On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects
Abstract
:1. Introduction
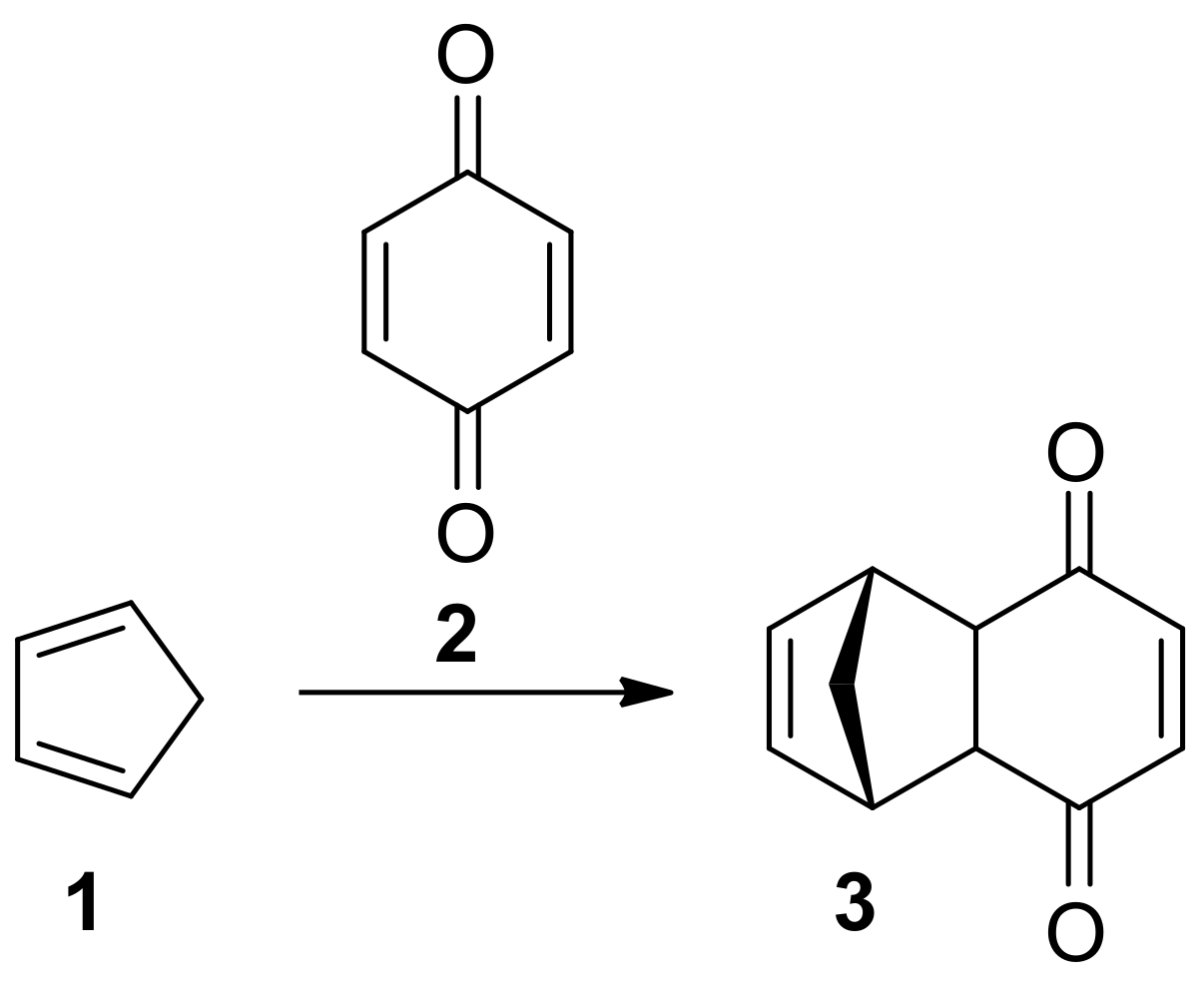
2. Diels-Alder Reactions
3. Hetero Diels-Alder Reactions Involving Heteroanalogs of Dienes
4. Hetero Diels-Alder Reactions Involving Heteroanalogs of Dienophiles
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Funel, J.-A.; Abele, S. Industrial Applications of the Diels-Alder Reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Juhl, M.; Tanner, D. Recent applications of intramolecular Diels-Alder reactions to natural product synthesis. Chem. Soc. Rev. 2009, 38, 2983–2992. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent progress in the field of cycloaddition reactions involving conjugated nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Yan, G.; Borah, A.J.; Wang, L. Recent advances in the synthesis of nitroolefin compounds. Org. Biomol. Chem. 2014, 12, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Halimehjani, A.Z.; Namboothiri, I.N.N.; Hooshmanda, S.E. Nitroalkenes in the synthesis of carbocyclic compounds. RSC Adv. 2014, 4, 31261–31299. [Google Scholar] [CrossRef]
- Lamri, S.; Heddam, A.; Kara, M.; Yahia, W.; Nacereddine, A.K. The Role of the Catalyst on the Reactivity and Mechanism in the Diels-Alder Cycloaddition Step of the Povarov Reaction for the Synthesis of a Biological Active Quinoline Derivative: Experimental and Theoretical Investigations. Organics 2021, 2, 57–71. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Understanding the uniqueness of the stepwise [4+1] cycloaddition reaction between conjugated nitroalkenes and electrophilic carbene systems with a molecular electron density theory perspective. Int. J. Quantum Chem. 2021, 121, e26440. [Google Scholar] [CrossRef]
- Lystsova, E.A.; Khramtsova, E.E.; Maslivets, M.A. Acyl(imidoyl)ketenes: Reactive Bidentate Oxa/Aza-Dienes for Organic Synthesis. Symmetry 2021, 13, 1509. [Google Scholar] [CrossRef]
- Khramtsova, E.E.; Dmitriev, M.; Maslivets, M.A. Synthesis of 1,4-benzothiazinones from acylpyruvic acids or furan-2,3-diones and o-aminothiophenol. Beilstein J. Org. Chem. 2020, 16, 2322–2331. [Google Scholar]
- Benhamed, L.; Mekelleche, S.M.; Charif, I.E.; Benchouk, W.; Ríos-Gutiérrez, M.; Domingo, L.R. Understanding the influence of the trifluoromethyl group on the chemo-, regio-, and stereoselectivity of [3+2]-cycloadditions of thiocarbonyl S-methanides with a,b-unsaturated ketones. A molecular electron density theory study. ChemistrySelect 2020, 5, 12791–12806. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk-Krygier, A. A DFT computational study on the [3+2] cycloaddition between parent thionitrone and nitroethene. Curr. Chem. Lett. 2018, 7, 27–34. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, Z.-L.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Aerobic radical-cascade cycloaddition of isocyanides, selenium and imidamides: Facile access to 1,2,4-selenadiazoles under metal-free conditions. Green Chem. 2017, 19, 1613–1618. [Google Scholar] [CrossRef]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry. Angew. Chem. Int. Ed. Engl. 1969, 8, 781–853. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular electron density theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Chamorro, E.; Pérez, P. Aromaticity in Pericyclic Transition State Structures? A Critical Rationalisation Based on the Topological Analysis of Electron Density. ChemistrySelect 2016, 1, 6026–6039. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Mechanism of Non-Polar Diels-Alder Reactions. A Comparative ELF Analysis of the Concerted and Stepwise Diradical Mechanisms. Org. Biomol. Chem. 2010, 8, 5495–5504. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Unveiling the Lewis Acid Catalysed Diels-Alder Reactions through the Molecular Electron Density Theory. Molecules 2020, 25, 2535. [Google Scholar] [CrossRef]
- Martin, J.G.; Hill, R.K. Stereochemistry of the Diels-Alder Reaction. Chem. Rev. 1961, 61, 537–562. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Jasiński, R.; Mirosław, B.; Demchuk, O.M.; Babyuk, D.; Łapczuk-Krygier, A. In the search for experimental and quantumchemical evidence for zwitterionic nature of (2E)-3-[4-(dimethylamino)phenyl]-2-nitroprop-2-enenitrile—An extreme example of donor-p-acceptor push-pull molecule. J. Mol. Struct. 2016, 1108, 689–697. [Google Scholar] [CrossRef]
- Kostiv, I.; Marshalok, O.; Marshalok, G.; Pyrig, I. Effect of the Reactants Molar Ratio on the Kinetics of the Reaction to Obtain 2-hydroxyethyl-1,3,4 trimethyl-cyclohex-3-encarboxylate. Chem. Chem. Tekh. 2015, 9, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Kostiv, I.S. Investigation of the reaction mechanism of [4+2] cyclization of 2,3 dimethylbuta-1,3-diene to methylacrylate using the Michaelis-Menten equation. French. Ukr. J. Chem. 2018, 6, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Demchuk, O.M.; Jasiński, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J.; Krasowska, D. A clean and simple method for deprotection of phosphines from borane complexes. Pure Appl. Chem. 2018, 90, 49–62. [Google Scholar] [CrossRef]
- Jasiński, R.; Mróz, K. Kinetic aspects of [3+2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones. React. Kinet. Mech. Catal. 2015, 116, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Singleton, D.A.; Schulmeier, B.E.; Hang, C.; Thomas, A.A.; Leung, S.-W.; Merrigan, S.R. Isotope effects and the distinction between synchronous, asynchronous, and stepwise Diels-Alder reactions. Tetrahedron 2001, 57, 5149–5160. [Google Scholar] [CrossRef]
- Saettel, N.J.; Wiest, O.; Singleton, D.A.; Meyer, M.P. Isotope effects and the mechanism of an electron-transfer-catalyzed Diels-Alder reaction. J. Am. Chem. Soc. 2002, 124, 11552–11559. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Zhong, L.P.; Lan, J.; Li, X.; Li, C.-C.; Chung, L.-W. Unusual KIE and dynamics effects in the Fe-catalyzed hetero-Diels-Alder reaction of unactivated aldehydes and dienes. Nat. Commun. 2020, 11, 1850. [Google Scholar] [CrossRef] [Green Version]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.; Jasiński, R. Experimental and theoretical mechanistic study on the thermal decomposition of 3,3-diphenyl-4-(trichloromethyl)-5-nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef]
- Barlett, P.D.; Schueller, K.E. Cycloaddition. VIII. Ethylene as a Dienophile. A minute Amount of 1,2 Cycloaddition of Etylene to Butadiene. J. Am. Chem. Soc. 1968, 90, 6071–6077. [Google Scholar] [CrossRef]
- Houk, K.N.; Lin, Y.-T.; Brown, F.K. Evidence for the Concerted Mechanism of the Diels-Alder reaction of Butadiene with Ethylene. J. Am. Chem. Soc. 1986, 108, 554–556. [Google Scholar] [CrossRef]
- Bernardi, F.; Bottoni, A.; Field, M.J.; Guest, M.F.; Hillier, I.H.; Robb, M.A.; Venturini, A. MC-SCF Study of the Diels-Alder Reaction between Ethylene and Butadiene. J. Am. Chem. Soc. 1988, 110, 3050–3055. [Google Scholar] [CrossRef]
- Goldstein, E.; Beno, B.; Houk, K.N. Density Functional Theory Prediction ofthe Relative Energies and Isotope Effects for the Concerted and Stepwise Mechanisms of the Diels-Alder Reaction of Butadiene and Ethylene. J. Am. Chem. Soc. 1996, 118, 6036–6043. [Google Scholar] [CrossRef]
- Sakai, S. Theoretical Analysis of Concerted and Stepwise Mechanims of Diels-Alder Reaction between Butadiene and Ethylene. J. Phys. Chem. A 2000, 104, 922–927. [Google Scholar] [CrossRef]
- Jursic, B.; Zdravkovski, Z. DFT study of the Diels-Alder reactions between ethylene with buta-1,3-diene and cyclopentadiene. J. Chem. Soc. Perkin Trans. 2 1995, 6, 1223–1226. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Lim, D.; Blake, J.F. Ab initio study of Diels-Alder reactions of cyclopentadiene with ethylene, isoprene, cyclopentadiene, acrylonitrile, and methyl vinyl ketone. J. Am. Chem. Soc. 1993, 115, 2936–2942. [Google Scholar] [CrossRef]
- Branchadell, A. Density functional study of Diels-Alder reactions between cyclopentadiene and substituted derivatives of ethylene. Int. J. Quantum Chem. 1998, 61, 381–388. [Google Scholar] [CrossRef]
- Drysdale, J.J.; Gilbert, W.W.; Sinclair, H.K.; Sharkey, W.H. A New Synthesis of Tropolone. J. Am. Chem. Soc. 1958, 80, 3672–3675. [Google Scholar] [CrossRef]
- Christova, N.B.; Pavlova, S.D.; Kostov, G.K. Kinetics of cycloaddition of tetrafluoroethylene to dicyclopentadiene. React. Kinet. Catal. Lett. 1993, 49, 393–402. [Google Scholar] [CrossRef]
- Jasiński, R. A reexamination of molecular mechanism of the Diels-Alder reaction between tetrafluoroethene and cyclopentadiene. React. Kinet. Mech. Catal. 2016, 119, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Klenz, O.; Miethchen, R.; Michalik, M. Organofluorine compounds and fluorinating agents. Part 16: Monoalkylations and cycloadditions with trans-3,3,3-trifluoro-1-nitropropene. J. Fluor. Chem. 1996, 81, 205–210. [Google Scholar] [CrossRef]
- Jasiński, R. β-Trifluoromethylated nitroethenes in Diels-Alder reaction with cyclopentadiene: A DFT computational study. J. Fluor. Chem. 2018, 206, 1–7. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Bell, A. β-Nitrostyrene in the Diene Synthesis. J. Org. Chem. 1943, 61, 521–522. [Google Scholar] [CrossRef]
- Bourguignon, J.; Le Nard, G.; Queguiner, G. Synthèse d’aryl nitronorbornènes par cycloaddition de Diels-Alder entre les aryl-nitroéthylènes et le cyclopentadiène. Justification de la stéréochimie et de la réactivité relative observées par la méthode CNDO/II. Obtention d’aryl aminonorbornènes. Can. J. Chem. 1985, 63, 2354–2361. [Google Scholar] [CrossRef]
- Jasiński, R.; Socha, J.; Mróz, K. Mechanizm [4+2] cykloaddycji cyklopentadienu do E-b-nitrostyrenu w świetle obliczeń B2LYP/6-311G**. Czas. Techn. 2010, 107/1Ch, 123–127. [Google Scholar]
- Łapczuk-Krygier, A.; Ponikiewski, L.; Jasiński, R. The Crystal Structure of (1RS,4RS,5RS,6SR)-5-Cyano-5-nitro-6-phenyl-bicyclo[2.2.1]hept-2-ne. Cryst. Rep. 2014, 59, 961–963. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Dresler, E.; Jasiński, R. Ionic liquids as an effective and eco-friendly medium for synthesis of nitrosubstituted norbornene analogs. Przem. Chem. 2016, 95, 1928–1931. [Google Scholar]
- Jasiński, R. First example of stepwise, zwitterionic mechanism for bicyclo[2.2.1]hept-5-ene (norbornene) formation process catalyzed by the 1-butyl-3-methylimidazolium cations. Monatsh. Chem. 2016, 147, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalyzed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Benhamed, L.; Mekelleche, S.M.; Benchouk, W. Theoretical Insight into the Reversal of Chemoselectivity in Diels-Alder Reactions of α,β-Unsaturated Aldehydes and Ketones Catalyzed by Brønsted and Lewis Acids. Organics 2021, 2, 38–49. [Google Scholar] [CrossRef]
- Jasiński, R. Solvent effect on the mecchanism of [4+2] cycloaddition of cyclopentadiene with methyl (Z)-p,-dinitrocinnamate. Czas. Techn. PK 2012, 109/2-M, 139–144. [Google Scholar]
- Huertas, D.; Florscher, M.; Dragojlovic, V. Solvent-free Diels-Alder reactions of in situ generated cyclopentadiene. Green Chem. 2009, 11, 91–95. [Google Scholar] [CrossRef]
- Silvestri, M.; Dills, C.E. A kinetic study of the Diels-Alder reaction: An experiment illustrating simple second-order reaction kinetic. J. Chem. Educ. 1989, 66, 690–691. [Google Scholar] [CrossRef]
- Kiesman, W.F.; Petter, R.C. Lewis-acid catalysis of the asymmetric Diels-Alder reaction of dimenthyl fumarate and cyclopentadiene. Tetrahedron Asymmetry 2002, 13, 957–960. [Google Scholar] [CrossRef]
- Mark, V. Nonstereospecific Diels-Alder reactions. I. Reaction of hexachlorocyclopentadiene with 1,2-disubstituted ethylenes. J. Org. Chem. 1974, 39, 3179–3181. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Pérez, P. Perfluorobicyclo[2.2.0]hex-1(4)-ene as unique partner for Diels-Alder reactions with benzene: A density functional theory study. Theor. Chem. Acc. 2021, 140, 17. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Kuzhaeva, A.A.; Berkova, G.A.; Deiko, L.I.; Berestovitskaya, V.M. Reactions of 2-Nitro- and 2-Bromo-2-nitroethenylphosphonates with Anthracene. Russ. J. Gen. Chem. 2005, 75, 689–693. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Participation of Phosphorylated Analogues of Nitroethene in Diels-Alder Reactions with Anthracene: A Molecular Electron Density Theory Study and Mechanistic Aspect. Organics 2020, 1, 36–48. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Kuznaeva, A.A.; Berkova, G.A.; Berestovitskaya, V.M.; Deiko, L.I. Specific Features of the Reaction of 2-Nitro- and 2-Bromo-2-Nitroethenylphosphonates with Furan. Russ. J. Gen. Chem. 2005, 75, 1750–1756. [Google Scholar] [CrossRef]
- Itoh, K.; Kitoh, K.; Kishimoto, S. Concerted and stepwise mechanisms in the Diels–Alder and Michael reactions of furans with methyl 3-nitroacrylate—Experimental and theoretical studies. Canad. J. Chem. 2006, 84, 392–406. [Google Scholar] [CrossRef]
- Lakhdar, S.; Terrier, F.; Vichard, D.; Berionni, G.; El-Guesmi, N.; Goumont, R.; Boubaker, T. The Diels-Alder Reaction of 4,6-Dinitrobenzofuroxan with 1-Trimethylsilyloxybuta-1,3-diene: A Case Example of a Stepwise Cycloaddition. Chem. Eur. J. 2010, 16, 5681–5690. [Google Scholar] [CrossRef]
- Linder, M.; Johansson, A.J.; Brinck, T. Mechanistic Insights into the Stepwise Diels-Alder Reaction of 4,6-Dinitrobenzofuroxan. Org. Lett. 2012, 14, 118–121. [Google Scholar] [CrossRef]
- Ormachea, C.M.; Mancini, P.M.E.; Kneeteman, M.N.; Domingo, L.R. A Understanding the participation of 3-nitropyridine in polar Diels-Alder reactions. A DFT study. Comput. Theor. Chem. 2015, 1072, 37–42. [Google Scholar] [CrossRef]
- Kurbatov, S.; Goumont, R.; Lakhdar, S.; Marrot, J.; Terrier, F. 4-Nitrobenzodifuroxan: A highly reactive nitroolefin in Diels–Alder reactions. Tetrahedron 2005, 61, 8167–8176. [Google Scholar] [CrossRef]
- Steglenko, D.V.; Kletsky, M.E.; Kurbatov, S.V.; Tatarov, A.V.; Minkin, V.I.; Goumont, R.; Terrier, F. A theoretical and experimental study of the polar Diels-Alder cycloaddition of cyclopentadiene with nitrobenzodifuroxan. J. Phys. Org. Chem. 2009, 22, 298–307. [Google Scholar] [CrossRef]
- Denmark, S.E.; Kesler, B.S.; Moon, Y.C. Inter- and intramolecular [4+2] cycloadditions of nitroalkenes with olefins. 2-Nitrostyrenes. J. Org. Chem. 1992, 57, 4912–4924. [Google Scholar] [CrossRef]
- Pipic, A.; Zeller, M.; Tsetsakos, P. Sequential Diels-Alder/[3,3]-sigmatropic rearrangement reactions of β-nitrostyrene with 3-methyl-1,3-pentadiene. Beilstein J. Org. Chem. 2013, 9, 2137–2146. [Google Scholar]
- Denmark, S.E.; Cramer, C.J.; Sternberg, J.A. Intermolecular [4+2]-Cycloadditions of Nitroalkenes with Cyclic Olefins. Transformations of Cyclic Nitronates. Helv. Chim. Acta 1986, 69, 1971–1989. [Google Scholar] [CrossRef]
- Fringuelli, F.; Matteucci, M.; Piermatti, O.; Pizzo, F.; Burla, M.C. [4+2] Cycloadditions of Nitroalkenes in Water. Highly Asymmetric Synthesis of Functionalized Nitronates. J. Org. Chem. 2001, 66, 4661–4666. [Google Scholar] [CrossRef]
- Jasiński, R.; Magdalena Kubik, M.; Łapczuk-Krygier, A.; Kącka, A.; Dresler, E.; Boguszewska-Czubara, A. An experimental and theoretical study of the hetero Diels-Alder reactions between (E)-2-aryl-1-cyano-1-nitroethenes and ethyl vinyl ether: One-step or zwitterionic, two-step mechanism? React. Kinet. Mech. Catal. 2014, 113, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Tohda, Y.; Yamawaki, N.; Matsui, H.; Kawashima, T.; Ariga, M.; Mori, Y. Synthesis and a novel fragmentation of 6-alkoxy-5,6-dihydro-4H-1,2-oxazine 2-oxide. Bull. Chem. Soc. Jpn. 1988, 61, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Jasiński, R. Searching for zwitterionic intermediates in Hetero Diels–Alder reactions between methyl α,p-dinitrocinnamate and vinyl-alkyl ethers. Comput. Theor. Chem. 2014, 1046, 93–98. [Google Scholar] [CrossRef]
- Korotaev, V.Y.; Barkov, A.Y.; Slepukhin, P.A.; Kodess, M.I.; Sosnovskikh, V.Y. Uncatalyzed reactions of a-(trihaloethylidene)nitroalkanes with push–pull enamines: A new type of ring–ring tautomerism in cyclobutane derivatives and the dramatic effect of the trihalomethyl group on the reaction pathway. Tetrahedron Lett. 2011, 52, 5764–5768. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Molecular mechanism of Hetero Diels-Alder reactions between (E)-1,1,1-trifluoro-3-nitrobut-2-enes and enamine systems in the light of Molecular Electron Density Theory. J. Mol. Graph. Model. 2020, 101, 107714. [Google Scholar] [CrossRef]
- Korotayev, Y.V.; Barkov, A.Y.; Slepukhin, P.A.; Kodess, M.I.; Sosnovskikh, V.Y. Diastereoselective reactions of 1,1,1-trichloro(trifluoro)-3-nitrobut-2-enes with morpholinoalk-1-enes. Mendeleev Commun. 2011, 21, 112–114. [Google Scholar] [CrossRef]
- Ernd, M.; Heuschmann, M.; Zipse, H. Cycloadditions of Aryl-Substituted 1,2,4-triazines with 2-cyclopropylidene-1,3-dimethylimidazolidine-Zwitterions as Discrete Intermediate. Helv. Chim. Acta. 2005, 88, 1491–1518. [Google Scholar] [CrossRef]
- Sakai, S. Theoretical analysis of concerted and stepwise mechanisms of the hetero-Diels-Alder reaction of butadiene with formaldehyde and thioformaldehyde. J. Mol. Struct. 2003, 630, 177–185. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Sobiecka, M.; Heimgartner, H.; Würthwein, E.-U.; Zimmer, R.; Lentz, D.; Reissig, H.-U. Hetero-Diels-Alder Reactions of In Situ-Generated Azoalkenes with Thioketones; Experimental and Theoretical Studies. Molecules 2021, 26, 2544. [Google Scholar] [CrossRef]
- Wilker, S.; Erker, G. Stereochemistry of the [4+2] Cycloaddition of Diarylselenoketones with Conjugated Dienes. J. Am. Chem. Soc. 1995, 117, 10922–10930. [Google Scholar] [CrossRef]
- Mlostoń, G.; Grzelak, P.; Linden, A.; Heimgartner, H. Thia-Diels-Alder reactions of hetaryl thioketones with nonactivated 1,3-dienes leading to 3,6-dihydro-2H-pyrans: Evidence for a diradical mechanism. Chem. Heterocycl. Compd. 2017, 53, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over Catalytic Surfaces. Phys. Chem. Phys. 2017, 18, 135–3141. [Google Scholar]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]

























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasiński, R. On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects. Symmetry 2021, 13, 1911. https://doi.org/10.3390/sym13101911
Jasiński R. On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects. Symmetry. 2021; 13(10):1911. https://doi.org/10.3390/sym13101911
Chicago/Turabian StyleJasiński, Radomir. 2021. "On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects" Symmetry 13, no. 10: 1911. https://doi.org/10.3390/sym13101911
APA StyleJasiński, R. (2021). On the Question of Stepwise [4+2] Cycloaddition Reactions and Their Stereochemical Aspects. Symmetry, 13(10), 1911. https://doi.org/10.3390/sym13101911





