What Are the Synergies between Paleoanthropology and Brain Imaging?
Abstract
:1. Introduction
Paleoanthropology and the Evolution of the Brain
2. A Synthesis on Past and Living Brains
Evolving Methodologies in the Study of Human Brain Morphology
3. Virtual Anthropology and Paleoneurology
3.1. Does the Endocast Reflects the Brain?
3.2. What Can Be Deduced about a Species’ Folding Pattern from a Few Samples?
3.3. How Are Brain Asymmetries Quantified in the Hominin Fossil Record?
3.4. The Complex Definition of Brain Features and of Their Application to the Fossil Record
3.5. How to Grow a Hominin Brain?
3.6. Brain Endocast and Function
4. Perspectives for Future Studies of the Evolution of the Human Brain
4.1. The Future of Neuroimaging
4.2. Endocast Side
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falk, D. Interpreting sulci on hominin endocasts: Old hypotheses and new findings. Front. Hum. Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Gall, F.J. On the Functions of the Brain and of Each of its Parts: With Observations on the Possibility of Determining the Instincts, Propensities, and Talents, or the Moral and Intellectual Dispositions of Men and Animals, by the Configuration of the Brain and Head; Marsh, Capen & Lyon: Boston, MA, USA, 1835. [Google Scholar]
- Parker Jones, O.; Alfaro-Almagro del, F.; Jbabdi, S. An empirical, 21st century evaluation of phrenology. Cortex 2018, 106, 26–35. [Google Scholar] [CrossRef]
- Dumoncel, J.; Subsol, G.; Durrleman, S.; Bertrand, A.; de Jager, E.; Oettlé, A.C.; Lockhat, Z.; Suleman, F.E.; Beaudet, A. Are endocasts reliable proxies for brains? A 3D quantitative comparison of the extant human brain and endocast. J. Anat. 2021, 238, 480–488. [Google Scholar] [CrossRef]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [Green Version]
- Broca, P. Mémoires d’Anthropologie; Reinwald: Paris, France, 1871. [Google Scholar]
- Brodmann, K. Physiologie des Gehirns. In Allgemeine Chirurgie der Gehirnkrankheiten; Knoblauch, A., Brodmann, K., Hauptmann, A., Eds.; Verlag von Ferdinand Enke: Stuttgart, Germany, 1914; pp. 86–426. [Google Scholar]
- Vogt, C.; Vogt, O. Die vergleichend-architektonische und die vergleichend reizphysiologische Felderung der Großhirnrinde unter besonderer Berucksichtigungder menschlichen. Naturwissenschaften 1926, 14, 1192–1195. [Google Scholar] [CrossRef]
- Amunts, K.; Mohlberg, H.; Bludau, S.; Zilles, K. Julich-Brain: A 3D probabilistic atlas of the human brain’s cytoarchitecture. Science 2020, 369, 988–992. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium W-MH. The WU-Minn Human Connectome Project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.T.; Sharp, K.; Alfaro-Almagro, F.; Shi, S.; Miller, K.L.; Douaud, G.; Marchini, J.; Smith, S.M. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 2018, 562, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Pääbo, S. The human condition-a molecular approach. Cell 2014, 157, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Tilot, A.K.; Khramtsova, E.A.; Liang, D.; Grasby, K.L.; Jahanshad, N.; Painter, J.; Colodro-Conde, L.; Bralten, J.; Hibar, D.P.; Lind, P.A.; et al. The Evolutionary History of Common Genetic Variants Influencing Human Cortical Surface Area. Cereb. Cortex 2020, 5, 1873–1887. [Google Scholar]
- Grasby, K.L.; Jahanshad, N.; Painter, J.N.; Colodro-Conde, L.; Bralten, J.; Hibar, D.P.; Lind, P.A.; Pizzagalli, F.; Ching, C.R.K.; McMahon, M.A.B.; et al. Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA)—Genetics working group. The genetic architecture of the human cerebral cortex. Science 2020, 367, 6484. [Google Scholar]
- Bozek, J.; Makropoulos, A.; Schuh, A.; Fitzgibbon, S.; Wright, R.; Glasser, M.F.; Coalson, T.S.; O’Muircheartaigh, J.; Hutter, J.; Price, A.N.; et al. Construction of a neonatal cortical surface atlas using Multimodal Surface Matching in the Developing Human Connectome Project. NeuroImage 2018, 179, 11–29. [Google Scholar] [CrossRef]
- Harms, M.P.; Somerville, L.H.; Ances, B.M.; Andersson, J.; Barch, D.M.; Bastiani, M.; Bookheimer, S.Y.; Brown, T.B.; Buckner, R.L.; Burgess, G.C.; et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage 2018, 183, 972–984. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Meguerditchian, A.; Coulon, O.; Bogart, S.; Mangin, J.F.; Sherwood, C.C.; Grabowski, M.W.; Bennett, A.J.; Pierre, P.J.; Fears, S.; et al. Evolution of the central sulcus morphology in primates. Brain Behav. Evol. 2014, 84, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, P.; Forkel, S.J.; Amiez, C.; Balsters, J.H.; Coulon, O.; Fan, L.; Goulas, A.; Hadj-Bouziane, F.; Hecht, E.E.; Heuer, K.; et al. Imaging evolution of the primate brain: The next frontier? NeuroImage 2021, 228, 117685. [Google Scholar] [CrossRef]
- Milham, M.; Petkov, C.I.; Margulies, D.S.; Schroeder, C.E.; Basso, M.A.; Belin, P.; Fair, D.A.; Fox, A.; Kastner, S.; Mars, R.; et al. Accelerating the Evolution of Nonhuman Primate Neuroimaging. Neuron 2020, 105, 600–603. [Google Scholar] [CrossRef] [Green Version]
- Trinkaus, E.; Churchill, S.E.; Ruff, C.B. Post-cranial robusticity in Homo II: Humeral bilateral asymmetry and bone plasticity. Am. J. Phys. Anthrop. 1994, 93, 1–34. [Google Scholar] [CrossRef]
- Balzeau, A.; Gilissen, E.; Grimaud-Hervé, D. Shared pattern of quantified endocranial shape asymmetries among anatomically modern humans, great apes and fossil hominins. PLoS ONE 2012, 7, e29581. [Google Scholar]
- Shaw, C.N.; Stock, J.T. Extreme mobility in the Late Pleistocene? Comparing limb biomechanics among fossil Homo, varsity athletes and Holocene foragers. J. Hum. Evol. 2013, 64, 242–249. [Google Scholar] [CrossRef]
- Davies, T.G.; Stock, J.T. Human variation in the periosteal geometry of the lower limb: Signatures of behaviour among human Holocene populations. In Reconstructing Mobility: Environmental, Behavioral, and Morphological Determinants; Carlson, K.J., Marchi, D., Eds.; Springer: New York, NY, USA, 2014; pp. 67–90. [Google Scholar]
- Goldberg, E.; Roediger, D.; Kucukboyaci, N.E.; Carlson, C.; Devinsky, O.; Kuzniecky, R.; Cash, S.; Thesen, T. Hemispheric asymmetries of cortical volume in the human brain. Cortex 2013, 49, 200–210. [Google Scholar] [CrossRef]
- Tobias, P.V. The brain of Homo habilis: A new level of organization in cerebral evolution. J. Hum. Evol. 1987, 16, 741–761. [Google Scholar] [CrossRef]
- Grimaud-Hervé, D. L’Evolution de l’Encéphale Chez Homo Erectus et Homo Sapiens: Exemples de l’Asie et de l’Europe; Cahiers de Paléoanthropologie, CNRS: Paris, France, 1997; 406p. [Google Scholar]
- Holloway, R.L.; Broadfield, D.C.; Yuan, M.S. The Human Fossil Record: Brain Endocasts, Paleoneurological Evidence; Wiley-Liss: New York, NY, USA, 2004; 315p. [Google Scholar]
- Balzeau, A.; Grimaud-Hervé, D.; Holloway, R.L.; Détroit, F.; Combès, B.; Prima, S. First description of the Cro-Magnon 1 endocast and study of brain variation an evolution in anatomically modern Homo Sapiens. Bull. Mémoires Société D’anthropologie Paris 2013, 25, 1–18. [Google Scholar] [CrossRef]
- Balzeau, A.; Gilissen, E.; Holloway, R.L.; Prima, S.; Grimaud-Hervé, D. Variations in size, shape and asymmetries of the third frontal convolution in hominids: Paleoneurological implications for hominin evolution and the origin of language. J. Hum. Evol. 2014, 76, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Galaburda, A.M.; Corsiglia, J.; Rosen, G.D.; Sherman, G.F. Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia 1987, 25, 853–868. [Google Scholar] [CrossRef]
- Mechelli, A.; Price, C.J.; Friston, K.J.; Ashburner, J. Voxel-based morphometry of the human brain: Methods and applications. Curr. Med. Imaging 2005, 1, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Bookstein, F.L. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage 2001, 14, 1454–1462. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Why voxel-based morphometry should be used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Mangin, J.F.; Lebenberg, J.; Lefranc, S.; Labra, N.; Auzias, G.; Labit, M.; Guevara, M.; Mohlberg, H.; Roca, P.; Guevara, P.; et al. Spatial normalization of brain images and beyond. Med. Image Anal. 2016, 33, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Mangin, J.F.; Jouvent, E.; Cachia, A. In-vivo measurement of cortical morphology: Means and meanings. Curr. Opin. Neurol. 2010, 23, 359–367. [Google Scholar] [CrossRef]
- Maier, W.O.; Nkini, A.T. The phylogenetic position of Olduvai Hominid 9, especially as determined from basicranial evidence. In Ancestors: The Hard Evidence; Delson, E., Alan, R., Eds.; Wiley-Liss: New York, NY, USA, 1985; pp. 249–254. [Google Scholar]
- Ruff, C.B.; Leo, F. Use of computed tomography in skeletal structure research. Yearb. Phys. Anthrop. 1986, 29, 181–196. [Google Scholar] [CrossRef]
- Weber, G.W. Virtual Anthropology (VA): A call for glasnost in paleoanthropology. Anat. Rec. 2001, 265, 193–201. [Google Scholar] [CrossRef]
- Immel, A.; Le Cabec, A.; Bonazzi, M.; Herbig, A.; Temming, H.; Schuenemann, V.J.; Bos, K.I.; Langbein, F.; Harvati, K.; Bridault, A.; et al. Effect of X-ray irradiation on ancient DNA in sub-fossil bones–Guidelines for safe X-ray imaging. Sci. Rep. 2016, 6, 32969. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Martín-Francés, L. Quantifying the impact of µCT-scanning of human fossil teeth on ESR age results. Am. J. Phys. Anthropol. 2017, 163, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastir, M.; Rosas, A.; Gunz, P.; Peña-Melian, A.; Manzi, G.; Harvati, K.; Kruszynski, R.; Stringer, C.; Hublin, J.-J. Evolution of the base of the brain in highly encephalized human species. Nat. Commun. 2011, 2, 588. [Google Scholar] [CrossRef] [Green Version]
- Fournier, M.; Combès, B.; Roberts, N.; Braga, J.; Prima, S. Mapping the distance between the brain and the inner surface of the skull and their global asymmetries. Med. Imaging 2011 Image Process. 2011, 7962, 79620Y. [Google Scholar]
- de Jager, E.J.; van Schoor, A.N.; Hoffman, J.W.; Oettlé, A.C.; Fonta, C.; Mescam, M.; Risser, L.; Beaudet, A. Sulcal pattern variation in extant human endocasts. J. Anat. 2019, 235, 803–810. [Google Scholar] [CrossRef]
- Bruner, E.; Grimaud-Hervé, D.; Wu, X.; Cuétara, J.M.; Holloway, R. A paleoneurological survey of Homo erectus endocranial metrics. Quat. Int. 2015, 368, 80–87. [Google Scholar] [CrossRef]
- Neubauer, S.; Hublin, J.-J.; Gunz, P. The evolution of modern human brain shape. Sci. Adv. 2018, 4, eaao5961. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, S.; Gunz, P.; Scott, N.A.; Hublin, J.-J.; Mitteroecker, P. Evolution of brain lateralization: A shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 2020, 6, eaax9935. [Google Scholar] [CrossRef] [Green Version]
- Germanaud, D.; Lefèvre, J.; Fischer, C.; Bintner, M.; Curie, A.; Portes, V.D.; Eliez, S.; Elmaleh-Bergès, M.; Lamblin, D.; Passemard, S.; et al. Simplified gyral pattern in severe developmental microcephalies? New insights from allometric modeling for spatial and spectral analysis of gyrification. NeuroImage 2014, 102, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.; Zollikofer, C.P.E.; Ponce de León, M.; Semendeferi, K.; Alatorre Warren, J.L.; Hopkins, W.D. Identification of in vivo Sulci on the External Surface of Eight Adult Chimpanzee Brains: Implications for Interpreting Early Hominin Endocasts. Brain. Behav. Evol. 2018, 91, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Alatorre Warren, J.L.; Ponce de León, M.; Hopkins, W.D.; Zollikofer, C.P.E. Evidence for independent brain and neurocranial reorganization during hominin evolution. Proc. Natl. Acad. Sci. USA 2019, 116, 22115–22121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangin, J.F.; Auzias, G.; Coulon, O.; Sun, Z.Y.; Rivière, D.; Régis, J. Sulci as landmarks. In Brain Mapping: An Encyclopedic Reference; Academic Press: Cambridge, MA, USA, 2015; Volume 2, pp. 45–52. [Google Scholar]
- Mangin, J.F.; Le Guen, Y.; Labra, N.; Grigis, A.; Frouin, V.; Guevara, M.; Fischer, C.; Rivière, D.; Hopkins, W.D.; Régis, J.; et al. “Plis de passage” deserve a role in models of the cortical folding process. Brain Topogr. 2019, 32, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Llinares-Benadero, C.; Borrell, V. Deconstructing cortical folding: Genetic, cellular and mechanical determinants. Nat. Rev. Neurosci. 2019, 20, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Tallinen, T.; Chung, J.Y.; Rousseau, G.; Girard, N.; Lefèvre, J.; Mahadevan, L. On the growth and form of cortical convolutions. Nat. Phys. 2016, 12, 588–593. [Google Scholar] [CrossRef]
- Van Essen, D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997, 385, 313–318. [Google Scholar] [CrossRef]
- Cachia, A.; Borst, G.; Jardri, R.; Raznahan, A.; Murray, G.K.; Mangin, J.F.; Plaze, M. Towards deciphering the fetal foundation of normal cognition and cognitive symptoms from sulcation of the cortex. Front. Neuroanat. 2021, in press. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Pinel, P.; Rivière, D.; Moreno, A.; Dehaene, S.; Mangin, J.F. Linking morphological and functional variability in hand movement and silent reading. Brain Struct. Funct. 2016, 221, 3361–3371. [Google Scholar] [CrossRef]
- Weaver, A.H. Reciprocal evolution of the cerebellum and neocortex in fossil humans. Proc. Natl. Acad. Sci. USA 2005, 102, 3576–3580. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Pan, L. Identification of Zhoukoudian Homo erectus brain asymmetry using 3D laser scanning. Chin. Sci. Bull. 2011, 56, 2215–2220. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry analyses revisited. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 279–319. [Google Scholar]
- Gómez-Robles, A.; Hopkins, W.D.; Sherwood, C.C. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130575. [Google Scholar] [CrossRef] [Green Version]
- Weaver, T.D.; Gunz, P. Using geometric morphometric visualizations of directional selection gradients to investigate morphological differentiation. Evolution 2018, 72, 838–850. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P. Advances in geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Richtsmeier, J.T.; Cole, T.M.; Lele, S.R. An invariant approach to the study of fluctuating asymmetry: Developmental instability in a mouse model for Down syndrome. In Modern Morphometrics in Physical Anthropology; Springer: Berlin, Germany, 2005; pp. 187–212. [Google Scholar]
- Kent, J.T.; Mardia, K.V. Shape, Procrustes tangent projections and bilateral symmetry. Biometrika 2001, 88, 469–485. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; McIntyre, G.S. Geometric Morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Mardia, K.V.; Bookstein, F.L.; Moreton, I.J. Statistical assessment of bilateral symmetry of shapes. Biometrika 2000, 87, 285–300. [Google Scholar] [CrossRef]
- Combès, B.; Hennessy, R.; Waddington, J.L.; Roberts, N.; Prima, S. Automatic symmetry plane estimation of bilateral objects in point clouds. In Proceedings of the 2008 IEEE Conference on Computer Vision and Pattern Recognition-CVPR’2008, Anchorage, AK, USA, 24–26 June 2008. [Google Scholar]
- Combès, B.; Fournier, M.; Kennedy, D.N.; Braga, J.; Roberts, N.; Prima, S. EM-ICP strategies for joint mean shape and correspondences estimation: Applications to statistical analysis of shape and of asymmetry. In Proceedings of the 8th IEEE International Symposium on Biomedical Imaging: From Nano to Macro (ISBI’2011), Chicago, IL, USA, 30 March–2 April 2011; pp. 1257–1263. [Google Scholar]
- Abdel Fatad, E.E.; Shirley, N.R.; Mahfouz, M.R.; Auerbach, B.M. A three-dimensional analysis of bilateral directional asymmetry in the human clavicles. Am. J. Phys. Anthrop. 2012, 49, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Balzeau, A.; Gilissen, E. Endocranial shape asymmetries in Pan paniscus, Pan troglodytes and Gorilla gorilla assessed via skull based landmark analysis. J. Hum. Evol. 2010, 59, 54–69. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Klöppel, S.; Rivière, D.; Perrot, M.; Frackowiak, R.; Siebner, H.; Mangin, J.F. The effect of handedness on the shape of the central sulcus. Neuroimage 2012, 60, 332–339. [Google Scholar] [CrossRef]
- Sprung-Much, T.; Eichert, N.; Nolan, E.; Petrides, M. Broca’s area and the search for anatomical asymmetry: Commentary and perspectives. Brain Struct. Funct. 2021, 1–9. [Google Scholar] [CrossRef]
- LeMay, M. Morphological cerebral asymmetries of modern man, fossil man and nonhuman primate. Ann. N. Y. Acad. Sci. 1976, 280, 349–366. [Google Scholar] [CrossRef]
- LeMay, M. Asymmetries of the skull and handedness. J. Neurol. Sci. 1977, 32, 243–253. [Google Scholar] [CrossRef]
- Holloway, R.L.; De La Coste-Lareymondie, M.C. Brain endocast asymmetry in pongids and hominids: Some preliminary findings on the paleontology of cerebral dominance. Am. J. Phys. Anthrop. 1982, 58, 101–110. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Pilcher, D.L.; Hammock, E.A.D.; Hopkins, W.D. Cerebral volumetric asymmetries in non-human primates: A magnetic resonance imaging study. Laterality 2001, 6, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001, 14, 685–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, K.E.; Paus, T.; Lerch, J.; Zijdenbos, A.; Collins, D.L.; Neelin, P.; Taylor, J.; Worsley, K.; Evans, A. Structural asymmetries in the human brain: A voxel-based statistical analysis of 142 MRI scans. Cereb. Cortex 2001, 11, 868–877. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Taglialatela, J.P.; Meguerditchian, A.; Nir, T.; Schenker-Ahmed, N.M.; Sherwood, C.C. Gray matter asymmetries in chimpanzees as revealed by voxel-based morphometry. Neuroimage 2008, 42, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Holloway, R.L. Volumetric and asymmetry determinations on recent hominid endocasts: Spy I and II, Djebel Ihroud I, and the Salé Homo erectus specimens. Am. J. Phys. Anthrop. 1981, 5, 385–393. [Google Scholar] [CrossRef] [PubMed]
- LeMay, M.; Kido, D.K. Asymmetries of the cerebral hemispheres on computed tomograms. J. Comput. Assist. Tomogr. 1978, 2, 471–476. [Google Scholar]
- LeMay, M.; Billig, M.S.; Geschwind, N. Asymmetries of the brains and skulls of nonhuman primates. In Primate Brain Evolution, Methods and Concepts; Falk, D., Armstrong, E., Eds.; Plenum Press: New York, NY, USA, 1976; pp. 263–277. [Google Scholar]
- Galaburda, A.M.; LeMay, M.; Kemper, T.L.; Geschwind, N. Right-left asymmetries in the brain. Science 1978, 199, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Black, S.E.; Polk, M.; Howell, J. Cerebral asymmetries on magnetic resonance imaging. Cortex 1986, 22, 117–127. [Google Scholar] [CrossRef]
- Falk, D.; Hildebolt, C.; Cheverud, J.; Vannier, M.W.; Helmkamp, R.C.; Konigsberg, L. Cortical asymmetries in frontal lobes of rhesus monkeys (Macaca mulatta). Brain Res. 1990, 512, 40–45. [Google Scholar] [CrossRef]
- LeMay, M. Asymmetries of the brains and skulls of nonhuman primates. In Cerebral Lateralization in Nonhuman Species; Glick, S.D., Ed.; Academic Press: New York, NY, USA, 1985; pp. 233–245. [Google Scholar]
- Cain, D.P.; Wada, J.A. An anatomical asymmetry in the baboon brain. Brain Behav. Evol. 1979, 16, 222–226. [Google Scholar] [CrossRef]
- Cheverud, J.M.; Falk, D.; Hildebolt, C.; Moore, A.J.; Helmkamp, R.C.; Vannier, M.W. Heritability and association of cortical petalias in rhesus macaques (Macaca mulatta). Brain Behav. Evol. 1990, 35, 368–372. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Phillips, K.; Bania, A.; Calcutt, S.E.; Gardner, M.; Russell, J.; Schaeffer, J.; Lonsdorf, E.V.; Ross, S.R.; Schapiro, S.J. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in hominins. J. Hum. Evol. 2011, 60, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Bogart, S.L.; Mangin, J.-F.; Schapiro, S.J.; Reamer, L.; Bennett, A.J.; Pierre, P.J.; Hopkins, W.D. Cortical sulci asymmetries in chimpanzees and macaques: A new look at an old idea. Neuroimage 2012, 61, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Corballis, M.C.; Badzakova-Trajkov, G.; Häberling, I.S. Right hand, left brain: Genetic and evolutionary bases of cerebral asymmetries for language and manual action. WIREs Cogn. Sci. 2012, 3, 1–17. [Google Scholar] [CrossRef]
- Xiang, L.; Crow, T.; Roberts, N. Cerebral torque is human specific and unrelated to brain size. Brain Struct. Funct. 2019, 224, 1141–1150. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.S.; Crow, T.; Foundas, A.; Amunts, K.; Roberts, N. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009, 109, 29–48. [Google Scholar] [CrossRef]
- Balzeau, A.; Grimaud-Hervé, D.; Jacob, T. Internal cranial features of the Mojokerto child fossil (East Java, Indonesia). J. Hum. Evol. 2005, 48, 535–553. [Google Scholar] [CrossRef]
- Gunz, P.; Neubauer, S.; Golovanova, L.; Doronichev, V.; Maureille, B.; Hublin, J.J. A uniquely modern human pattern of endocranial development. Insights from a new cranial reconstruction of the Neandertal newborn from Mezmaiskaya. J. Hum. Evol. 2012, 62, 300–313. [Google Scholar] [CrossRef]
- Gunz, P.; Neubauer, S.; Maureille, B.; Hublin, J.J. Brain development after birth differs between Neanderthals and modern humans. Curr. Biol. 2010, 20, R921–R922. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, S.; Gunz, P.; Hublin, J.J. Endocranial shape changes during growth in chimpanzees and humans: A morphometric analysis of unique and shared aspects. J. Hum. Evol. 2010, 59, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León, M.S.; Bienvenu, T.; Akazawa, T.; Zollikofer, C.P.E. Brain development is similar in Neanderthals and modern humans. Curr. Biol. 2016, 26, R665–R666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzeau, A.; Holloway, R.L.; Grimaud-Hervé, D. Variations and asymmetries in regional brain surface in the genus Homo. J. Hum. Evol. 2012, 62, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Coulon, O.; Sein, J.; Auzias, G.; Nazarian, B.; Anton, J.L.; Rousseau, F.; Velly, L.; Girard, N. High temporal resolution longitudinal observation of fetal brain development. A baboon pilot study. In Proceedings of the 26th Annual Meeting of the Organization for Human Brain Mapping, Montreal, QC, Canada, 23 June–3 July 2020. [Google Scholar]
- Pearce, E.; Stringer, C.; Dunbar, R.I.M. New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130168. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, H.; Le Guen, Y.; Tilot, A.K.; Stein, J.L.; Philippe, C.; Mangin, J.-F.; Fisher, S.E.; Frouin, V. Genetic variations within human gained enhancer elements affect human brain sulcal morphology. bioRxiv 2021, in press. [Google Scholar]
- Ferran, J.L. Architect genes of the brain a look at brain evolution through genoarchitrecture. Metode Sci. Stud. J. 2017, 7, 17–23. [Google Scholar]
- Kochiyama, T.; Ogihara, N.; Tanabe, H.C.; Kondo, O.; Amano, H.; Hasegawa, K.; Suzuki, H.; De León, M.S.P.; Zollikofer, C.P.E.; Bastir, M.; et al. Reconstructing the Neanderthal brain using computational anatomy. Sci. Rep. 2018, 8, 6296. [Google Scholar] [CrossRef] [PubMed]
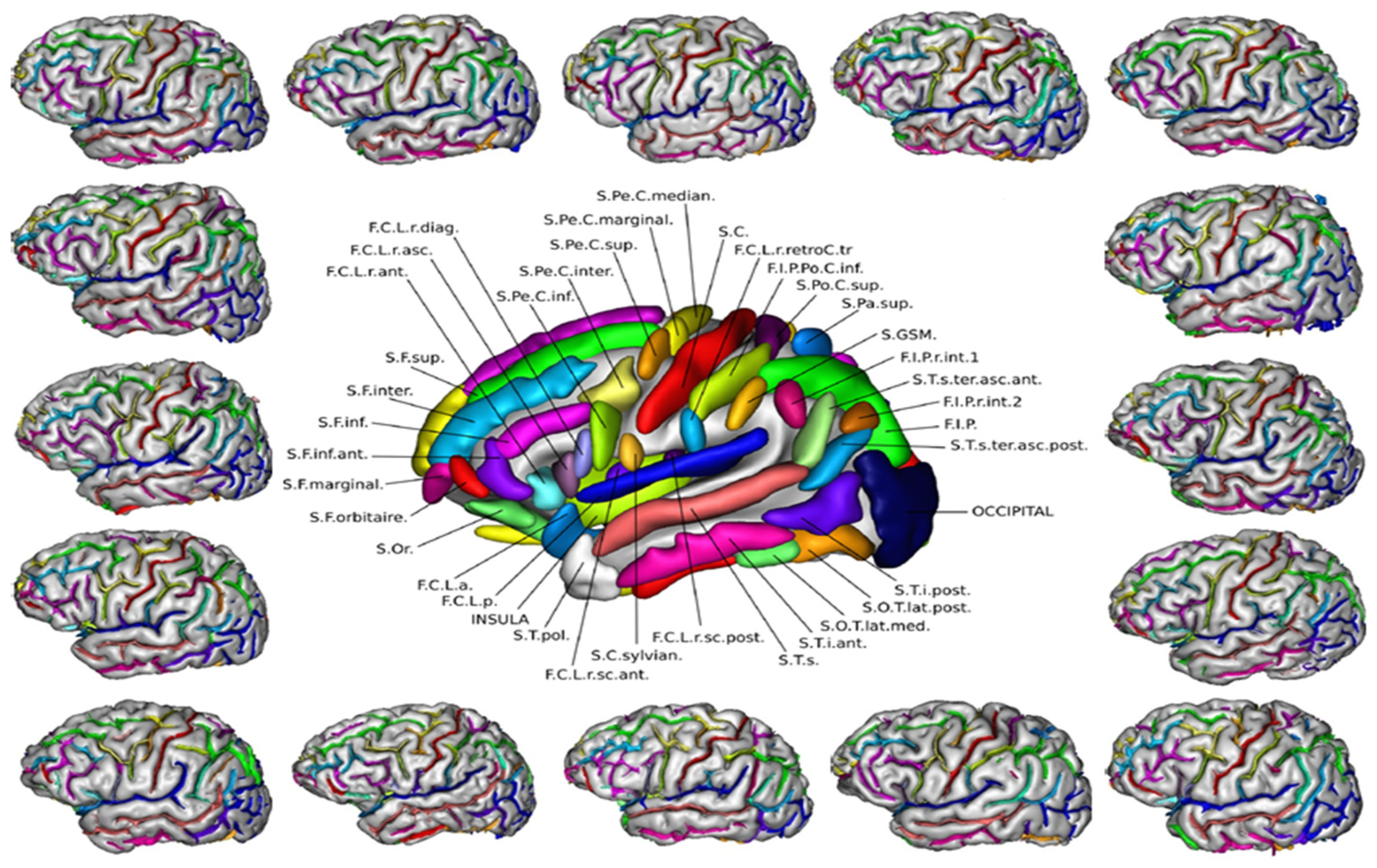
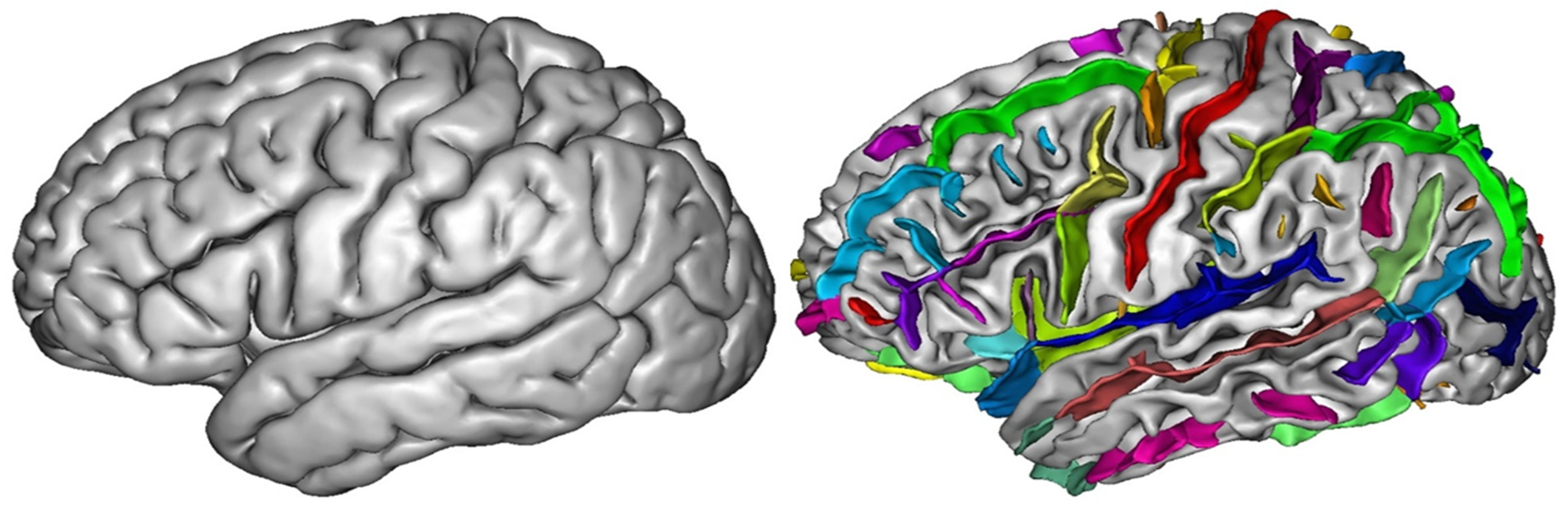




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzeau, A.; Mangin, J.-F. What Are the Synergies between Paleoanthropology and Brain Imaging? Symmetry 2021, 13, 1974. https://doi.org/10.3390/sym13101974
Balzeau A, Mangin J-F. What Are the Synergies between Paleoanthropology and Brain Imaging? Symmetry. 2021; 13(10):1974. https://doi.org/10.3390/sym13101974
Chicago/Turabian StyleBalzeau, Antoine, and Jean-François Mangin. 2021. "What Are the Synergies between Paleoanthropology and Brain Imaging?" Symmetry 13, no. 10: 1974. https://doi.org/10.3390/sym13101974
APA StyleBalzeau, A., & Mangin, J.-F. (2021). What Are the Synergies between Paleoanthropology and Brain Imaging? Symmetry, 13(10), 1974. https://doi.org/10.3390/sym13101974






