Biomechanical Evaluation on the Bilateral Asymmetry of Complete Humeral Diaphysis in Chinese Archaeological Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Data Collecting and Processing
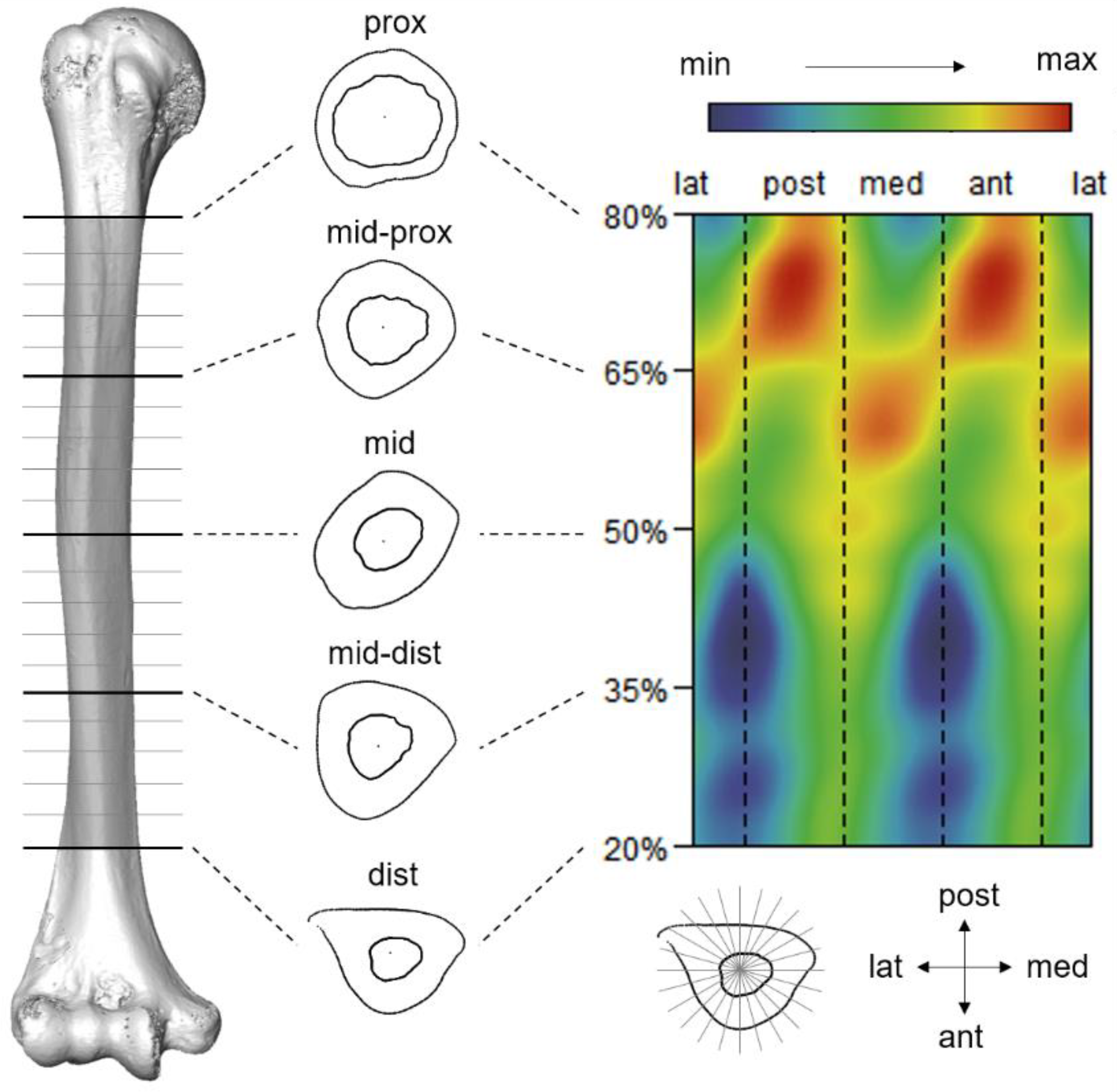
2.3. Cross-Sectional Geometric Parameters Calculation
2.4. Bilateral Asymmetry Quantification
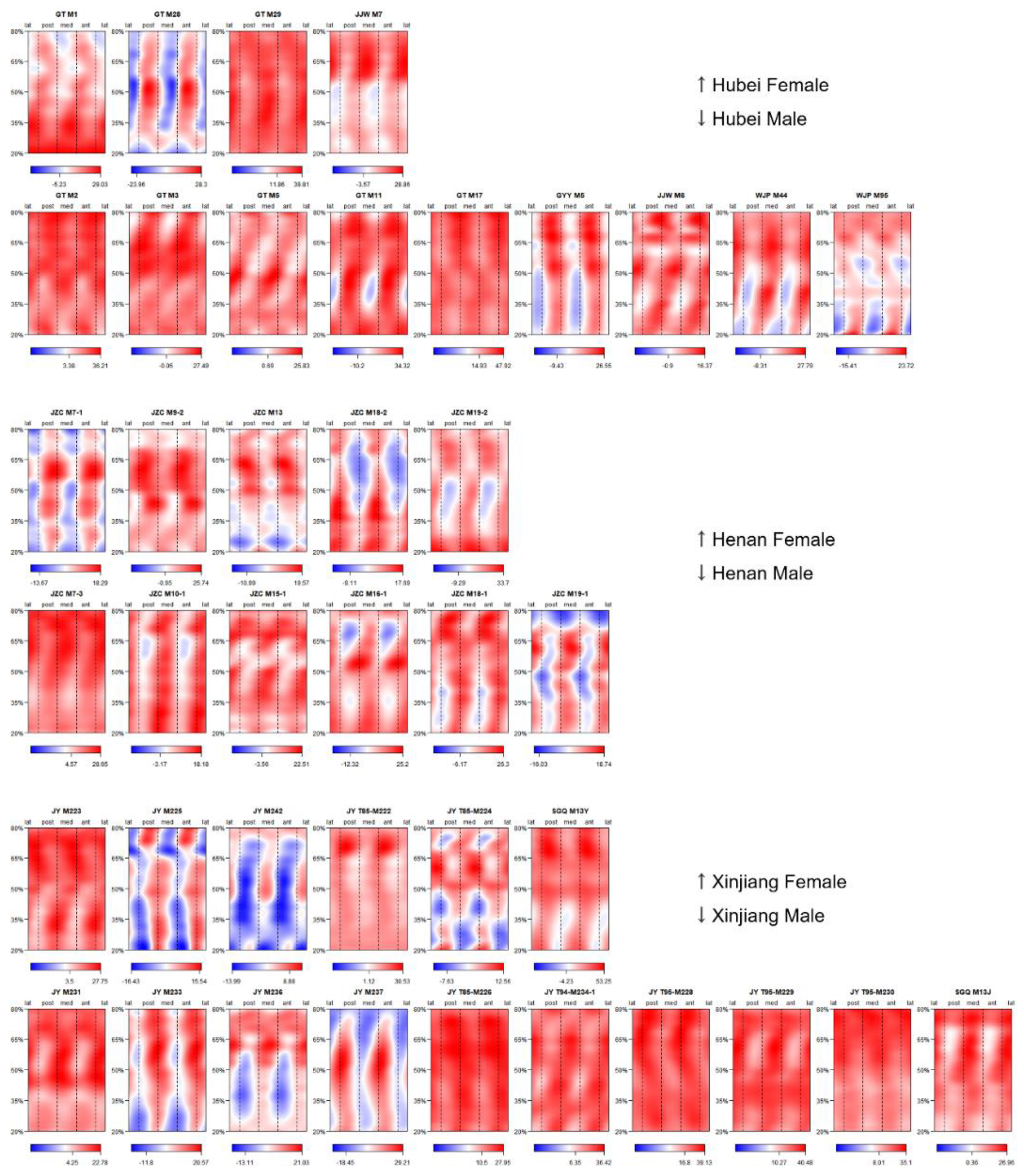
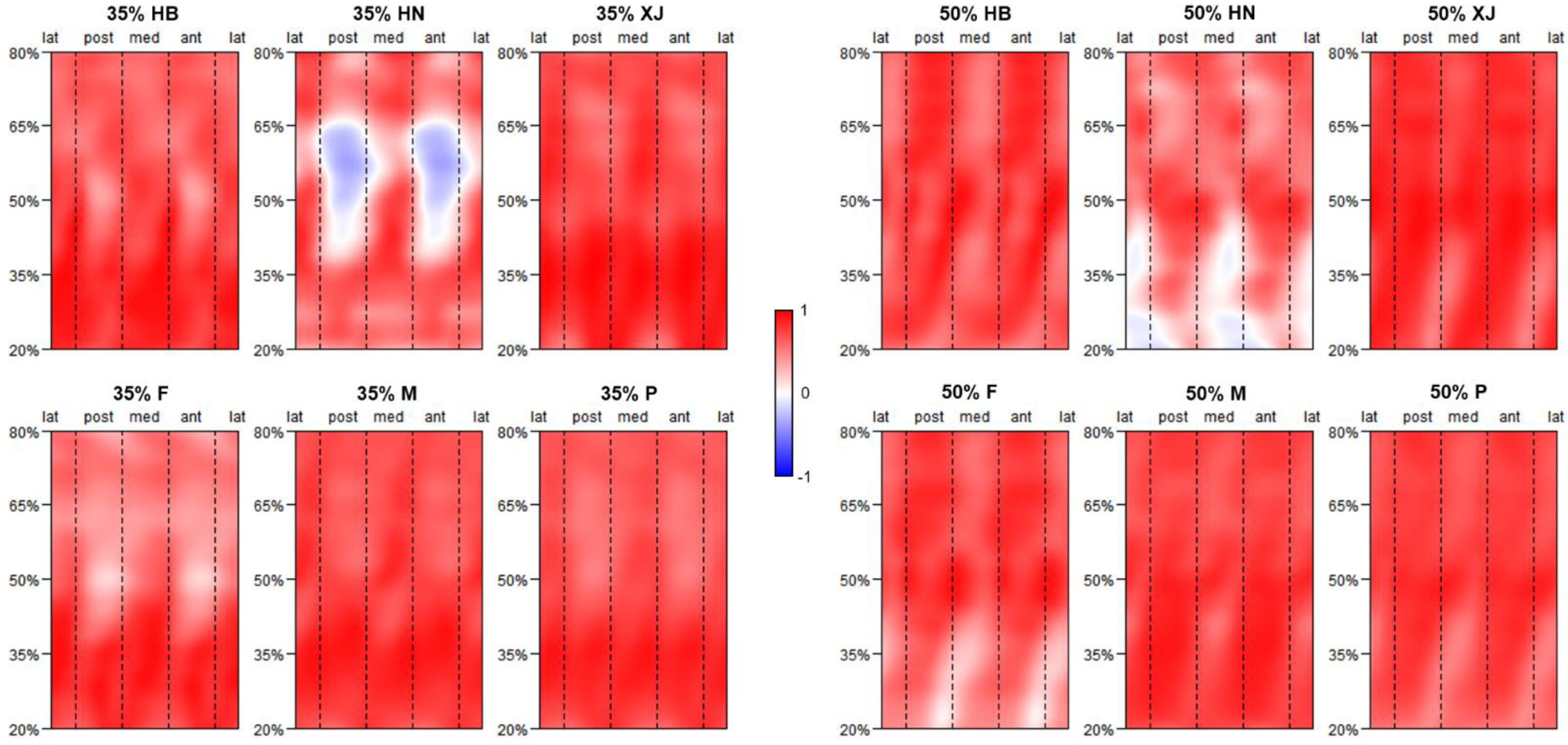
2.5. Morphometric Mapping
2.6. Methods to Estimate the Variation of Humeral Biomechanical Asymmetry
2.7. Methods to Test the Representativeness of J35 or J50 Asymmetry
3. Results
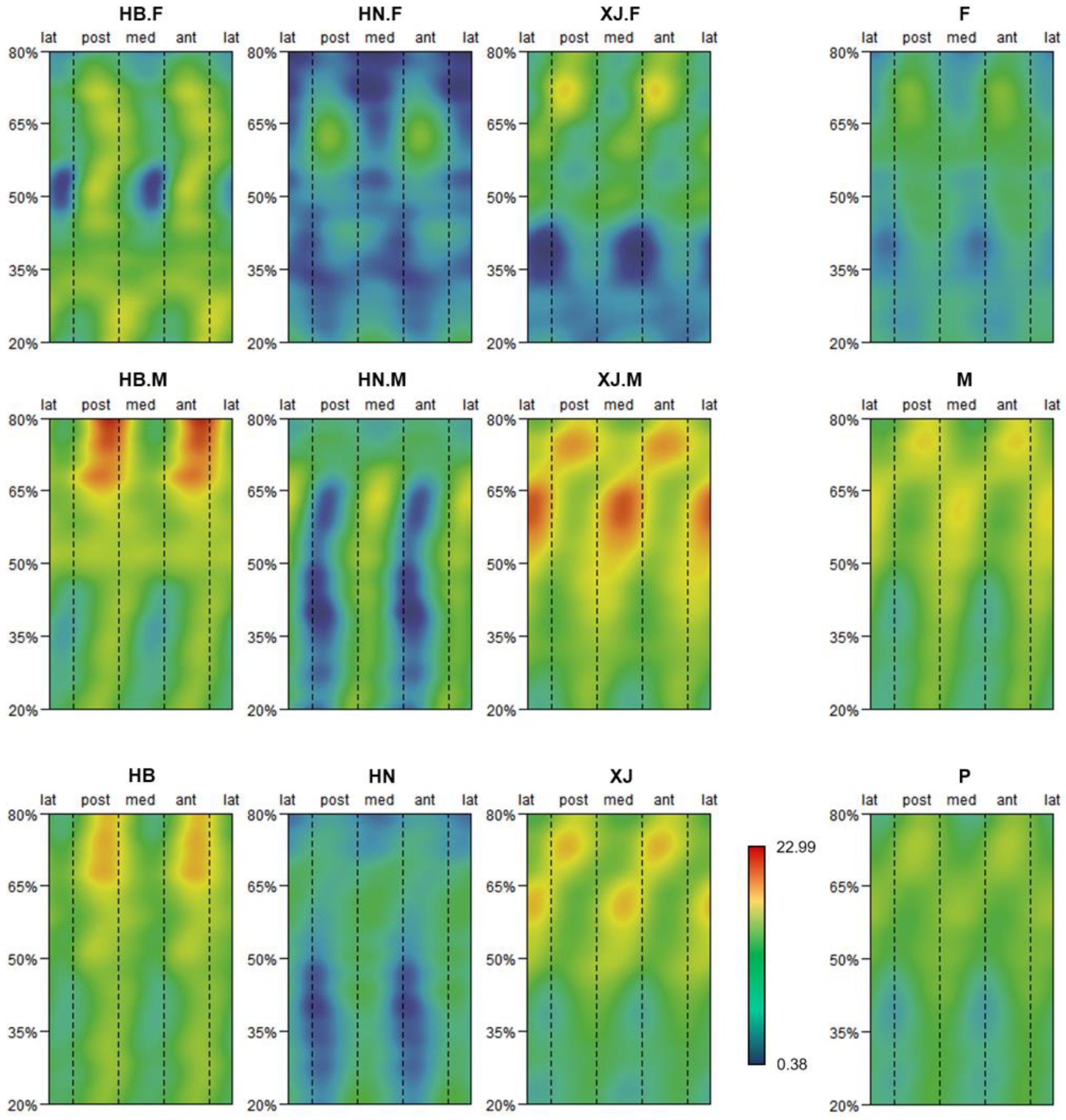
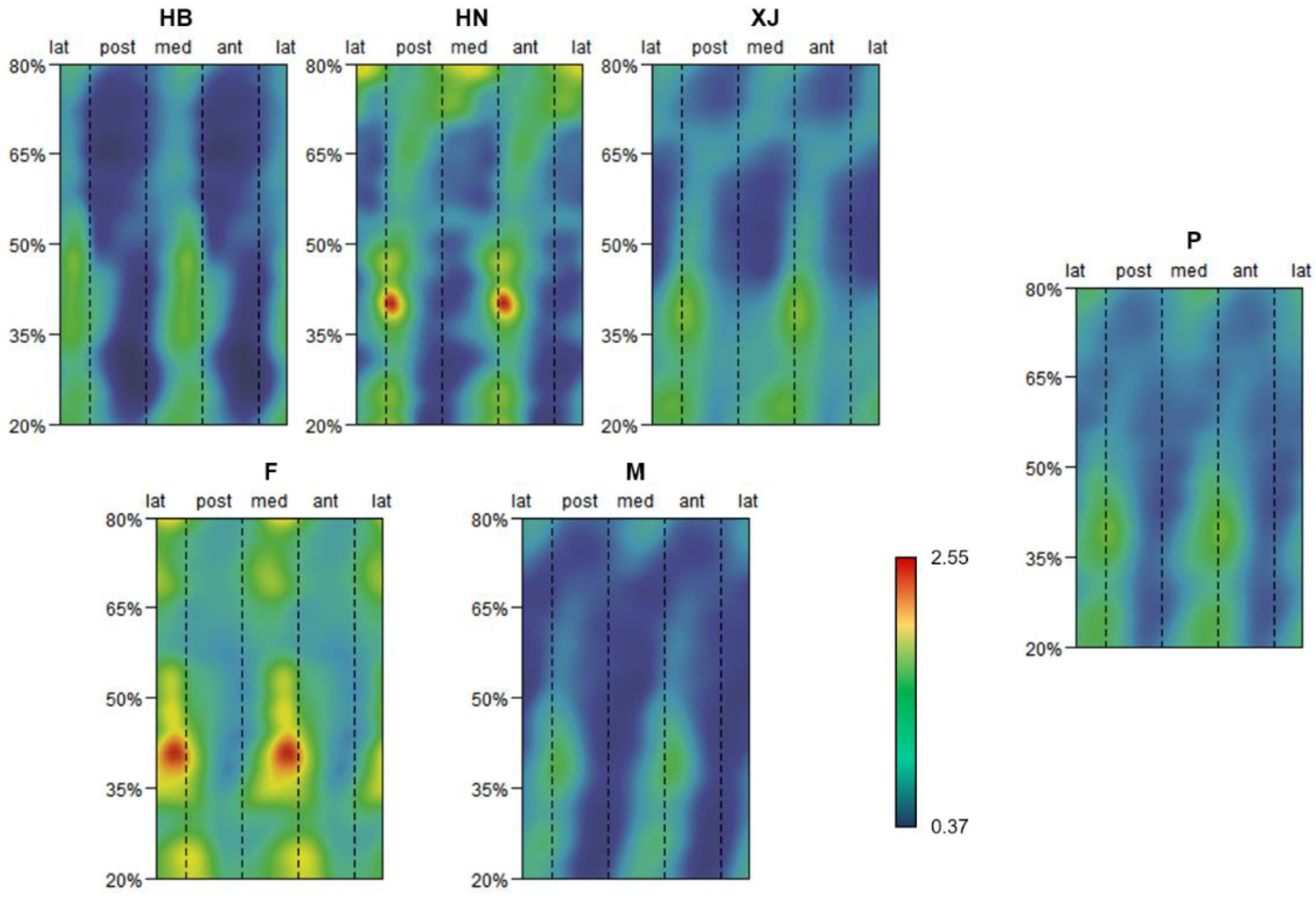
3.1. Pattern of Humeral Biomechanical Asymmetry in Modern Humans
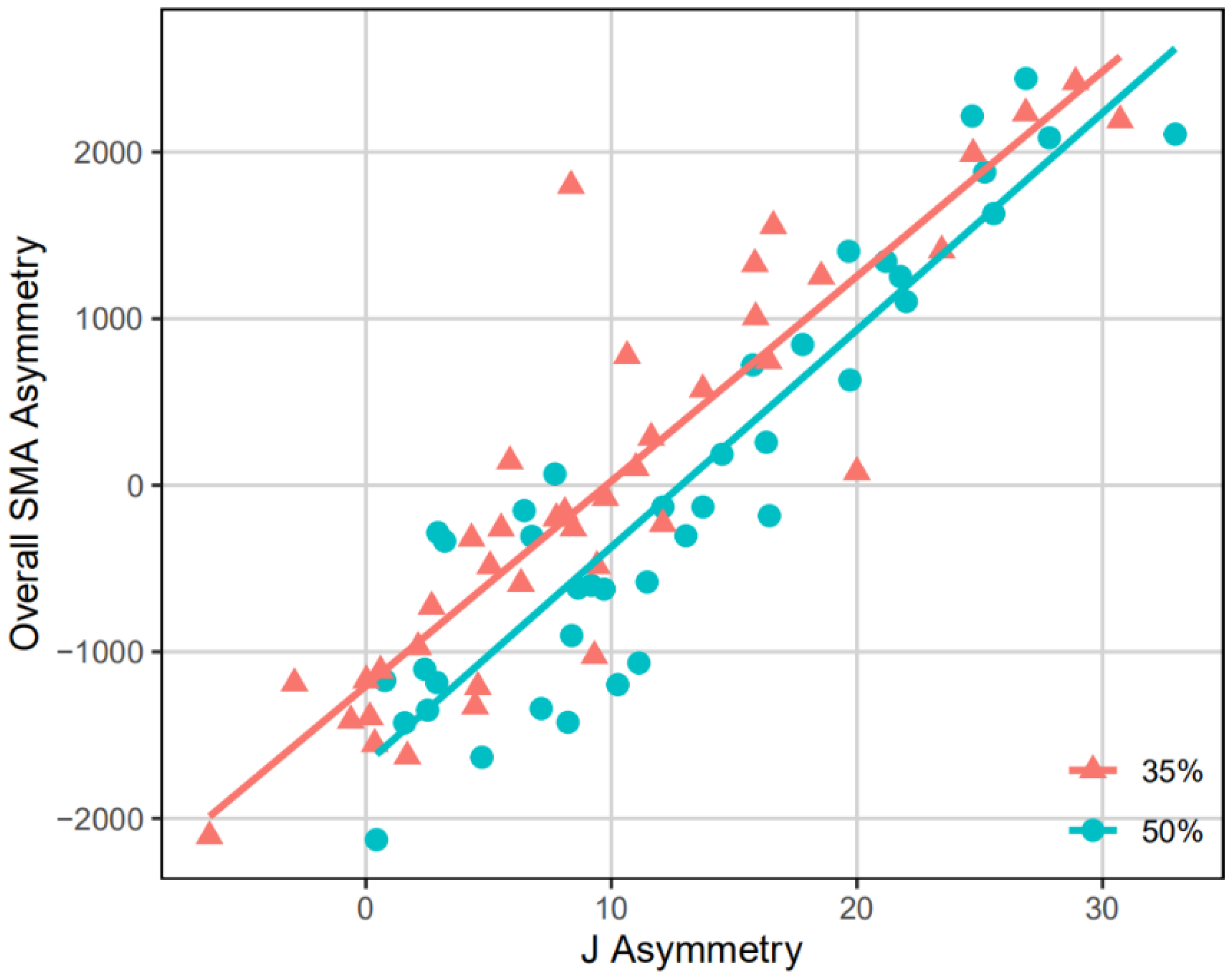
3.2. Representativeness of J35 and J50 Bilateral Asymmetry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- DeLude, J.A.; Bicknell, R.T.; MacKenzie, G.A.; Ferreira, L.M.; Dunning, C.E.; King, G.J.W.; Johnson, J.A.; Drosdowech, D.S. An anthropometric study of the bilateral anatomy of the humerus. J. Shoulder Elbow Surg. 2007, 16, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ubelaker, D.H.; Zarenko, K.M. Can handedness be determined from skeletal remains? A chronological review of the literature. J. Forensic Sci. 2012, 57, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.B. Biomechanical analyses of archaeological human skeletons. In Biological Anthropology of the Human Skeleton, 3rd ed.; Katzenberg, M.A., Grauer, A.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 189–224. [Google Scholar]
- Faurie, C.; Raymond, M. Handedness, homicide and negative frequency-dependent selection. Proc. R. Soc. B 2005, 272, 25–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, C.N. Is ‘hand preference’ coded in the hominin skeleton? An in-vivo study of bilateral morphological variation. J. Hum. Evol. 2011, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ozener, B. Extreme behavioral lateralization and the remodeling of the distal humerus. Am. J. Hum. Biol. 2012, 24, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, H.; Kannus, P.; Sievänen, H.; Pasanen, M.; Uusi-Rasi, K.; Heinonen, A.; Oja, P.; Vuori, I. Effect of long-term unilateral activity on bone mineral density of female junior tennis players. J. Bone Miner. Res. 1998, 13, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bass, S.L.; Saxon, L.; Daly, R.M.; Turner, C.H.; Robling, A.G.; Seeman, E.; Stuckey, S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J. Bone Miner. Res. 2002, 17, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.N.; Stock, J.T. Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern human athletes. Am. J. Phys. Anthropol. 2009, 140, 160–172. [Google Scholar] [CrossRef]
- Balzeau, A.; Ball-Albessard, L.; Kubicka, A.M. Variation and correlations in departures from symmetry of brain torque, humeral morphology and handedness in an archaeological sample of Homo sapiens. Symmetry 2020, 12, 432. [Google Scholar] [CrossRef] [Green Version]
- Shackelford, L.L. Regional variation in the postcranial robusticity of late upper paleolithic humans. Am. J. Phys. Anthropol. 2007, 133, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Sparacello, V.S.; d’Ercole, V.; Coppa, A. A bioarchaeological approach to the reconstruction of changes in military organization among Iron Age Samnites (Vestini) From Abruzzo, Central Italy. Am. J. Phys. Anthropol. 2015, 156, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sládek, V.; Ruff, C.B.; Berner, M.; Holt, B.; Niskanen, M.; Schuplerová, E.; Hora, M. The impact of subsistence changes on humeral bilateral asymmetry in Terminal Pleistocene and Holocene Europe. J. Hum. Evol. 2016, 92, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparacello, V.S.; Villotte, S.; Shackelford, L.L.; Trinkaus, E. Patterns of humeral asymmetry among Late Pleistocene humans. Comptes Rendus Palevol 2017, 16, 680–689. [Google Scholar] [CrossRef]
- Wei, P.; Lu, H.; Carlson, K.J.; Zhang, H.; Hui, J.; Zhu, M.; He, K.; Jashashvili, T.; Zhang, X.; Yuan, H.; et al. The upper limb skeleton and behavioral lateralization of modern humans from Zhaoguo Cave, southwestern China. Am. J. Phys. Anthropol. 2020, 173, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, Y.; Walker, C.S.; He, J.; Lu, X.; Hui, J.; Shui, W.; Jin, L.; Liu, W. Internal structural properties of the humeral diaphyses in an early modern human from Tianyuan Cave, China. Quat. Int. 2021, 591, 107–118. [Google Scholar] [CrossRef]
- Hsieh, Y.-F.; Robling, A.G.; Ambrosius, W.T.; Burr, D.B.; Turner, C.H. Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J. Bone Miner. Res. 2001, 16, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Robling, A.G.; Turner, C.H. Effects of biomechanical stress on bones in animals. Bone 2002, 30, 781–786. [Google Scholar] [CrossRef]
- Pearson, O.M.; Lieberman, D.E. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthropol. 2004, 125, 63–99. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.; Holt, B.; Trinkaus, E. Who’s afraid of the big bad wolff? “Wolff is law” and bone functional adaptation. Am. J. Phys. Anthropol. 2006, 129, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Gosman, J.H.; Hubbell, Z.R.; Shaw, C.N.; Ryan, T.M. Development of cortical bone geometry in the human femoral and tibial diaphysis. Anat. Rec. 2013, 296, 774–787. [Google Scholar] [CrossRef]
- Sládek, V.; Berner, M.; Holt, B.; Niskanen, M.; Ruff, C.B. Past human manipulative behavior in the European Holocene as assessed through upper limb asymmetry. In Skeletal Variation and Adaptation in Europeans; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 163–208. [Google Scholar]
- Perchalski, B.; Placke, A.; Sukhdeo, S.M.; Shaw, C.N.; Gosman, J.H.; Raichlen, D.A.; Ryan, T.M. Asymmetry in the cortical and trabecular bone of the human humerus during development. Anat. Rec. 2018, 301, 1012–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.; Kwak, D.-S.; Kim, I.-B. Morphometric evaluation of detailed asymmetry for the proximal humerus in Korean population. Symmetry 2021, 13, 862. [Google Scholar] [CrossRef]
- Ruff, C.B.; Burgess, M.L.; Bromage, T.G.; Mudakikwa, A.; McFarlin, S.C. Ontogenetic changes in limb bone structural proportions in mountain gorillas (Gorilla beringei beringei). J. Hum. Evol. 2013, 65, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.B. Long bone articular and diaphyseal structure in old world monkeys and apes. I: Locomotor effects. Am. J. Phys. Anthropol. 2002, 119, 305–342. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E. Humeral cross-sectional morphology from 18th century Quebec prisoners of war: Limits to activity reconstruction. Am. J. Phys. Anthropol. 2005, 126, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Wescott, D.J.; Cunningham, D.L. Temporal changes in Arikara humeral and femoral cross-sectional geometry associated with horticultural intensification. J. Archaeol. Sci. 2006, 33, 1022–1036. [Google Scholar] [CrossRef]
- Sládek, V.; Berner, M.; Sosna, D.; Sailer, R. Human manipulative behavior in the Central European Late Eneolithic and Early Bronze Age: Humeral bilateral asymmetry. Am. J. Phys. Anthropol. 2007, 133, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.A.; Knüsel, C.J. Activity-related skeletal change in medieval humeri: Cross-sectional and architectural alterations. Am. J. Phys. Anthropol. 2005, 128, 536–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, E.C.; Pearson, O.M.; Durband, A.C.; Walshe, K.; Carlson, K.J.; Grine, F.E. An examination of the cross-sectional geometrical properties of the long bone diaphyses of Holocene foragers from Roonka, South Australia. Am. J. Phys. Anthropol. 2020, 172, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, L.W.; Mednikova, M.B.; Buzhilova, A.P.; Trinkaus, E. The Sunghir 3 Upper Paleolithic juvenile: Pathology versus persistence in the Paleolithic. Int. J. Osteoarchaeol. 2015, 25, 176–187. [Google Scholar] [CrossRef]
- Shaw, C.N.; Stock, J.T. Extreme mobility in the Late Pleistocene? Comparing limb biomechanics among fossil Homo, varsity athletes and Holocene foragers. J. Hum. Evol. 2013, 64, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Trinkaus, E. Epipaleolithic human appendicular remains from Ein Gev I, Israel. Comptes Rendus Palevol 2018, 17, 616–627. [Google Scholar] [CrossRef]
- Kubicka, A.M.; Nowaczewska, W.; Balzeau, A.; Piontek, J. Bilateral asymmetry of the humerus in Neandertals, Australian aborigines and medieval humans. Am. J. Phys. Anthropol. 2018, 167, 46–60. [Google Scholar] [CrossRef]
- Ogilvie, M.D.; Hilton, C.E. Cross-sectional geometry in the humeri of foragers and farmers from the prehispanic American Southwest: Exploring patterns in the sexual division of labor. Am. J. Phys. Anthropol. 2011, 144, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sparacello, V.S.; Pearson, O.M.; Coppa, A.; Marchi, D. Changes in skeletal robusticity in an iron age agropastoral group: The samnites from the Alfedena necropolis (Abruzzo, Central Italy). Am. J. Phys. Anthropol. 2011, 144, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Mantila Roosa, S.M.; Kersh, M.E.; Hurd, A.L.; Fleisig, G.S.; Pandy, M.G.; Fuchs, R.K. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc. Natl. Acad. Sci. USA 2014, 111, 5337–5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.A.B.; Humphrey, L.T. A Virtual geometric morphometric approach to the quantification of long bone bilateral asymmetry and cross-sectional shape. Am. J. Phys. Anthropol. 2015, 158, 541–556. [Google Scholar] [CrossRef]
- Bondioli, L.; Bayle, P.; Dean, C.; Mazurier, A.; Puymerail, L.; Ruff, C.; Stock, J.T.; Volpato, V.; Zanolli, C.; Macchiarelli, R. Technical note: Morphometric maps of long bone shafts and dental roots for imaging topographic thickness variation. Am. J. Phys. Anthropol. 2010, 142, 328–334. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Wei, P.; Xing, S. 2D visualization and quantitative analysis of the humeral diaphysis cortical thickness. Acta Anthropol. Sin. 2020, 39, 632–647. [Google Scholar] [CrossRef]
- Puymerail, L.; Ruff, C.B.; Bondioli, L.; Widianto, H.; Trinkaus, E.; Macchiarelli, R. Structural analysis of the Kresna 11 Homo erectus femoral shaft (Sangiran, Java). J. Hum. Evol. 2012, 63, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Puymerail, L.; Volpato, V.; Debénath, A.; Mazurier, A.; Tournepiche, J.-F.; Macchiarelli, R. A Neanderthal partial femoral diaphysis from the "grotte de la Tour", La Chaise-de-Vouthon (Charente, France): Outer morphology and endostructural organization. Comptes Rendus Palevol 2012, 11, 581–593. [Google Scholar] [CrossRef]
- Morimoto, N.; Ponce de León, M.S.; Zollikofer, C.P.E. Exploring femoral diaphyseal shape variation in wild and captive chimpanzees by means of morphometric mapping: A test of Wolff’s law. Anat. Rec. 2011, 294, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Jashashvili, T.; Dowdeswell, M.R.; Lebrun, R.; Carlson, K.J. Cortical structure of hallucal metatarsals and locomotor adaptations in hominoids. PLoS ONE 2015, 10, e0117905. [Google Scholar] [CrossRef]
- Cameron, M.E.; Pfeiffer, S. Long bone cross-sectional geometric properties of Later Stone Age foragers and herder-foragers. S. Afr. J. Sci. 2014, 110, 01–11. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Tang, L.; Shi, D.; Luo, Y.; Zhao, Z. Research on the charred plant remains unearthed from the Jijiawan site in Fang County, Hubei Province. Cult. Relics South. China 2019, 5, 180–188. (In Chinese) [Google Scholar] [CrossRef]
- Xing, S.; Carlson, K.J.; Wei, P.; He, J.; Liu, W. Morphology and structure of Homo erectus humeri from Zhoukoudian, Locality 1. PeerJ 2018, 6, e4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xiao, G.; Liu, Z.; Lv, E.; Wu, Y. Brief report on excavation of Jiayi cemeteries in Turpan, Xinjiang. Turfanological Res. 2014, 1, 1–19. [Google Scholar] [CrossRef]
- Xing, K.; Zhang, Y.; Li, Z. 新疆鄯善三个桥墓葬发掘简报. Cult. Relics 2002, 6, 46–56. (In Chinese). Available online: http://www.cqvip.com/qk/97337x/2002006/11400528.html (accessed on 27 August 2021).
- Xiao, G. The Review and Study of Jia Yi Cemetery in Turfan, Xinjiang. Master’s Thesis, Northwest University, Xi’an, China, 2018. [Google Scholar]
- Profico, A.; Bondioli, L.; Raia, P.; O’Higgins, P.; Marchi, D. morphomap: An R package for long bone landmarking, cortical thickness, and cross-sectional geometry mapping. Am. J. Phys. Anthropol. 2021, 174, 129–139. [Google Scholar] [CrossRef]
- Collyer, M.L.; Adams, D.C. RRPP: An r package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol. Evol. 2018, 9, 1772–1779. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.C.; Otárola-Castillo, E. geomorph: An r package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Zelazny, K.G.; Sylvester, A.D.; Ruff, C.B. Bilateral asymmetry and developmental plasticity of the humerus in modern humans. Am. J. Phys. Anthropol. 2021, 174, 418–433. [Google Scholar] [CrossRef] [PubMed]
- White, T.D.; Black, M.T.; Folkens, P.A. Arm: Humerus, Radius, and Ulna. In Human Osteology, 3rd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 175–198. [Google Scholar]
- Ruff, C. Growth in bone strength, body size, and muscle size in a juvenile longitudinal sample. Bone 2003, 33, 317–329. [Google Scholar] [CrossRef]
- Schoenau, E.; Neu, C.M.; Mokov, E.; Wassmer, G.; Manz, F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J. Clin. Endocrinol. Metab. 2000, 85, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Niinimäki, S. The relationship between musculoskeletal stress markers and biomechanical properties of the humeral diaphysis. Am. J. Phys. Anthropol. 2012, 147, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E.; Devlin, M.J.; Pearson, O.M. Articular area responses to mechanical loading: Effects of exercise, age, and skeletal location. Am. J. Phys. Anthropol. 2001, 116, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Plochocki, J.H. Bilateral variation in limb articular surface dimensions. Am. J. Hum. Biol. 2004, 16, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Hallgrímsson, B.; Willmore, K.; Hall, B.K. Canalization, developmental stability, and morphological integration in primate limbs. Am. J. Phys. Anthropol. 2002, 119, 131–158. [Google Scholar] [CrossRef] [Green Version]
| Df | SS | MS | Rsq | F | Z | P (>F) | |
|---|---|---|---|---|---|---|---|
| Sex | 1 | 5,309,415 | 5,309,415 | 0.05494 | 2.1778 | 1.31140 | 0.110 |
| Population | 2 | 5,792,218 | 2,896,109 | 0.05993 | 1.1879 | 0.58014 | 0.296 |
| Sex:Population | 2 | 2,652,077 | 1,326,039 | 0.02744 | 0.5439 | −0.78875 | 0.783 |
| Residuals | 34 | 82,891,403 | 2,437,982 | 0.85769 | |||
| Total | 39 | 96,645,113 |
| Df | SS | MS | Rsq | F | Z | P (>F) | |
|---|---|---|---|---|---|---|---|
| J35 asymmetry | 1 | 47,026,558 | 47,026,558 | 0.48659 | 36.015 | 3.5479 | 0.001 ** |
| Residuals | 38 | 49,618,555 | 1,305,751 | 0.51341 | |||
| Total | 39 | 96,645,113 | |||||
| J50 asymmetry | 1 | 49,218,068 | 49,218,068 | 0.50927 | 39.435 | 3.7475 | 0.001 ** |
| Residuals | 38 | 47,427,046 | 1,248,080 | 0.49073 | |||
| Total | 39 | 96,645,113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhou, M.; Li, H.; He, J.; Wei, P.; Xing, S. Biomechanical Evaluation on the Bilateral Asymmetry of Complete Humeral Diaphysis in Chinese Archaeological Populations. Symmetry 2021, 13, 1843. https://doi.org/10.3390/sym13101843
Zhao Y, Zhou M, Li H, He J, Wei P, Xing S. Biomechanical Evaluation on the Bilateral Asymmetry of Complete Humeral Diaphysis in Chinese Archaeological Populations. Symmetry. 2021; 13(10):1843. https://doi.org/10.3390/sym13101843
Chicago/Turabian StyleZhao, Yuhao, Mi Zhou, Haijun Li, Jianing He, Pianpian Wei, and Song Xing. 2021. "Biomechanical Evaluation on the Bilateral Asymmetry of Complete Humeral Diaphysis in Chinese Archaeological Populations" Symmetry 13, no. 10: 1843. https://doi.org/10.3390/sym13101843






