Rectified Diffusion of Gas Bubbles in Molten Metal during Ultrasonic Degassing
Abstract
1. Introduction
2. Theoretical Analysis
- The involved sizes of the bubble is far beyond the molecular level [15].
- The viscosity and the compressibility of molten metal are both considered.
- The relationship between the volume and the internal pressure of gas bubbles is described by the polytropic model [21].
- Thermal damping is currently ignored. As shown in our previous work [21], this damping mechanism often does not serve as a dominant one.
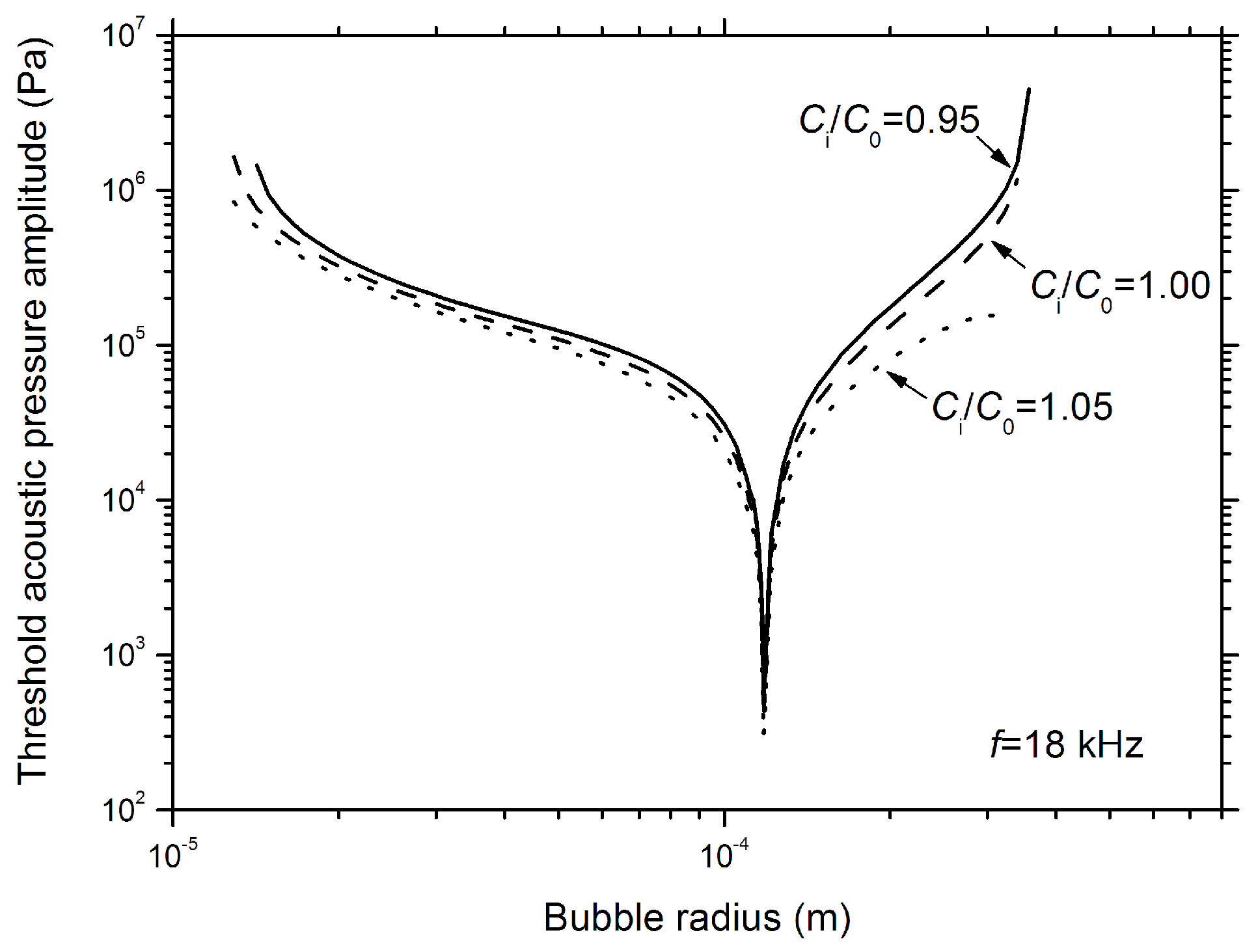
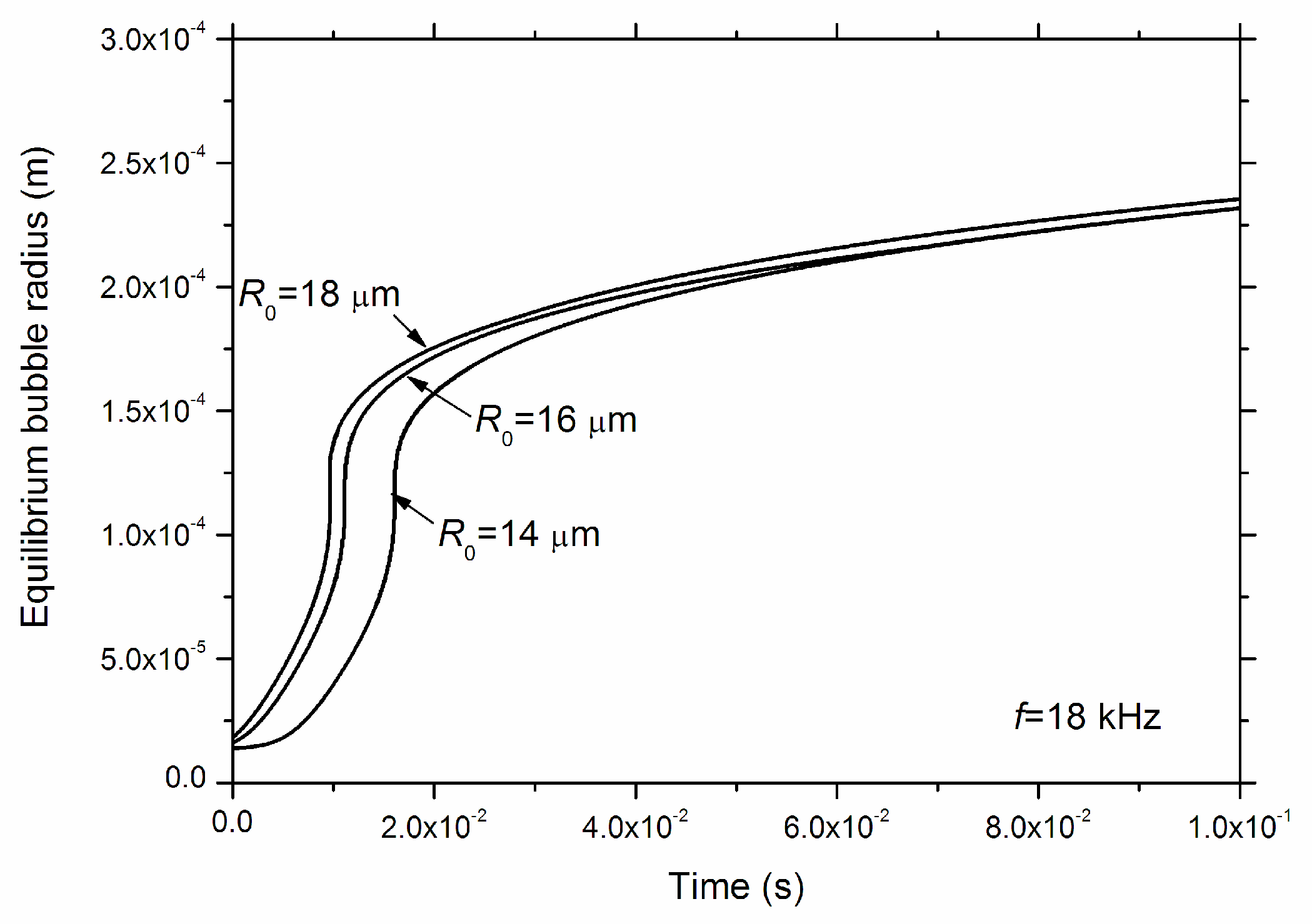
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eskin, D.; Alba-Baena, N.; Pabel, T.; Silva, M. Ultrasonic degassing of aluminium alloys: basic studies and practical implementation. Mater. Sci. Technol. 2015, 31, 79–84. [Google Scholar] [CrossRef]
- Xu, H.; Han, Q.; Meek, T.T. Effects of ultrasonic vibration on degassing of aluminum alloys. Mater. Sci. Eng. A 2008, 473, 96–104. [Google Scholar] [CrossRef]
- Meidani, A.N.; Meidani, A.N.; Hasan, M. A study of hydrogen bubble growth during ultrasonic degassing of Al–Cu alloy melts. J. Mater. Process. Technol. 2004, 147, 311–320. [Google Scholar] [CrossRef]
- Meidani, A.N.; Hasan, M. Mathematical and physical modelling of bubble growth due to ultrasound. Appl. Math. Model. 2004, 28, 333–351. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Pericleous, K.; Tzanakis, I.; Eskin, D.G. Dynamics of two interacting hydrogen bubbles in liquid aluminum under the influence of a strong acoustic field. Phys. Rev. E 2015, 92, 043004. [Google Scholar] [CrossRef]
- Eskin, G. Cavitation mechanism of ultrasonic melt degassing. Ultrason. Sonochemistry 1995, 2, 137. [Google Scholar] [CrossRef]
- Xu, W.; Tzanakis, I.; Srirangam, P.; Mirihanage, W.; Eskin, D.; Bodey, A.; Lee, P.; Xu, W.; Mirihanage, W.; Lee, P. Synchrotron quantification of ultrasound cavitation and bubble dynamics in Al–10Cu melts. Ultrason. Sonochemistry 2016, 31, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Eskin, G.I. Prospects of ultrasonic (cavitational) treatment of the melt in the manufacture of aluminum alloy products. Metallurgist 1998, 42, 284–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S. Mass transfer during radial oscillations of gas bubbles in viscoelastic mediums under acoustic excitation. Int. J. Heat Mass Transf. 2014, 69, 106–116. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, F.; Jia, H.; Li, Y.; Yang, Y. Numerical simulation of non-dendritic structure formation in Mg-Al alloy solidified with ultrasonic field. Ultrason. Sonochemistry 2018, 40, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Waly, M.; Amin, M. Effect of high intensity ultrasonic treatment on microstructural modification and hardness of a nickel-aluminum bronze alloy. J. Alloy. Compd. 2018, 741, 804–813. [Google Scholar] [CrossRef]
- Nagasivamuni, B.; Wang, G.; StJohn, D.H.; Dargusch, M.S. The effect of ultrasonic treatment on the mechanisms of grain formation in as-cast high purity zinc. J. Cryst. 2018, 495, 20–28. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, F.; Jia, H.; Zhou, J.; Li, Y.; Li, W.; Yang, Y. Effect of temperature conditions on grain refinement of Mg–Al alloy under ultrasonic field. Int. J. Cast Met. Res. 2017, 30, 341–347. [Google Scholar] [CrossRef]
- Mills, K.; Wang, G.; StJohn, D.; Dargusch, M. Ultrasonic Processing of Aluminum–Magnesium Alloys. Materials 2018, 11, 1994. [Google Scholar] [CrossRef]
- Wang, H.-S.; Fu, G.-S.; Cheng, C.-Z.; Song, L.-L.; Wang, L.-D. Molecular mechanics and dynamics simulation of hydrogen diffusion in aluminum melt. China Foundry 2017, 14, 478–484. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Zhang, Y.; Du, X. Experimental investigations of interactions between a laser-induced cavitation bubble and a spherical particle. Exp. Therm. Sci. 2018, 98, 645–661. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Zhang, Y.; Zhang, Y. High-speed experimental photography of collapsing cavitation bubble between a spherical particle and a rigid wall. J. Hydrodyn. 2018, 30, 1012–1021. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Zhang, Y.; Zhang, Y.; Du, X. Experimental study of influences of a particle on the collapsing dynamics of a laser-induced cavitation bubble near a solid wall. Exp. Therm. Sci. 2019. [Google Scholar] [CrossRef]
- Klapcsik, K.; Hegedűs, F. Study of non-spherical bubble oscillations under acoustic irradiation in viscous liquid. Ultrason. Sonochemistry 2019. [Google Scholar] [CrossRef]
- Klapcsik, K.; Hegedűs, F. The effect of high viscosity on the evolution of the bifurcation set of a periodically excited gas bubble. Chaos Solitons Fractals 2017, 104, 198–208. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Li, S.C. Notes on radial oscillations of gas bubbles in liquids: Thermal effects. J. Acoust. Soc. Am. 2010, 128, EL306–EL309. [Google Scholar] [CrossRef]
- Eller, A.I.; Flynn, H.G. Rectified diffusion during nonlinear pulsations of cavitation bubbles. J. Acoust. Soc. Am. 1965, 37, 493–503. [Google Scholar] [CrossRef]
- Gupta, C.K. Chemical Metallurgy: Priciples and Practice; Viley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2003; p. 273. ISBN 3-527-30376-6. [Google Scholar]
- Hatch, J.E. Aluminum: properties and physical metallurgy. Am. Soc. Met. 1984, 1, 1–24. [Google Scholar]
- Opie, W.R.; Grant, N.J. Hydrogen solubility in aluminum and some aluminum alloys. JOM 1950, 2, 1237–1241. [Google Scholar] [CrossRef]
- Eichenauer, W.; Markopoulos, J. Measurement of the diffusion coefficients of hydrogen in liquid aluminum. WAA Trans. Z. Met. 1974, 65, 649–652. [Google Scholar]
- Lv, L.; Zhang, Y.; Zhang, Y.; Zhang, Y. Experimental investigations of the particle motions induced by a laser-generated cavitation bubble. Ultrason. Sonochemistry 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, X.; Chen, F.; Liu, K.; Zhang, Y.; Liu, C. A selected review of vortex identification methods with applications. J. Hydrodyn. 2018, 30, 767–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhang, Y.; Liu, C. Comparisons of and analyses of vortex identification between omega method and Q criterion. J. Hydrodyn. 2019, 31, 224–230. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Chen, X.; Dong, Y.; Zhang, Y.; Liu, C. Determination of epsilon for Omega vortex identification method. J. Hydrodyn. 2018, 30, 541–548. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T. Physics of bubble oscillations. Rep. Prog. Phys. 2010, 73, 106501. [Google Scholar] [CrossRef]
- Wang, S.-P.; Zhang, A.-M.; Liu, Y.-L.; Zhang, S.; Cui, P. Bubble dynamics and its applications. J. Hydrodyn. 2018, 30, 975–991. [Google Scholar] [CrossRef]
- Ganesh, H.; Mäkiharju, S.A.; Ceccio, S.L. Bubbly shock propagation as a mechanism of shedding in separated cavitating flows. J. Hydrodyn. 2017, 29, 907–916. [Google Scholar] [CrossRef]
- Yuan, Y.; Miao, B.; An, Y. Cavitation clouds in gas-containing liquids block low-frequency components of ultrasonic waves. J. Appl. Phys. 2018, 124, 224902. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Yuan, J.; Chen, T.; Tang, N.; Du, X. Influences of bubble size distribution on propagation of acoustic waves in dilute polydisperse bubbly liquids. J. Hydrodyn. 2019, 31, 50–57. [Google Scholar] [CrossRef]
- Chen, H.; Lai, Z.; Chen, Z.; Li, Y. The secondary Bjerknes force between two oscillating bubbles in Kelvin-Voigt-type viscoelastic fluids driven by harmonic ultrasonic pressure. Ultrason. Sonochemistry 2018. [Google Scholar] [CrossRef]
- Baresch, D.; Garbin, V. Acoustic manipulation and actuation of bubbles in complex environments: Beyond the Bjerknes force. J. Acoust. Soc. Am. 2018, 144, 1933. [Google Scholar] [CrossRef]
- Xu, Z. Numerical simulation of the coalescence of two bubbles in an ultrasound field. Ultrason. Sonochemistry 2018, 49, 277–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, S. The secondary Bjerknes force between two gas bubbles under dual-frequency acoustic excitation. Ultrason. Sonochemistry 2016, 29, 129–145. [Google Scholar] [CrossRef]
- Klapcsik, K.; Varga, R.; Hegedűs, F. Bi-parametric topology of subharmonics of an asymmetric bubble oscillator at high dissipation rate. Nonlinear Dyn. 2018, 94, 2373–2389. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, S. Combination and simultaneous resonances of gas bubbles oscillating in liquids under dual-frequency acoustic excitation. Ultrason. Sonochemistry 2017, 35, 431–439. [Google Scholar] [CrossRef]
| Parameters | Values |
|---|---|
| 2375 kg/m3 | |
| 933.4 K | |
| 8.314 J/mol/K | |
| 1073.15 K | |
| 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y. Rectified Diffusion of Gas Bubbles in Molten Metal during Ultrasonic Degassing. Symmetry 2019, 11, 536. https://doi.org/10.3390/sym11040536
Zhang Y, Zhang Y. Rectified Diffusion of Gas Bubbles in Molten Metal during Ultrasonic Degassing. Symmetry. 2019; 11(4):536. https://doi.org/10.3390/sym11040536
Chicago/Turabian StyleZhang, Yuning, and Yuning Zhang. 2019. "Rectified Diffusion of Gas Bubbles in Molten Metal during Ultrasonic Degassing" Symmetry 11, no. 4: 536. https://doi.org/10.3390/sym11040536
APA StyleZhang, Y., & Zhang, Y. (2019). Rectified Diffusion of Gas Bubbles in Molten Metal during Ultrasonic Degassing. Symmetry, 11(4), 536. https://doi.org/10.3390/sym11040536




