Unsubstituted Oximes as Potential Therapeutic Agents
Abstract
:1. Introduction
2. The Anti-Inflammatory Activity
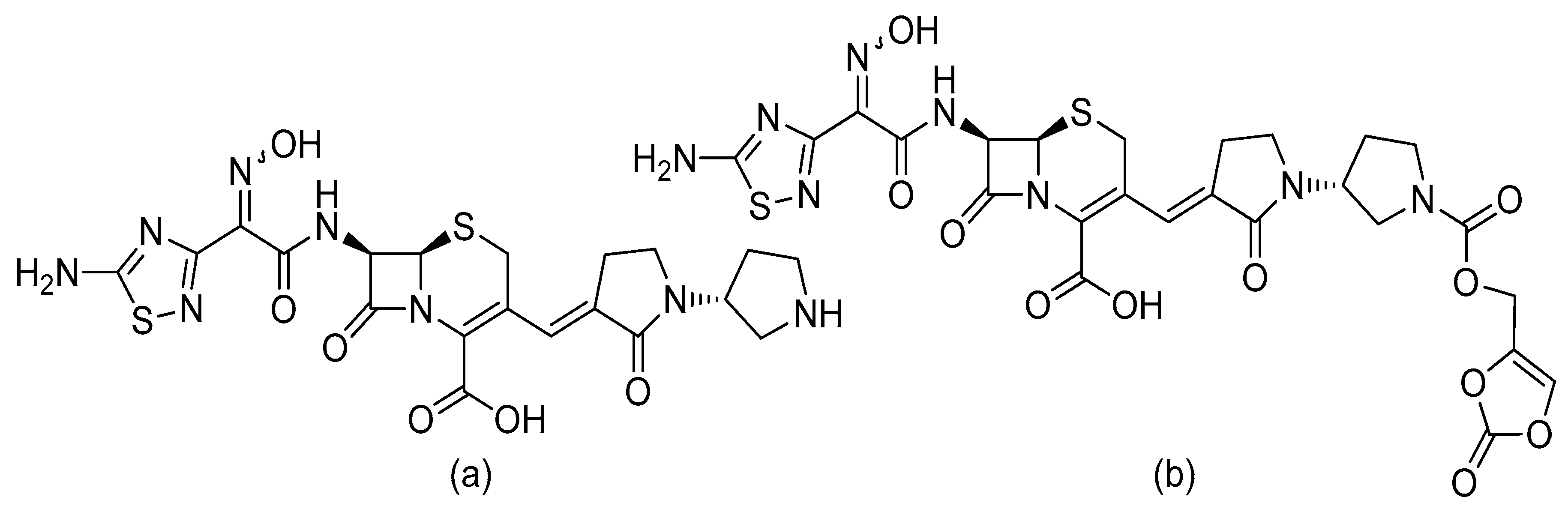
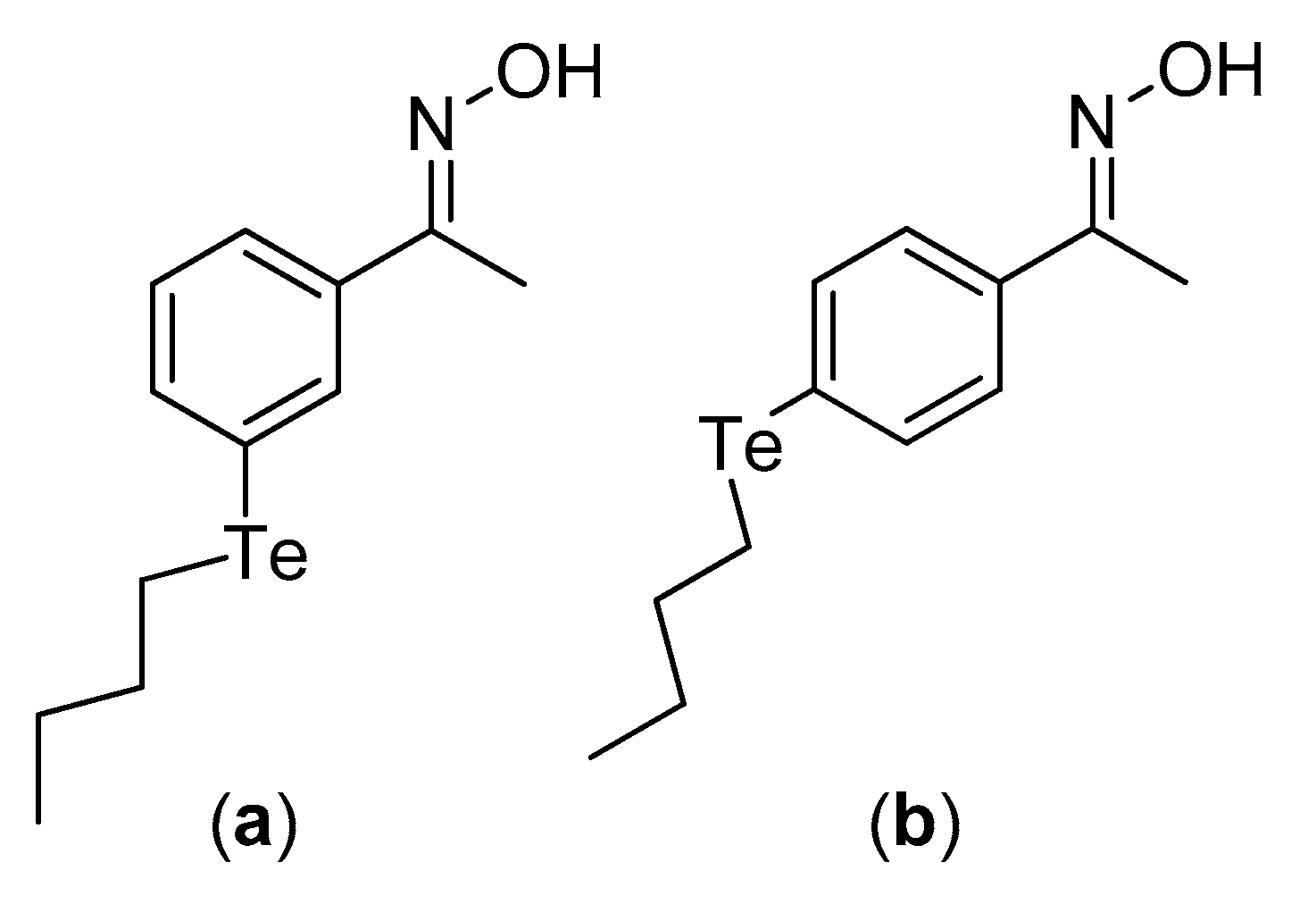
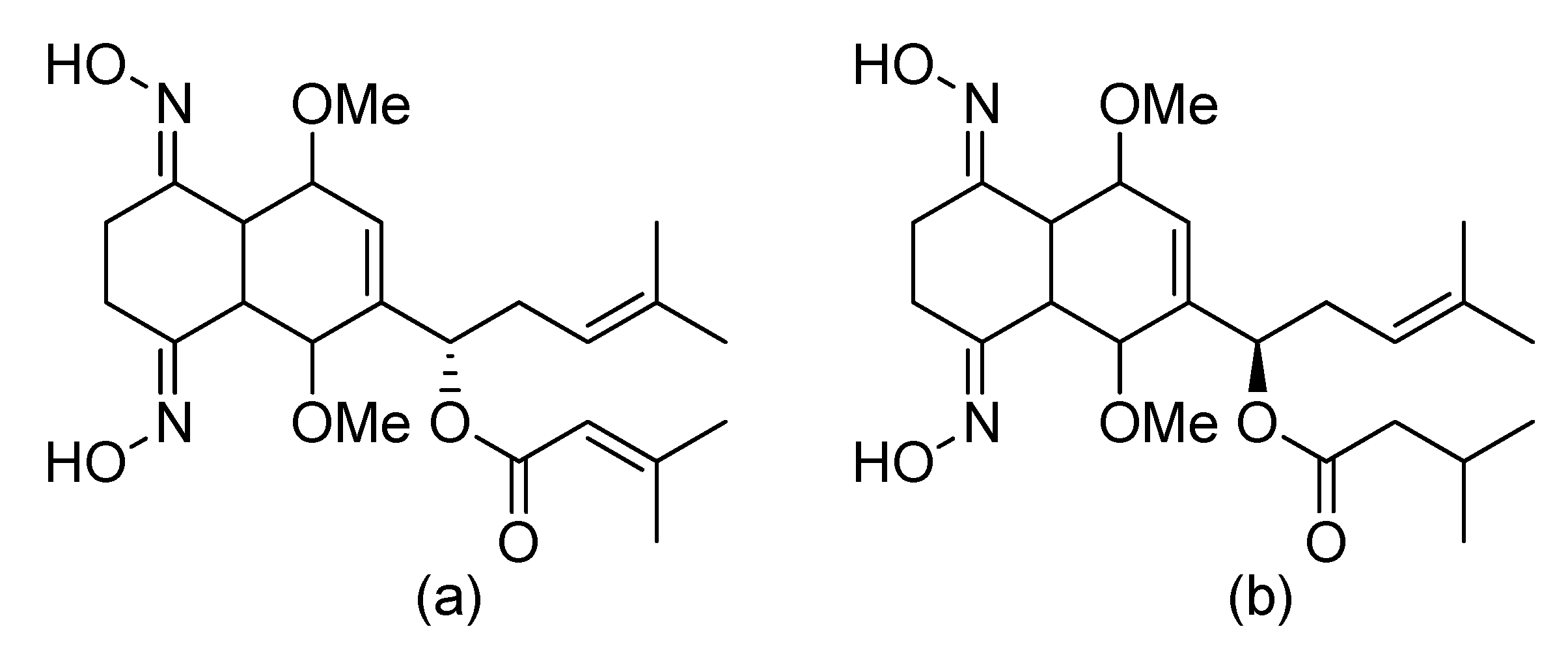
3. The Antimicrobial Activity
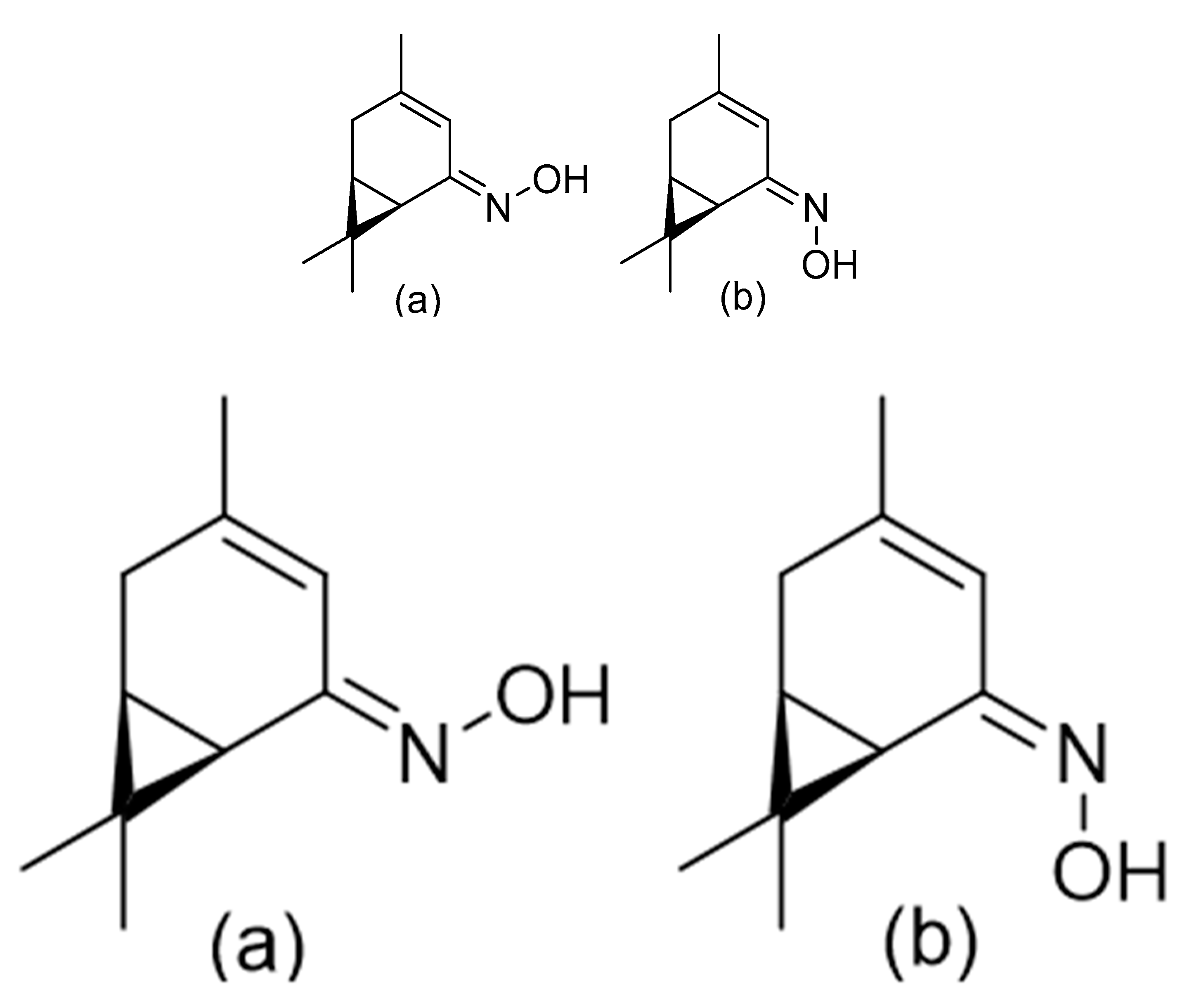
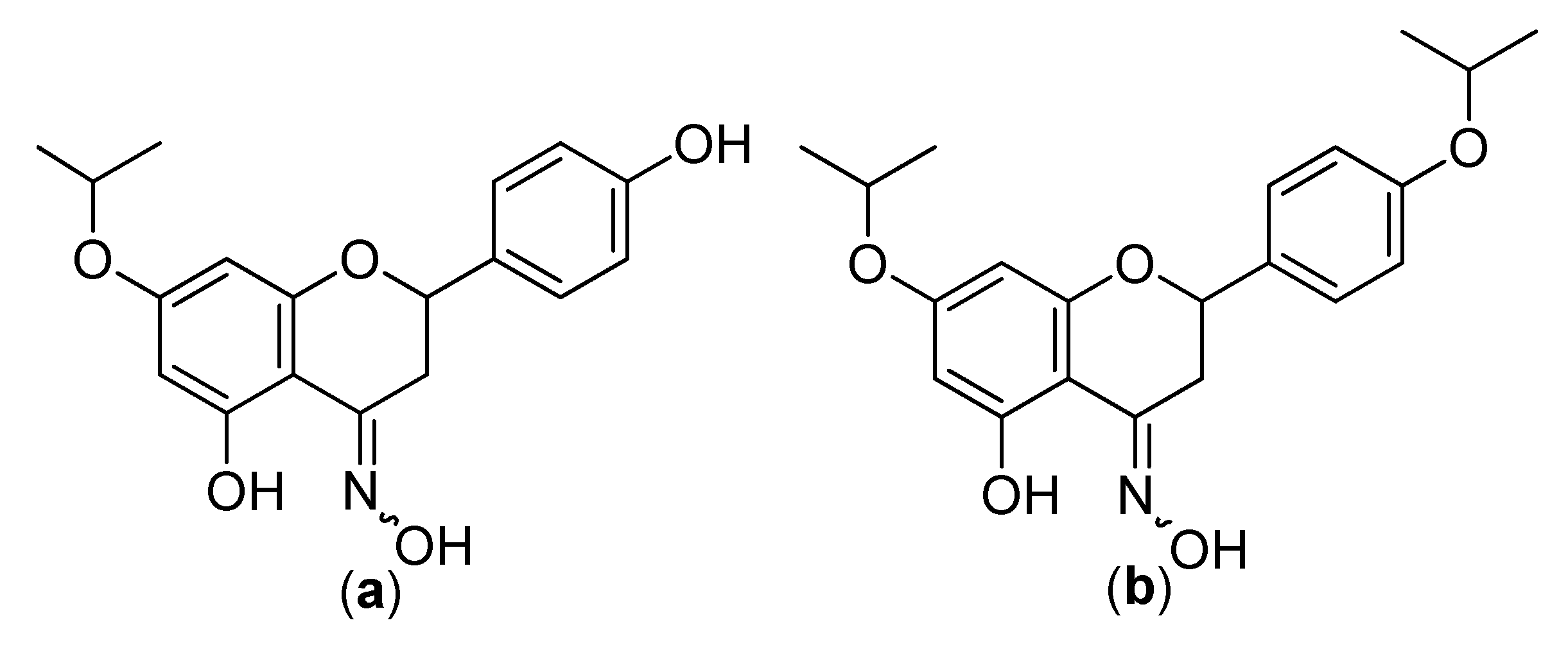
4. The Antioxidant Activity
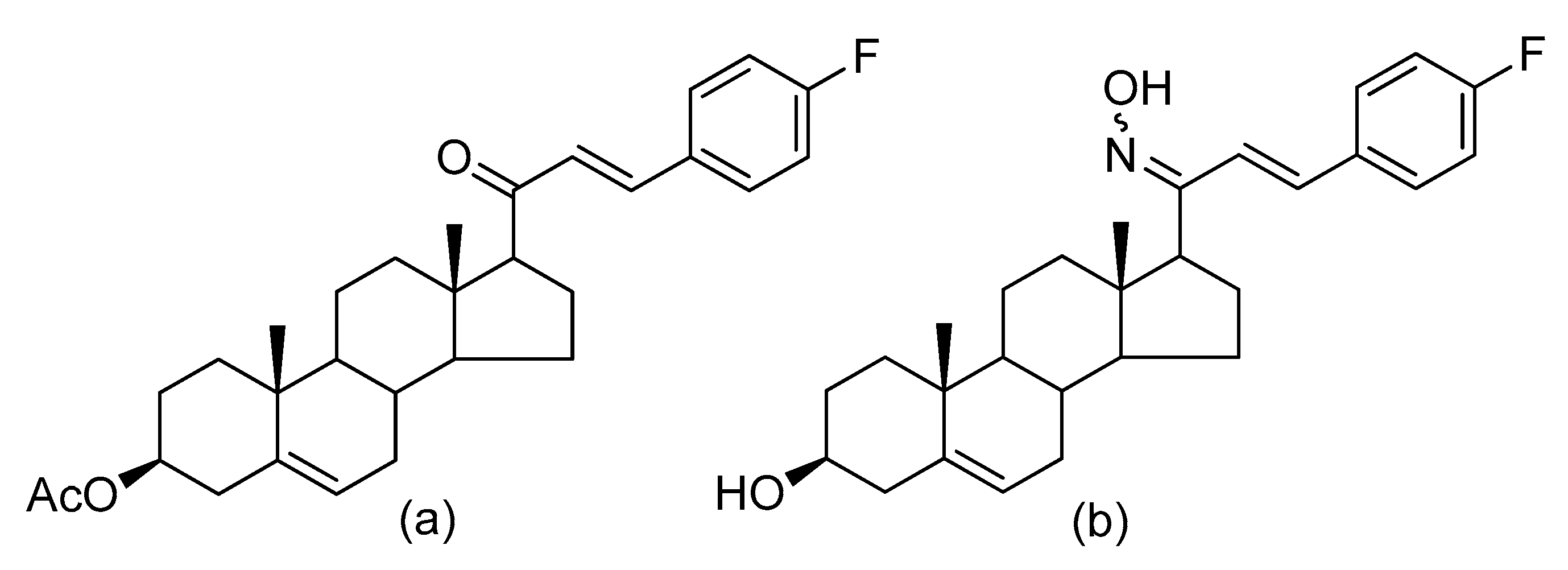
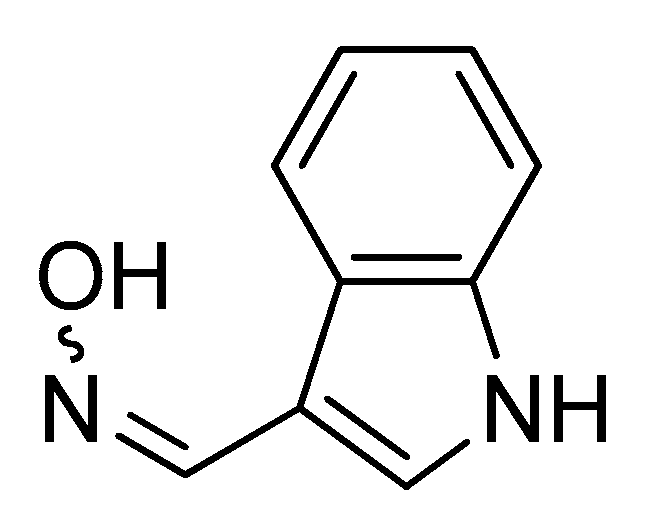
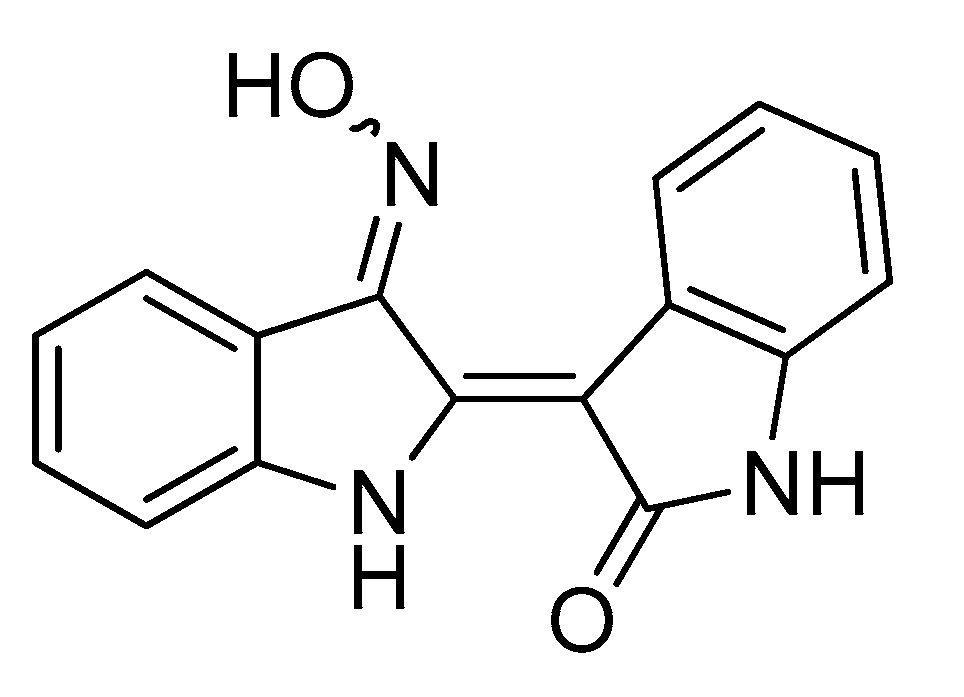
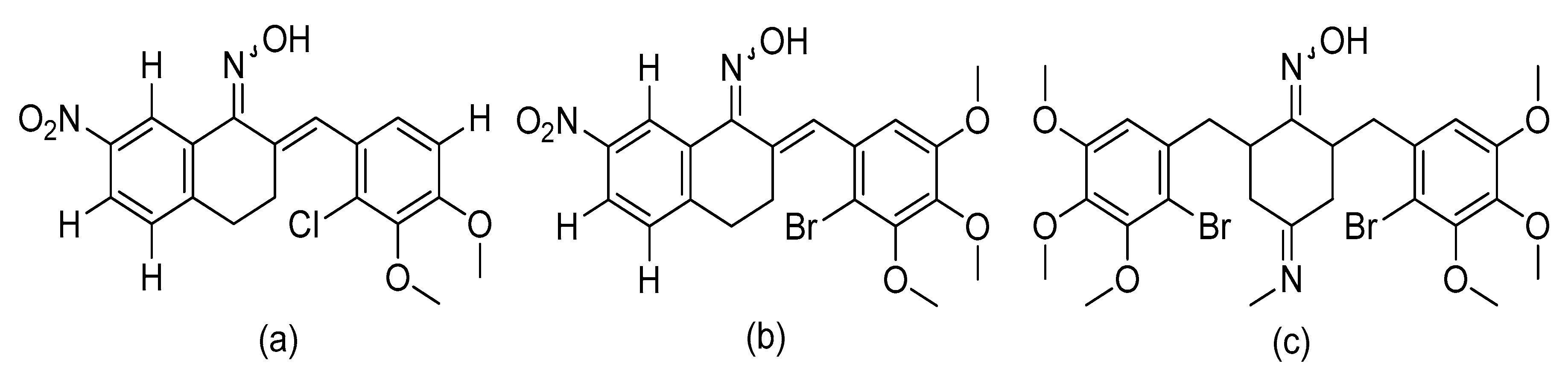
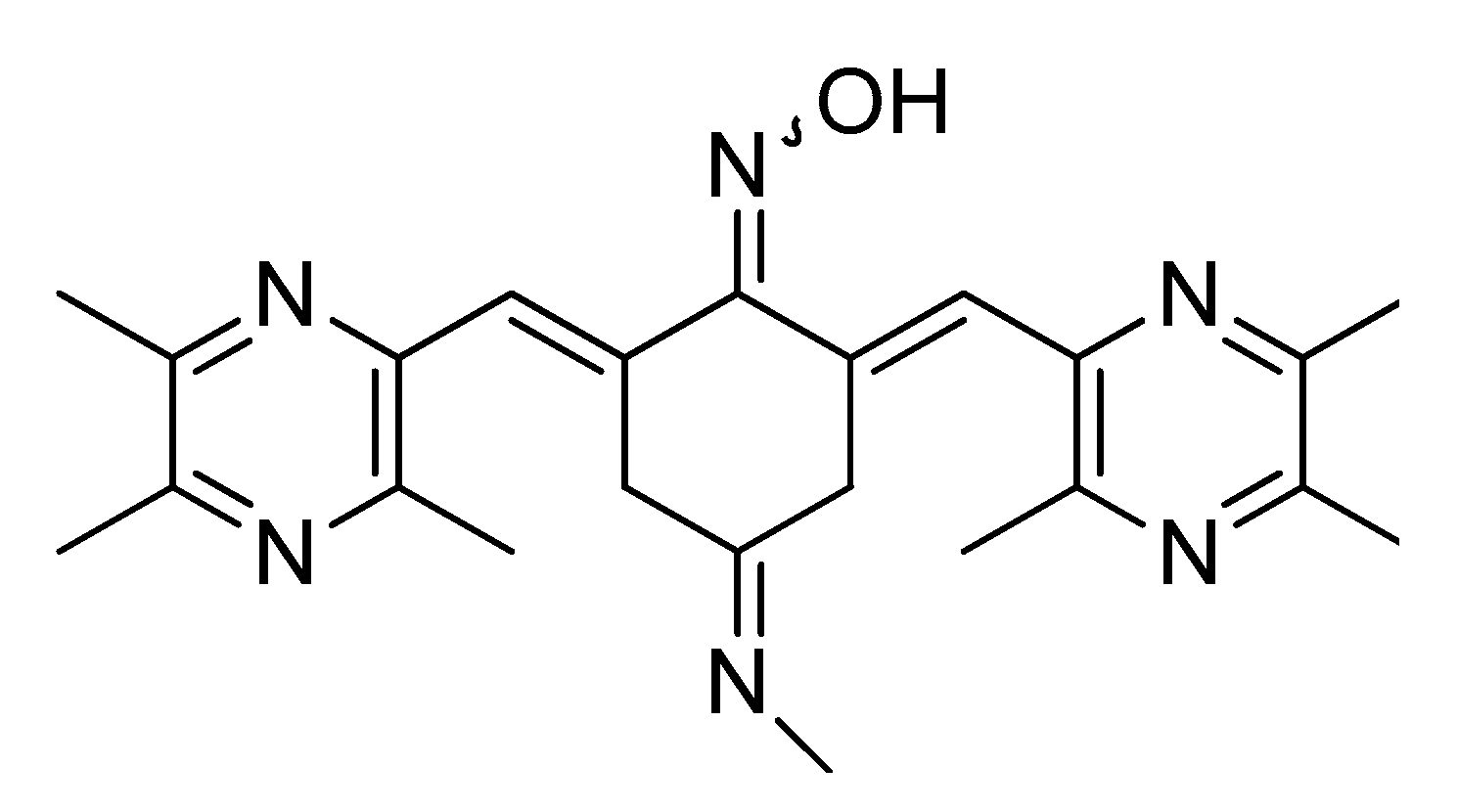
5. The Anticancer Activity
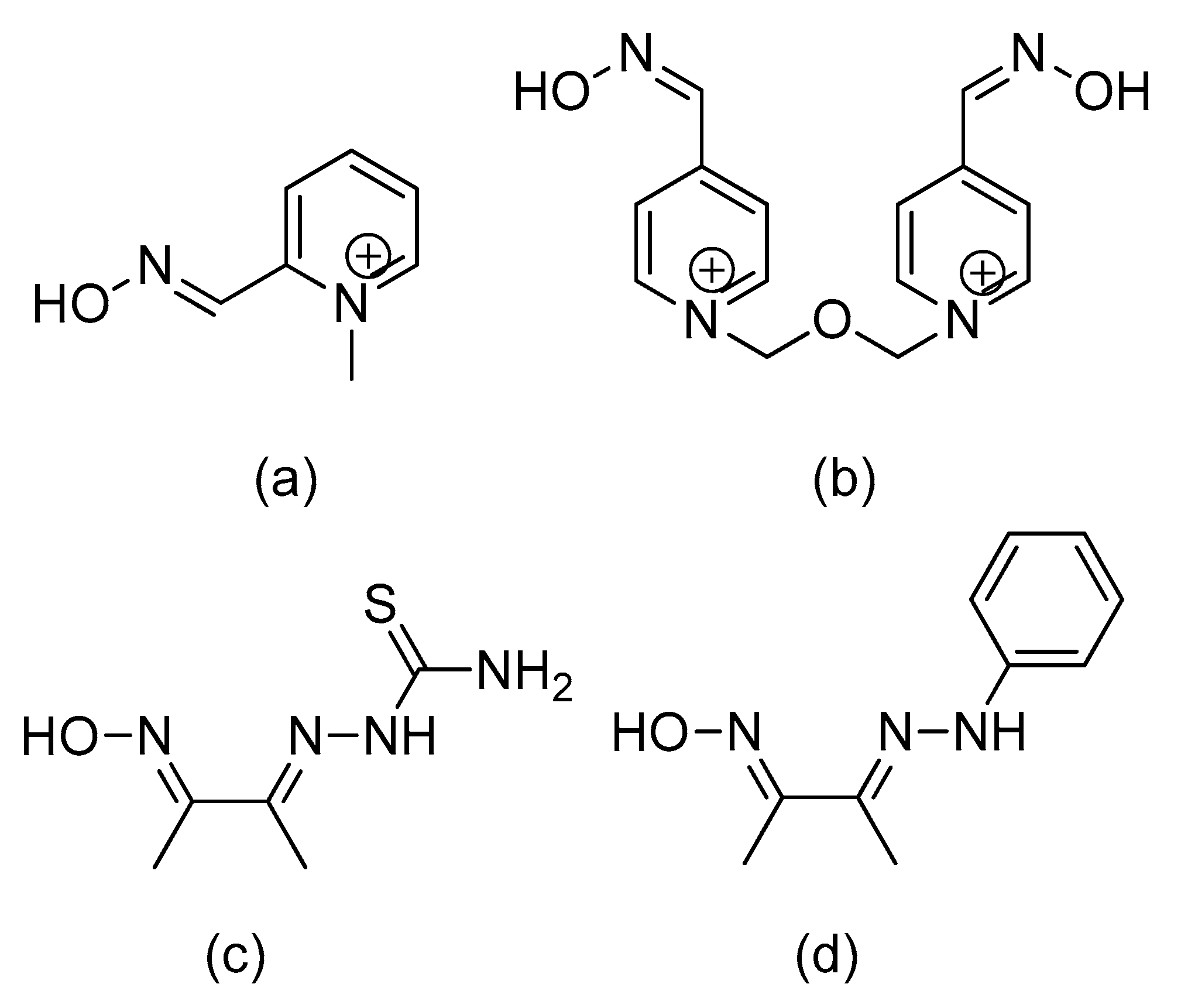
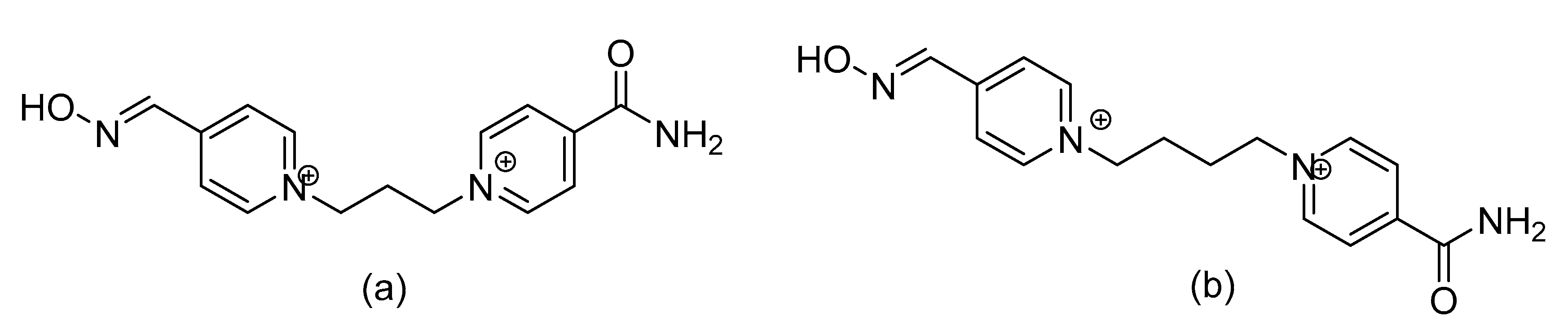
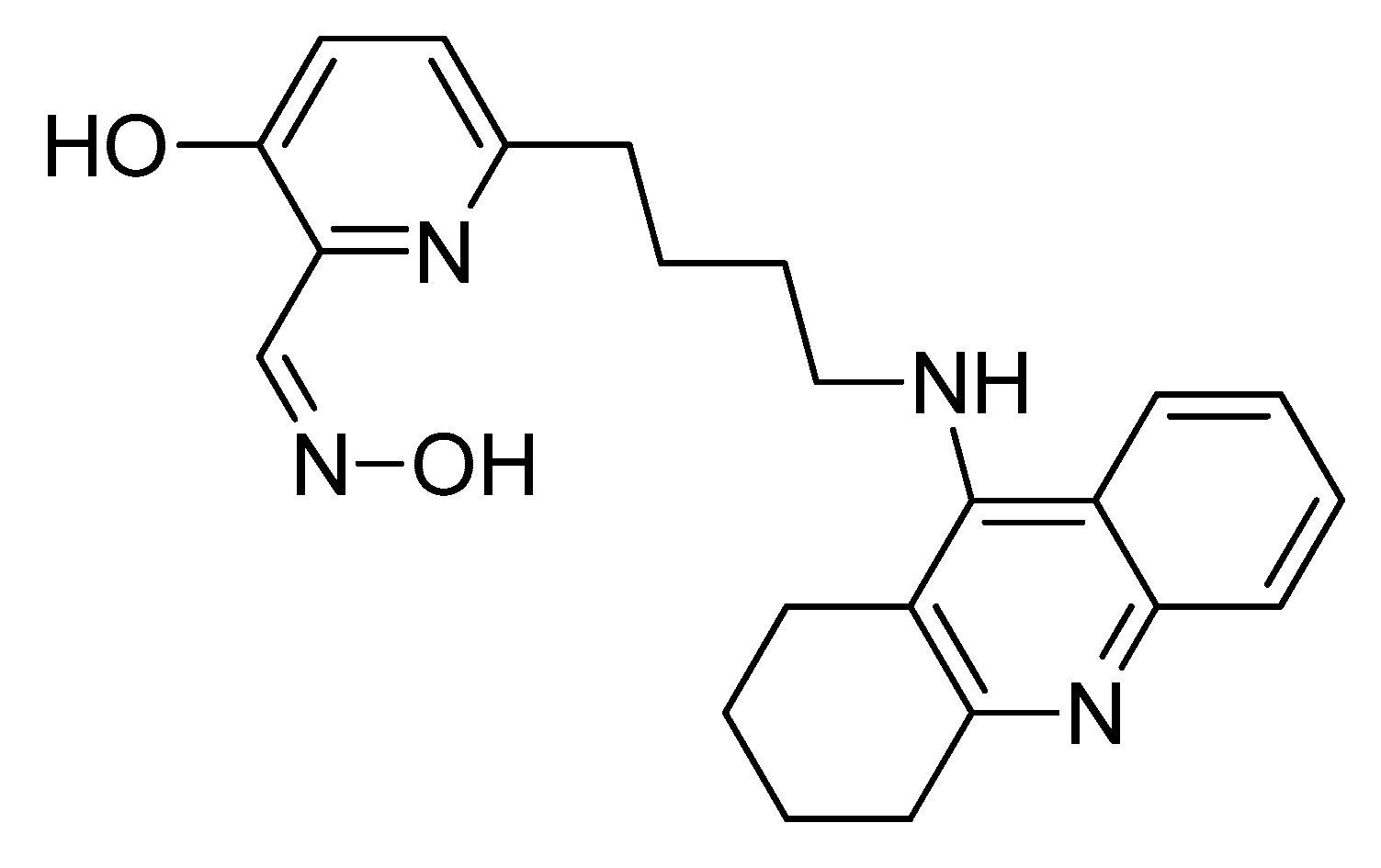
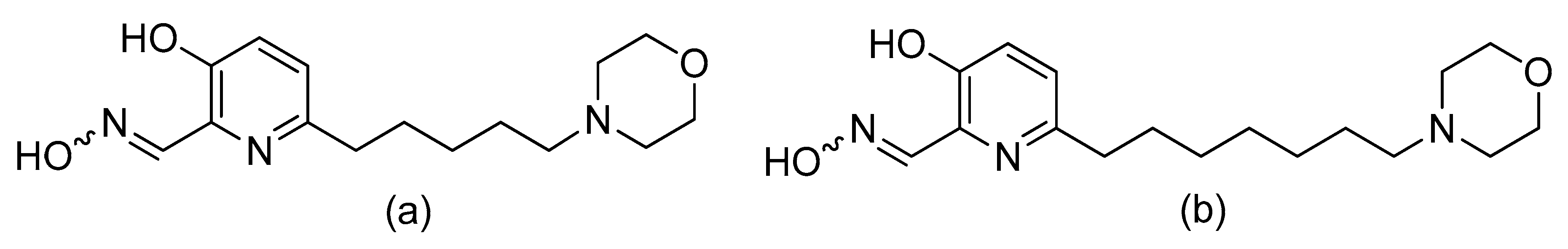
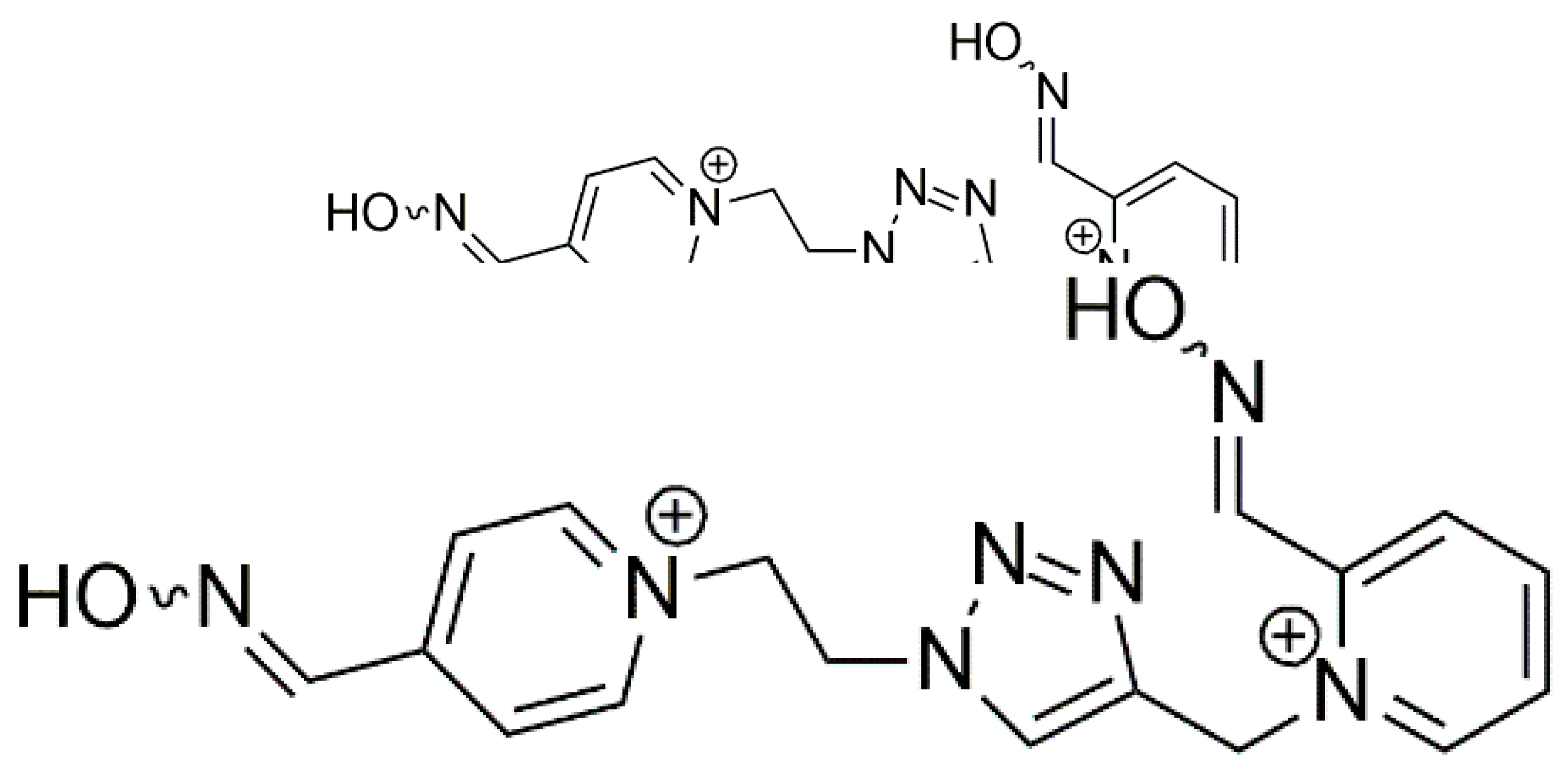
6. A Counteractive Agent to Organophosphorus Compound Poisoning
7. Conclusions
Funding
Conflicts of Interest
References
- Sørensen, M.; Neilson, E.H.J.; Møller, B.L. Oximes: Unrecognized Chameleons in General and Specialized Plant Metabolism. Mol. Plant 2018, 11, 95–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politzer, P.; Murray, J.S. Structural analysis of hydroxyloamines, oximes and hydroxamic acids: Trends and patterns. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Part 1; Rappoport, Z., Liebman, J.F., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 29–52. [Google Scholar]
- Porcheddu, A.; Giacomelli, G. Synthesis of oximes and hydroxamic acids. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Part 1; Rappoport, Z., Liebman, J.F., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 163–232. [Google Scholar]
- Ashani, Y.; Silman, I. Hydroxylamines and oximes: Biological properties and potential uses as therapeutic agents. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Part 1; Rappoport, Z., Liebman, J.F., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 609–653. [Google Scholar]
- Okolotowicz, K.J.; Dwyer, M.; Smith, E.; Cashman, J.R. Preclinical Studies of Noncharged Oxime Reactivators for Organophosphate Exposure. J. Biochem. Mol. Toxicol. 2014, 28, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kölmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-K.; Ko, D.-H.; You, Z.; Khan, M.O.F.; Lee, H.J. In vitro anti-inflammatory activities of new steroidal antedrugs: [16α,17α-d] Isoxazoline and [16α,17α-d]-3′-hydroxy-iminoformyl isoxazoline derivatives of prednisolone and 9α-fluoroprednisolone. Steroids 2006, 71, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Chen, L.Z.; Wang, J.Q.; Zhou, H.P.; Tang, W.J.; Xue, W.; Liu, X.H. New pentadienone oxime ester derivatives: Synthesis and anti-inflammatory activity. J. Enzyme Inhib. Med. Chem. 2018, 33, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
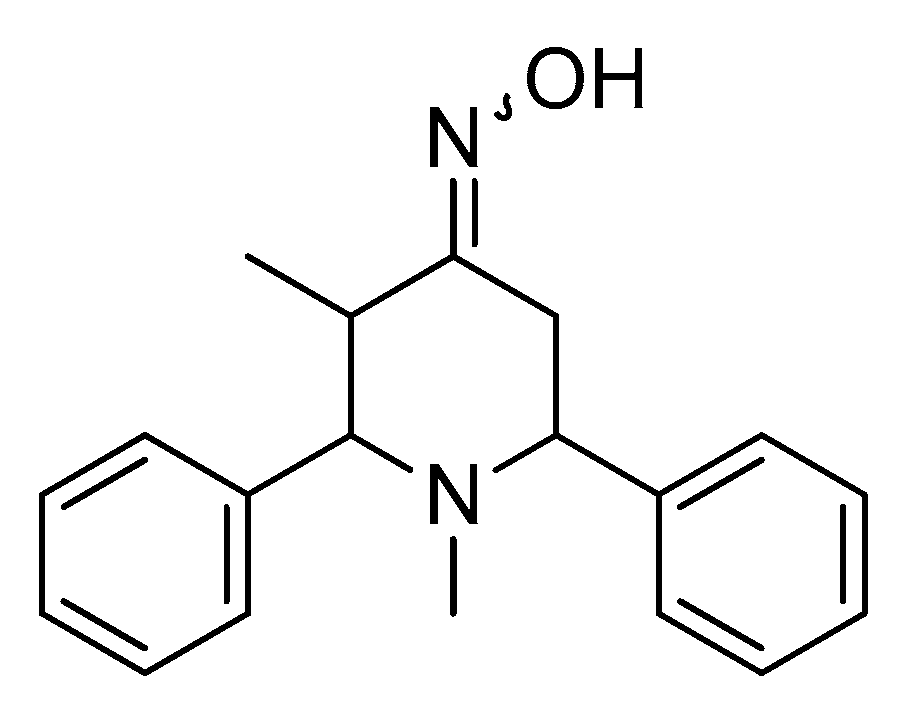
- Tharini, K.; Sangeetha, P. Antioxidant and anti-inflammatory activity of 3,3-dimethyl 2,6- dimethyl piperidine 4-one oxime. Int. J. Chem. Sci. 2015, 13, 1794–1804. [Google Scholar]
- Zeferino-Díaz, R.; Olivera-Castillo, L.; Dávalos, A.; Grant, G.; Kantún-Moreno, N.; Rodriguez-Canul, R.; Bernès, S.; Sandoval-Ramírez, J.; Fernández-Herrera, M.A. 22-Oxocholestane oximes as potential anti-inflammatory drug candidates. Eur. J. Med. Chem. 2019, 168, 78–86. [Google Scholar] [CrossRef]
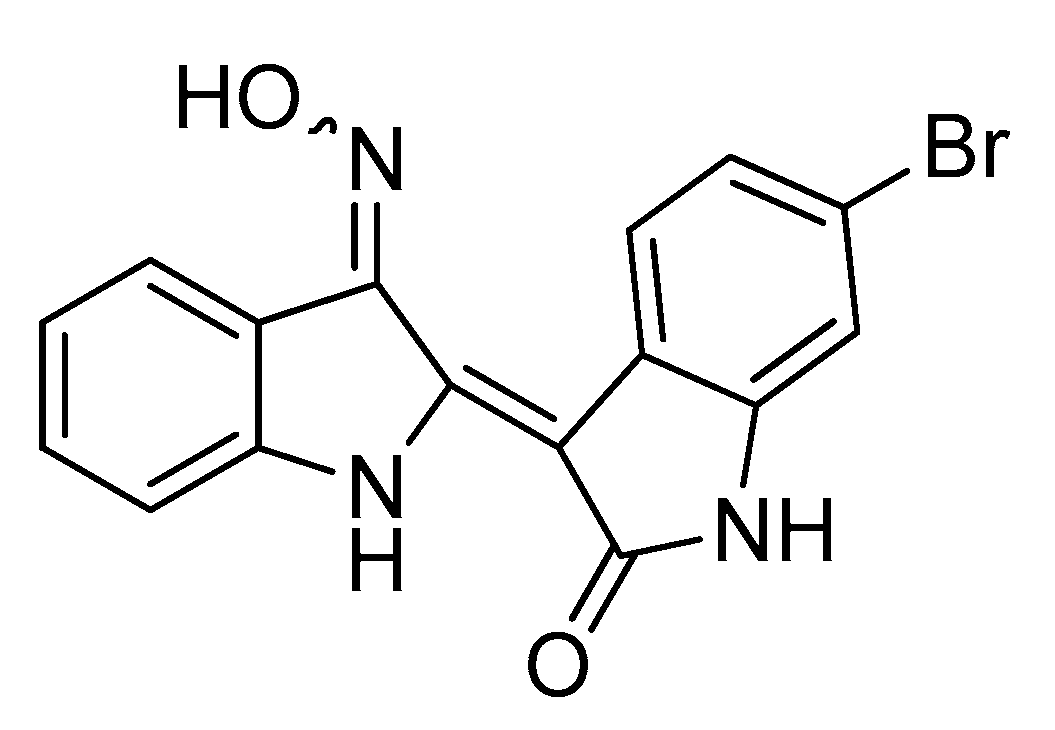
- Liu, C.; Tang, X.; Zhang, W.; Li, G.; Chen, Y.; Guo, A.; Hu, C. 6-Bromoindirubin-3′-Oxime Suppresses LPS-Induced Inflammation via Inhibition of the TLR4/NF-κB and TLR4/MAPK Signaling Pathways. Inflammation 2019, 42, 2192–2204. [Google Scholar] [CrossRef]
- Kasare, M.S.; Dhavan, P.P.; Jadhav, B.L.; Pawar, S.D. In-vitro antibacterial activity of Ni(II), Cu(II), and Zn(II) complexes incorporating new azo-azomethine ligand possessing excellent antioxidant, anti-inflammatory activity and protective effect of free radicals against plasmid DNA. Synth. Commun. 2019, 49, 3311–3323. [Google Scholar] [CrossRef]
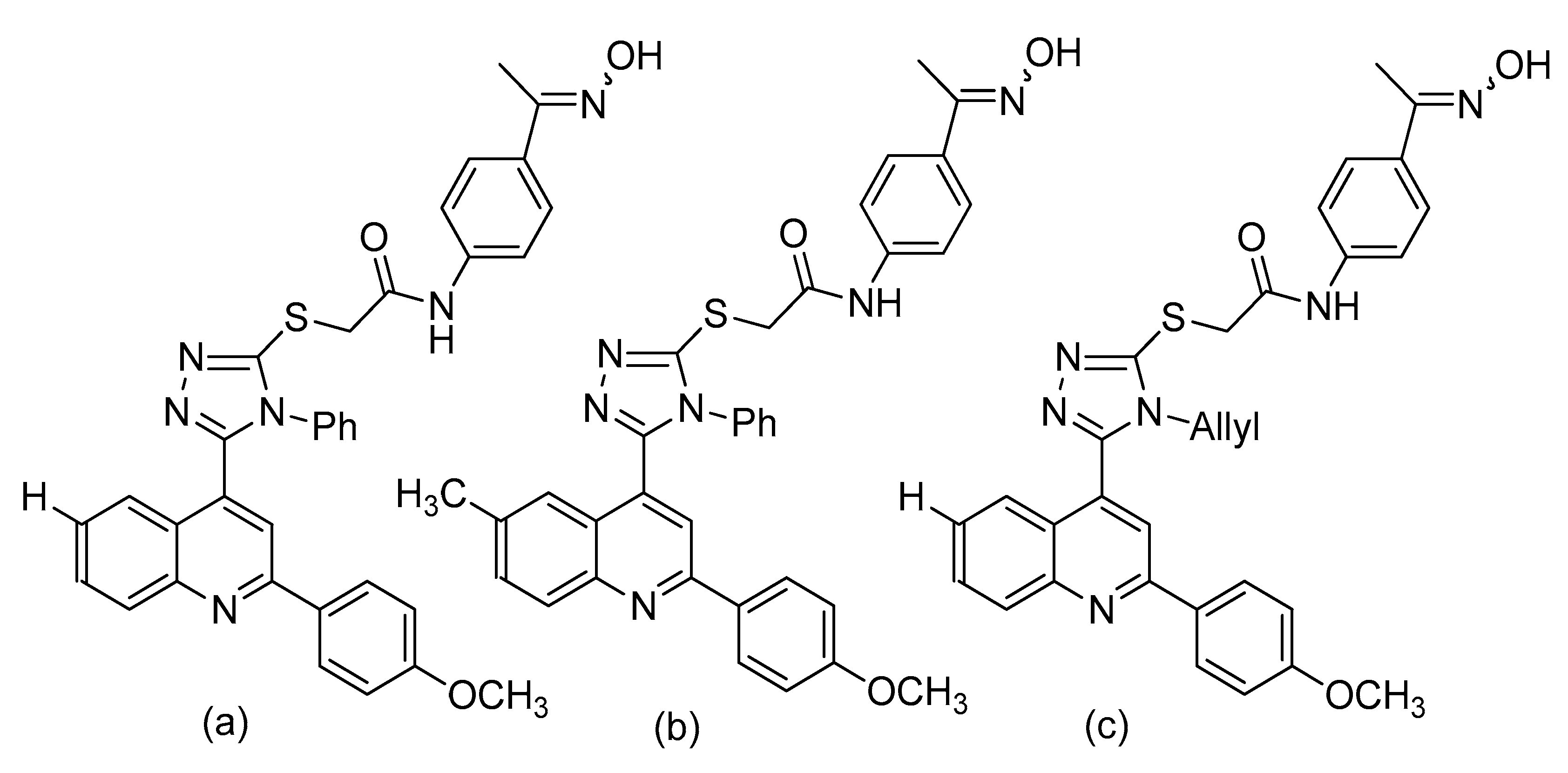
- Mohassab, A.M.; Hassan, H.; Abdelhamid, D.; Abdel-Aziz, M.; Dalby, K.; Kaoud, T. Novel quinoline incorporating 1,2,4-triazole/oxime hybrids: Synthesis, molecular docking, anti-inflammatory, COX inhibition, ulceroginicity and histopathological investigations. Bioorg. Chem. 2017, 75, 242–259. [Google Scholar]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.-S.F.F. Novel 1,3,4-oxadiazole/oxime hybrids: Synthesis, docking studies and investigation of anti-inflammatory, ulcerogenic liability and analgesic activities. Bioorg. Chem. 2016, 69, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yasukawa, K.; Tokuda, H. Potentially Cancer Chemopreventive and Anti-Inflammatory Terpenoids from Natural Sources. In Bioactive Natural Products; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 73–126. [Google Scholar]
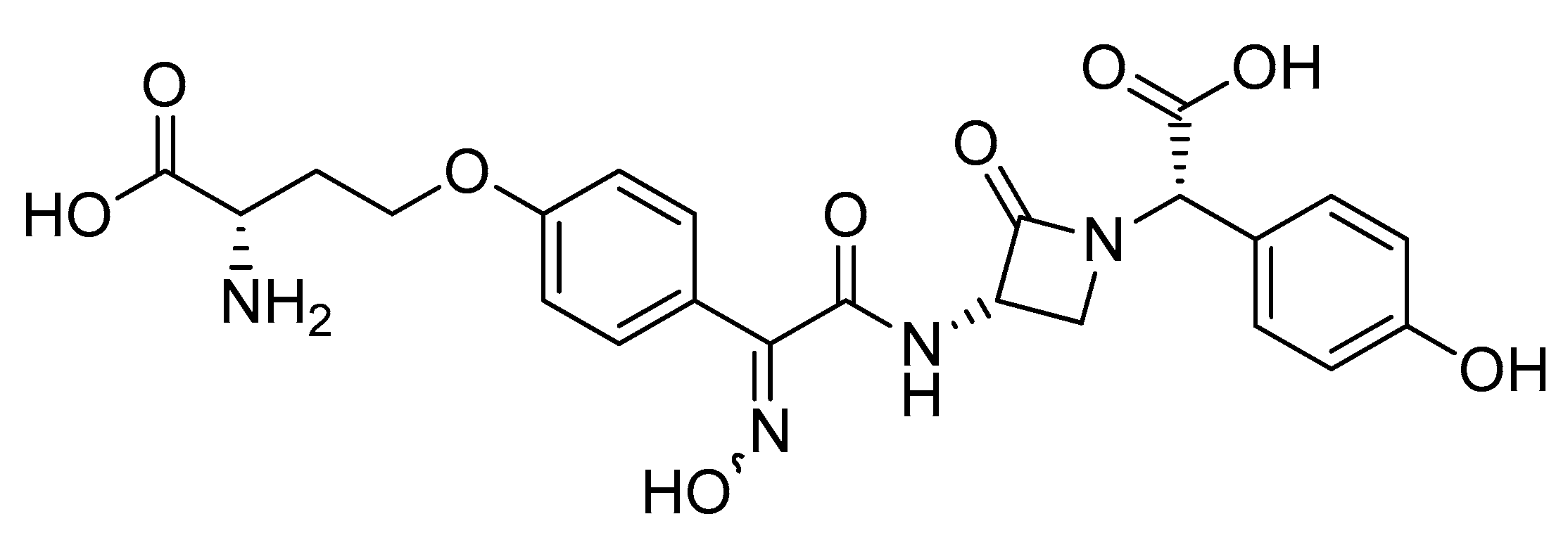
- Kojo, H.; Mine, Y.; Nishida, M.; Goto, S.; Kuwahara, S. Nature of Monocyclic β-Lactam Antibiotic Nocardicin A to β-Lactamases. Microbiol. Immunol. 1988, 32, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L. Patent Review of Manufacturing Routes to Fifth-Generation Cephalosporin Drugs. Part 2, Ceftaroline Fosamil and Ceftobiprole Medocaril. Org. Process Res. Dev. 2017, 21, 800–815. [Google Scholar] [CrossRef]
- Syed, Y.Y. Ceftobiprole Medocaril: A Review of Its Use in Patients with Hospital- or Community-Acquired Pneumonia. Drugs 2014, 74, 1523–1542. [Google Scholar] [CrossRef]
- Paulsen, B.; Fredriksen, K.A.; Petersen, D.; Maes, L.; Matheeussen, A.; Naemi, A.-O.; Scheie, A.A.; Simm, R.; Ma, R.; Wan, B.; et al. Synthesis and antimicrobial activities of N6-hydroxyagelasine analogs and revision of the structure of ageloximes. Bioorg. Med. Chem. 2019, 27, 620–629. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef]
- Ilboudo, O.; Bonzi, S.; Tapsoba, I.; Somda, I.; Bonzi-Coulibaly, Y.L. In vitro antifungal activity of flavonoid diglycosides of Mentha piperita and their oxime derivatives against two cereals fungi. Comptes Rendus Chim. 2016, 19, 857–862. [Google Scholar] [CrossRef]
- Huang, M.; Duan, W.-G.; Lin, G.-S.; Li, K.; Hu, Q. Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters. Molecules 2017, 22, 1538. [Google Scholar] [CrossRef] [Green Version]
- Kozłowska, J.; Grela, E.; Baczyńska, D.; Grabowiecka, A.; Anioł, M. Novel O-alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity. Molecules 2019, 24, 679. [Google Scholar] [CrossRef] [Green Version]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef] [PubMed]
- Lone, I.H.; Khan, K.Z.; Fozdar, B.I.; Hussain, F. Synthesis antimicrobial and antioxidant studies of new oximes of steroidal chalcones. Steroids 2013, 78, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Kakati, D.; Sarma, R.K.; Saikia, R.; Barua, N.C.; Sarma, J.C. Rapid microwave assisted synthesis and antimicrobial bioevaluation of novel steroidal chalcones. Steroids 2013, 78, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, A.; Grela, E.; Macegoniuk, K.; Grabowiecka, A.; Lochyński, S. Synthesis of nitrogen-containing monoterpenoids with antibacterial activity. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Zhou, X.; He, Y.; Chen, Q. Antiviral activities of Janus-type nucleosides and their related oxime-intermediates. Bioorg. Med. Chem. 2019, 27, 2332–2339. [Google Scholar] [CrossRef]
- Chan, M.; Chan, R.; Mok, C.; Mak, N.; Wong, R. Indirubin-3′-oxime as an antiviral and immunomodulatory agent in treatment of severe human influenza virus infection. Hong Kong Med. J. 2018, 24 (Suppl. 6), 45–47. [Google Scholar]
- Özyürek, M.; Akpınar, D.; Bener, M.; Türkkan, B.; Güçlü, K.; Apak, R. Novel oxime based flavanone, naringin-oxime: Synthesis, characterization and screening for antioxidant activity. Chem. Biol. Interact. 2014, 212, 40–46. [Google Scholar] [CrossRef]
- Potaniec, B.; Grabarczyk, M.; Stompor, M.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.K.; Anioł, M. Antioxidant activity and spectroscopic data of isoxanthohomol oxime and related compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 716–723. [Google Scholar] [CrossRef]
- Kaur, N.; Chahal, K.K.; Kumar, A.; Singh, R.; Bhardwaj, U. Antioxidant activity of Anethum graveolens L. essential oil constituents and their chemical analogues. J. Food Biochem. 2019, 43, e12782. [Google Scholar] [CrossRef]
- Bandeira, P.T.; Dalmolin, M.C.; de Oliveira, M.M.; Nunes, K.C.; Garcia, F.P.; Nakamura, C.V.; de Oliveira, A.R.M.; Piovan, L. Synthesis, Antioxidant Activity and Cytotoxicity of N-Functionalized Organotellurides. Bioorg. Med. Chem. 2019, 27, 410–415. [Google Scholar] [CrossRef]
- Bensegueni, R.; Guergouri, M.; Bensouici, C.; Bencharif, M. Synthesis, antioxidant, and anti-tyrosinase activity of some aromatic oximes: An experimental and theoretical study. J. Rep. Pharm. Sci. 2019, 8, 195–203. [Google Scholar]
- Siddiqui, R.; Saify, Z.; Akhter, S.; Saeed, G.; Haider, S.; Leghari, Q. Synthesis, Characterization and evaluation of antioxidant potential of 2, 6-diphenylpiperidine-4-one compounds and their novel imine derivatives. Pak. J. Pharm. Sci. 2018, 31, 2361–2365. [Google Scholar] [PubMed]
- Banday, A.H.; Akram, S.M.M.; Shameem, S.A. Benzylidine pregnenolones and their oximes as potential anticancer agents: Synthesis and biological evaluation. Steroids 2014, 84, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, Y.; Saito, H.; Uchiyama, T.; Metori, K.; Tabata, K.; Suzuki, T.; Miyairi, S. Indirubin 3′-(O-oxiran-2-ylmethyl)oxime: A novel anticancer agent. Bioorg. Med. Chem. Lett. 2015, 25, 1403–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, X.; Song, H.; Zhou, S.; Li, S. Synthesis and evaluation of novel alkannin and shikonin oxime derivatives as potent antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 4304–4307. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-L.; Leng, J.; Zhang, C.-P.; Jantan, I.; Amjad, M.W.; Sher, M.; Naeem-ul-Hassan, M.; Hussain, M.A.; Bukhari, S.N.A. Synthesis of α,β-Unsaturated Carbonyl-Based Compounds, Oxime and Oxime Ether Analogs as Potential Anticancer Agents for Overcoming Cancer Multidrug Resistance by Modulation of Efflux Pumps in Tumor Cells. J. Med. Chem. 2016, 59, 3549–3561. [Google Scholar] [CrossRef]
- Zha, G.-F.; Qin, H.-L.; Youssif, B.G.M.; Amjad, M.W.; Raja, M.A.G.; Abdelazeem, A.H.; Bukhari, S.N.A. Discovery of potential anticancer multi-targeted ligustrazine based cyclohexanone and oxime analogs overcoming the cancer multidrug resistance. Eur. J. Med. Chem. 2017, 135, 34–48. [Google Scholar] [CrossRef]
- Petersen, A.B.; Konotop, G.; Hanafiah, N.H.M.; Hammershøj, P.; Raab, M.S.; Krämer, A.; Clausen, M.H. Strategies for improving the solubility and metabolic stability of griseofulvin analogues. Eur. J. Med. Chem. 2016, 116, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Rønnest, M.H.; Raab, M.S.; Anderhub, S.; Boesen, S.; Krämer, A.; Larsen, T.O.; Clausen, M.H. Disparate SAR Data of Griseofulvin Analogues for the Dermatophytes Trichophyton mentagrophytes, T. rubrum, and MDA-MB-231 Cancer Cells. J. Med. Chem. 2012, 55, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Worek, F.; Thiermann, H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol. Ther. 2013, 139, 249–259. [Google Scholar] [CrossRef]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Reactivation kinetics of a series of related bispyridinium oximes with organophosphate-inhibited human acetylcholinesterase—Structure–activity relationships. Biochem. Pharmacol. 2012, 83, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.D.; Blecha, J.E.; Hayes, T.R.; Huynh, T.; Chao, C.-K.; Guilloteau, N.; Zinn, K.R.; VanBrocklin, H.F.; Thompson, C.M.; Gerdes, J.M. Radiosynthesis, ex Vivo Biodistribution, and in Vivo Positron Emission Tomography Imaging Evaluations of [11C]2-Pyridinealdoxime Methiodide ([11C]2-PAM): A First-In-Class Antidote Tracer for Organophosphate Intoxication. ACS Chem. Neurosci. 2018, 9, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.M.; Snider, T.H.; Babin, M.C.; Jett, D.A.; Platoff, G.E.; Yeung, D.T. A comprehensive evaluation of the efficacy of leading oxime therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Toxicol. Appl. Pharmacol. 2014, 281, 254–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.D.; Freitas, M.L.; Soares, F.A.A.; Carratu, V.S.; Brandão, R. Potential of two new oximes in reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by organophosphate compounds: An in vitro study. Toxicol. Vitr. 2011, 25, 2120–2123. [Google Scholar] [CrossRef] [Green Version]
- Quinn, D.M. Resurrection Biology: Aged Acetylcholinesterase Brought Back to Life. J. Med. Chem. 2018, 61, 7032–7033. [Google Scholar] [CrossRef] [Green Version]
- Žunec, S.; Radić, B.; Kuča, K.; Musilek, K.; Vrdoljak, A.L. Comparative determination of the efficacy of bispyridinium oximes in paraoxon poisoning/Usporedno određivanje učinkovitosti bispiridinijevih oksima pri trovanju paraoksonom. Arch. Ind. Hyg. Toxicol. 2015, 66, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Kuca, K.; Nepovimova, E.; Wu, Q.; de Souza, F.R.; de Ramalho, T.C.; Franca, T.C.C.; Musilek, K. Experimental hydrophilic reactivator: Bisoxime with three positive charges. Chem. Pap. 2019, 73, 777–782. [Google Scholar] [CrossRef]
- Santoni, G.; de Sousa, J.; de la Mora, E.; Dias, J.; Jean, L.; Sussman, J.L.; Silman, I.; Renard, P.-Y.; Brown, R.C.D.; Weik, M.; et al. Structure-Based Optimization of Nonquaternary Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. J. Med. Chem. 2018, 61, 7630–7639. [Google Scholar] [CrossRef] [Green Version]
- Zorbaz, T.; Braïki, A.; Maraković, N.; Renou, J.; de la Mora, E.; Maček Hrvat, N.; Katalinić, M.; Silman, I.; Sussman, J.L.; Mercey, G.; et al. Potent 3-Hydroxy-2-Pyridine Aldoxime Reactivators of Organophosphate-Inhibited Cholinesterases with Predicted Blood–Brain Barrier Penetration. Chem. A Eur. J. 2018, 24, 9675–9691. [Google Scholar] [CrossRef]
- Kovarik, Z.; Kalisiak, J.; Hrvat, N.M.; Katalinić, M.; Zorbaz, T.; Žunec, S.; Green, C.; Radić, Z.; Fokin, V.V.; Sharpless, K.B.; et al. Reversal of Tabun Toxicity Enabled by a Triazole-Annulated Oxime Library—Reactivators of Acetylcholinesterase. Chem. A Eur. J. 2019, 25, 4100–4114. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surowiak, A.K.; Lochyński, S.; Strub, D.J. Unsubstituted Oximes as Potential Therapeutic Agents. Symmetry 2020, 12, 575. https://doi.org/10.3390/sym12040575
Surowiak AK, Lochyński S, Strub DJ. Unsubstituted Oximes as Potential Therapeutic Agents. Symmetry. 2020; 12(4):575. https://doi.org/10.3390/sym12040575
Chicago/Turabian StyleSurowiak, Alicja K., Stanisław Lochyński, and Daniel J. Strub. 2020. "Unsubstituted Oximes as Potential Therapeutic Agents" Symmetry 12, no. 4: 575. https://doi.org/10.3390/sym12040575
APA StyleSurowiak, A. K., Lochyński, S., & Strub, D. J. (2020). Unsubstituted Oximes as Potential Therapeutic Agents. Symmetry, 12(4), 575. https://doi.org/10.3390/sym12040575




