Resting EEG Asymmetry Markers of Multiple Facets of the Behavioral Approach System: A LORETA Analysis
Abstract
1. Introduction
1.1. Personality Traits and EEG Alpha Asymmetry
1.2. Aims and Hypotheses
2. Methods
2.1. Participants and Questionnaires
2.2. Resting Condition
2.3. EEG Assessment and Alpha Asymmetry
2.4. Cortical Source Analysis by eLORETA
2.5. Isolated Effective Coherence (iCOH)
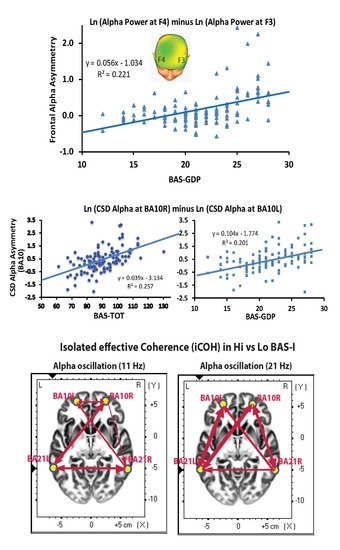
3. Results
3.1. Alpha-Asymmetry Correlates of BAS and its Facets
3.2. Functional Source Localization Differences Between High and Low BAS Individuals
3.3. BAS Differences on iCOH of Significant ROIs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gray, J.A. The psychophysiological basis of introversion-extraversion. Behav. Res. Ther. 1970, 8, 249–266. [Google Scholar] [CrossRef]
- Gray, J.A. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Fowles, D.C. Biological Variables in Psychopathology: A Psychobiological Perspective. In Comprehensive Handbook of Psychopathology, 3rd ed.; Sutker, P.B., Adams, H.E., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 85–104. [Google Scholar]
- Fowles, D.C. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. J. Res. Personal. 1987, 21, 417–435. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F. Feedback processes in the simultaneous regulation of action and affect. In Handbook of Motivation Science; Shah, J.Y., Gardner, W.L., Eds.; Guilford Press: New York, NY, USA, 2008; pp. 308–324. [Google Scholar]
- Kennis, M.; Rademaker, A.R.; Geuze, E. Neural correlates of personality: An integrative review. Neurosci. Biobehav. Rev. 2013, 37, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Corr, P.J. Reinforcement sensitivity theory of personality questionnaires: Structural survey with recommendations. Personal. Individ. Differ. 2016, 89, 60–64. [Google Scholar] [CrossRef]
- Corr, P.J.; McNaughton, N. Neuroscience and approach/avoidance personality traits: A two stage (valuation–motivation) approach. Neurosci. Biobehav. Rev. 2012, 36, 2339–2354. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System, 2nd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- McNaughton, N.; Corr, P.J. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004, 28, 285–305. [Google Scholar] [CrossRef]
- McNaughton, N.; Corr, P.J. The neuropsychology of fear and anxiety: A foundation for reinforcement sensitivity theory. In The Reinforcement Sensitivity Theory of Personality; Corr, P.J., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 44–94. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Personal. Soc. Psychol. 1994, 67, 319. [Google Scholar] [CrossRef]
- De Pascalis, V.; Sommer, K.; Scacchia, P. Resting Frontal Asymmetry and Reward Sensitivity Theory Motivational Traits. Sci. Rep. 2018, 8, 13154. [Google Scholar] [CrossRef]
- Corr, P.J. (Ed.) The Reinforcement Sensitivity Theory of Personality; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Smillie, L.D.; Cooper, A.J.; Wilt, J.; Revelle, W. Do extraverts get more bang for the buck? Refining the affective-reactivity hypothesis of extraversion. J. Personal. Soc. Psychol. 2012, 103, 306. [Google Scholar] [CrossRef]
- Carver, C.S. Impulse and constraint: Perspectives from personality psychology, convergence with theory in other areas, and potential for integration. Personal. Soc. Psychol. Rev. 2005, 9, 312–333. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J.B. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology 2003, 40, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.A.; Allen, J.J.B. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004, 67, 7–50. [Google Scholar] [CrossRef] [PubMed]
- Gable, P.A.; Neal, L.B.; Threadgill, A.H. Regulatory behavior and frontal activity: Considering the role of revised-BIS in relative right frontal asymmetry. Psychophysiology 2018, 55, e12910. [Google Scholar] [CrossRef]
- Lindsley, D.B.; Wicke, J.D. The Electroencephalogram: Autonomous electrical activity in man and animals. In Bioelectric Recording Techniques; Thompson, R., Patterson, M.N., Eds.; Academic Press: New York, NY, USA, 1974; pp. 3–79. [Google Scholar]
- Allen, J.J.B.; Urry, H.L.; Hitt, S.K.; Coan, J.A. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 2004, 41, 269–280. [Google Scholar] [CrossRef]
- Davidson, R.J. EEG Measures of Cerebral Asymmetry: Conceptual and Methodological Issues. Int. J. Neurosci. 1988, 39, 71–89. [Google Scholar] [CrossRef]
- Gable, P.A.; Mechin, N.C.; Hicks, J.A.; Adams, D.L. Supervisory control system and frontal asymmetry: Neurophysiological traits of emotion-based impulsivity. Soc. Cogn. Affect. Neurosci. 2015, 10, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Hewig, J.; Hagemann, D.; Seifert, J.; Naumann, E.; Bartussek, D. The relation of cortical activity and BIS/BAS on the trait level. Biol. Psychol. 2006, 71, 42–53. [Google Scholar] [CrossRef]
- Shackman, A.J.; McMenamin, B.W.; Maxwell, J.S.; Greischar, L.L.; Davidson, R.J. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol. Sci. 2009, 20, 1500–1506. [Google Scholar] [CrossRef]
- Amodio, D.M.; Master, S.L.; Yee, C.M.; Taylor, S.E. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology 2008, 45, 11–19. [Google Scholar] [CrossRef]
- Boksem, M.A.S.; Smolders, R.; Cremer, D.D. Social power and approach-related neural activity. Soc. Cogn. Affect. Neurosci. 2009, 7, 516–520. [Google Scholar] [CrossRef]
- De Pascalis, V.; Cozzuto, G.; Caprara, G.V.; Alessandri, G. Relations among EEG-alpha asymmetry, BIS/BAS, and dispositional optimism. Biol. Psychol. 2013, 94, 198–209. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.K.; Davidson, R.J. Prefrontal Brain Asymmetry: A Biological Substrate of the Behavioral Approach and Inhibition Systems. Psychol. Sci. 1997, 8, 204–210. [Google Scholar] [CrossRef]
- Balconi, M. Frontal brain oscillation modulation in facial emotion comprehension: The role of reward and inhibitory systems in subliminal and supraliminal processing. J. Cogn. Psychol. 2011, 23, 723–735. [Google Scholar] [CrossRef]
- Neal, L.B.; Gable, P.A. Regulatory Control and Impulsivity Relate to Resting Frontal Activity. Soc. Cogn. Affect. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.B.; Gable, P.A. Shifts in frontal asymmetry underlying impulsive and controlled decision-making. Biol. Psychol. 2019, 140, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.A.; Allen, J.J.B.; Harmon-Jones, E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology 2001, 38, 912–925. [Google Scholar] [CrossRef]
- Gable, P.; Harmon-Jones, E. Relative left frontal activation to appetitive stimuli: Considering the role of individual differences. Psychophysiology 2008, 45, 275–278. [Google Scholar] [CrossRef]
- Poole, B.D.; Gable, P.A. Affective motivational direction drives asymmetric frontal hemisphere activation. Exp. Brain Res. 2014, 232, 2121–2130. [Google Scholar] [CrossRef]
- Neal, L.B.; Gable, P.A. Neurophysiological markers of multiple facets of impulsivity. Biol. Psychol. 2016, 115, 64–68. [Google Scholar] [CrossRef]
- Wacker, J.; Chavanon, M.-L.; Stemmler, G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. J. Res. Personal. 2010, 44, 167–179. [Google Scholar] [CrossRef]
- Corr, P.J.; Cooper, A.J. The Reinforcement Sensitivity Theory of Personality Questionnaire (RST-PQ): Development and Validation. Psychol. Assess. 2016. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G. Personality Neuroscience and the Biology of Traits. Soc. Personal. Psychol. Compass 2010, 4, 1165–1180. [Google Scholar] [CrossRef]
- Yacubian, J.; Sommer, T.; Schroeder, K.; Gläscher, J.; Braus, D.F.; Büchel, C. Subregions of the ventral striatum show preferential coding of reward magnitude and probability. NeuroImage 2007, 38, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Corr, P.J. Approach and avoidance behaviour: Multiple systems and their interactions. Emot. Rev. 2013, 5, 285–290. [Google Scholar] [CrossRef]
- Drago, F.; Caccamo, G.; Continella, G.; Scapagnini, U. Amphetamine-induced analgesia does not involve brain opioids. Eur. J. Pharmacol. 1984, 101, 267–269. [Google Scholar] [CrossRef]
- Schweinhardt, P.; Seminowicz, D.A.; Jaeger, E.; Duncan, G.H.; Bushnell, M.C. The anatomy of the mesolimbic reward system: A link between personality and the placebo analgesic response. J. Neurosci. 2009, 29, 4882–4887. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Iwata, S.-I.; Morioka, H.; Masuyama, T.; Fukuda, T.; Nomoto, M. Antinociceptive mechanism of l-DOPA. Pain 2004, 110, 246–249. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the Statetrait Anxiety Inventory (Form Y); Consulting Psychologist Press: Palo Alto, CA, USA, 1988. [Google Scholar]
- Schneider, M.; Chau, L.; Mohamadpour, M.; Stephens, N.; Arya, K.; Grant, A. EEG asymmetry and BIS/BAS among healthy adolescents. Biol. Psychol. 2016, 120, 142–148. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp. Clin. Pharm. 2002, 24, 5–12. [Google Scholar]
- Hagemann, D.; Naumann, E.; Becker, G.; Maier, S.; Bartussek, D. Frontal brain asymmetry and affective style: A conceptual replication. Psychophysiology 1998, 35, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, D.; Naumann, E.; Thayer, J.F. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology 2001, 38, 847–857. [Google Scholar] [CrossRef]
- Hagemann, D. Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biol. Psychol. 2004, 67, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage 2007, 34, 1600–1611. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Sherwood, R.J.; Henriques, J.B.; Davidson, R.J. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychol. Sci. 2005, 16, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Biscay, R.J.; Bosch-Bayard, J.; Lehmann, D.; Kochi, K.; Yamada, N.; Kinoshita, T.; Sadato, N. Isolated effective coherence (iCoh): Causal information flow excluding indirect paths. arXiv 2014, arXiv:1402.4887. [Google Scholar]
- Salmaso, D.; Longoni, A.M. Problems in the assessment of hand preference. Cortex 1985, 21, 533–549. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Allen, J.J. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. J. Abnorm. Psychol. 1997, 106, 159. [Google Scholar] [CrossRef]
- Allen, J.J.B.; Coan, J.A.; Nazarian, M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004, 67, 183–218. [Google Scholar] [CrossRef] [PubMed]
- Tomarken, A.J.; Davidson, R.J.; Wheeler, R.E.; Kinney, L. Psychometric Properties of Resting Anterior EEG Asymmetry: Temporal Stability and Internal Consistency. Psychophysiology 1992, 29, 576–592. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic. 1981, 86, 127–137. [Google Scholar]
- Pascual-Marqui, R.D. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: Exact, zero error localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Pascual-Marqui, R.D. Theory of the EEG inverse problem. In Quantitative EEG Analysis: Methods and Clinical Applications; Tong, S., Thakor, N.V., Eds.; Artech House: Boston, MA, USA, 2009; pp. 121–140. [Google Scholar]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Siclari, F.; Bernardi, G.; Riedner, B.A.; LaRocque, J.J.; Benca, R.M.; Tononi, G. Two Distinct Synchronization Processes in the Transition to Sleep: A High-Density Electroencephalographic Study. Sleep 2014, 37, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Grech, R.; Cassar, T.; Muscat, J.; Camilleri, K.P.; Fabri, S.G.; Zervakis, M.; Xanthopoulos, P.; Sakkalis, V.; Vanrumste, B. Review on solving the inverse problem in EEG source analysis. J. Neuroeng. Rehabil. 2008, 5, 25. [Google Scholar] [CrossRef]
- Lehmann, D.; Faber, P.L.; Tei, S.; Pascual-Marqui, R.D.; Milz, P.; Kochi, K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. NeuroImage 2012, 60, 1574–1586. [Google Scholar] [CrossRef]
- Jatoi, M.A.; Kamel, N.; Malik, A.S.; Faye, I. EEG based brain source localization comparison of sLORETA and eLORETA. Australas. Phys. Eng. Sci. Med. 2014, 37, 713–721. [Google Scholar] [CrossRef]
- Martín-Buro, M.C.; Garcés, P.; Maestú, F. Test-retest reliability of resting-state magnetoencephalography power in sensor and source space. Hum. Brain Mapp. 2016, 37, 179–190. [Google Scholar] [CrossRef]
- Candelaria-Cook, F.T.; Schendel, M.E.; Ojeda, C.J.; Bustillo, J.R.; Stephen, J.M. Reduced parietal alpha power and psychotic symptoms: Test-retest reliability of resting-state magnetoencephalography in schizophrenia and healthy controls. Schizophr. Res. 2020, 215, 229–240. [Google Scholar] [CrossRef]
- Hagemann, D.; Naumann, E.; Thayer, J.F.; Bartussek, D. Does resting electroencephalograph asymmetry reflect a trait? An application of latent state-trait theory. J. Personal. Soc. Psychol. 2002, 82, 619–641. [Google Scholar] [CrossRef]
- Hagemann, D.; Hewig, J.; Seifert, J.; Naumann, E.; Bartussek, D. The latent state-trait structure of resting EEG asymmetry: Replication and extension. Psychophysiology 2005, 42, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cogn. Emot. 1993, 7, 115–138. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology 1998, 35, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.A.; Allen, J.J.B. The state and trait nature of frontal EEG asymmetry in emotion. In The Asymmetrical Brain; Hugdahl, K., Davidson, R.J., Eds.; MIT Press: Cambridge, MA, USA, 2003; pp. 565–615. [Google Scholar]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shimojo, S.; O’Doherty, J.P. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb. Cortex 2011, 21, 769–776. [Google Scholar] [CrossRef]
- Montague, P.R.; Berns, G.S. Neural Economics and the Biological Substrates of Valuation. Neuron 2002, 36, 265–284. [Google Scholar] [CrossRef]
- Levy, D.J.; Glimcher, P.W. The root of all value: A neural common currency for choice. Curr. Opin. Neurobiol. 2012, 22, 1027–1038. [Google Scholar] [CrossRef]
- Zhu, J. Neural Representation of Social, Monetary and Chocolate Reinforcer Processing; Aston University: Birmingham, UK, 2016. [Google Scholar]
- Elliott, R.; Friston, K.J.; Dolan, R.J. Dissociable Neural Responses in Human Reward Systems. J. Neurosci. 2000, 20, 6159. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex and reward. Cereb. Cortex 2000, 10, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.T.; Price, J.L. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1995, 363, 642–664. [Google Scholar] [CrossRef] [PubMed]
- Montague, P.R.; McClure, S.M.; Baldwin, P.R.; Phillips, P.E.M.; Budygin, E.A.; Stuber, G.D.; Kilpatrick, M.R.; Wightman, R.M. Dynamic Gain Control of Dopamine Delivery in Freely Moving Animals. J. Neurosci. 2004, 24, 1754. [Google Scholar] [CrossRef][Green Version]
- Tanji, J.; Hoshi, E. Behavioral planning in the prefrontal cortex. Curr. Opin. Neurobiol. 2001, 11, 164–170. [Google Scholar] [CrossRef]
- Schmidt, L.; d’Arc, B.F.; Lafargue, G.; Galanaud, D.; Czernecki, V.; Grabli, D.; Schüpbach, M.; Hartmann, A.; Lévy, R.; Dubois, B. Disconnecting force from money: Effects of basal ganglia damage on incentive motivation. Brain 2008, 131, 1303–1310. [Google Scholar] [CrossRef]
- Peters, J.; Büchel, C. Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Mediotemporal Interactions. Neuron 2010, 66, 138–148. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef]
- Pochon, J.B.; Levy, R.; Fossati, P.; Lehericy, S.; Poline, J.B.; Pillon, B.; Le Bihan, D.; Dubois, B. The neural system that bridges reward and cognition in humans: An fMRI study. Proc. Natl. Acad. Sci. USA 2002, 99, 5669–5674. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef]
- Benoit, R.G.; Gilbert, S.J.; Burgess, P.W. A Neural Mechanism Mediating the Impact of Episodic Prospection on Farsighted Decisions. J. Neurosci. 2011, 31, 6771. [Google Scholar] [CrossRef] [PubMed]
- Kwan, D.; Craver, C.F.; Green, L.; Myerson, J.; Rosenbaum, R.S. Dissociations in future thinking following hippocampal damage: Evidence from discounting and time perspective in episodic amnesia. J. Exp. Psychol. Gen. 2013, 142, 1355–1369. [Google Scholar] [CrossRef]
- Race, E.; Keane, M.M.; Verfaellie, M. Medial Temporal Lobe Damage Causes Deficits in Episodic Memory and Episodic Future Thinking Not Attributable to Deficits in Narrative Construction. J. Neurosci. 2011, 31, 10262. [Google Scholar] [CrossRef]
- Race, E.; Keane, M.M.; Verfaellie, M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus 2013, 23, 268–277. [Google Scholar] [CrossRef]
- Tulving, E. Memory and consciousness. Can. Psychol. Psychol. Can. 1985, 26, 1–12. [Google Scholar] [CrossRef]
- Squire, L.R.; van der Horst, A.S.; McDuff, S.G.R.; Frascino, J.C.; Hopkins, R.O.; Mauldin, K.N. Role of the hippocampus in remembering the past and imagining the future. Proc. Natl. Acad. Sci. USA 2010, 107, 19044–19048. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.J.; Keane, M.M.; Verfaellie, M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus 2015, 25, 345–353. [Google Scholar] [CrossRef]
- Xu, J.; Lyu, H.; Li, T.; Xu, Z.; Fu, X.; Jia, F.; Wang, J.; Hu, Q. Delineating functional segregations of the human middle temporal gyrus with resting-state functional connectivity and coactivation patterns. Hum. Brain Mapp. 2019, 40, 5159–5171. [Google Scholar] [CrossRef]
- Goel, V.; Grafman, J.; Sadato, N.; Hallett, M. Modeling other minds. Neuroreport 1995, 6, 1741–1746. [Google Scholar] [CrossRef]
- Carver, C.S.; Johnson, S.L.; Joormann, J. Two-Mode Models of Self-Regulation as a Tool for Conceptualizing Effects of the Serotonin System in Normal Behavior and Diverse Disorders. Curr. Dir. Psychol. Sci. 2009, 18, 195–199. [Google Scholar] [CrossRef]
- MacDonald, K.B. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol. Rev. 2008, 115, 1012–1031. [Google Scholar] [CrossRef]
- Raine, A. From Genes to Brain to Antisocial Behavior. Curr. Dir. Psychol. Sci. 2008, 17, 323–328. [Google Scholar] [CrossRef]
- Knutson, B.; Adams, C.M.; Fong, G.W.; Hommer, D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001, 21, RC159. [Google Scholar] [CrossRef] [PubMed]
- Basar, K.; Sesia, T.; Groenewegen, H.; Steinbusch, H.W.M.; Visser-Vandewalle, V.; Temel, Y. Nucleus accumbens and impulsivity. Prog. Neurobiol. 2010, 92, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.L.; Yang, Y. The Potential Role of the Striatum in Antisocial Behavior and Psychopathy. Biol. Psychiatry 2012, 72, 817–822. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; Smith, C.E.; et al. Dopaminergic Network Differences in Human Impulsivity. Science 2010, 329, 532. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Benning, S.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010, 13, 419–421. [Google Scholar] [CrossRef]
- Bjork, J.M.; Chen, G.; Hommer, D.W. Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biol. Psychol. 2012, 89, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Geurts, D.E.M.; von Borries, K.; Volman, I.; Bulten, B.H.; Cools, R.; Verkes, R.-J. Neural connectivity during reward expectation dissociates psychopathic criminals from non-criminal individuals with high impulsive/antisocial psychopathic traits. Soc. Cogn. Affect. Neurosci. 2016, 11, 1326–1334. [Google Scholar] [CrossRef]
- Angelides, N.H.; Gupta, J.; Vickery, T.J. Associating resting-state connectivity with trait impulsivity. Soc. Cogn. Affect. Neurosci. 2017, 12, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Affect, cognition, and hemispheric specialization. In Emotions, Cognition, and Behavior; Izard, C.E., Kagan, J., Eds.; Cambridge University Press: New York, NY, USA, 1985; pp. 320–365. [Google Scholar]
- Nusslock, R.; Harmon-Jones, E.; Alloy, L.B.; Urosevic, S.; Goldstein, K.; Abramson, L.Y. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. J. Abnorm. Psychol. 2012, 121, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A.; Dzemidzic, M.; Eiler, W.J.; Coskunpinar, A.; Karyadi, K.A.; Kareken, D.A. Negative Urgency Mediates the Relationship between Amygdala and Orbitofrontal Cortex Activation to Negative Emotional Stimuli and General Risk-Taking. Cereb. Cortex 2015, 25, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Berretz, G.; Packheiser, J.; Friedrich, P. Laterality 2020: Entering the next decade. Laterality 2020, 1–33. [Google Scholar] [CrossRef] [PubMed]


| Brodmann Areas | X (mm) | Y (mm) | Z (mm) | Lobe | Structure |
|---|---|---|---|---|---|
| BA10L | −25 | 55 | 5 | Left-Frontal Lobe | Superior Frontal Gyrus |
| BA10R | 25 | 55 | 5 | Right-Frontal Lobe | |
| BA11L | −20 | 40 | −15 | Left-Frontal Lobe | Middle Frontal Gyrus |
| BA11R | 20 | 40 | −15 | Right-Frontal Lobe | |
| BA21L | −65 | −50 | 5 | Left-Temporal Lobe | Middle Temporal Gyrus |
| BA21R | 65 | −50 | 5 | Right-Temporal Lobe | |
| BA31L | −10 | −54 | 19 | Left-Limbic Lobe | Posterior Cingulate |
| BA31R | 10 | −54 | 19 | Right-Limbic Lobe | |
| BA7L | −20 | −65 | 50 | Left-Parietal Lobe | Precuneus |
| BA7R | 20 | −65 | 50 | Right-Parietal Lobe |
| BAS–TOT | BAS–GDP | BAS–RI | BAS–RR | BAS–I | BIS | FFFS | STAI-Y1 | |
|---|---|---|---|---|---|---|---|---|
| BAS–TOT | 1 | |||||||
| BAS–GDP | 0.518 ‡ | 1 | ||||||
| BAS–RI | 0.665 ‡ | 0.330 • | 1 | |||||
| BAS–RR | 0.774 ‡ | 0.350 ‡ | 0.319 ‡ | 1 | ||||
| BAS–I | 0.587 ‡ | −0.083 | 0.191 | 0.338 ‡ | 1 | |||
| BIS | 0.195 | −0.019 | −0.047 | 0.272 • | 0.179 | 1 | ||
| FFFS | 0.148 | 0.057 | −0.135 | 0.275 • | 0.054 | 0.438 ‡ | 1 | |
| STAI-Y1 | −0.011 | −0.057 | 0.003 | −0.064 | 0.032 | 0.044 | 0.02801 | 1 |
| Mean | 89.7 | 21.4 | 19.8 | 29.5 | 18.9 | 55.8 | 25.0 | 38.3 |
| SD | 11.6 | 3.8 | 4.1 | 4.5 | 4.4 | 12.1 | 6.0 | 6.1 |
| Range | 61–130 | 12–28 | 10–28 | 19–39 | 3–32 | 29–87 | 13–38 | 25–52 |
| EEG Alpha | BAS–TOT | BAS–GDP | BAS–RI | BAS–RR | BAS–I | BIS | FFFS |
|---|---|---|---|---|---|---|---|
| Alpha Asymmetry (Average Reference) | |||||||
| Frontopolar | 0.254 * | 0.088 | 0.278 • | 0.082 | 0.179 | −0.002 | −0.152 |
| Frontal | 0.241 * | 0.472 ‡ | 0.164 | 0.176 | −0.121 | 0.063 | −0.033 |
| Frontocentral | −0.023 | 0.007 | −0.008 | −0.034 | −0.092 | 0.082 | −0.089 |
| Inferior Frontal | 0.005 | −0.094 | −0.014 | 0.033 | 0.048 | 0.113 | −0.031 |
| Central | −0.010 | 0.147 | −0.041 | −0.027 | −0.151 | −0.013 | −0.080 |
| Centroparietal | −0.023 | 0.042 | −0.059 | −0.011 | −0.124 | −0.005 | −0.030 |
| Parietal | −0.045 | −0.073 | −0.002 | −0.152 | −0.004 | 0.011 | −0.033 |
| CSD Alpha Asymmetry | |||||||
| BA10 | 0.479‡ | 0.471 ‡ | 0.336 ‡ | 0.330 • | 0.175 | 0.107 | 0.189 |
| BA11 | 0.035 | 0.028 | −0.040 | 0.009 | 0.111 | −0.019 | 0.006 |
| BA7 | 0.125 | 0.075 | 0.155 | 0.045 | 0.099 | −0.069 | −0.051 |
| BA31 | 0.088 | 0.128 | 0.043 | 0.122 | 0.004 | −0.045 | 0.017 |
| BA21 | 0.309 • | 0.072 | 0.099 | 0.296 • | 0.301 • | 0.189 | −0.112 |
| Variable | Adjusted R2 | Parameter Estimate (B) | Standard Error | T | p(FDR) | Standardized Estimate (β) | 95% Confidence Limits | |
|---|---|---|---|---|---|---|---|---|
| EEG Alpha Asymmetry | ||||||||
| Frontopolar | ||||||||
| BAS–TOT | 0.047 | 0.017 | 0.006 | 2.77 | 0.028 | 0.233 | 0.005 | 0.030 |
| BAS–GDP | 0.003 | 0.016 | 0.020 | 0.81 | 0.691 | 0.070 | −0.023 | 0.055 |
| BAS–RI | 0.078 | 0.062 | 0.018 | 3.51 | 0.006 | 0.291 | 0.027 | 0.098 |
| BAS–RR | 0.006 | 0.006 | 0.017 | 0.38 | 0.907 | 0.033 | −0.026 | 0.039 |
| BAS–I | 0.023 | 0.034 | 0.017 | 2.03 | 0.153 | 0.173 | 0.001 | 0.068 |
| Frontal | ||||||||
| BAS–TOT | 0.048 | 0.009 | 0.003 | 2.79 | 0.028 | 0.234 | 0.003 | 0.016 |
| BAS–GDP | 0.209 | 0.057 | 0.009 | 6.05 | 0.001 | 0.463 | 0.038 | 0.076 |
| BAS–RI | 0.023 | 0.020 | 0.010 | 2.04 | 0.153 | 0.174 | 0.001 | 0.040 |
| BAS–RR | 0.016 | 0.016 | 0.009 | 1.78 | 0.232 | 0.152 | −0.002 | 0.033 |
| BAS–I | 0.007 | −0.013 | 0.009 | −1.42 | 0.393 | −0.122 | −0.031 | 0.005 |
| CSD Alpha Asymmetry | ||||||||
| BA10 | ||||||||
| BAS–TOT | 0.226 | 0.038 | 0.006 | 6.36 | 0.001 | 0.482 | 0.026 | 0.050 |
| BAS–GDP | 0.217 | 0.113 | 0.018 | 6.19 | 0.001 | 0.471 | 0.077 | 0.149 |
| BAS–RI | 0.093 | 0.072 | 0.019 | 3.85 | 0.002 | 0.315 | 0.035 | 0.108 |
| BAS–RR | 0.110 | 0.069 | 0.016 | 4.21 | 0.001 | 0.342 | 0.037 | 0.102 |
| BAS–I | 0.025 | 0.038 | 0.018 | 2.10 | 0.152 | 0.179 | 0.002 | 0.073 |
| BA21 | ||||||||
| BAS–TOT | 0.088 | 0.031 | 0.008 | 3.74 | <0.0001 | 0.307 | 0.014 | 0.047 |
| BAS–GDP | 0.003 | 0.021 | 0.026 | 0.80 | 0.691 | 0.069 | −0.031 | 0.073 |
| BAS–RI | 0.003 | 0.029 | 0.025 | 1.16 | 0.534 | 0.100 | −0.020 | 0.077 |
| BAS–RR | 0.075 | 0.073 | 0.021 | 3.46 | 0.006 | 0.286 | 0.031 | 0.115 |
| BAS–I | 0.084 | 0.080 | 0.022 | 3.65 | <0.0001 | 0.301 | 0.037 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascalis, V.D.; Cirillo, G.; Vecchio, A. Resting EEG Asymmetry Markers of Multiple Facets of the Behavioral Approach System: A LORETA Analysis. Symmetry 2020, 12, 1794. https://doi.org/10.3390/sym12111794
Pascalis VD, Cirillo G, Vecchio A. Resting EEG Asymmetry Markers of Multiple Facets of the Behavioral Approach System: A LORETA Analysis. Symmetry. 2020; 12(11):1794. https://doi.org/10.3390/sym12111794
Chicago/Turabian StylePascalis, Vilfredo De, Giuliana Cirillo, and Arianna Vecchio. 2020. "Resting EEG Asymmetry Markers of Multiple Facets of the Behavioral Approach System: A LORETA Analysis" Symmetry 12, no. 11: 1794. https://doi.org/10.3390/sym12111794
APA StylePascalis, V. D., Cirillo, G., & Vecchio, A. (2020). Resting EEG Asymmetry Markers of Multiple Facets of the Behavioral Approach System: A LORETA Analysis. Symmetry, 12(11), 1794. https://doi.org/10.3390/sym12111794