Pyrroloindole-Based Dynamic Combinatorial Chemistry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Building Block Design and Properties
3.1.1. Synthesis
3.1.2. Optoelectronic and structural properties
3.2. Behaviour of l-PI in DCLs
3.2.1. DCLs of l-PI
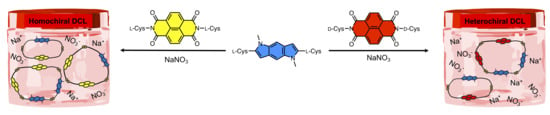
3.2.2. Homochiral DCLs of l-PI and l-NDI
3.2.3. Heterochiral DCLs of l-PI and d-NDI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.-L.; Sanders, J.K.; Otto, S. Dynamic combinatorial chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef]
- Moulin, E.; Cormos, G.; Giuseppone, N. Dynamic combinatorial chemistry as a tool for the design of functional materials and devices. Chem. Soc. Rev. 2012, 41, 1031–1049. [Google Scholar] [CrossRef] [PubMed]
- Buryak, A.; Severin, K. Dynamic combinatorial libraries of dye complexes as sensors. Angew. Chem. Int. Ed. 2005, 44, 7935–7938. [Google Scholar] [CrossRef] [PubMed]
- Carnall, J.M.A.; Waudby, C.A.; Belenguer, A.M.; Stuart, M.C.A.; Yang, X.; Peyralans, J.J.-P.; Otto, S. Mechanosensitive self-replication driven by self-organisation. Science 2010, 327, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Peyralans, J.J.-P.; Colomb-Delsuc, M.; Fanlo-Virgós, H.; Stuart, M.C.A.; Otto, S. Uncovering the selection criteria for the emergence of multi-building-block replicators from dynamic combinatorial libraries. J. Am. Chem. Soc. 2013, 135, 18406–18417. [Google Scholar] [CrossRef]
- Li, J.; Nowak, P.; Otto, S. Dynamic combinatorial libraries: From exploring molecular recognition to systems chemistry. J. Am. Chem. Soc. 2013, 135, 9222–9239. [Google Scholar] [CrossRef]
- Sadownik, J.W.; Mattia, E.; Nowak, P.; Otto, S. Diversification of self-replicating molecules. Nat. Chem. 2016, 8, 264–269. [Google Scholar] [CrossRef]
- James, L.I.; Beaver, J.E.; Rice, N.W.; Waters, M.L. A synthetic receptor for asymmetric dimethyl arginine. J. Am. Chem. Soc. 2013, 135, 6450–6455. [Google Scholar] [CrossRef]
- Mullins, A.G.; Pinkin, N.K.; Hardin, J.A.; Waters, M.L. Achieving High affinity and selectivity for asymmetric Dimethylarginine by putting a lid on a box. Angew. Chem. Int. Ed. 2019, 58, 5282–5285. [Google Scholar] [CrossRef]
- Lam, R.T.S.; Belenguer, A.; Roberts, S.L.; Naumann, C.; Jarrosson, T.; Otto, S.; Sanders, J.K.M. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science 2005, 308, 667–669. [Google Scholar] [CrossRef]
- Bugaut, A.; Jantos, K.; Wietor, J.-L.; Rodriguez, R.; Sanders, J.K.M.; Balasubramanian, S. Exploring the differential recognition of DNA G-Quadruplex targets by small molecules using dynamic combinatorial chemistry. Angew. Chem. Int. Ed. 2008, 47, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Exploring the formation pathways of donor—Acceptor catenanes in aqueous dynamic combinatorial libraries. J. Am. Chem. Soc. 2011, 133, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, M.E.; Luxami, V.; Pantoş, G.D. High-yielding synthesis of chiral donor—Acceptor catenanes. J. Org. Chem. 2018, 83, 11654–11660. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, N.; Cougnon, F.B.L.; Pantoş, G.D.; Sanders, J.K.M. Homochiral and meso figure eight knots and a Solomon link. J. Am. Chem. Soc. 2014, 136, 8243–8251. [Google Scholar] [CrossRef]
- Ponnuswamy, N.; Cougnon, F.B.L.; Clough, J.M.; Pantoş, G.D.; Sanders, J.K.M. Discovery of an organic trefoil knot. Science 2012, 338, 783–785. [Google Scholar] [CrossRef]
- Stefankiewicz, A.R.; Sambrook, M.R.; Sanders, J.K.M. Template-directed synthesis of multi-component organic cages in water. Chem. Sci. 2012, 3, 2326. [Google Scholar] [CrossRef]
- Stefankiewicz, A.R.; Sanders, J.K.M. Diverse topologies in dynamic combinatorial libraries from tri- and mono-thiols in water: Sensitivity to weak supramolecular interactions. Chem. Commun. 2013, 49, 5820. [Google Scholar] [CrossRef]
- Furusho, Y.; Oku, T.; Hasegawa, T.; Tsuboi, A.; Kihara, N.; Takata, T. Dynamic covalent approach to [2]- and [3]Rotaxanes by Utilizing a reversible Thiol–Disulfide interchange reaction. Chem.-Eur. J. 2003, 9, 2895–2903. [Google Scholar] [CrossRef]
- Kassem, S.; Lee, A.T.L.; Leigh, D.A.; Markevicius, A.; Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 2015, 8, 138–143. [Google Scholar] [CrossRef]
- Sun, J.; Patrick, B.O.; Sherman, J.C. A new [4]carceplex, and a crystal structure and dynamic combinatorial chemistry of a [5]carceplex. Tetrahedron 2009, 65, 7296–7302. [Google Scholar] [CrossRef]
- Chichak, K.S.; Cantrill, S.J.; Pease, A.R.; Chiu, S.-H.; Cave, G.W.V.; Atwood, J.L.; Stoddart, J.F. Molecular borromean rings. Science 2004, 304, 1308–1312. [Google Scholar] [CrossRef]
- Meyer, C.D.; Forgan, R.S.; Chichak, K.S.; Peters, A.J.; Tangchaivang, N.; Cave, G.W.V.; Khan, S.I.; Cantrill, S.J.; Stoddart, J.F. The dynamic chemistry of molecular borromean rings and solomon knots. Chem.-Eur. J. 2010, 16, 12570–12581. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, C.D.; Chichak, K.S.; Peters, A.J.; Cave, G.W.V.; Cantrill, S.J.; Stoddart, J.F. A molecular Solomon link. Angew. Chem. Int. Ed. 2007, 46, 218–222. [Google Scholar] [CrossRef]
- Avestro, A.-J.; Gardner, D.M.; Vermeulen, N.A.; Wilson, E.A.; Schneebeli, S.T.; Whalley, A.C.; Belowich, M.E.; Carmieli, R.; Wasielewski, M.R.; Stoddart, J.F. Gated electron sharing within dynamic naphthalene Diimide-based Oligorotaxanes. Angew. Chem. Int. Ed. 2014, 53, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Valente, C.; Stoddart, J.F. Template-directed syntheses of rigid oligorotaxanes under thermodynamic control. Angew. Chem. Int. Ed. 2010, 49, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Bilbeisi, R.A.; Ronson, T.K.; Nitschke, J.R. A self-assembled [Fe II 12 L 12] capsule with an icosahedral framework. Angew. Chem. Int. Ed. 2013, 52, 9027–9030. [Google Scholar] [CrossRef]
- Black, S.P.; Stefankiewicz, A.R.; Smulders, M.M.J.; Sattler, D.; Schalley, C.A.; Nitschke, J.R.; Sanders, J.K.M. Generation of a Dynamic system of three-dimensional tetrahedral Polycatenanes. Angew. Chem. Int. Ed. 2013, 52, 5749–5752. [Google Scholar] [CrossRef]
- Kieffer, M.; Pilgrim, B.S.; Ronson, T.K.; Roberts, D.A.; Aleksanyan, M.; Nitschke, J.R. Perfluorinated ligands induce Meridional metal stereochemistry to generate M8L12, M10L15, and M12L18 prisms. J. Am. Chem. Soc. 2016, 138, 6813–6821. [Google Scholar] [CrossRef]
- Jansze, S.M.; Cecot, G.; Wise, M.D.; Zhurov, K.O.; Ronson, T.K.; Castilla, A.M.; Finelli, A.; Pattison, P.; Solari, E.; Scopelliti, R.; et al. Ligand aspect ratio as a decisive factor for the self-assembly of coordination cages. J. Am. Chem. Soc. 2016, 138, 2046–2054. [Google Scholar] [CrossRef]
- Hasell, T.; Wu, X.; Jones, J.T.A.; Bacsa, J.; Steiner, A.; Mitra, T.; Trewin, A.; Adams, D.J.; Cooper, A.I. Triply interlocked covalent organic cages. Nat. Chem. 2010, 2, 750–755. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef]
- Black, S.P.; Sanders, J.K.M.; Stefankiewicz, A.R. Disulfide exchange: Exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 2014, 43, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Sanders, J.K.M. Evolution of dynamic combinatorial chemistry. Acc. Chem. Res. 2012, 45, 2211–2221. [Google Scholar] [CrossRef]
- Beeren, S.R.; Sanders, J.K.M. History and principles of dynamic combinatorial chemistry. In Dynamic Combinatorial Chemistry; Reek, J.N.H., Otto, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–22. [Google Scholar]
- Christinat, N.; Scopelliti, R.; Severin, K. Boron-based rotaxanes by multicomponent self-assembly. Chem. Commun. 2008, 31, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; James, T.D.; Kubo, Y. Ion pair-driven Heterodimeric capsule based on Boronate esterification: Construction and the dynamic behavior. J. Am. Chem. Soc. 2007, 129, 15126–15127. [Google Scholar] [CrossRef]
- Nishimura, N.; Kobayashi, K. Self-assembly of a cavitand-based capsule by dynamic boronic ester formation. Angew. Chem. Int. Ed. 2008, 47, 6255–6258. [Google Scholar] [CrossRef] [PubMed]
- von Delius, M.; Geertsema, E.M.; Leigh, D.A. A synthetic small molecule that can walk down a track. Nat. Chem. 2010, 2, 96–101. [Google Scholar] [CrossRef] [PubMed]
- von Delius, M.; Geertsema, E.M.; Leigh, D.A.; Tang, D.-T.D. Design, synthesis, and operation of small molecules that walk along tracks. J. Am. Chem. Soc. 2010, 132, 16134–16145. [Google Scholar] [CrossRef] [PubMed]
- Pantoş, G.D.; Wietor, J.L.; Sanders, J.K.M. Filling helical nanotubes with C-60. Angew. Chem. Int. Ed. 2007, 46, 2238–2240. [Google Scholar] [CrossRef]
- Pantoş, G.D.; Pengo, P.; Sanders, J.K.M. Hydrogen-bonded helical organic nanotubes. Angew. Chem. Int. Ed. 2007, 46, 194–197. [Google Scholar] [CrossRef]
- Wietor, J.-L.; Pantoş, G.D.; Sanders, J.K.M. Templated amplification of an unexpected receptor for C70. Angew. Chem. Int. Ed. 2008, 47, 2689–2692. [Google Scholar] [CrossRef]
- Cacciapaglia, R.; Di Stefano, S.; Ercolani, G.; Mandolini, L. Combinatorial macrocyclizations under thermodynamic control: The two-monomer case. Macromolecules 2009, 42, 4077–4083. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Dynamic combinatorial donor−acceptor catenanes in water: Access to unconventional and unexpected structures. J. Org. Chem. 2011, 76, 1257–1268. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Dynamic combinatorial synthesis of a catenane based on donor-acceptor interactions in water. Proc. Natl. Acad. Sci. USA 2009, 106, 10466–10470. [Google Scholar] [CrossRef]
- Fass, D.; Thorpe, C. Chemistry and enzymology of disulfide cross-linking in proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef]
- Bosnjak, I.; Bojovic, V.; Segvic-Bubic, T.; Bielen, A. Occurrence of protein disulfide bonds in different domains of life: A comparison of proteins from the Protein Data Bank. Protein Eng. Des. Sel. 2014, 27, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Ponnuswamy, N.; Jenkins, N.A.; Pantoş, G.D.; Sanders, J.K.M. Structural parameters Governing the dynamic combinatorial synthesis of catenanes in water. J. Am. Chem. Soc. 2012, 134, 19129–19135. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Allouche, A.-R. Gabedit-A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Samsoniya, S.A.; Targamadze, N.L.; Suvorov, N.N. The chemistry of pyrroloindoles. Russ. Chem. Rev. 1994, 63, 815–832. [Google Scholar] [CrossRef]
- Manjal, S.K.; Pathania, S.; Bhatia, R.; Kaur, R.; Kumar, K.; Rawal, R.K. Diversified synthetic strategies for pyrroloindoles: An overview. J. Heterocycl. Chem. 2019, 56, 2318–2332. [Google Scholar] [CrossRef]
- Heaner, W.L., IV; Gelbaum, C.S.; Gelbaum, L.; Pollet, P.; Richman, K.W.; DuBay, W.; Butler, J.D.; Wells, G.; Liotta, C.L. Indoles via Knoevenagel–Hemetsberger reaction sequence. RSC Adv. 2013, 3, 13232–13242. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral properties of substituted coumarins in solution and polymer matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef]
- Gianga, T.-M. Topologically Complex Molecules: Synthesis and Properties. PhD Thesis, University of Bath, Bath, UK, 2020. [Google Scholar]
- Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Efficient and mild microwave-assisted stepwise functionalization of Naphthalenediimide with α-amino acids. J. Org. Chem. 2006, 71, 7063–7066. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Templated amplification of a naphthalenediimide-based receptor from a donor–acceptor dynamic combinatorial library in water. Chem. Commun. 2009, 4, 419–421. [Google Scholar] [CrossRef]








| Absorbance & CD | Emission | |||
|---|---|---|---|---|
| λmax (nm) | ε (L × mol−1 × cm−1) | Molar Ellipticity (deg × cm2 × dmol−1) | λmax (nm) | Φ (%) |
| 327 | 21,600 ± 1.8 | 2.11 × 104 | 394 | 48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianga, T.-M.; Răsădean, D.-M.; Pantoș, G.D. Pyrroloindole-Based Dynamic Combinatorial Chemistry. Symmetry 2020, 12, 726. https://doi.org/10.3390/sym12050726
Gianga T-M, Răsădean D-M, Pantoș GD. Pyrroloindole-Based Dynamic Combinatorial Chemistry. Symmetry. 2020; 12(5):726. https://doi.org/10.3390/sym12050726
Chicago/Turabian StyleGianga, Tiberiu-Marius, Dora-Maria Răsădean, and G. Dan Pantoș. 2020. "Pyrroloindole-Based Dynamic Combinatorial Chemistry" Symmetry 12, no. 5: 726. https://doi.org/10.3390/sym12050726
APA StyleGianga, T.-M., Răsădean, D.-M., & Pantoș, G. D. (2020). Pyrroloindole-Based Dynamic Combinatorial Chemistry. Symmetry, 12(5), 726. https://doi.org/10.3390/sym12050726