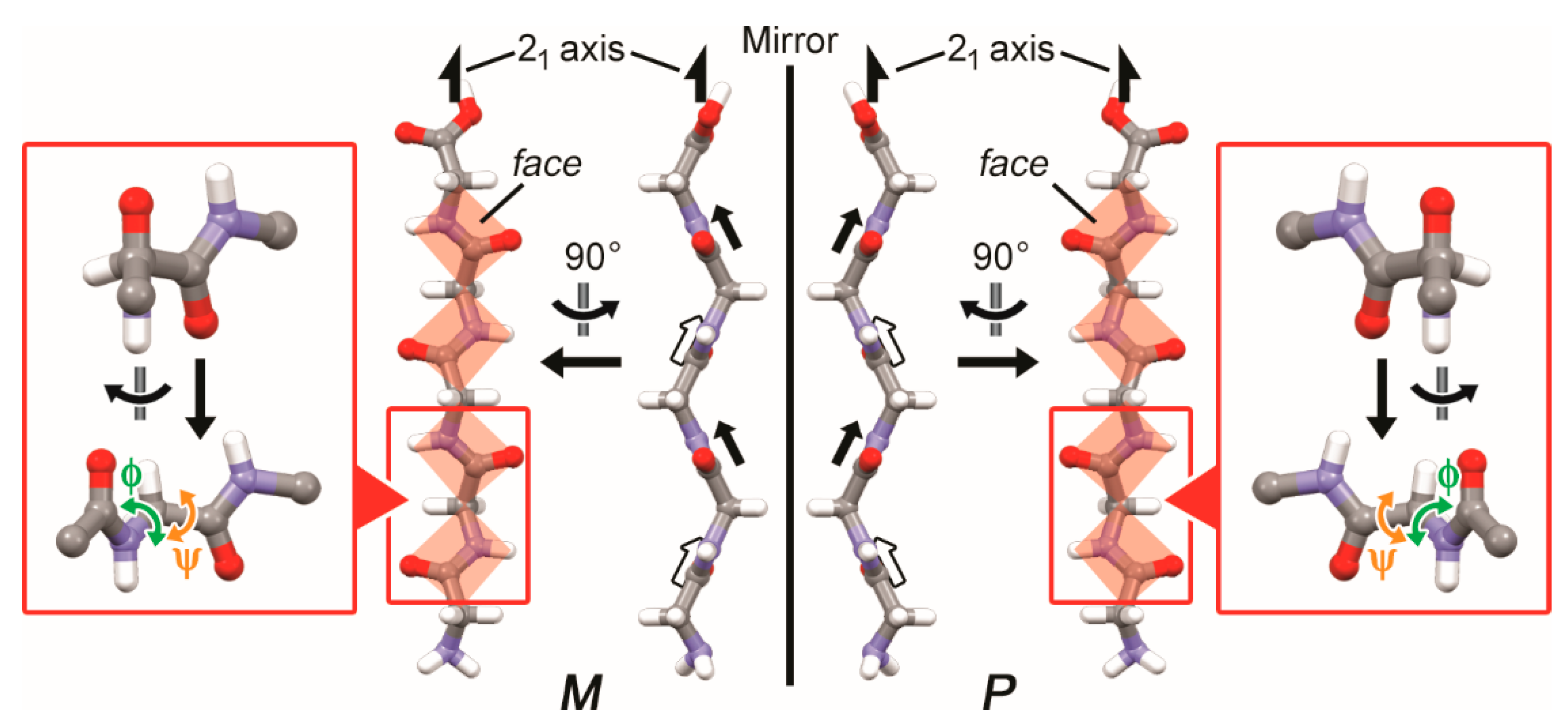
3.2. Two-Fold Helicity of Zig-Zag Pentaglycine
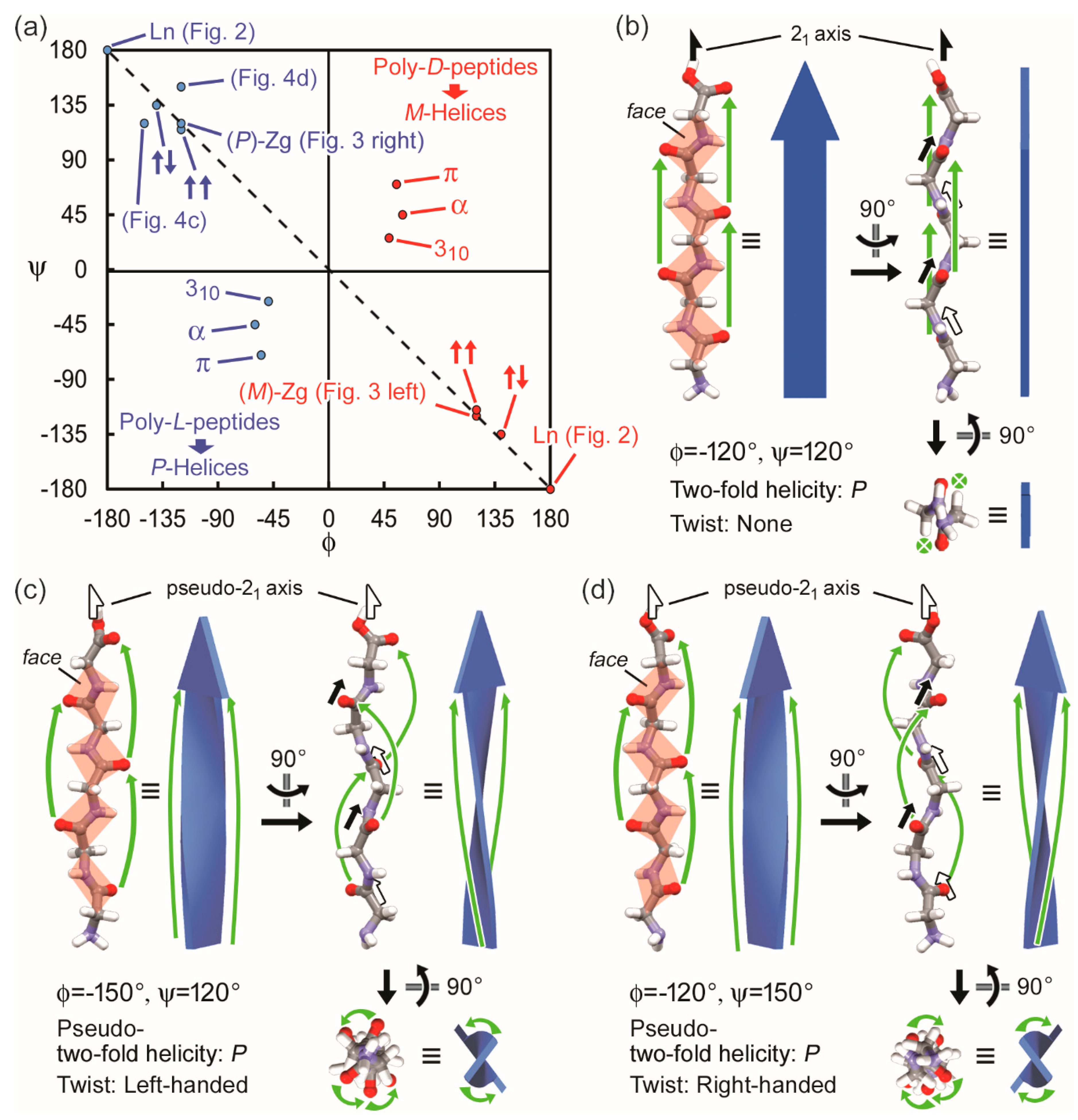
4. Discussion
4.1. Two-Fold Helicity and Twists of β-Strands in Real Polypeptides
4.2. Correlation Between Molecular Chirality and Chirality of High Dimensional Structures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mason, S.F. Origins of Biomolecular Handedness. Nature 1984, 311, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L.; van Delden, R.A. Absolute Asymmetric Synthesis: the Origin, Control, and Amplification of Chirality. Angew. Chem. Int. Ed. 1999, 38, 3418–3438. [Google Scholar] [CrossRef]
- Okano, K.; Taguchi, M.; Fujiki, M.; Yamashita, T. Circularly Polarized Luminescence of Rhodamine B in a Supramolecular Chiral Medium Formed by a Vortex Flow. Angew. Chem. Int. Ed. 2011, 50, 12474–12477. [Google Scholar] [CrossRef]
- Inoue, Y. Asymmetric Photochemical Reactions in Solution. Chem. Rev. 1992, 92, 741–770. [Google Scholar] [CrossRef]
- Lowry, T.M. Optical Rotatory Power; Dover Publications: New York, NY, USA; London, UK, 1964. [Google Scholar]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Krieger: Malabar, India, 1991. [Google Scholar]
- Matsuura, T.; Koshima, H. Introduction to Chiral Crystallization of Achiral Organic Compounds Spontaneous Generation of Chirality. J. Photochem. Photobiol. C 2005, 6, 7–24. [Google Scholar] [CrossRef]
- Vogl, O. Chiral Crystallization and the Origin of Chiral Life on Earth. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1299–1308. [Google Scholar] [CrossRef]
- Weissbuch, I.; Leiserowitz, L.; Lahav, M. Stochastic “Mirror Symmetry Breaking” via Self-Assembly, Reactivity and Amplification of Chirality: Relevance to Abiotic Conditions. Top. Curr. Chem. 2005, 259, 123–165. [Google Scholar]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric Autocatalysis and Amplification of Enantiomeric Excess of a Chiral Molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Viedma, C. Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Green, B.S.; Lahav, M.; Rabinovich, D. Asymmetric Synthesis via Reactions in Chiral Crystals. Acc. Chem. Res. 1979, 12, 191–197. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sholl, D.S. Chiral Selection on Inorganic Crystalline Surfaces. Nat. Mater. 2003, 2, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Azumaya, I.; Yamaguchi, K.; Okamoto, I.; Kagechika, H.; Shudo, K. Total Asymmetric Transformation of an N-Methylbenzamide. J. Am. Chem. Soc. 1995, 117, 9083–9084. [Google Scholar] [CrossRef]
- Iitaka, Y. The Crystal Structure of β-Glycine. Acta Cryst. 1960, 13, 35–45. [Google Scholar] [CrossRef]
- Iitaka, Y. The Crystal Structure of γ-Glycine. Acta Cryst. 1961, 14, 1–10. [Google Scholar] [CrossRef]
- Sasaki, T.; Ida, Y.; Tanaka, A.; Hisaki, I.; Tohnai, N.; Miyata, M. Chiral Crystallization by Non-Parallel Face Contacts on the Basis of Three-Axially Asymmetric Twofold Helices. Cryst. Eng. Comm. 2013, 15, 8237–8240. [Google Scholar] [CrossRef]
- Sasaki, T.; Miyata, M.; Tsuzuki, S.; Sato, H. Experimental and Theoretical Analysis of Two-fold Helix-Based Chiral Crystallization by Confined Interhelical CH/π Contacts. Cryst. Growth Des. 2019, 19, 1411–1417. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Molecular Crystals and Molecules; Academic Press: London, UK, 1973. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- International Tables for Crystallography Volume A: Space-Group Symmetry. 2016. Available online: https://it.iucr.org/A/ (accessed on 27 March 2019).
- Hisaki, I.; Sasaki, T.; Sakaguchi, K.; Liu, W.-L.; Tohnai, N.; Miyata, M. Right- and Left-Handedness of 21 Symmetrical Herringbone Assemblies of Benzene. Chem. Commun. 2012, 48, 2219–2221. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Sasaki, T.; Tohnai, N.; Miyata, M. Supramolecular-Tilt-Chirality on Twofold Helical Assemblies. Chem. Eur. J. 2012, 18, 10066–10073. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Tohnai, N.; Hisaki, I.; Sasaki, T. Generation of Supramolecular Chirality around Twofold Rotational or Helical Axes in Crystalline Assemblies of Achiral Components. Symmetry 2015, 7, 1914–1928. [Google Scholar] [CrossRef]
- Miyata, M.; Tohnai, N.; Hisaki, I. Crystalline Host–Guest Assemblies of Steroidal and Related Molecules: Diversity, Hierarchy, and Supramolecular Chirality. Acc. Chem. Res. 2007, 40, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Hisaki, I. Twofold Helical Molecular Assemblies in Organic Crystals: Chirality Generation and Handedness Determination. In Advances in Organic Crystal Chemistry: Comprehensive Reviews; Tamura, R., Miyata, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 371–390. [Google Scholar]
- Sasaki, T.; Miyata, M.; Sato, H. Helicity and Topological Chirality in Hydrogen-Bonded Supermolecules Characterized by Advanced Graph Set Analysis and Solid-State Vibrational Circular Dichroism Spectroscopy. Cryst. Growth Des. 2018, 18, 4621–4627. [Google Scholar] [CrossRef]
- Kawasaki, T.; Hakoda, Y.; Mineki, H.; Suzuki, k.; Soai, K. Generation of Absolute Controlled Crystal Chirality by the Removal of Crystal Water from Achiral Crystal of Nucleobase Cytosine. J. Am. Chem. Soc. 2010, 132, 2874–2875. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ida, Y.; Hisaki, I.; Yuge, T.; Uchida, Y.; Tohnai, N.; Miyata, M. Characterization of Supramolecular Hidden Chirality of Hydrogen-Bonded Networks by Advanced Graph Set Analysis. Chem. Eur. J. 2014, 20, 2478–2487. [Google Scholar] [CrossRef]
- Chothia, C. Conformation of Twisted β-Pleated Sheets in Proteins. J. Mol. Biol. 1973, 75, 295–302. [Google Scholar] [CrossRef]
- Weatherford, D.W.; Salemme, F.R. Conformations of Twisted Parallel β-Sheets and the Origin of Chirality in Protein Structures. Proc. Natl. Acad. Sci. USA 1979, 76, 19–23. [Google Scholar] [CrossRef]
- Ho, B.K.; Curmi, P.M.G. Twist and Shear in β-Sheets and β-Ribbons. J. Mol. Biol. 2002, 317, 291–308. [Google Scholar] [CrossRef]
- Salemme, F.R. Structural Properties of Protein β-Sheets. Prog. Biophys. Mol. Biol. 1983, 42, 95–133. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of Polypeptide Chain Configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Dennington, R.D., II; Keith, T.A.; Millam, J.M. Gauss View, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B. Configurations of Polypeptide Chains with Favored Orientations Around Single Bonds: Two New Pleated Sheets. Proc. Natl. Acad. Sci. USA 1951, 37, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Karplus, A. A Fresh Look at the Ramachandran Plot and the Occurrence of Standard Structures in Proteins. Biomol. Concepts 2010, 1, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hisaki, I.; Miyano, T.; Tohnai, N.; Morimoto, K.; Sato, H.; Tsuzuki, S.; Miyata, M. Linkage Control between Molecular and Supramolecular Chirality in 21−Helical Hydrogen-Bonded Networks using Achiral Components. Nat. Commun. 2013, 4, 1787. [Google Scholar] [CrossRef]
- Kurouski, D. Advances of Vibrational Circular Dichroism (VCD) in Bioanalytical Chemistry. A Review. Anal. Chim. Acta 2017, 990, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Nafie, L.A.; Freedman, T.A. Biological and Pharmaceutical Applications of Vibrational Optical Activity. Pract. Spectrosc. 2001, 24, 15–54. [Google Scholar]
- Keiderling, T.A. Vibrational Circular Dichroism of Peptides and Proteins. Survey of Techniques, Qualitative and Quantitative Analyses, and Applications. Pract. Spectrosc. 2001, 24, 55–100. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984. [Google Scholar]
- Rosa, C.D.; Auriemma, F. Structure and Physical Properties of Syndiotactic Polypropylene: A Highly Crystalline Thermoplastic Elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar]
- Sipe, J.D.; Cohen, A.S. Review: History of the Amyloid Fibril. J. Struct. Biol. 2000, 130, 88–98. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic Chemistry and the Origin of the RNA World. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Krishnamurthy, R. Giving Rise to Life: Transition from Prebiotic Chemistry to Protobiology. Acc. Chem. Res. 2017, 50, 455–459. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T.; Miyata, M. Characterization of Hidden Chirality: Two-Fold Helicity in β-Strands. Symmetry 2019, 11, 499. https://doi.org/10.3390/sym11040499
Sasaki T, Miyata M. Characterization of Hidden Chirality: Two-Fold Helicity in β-Strands. Symmetry. 2019; 11(4):499. https://doi.org/10.3390/sym11040499
Chicago/Turabian StyleSasaki, Toshiyuki, and Mikiji Miyata. 2019. "Characterization of Hidden Chirality: Two-Fold Helicity in β-Strands" Symmetry 11, no. 4: 499. https://doi.org/10.3390/sym11040499
APA StyleSasaki, T., & Miyata, M. (2019). Characterization of Hidden Chirality: Two-Fold Helicity in β-Strands. Symmetry, 11(4), 499. https://doi.org/10.3390/sym11040499




