Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology
Abstract
:1. Introduction
2. The Hierarchies of Chiral Structures in Proteins and Nucleic Acids
3. Structure Formation in Homochiral Systems of Nonbiological Origin
4. Physical Bases and Biological Functionality of Sign-Alternating Chiral Hierarchical Structures
4.1. Protein Folding
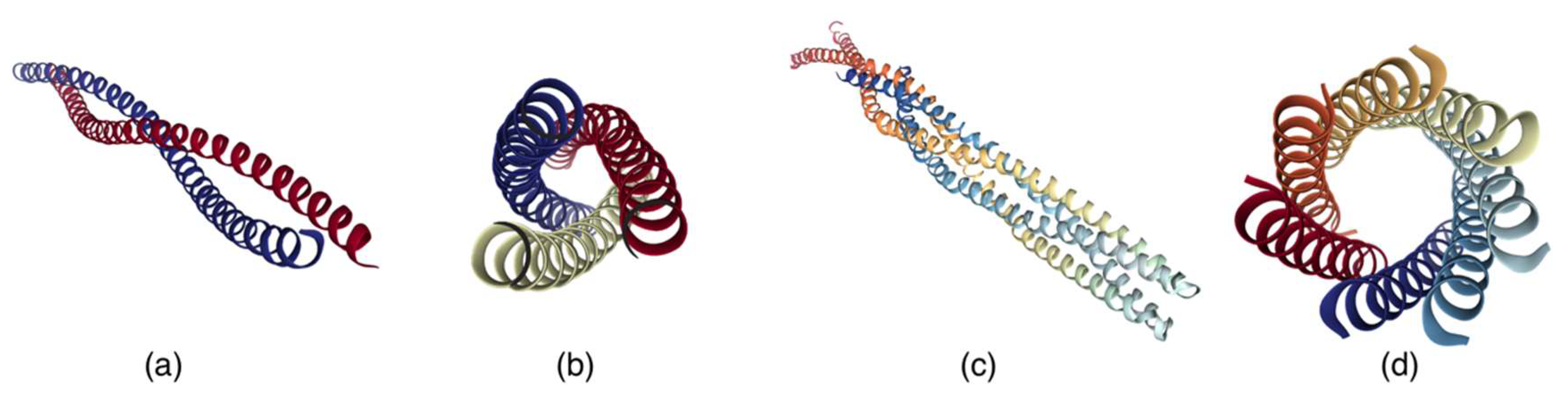
4.2. Molecular Machines
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Schwartz, A.W. Origin of life. The origin of macromolecular chirality. Curr. Biol. 1994, 4, 758–760. [Google Scholar] [CrossRef]
- Kojić-Prodić, B.; Štefanić, Z. Symmetry versus Asymmetry in the Molecules of Life: Homomeric Protein Assemblies. Symmetry 2010, 2, 884–906. [Google Scholar] [CrossRef] [Green Version]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left-right asymmetric development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlech, J. Origin of organic molecules and biomolecular homochirality. Cell Mol. Life Sci. 2001, 58, 44–60. [Google Scholar] [CrossRef]
- Hein, J.E.; Blackmond, D.G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef]
- Dorta-Urra, A.; Bargueño, P. Homochirality: A Perspective from Fundamental Physics. Symmetry 2019, 11, 661. [Google Scholar] [CrossRef] [Green Version]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2019, 11, a032540. [Google Scholar] [CrossRef] [Green Version]
- Famiano, M.; Boyd, R.; Kajino, T.; Onaka, T.; Mo, Y. Astrophysical Sites that Can Produce Enantiomeric Amino Acids. Symmetry 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Itabashi, Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry 2019, 11, 966. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.-I.; Kobayashi, K. Origin of Terrestrial Bioorganic Homochirality and Symmetry Breaking in the Universe. Symmetry 2019, 11, 919. [Google Scholar] [CrossRef] [Green Version]
- Sang, Y.; Liu, M. Symmetry Breaking in Self-Assembled Nanoassemblies. Symmetry 2019, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Aav, R.; Mishra, K.A. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Tverdislov, V.A.; Yakovenko, L.V. Physical Aspects of the Emergence of Living Cell Precursors: The Ion and Chiral Asymmetries as Two Fundamental Asymmetry Types. Mosc. Univ. Phys. Bull. 2008, 63, 151–163. [Google Scholar] [CrossRef]
- Zlenko, D.; Zanin, A.; Skoblin, A.; Tverdislov, V.; Stovbun, S. Spontaneous resolution in racemic solutions of N-trifluoroacetylated α-aminoalcohols. J. Mol. Struct. 2019, 1183, 8–13. [Google Scholar] [CrossRef]
- Hirose, K.; Ukimi, M.; Ueda, S.; Onoda, C.; Kano, R.; Tsuda, K.; Hinohara, Y.; Tobe, Y. The Asymmetry is Derived from Mechanical Interlocking of Achiral Axle and Achiral Ring Components—Syntheses and Properties of Optically Pure [2]Rotaxanes. Symmetry 2018, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Ustrnul, L.; Kaabel, S.; Burankova, T.; Martõnova, J.; Adamson, J.; Konrad, N.; Burk, P.; Borovkov, V.; Aav, R. Supramolecular chirogenesis in zinc porphyrins by enantiopure hemicucurbit[n]urils (n = 6, 8). Chem. Commun. 2019, 55, 14434–14437. [Google Scholar] [CrossRef] [Green Version]
- Rickhaus, M.; Mayor, M.; Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Choi, C.K.K.; Wang, Q. Origin of the Plasmonic Chirality of Gold Nanorod Trimers Templated by DNA Origami. ACS Appl. Mater. Interfaces 2018, 10, 26835–26840. [Google Scholar] [CrossRef]
- Tverdislov, V.A. Chirality as an Instrument of Stratification of Hierarchical Systems in Animate and Inanimate Nature. 2012. Available online: https://arxiv.org/abs/1212.1677 (accessed on 7 January 2020).
- Tverdislov, V.A. Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 2013, 58, 128–132. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V.; Ilchenko, S.A.; Zhulyabina, O.A.; Yakovenko, L.V. A periodic system of chiral structures in molecular biology. Biophysics 2017, 62, 421–432. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V. On regularities of spontaneous formation of structural hierarchies in chiral systems of non-living and living nature. Phys. Uspekhi 2019, 189, 375–385. [Google Scholar] [CrossRef]
- Burkhard, P.; Kammerer, R.A.; Steinmetz, M.O.; Bourenkov, G.P.; Aebi, U. The coiled-coil trigger site of the rod domain of cortexillin I unveils a distinct network of interhelical and intrahelical salt bridges. Structure 2000, 8, 223–230. [Google Scholar] [CrossRef]
- Garcia, P.; Ucurum, Z.; Bucher, R.; Svergun, D.I.; Huber, T.; Lustig, A.; Konarev, P.V.; Marino, M.; Mayans, O. Molecular insights into the self-assembly mechanism of dystrophia myotonica kinase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Yokota, H.; Kim, S.H. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 1999, 400, 787–792. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Q.; Deng, Y.; Cheng, C.S.; Kallenbach, N.R.; Lu, M. A seven-helix coiled coil. Proc. Natl. Acad. Sci. USA 2006, 103, 15457–15462. [Google Scholar] [CrossRef] [Green Version]
- RCSB PDB. Available online: http://www.rcsb.org (accessed on 15 January 2020).
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Cross, L.C.; Klyne, W. Rules for the Nomenclature of Organic Chemistry: Section E: Stereochemistry. Pure Appl. Chem. 1974, 45, 11–30. [Google Scholar]
- Chothia, C. Conformation of twisted beta-pleated sheets in proteins. J. Mol. Biol. 1973, 75, 295–302. [Google Scholar] [CrossRef]
- Mandelkow, E.-M.; Schultheiss, R.; Rapp, R.; Müller, M.; Mandelkow, E. On the surface lattice of microtubules: Helix starts, protofilament number, seam, and handedness. J. Cell Biol. 1986, 102, 1067–1073. [Google Scholar] [CrossRef]
- Amabilino, D. Chirality at the Nanoscale, Nanoparticles, Surfaces, Materials and More; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; 440p. [Google Scholar] [CrossRef]
- Danila, I.; Riobé, F.; Piron, F.; Puigmartí-Luis, J.; Wallis, J.D.; Linares, M.; Ågren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical chiral expression from the nano- to mesoscale in synthetic supramolecular helical fibers of a nonamphiphilic C3-symmetrical π-functional molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.; Fischer, M.; Sommerdijk, N.A.J.M.; Nolte, R.J.M. Helical superstructures from charged Poly(styrene)-Poly(isocyanodipeptide) block copolymers. Science 1998, 280, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.; Rowan, A.E.; Nolte, R.J.M. Mastering Molecular Matter. Supramolecular Architectures by Hierarchical Self-Assembly. J. Mater. Chem. 2003, 13, 2661–2670. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Zanin, A.M.; Skoblin, A.A.; Mikhaleva, M.G.; Zlenko, D.V.; Tverdislov, V.A. Self Assembly of Supramolecular Homochiral Structures in Solutions of Chiral Biomimetics. Mosc. Univ. Phys. Bull. 2015, 70, 51–56. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Zelenovskiy, P.S.; Nuraeva, A.S.; Kopyl, S.; Zhulyabina, O.A.; Tverdislov, V.A. Molecular modeling and computational study of the chiral-dependent structures and properties of self-assembling diphenylalanine peptide nanotubes. J. Mol. Model 2019, 25, 199. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Chen, J.; Liu, M. Hierarchical Self-Assembly of a Porphyrin into Chiral Macroscopic Flowers with Superhydrophobic and Enantioselective Property. ACS Nano. 2017, 11, 12453–12460. [Google Scholar] [CrossRef]
- Nandi, N.; Vollhardt, D. Effect of Molecular Chirality on the Morphology of Biomimetic Langmuir Monolayers. Chem. Rev. 2003, 103, 4033–4076. [Google Scholar] [CrossRef]
- Levinthal, C. How to Fold Graciously. In Mossbauer Spectroscopy in Biological Systems: Proceedings of the meeting held at Allerton House, Monticello, IL, USA; DeBrunner, J.T.P., Munck, E., Eds.; University of Illinois: Champaign, IL, USA, 1969; pp. 22–24. [Google Scholar]
- Wächtershäuser, G. From pre-cells to Eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Blumenfeld, L.A. Problems of Biological Physics; Springer: New York, NY, USA, 1981; 222p. [Google Scholar]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; 552p. [Google Scholar]
- Feynman, R. The Feynman Lectures on Physics. Volume I; Basic Books; California Institute of Technology: Pasadena, CA, USA, 2010; 968p. [Google Scholar]
- Gottarelli, G.; Lena, S.; Masiero, S.; Pieraccini, S.; Spada, G.P. The use of circular dichroism spectroscopy for studying the chiral molecular self-assembly: An overview. Chirality 2008, 20, 471–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alù, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.T. Circular dichroism and its use in protein-folding studies. Methods Mol. Biol. 2011, 752, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, M. Chirality and Symmetry Measures: A Transdisciplinary Review. Entropy 2003, 5, 271–312. [Google Scholar] [CrossRef]
- Randić, M.; Razinger, M. Molecular shapes and chirality. J. Chem. Inf. Comput. Sci. 1996, 36, 429–441. [Google Scholar] [CrossRef]
- Yewande, E.O.; Neal, M.P.; Low, R. The Hausdorff chirality measure and a proposed Hausdorff structure measure. Mol. Phys. 2009, 107, 281–291. [Google Scholar] [CrossRef]
- Raos, G. Degrees of chirality in helical structures. Macromol. Theory Simul. 2002, 11, 739–750. [Google Scholar] [CrossRef]
- Dryzun, C.; Zait, A.; Avnir, D. Quantitative symmetry and chirality—A fast computational algorithm for large structures: Proteins, macromolecules, nanotubes, and unit cells. J. Comput. Chem. 2011, 32, 2526–2538. [Google Scholar] [CrossRef]
- Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on earth. J. Theor. Biol. 1966, 11, 495–498. [Google Scholar] [CrossRef]
- Letokhov, V.S. Difference of energy-levels of left and right molecules due to weak interactions. Phys. Lett. A 1975, 53, 275–276. [Google Scholar] [CrossRef]
- Berger, R.; Quack, M.; Tschumper, G.S. Electroweak quantum chemistry for possible precursor molecules in the evolution of biomolecular homochirality. Helv. Chim. Acta 2000, 83, 1919–1950. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tverdislov, V.A.; Malyshko, E.V. Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry 2020, 12, 587. https://doi.org/10.3390/sym12040587
Tverdislov VA, Malyshko EV. Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry. 2020; 12(4):587. https://doi.org/10.3390/sym12040587
Chicago/Turabian StyleTverdislov, Vsevolod A., and Ekaterina V. Malyshko. 2020. "Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology" Symmetry 12, no. 4: 587. https://doi.org/10.3390/sym12040587
APA StyleTverdislov, V. A., & Malyshko, E. V. (2020). Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry, 12(4), 587. https://doi.org/10.3390/sym12040587




