Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of 4-[(4-(n-Alkoxy)phenylimino)methyl] Benzoic Acid (Im & In)
2.2. Formation of SM H-Bonded Complexes (Im/In)
3. Results and Discussion
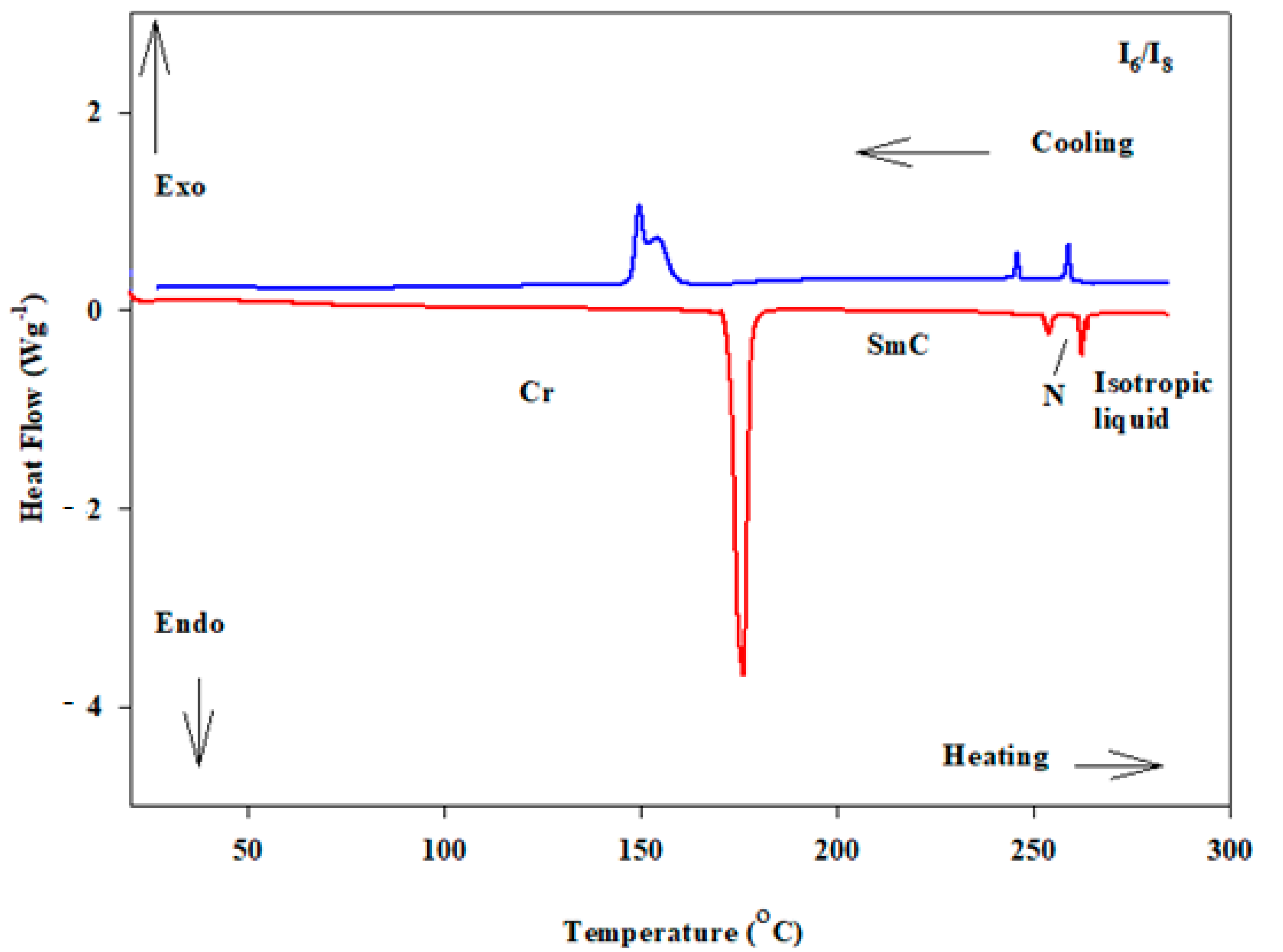
3.1. Effect of Total Terminal Chain Length on Mesomorphic Behaviours of 1:1 Molar Ratios of SMCs
3.2. Effect of Mesogenic Cores on the Mesophase Stability of SMCs

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demus, D.; Goodby, J.W.; Gray, G.W.; Spiess, H.W.; Vill, V. Handbook of Liquid Crystals, Volume 2A: Low Molecular Weight Liquid Crystals I: Calamitic Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kato, T.; Fréchet, J.M.J. Hydrogen bonding and the self-assembly of supramolecular liquid-crystalline materials. Macromol. Symp. 1995, 98, 311–326. [Google Scholar] [CrossRef]
- Kato, T.; Hirota, N.; Fujishima, A.; Fréchet, J.M. Supramolecular hydrogen-bonded liquid–crystalline polymer complexes. Design of side-chain polymers and a host–guest system by noncovalent interaction. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 57–62. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular chemistry—Molecular information and the design of supramolecular materials. Makromol. Chemie. Macromol. Symp. 1993, 69, 1–17. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Thermotropic Liquid Crystals Formed by Intermolecular Hydrogen Bonding Interactions. Angew. Chem. Int. Ed. 1995, 34, 1696–1711. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, H.A.; Shaban, M.; Abolibda, T.Z.; Alharbi, K.A.; Alalawy, H.H. New nematogenic conical-shaped supramolecular H-bonded complexes for solar energy investigations. Sci. Rep. 2021, 11, 17622. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zou, Y.; Wu, J.; Zhou, Y.; Yi, T.; Li, F.; Huang, C. Hydrogen bonding assisted switchable fluorescence in self-assembled complexes containing diarylethene: Controllable fluorescent emission in the solid state. J. Mater. Chem. 2007, 17, 2483–2489. [Google Scholar] [CrossRef]
- Kohmoto, S.; Someya, Y.; Kishikawa, K. Liquid crystalline molecules with hydrogen-bonding networks in the direction of molecular short axes. Liq. Cryst. 2010, 37, 209–216. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Naoum, M.M.; Mostafa, A.M.; Alserehi, A.A. Induced Smectic Phases from Supramolecular H-Bonded Complexes Based on Non-Mesomorphic Components. Crystals 2021, 11, 940. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Naoum, M. Mesophase behavior of binary and ternary mixtures of benzoic acids bearing terminal substituents of different polarity and chain-lengths. Thermochim. Acta 2014, 575, 122–128. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Tsuji, H. Supramolecular hydrogen-bonded liquid crystals based on 4-n-alkylthiobenzoic acids and 4,4′-bipyridine: Their mesomorphic behavior with comparative study including alkyl and alkoxy counterparts. J. Mol. Liq. 2019, 280, 153–159. [Google Scholar] [CrossRef]
- Vasanthi, T.; Subhasri, P.; Jayaprakasam, R.; Vijayakumar, V.N. Experimental and computational studies on induced thermochromic effect and re-entrant smectic phase in linear double hydrogen-bonded binary liquid crystal mixtures. Phase Transit. 2019, 92, 229–248. [Google Scholar] [CrossRef]
- Kishor, M.H.; Mohan, M.M. Investigations on smectic X* and re-entrant smectic C* orderings in hydrogen bonded ferroelectric liquid crystals. J. Mol. Liq. 2019, 273, 504–524. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New Nitro-Laterally Substituted Azomethine Derivatives; Synthesis, Mesomorphic and Computational Characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.; Alshabanah, L.; Ahmed, H.; Alalawy, H.; Al Alwani, M. Synthesis, Mesomorphic and Computational Characterizations of Nematogenic Schiff Base Derivatives in Pure and Mixed State. Molecules 2021, 26, 2038. [Google Scholar] [CrossRef] [PubMed]
- Weissflog, W.; Lischka, C.; Diele, S.; Pelzl, G.; Wirth, I.; Grande, S.; Kresse, H.; Schmalfuss, H.; Hartung, H.; Stettler, A. Banana-Shaped or Rod-Like Mesogens? Molecular Structure, Crystal Structure and Mesophase Behaviour of 4,6-Dichloro-1,3-Phenylene Bis[4-(4-n-Subst.-Phenyliminomethyl) Benzoates]. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1999, 333, 203–235. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Lu, Z.; Henderson, P.A.; Picken, S.J.; Norder, B.; Imrie, C.T.; Ribes-Greus, A. Synthesis and characterisation of side chain liquid crystal copolymers containing sulfonic acid groups. Polymers 2012, 53, 2604–2612. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry-scope and prespectives, molecules, supermolecules and molecular devices. Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Constantinos, M.P.; Dimitris, T. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar]
- Thote, A.J.; Gupta, R.B. Hydrogen-Bonding Effects in Liquid Crystals for Application to LCDs. Ind. Eng. Chem. Res. 2003, 42, 1129–1136. [Google Scholar] [CrossRef]
- Gabard, J.; Lehn, J.-M.; Stibor, I. Macroscopic expression of molecular recognition. Supramolecular liquid crystalline phases induced by association of complementary heterocyclic components. J. Chem. Soc., Chem. Commun. 1989, 24, 1868–1870. [Google Scholar] [CrossRef]
- Bradfield, A.E.; Jones, B.J. Two apparent cases of liquid crystal formation. J. Chem. Soc. 1929, 1, 2660–2661. [Google Scholar] [CrossRef]
- Sastry, S.S.; Sarada, K.L.; Mallika, K.; Rao, C.N.; Lakhminarayana, S.; Tiong, H.S. Eigen value analysis studies on hydrogen-bonded mesogens. Liq. Cryst. 2014, 41, 1483–1494. [Google Scholar] [CrossRef]
- Miranda, M.D.; Chavez, F.V.; Maria, T.M.; Eusébio, M.E.S.; Sebastião, P.; Silva, M. Self-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: Solvent-free synthesis by mechanochemistry. Liq. Cryst. 2014, 41, 1743–1751. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Choudhury, B. Study of binary mixtures of two liquid crystallinesamples showing induced smectic phase. Assam Univ. J. Sci. Technol. Phys. Sci. Technol. 2010, 5, 167–168. [Google Scholar]
- Lohar, J.M. A study of mixed liquid crystal formation in mix-ture of p-methoxy and p-ethoxy benzoic acids. J. Phys. 1975, 36, 399–401. [Google Scholar]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic Press: London, UK, 1962; pp. 239–298. [Google Scholar]
- Yeap, G.-Y.; Hng, T.-C.; Yeap, S.-Y.; Górecka, E.; Ito, M.M.; Ueno, K.; Okamoto, M.; Mahmood, W.; Imrie, C. Why do nonsymmetric dimers intercalate? The synthesis and characterization of the α-(4-benzylidene-substituted-aniline-4′-oxy)-ω-(2-methylbutyl-4′-(4 ″-phenyl) benzoateoxy)alkanes. Liq. Cryst. 2009, 36, 1431–1441. [Google Scholar] [CrossRef]
- Attard, G.; Date, R.; Imrie, C.T.; Luckhurst, G.R.; Roskilly, S.J.; Seddon, J.; Taylor, L. Non-symmetric dimeric liquid crystals the preparation and properties of the α-(4-cyanobiphenyl-4′-yloxy)-ω-(4-n-alkylanilinebenzylidene-4′-oxy) alkanes. Liq. Cryst. 1994, 16, 529–581. [Google Scholar] [CrossRef]
- Lizu, M.; Lutfor, M.R.; Surugau, N.L.; How, S.-E.; Arshad, S.E. Synthesis and Characterization of Ethyl Cellulose–Based Liquid Crystals Containing Azobenzene Chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Date, R.W.; Imrie, C.T.; Luckhurst, G.R.; Seddon, J. Smectogenic dimeric liquid crystals. The preparation and properties of the α,ω-bis(4-n-alkylanilinebenzylidine-4′-oxy)alkanes. Liq. Cryst. 1992, 12, 203–238. [Google Scholar] [CrossRef]
- Lee, H.-C.; Lu, Z.; Henderson, P.A.; Achard, M.F.; Mahmood, W.A.K.; Yeap, G.Y.; Imrie, C.T. Cholesteryl-based liquid crystal dimers containing a sulfur–sulfur link in the flexible spacer. Liq. Cryst. 2012, 39, 259–268. [Google Scholar] [CrossRef]






| System | ñ | TCr-SmC | ∆HCr-SmC | TSmC-I | ∆HSmC-I | TSmC-N | ∆HSmC-N | TN-I | ∆HN-I | ∆S/R |
|---|---|---|---|---|---|---|---|---|---|---|
| I6/I6 | 12 | 189.0 | 55.7 | 257.4 | 2.7 | 265.9 | 1.8 | 0.40 | ||
| I6/I8 | 14 | 175.4 | 40.1 | 260.2 | 3.2 | 262.8 | 1.9 | 0.43 | ||
| I6/I16 | 22 | 158.9 | 39.6 | 174.7 | 2.4 | 252.0 | 1.7 | 0.39 | ||
| I8/I8 | 16 | 182.0 | 49.8 | 258.0 | 2.1 | 261.0 | 1.9 | 0.43 | ||
| I8/I16 | 24 | 158.2 | 36.3 | 175.8 | 2.6 | 246.8 | 1.1 | 0.25 | ||
| I16/I16 | 32 | 157.0 | 56.9 | 239.7 | 3.7 | 0.87 |
| ñ | System -N=CH- | TC | System -N=N- | TC | System -- | TC |
|---|---|---|---|---|---|---|
| 12 | I6/I6 | 265.9 | II6/II6 | 264.0 | III6/III6 | 158.0 |
| 14 | I6/I8 | 262.8 | II6/II8 | 266.0 | III6/III8 | 151.6 |
| 16 | I8/I8 | 261.0 | II8/II8 | 260.0 | III8/III8 | 147.0 |
| 22 | I6/I16 | 252.0 | II6/II16 | 241.0 | III6/III16 | 130.2 |
| 24 | I8/I16 | 246.8 | II8/II16 | 242.9 | III8/III16 | 133.0 |
| 32 | I16/I16 | 239.7 | II16/II16 | 240.0 | III16/III16 | 132.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Ahmed, H.A.; Mostafa, A.M.; Naoum, M.M. Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths. Symmetry 2021, 13, 1785. https://doi.org/10.3390/sym13101785
Alamro FS, Ahmed HA, Mostafa AM, Naoum MM. Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths. Symmetry. 2021; 13(10):1785. https://doi.org/10.3390/sym13101785
Chicago/Turabian StyleAlamro, Fowzia S., Hoda A. Ahmed, Ayman M. Mostafa, and Magdi M. Naoum. 2021. "Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths" Symmetry 13, no. 10: 1785. https://doi.org/10.3390/sym13101785
APA StyleAlamro, F. S., Ahmed, H. A., Mostafa, A. M., & Naoum, M. M. (2021). Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy)phenylimino)methyl]benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths. Symmetry, 13(10), 1785. https://doi.org/10.3390/sym13101785








