The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception
Abstract
1. Introduction
“The universe is asymmetric and I am persuaded that life, as it is known to us, is a direct result of the asymmetry of the universe or of its indirect consequences.”—Louis Pasteur
2. Global vs. Local Perception
2.1. Behavioral Evidence
2.2. Neuropsychological Evidence
2.3. Neuroimaging Evidence
2.4. Comparative Evidence
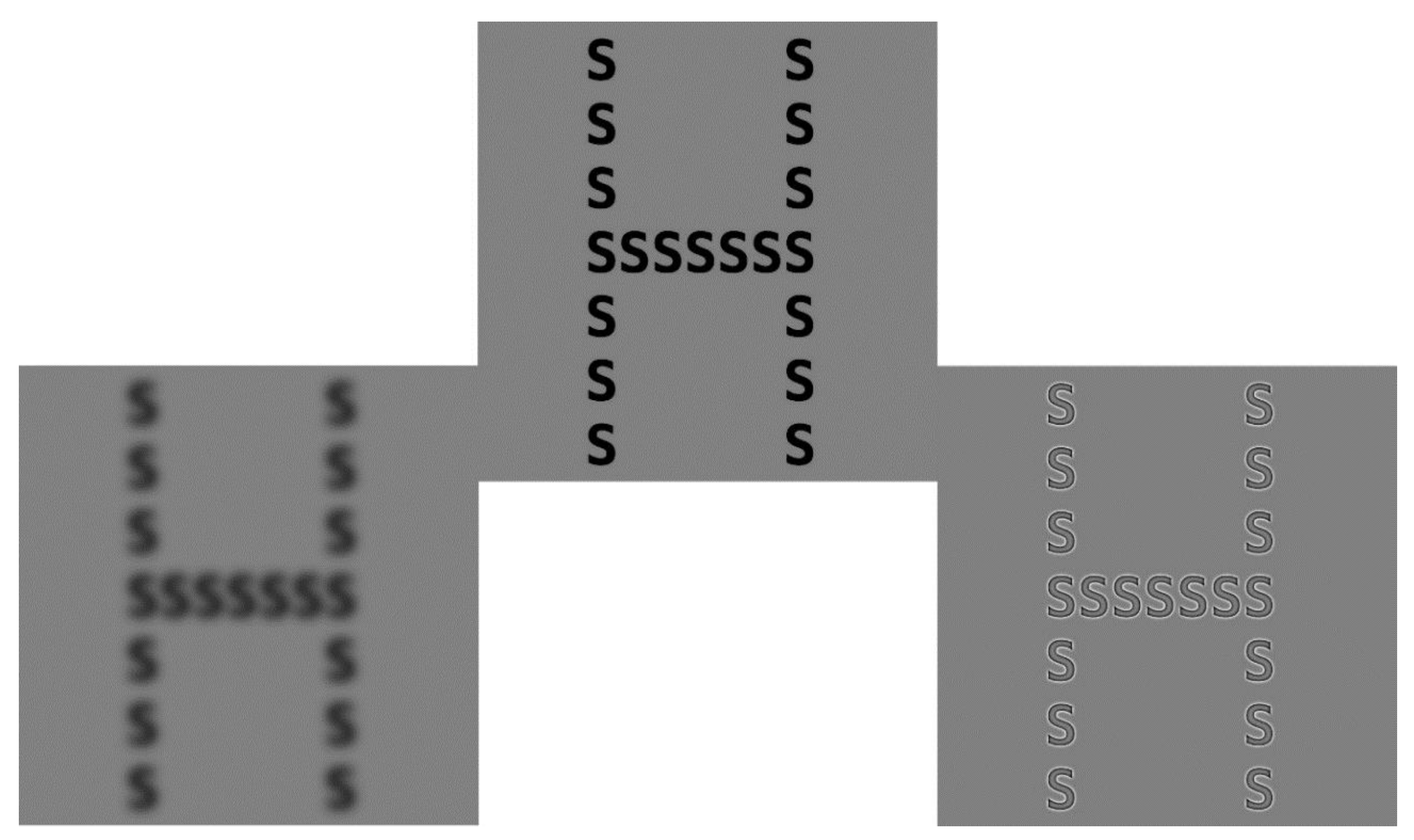
3. Global/Local Processing and Spatial Frequencies
3.1. Hemispheric Lateralization for SF Processing and Previous Models
3.1.1. Sergent’s Model
3.1.2. Dual Filtering by Frequency (DFF)
3.1.3. Reverse Hierarchy Theory (RHT)
3.1.4. Coarse-To-Fine Processing (CTF)
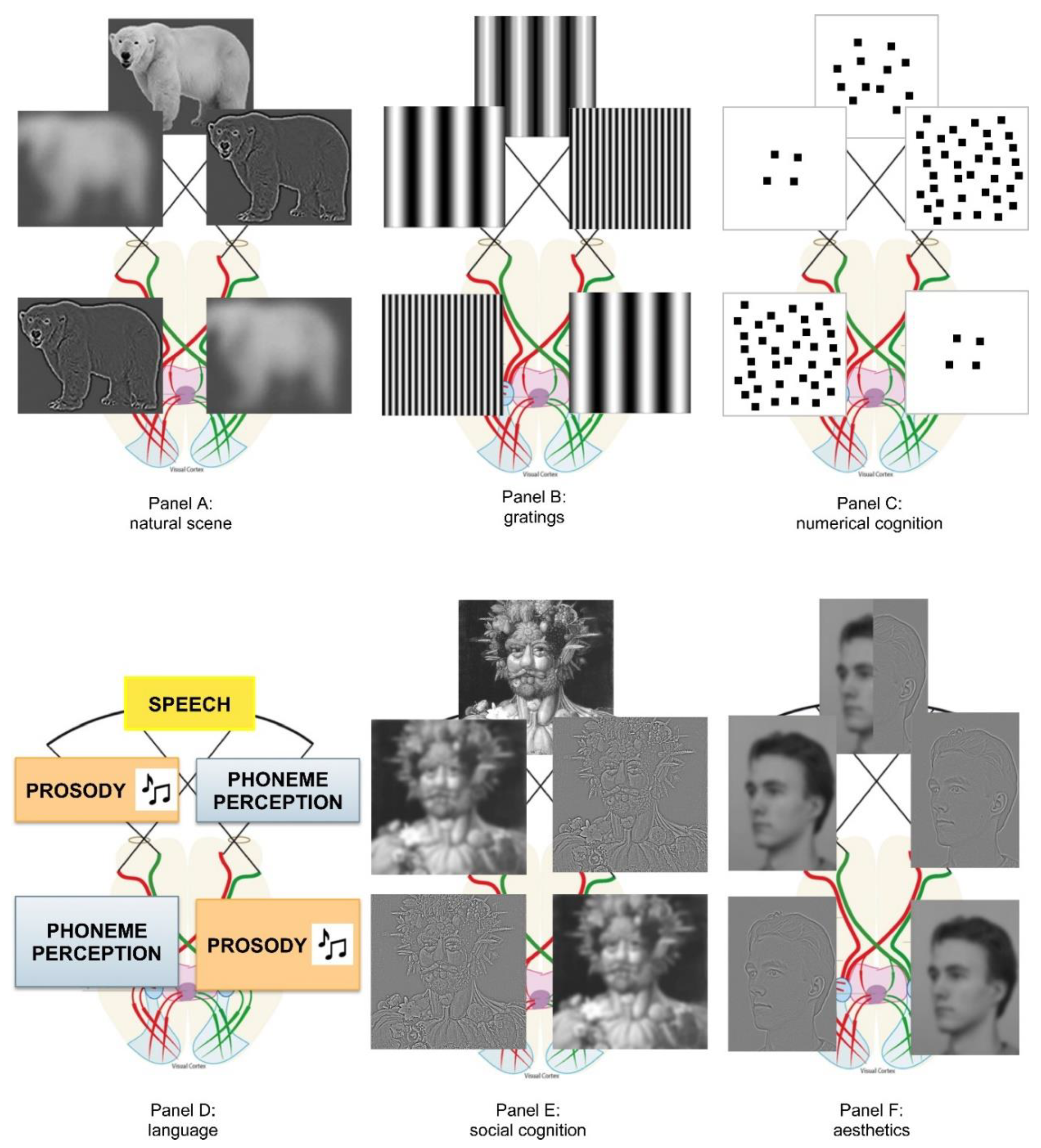
3.2. Brain’s Asymmetric Frequency Tuning (BAFT) as Generalized Account of a Perceptual Processing
4. Extending BAFT to Other Cognitive Domains
4.1. Numerical Cognition and Dyscalculia
4.2. Language, Dyslexia, and Music
4.3. Social Cognition and Emotion
4.4. Aesthetics
- (a)
- (b)
- similar asymmetrical spatial preferences for larger salient content on the right side of paintings [199],
- (c)
- the scanning motion bias, according to which we tend to scan our perceptual field left-to-right [201,202], which is in accordance with a preference for stimuli with left-to-right directionality [203]. Moreover, Maass, Pagani, and Berta (2007) [204] found that left-to-right readers rated movie clips of lateral actions as stronger, faster, and more beautiful when actions had left-to-right directionality, while right-to-left readers showed the reverse pattern (see Reference [205]),
- (d)
- a leftward attractiveness bias, according to which participants judge the left side of abstract visual patterns as more attractive than the right side [206].
5. Implications and Outstanding Questions—Experimental Avenues
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAFT | Brain’s Asymmetric Frequency Tuning |
| CTF | Coarse-To-Fine |
| DD | Developmental Dyslexia |
| DDs | Developmental Dyslexics |
| DFF | Dual Filtering by Frequency |
| HSFs | High Spatial Frequencies |
| LSFs | Low Spatial Frequencies |
| RHT | Reverse Hierarchy Theory |
| SFs | Spatial Frequencies |
| SNAs | Spatial Numerical Associations |
| TFs | Temporal Frequencies |
References
- Sergent, J. The cerebral balance of power: Confrontation or cooperation? J. Exp. Psychol. Human Percept. Perform. 1982, 8, 253–272. [Google Scholar] [CrossRef]
- Hellige, J.B. Hemispheric asymmetry for visual information processing. Acta Neurobiol. Exp. 1969, 56, 485–497. [Google Scholar]
- Kauffmann, L.; Ramanoël, S.; Peyrin, C. The neural bases of spatial frequency processing during scene perception. Front. Integr. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Navon, D. Forest before trees: The precedence of global features in visual perception. Cogn. Psychol. 1977, 9, 353–383. [Google Scholar] [CrossRef]
- Van Kleeck, M.H. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: New data and a meta-analysis of previous studies. Neuropsychologia 1989, 27, 1165–1178. [Google Scholar] [CrossRef]
- Sergent, J.; Hellige, J.B. Role of input factors in visual-field asymmetries. Brain Cogn. 1986, 5, 174–199. [Google Scholar] [CrossRef]
- Kitterle, F.L.; Christman, S.; Hellige, J.B. Hemispheric differences are found in the identification, but not the detection, of low versus high spatial frequencies. Percept. Psychophys. 1990, 48, 297–306. [Google Scholar] [CrossRef]
- Christman, S.; Kitterle, F.L.; Hellige, J. Hemispheric asymmetry in the processing of absolute versus relative spatial frequency. Brain Cogn. 1991, 16, 62–73. [Google Scholar] [CrossRef]
- Peyrin, C.; Chauvin, A.; Chokron, S.; Marendaz, C. Hemispheric specialization for spatial frequency processing in the analysis of natural scenes. Brain Cogn. 2003, 53, 278–282. [Google Scholar] [CrossRef]
- Peyrin, C.; Chokron, S.; Guyader, N.; Gout, O.; Moret, J.; Marendaz, C. Neural correlates of spatial frequency processing: A neuropsychological approach. Brain Res. 2006, 1073–1074, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Piazza, E.A.; Silver, M.A. Persistent Hemispheric Differences in the Perceptual Selection of Spatial Frequencies. J. Cogn. Neurosci. 2014, 26, 2021–2027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guiard, Y. Asymmetric Division of Labor in Human Skilled Bimanual Action: The Kinematic Chain as a Model. J. Mot. Behav. 1987, 19, 486–517. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Nešić, M. Contralateral Hemisphere Activation by Unilateral Hand Contraction: ReExamining of Global and Local Attention. Percept. Mot. Ski. 2018. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, M.; Plaut, D.C. A vision of graded hemispheric specialization: Graded hemispheric specialization. Ann. N. Y. Acad. Sci. 2015, 1359, 30–46. [Google Scholar] [CrossRef]
- Brederoo, S.G.; Nieuwenstein, M.R.; Cornelissen, F.W.; Lorist, M.M. Reproducibility of visual-field asymmetries: Nine replication studies investigating lateralization of visual information processing. Cortex 2019, 111, 100–126. [Google Scholar] [CrossRef]
- Vingerhoets, G. Phenotypes in hemispheric functional segregation? Perspectives and challenges. Phys. Life Rev. 2019, 30, 1–18. [Google Scholar] [CrossRef]
- Brederoo, S.G.; Van der Haegen, L.; Brysbaert, M.; Nieuwenstein, M.R.; Cornelissen, F.W.; Lorist, M.M. Towards a unified understanding of lateralized vision: A large-scale study investigating principles governing patterns of lateralization using a heterogeneous sample. Cortex 2020, 133, 201–214. [Google Scholar] [CrossRef]
- Ventura, P.; Delgado, J.; Ferreira, M.; Farinha-Fernandes, A.; Guerreiro, J.C.; Faustino, B.; Leite, I.; Wong, A.C.-N. Hemispheric asymmetry in holistic processing of words. Laterality Asymmetries Body Brain Cogn. 2019, 24, 98–112. [Google Scholar] [CrossRef]
- Dundas, E.M.; Plaut, D.C.; Behrmann, M. Variable Left-hemisphere Language and Orthographic Lateralization Reduces Right-hemisphere Face Lateralization. J. Cogn. Neurosci. 2015, 27, 913–925. [Google Scholar] [CrossRef]
- Karlsson, E.M.; Johnstone, L.T.; Carey, D.P. The depth and breadth of multiple perceptual asymmetries in right handers and non-right handers. Laterality Asymmetries Body Brain Cogn. 2019, 24, 707–739. [Google Scholar] [CrossRef]
- Gerrits, R.; Van der Haegen, L.; Brysbaert, M.; Vingerhoets, G. Laterality for recognizing written words and faces in the fusiform gyrus covaries with language dominance. Cortex 2019, 117, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, R.; Verhelst, H.; Vingerhoets, G. Mirrored brain organization: Statistical anomaly or reversal of hemispheric functional segregation bias? Proc. Natl. Acad. Sci. USA 2020, 117, 14057–14065. [Google Scholar] [CrossRef] [PubMed]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Fischer, M.H. Cognition in the bisection task. Trends Cogn. Sci. 2001, 5, 460–462. [Google Scholar] [CrossRef]
- Fischer, M.H. Bisection performance indicates spatial word representation. Cogn. Brain Res. 1996, 4, 163–170. [Google Scholar] [CrossRef]
- Fischer, M.H. Word Centre is Misperceived. Perception 2000, 29, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M. Orthographic contributions to perceived word center. Brain Lang. 2004, 88, 321–330. [Google Scholar] [CrossRef]
- Fischer, M.H. Number processing induces spatial performance biases. Neurology 2001, 57, 822–826. [Google Scholar] [CrossRef]
- Girelli, L.; Marinelli, C.V.; Grossi, G.; Arduino, L.S. Cultural and biological factors modulate spatial biases over development. Laterality Asymmetries Body Brain Cogn. 2017, 22, 725–739. [Google Scholar] [CrossRef]
- Rinaldi, L.; Di Luca, S.; Henik, A.; Girelli, L. Reading direction shifts visuospatial attention: An Interactive Account of attentional biases. Acta Psychol. 2014, 151, 98–105. [Google Scholar] [CrossRef]
- Maass, A.; Suitner, C.; Nadhmi, F. What drives the spatial agency bias? An Italian-Malagasy-Arabic comparison study. J. Exp. Psychol. Gen. 2014, 143, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Rosenich, E.; Shaki, S.; Loetscher, T. Unstable world: Recent experience affects spatial perception. Psychon. Bull. Rev. 2020, 27, 286–292. [Google Scholar] [CrossRef] [PubMed]
- McManus, I.C.; Edmondson, D.; Rodger, J. Balance in pictures. Br. J. Psychol. 1985, 76, 311–324. [Google Scholar] [CrossRef]
- Robertson, L.C.; Lamb, M.R. Neuropsychological contributions to theories of part/whole organization. Cogn. Psychol. 1991, 23, 299–330. [Google Scholar] [CrossRef]
- Bowers, D.; Heilman, K.M. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia 1980, 18, 491–498. [Google Scholar] [CrossRef]
- Cazzoli, D.; Chechlacz, M. A matter of hand: Causal links between hand dominance, structural organization of fronto-parietal attention networks, and variability in behavioural responses to transcranial magnetic stimulation. Cortex 2017, 86, 230–246. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Dell’Acqua, F.; Forkel, S.; Simmons, A.; Vergani, F.; Murphy, D.G.M.; Catani, M. A Lateralized Brain Network for Visuo-Spatial Attention. Nat. Prec. 2011. [Google Scholar] [CrossRef]
- Dos Santos, N.A.; Andrade, S.M.; Fernandez Calvo, B. Detection of spatial frequency in brain-damaged patients: Influence of hemispheric asymmetries and hemineglect. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Chechlacz, M.; Mantini, D.; Gillebert, C.R.; Humphreys, G.W. Asymmetrical white matter networks for attending to global versus local features. Cortex 2015, 72, 54–64. [Google Scholar] [CrossRef]
- Berardi, N.; Bodis-Wollner, I.; Fiorentini, A.; Giuffré, G.; Morelli, M. Electrophysiological evidence for interhemispheric transmission of visual information in man. J. Physiol. 1989, 411, 207–225. [Google Scholar] [CrossRef]
- Nowicka, A.; Grabowska, A.; Fersten, E. Interhemispheric transmission of information and functional asymmetry of the human brain. Neuropsychologia 1996, 34, 147–151. [Google Scholar] [CrossRef]
- Robertson, L.C.; Lamb, M.R.; Zaidel, E. Interhemispheric relations in processing hierarchical patterns: Evidence from normal and commissurotomized subjects. Neuropsychology 1993, 7, 325–342. [Google Scholar] [CrossRef]
- Kleinman, J.T.; Gupta, A. The Right Hand Draws the Trees, But the Left Draws the Forest? Behav. Neurol. 2008, 20, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Putnam, M.C.; Steven, M.S.; Doron, K.W.; Riggall, A.C.; Gazzaniga, M.S. Cortical Projection Topography of the Human Splenium: Hemispheric Asymmetry and Individual Differences. J. Cogn. Neurosci. 2010, 22, 1662–1669. [Google Scholar] [CrossRef]
- Koch, G.; Cercignani, M.; Bonni, S.; Giacobbe, V.; Bucchi, G.; Versace, V.; Caltagirone, C.; Bozzali, M. Asymmetry of Parietal Interhemispheric Connections in Humans. J. Neurosci. 2011, 31, 8967–8975. [Google Scholar] [CrossRef]
- Warrington, E.K.; Broadbent, D.E.; Weiskrantz, L. Neuropsychological studies of object recognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 15–33. [Google Scholar] [CrossRef]
- Damasio, A.R. Prosopagnosia. Trends Neurosci. 1985, 8, 132–135. [Google Scholar] [CrossRef]
- Farah, M.J. Cognitive Neuropsychology: Patterns of Co-occurrence Among the Associative Agnosias: Implications for Visual Object Representation. Cogn. Neuropsychol. 1991, 8, 1–19. [Google Scholar] [CrossRef]
- Musel, B.; Bordier, C.; Dojat, M.; Pichat, C.; Chokron, S.; Le Bas, J.-F.; Peyrin, C. Retinotopic and Lateralized Processing of Spatial Frequencies in Human Visual Cortex during Scene Categorization. J. Cogn. Neurosci. 2013, 25, 1315–1331. [Google Scholar] [CrossRef]
- Woodhead, Z.V.J.; Wise, R.J.S.; Sereno, M.; Leech, R. Dissociation of Sensitivity to Spatial Frequency in Word and Face Preferential Areas of the Fusiform Gyrus. Cereb. Cortex 2011, 21, 2307–2312. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Güntürkün, O. Hemispheric Asymmetries: The Comparative View. Front. Psychol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Corballis, M.C. What’s left in language? Beyond the classical model: What’s left in language? Ann. N. Y. Acad. Sci. 2015, 1359, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.J.; Bowers, C.A.; Vogel, D.S. Cerebral lateralization of spatial abilities: A meta-analysis. Brain Cogn. 2003, 52, 197–204. [Google Scholar] [CrossRef]
- Concha, M.L.; Bianco, I.H.; Wilson, S.W. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 2012, 13, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. A Matter of Degree: Strength of Brain Asymmetry and Behaviour. Symmetry 2017, 9, 57. [Google Scholar] [CrossRef]
- Karenina, K.; Giljov, A.; Ivkovich, T.; Malashichev, Y. Evidence for the perceptual origin of right-sided feeding biases in cetaceans. Anim. Cogn. 2016, 19, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.; Rogers, L.J. Lateralized prey-catching responses in the cane toad, Bufo marinus: Analysis of complex visual stimuli. Anim. Behav. 2004, 68, 767–775. [Google Scholar] [CrossRef]
- Andrew, R.J.; Tommasi, L.; Ford, N. Motor Control by Vision and the Evolution of Cerebral Lateralization. Brain Lang. 2000, 73, 220–235. [Google Scholar] [CrossRef]
- Rogers, L.J. Lateralization in vertebrates: Its early evolution, general pattern, and development. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 2002; Volume 31, pp. 107–161. ISBN 978-0-12-004531-0. [Google Scholar]
- Vallortigara, G. The evolutionary psychology of left and right: Costs and benefits of lateralization. Dev. Psychobiol. 2006, 48, 418–427. [Google Scholar] [CrossRef]
- Koboroff, A.; Kaplan, G.; Rogers, L.J. Hemispheric specialization in Australian magpies (Gymnorhina tibicen) shown as eye preferences during response to a predator. Brain Res. Bull. 2008, 76, 304–306. [Google Scholar] [CrossRef]
- Sovrano, V.A. Visual lateralization in response to familiar and unfamiliar stimuli in fish. Behav. Brain Res. 2004, 152, 385–391. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [PubMed]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal): Brain asymmetry. WIREs Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef]
- Güntürkün, O.; Diekamp, B.; Manns, M.; Nottelmann, F.; Prior, H.; Schwarz, A.; Skiba, M. Asymmetry pays: Visual lateralization improves discrimination success in pigeons. Curr. Biol. 2000, 10, 1079–1081. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. Lond. B 2004, 271, S420–S422. [Google Scholar] [CrossRef]
- Rezvani, Z.; Katanforoush, A.; Pouretemad, H. Global precedence changes by environment: A systematic review and meta-analysis on effect of perceptual field variables on global-local visual processing. Atten. Percept. Psychophys. 2020, 82, 2348–2359. [Google Scholar] [CrossRef]
- Campbell, F.W.; Maffei, L. Contrast and Spatial Frequency. Sci. Am. 1974, 231, 106–115. [Google Scholar] [CrossRef]
- Lamb, M.R.; Yund, E.W. The role of spatial frequency in the processing of hierarchically organized stimuli. Percept. Psychophys. 1993, 54, 773–784. [Google Scholar] [CrossRef]
- Shulman, G.L.; Sullivan, M.A.; Gish, K.; Sakoda, W.J. The Role of Spatial-Frequency Channels in the Perception of Local and Global Structure. Perception 1986, 15, 259–273. [Google Scholar] [CrossRef]
- Shulman, G.L.; Wilson, J. Spatial Frequency and Selective Attention to Local and Global Information. Perception 1987, 16, 89–101. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yamins, D.L.K.; Hong, H.; Cadieu, C.F.; Solomon, E.A.; Seibert, D.; DiCarlo, J.J. Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc. Natl. Acad. Sci. USA 2014, 111, 8619–8624. [Google Scholar] [CrossRef]
- Dreher, B.; Fukada, Y.; Rodieck, R.W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J. Physiol. 1976, 258, 433–452. [Google Scholar] [CrossRef]
- Lawton, T.; Shelley-Tremblay, J. Training on Movement Figure-Ground Discrimination Remediates Low-Level Visual Timing Deficits in the Dorsal Stream, Improving High-Level Cognitive Functioning, Including Attention, Reading Fluency, and Working Memory. Front. Hum. Neurosci. 2017, 11, 236. [Google Scholar] [CrossRef]
- Bullier, J. Integrated model of visual processing. Brain Res. Rev. 2001, 36, 96–107. [Google Scholar] [CrossRef]
- Bar, M. A Cortical Mechanism for Triggering Top-Down Facilitation in Visual Object Recognition. J. Cogn. Neurosci. 2003, 15, 600–609. [Google Scholar] [CrossRef]
- Peyrin, C.; Michel, C.M.; Schwartz, S.; Thut, G.; Seghier, M.; Landis, T.; Marendaz, C.; Vuilleumier, P. The Neural Substrates and Timing of Top-Down Processes during Coarse-to-Fine Categorization of Visual Scenes: A Combined fMRI and ERP Study. J. Cogn. Neurosci. 2010, 22, 2768–2780. [Google Scholar] [CrossRef]
- Musel, B.; Kauffmann, L.; Ramanoël, S.; Giavarini, C.; Guyader, N.; Chauvin, A.; Peyrin, C. Coarse-to-fine Categorization of Visual Scenes in Scene-selective Cortex. J. Cogn. Neurosci. 2014, 26, 2287–2297. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Larson, A.M.; Loschky, L.C. The contributions of central versus peripheral vision to scene gist recognition. J. Vis. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.M.; Gilchrist, I.D. Active Vision: The Psychology of Looking and Seeing; Oxford University Press: Oxford, UK, 2003; ISBN 978-0-19-852479-3. [Google Scholar]
- Laubrock, J.; Cajar, A.; Engbert, R. Control of fixation duration during scene viewing by interaction of foveal and peripheral processing. J. Vis. 2013, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.D.; Kahn, I.; Wig, G.S.; Schacter, D.L. Hemispheric Asymmetry of Visual Scene Processing in the Human Brain: Evidence from Repetition Priming and Intrinsic Activity. Cereb. Cortex 2012, 22, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Pisella, L.; Alahyane, N.; Blangero, A.; Thery, F.; Blanc, S.; Pelisson, D. Right-hemispheric dominance for visual remapping in humans. Phil. Trans. R. Soc. B 2011, 366, 572–585. [Google Scholar] [CrossRef]
- Sheremata, S.L.; Bettencourt, K.C.; Somers, D.C. Hemispheric Asymmetry in Visuotopic Posterior Parietal Cortex Emerges with Visual Short-Term Memory Load. J. Neurosci. 2010, 30, 12581–12588. [Google Scholar] [CrossRef]
- Stephan, K.E.; Marshall, J.C.; Penny, W.D.; Friston, K.J.; Fink, G.R. Interhemispheric Integration of Visual Processing during Task-Driven Lateralization. J. Neurosci. 2007, 27, 3512–3522. [Google Scholar] [CrossRef]
- Robertson, L.C.; Ivry, R. Hemispheric Asymmetries: Attention to Visual and Auditory Primitives. Curr. Dir. Psychol. Sci. 2000, 9, 59–63. [Google Scholar] [CrossRef]
- Poeppel, D. The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time’. Speech Commun. 2003, 41, 245–255. [Google Scholar] [CrossRef]
- Flinker, A.; Doyle, W.K.; Mehta, A.D.; Devinsky, O.; Poeppel, D. Spectrotemporal modulation provides a unifying framework for auditory cortical asymmetries. Nat. Hum. Behav. 2019, 3, 393–405. [Google Scholar] [CrossRef]
- Telkemeyer, S.; Rossi, S.; Koch, S.P.; Nierhaus, T.; Steinbrink, J.; Poeppel, D.; Obrig, H.; Wartenburger, I. Sensitivity of Newborn Auditory Cortex to the Temporal Structure of Sounds. J. Neurosci. 2009, 29, 14726–14733. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, T.; Foster, N.E.V.; Tryfon, A.; Hyde, K.L. Auditory-musical processing in autism spectrum disorders: A review of behavioral and brain imaging studies. Ann. N. Y. Acad. Sci. 2012, 1252, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, L.; Simard-Meilleur, A.-A.; Paignon, A.; Mottron, L.; Donnadieu, S. Auditory local bias and reduced global interference in autism. Cognition 2014, 131, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Haigh, S.M. Variable sensory perception in autism. Eur. J. Neurosci. 2018, 47, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Di Lollo, V. Hemispheric symmetry in duration of visible persistence. Percept. Psychophys. 1981, 29, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.; Nowicka, A. Visual-spatial-frequency model of cerebral asymmetry: A critical survey of behavioral and electrophysiological studies. Psychol. Bull. 1996, 120, 434–449. [Google Scholar] [CrossRef]
- Ivry, R.B.; Robertson, L.C.; Robertson, L.C. The Two Sides of Perception; MIT Press: Cambridge, MA, USA, 1998; ISBN 978-0-262-09034-6. [Google Scholar]
- Hochstein, S.; Ahissar, M. View from the Top: Hierarchies and Reverse Hierarchies in the Visual System. Neuron 2002, 36, 791–804. [Google Scholar] [CrossRef]
- Ahissar, M.; Hochstein, S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn. Sci. 2004, 8, 457–464. [Google Scholar] [CrossRef]
- Ahissar, M.; Nahum, M.; Nelken, I.; Hochstein, S. Reverse hierarchies and sensory learning. Phil. Trans. R. Soc. B 2009, 364, 285–299. [Google Scholar] [CrossRef]
- Hegde, J. Time course of visual perception: Coarse-to-fine processing and beyond. Prog. Neurobiol. 2008, 84, 405–439. [Google Scholar] [CrossRef]
- Flevaris, A.V.; Robertson, L.C. Spatial frequency selection and integration of global and local information in visual processing: A selective review and tribute to Shlomo Bentin. Neuropsychologia 2016, 83, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Piazza, E.A.; Silver, M.A. Relative Spatial Frequency Processing Drives Hemispheric Asymmetry in Conscious Awareness. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Cueva, C.J.; Tsodyks, M.; Qian, N. Visual perception as retrospective Bayesian decoding from high- to low-level features. Proc. Natl. Acad. Sci. USA 2017, 114, E9115–E9124. [Google Scholar] [CrossRef] [PubMed]
- Barsalou, L.W. Grounded Cognition. Annu. Rev. Psychol. 2008, 59, 617–645. [Google Scholar] [CrossRef] [PubMed]
- Matheson, H.E.; Barsalou, L.W. Embodiment and Grounding in Cognitive Neuroscience. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience; Wixted, J.T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–27. ISBN 978-1-119-17016-7. [Google Scholar]
- Fischer, M.H.; Coello, Y.E. Foundations of Embodied Cognition: Conceptual and Interactive Embodiment; Routledge/Taylor & Francis Group: New York, NY, USA, 2016; ISBN 978-1-138-80582-8. [Google Scholar]
- Newen, A.; Bruin, L.D.; Gallagher, S. The Oxford Handbook of 4E Cognition; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-105435-8. [Google Scholar]
- Felisatti, A.; Laubrock, J.; Shaki, S.; Fischer, M.H. A biological foundation for spatial–numerical associations: The brain’s asymmetric frequency tuning. Ann. N. Y. Acad. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.H. A hierarchical view of grounded, embodied, and situated numerical cognition. Cogn. Process. 2012, 13, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Pezzulo, G.; Barsalou, L.W.; Cangelosi, A.; Fischer, M.H.; McRae, K.; Spivey, M.J. Computational Grounded Cognition: A new alliance between grounded cognition and computational modeling. Front. Psychol. 2013, 3. [Google Scholar] [CrossRef]
- Myachykov, A.; Scheepers, C.; Fischer, M.H.; Kessler, K. TEST: A Tropic, Embodied, and Situated Theory of Cognition. Top Cogn. Sci. 2014, 6, 442–460. [Google Scholar] [CrossRef]
- Panichello, M.F.; Cheung, O.S.; Bar, M. Predictive Feedback and Conscious Visual Experience. Front. Psychol. 2013, 3. [Google Scholar] [CrossRef]
- Park, J.; Spence, C.; Ishii, H.; Togawa, T. Turning the other cheek: Facial orientation influences both model attractiveness and product evaluation. Psychol. Mark. 2020. [Google Scholar] [CrossRef]
- Larsson, M.L. Binocular vision, the optic chiasm, and their associations with vertebrate motor behavior. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef]
- Shaki, S.; Fischer, M.H. Systematic spatial distortion of quantitative estimates. Psychol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.H.; Shaki, S. Spatial Associations in Numerical Cognition—From Single Digits to Arithmetic. Q. J. Exp. Psychol. 2014, 67, 1461–1483. [Google Scholar] [CrossRef] [PubMed]
- Göbel, S.M.; Shaki, S.; Fischer, M.H. The Cultural Number Line: A Review of Cultural and Linguistic Influences on the Development of Number Processing. J. Cross-Cult. Psychol. 2011, 42, 543–565. [Google Scholar] [CrossRef]
- Rugani, R.; de Hevia, M.-D. Number-space associations without language: Evidence from preverbal human infants and non-human animal species. Psychon. Bull. Rev. 2017, 24, 352–369. [Google Scholar] [CrossRef]
- Cipora, K.; Schroeder, P.A.; Soltanlou, M.; Nuerk, H.-C. More Space, Better Mathematics: Is Space a Powerful Tool or a Cornerstone for Understanding Arithmetic? In Visualizing Mathematics; Mix, K.S., Battista, M.T., Eds.; Research in Mathematics Education; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–116. ISBN 978-3-319-98766-8. [Google Scholar]
- Di Giorgio, E.; Lunghi, M.; Rugani, R.; Regolin, L.; Dalla Barba, B.; Vallortigara, G.; Simion, F. A mental number line in human newborns. Dev. Sci. 2019, 22. [Google Scholar] [CrossRef]
- Rugani, R.; Vallortigara, G.; Priftis, K.; Regolin, L. Number-space mapping in the newborn chick resembles humans’ mental number line. Science 2015, 347, 534–536. [Google Scholar] [CrossRef]
- Nemeh, F.; Humberstone, J.; Yates, M.J.; Reeve, R.A. Non-symbolic magnitudes are represented spatially: Evidence from a non-symbolic SNARC task. PLoS ONE 2018, 13, e0203019. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, C.; Li, L.; Li, D.; Cui, J. Mental Numerosity Line in the Human’s Approximate Number System. Exp. Psychol. 2016, 63, 169–179. [Google Scholar] [CrossRef]
- Mitchell, T.; Bull, R.; Cleland, A.A. Implicit response-irrelevant number information triggers the SNARC effect: Evidence using a neural overlap paradigm. Q. J. Exp. Psychol. 2012, 65, 1945–1961. [Google Scholar] [CrossRef]
- Dehaene, S.; Bossini, S.; Giraux, P. The mental representation of parity and number magnitude. J. Exp. Psychol. Gen. 1993, 122, 371–396. [Google Scholar] [CrossRef]
- Rugani, R.; Vallortigara, G.; Priftis, K.; Regolin, L. Numerical magnitude, rather than individual bias, explains spatial numerical association in newborn chicks. eLife 2020, 9, e54662. [Google Scholar] [CrossRef] [PubMed]
- Bulf, H.; de Hevia, M.D.; Cassia, V.M. Small on the left, large on the right: Numbers orient visual attention onto space in preverbal infants. Dev. Sci. 2016, 19, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Bulf, H.; Cassia, V.M.; de Hevia, M.D. Are Numbers, Size and Brightness Equally Efficient in Orienting Visual Attention? Evidence from an Eye-Tracking Study. PLoS ONE 2014, 9, e99499. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Zorzi, M.; Ziegler, J.C. Understanding Dyslexia Through Personalized Large-Scale Computational Models. Psychol. Sci. 2019, 30, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.B.; Pennington, B.F.; Peterson, R.L.; Willcutt, E.G.; DeFries, J.C.; Olson, R.K. Explaining the sex difference in dyslexia. J. Child Psychol. Psychiatr. 2017, 58, 719–727. [Google Scholar] [CrossRef]
- Badian, N.A. Persistent arithmetic, reading, or arithmetic and reading disability. Ann. Dyslexia 1999, 49, 43–70. [Google Scholar] [CrossRef]
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Jacobsen, S.J. Math Learning Disorder: Incidence in a Population-Based Birth Cohort, 1976–82, Rochester, Minn. Ambul. Pediatrics 2005, 5, 281–289. [Google Scholar] [CrossRef]
- Dirks, E.; Spyer, G.; van Lieshout, E.C.D.M.; de Sonneville, L. Prevalence of Combined Reading and Arithmetic Disabilities. J. Learn. Disabil. 2008, 41, 460–473. [Google Scholar] [CrossRef]
- Lewis, C.; Hitch, G.J.; Walker, P. The Prevalence of Specific Arithmetic Difficulties and Specific Reading Difficulties in 9- to 10-year-old Boys and Girls. J. Child Psychol. Psychiat. 1994, 35, 283–292. [Google Scholar] [CrossRef]
- Landerl, K.; Fussenegger, B.; Moll, K.; Willburger, E. Dyslexia and dyscalculia: Two learning disorders with different cognitive profiles. J. Exp. Child Psychol. 2009, 103, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.; Bryant, P.E. Categorizing sounds and learning to read—A causal connection. Nature 1983, 301, 419–421. [Google Scholar] [CrossRef]
- Boets, B.; de Beeck, H.P.O.; Vandermosten, M.; Scott, S.K.; Gillebert, C.R.; Mantini, D.; Bulthe, J.; Sunaert, S.; Wouters, J.; Ghesquiere, P. Intact But Less Accessible Phonetic Representations in Adults with Dyslexia. Science 2013, 342, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Stein, J. What is Developmental Dyslexia? Brain Sci. 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Bertoni, S.; Gianesini, T.; Gori, S.; Facoetti, A. A different vision of dyslexia: Local precedence on global perception. Sci. Rep. 2017, 7, 17462. [Google Scholar] [CrossRef]
- Franceschini, S.; Bertoni, S.; Puccio, G.; Mancarella, M.; Gori, S.; Facoetti, A. Local perception impairs the lexical reading route. Psychol. Res. 2020. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef]
- Sammler, D.; Grosbras, M.-H.; Anwander, A.; Bestelmeyer, P.E.G.; Belin, P. Dorsal and Ventral Pathways for Prosody. Curr. Biol. 2015, 25, 3079–3085. [Google Scholar] [CrossRef]
- Skottun, B.C. On the use of spatial frequency to isolate contributions from the magnocellular and parvocellular systems and the dorsal and ventral cortical streams. Neurosci. Biobehav. Rev. 2015, 56, 266–275. [Google Scholar] [CrossRef]
- Grinter, E.J.; Maybery, M.T.; Badcock, D.R. Vision in developmental disorders: Is there a dorsal stream deficit? Brain Res. Bull. 2010, 82, 147–160. [Google Scholar] [CrossRef]
- Van der Hallen, R.; Evers, K.; Brewaeys, K.; Van den Noortgate, W.; Wagemans, J. Global processing takes time: A meta-analysis on local–global visual processing in ASD. Psychol. Bull. 2015, 141, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N. Disconnexion Syndromes in Animals and Man. Brain 1965, 88, 585. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S. Right hemisphere language following brain bisection: A 20-year perspective. Am. Psychol. 1983, 38, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Hofstadter, D.R. Gödel, Escher, Bach: An Eternal Golden Braid; Penguin Books Ltd.: Middlesex, UK, 1979. [Google Scholar]
- Peretz, I. Neurobiology of Congenital Amusia. Trends Cogn. Sci. 2016, 20, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Hyde, K.L. What is specific to music processing? Insights from congenital amusia. Trends Cogn. Sci. 2003, 7, 362–367. [Google Scholar] [CrossRef]
- Friederici, A.D.; Alter, K. Lateralization of auditory language functions: A dynamic dual pathway model. Brain Lang. 2004, 89, 267–276. [Google Scholar] [CrossRef]
- Norton, A.; Zipse, L.; Marchina, S.; Schlaug, G. Melodic Intonation Therapy: Shared Insights on How It Is Done and Why It Might Help. Ann. N. Y. Acad. Sci. 2009, 1169, 431–436. [Google Scholar] [CrossRef]
- Albouy, P.; Benjamin, L.; Morillon, B.; Zatorre, R.J. Distinct sensitivity to spectrotemporal modulation supports brain asymmetry for speech and melody. Science 2020, 367, 1043–1047. [Google Scholar] [CrossRef]
- Happé, F.; Cook, J.L.; Bird, G. The Structure of Social Cognition: In(ter)dependence of Sociocognitive Processes. Annu. Rev. Psychol. 2017, 68, 243–267. [Google Scholar] [CrossRef]
- Kennedy, D.P.; Adolphs, R. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 2012, 16, 559–572. [Google Scholar] [CrossRef]
- McCarthy, G.; Puce, A.; Gore, J.C.; Allison, T. Face-Specific Processing in the Human Fusiform Gyrus. J. Cogn. Neurosci. 1997, 9, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S.; Smylie, C.S. Facial recognition and brain asymmetries: Clues to underlying mechanisms. Ann. Neurol. 1983, 13, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cherian, T.; Singal, G.; Sinha, P. Lateralization of face processing in the human brain. Proc. R. Soc. B 2012, 279, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Caharel, S.; Leleu, A.; Bernard, C.; Viggiano, M.-P.; Lalonde, R.; Rebaï, M. Early holistic face-like processing of Arcimboldo paintings in the right occipito-temporal cortex: Evidence from the N170 ERP component. Int. J. Psychophysiol. 2013, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Otsuka, Y.; Nakato, E.; Kanazawa, S.; Yamaguchi, M.K.; Kakigi, R. Do infants recognize the Arcimboldo images as faces? Behavioral and near-infrared spectroscopic study. J. Exp. Child Psychol. 2012, 111, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, V.; Rossion, B. Faces are “spatial”—Holistic face perception is supported by low spatial frequencies. J. Exp. Psychol. Human Percept. Perform. 2006, 32, 1023–1039. [Google Scholar] [CrossRef]
- Stein, T.; Seymour, K.; Hebart, M.N.; Sterzer, P. Rapid Fear Detection Relies on High Spatial Frequencies. Psychol. Sci. 2014, 25, 566–574. [Google Scholar] [CrossRef]
- Johnson, M.H. Subcortical face processing. Nat. Rev. Neurosci. 2005, 6, 766–774. [Google Scholar] [CrossRef]
- Öhman, A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology 2005, 30, 953–958. [Google Scholar] [CrossRef]
- Tamietto, M.; de Gelder, B. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 2010, 11, 697–709. [Google Scholar] [CrossRef]
- Blom, S.S.A.H.; Aarts, H.; Semin, G.R. Lateralization of facial emotion processing and facial mimicry. Laterality 2020, 25, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Bourne, V.J. How are emotions lateralised in the brain? Contrasting existing hypotheses using the Chimeric Faces Test. Cogn. Emot. 2010, 24, 903–911. [Google Scholar] [CrossRef]
- Demaree, H.A.; Everhart, D.E.; Youngstrom, E.A.; Harrison, D.W. Brain Lateralization of Emotional Processing: Historical Roots and a Future Incorporating “Dominance”. Behav. Cogn. Neurosci. Rev. 2005, 4, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.L.; Schwartz, G.E. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia 1985, 23, 745–755. [Google Scholar] [CrossRef]
- Davidson, R.J. Well-being and affective style: Neural substrates and biobehavioural correlates. Phil. Trans. R. Soc. Lond. B 2004, 359, 1395–1411. [Google Scholar] [CrossRef]
- Packheiser, J.; Rook, N.; Dursun, Z.; Mesenhöller, J.; Wenglorz, A.; Güntürkün, O.; Ocklenburg, S. Embracing your emotions: Affective state impacts lateralisation of human embraces. Psychol. Res. 2019, 83, 26–36. [Google Scholar] [CrossRef]
- Gordon, I.E. Left and right in Goya’s portraits. Nature 1974, 249, 197–198. [Google Scholar] [CrossRef]
- Mcmanus, I.C.; Humphrey, N.K. Turning the Left Cheek. Nature 1973, 243, 271–272. [Google Scholar] [CrossRef]
- Pérez González, C. Lateral organisation in nineteenth-century studio photographs is influenced by the direction of writing: A comparison of Iranian and Spanish photographs. Laterality Asymmetries Body Brain Cogn. 2012, 17, 515–532. [Google Scholar] [CrossRef]
- Chahboun, S.; Flumini, A.; González, C.P.; McManus, I.C.; Santiago, J. Reading and writing direction effects on the aesthetic appreciation of photographs. Laterality Asymmetries Body Brain Cogn. 2017, 22, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.R.; Schirillo, J.A. Asymmetrical facial expressions in portraits and hemispheric laterality: A literature review. Laterality Asymmetries Body Brain Cogn. 2009, 14, 545–572. [Google Scholar] [CrossRef] [PubMed]
- Lindell, A.K. The silent social/emotional signals in left and right cheek poses: A literature review. Laterality Asymmetries Body Brain Cogn. 2013, 18, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Lindell, A.K. Consistently Showing Your Best Side? Intra-individual Consistency in# Selfie Pose Orientation. Front. Psychol. 2017, 8, 26. [Google Scholar] [CrossRef]
- Manovich, L.; Ferrari, V.; Bruno, N. Selfie-Takers Prefer Left Cheeks: Converging Evidence from the (Extended) selfiecity Database. Front. Psychol. 2017, 8, 1460. [Google Scholar] [CrossRef]
- Wickens, T.; Palmer, S.; Gardner, J. Aesthetic issues in spatial composition: Effects of position and direction on framing single objects. Spat. Vis. 2008, 21, 421–449. [Google Scholar] [CrossRef]
- Benjafield, J.; Segalowitz, S.J. Left and Right in Leonardo’s Drawings of Faces. Empir. Stud. Arts 1993, 11, 25–32. [Google Scholar] [CrossRef]
- Christman, S.; Pinger, K. Lateral Biases in Aesthetic Preferences: Pictorial Dimensions and Neural Mechanisms. Laterality Asymmetries Body Brain Cogn. 1997, 2, 155–175. [Google Scholar] [CrossRef]
- Busin, Y.; Lukasova, K.; Asthana, M.K.; Macedo, E.C. Hemiface Differences in Visual Exploration Patterns When Judging the Authenticity of Facial Expressions. Front. Psychol. 2018, 8, 2332. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kowatari, Y.; Ueno, S.; Yamane, S.; Kitazawa, S. Accelerated recognition of left oblique views of faces. Exp. Brain Res. 2005, 161, 27–33. [Google Scholar] [CrossRef]
- Hayes, T.; Muday, J.A.; Schirillo, J.A. Portrait hemispheric laterality measured using pupil diameter and aesthetic judgments. Psychol. Aesthet. Creat. Arts 2013, 7, 276–284. [Google Scholar] [CrossRef][Green Version]
- Grüsser, O.-J.; Selke, T.; Zynda, B. Cerebral Lateralization and Some Implications for Art, Aesthetic Perception, and Artistic Creativity. In Beauty and the Brain: Biological Aspects of Aesthetics; Rentschler, I., Herzberger, B., Epstein, D., Eds.; Birkhäuser: Basel, Switzerland, 1988; pp. 257–293. ISBN 978-3-0348-6350-6. [Google Scholar]
- Lindell, A.K.; Savill, N.J. Time to turn the other cheek? The influence of left and right poses on perceptions of academic specialisation. Laterality Asymmetries Body Brain Cogn. 2010, 15, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.E.R.; Clode, D.; Wood, S.J.; Wood, A.G. Laterality of expression in portraiture: Putting your best cheek forward. Proc. R. Soc. Lond. B 1999, 266, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.E.; Roberts, G.R. Can Free-Viewing Perceptual Asymmetries be Explained by Scanning, Pre-Motor or Attentional Biases? Cortex 2002, 38, 113–136. [Google Scholar] [CrossRef]
- Roether, C.L.; Omlor, L.; Giese, M.A. Lateral asymmetry of bodily emotion expression. Curr. Biol. 2008, 18, R329–R330. [Google Scholar] [CrossRef] [PubMed]
- Chokron, S.; De Agostini, M. Reading habits influence aesthetic preference. Cogn. Brain Res. 2000, 10, 45–49. [Google Scholar] [CrossRef]
- Friedrich, T.E.; Elias, L.J. The write bias: The influence of native writing direction on aesthetic preference biases. Psychol. Aesthet. Creat. Arts 2016, 10, 128–133. [Google Scholar] [CrossRef]
- Nachson, I.; Argaman, E.; Luria, A. Effects of Directional Habits and Handedness on Aesthetic Preference for Left and Right Profiles. J. Cross-Cult. Psychol. 1999, 30, 106–114. [Google Scholar] [CrossRef]
- Smith, A.K.; Duerksen, K.N.; Gutwin, C.; Elias, L.J. Lateral biases in aesthetic and spatial location judgments: Differences between tasks and native reading directions. Laterality 2020, 25, 5–21. [Google Scholar] [CrossRef]
- Page, A.G.; McManus, C.; González, C.P.; Chahboun, S. Is Beauty in the Hand of the Writer? Influences of Aesthetic Preferences through Script Directions, Cultural, and Neurological Factors: A Literature Review. Front. Psychol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Beaumont, J.G. Lateral organization and aesthetic preference: The importance of peripheral visual asymmetries. Neuropsychologia 1985, 23, 103–113. [Google Scholar] [CrossRef]
- Levy, J. Lateral dominance and aesthetic preference. Neuropsychologia 1976, 14, 431–445. [Google Scholar] [CrossRef]
- Gaffron, M. Left and right in pictures. Art Q. 1950, 13, 312–321. [Google Scholar]
- Kliegl, R.; Laubrock, J.; Köstler, A. Augenblicke bei der Bildbetrachtung. Eine kognitionswissenschaftliche Spekulation zum Links und Rechts im Bild. In Räume-Bilder-Kulturen; Lepper, V.M., Deuflhard, P., Markschies, C., Eds.; de Gruyter: Berlin, Germany, 2015; pp. 77–90. [Google Scholar]
- Mead, A.M.; McLaughlin, J.P. The roles of handedness and stimulus asymmetry in aesthetic preference. Brain Cogn. 1992, 20, 300–307. [Google Scholar] [CrossRef]
- Maass, A.; Pagani, D.; Berta, E. How Beautiful is the Goal and How Violent is the Fistfight? Spatial Bias in the Interpretation of Human Behavior. Soc. Cogn. 2007, 25, 833–852. [Google Scholar] [CrossRef]
- Flath, M.E.; Smith, A.K.; Elias, L.J. Cultural differences in lateral biases on aesthetic judgments: The effect of native reading direction. Cult. Brain 2019, 7, 57–66. [Google Scholar] [CrossRef]
- Rodway, P.; Schepman, A.; Crossley, B.; Lee, J. A leftward perceptual asymmetry when judging the attractiveness of visual patterns. Laterality Asymmetries Body Brain Cogn. 2019, 24, 1–25. [Google Scholar] [CrossRef]
- Cajar, A.; Engbert, R.; Laubrock, J. How spatial frequencies and color drive object search in real-world scenes: A new eye-movement corpus. J. Vis. 2020, 20, 8. [Google Scholar] [CrossRef]
- Cajar, A.; Engbert, R.; Laubrock, J. Spatial frequency processing in the central and peripheral visual field during scene viewing. Vis. Res. 2016, 127, 186–197. [Google Scholar] [CrossRef]
- Cajar, A.; Schneeweiß, P.; Engbert, R.; Laubrock, J. Coupling of attention and saccades when viewing scenes with central and peripheral degradation. J. Vis. 2016, 16, 8. [Google Scholar] [CrossRef]
- Engbert, R.; Nuthmann, A.; Richter, E.M.; Kliegl, R. SWIFT: A Dynamical Model of Saccade Generation during Reading. Psychol. Rev. 2005, 112, 777–813. [Google Scholar] [CrossRef]
- Kliegl, R.; Risse, S.; Laubrock, J. Preview benefit and parafoveal-on-foveal effects from word n + 2. J. Exp. Psychol. Human Percept. Perform. 2007, 33, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yan, M.; Laubrock, J. Semantic preview benefit and cost: Evidence from parafoveal fast-priming paradigm. Cognition 2020, 205, 104452. [Google Scholar] [CrossRef] [PubMed]
- Reichle, E.D.; Pollatsek, A.; Fisher, D.L.; Rayner, K. Toward a Model of Eye Movement Control in Reading. Psychol. Rev. 1998, 105, 125. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Facoetti, A.; Trussardi, A.N.; Berteletti, I.; Conte, S.; Lucangeli, D.; Dehaene, S.; Zorzi, M. Developmental trajectory of number acuity reveals a severe impairment in developmental dyscalculia. Cognition 2010, 116, 33–41. [Google Scholar] [CrossRef]
- Zorzi, M.; Testolin, A. An emergentist perspective on the origin of number sense. Phil. Trans. R. Soc. B 2018, 373. [Google Scholar] [CrossRef]
- De Hevia, M.D.; Veggiotti, L.; Streri, A.; Bonn, C.D. At Birth, Humans Associate “Few” with Left and “Many” with Right. Curr. Biol. 2017, 27, 3879–3884. [Google Scholar] [CrossRef]
- Märker, G.; Learmonth, G.; Thut, G.; Harvey, M. Intra- and inter-task reliability of spatial attention measures in healthy older adults. PLoS ONE 2019, 14, e0226424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felisatti, A.; Aagten-Murphy, D.; Laubrock, J.; Shaki, S.; Fischer, M.H. The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception. Symmetry 2020, 12, 2083. https://doi.org/10.3390/sym12122083
Felisatti A, Aagten-Murphy D, Laubrock J, Shaki S, Fischer MH. The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception. Symmetry. 2020; 12(12):2083. https://doi.org/10.3390/sym12122083
Chicago/Turabian StyleFelisatti, Arianna, David Aagten-Murphy, Jochen Laubrock, Samuel Shaki, and Martin H. Fischer. 2020. "The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception" Symmetry 12, no. 12: 2083. https://doi.org/10.3390/sym12122083
APA StyleFelisatti, A., Aagten-Murphy, D., Laubrock, J., Shaki, S., & Fischer, M. H. (2020). The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception. Symmetry, 12(12), 2083. https://doi.org/10.3390/sym12122083



