Super Stability of Ag Nanoparticle in Crystalline Lamellar (Lc) Liquid Crystal Matrix at Different pH Environment
Abstract
:1. Introduction
2. Materials and Methods
- (i)
- Pure LC. The solution of HDTABr:1-pentanol:water was prepared by the mass ratio of 0.33:0.22:0.45.
- (ii)
- LC containing AgNPs (LC-AgNPs). In this case, the AgNPs were produced by an in situ method in the LC system. Firstly, the mixture of HDTABr:1-pentanol was prepared by the mass ratio of 0.33:0.22. Then 0.01 M AgNO3 solution and 0.04 M NaBH4 solution in a volume ratio 1:1 were added to the HDTABr:1-pentanol mixture. The specific components ratio of HDTABr:1-pentanol:water (or Ag solution) was referred to in the ternary diagram illustrated earlier by Patakfalvi et al. [14] that also stated that AgNPs content, using this method, is 0.005 M.
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Setia, S.; Sidiq, S.; De, J.; Pani, I.; Pal, S.K. Apllication of liquid crystals in biosensing and organic light-emiting devices. Liq. Cryst. 2016, 43, 2009–2050. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspective in liquid-crystal-aided nanotechnology and nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Bharti, A.; Kumar, V. Structural, thermal, zeta potential and electrical properties of disaccharide reduced silver nanoparticles. J. Mater. Sci. Mater. 2014, 25, 3747–3752. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapou, A.; Amani, A.M.; Dashtak, A.S.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Shutsko, I.; Böttge, C.M.; Von Bargen, J.; Henkel, A.; Meudt, M.; Görrn, P. Enhanced hybrid optics by growing silver nanoparticles at local intensity hot spots. Nanophotonics 2019, 8, 1457–1464. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver nanoparticles in cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar] [CrossRef] [Green Version]
- De Aberasturi, D.J.; Serrano-montes, A.B.; Liz-marzán, L.M. Modern applications of plasmonic nanoparticles: From energy to health. Adv. Opt. Mater. 2015, 3, 602–617. [Google Scholar] [CrossRef]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Dellinger, T.M.; Braun, P.V. Lyotropic liquid crystals as nanoreactors for nanoparticle synthesis. Chem. Mater. 2004, 16, 2201–2207. [Google Scholar] [CrossRef]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid crystalline thermotropic and lyotropic nanohybrids. Nanoscale 2013, 5, 6641–6661. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Davidson, P.; Imp, M.; Mingotaud, C.; Kahn, L.; Marty, J. Facile direct synthesis of ZnO nanoparticles within lyotropic liquid crystals: Towards organized hybrid materials. J. Mater. Chem. 2011, 21, 18191–18194. [Google Scholar] [CrossRef]
- Umadevi, S.; Umamaheswari, R.; Ganesh, V. Lyotropic liquid crystal-assisted synthesis of micro- and nanoparticles of silver. Liq. Cryst. 2017, 44, 1409–1420. [Google Scholar] [CrossRef]
- Patakfalvi, R.; Dékány, I. Preparation of silver nanoparticles in liquid crystalline systems. Colloid Polym. Sci. 2002, 280, 461–470. [Google Scholar] [CrossRef]
- Qi, L.; Gao, Y.; Ma, J. Synthesis of ribbons of silver nanoparticles in lamellar liquid crystals. Colloids Surf. A Physicochem. Eng. Asp. 1999, 157, 285–294. [Google Scholar] [CrossRef]
- Wang, W.; Efrima, S.; Regev, O. Directing silver nanoparticles into colloid−surfactant lyotropic lamellar systems. J. Phys. Chem. B 1999, 103, 5613–5621. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M. Gummy gold and silver nanoparticles of apricot (prunus armeniaca) confer high stability and biological activity. Arab. J. Chem 2016, in press. [Google Scholar] [CrossRef] [Green Version]
- Abidi, W.; Pansu, B.; Krishnaswamy, R.; Beaunier, P.; Remita, H.; Impéror-Clerc, M. Gold nanoparticles confined in lamellar mesophases. RSC Adv. 2011, 1, 434–439. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdo¨rster, G.; Biswas, P. Characterization of size, surface charge and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhu, H.P.; Liu, G.K.; Wu, D.Y.; Ren, B.; Tian, Z.Q. When the signal is not from the original molecule to be detected: Chemical transformation of para-Aminothiophenol on Ag during the SERS measurement. J. Am. Chem. Soc. 2010, 132, 9244–9246. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.-Y.; Lee, H.B.; Shin, K.S. Raman scattering of 4-aminobenzenethiol sandwiched between Ag nanoparticle and macroscopically smooth Au substrate: Effects of size of Ag nanoparticles and the excitation wavelength. J. Chem. Phys. 2013, 135, 124705. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yasin, S.M.; Johan, M.R. Optical and thermal characterization of silver nanoparticles dispersion in lamellar liquid crystal matrix. Optik 2019, 176, 593–599. [Google Scholar] [CrossRef]
- Kulkarni, C.V. Lipid crystallization: From self-assembly to hierarchical and biological ordering. Nanoscale 2012, 4, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.M.E.L.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T. Impact of environmental conditions (pH, ionic strength and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Arceo, E.; Goulet, P.J.G.; Garrido, J.J.; Aroca, R.F. Role of nanoparticle surface charge in surface-enhanced raman scattering. J. Phys. Chem. B 2005, 109, 3787–3792. [Google Scholar] [CrossRef]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced Raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark extract mediated green synthesis of silver nanoparticles: Evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Soo, K.; Kwan, Y.; Kim, K. Surface-enhanced Raman scattering characteristics of 4-nitrobenzenethiol adsorbed on palladium and silver thin films. Vib. Spectrosc. 2014, 70, 120–124. [Google Scholar]
- Kim, K.; Shin, D.; Choi, J.; Kim, K.L.; Shin, K.S. Surface enhanced Raman scattering characteristics of 4-Aminobenzenethiol derivatives adsorbed on silver. J. Phys. Chem. C 2011, 115, 24960–24966. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J.K.; Lee, H.B.; Shin, D.; Shin, K.S. Surface-enhanced Raman scattering of 4-Aminobenzenethiol in Ag sol: Relative intensity of a1- and b2-type bands invariant against aggregation of Ag nanoparticles. Langmuir 2011, 27, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.L.; Shin, D.; Choi, J.; Shin, K.S. Surface enhanced Raman scattering of 4-Aminobenzenethiol on Ag and Au: pH dependence of b2 -type bands. J. Phys. Chem. C 2012, 116, 4776–4779. [Google Scholar] [CrossRef]
- Seney, C.S.; Gutzman, B.M.; Goddard, R.H. Correlation of size and surface-enhanced Raman scattering activity of optical and spectroscopic properties for silver nanoparticles. J. Phys. Chem. C 2009, 113, 74–80. [Google Scholar] [CrossRef]
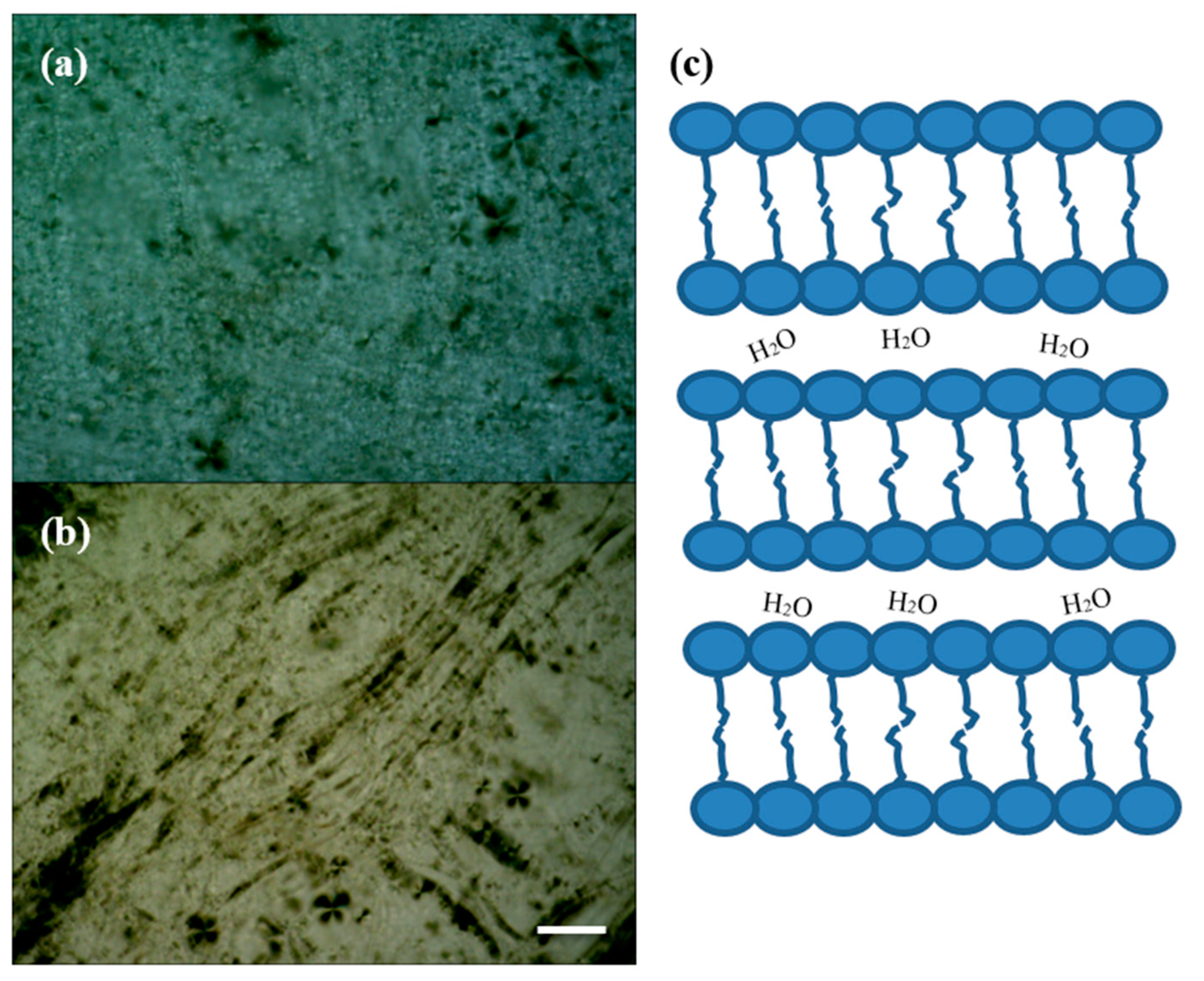
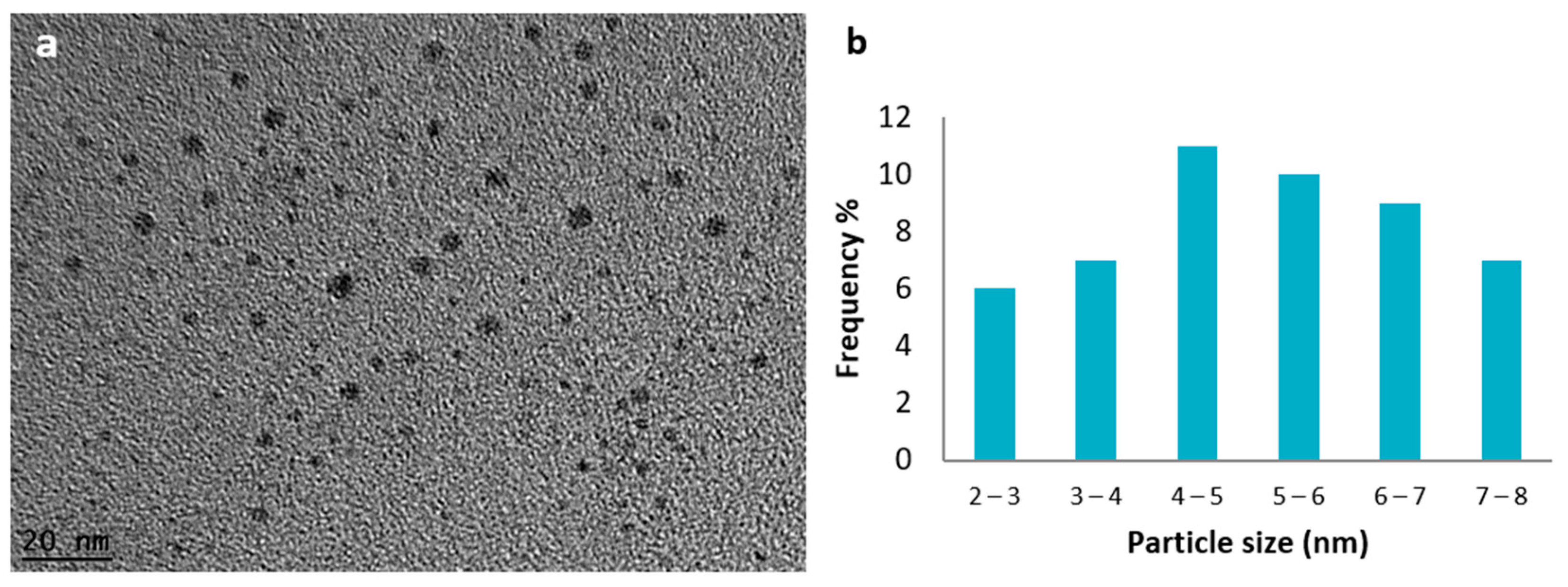
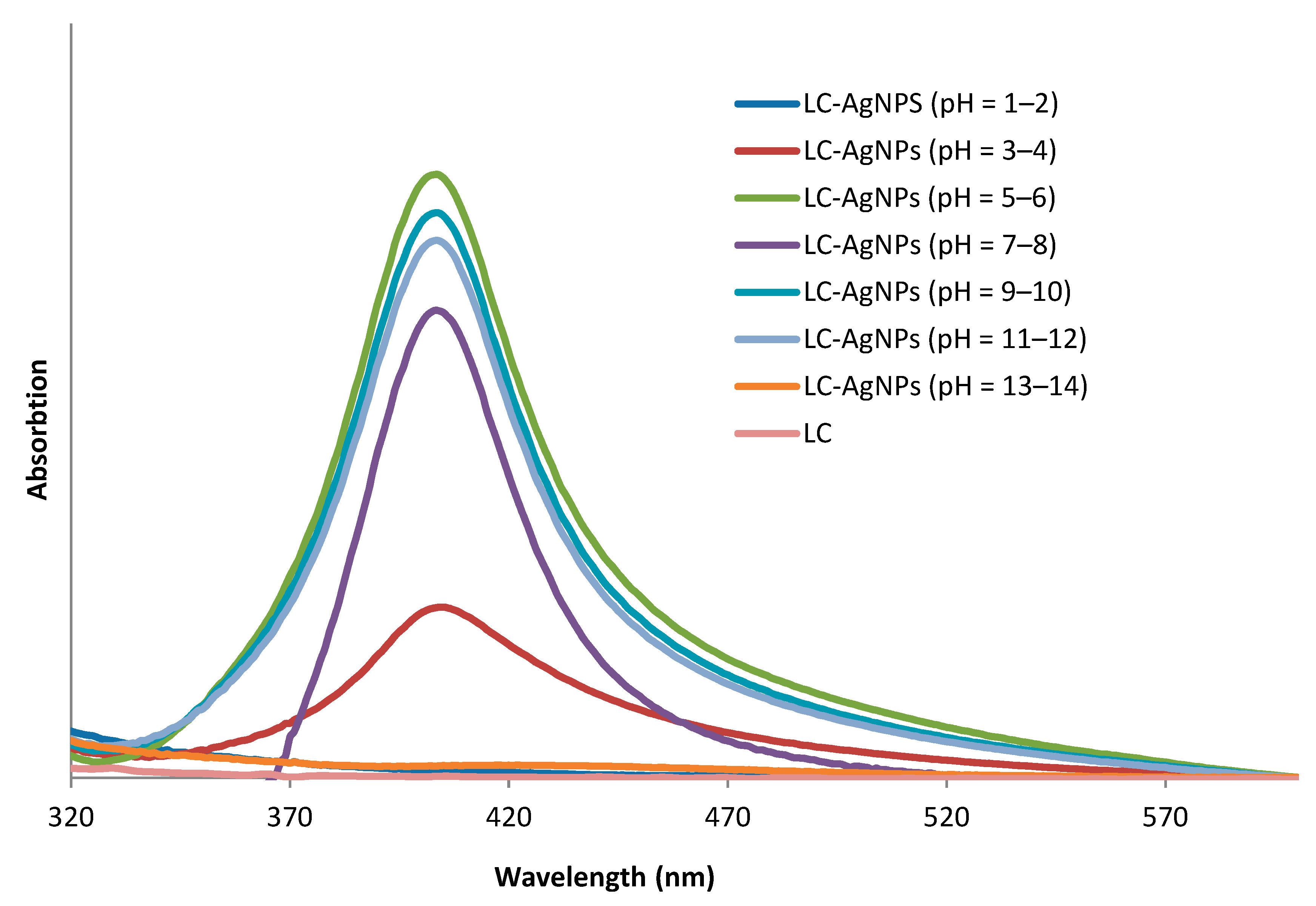

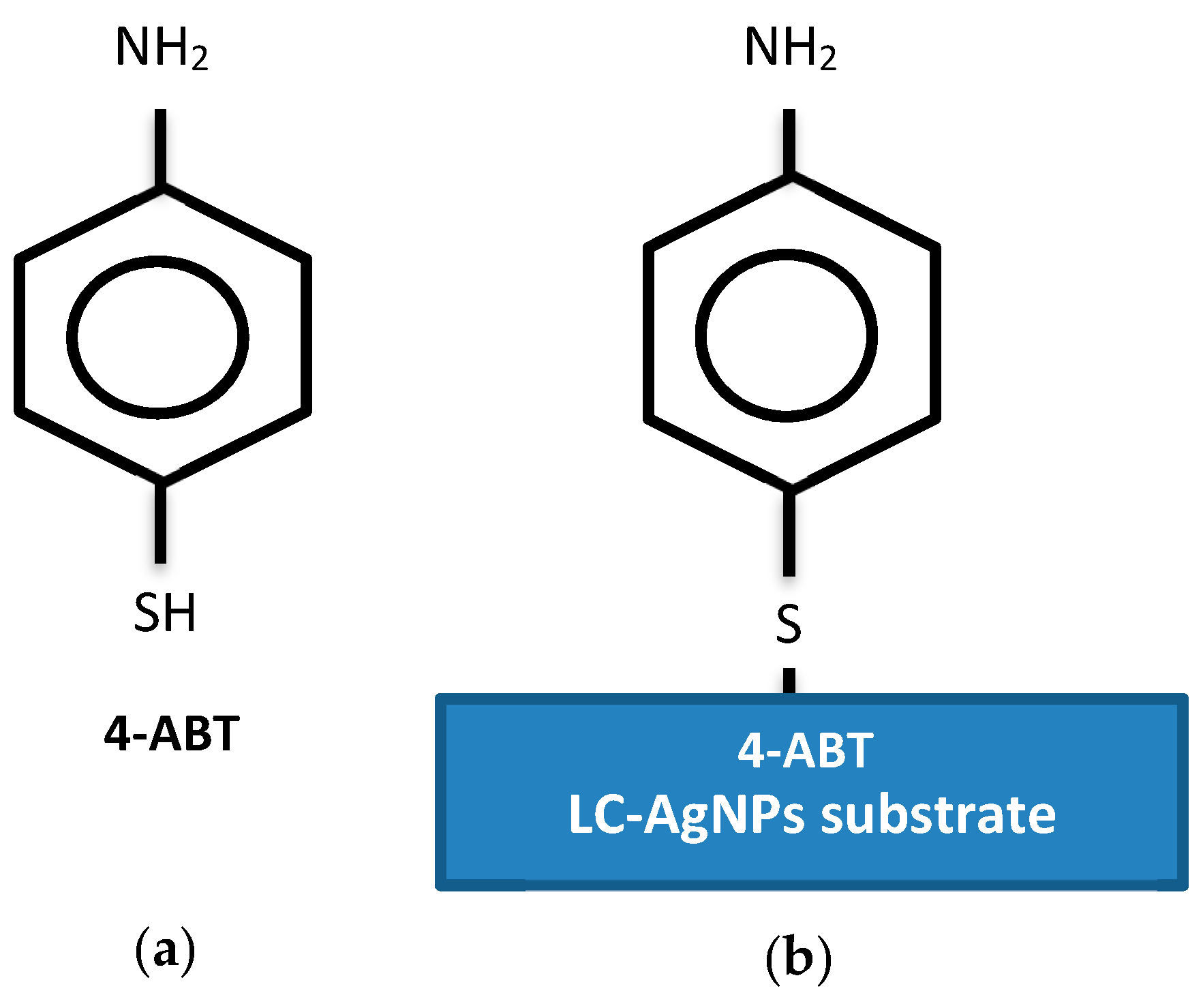
| LC-AgNPs (pH) | Wavelength, λ (nm) | Absorption | FWHM (nm) | Z-Average, d (nm) | Z-Potential, ζ (mV) | Polydispersity Index, PdI |
|---|---|---|---|---|---|---|
| 1–2 | - | - | - | 40.00 | 36.0 | 0.55 |
| 3–4 | 404 | 0.22 | 46.65 | 44.39 | 68.6 | 0.55 |
| 5–6 | 403 | 0.80 | 41.65 | 39.30 | 70.7 | 0.53 |
| 7–8 | 403 | 0.80 | 41.65 | 45.04 | 69.0 | 0.53 |
| 9–10 | 403 | 0.72 | 41.65 | 43.98 | 65.9 | 0.53 |
| 11–12 | 403 | 0.68 | 41.65 | 43.82 | 52.5 | 0.53 |
| 13–14 | - | - | - | 722.97 | 36.0 | 0.43 |
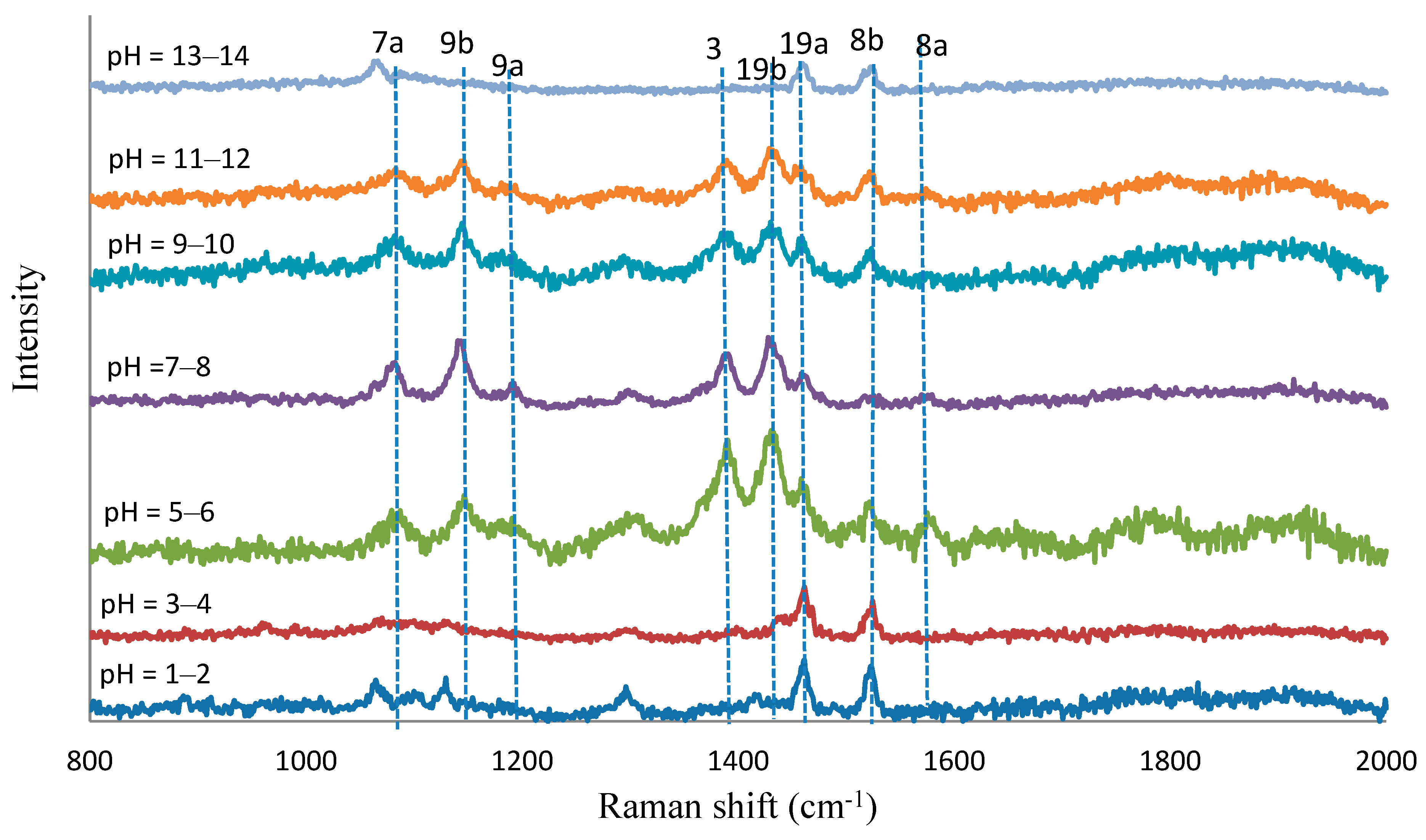
| Normal Raman | SERS (cm−1) | Assignment | ||||||
|---|---|---|---|---|---|---|---|---|
| 4-ABT | pH = 1–2 | pH = 3–4 | pH = 5–6 | pH = 7–8 | pH = 9–10 | pH = 11–12 | pH = 13–14 | |
| 1583st | 1566st | 1560vw | 1560vw | 1560vw | ύCC, (8a) | |||
| 1518me | 1516we | 1512we | 1510vwe | 1512we | 1512we | 1511vwe | ύCC, (8b) | |
| 1458me | 1457me | 1446we | 1444we | 1444we | 1446vwe | 1455vwe | ύCC + φCH, (19a) | |
| 1426st | 1426me | 1422me | 1421me | ύCC + φCH, (19b) | ||||
| 1388st | 1382me | 1381me | 1381me | ύCC + φCH, (3) | ||||
| 117me | 1175we | 1175we | 1170vwe | φCH, (9a) | ||||
| 1126vwe | 1126vwe | 1141st | 1139st | 1137me | 1137me | φCH, (9b) | ||
| 1079st | 1062we | 1062vwe | 1076st | 1074st | 1069me | 1067me | 1055we | ύCS, (7a) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Yasin, S.M.; Badruddin, I.A.; Johan, M.R. Super Stability of Ag Nanoparticle in Crystalline Lamellar (Lc) Liquid Crystal Matrix at Different pH Environment. Symmetry 2020, 12, 31. https://doi.org/10.3390/sym12010031
Mohd Yasin SM, Badruddin IA, Johan MR. Super Stability of Ag Nanoparticle in Crystalline Lamellar (Lc) Liquid Crystal Matrix at Different pH Environment. Symmetry. 2020; 12(1):31. https://doi.org/10.3390/sym12010031
Chicago/Turabian StyleMohd Yasin, Siti Mariah, Irfan Anjum Badruddin, and Mohd Rafie Johan. 2020. "Super Stability of Ag Nanoparticle in Crystalline Lamellar (Lc) Liquid Crystal Matrix at Different pH Environment" Symmetry 12, no. 1: 31. https://doi.org/10.3390/sym12010031






