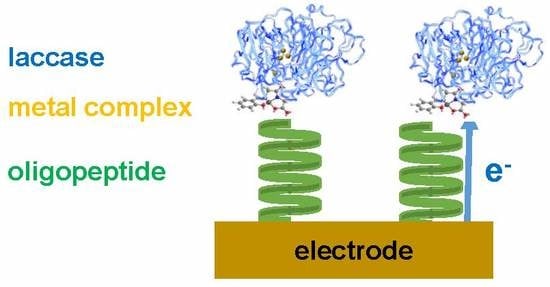
Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
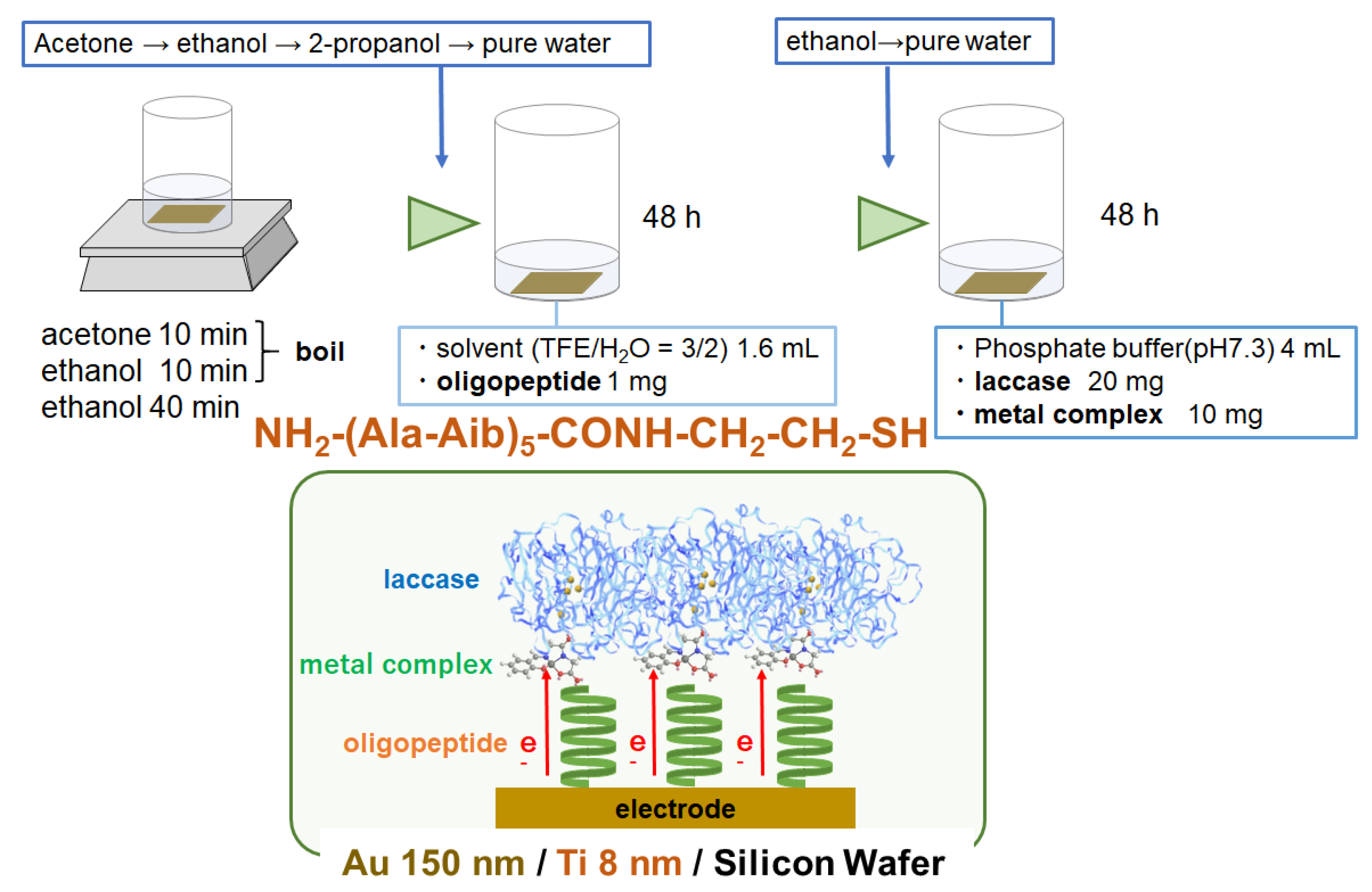
2.2. Preparation
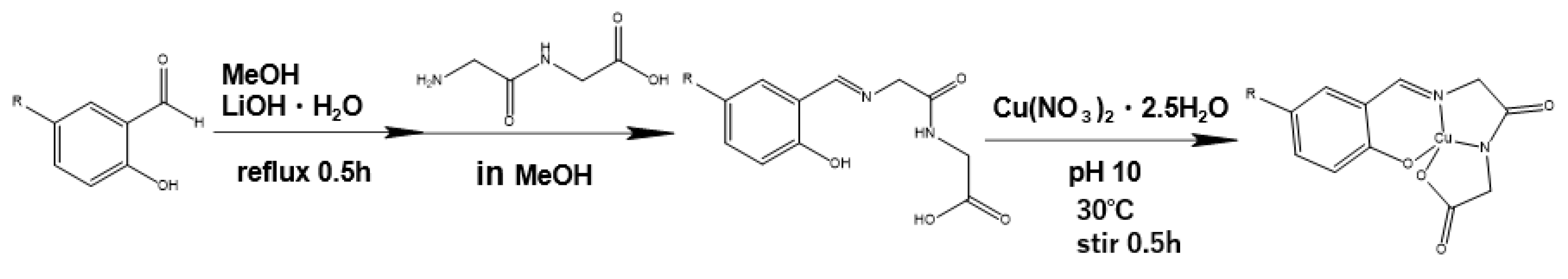
2.2.1. Preparation of 1
2.2.2. Preparation of 2
2.2.3. Electrode Fabrication
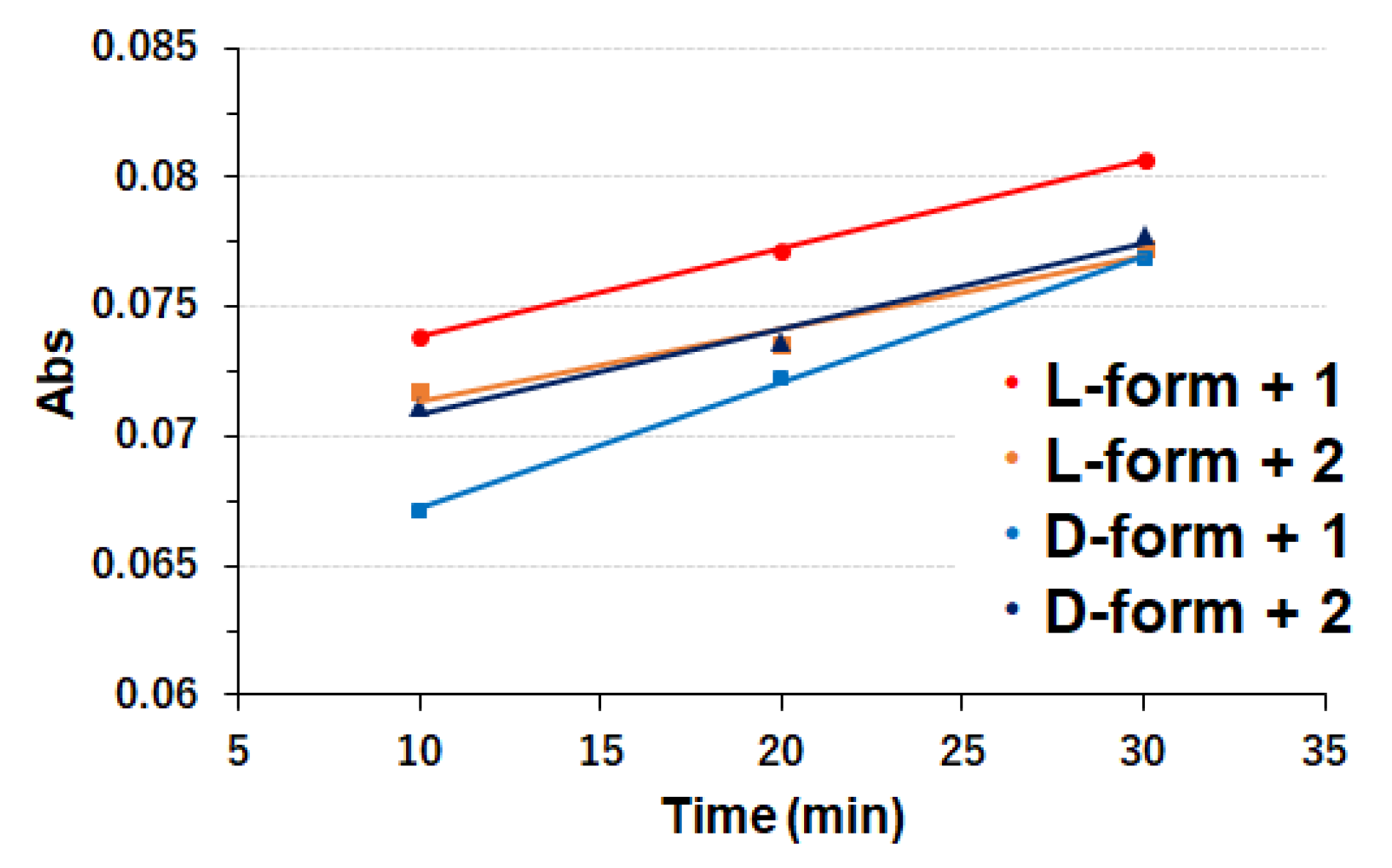
2.2.4. ABTS Assay Procedures
2.3. Physical or Instrumental Measurements
2.4. x-ray Crystal Structure Analysis
2.5. Computational Calculations
3. Results and Discussion
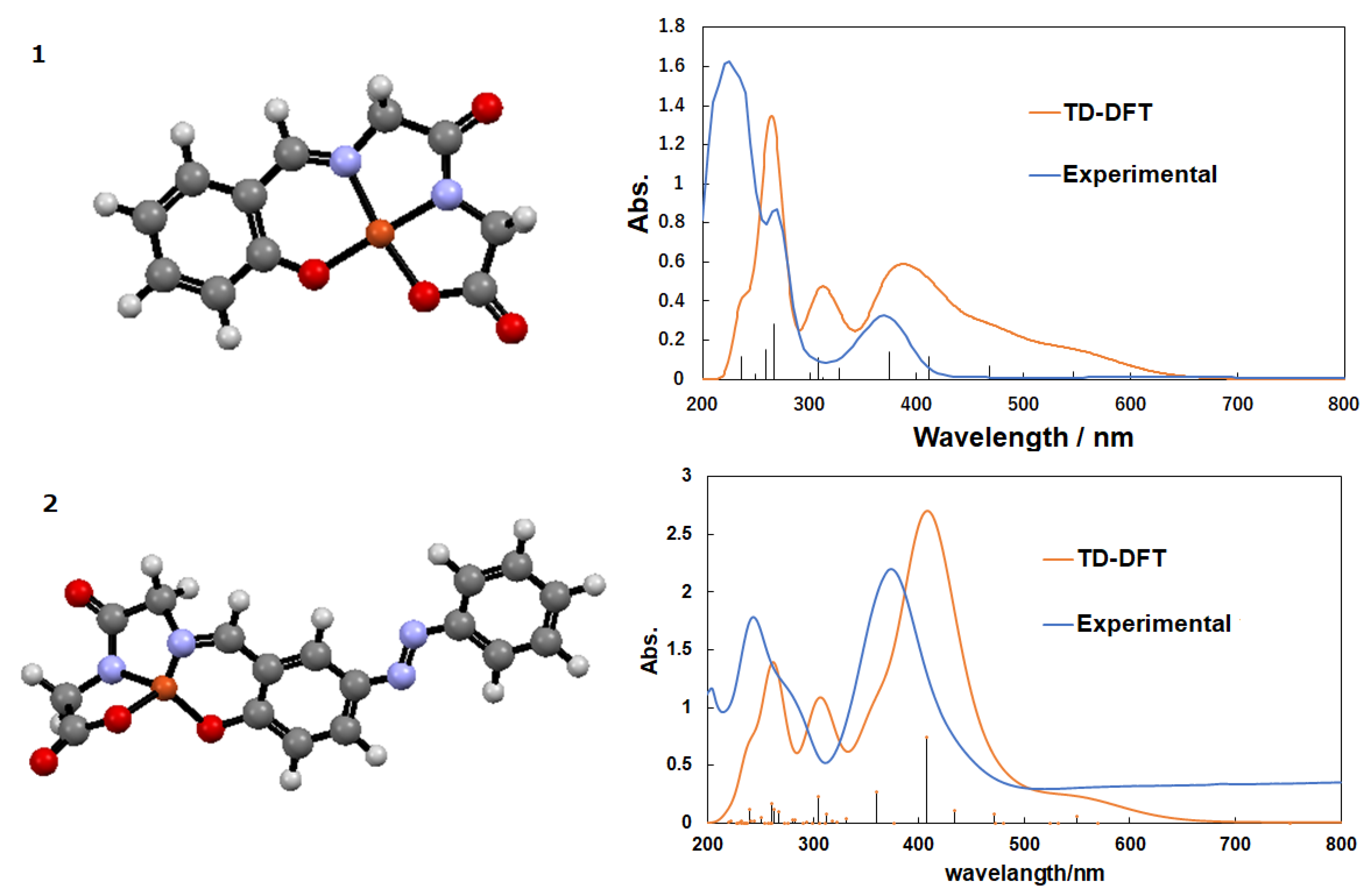
3.1. Copper Complexes
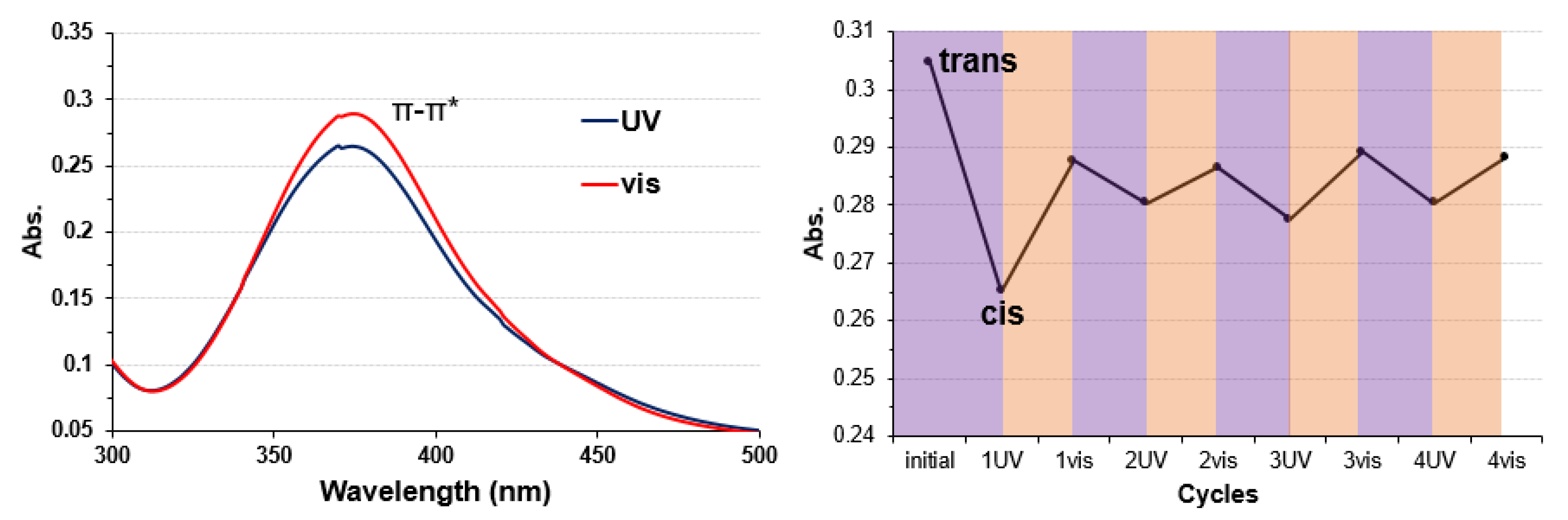
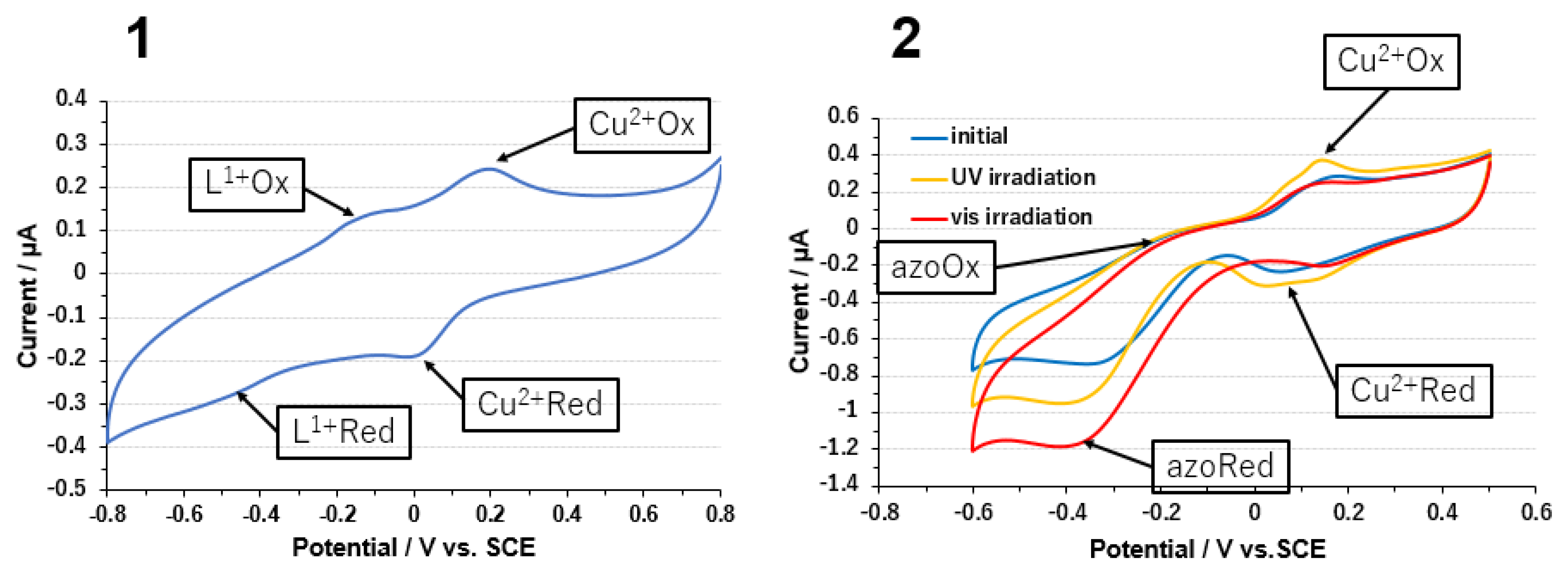
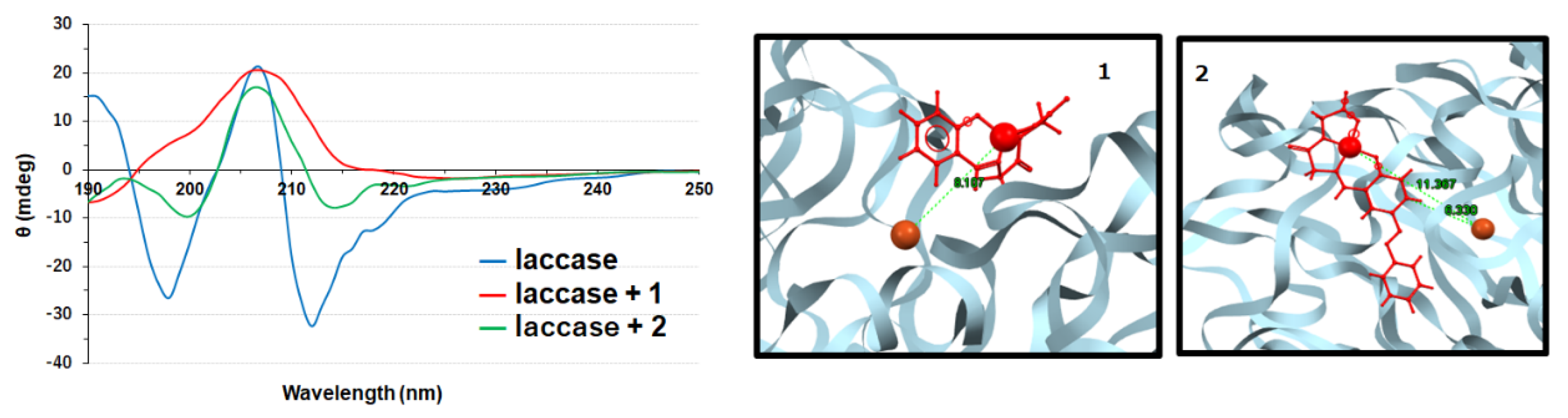
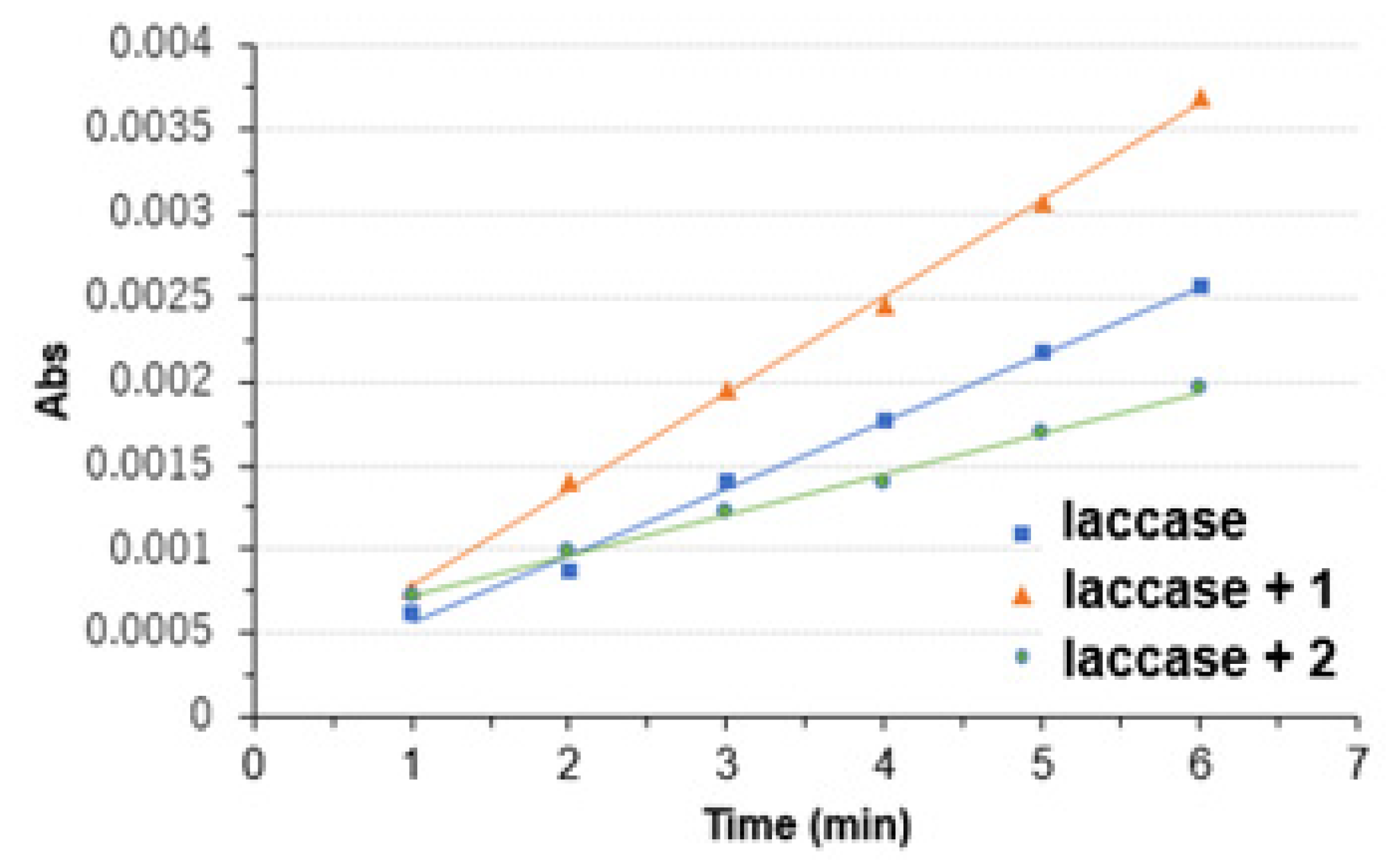
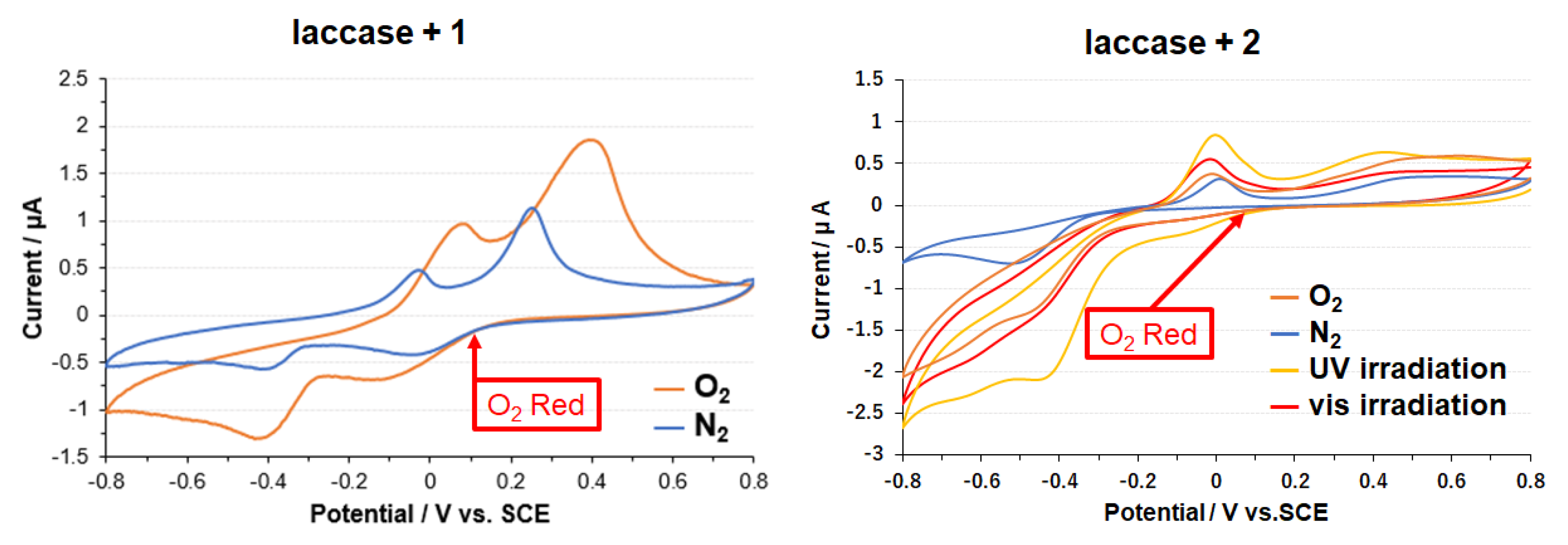
3.2. Copper Complexes and Laccase
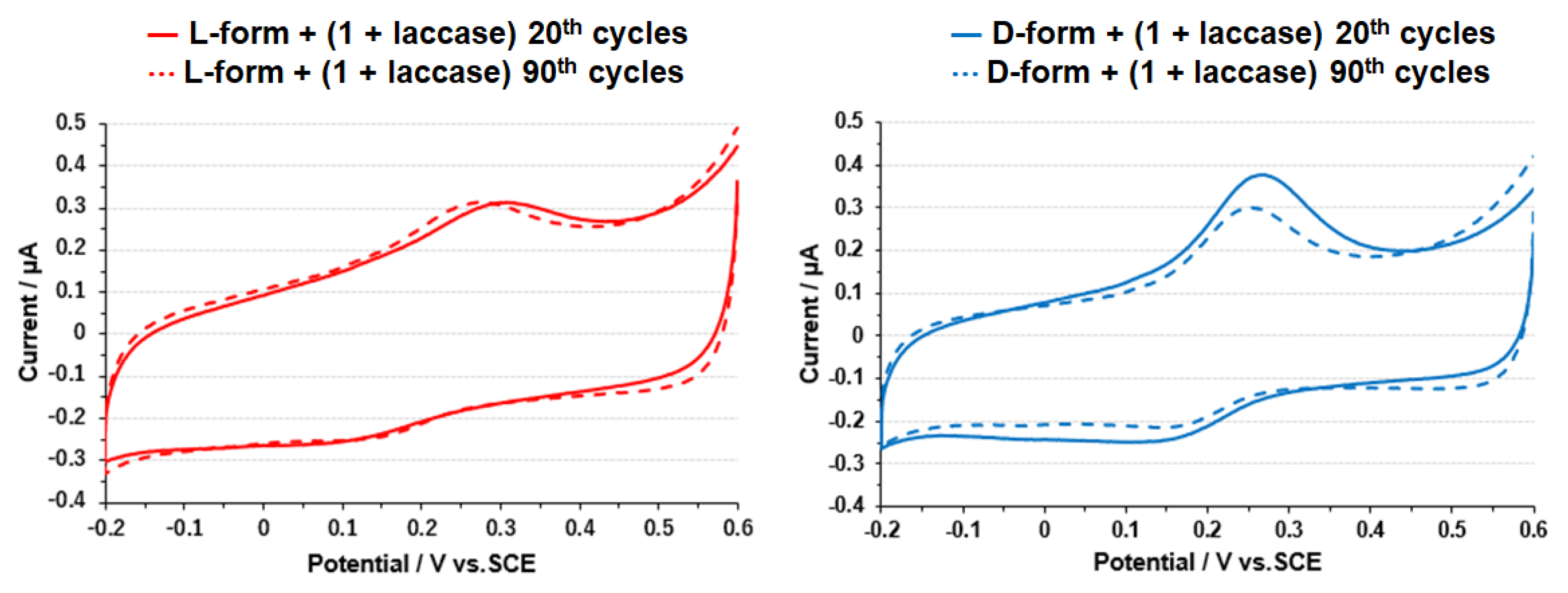
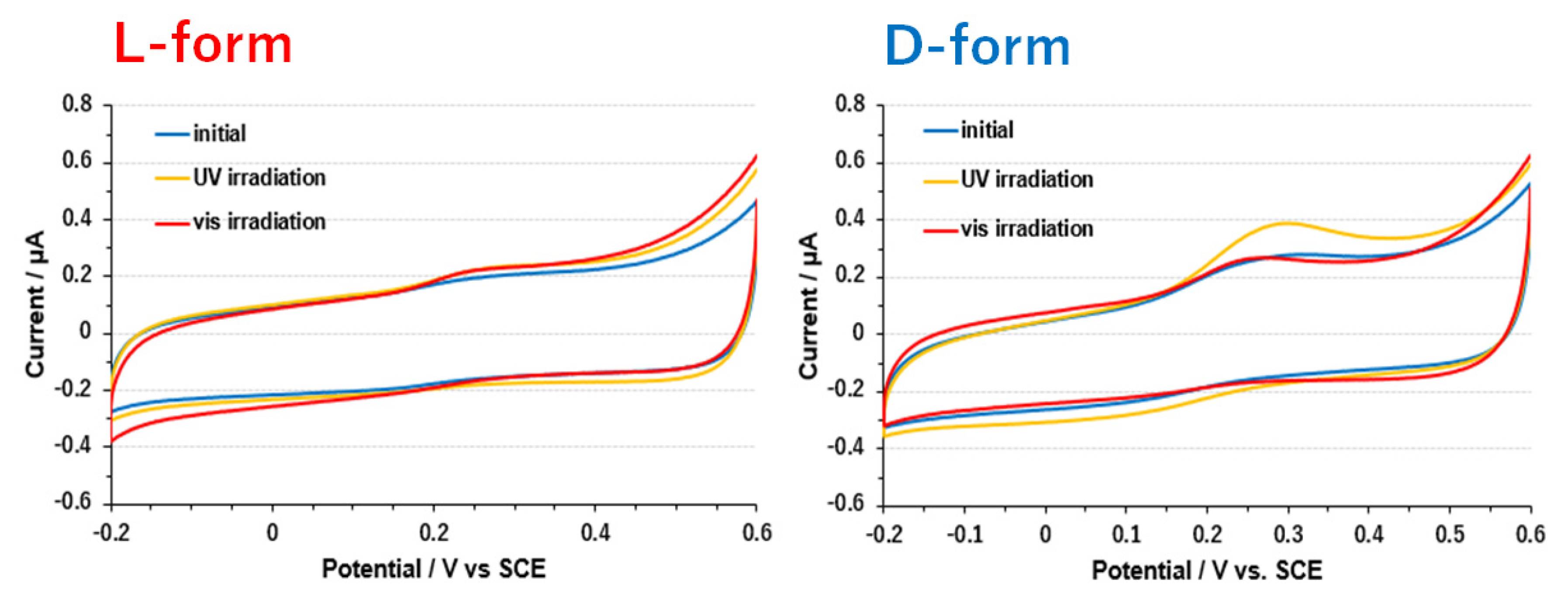
3.3. Electrode on Gold Substrate Composed of Oligopeptide, Copper Complexes, and Laccase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mano, N.; de Poulpiquet, A. O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, A.; Holzinger, M.; Cosnier, S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell. Mol. Life Sci. 2015, 72, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Mita, H.; Sugiyama, T.; Tokita, Y.; Shirai, S.; Kano, K. Construction of a Multi-stacked Sheet-type Enzymatic Biofuel Cell. Electrochemistry 2014, 82, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Solomon, E.I.; Szilagyi, R.K.; DeBeer George, S.; Basumallick, L. Electronic structures of metal sites in proteins and models: Contributions to function in blue copper proteins. Chem. Rev. 2004, 104, 419–458. [Google Scholar] [CrossRef]
- Quintanar, L.; Stoj, C.; Taylor, A.B.; Hart, P.J.; Kosman, D.J.; Solomon, E.I. Shall we dance? how a multicopper oxidase chooses its electron transfer partner. Chem. Rev. 2007, 40, 445–452. [Google Scholar]
- Mehra, R.; Muschiol, J.; Meyer, A.S.; Kepp, K.P. A structural-chemical explanation of fungal laccase activity. Sci. Rep. 2018, 8, 17285. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shimada, A. Reaction Mechanism of Cytochrome c Oxidase. Chem. Rev. 2015, 115, 1936–1989. [Google Scholar] [CrossRef]
- Tsukihara, T.; Shimokata, K.; Katayama, Y.; Shimada, H.; Muramoto, K.; Aoyama, H.; Mochizuki, M.; Shinzawa-Itoh, K.; Yamashita, E.; Yao, M.; et al. The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process. Proc. Natl. Acad. Sci. USA 2003, 100, 15304–15309. [Google Scholar] [CrossRef] [Green Version]
- Kruse, F.; Nguyen, A.D.; Dragelj, J.; Schlesinger, R.; Heberle, J.; Mroginski, M.A.; Weidinger, I.M. Characterization of the Cyanate Inhibited State of Cytochrome c Oxidase. Sci. Rep. 2020, 10, 3863. [Google Scholar] [CrossRef] [PubMed]
- Hitaishi, V.P.; Clément, R.; Quattrocchi, L.; Parent, P.; Duché, D.; Zuily, L.; Ilbert, M.; Lojou, E.; Mazurenko, I. Interplay between Orientation at Electrodes and Copper Activation of Thermus thermophilus Laccase for O2 Reduction. J. Am. Chem. Soc. 2020, 142, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Agbo, P.; Heath, J.R.; Gray, H.B. Modeling Dioxigen Reduction at Multicopper Oxidase Cathodes. J. Am. Chem. Soc. 2014, 136, 13882–13887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateljak, I.; Monza, E.; Lucas, M.F.; Guallar, V.; Aleksejeva, O.; Ludwig, R.; Leech, D.; Shleev, S.; Alcalde, M. Increasing redox potential, redox mediator activity, and stability in a fungal laccase by computer-guided mutagenesis and directed evolution. ACS Catal. 2019, 9, 4561–4572. [Google Scholar] [CrossRef] [Green Version]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Yin, H.; Tnag, Z. Electron highways. Nat. Energy 2018, 3, 543–544. [Google Scholar] [CrossRef]
- Tylkowski, B.; Trojanowska, A.; Marturano, V.; Nowak, M.; Marciniak, L.; Giamberini, M.; Ambrogi, V.; Cerruti, P. Power of light—Functional complexes based on azobenzene molecules. Coord. Chem. Rev. 2017, 351, 205–217. [Google Scholar] [CrossRef]
- Naaman, R.; Paltiel, Y.; Waldeck, D.H. Chiral molecules and the electron spin. Nat. Rev. Chem. 2019, 3, 250–260. [Google Scholar] [CrossRef]
- Kettner, M.; Göhler, B.; Zacharias, H.; Mishra, D.; Kiran, V.; Naaman, R.; Waldeck, D.H.; Sek, S.; Pawlowski, J.; Juhaniewicz, J. Spin Filtering in Electron Transport Through Chiral Oligopeptides. J. Phys. Chem. C 2015, 119, 14542–14547. [Google Scholar] [CrossRef]
- Onami, Y.; Koya, R.; Kawasaki, T.; Aizawa, H.; Nakagame, R.; Miyagawa, Y.; Haraguchi, T.; Akitsu, T.; Tsukiyama, K.; Palafox, M.A. Investigation by DFT methods of the damage of human serum albumin including amino acid derivative schiff base Zn(II) complexes by IR-FEL irradiation. Int. J. Mol. Sci. 2019, 20, 2846. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Yin, F.; Zhou, X.-J.; Chen, J.; Meng, Q.-J. A CuII–GdIII–CuII heterometallic coordination polymer constructed by gadolinium(III) ion and copper(II) Schiff-base building block: Structure and magnetic property. Inorg. Chem. Commun. 2014, 45, 25–29. [Google Scholar] [CrossRef]
- Mitsumoto, Y.; Sunaga, N.; Akitsu, T. Polarized light induced molecular orientation in laccase for chiral azosalen Mn(II), Co(II), Ni(II), Cu(II), Zn(II) mediators toward application for biofuel cell. SciFed J. Chem. Res. 2017, 1, 1. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- GOLD has Proven Success in Virtual Screening, Lead Optimisation, and Identifying the Correct Binding Mode of Active Molecules. Available online: https://www.ccdc.cam.ac.uk/solutions/csd-discovery/Components/Gold/ (accessed on 8 May 2020).
- A Structural View of Biology. Available online: http://www.rcsb.org/ (accessed on 8 May 2020).
- Takeshita, Y.; Takakura, K.; Akitsu, T. Multifunctional composites of chiral valine derivative schiff base Cu(II) complexes and TiO2. Int. J. Mol. Sci. 2015, 16, 3955–3969. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, R.; Tsaturyan, A.; Haraguchi, T.; Pimonova, Y.; Lastovina, T.; Akitsu, T.; Shcherbakov, I. Photochemical reaction of amino acid Schiff base derived Cu complexes with extended π-system and their titanium oxide composites. Inorg. Chim. Acta 2019, 486, 221–231. [Google Scholar] [CrossRef]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Akitsu, T. Photofunctional supramolecular solution systems of chiral Schiff base nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes. Polyhedron 2007, 26, 2527–2535. [Google Scholar] [CrossRef]
- Sano, A.; Yagi, S.; Haraguchi, T.; Akitsu, T. Synthesis of Mn (II) and, Cu (II) complexes including azobenzene and its application to mediators of laccase for biofuel cells. J. Indian Chem. Soc. 2018, 95, 487–494. [Google Scholar]
- Kunitake, F.; Kim, J.-Y.; Yagi, S.; Yamazaki, S.; Haraguchi, T.; Akitsu, T. Chiral recognition of azo-Schiff base ligands, their Cu(II) complexes and their docking to laccase as mediators. Symmetry 2019, 11, 666. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, Y.; Tsuda, E.; Takase, M.; Yoshida, N.; Takeuchi, Y.; Mitsumoto, Y.; Akitsu, T. Spectroscopic and electrochemical studies on metalloprotein (laccase) and Cu(II) complex mediators as model systems for biofuel cell cathodes. In Threonine: Food Sources, Functions and Health Benefits; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; Chapter 4; pp. 73–86. [Google Scholar]
- Takeuchi, Y.; Akitsu, T. Anthraquinone derivative chiral schiff base Copper(II) complexes for enzyme type bio-fuel cell mediators. J. Electr. Eng. 2016, 4, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Sunaga, N.; Akitsu, T. Anthraquinone and L-amino acid derivatives schiff base Cu(II) complexes as a mediator between cathode of biofuel cell and oxygen-reducing laccase. Trend Green Chem. 2017, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-Y.; Liao, C.-I.; Lin, S.-F. Borate-fructose complex: A novel mediator for laccase and its new function for fructose determination. FEBS Lett. 2015, 589, 3107–3112. [Google Scholar] [CrossRef] [Green Version]
- Kominato, C.; Akitsu, T. Photoinduced molecular orientation of catalytic-like chiral azo-Schiff base complexes in PMMA or laccase matrices. Lett. Appl. NanoBioSci 2015, 2, 264–270. [Google Scholar]
- Kiran, V.; Cohen, S.R.; Naaman, R. Structure dependent spin selectivity in electron transport through oligopeptides. J. Chem. Phys. 2016, 146, 092302. [Google Scholar] [CrossRef]
- Mondal, P.C.; Fontanesi, C.; Waldeck, D.H.; Naaman, R. Spin-dependent transport through chiral molecules studied by spin-dependent electrochemistry. Acc. Chem. Res. 2016, 49, 2560–2568. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Chiral-Induced Spin Selectivity Effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kantor-Uriel, N.; Mathew, S.P.; Guliamov, R.; Naaman, R. A device for measuring spin selectivity in electron transfer. Phys. Chem. Chem. Phys. 2013, 15, 18357–18362. [Google Scholar] [CrossRef]
- Michaeli, K.; Naaman, R. Origin of spin dependent tunneling through chiral molecules. arXiv 2016, arXiv:1512.03435v2. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; van der Wal, C.H.; van Wees, B.J. Spin-dependent electron transmission model for chiral molecules in mesoscopic devices. Phys. Rev. B 2019, 99, 024418. [Google Scholar] [CrossRef] [Green Version]
- Göhler, B.; Hamelbeck, V.; Markus, T.Z.; Kettner, M.; Hanne, G.F.; Vager, Z.; Naaman, R.; Zacharias, H. Spin selectivity in electron transmission through self-assembled monolayers of double-stranded DNA. Science 2011, 331, 894–897. [Google Scholar] [CrossRef]
- Kumar, A.; Capua, E.; Kesharwani, M.K.; Martin, J.M.L.; Sitbon, E.; Waldeck, D.H.; Naaman, R. Chirality-induced spin polarization places symmetry constrains on bimolecular interaction. Proc. Natl. Acad. Sci. USA 2017, 114, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Shinzawa-Itoh, K.; Baba, J.; Aoe, S.; Shimada, A.; Yamashita, E.; Kang, J.; Tateno, M.; Yoshikawa, S.; Tsukihara, T. Complex structure of cytochrome c–cytochrome c oxidase reveals a novel protein–protein interaction mode. EMBO J. 2017, 36, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Eckshtain-Levi, M.; Capua, E.; Refaely-Abramson, S.; Sarkar, S.; Gavrilov, Y.; Mathew, S.P.; Paltiel, Y.; Levy, Y.; Kronik, L.; Naaman, R. Cold denaturation induces inversion of dipole and spin transfer in chiral peptide monolayers. Nat. Commun. 2016, 7, 10744. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Minteer, S.D. Direct enzymatic bioelectrocatalysis: Differentiating between myth and reality. J. R. Soc. Interface 2017, 14, 20170253. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashiwagi, K.; Tassinari, F.; Haraguchi, T.; Banerjee-Gosh, K.; Akitsu, T.; Naaman, R. Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators. Symmetry 2020, 12, 808. https://doi.org/10.3390/sym12050808
Kashiwagi K, Tassinari F, Haraguchi T, Banerjee-Gosh K, Akitsu T, Naaman R. Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators. Symmetry. 2020; 12(5):808. https://doi.org/10.3390/sym12050808
Chicago/Turabian StyleKashiwagi, Kumpei, Francesco Tassinari, Tomoyuki Haraguchi, Koyel Banerjee-Gosh, Takashiro Akitsu, and Ron Naaman. 2020. "Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators" Symmetry 12, no. 5: 808. https://doi.org/10.3390/sym12050808
APA StyleKashiwagi, K., Tassinari, F., Haraguchi, T., Banerjee-Gosh, K., Akitsu, T., & Naaman, R. (2020). Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators. Symmetry, 12(5), 808. https://doi.org/10.3390/sym12050808