X-ray Crystal Structure and Hirshfeld Analysis of Gem-Aminals-Based Morpholine, Pyrrolidine, and Piperidine Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 1,2-Dimorpholinoethane 1 and 1-Morpholino-3-Morpholinium Bromide Propane 2
2.2. X-ray Structure Determinations of 1 and 2
3. Results
3.1. Chemistry
3.2. X-ray Structure Description of 1 and 2
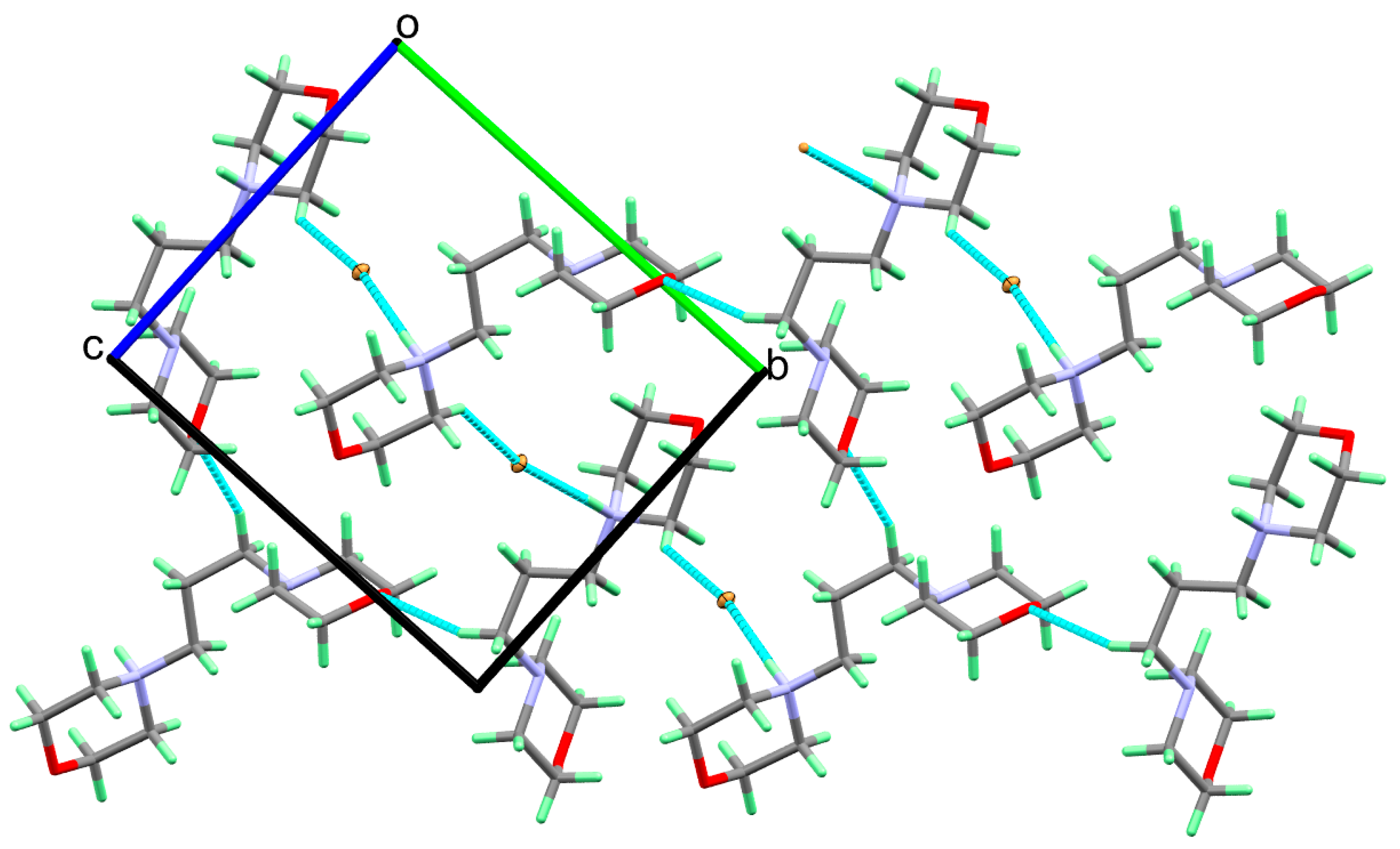
3.3. Analysis of Molecular Packing
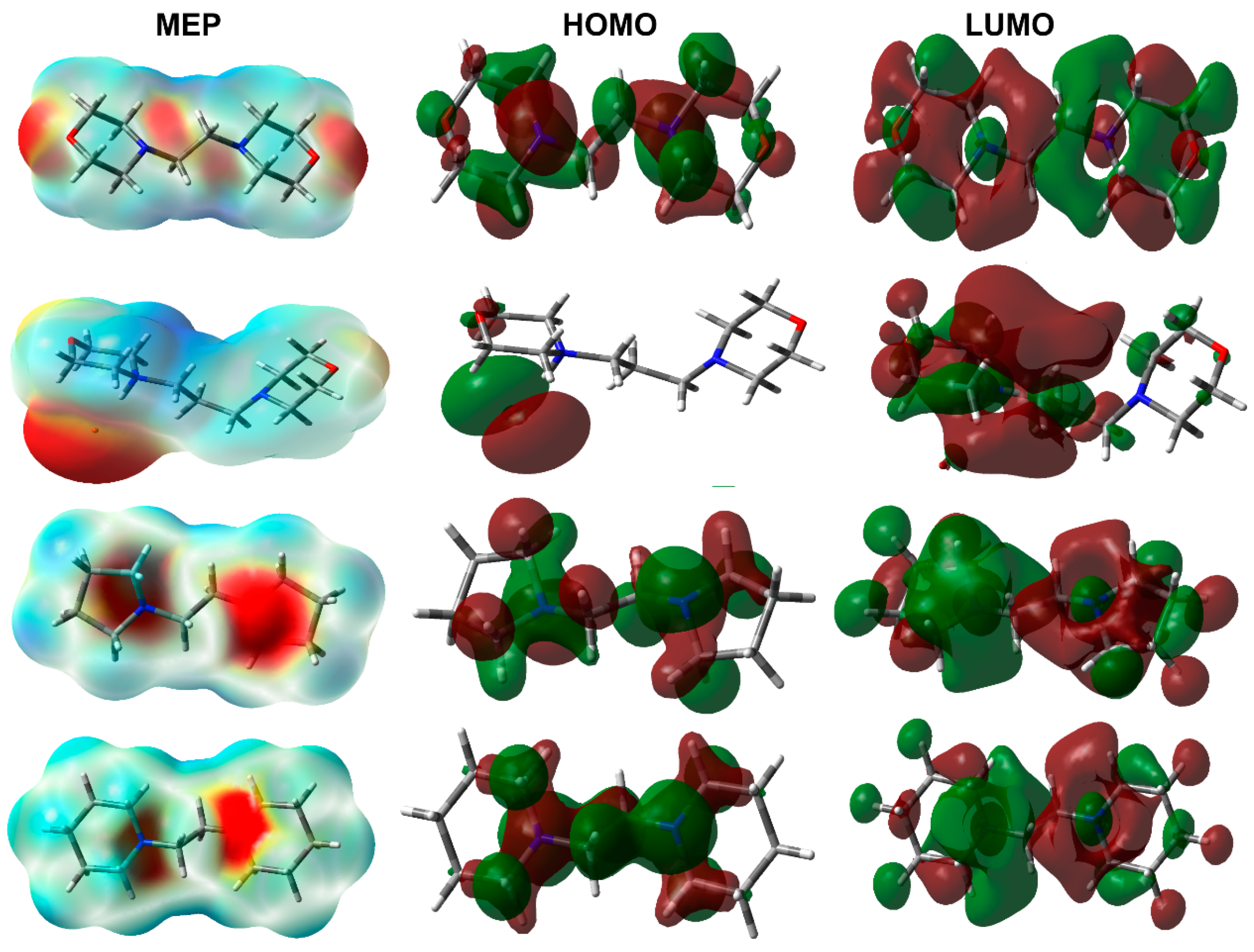
3.4. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Kaur, S.; Sapehiyia, V.; Singh, J.; Kad, G.L. Microwave accelerated preparation of [bmim][HSO4] ionic liquid: An acid catalyst for improved synthesis of coumarins. Catal. Commun. 2005, 6, 57–60. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, X.; Liu, Z.; Meng, J.; Xu, C. Isomerization of n-pentane catalyzed by acidic chloroaluminate ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 8205–8210. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F.; Ruoho, A.E. Oxidation of benzylic alcohols to their corresponding carbonyl compounds using KIO4 in ionic liquid by microwave irradiation. Synth. Commun. 2006, 36, 2563–2568. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F.; Ruoho, A.E. Facile and selective oxidation of benzylic alcohols to their corresponding carbonyl compounds with sodium nitrate in the presence of Brønsted acidic ionic liquids. Synlett 2007, 2007, 1118–1120. [Google Scholar] [CrossRef]
- Bastrzyk, A.; Feder-Kubis, J. Pyrrolidinium and morpholinium ionic liquids as a novel effective destabilising agent of mineral suspension. Colloids Surf. A 2018, 557, 58–65. [Google Scholar] [CrossRef]
- Dutta, A.; Damarla, K.; Kumar, A.; Saikia, P.J.; Sarma, D. Gemini basic ionic liquid as bi-functional catalyst for the synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones at room temperature. Tetrahedron Lett. 2020, 61, 151587. [Google Scholar] [CrossRef]
- Daniele, S.; Hubert-Pfalzgraf, L.G.; Bavoux, C. Calcium tetramethylheptanedionate adducts with N-donor ligands. Molecular structure of a dimeric and volatile adduct Ca2 (η2-thd)(μ, η2-thd) 3 (η2-bipy). Polyhedron 2001, 20, 1065–1070. [Google Scholar] [CrossRef]
- Awalt, J.K.; Lam, R.; Kellam, B.; Graham, B.; Scammells, P.J.; Singer, R.D. Utility of iron nanoparticles and a solution-phase iron species for the N-demethylation of alkaloids. Green Chem. 2017, 19, 2587–2594. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Wang, L. Silver-catalyzed amidation of benzoylformic acids with tertiary amines via selective carbon–nitrogen bond cleavage. Org. Biomol. Chem. 2013, 11, 3649–3654. [Google Scholar] [CrossRef]
- Yu, H.; Gao, B.; Hu, B.; Huang, H. Charge-transfer complex promoted C–N bond activation for Ni-catalyzed carbonylation. Org. Lett. 2017, 19, 3520–3523. [Google Scholar] [CrossRef]
- Gao, B.; Huang, H. Palladium-catalyzed hydroaminocarbonylation of alkynes with tertiary amines via C–N bond cleavage. Org. Lett. 2017, 19, 6260–6263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, J.; Wu, H.; Jiao, N. Rh-catalyzed aerobic oxidative cyclization of anilines, alkynes, and CO. Chem. Sci. 2017, 8, 6266–6273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Xu, Q.S.; Shi, C.Z.; Du, Z.; Xiao, C. Charge-Transfer Complex Promoted Regiospecific C−N Bond Cleavage of Vicinal Tertiary Diamines. Adv. Synth. Catal. 2018, 360, 3502–3506. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of Compounds That Cross the Blood-Brain Barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayol-Llinàs, J.; Nelson, A.; Farnaby, W.; Ayscough, A. Assessing Molecular Scaffolds for CNS Drug Discovery. Drug Discov. Today 2017, 22, 965–969. [Google Scholar]
- Yu, L.F.; Zhang, H.K.; Caldarone, B.J.; Eaton, J.B.; Lukas, R.J.; Kozikowski, A.P. Recent Developments in Novel Antidepressants Targeting α4β2-Nicotinic Acetylcholine Receptors. J. Med. Chem. 2014, 57, 8204–8223. [Google Scholar] [CrossRef]
- Hocine, S.; Berger, G.; Hanessian, S. Design and Synthesis of Backbone-Fused, Conformationally Constrained Morpholine-Proline Chimeras. J. Org. Chem. 2020, 85, 4237–4247. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17. University of Western Australia. 2017. Available online: http://hirshfeldsurface.net (accessed on 12 June 2017).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R., II; Keith, T.; Millam, J. (Eds.) GaussView; Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology, Volume 276, Macromolecular Crystallography, Part A; Carter, C.W., Sweet, J., Eds.; Academic Press: New York, NY, USA, 1997; pp. 307–326. [Google Scholar]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Yarnton, UK, 2013. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Acta Cryst. 2015, C71, 3–8.
- Talybov, A.; Mamedbeyli, E.; Abbasov, V.; Abdullayev, Y.; Kochetkov, K. Synthesis of Substituted N-Alkylamines in Aqueous Media. Green Sustain. Chem. 2013, 3, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Katritzky, A.R.; Fan, W.; Fu, C. The chemistry of benzotriazole. A novel method for the synthesis of symmetrical vicinal tertiary and secondary diamines. J. Org. Chem. 1990, 55, 3209–3213. [Google Scholar] [CrossRef]
- Markosyan, A.J.; Baghdasaryan, G.A.; Oganesyan, G.P.; Attaryan, A.O.; Asratyan, G.V. Phase-Transfer Catalyzed Alkylation of Morpholine with 1,2-Dichloroethane. Russ. J. Gen. Chem. 2012, 82, 344–345. [Google Scholar] [CrossRef]
- Lennartson, A.; Hakansson, M. CCDC 840299: Experimental Crystal Structure Determination 2011. CCDC 2011, 840299. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, Æ. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]













| 1 | 2 | |
|---|---|---|
| empirical formula | C10H20N2O2 | C11H23BrN2O2 |
| fw | 200.28 | 295.22 |
| temp (K) | 170(2) | 120(2) |
| λ (Å) | 0.71073 | 1.54184 |
| cryst syst | Monoclinic | Monoclinic |
| space group | P21/n | P21 |
| a (Å) | 6.0430(3) | 6.37450(10) |
| b (Å) | 8.0805(3) | 11.1378(2) |
| c (Å) | 11.1700(4) | 9.6549(2) |
| β (deg) | 97.475(2) | 93.358(2) |
| V (Å3) | 540.80(4) | 684.30(2) |
| Z | 2 | 2 |
| ρcalc (Mg/m3) | 1.230 | 1.433 |
| μ (Mo Kα) (mm−1) | 0.086 | 4.021 |
| No. reflns. | 12461 | 14253 |
| Unique reflns. | 1592 | 2811 |
| GOOF (F2) | 1.074 | 1.054 |
| Rint | 0.0310 | 0.0266 |
| R1 a (I ≥ 2σ) | 0.0419 | 0.0195 |
| wR2 b (I ≥ 2σ) | 0.1096 | 0.0491 |
| CCDC | 2,022,360 | 2,039,255 |
| Atoms | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| N2-H2...Br1 | 0.92(3) | 2.29(3) | 3.205(2) | 178(2) |
| C5-H5B…O1 | 0.99 | 2.52 | 3.457(3) | 158 |
| C11-H11B...Br1 | 0.99 | 2.75 | 3.580(3) | 142 |
| Parameter | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| I | 5.889 | 5.422 | 5.566 | 5.594 |
| A | −2.323 | −1.361 | −2.305 | −2.337 |
| η | 8.212 | 6.783 | 7.872 | 7.931 |
| μ | −1.783 | −2.031 | −1.630 | −1.629 |
| ω | 0.194 | 0.304 | 0.169 | 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Majid, A.M.; Haukka, M.; Soliman, S.M.; Alamary, A.S.; Alshahrani, S.; Ali, M.; Islam, M.S.; Barakat, A. X-ray Crystal Structure and Hirshfeld Analysis of Gem-Aminals-Based Morpholine, Pyrrolidine, and Piperidine Moieties. Symmetry 2021, 13, 20. https://doi.org/10.3390/sym13010020
Al-Majid AM, Haukka M, Soliman SM, Alamary AS, Alshahrani S, Ali M, Islam MS, Barakat A. X-ray Crystal Structure and Hirshfeld Analysis of Gem-Aminals-Based Morpholine, Pyrrolidine, and Piperidine Moieties. Symmetry. 2021; 13(1):20. https://doi.org/10.3390/sym13010020
Chicago/Turabian StyleAl-Majid, Abdullah Mohammed, Matti Haukka, Saied M. Soliman, Abdullah Saleh Alamary, Saeed Alshahrani, M. Ali, Mohammad Shahidul Islam, and Assem Barakat. 2021. "X-ray Crystal Structure and Hirshfeld Analysis of Gem-Aminals-Based Morpholine, Pyrrolidine, and Piperidine Moieties" Symmetry 13, no. 1: 20. https://doi.org/10.3390/sym13010020
APA StyleAl-Majid, A. M., Haukka, M., Soliman, S. M., Alamary, A. S., Alshahrani, S., Ali, M., Islam, M. S., & Barakat, A. (2021). X-ray Crystal Structure and Hirshfeld Analysis of Gem-Aminals-Based Morpholine, Pyrrolidine, and Piperidine Moieties. Symmetry, 13(1), 20. https://doi.org/10.3390/sym13010020








