An Intelligent Iris Based Chronic Kidney Identification System
Abstract
:1. Introduction
2. Related Work
3. Methodology: An Intelligent Iris Based Chronic Kidney Identification System
3.1. Kidney Pathology
3.1.1. Conventional Method
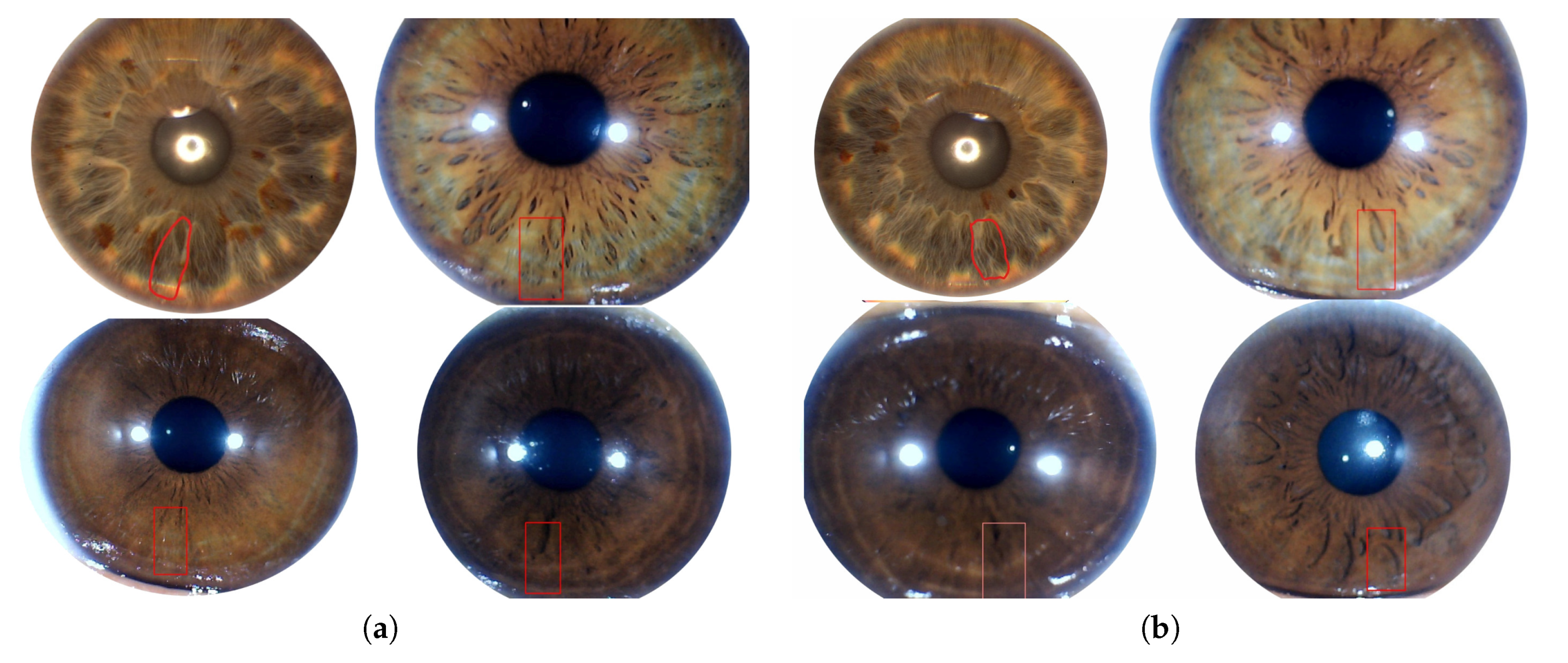
3.1.2. Iridology
3.2. Iris-Acquisition System (IAS)
3.3. Processing System
3.4. Artificial Intelligence (AI) Algorithm
4. Results and Discussion
4.1. Iris Data-Set
4.2. Training Methodology
4.3. Validation and Accuracy
4.4. Comparison with Other Proposed Algorithms
5. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, T.C.; Hard, G.C.; Mohr, U. Urinary System; Springer: Berlin/Heisenberg, Germany, 2013. [Google Scholar]
- Kurtz, I.; Maher, T.; Hulter, H.N.; Schambelan, M.; Sebastian, A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983, 24, 670–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska, I.; Płonka, J. Determination of biogenic amines and vitamins in urine samples with HPLC. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2974–2987. [Google Scholar] [CrossRef] [Green Version]
- Kirchmann, H.; Pettersson, S. Human urine-chemical composition and fertilizer use efficiency. Fertil. Res. 1994, 40, 149–154. [Google Scholar] [CrossRef]
- Veeralingam, S.; Sahatiya, P.; Badhulika, S. Low cost, flexible and disposable SnSe2 based photoresponsive ammonia sensor for detection of ammonia in urine samples. Sens. Actuators B Chem. 2019, 297, 126725. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 107–213. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Abbrecht, P.H.; Malvin, R.L. Effects of GFR and renal plasma flow on urine osmolarity. Am. J. Physiol. Leg. Content 1961, 201, 754–758. [Google Scholar] [CrossRef] [Green Version]
- Griggers, S.; Paccamonti, D.; Thompson, R.; Eilts, B. The effects of pH, osmolarity and urine contamination on equine spermatozoal motility. Theriogenology 2001, 56, 613–622. [Google Scholar] [CrossRef]
- Schrier, R.W. Diseases of the Kidney and Urinary Tract; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1. [Google Scholar]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Prim. 2017, 3, 17088. [Google Scholar] [CrossRef]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.; Jardine, M. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef]
- Levin, A. The clinical epidemiology of cardiovascular diseases in chronic kidney disease: Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. In Seminars in Dialysis; Wiley Online Library; Blackwell Science Inc.: Oxford, UK, 2003; Volume 16, pp. 101–105. [Google Scholar]
- Wilson, B.J.; Watson, M.S.; Prescott, G.J.; Sunderland, S.; Campbell, D.M.; Hannaford, P.; Smith, W.C.S. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ 2003, 326, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauci, A.S. The human immunodeficiency virus: Infectivity and mechanisms of pathogenesis. Science 1988, 239, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.h.; Lv, J.; Garg, A.X.; Knight, J. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414. [Google Scholar] [CrossRef]
- Mischak, H. Autosomal-Dominant Polycystic Kidney Disease (ADPKD). US Patent App. 13/140,106, 6 July 2011. [Google Scholar]
- Cattran, D.C.; Feehally, J.; Cook, H.T.; Liu, Z.H.; Fervenza, F.C.; Mezzano, S.A.; Floege, J.; Nachman, P.H.; Gipson, D.S.; Praga, M. Kidney disease: Improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012, 2, 139–274. [Google Scholar]
- Murray, T.; Goldberg, M. Chronic interstitial nephritis: Etiologic factors. Ann. Intern. Med. 1975, 82, 453–459. [Google Scholar] [CrossRef]
- Foley, R.N.; Murray, A.M.; Li, S.; Herzog, C.A.; McBean, A.M.; Eggers, P.W.; Collins, A.J. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. 2005, 16, 489–495. [Google Scholar] [CrossRef]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tim Newman. Symptoms, Causes, and Treatment of Chronic Kidney Disease. 2017. Available online: https://www.medicalnewstoday.com/articles/172179 (accessed on 3 October 2020).
- Stevens, L.A.; Levey, A.S. Measured GFR as a confirmatory test for estimated GFR. J. Am. Soc. Nephrol. 2009, 20, 2305–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avorn, J.; Winkelmayer, W.C.; Bohn, R.L.; Levin, R.; Glynn, R.J.; Levy, E.; Owen, W., Jr. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J. Clin. Epidemiol. 2002, 55, 711–716. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E. Early detection of CKD: The benefits, limitations and effects on prognosis. Nat. Rev. Nephrol. 2011, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Jungers, P.; Zingraff, J.; Albouze, G.; Chauveau, P.; Page, B.; Hannedouche, T.; Man, N. Late referral to maintenance dialysis: Detrimental consequences. Nephrol. Dial. Transplant. 1993, 8, 1089–1093. [Google Scholar] [PubMed]
- Perazella, M.A.; Khan, S. Increased mortality in chronic kidney disease: A call to action. Am. J. Med. Sci. 2006, 331, 150–153. [Google Scholar] [CrossRef]
- Roubicek, C.; Brunet, P.; Huiart, L.; Thirion, X.; Leonetti, F.; Dussol, B.; Jaber, K.; Andrieu, D.; Ramananarivo, P.; Berland, Y. Timing of nephrology referral: Influence on mortality and morbidity. Am. J. Kidney Dis. 2000, 36, 35–41. [Google Scholar] [CrossRef]
- Jonas, W.B. Alternative medicine—Learning from the past, examining the present, advancing to the future. JAMA 1998, 280, 1616–1618. [Google Scholar] [CrossRef]
- Shon, H.S.; Batbaatar, E.; Kim, K.O.; Cha, E.J.; Kim, K.A. Classification of Kidney Cancer Data Using Cost-Sensitive Hybrid Deep Learning Approach. Symmetry 2020, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, A.H.; Wilson, D.H.; Taylor, A.W. Prevalence and cost of alternative medicine in Australia. Lancet 1996, 347, 569–573. [Google Scholar] [CrossRef]
- Jensen, B. Iridology Simplified, 5th ed.; Book Pub Co.: Summertown, TN, USA, 2012. [Google Scholar]
- Lodin, A.; Demea, S. Design of an iris-based medical diagnosis system. In Proceedings of the 2009 International Symposium on Signals, Circuits and Systems, Iasi, Romania, 9–10 July 2009; pp. 1–4. [Google Scholar]
- Hussein, S.E.; Hassan, O.A.; Granat, M.H. Assessment of the potential iridology for diagnosing kidney disease using wavelet analysis and neural networks. Biomed. Signal Process. Control 2013, 8, 534–541. [Google Scholar] [CrossRef]
- Hussain, T.; Haider, A.; Muhammad, A.M.; Agha, A.; Khan, B.; Rashid, F.; Raza, M.S.; Din, M.; Khan, M.; Ullah, S. An Iris based Lungs Pre-diagnostic System. In Proceedings of the 2019 2nd International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 30–31 January 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Li, Y.H.; Aslam, M.S.; Yang, K.L.; Kao, C.A.; Teng, S.Y. Classification of Body Constitution Based on TCM Philosophy and Deep Learning. Symmetry 2020, 12, 803. [Google Scholar] [CrossRef]
- Jha, V. Current status of end-stage renal disease care in India and Pakistan. Kidney Int. Suppl. 2013, 3, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.J.K. Circular Hough Transform; Aalb. Univ. Vision, Graph. Interact. Syst.; University in Aalborg: Aalborg, Denmark, 2007; Volume 123. [Google Scholar]
- Amerifar, S.; Targhi, A.T.; Dehshibi, M.M. Iris the picture of health: Towards medical diagnosis of diseases based on iris pattern. In Proceedings of the 2015 Tenth International Conference on Digital Information Management (ICDIM), Jeju, Korea, 21–23 October 2015; pp. 120–123. [Google Scholar]
- You, J.; Chen, B. New approach to retinal image enhancement based on Hessian matrix. Jisuanji Yingyong/ J. Comput. Appl. 2011, 31, 1560–1562. [Google Scholar] [CrossRef]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Cambridge, MA, USA, 11–13 October 1998; pp. 130–137. [Google Scholar]
- Prayitno, A.; Wibawa, A.D.; Purnomo, M.H. Early detection study of Kidney Organ Complication caused by Diabetes Mellitus using iris image color constancy. In Proceedings of the 2016 International Conference on Information & Communication Technology and Systems (ICTS), Surabaya, Indonesia, 12 October 2016; pp. 146–149. [Google Scholar]
- Ebner, M. Color Constancy; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 7. [Google Scholar]
- Comon, P. Independent component analysis, a new concept? Signal Process. 1994, 36, 287–314. [Google Scholar] [CrossRef]
- Sitorus, M.A.; Purnomo, M.H.; Wibawa, A.D. Iris image analysis of patient Chronic Renal Failure (CRF) using watershed algorithm. In Proceedings of the 2015 4th International Conference on Instrumentation, Communications, Information Technology, and Biomedical Engineering (ICICI-BME), Bandung, Indonesia, 2–3 November 2015; pp. 54–58. [Google Scholar]
- Samant, P.; Agarwal, R. Machine learning techniques for medical diagnosis of diabetes using iris images. Comput. Methods Programs Biomed. 2018, 157, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Passarella, R.; Erwin, E.; Fachrurrozi, M.; Sutarno, S. Development of iridology system database for colon disorders identification using Image processing. Indian J. Bioinform. Biotechnol. 2013, 2, 100–103. [Google Scholar]
- Banzi, J.F.; Xue, Z. An automated tool for non-contact, real time early detection of diabetes by computer vision. Int. J. Mach. Learn. Comput. 2015, 5, 225. [Google Scholar] [CrossRef]
- About: UCERD Private Limited Islamabad. 2018. Available online: http://ucerd.com/my_uploads/pdfs/info/UCERD.pdf (accessed on 17 September 2020).
- Iridology Research Group (IRG). 2018. Available online: http://ucerd.com/Iridology_Research_Group.php (accessed on 22 September 2020).
- Hussain, T.; Haider, A.; Taleb-Ahmed, A. A Heterogeneous Multi-Core Based Biomedical Application Processing System and Programming Toolkit. J. Signal Process. Syst. 2019, 91, 963–978. [Google Scholar] [CrossRef]
- Haider, A.; Hussain, T.; Agha, A.; Khan, B.; Rashid, F.; Muzamil, S.; Ahmed, A.T.; Alharbi, S.A.; Ayguade, E. An Iris based Smart System for Stress Identification. In Proceedings of the 2019 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), Swat, Pakistan, 24–25 July 2019; pp. 1–5. [Google Scholar]
- Hussain, T.; Palomar, O.; Cristal, A.; Ayguade, E.; Amna, H. ViPS: Visual Processing System for Medical Imaging. In Proceedings of the 2015 8th International Congress on Image and Signal Processing (CISP 2015) and the 2015 8th International Conference on BioMedical Engineering and Informatics (BMEI 2015), Shenyang, China, 14–16 October 2015. [Google Scholar]
- Hussain, T.; Haider, A. PGC: A pattern-based graphics controller. Int. J. Circuits Archit. Des. 2014, 1, 117–140. [Google Scholar] [CrossRef]
- Hussain, T. ViPS: A novel visual processing system architecture for medical imaging. Biomed. Signal Process. Control 2017, 38, 293–301. [Google Scholar] [CrossRef]
- Hussain, T.; Haider, A.; Cristal, A.; Ayguadé, E. EMVS: Embedded Multi Vector-core System. J. Syst. Archit. 2018, 87, 12–22. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Oh, S.L.; Vicnesh, J.; Ciaccio, E.J.; Yuvaraj, R.; Acharya, U.R. Deep convolutional neural network model for automated diagnosis of schizophrenia using EEG signals. Appl. Sci. 2019, 9, 2870. [Google Scholar] [CrossRef] [Green Version]
- Jaderberg, M.; Vedaldi, A.; Zisserman, A. Speeding up convolutional neural networks with low rank expansions. arXiv 2014, arXiv:1405.3866. [Google Scholar]
- Sergeev, A.; Del Balso, M. Horovod: Fast and easy distributed deep learning in TensorFlow. arXiv 2018, arXiv:1802.05799. [Google Scholar]
- Shankar, K.; Manickam, P.; Devika, G.; Ilayaraja, M. Optimal Feature Selection for Chronic Kidney Disease Classification using Deep Learning Classifier. In Proceedings of the 2018 IEEE International Conference on Computational Intelligence and Computing Research (ICCIC), Madurai, India, 13–15 December 2018; pp. 1–5. [Google Scholar]
- Putra, A.P.; Sutojo, T. Identifikasi Penurunan Kondisi Fungsi Organ Ginjal Melalui Iris Mata Menggunakan Metode Jaringan Syaraf Tiruan Learning Vector Quantization. Fakultas Ilmu Komputer Universitas Dian Nuswantoro 2014, 13, 45–52. [Google Scholar]
- Rahayu, D.N.P.; Isnanto, R.R.; Hidayatno, A. Aplikasi Pendiagnosis Gangguan Ginjal Melalui Citra Iris Mata Menggunakan Metode Segmentasi Berdasar Deteksi Tepi. Transient J. Ilm. Tek. Elektro 2013, 2, 283–288. [Google Scholar]






| Data-Set | Healthy | Chronic | Total |
|---|---|---|---|
| Subjects/Images | Subjects/Images | Subjects/Images | |
| Training | 1000/10,000 | 1000/10,000 | 2000/20,000 |
| Testing | 1000/10,000 | 1000/10,000 | 2000/20,000 |
| Total | 2000/20,000 | 2000/20,000 | 4000/40,000 |
| Healthy Kidney | Chronic Kidney | |
|---|---|---|
| Number of tested Subjects | 1000 | 1000 |
| Correct Classified | 954 | 982 |
| False Classified | 46 | 18 |
| Author | Data-Set [Total/Chronic kidney] | Accuracy |
|---|---|---|
| Hussein et al. [39] | 340/172 | 82% |
| S. Amerifar er al. [44] | 116/56 | 82% |
| Prayitno et al. [47] | 47/31 | 76% |
| Sitorus et al. [50] | 33/33 | 90%R, 94%L |
| Shankar et al. [67] | 400/250 | 96.63% |
| Putra AP et al. [68] | 27/12 | 93.75% |
| DNP Rahayu et al. [69] | 20/20 | 95% |
| Proposed Method | 4000/2000 | 96.80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzamil, S.; Hussain, T.; Haider, A.; Waraich, U.; Ashiq, U.; Ayguadé, E. An Intelligent Iris Based Chronic Kidney Identification System. Symmetry 2020, 12, 2066. https://doi.org/10.3390/sym12122066
Muzamil S, Hussain T, Haider A, Waraich U, Ashiq U, Ayguadé E. An Intelligent Iris Based Chronic Kidney Identification System. Symmetry. 2020; 12(12):2066. https://doi.org/10.3390/sym12122066
Chicago/Turabian StyleMuzamil, Sohail, Tassadaq Hussain, Amna Haider, Umber Waraich, Umair Ashiq, and Eduard Ayguadé. 2020. "An Intelligent Iris Based Chronic Kidney Identification System" Symmetry 12, no. 12: 2066. https://doi.org/10.3390/sym12122066
APA StyleMuzamil, S., Hussain, T., Haider, A., Waraich, U., Ashiq, U., & Ayguadé, E. (2020). An Intelligent Iris Based Chronic Kidney Identification System. Symmetry, 12(12), 2066. https://doi.org/10.3390/sym12122066






