Resonance in Chirogenesis and Photochirogenesis: Colloidal Polymers Meet Chiral Optofluidics
Abstract
1. Introduction—Historical Backgrounds, Knowledge, Then, and Now
1.1. What Is the Life?—Open System, Negative Entropy, Non-Equilibrium Thermodynamics
1.2. Physical Advantage Factors for Left–Right Asymmetry
1.2.1. Circularly Polarized Light Source
1.2.2. Static Electric Field
1.2.3. Static Magnetic Field
1.2.4. Hydrodynamic Flowing as a Model of Coriolis Force with Gravitational Force
1.2.5. Static Magnetic Field with Polarized Light
1.2.6. Longitudinally Polarized Left-Handed β-Electron and Right-Handed β-Positron
1.2.7. Handed Weak Neutral Current Mediated by Z0 Boson
1.3. Extraterrestrial Origins of Life and Chirality—Panspermia Hypotheses
1.4. Terrestrial Origins of Life and Chirality
1.5. Extraterrestrial Origins of Circularly Polarized Radiation Sources
1.6. Coacervate Hypothesis
1.7. Colloids
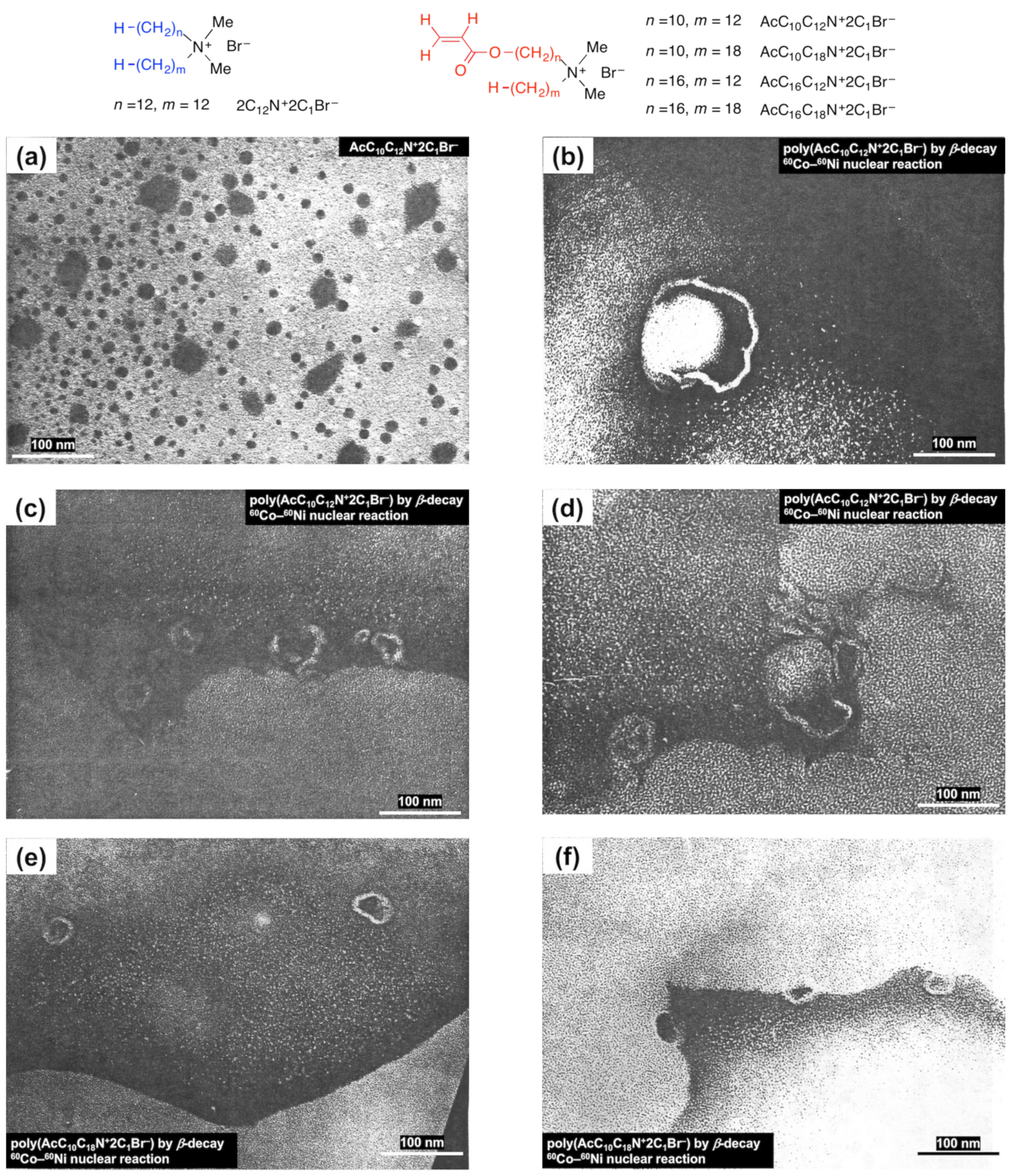
1.8. Ostwald Ripening and Viedma Ripening
1.9. Optofluidics Connecting to Colloids and Circularly Polarized Light
1.10. Microdroplets and Aerosols—Prebiotic Chemical Reactors
1.11. Our Hypothesis for Chirogenesis and Photochirogenesis
1.12. Resonance Effects from Colloidal Systems in Optofluidic Media in the Ground and Photoexcited States
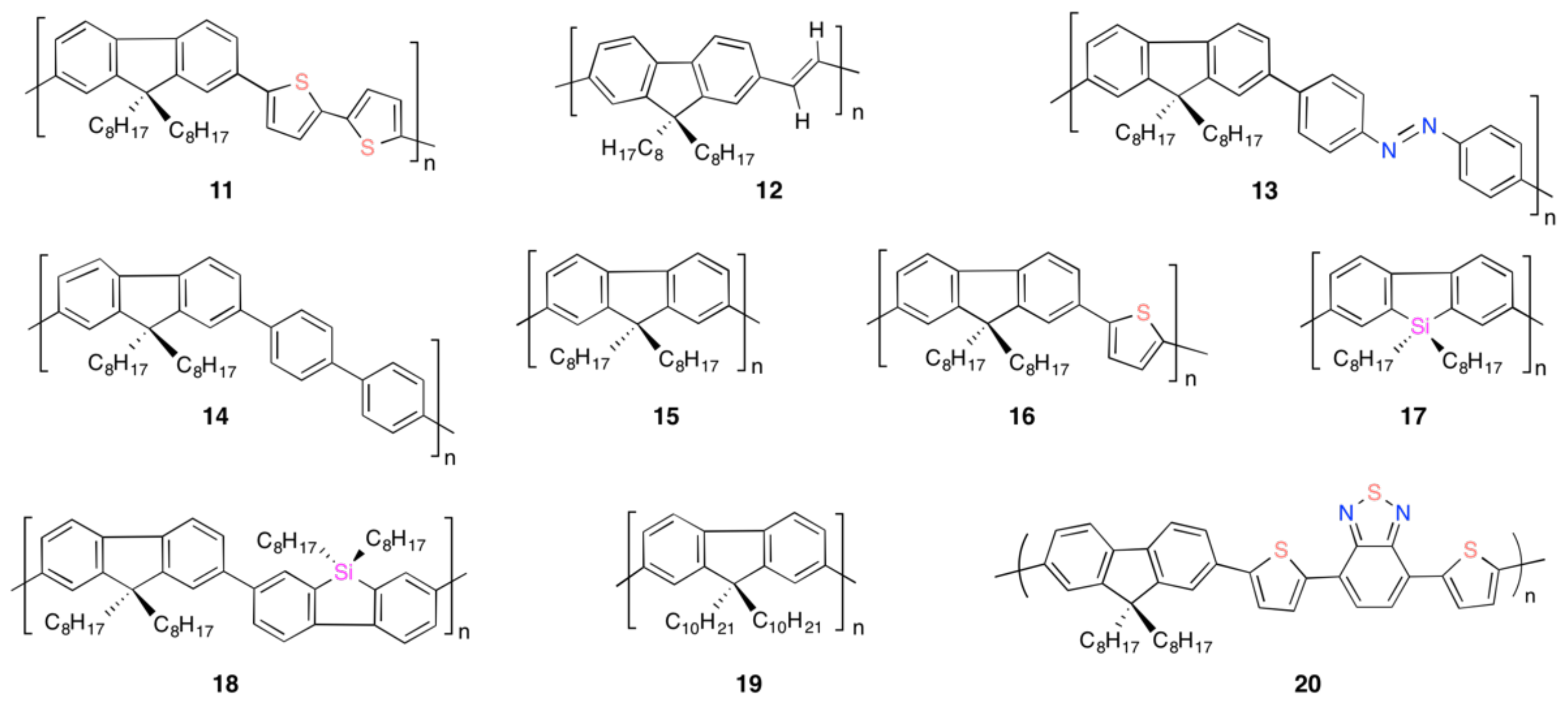
2. Colloid-Induced Chiroptical Enhancement and Aggregation-Induced Emission
3. Open-Flow, Non-Equilibrium Coacervate Hypothesis Meets Optofluidics
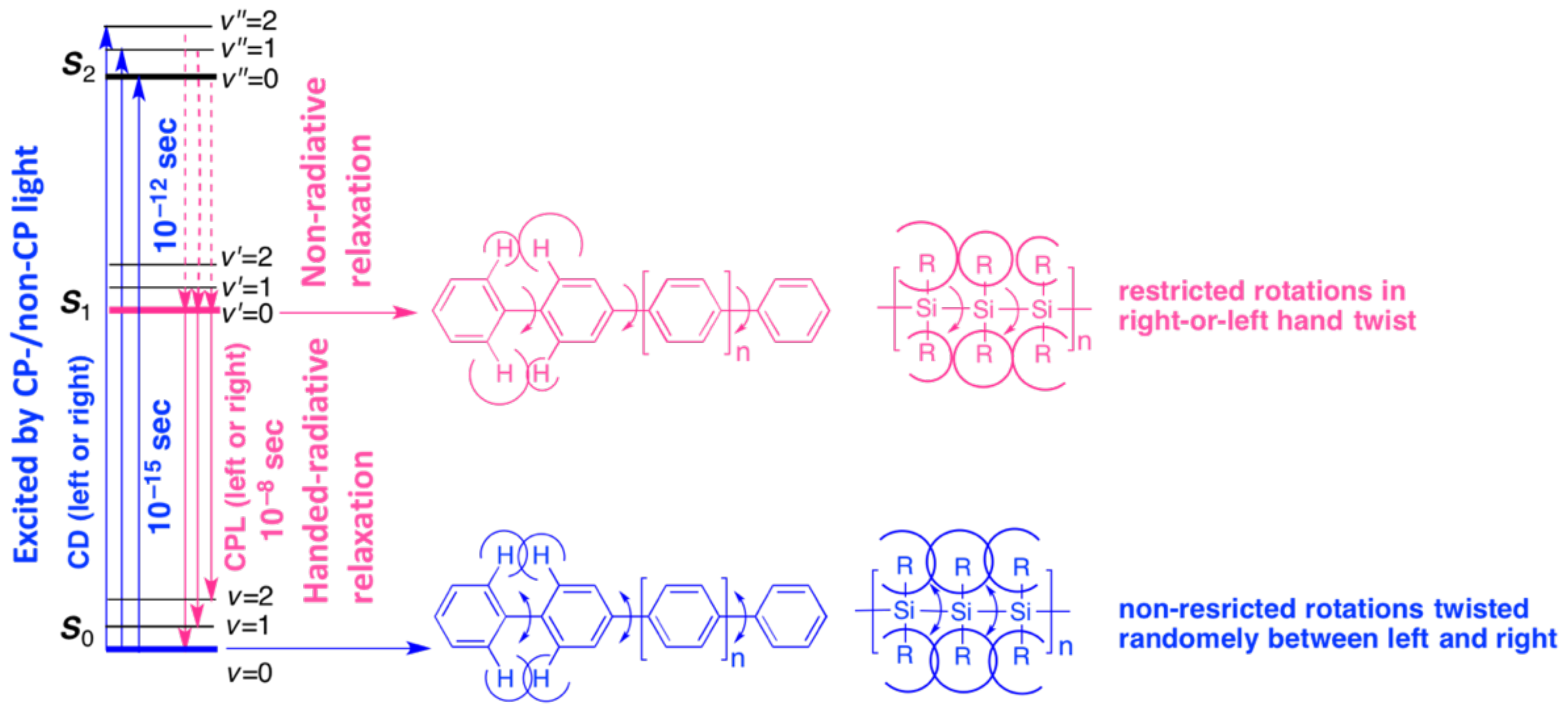
4. CIE-CD in the Ground State and CIE-CPL in the Photoexcited States
4.1. Steady-State CD and CPL Spectroscopies
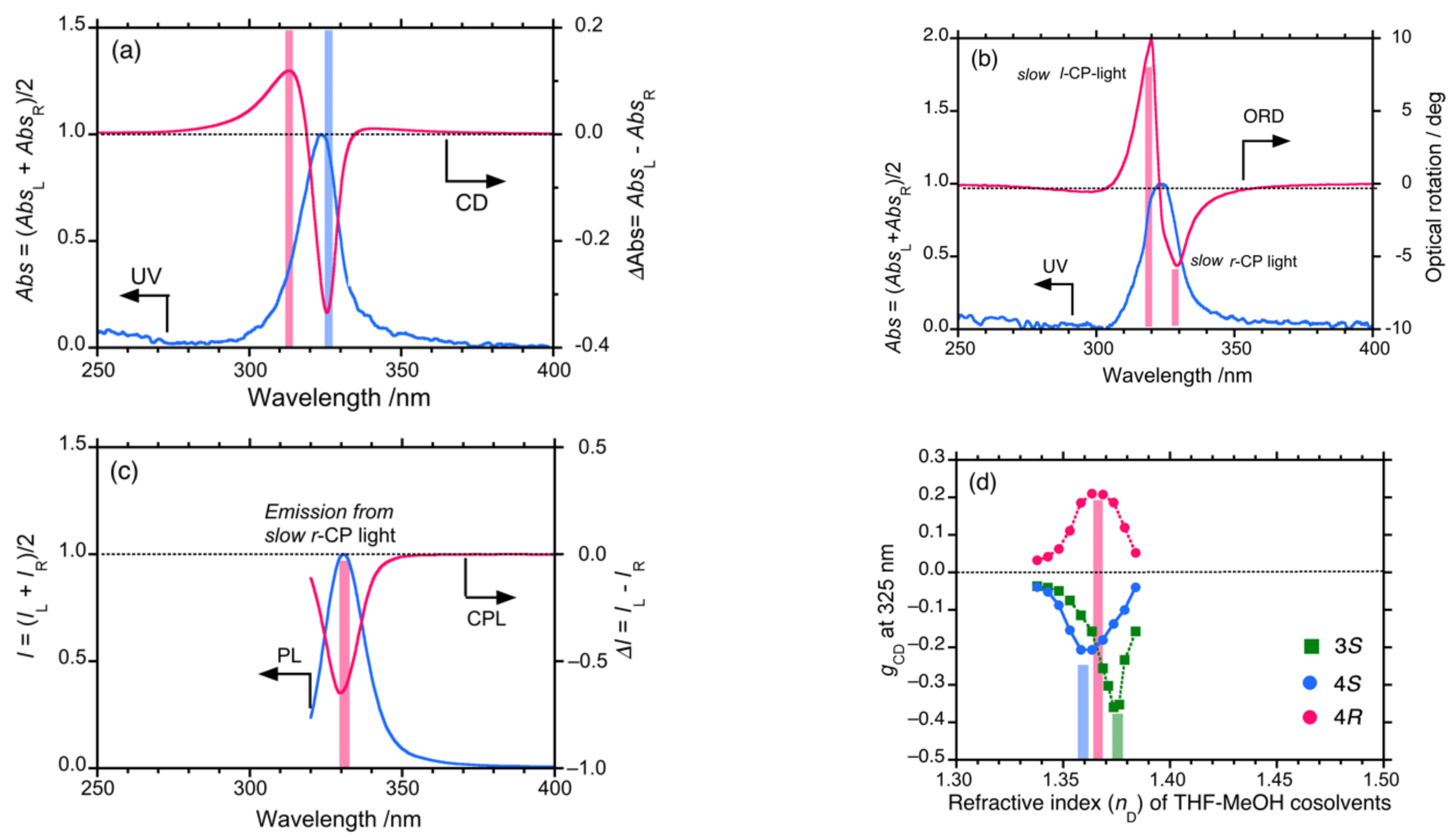
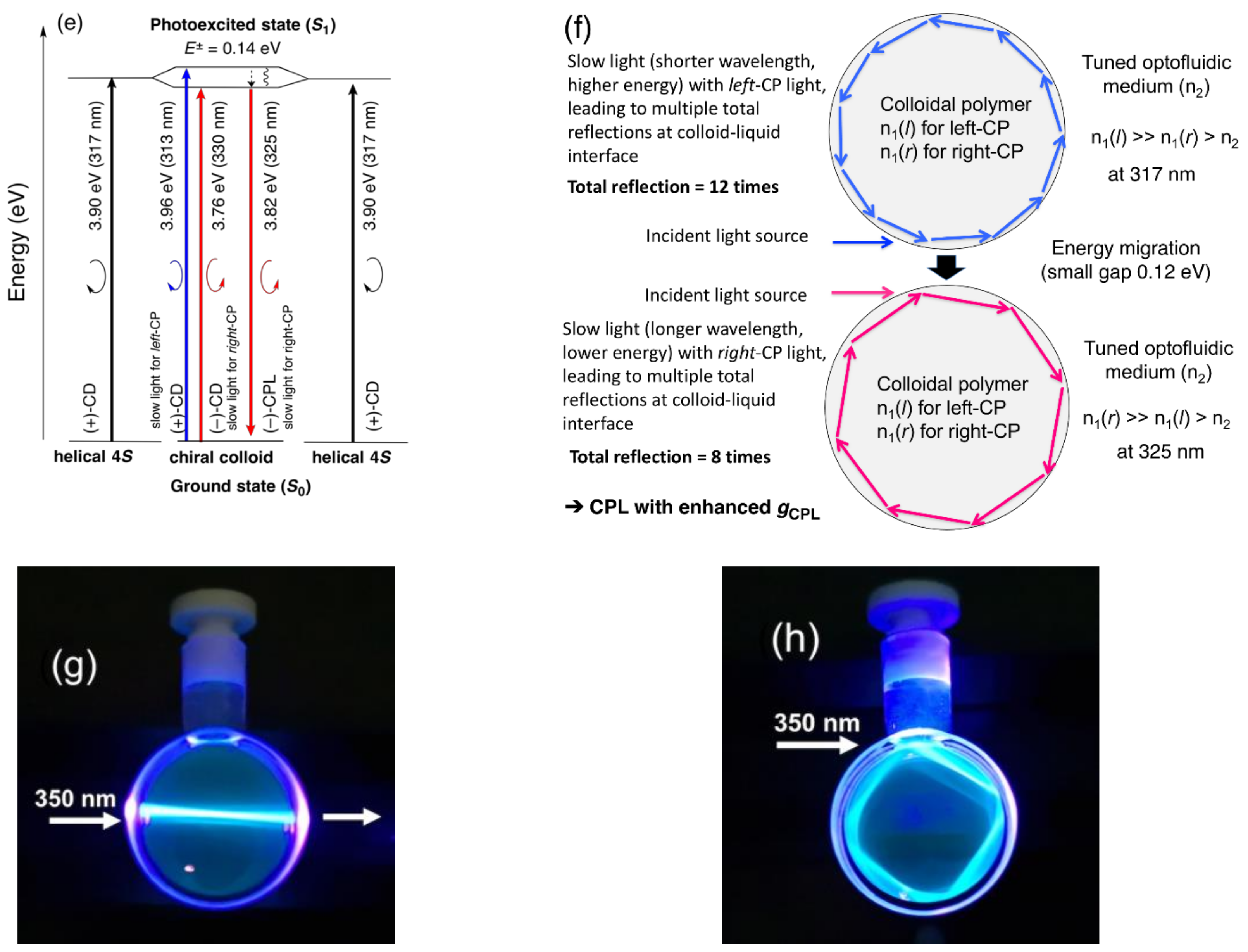
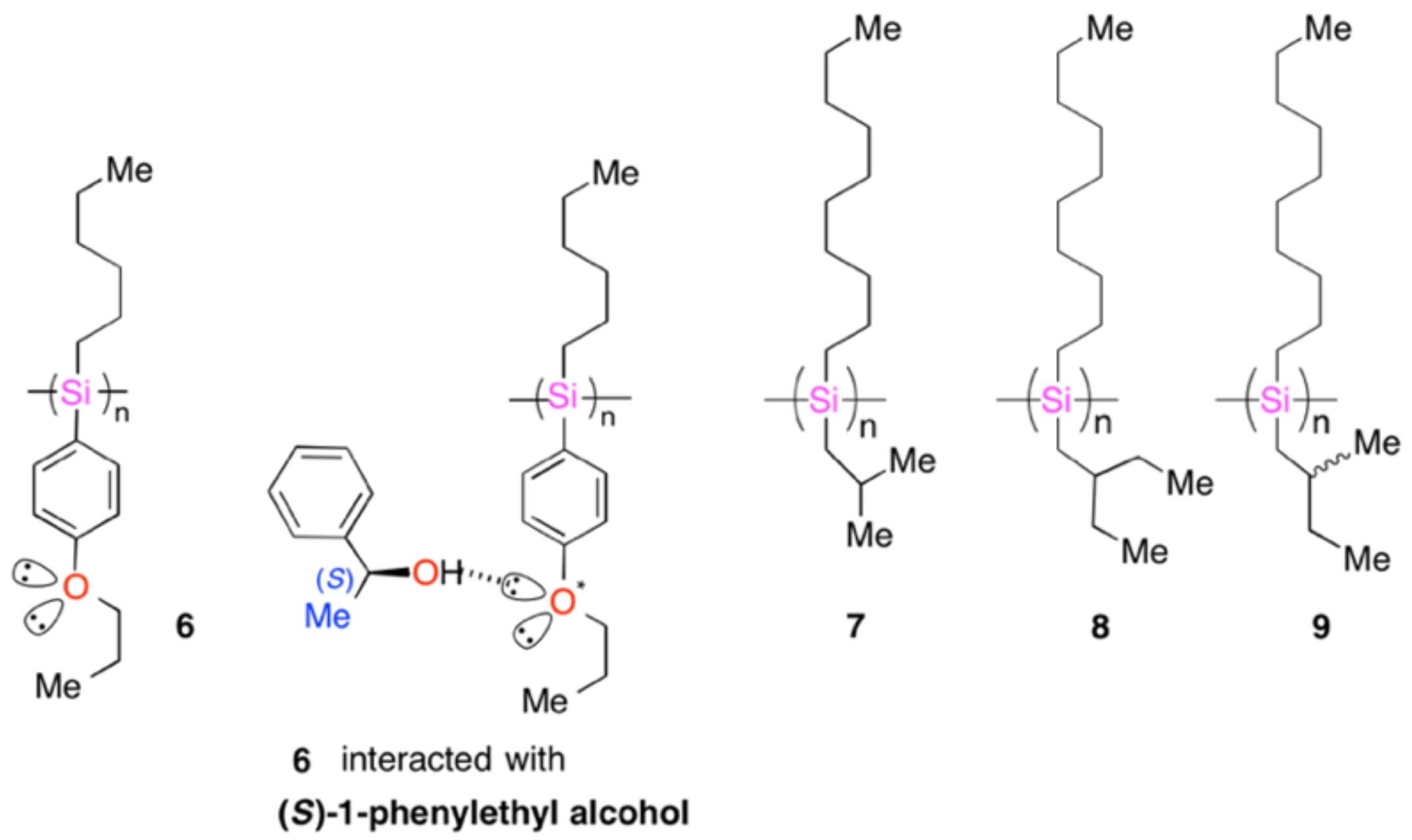
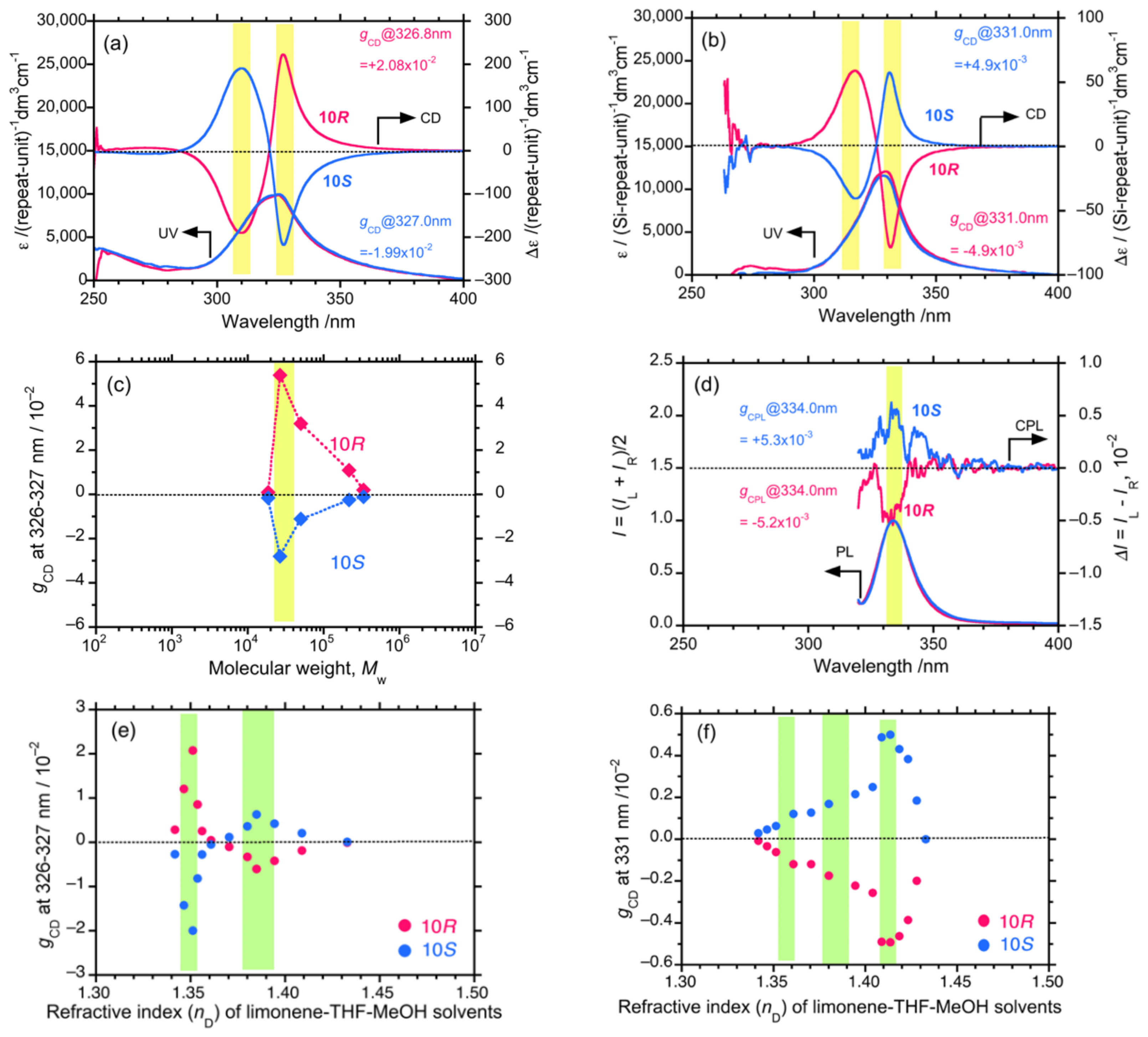
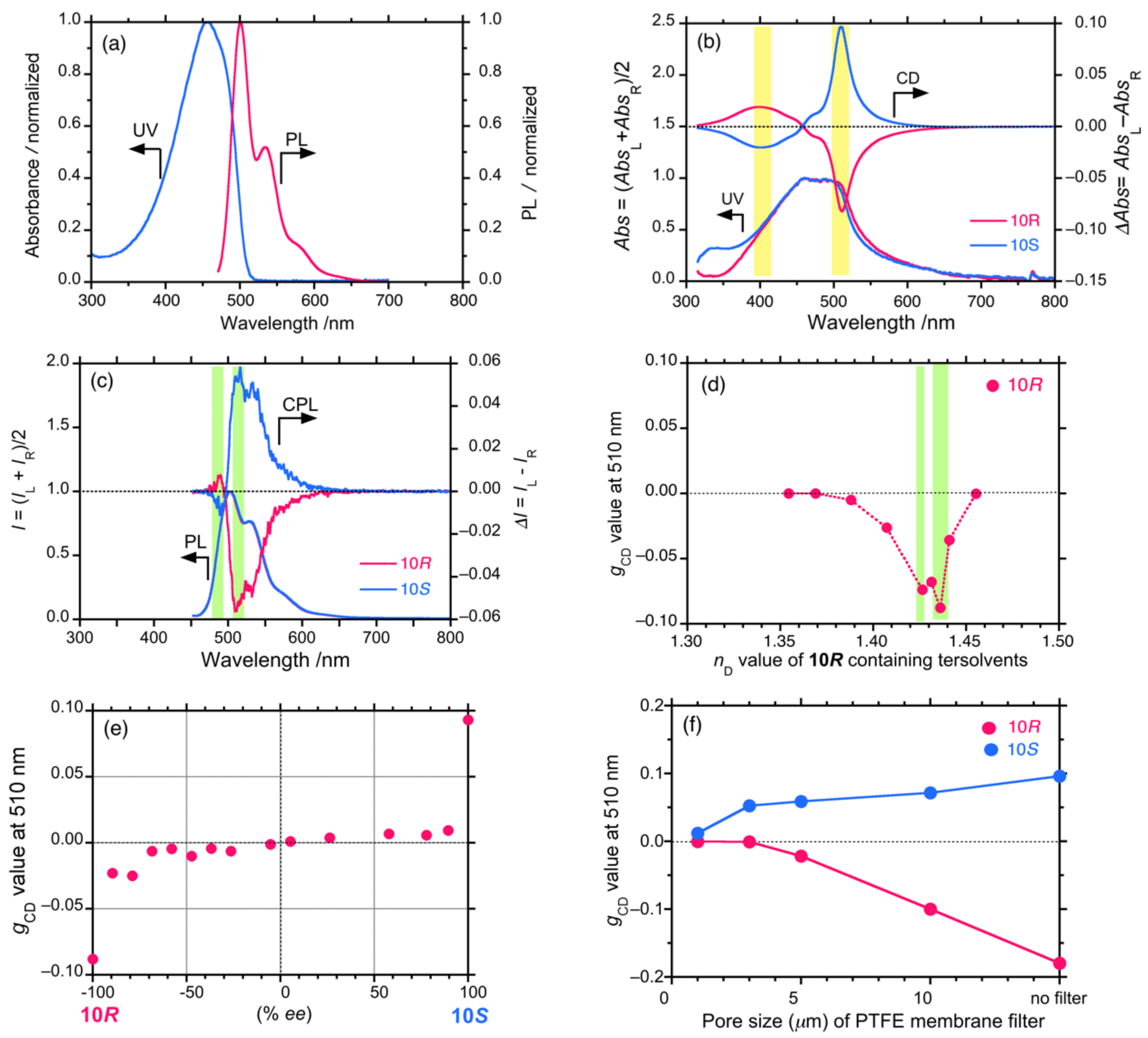
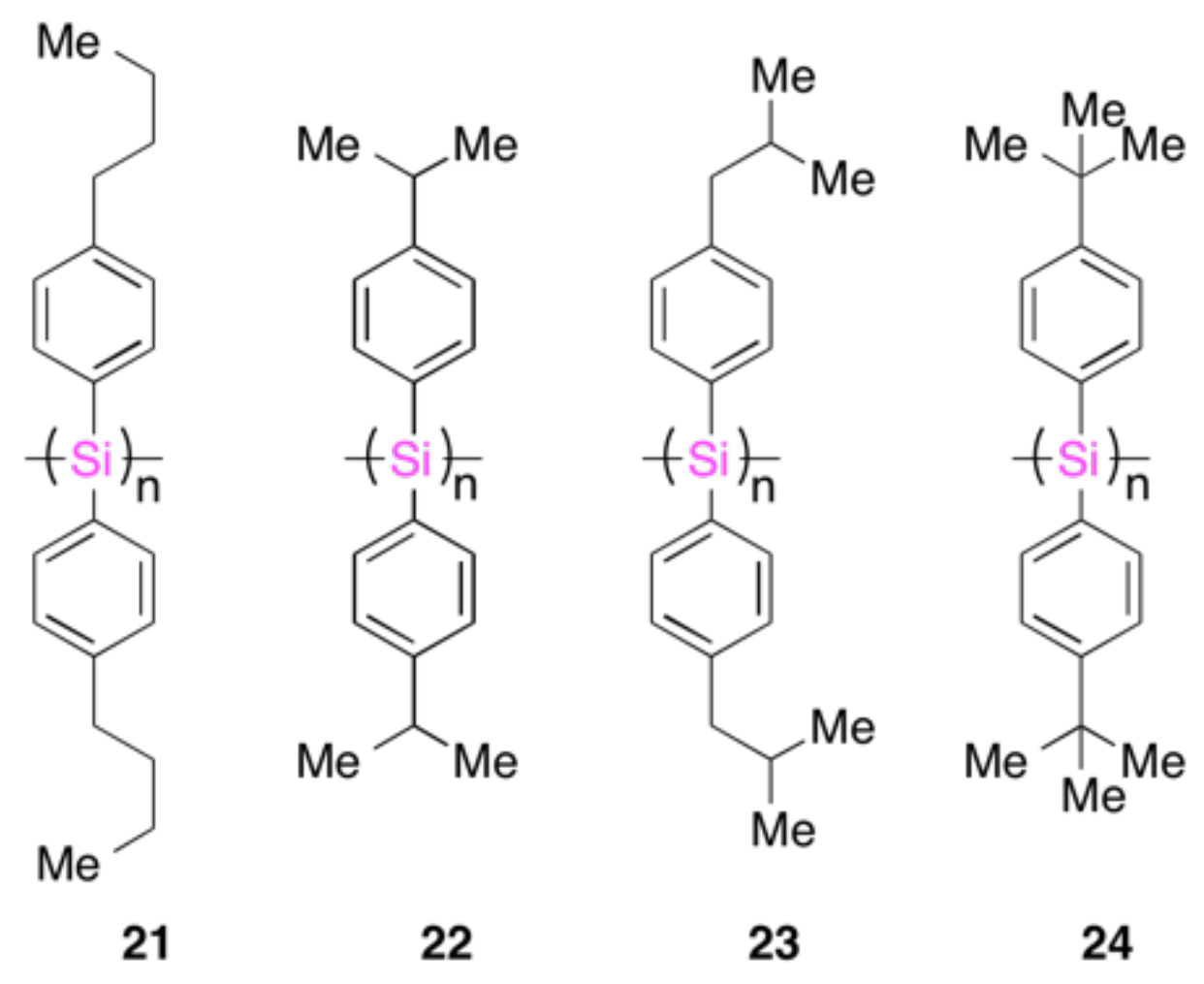
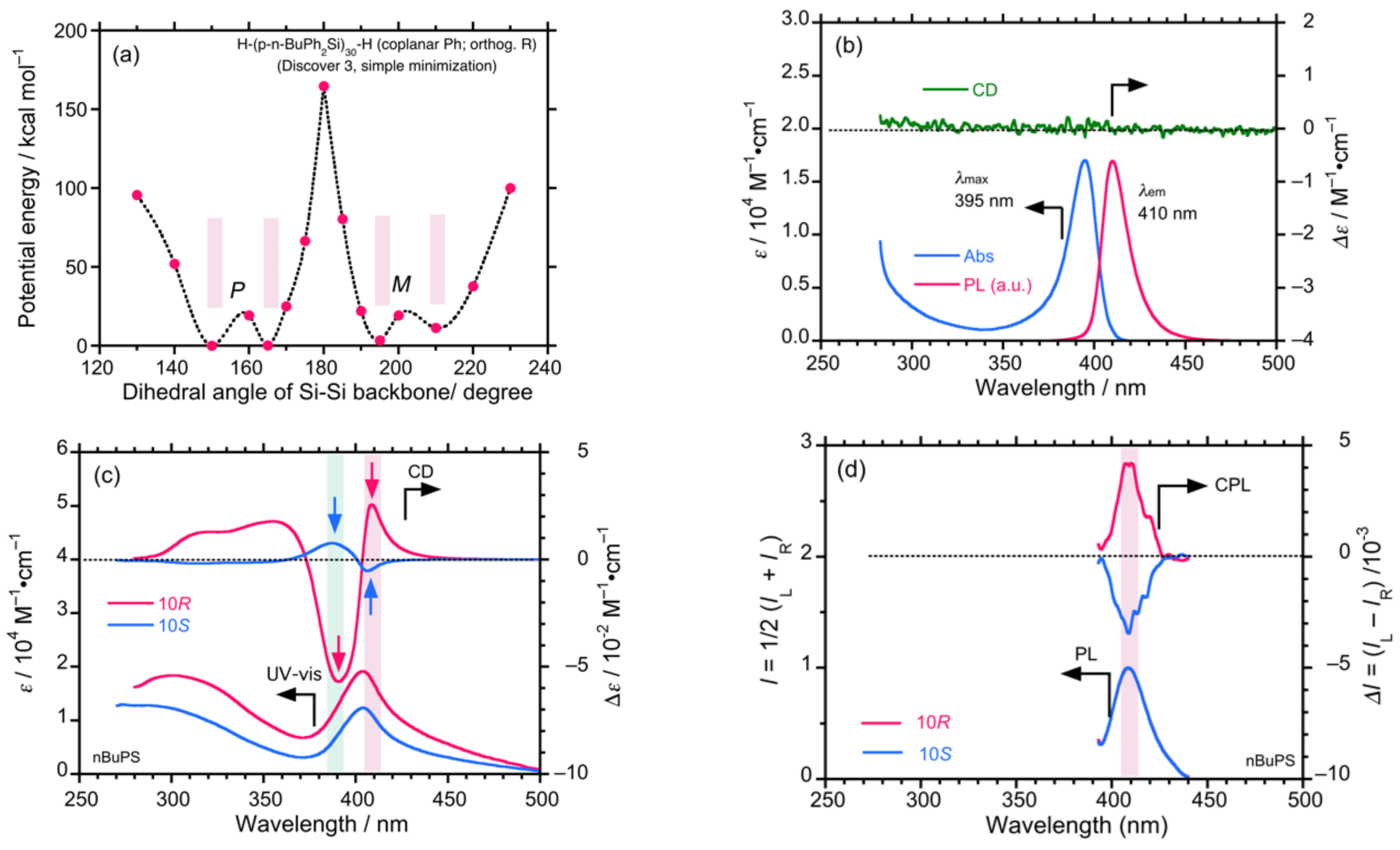
4.2. Gigantic Enhanced CD, ORD, and CPL from Colloidal Optically Active Helical Poly-silanes—Importance of Controlled RI in Optofluidic Medium
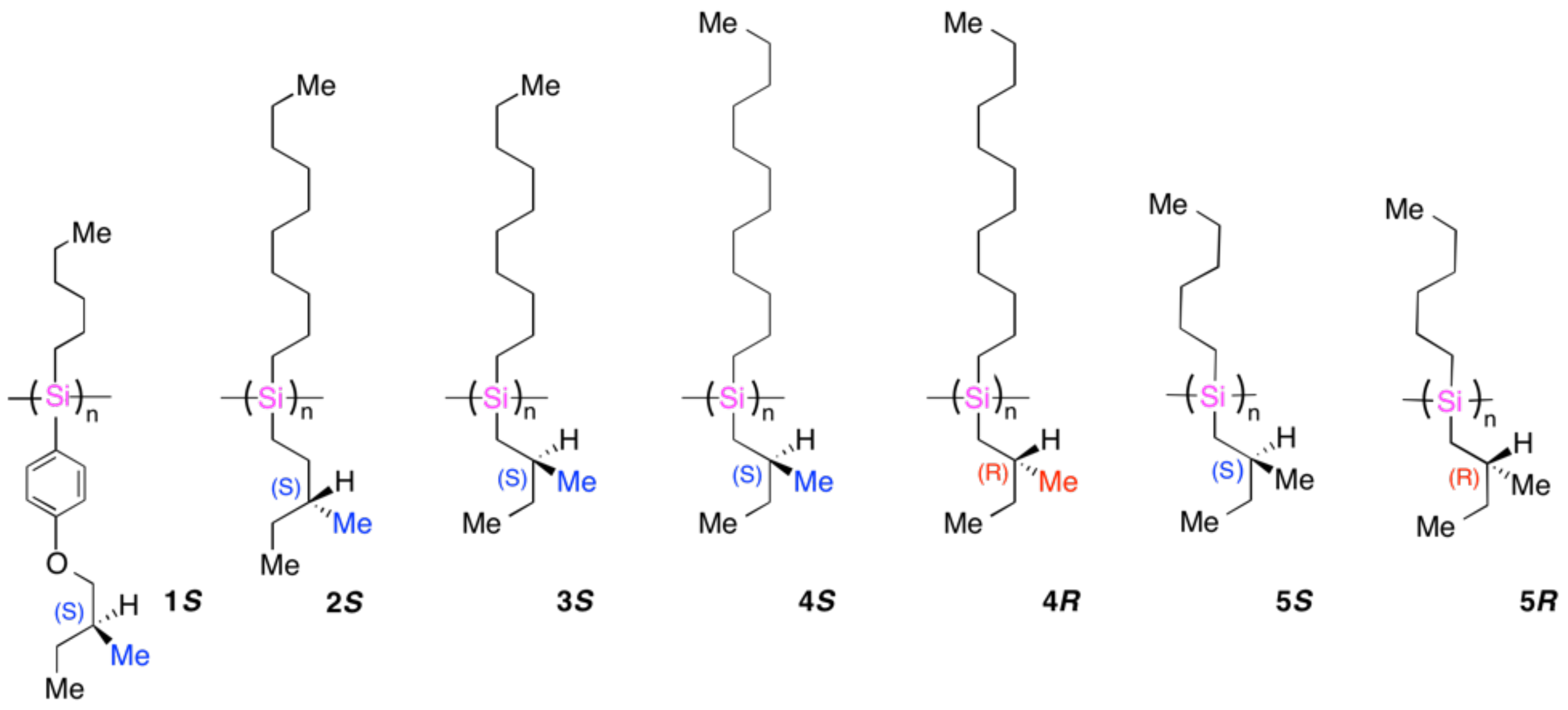
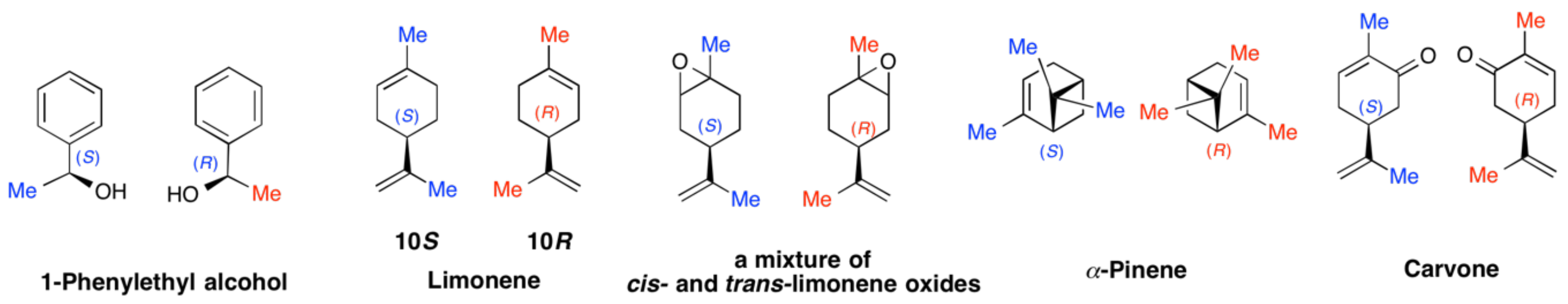
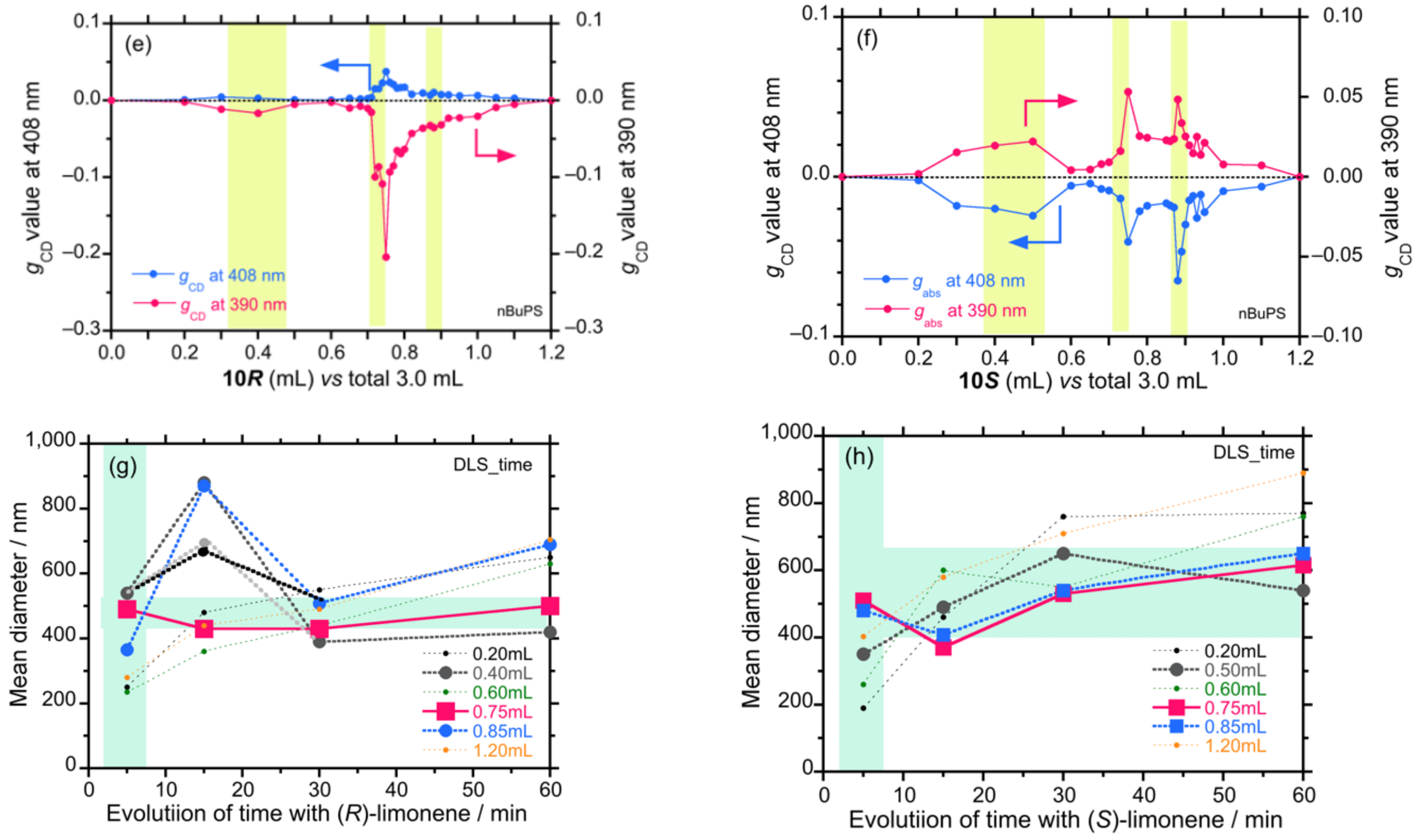
4.3. Controlled Chirogenesis from Optically Inactive Helical Polysilanes Endowed with Limonene Chirality
4.4. Chirogenesis from Achiral π-Conjugated Polymers Endowed with Limonene Chirality
4.5. Fully Controlled Absolute Photochirogenesis from Achiral π-Conjugated Polymers Endowed with Excitation Wavelength Dependent Circularly Polarized Light Chirality
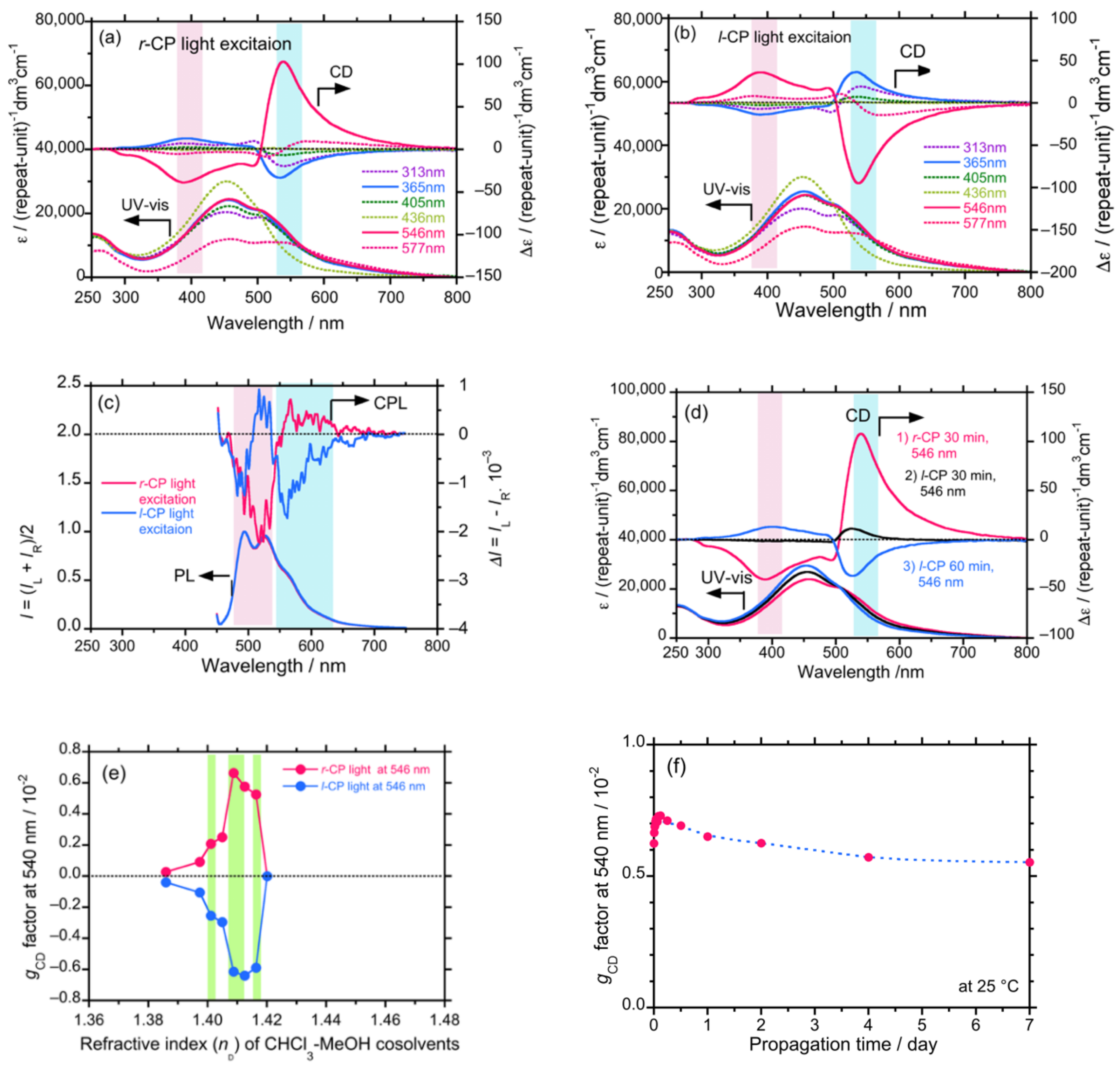
4.5.1. Achiral π-Conjugated Polymer Containing Azobenzene Unit as a Backbone Endowed with Circularly Polarized Light Chirality
4.5.2. Achiral Luminescent π-Conjugated Polymer Endowed with Excitation Wavelength Dependent Circularly Polarized Light Chirality
4.5.3. Achiral Polymethacrylate Carrying Azobenzene Pendants Endowed with Excitation Wavelength Dependent Circularly Polarized Light Chirality
4.6. Tempo-Spatial Chirogenesis
4.6.1. Changes in Colloidal Sizes of Diaryl Polysilane with Propagation Time
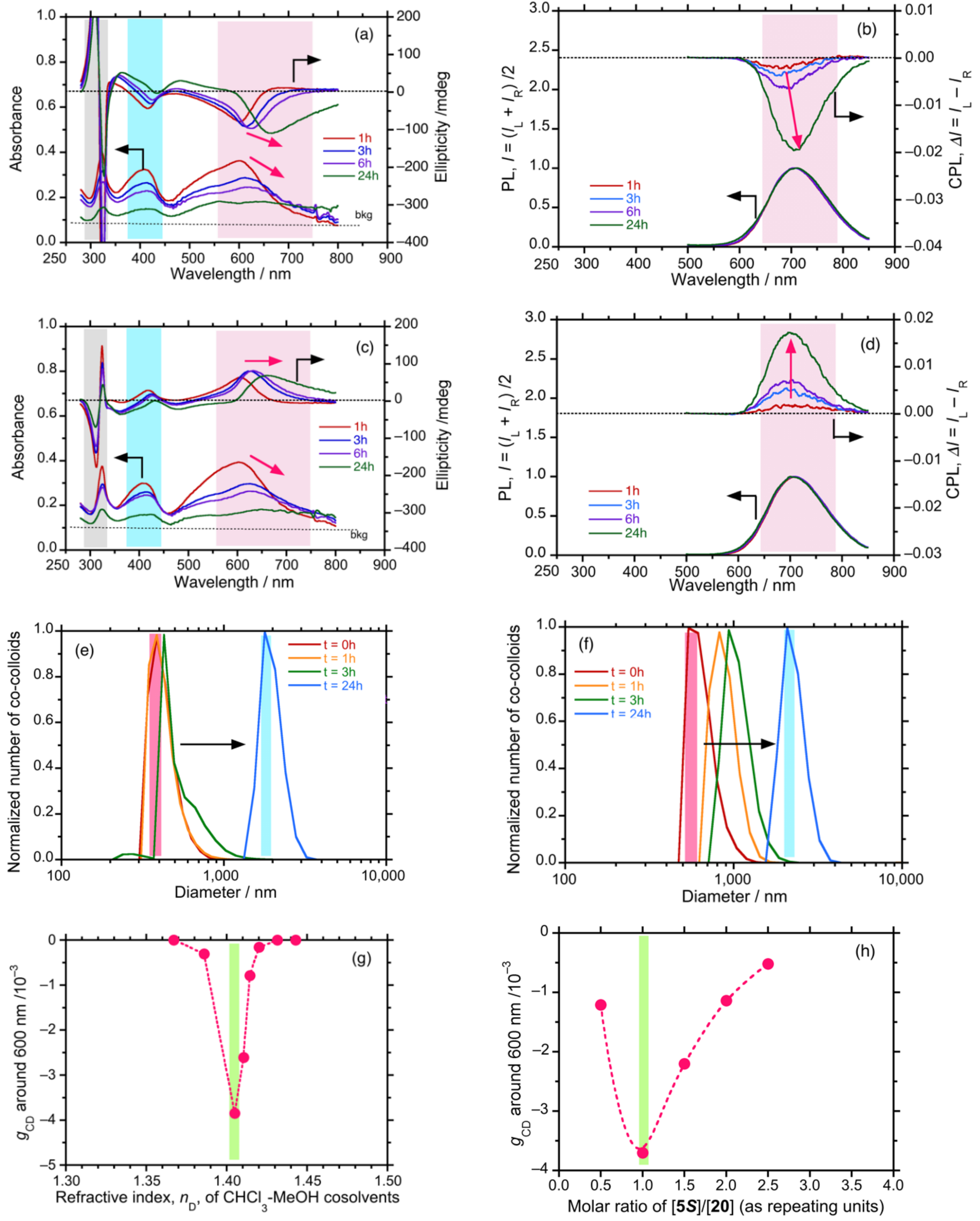
4.6.2. Time-Dependent Evolution of CIE-CD and CIE-CPL in Co-Colloids of Achiral π-Conjugated Polymer and Helical Dialkyl Polysilanes
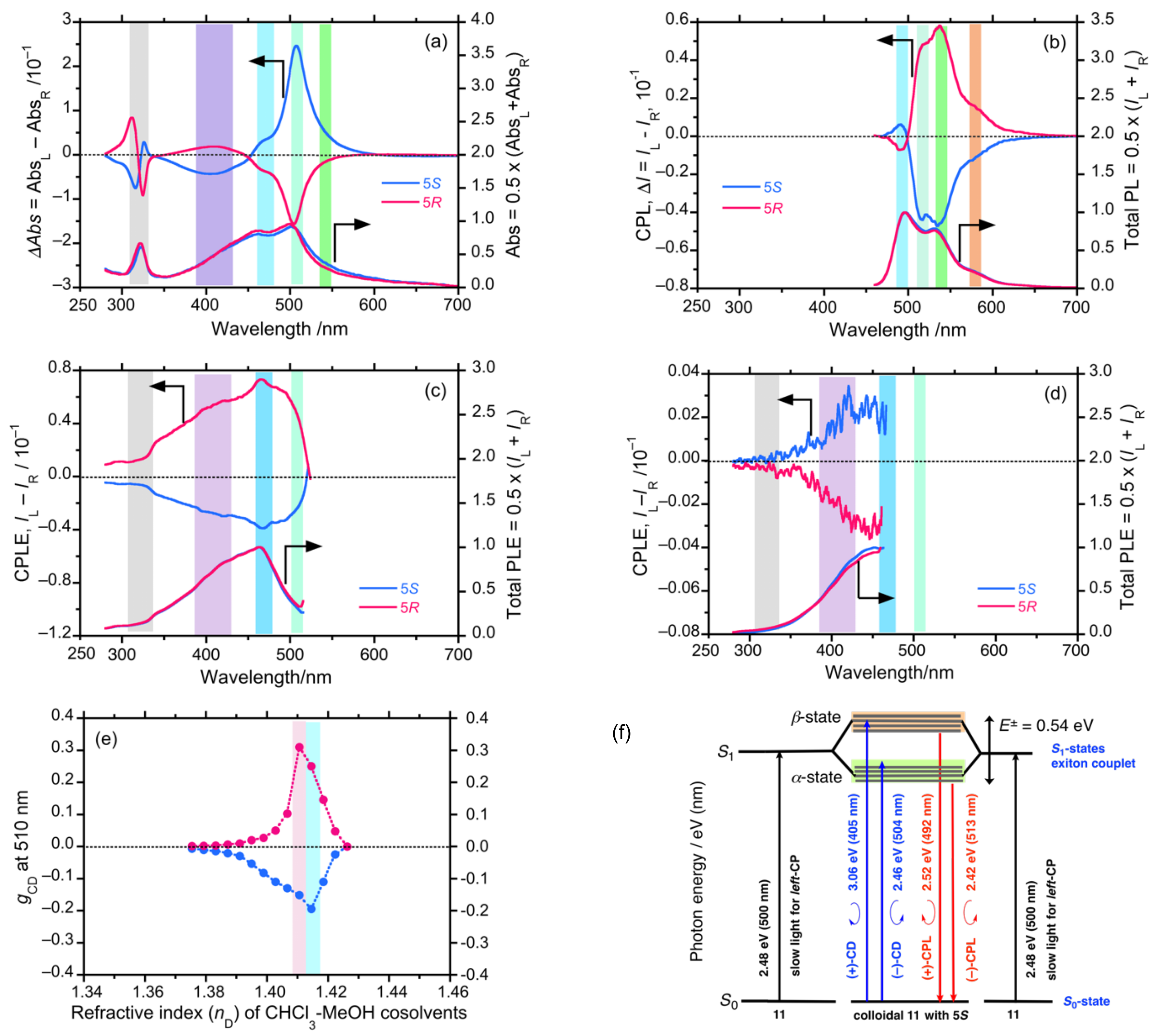
4.7. Unveiling Anti-Kasha’s Rule from CIE-CPL, CIE-CPLE, and CIE-CD in Co-Colloids of Achiral π-Conjugated Polymer and Helical Polysilanes
5. Perspectives—Colloids Connecting to Light, Helix, Coacervate, Panspermia, Microoptics, Chiroptics, Radioisotopes, Nuclear Physics, Biology, Homochirality Question, and Cosmology
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrodinger, E. What Is Life?: With Mind and Matter and Autobiographical Sketches; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Mason, S.F. Molecular Optical Activity and the Chiral Discriminations; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Mason, S.F.; Tranter, G.E. The Electroweak Origin of Biomolecular Handedness. Proc. R. Soc. Lond. A 1985, 397, 45–65. [Google Scholar]
- Bonner, W.A. Terrestrial and Extraterrestrial Sources of Molecular Homochirality. Orig. Life Evol. Biosph. 1992, 21, 407–420. [Google Scholar] [CrossRef]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular Polarization in Star-Formation Regions: Implications for Biomolecular Homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C. From Parity to Chirality: Chemical Implications Revisited. Tetrahedron Asymmetry 2000, 11, 2845–2874. [Google Scholar] [CrossRef]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Aoki, F.; Miyauchi, K.; Shitomi, H.; Onuki, H.; Inoue, Y. Absolute Asymmetric Photoreactions of Aliphatic Amino Acids by Circularly Polarized Synchrotron Radiation: Critically pH-Dependent Photobehavior. J. Am. Chem. Soc. 2002, 124, 11618–11627. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Sato, M.; Ishiguro, S.; Saito, T.; Morishita, Y.; Sato, I.; Nishino, H.; Inoue, Y.; Soai, K. Enantioselective Synthesis of Near Enantiopure Compound by Asymmetric Autocatalysis Triggered by Asymmetric Photolysis with Circularly Polarized Light. J. Am. Chem. Soc. 2005, 127, 3274–3275. [Google Scholar] [CrossRef]
- Guijarro, A.; Yus, M. Origin of Chirality in the Molecules of Life: A Revision from Awareness to the Current Theories and Perspectives of this Unsolved Problem; RSC Publishing: Cambridge, UK, 2008; ISBN 978-0-85404-156-5. [Google Scholar]
- Plasson, R.; Kondepudi, D.K.; Bersini, H.; Commeyras, A.; Asakura, K. Emergence of Homochirality in Far-From-Equilibrium Systems: Mechanisms and Role in Prebiotic Chemistry. Chirality 2007, 19, 589–600. [Google Scholar] [CrossRef]
- Macdermott, A.J. Chiroptical Signatures of Life and Fundamental Physics. Chirality 2012, 24, 764–769. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Davankov, V.A. Biological Homochirality on the Earth, or in the Universe? A Selective Review. Symmetry 2018, 10, 749. [Google Scholar] [CrossRef]
- Famiano, M.; Boyd, R.; Kajino, T.; Onaka, T.; Mo, Y. Astrophysical Sites that Can Produce Enantiomeric Amino Acids. Symmetry 2019, 11, 23. [Google Scholar] [CrossRef]
- Tamura, M.; Kandori, R.; Kusakabe, N.; Nakajima, Y.; Hashimoto, J.; Nagashima, C.; Nagata, T.; Nagayama, T.; Kimura, H.; Yamamoto, T.; et al. Near-infrared polarization images of the Orion nebula. Astrophys. J. Lett. 2006, 649, L29–L32. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Kobayashi, K. Origin of Terrestrial Bioorganic Homochirality and Symmetry Breaking in the Universe. Symmetry 2019, 11, 919. [Google Scholar] [CrossRef]
- Ribó, J.M.; Hochberg, D. Chemical Basis of Biological Homochirality during the Abiotic Evolution Stages on Earth. Symmetry 2019, 11, 814. [Google Scholar] [CrossRef]
- Suzuki, N.; Itabashi, Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry 2019, 11, 966. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Symmetry Breaking in Self-Assembled Nanoassemblies. Symmetry 2019, 11, 950. [Google Scholar] [CrossRef]
- Soai, K.; Kawasaki, T.; Matsumoto, A. Role of Asymmetric Autocatalysis in the Elucidation of Origins of Homochirality of Organic Compounds. Symmetry 2019, 11, 694. [Google Scholar] [CrossRef]
- Fujiki, M. Mirror Symmetry Breaking in Helical Polysilanes: Preference Between Left and Right of Chemical and Physical Origin. Symmetry 2010, 2, 1625–1652. [Google Scholar] [CrossRef]
- Akabori, S.; Okawa, K.; Sato, M. Introduction of Side Chains into Polyglycine Dispersed on Solid Surface. I. Bull. Chem. Soc. Jpn. 1956, 29, 608–611. [Google Scholar] [CrossRef]
- Rohlfing, D.L.; Oparin, A.I. (Eds.) Molecular Evolution: Prebiological and Biological; Plenum Press: New York, NY, USA, 1972. [Google Scholar]
- Sparks, W.B.; Hough, J.H.; Kolokolova, L.; Germer, T.A.; Chen, F.; DasSarma, S.; DasSarma, P.; Robb, F.T.; Manset, N.; Reid, I.N.; et al. Circular Polarization in Scattered Light as a Possible Biomarker. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1771–1779. [Google Scholar] [CrossRef]
- Pizzarello, S. Molecular Asymmetry in Prebiotic Chemistry: An Account from Meteorites. Life 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.M.; Runyon, S.E.; Hazen, R.M. The Paleomineralogy of the Hadean Eon Revisited. Life 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L.; Gaidos, E.J.; Bertani, L.E.; Beukes, N.J.; Gutzmer, J.; Maepa, L.N.; Steinberger, R.E. Paleoproterozoic Snowball Earth: Extreme Climatic and Geochemical Global Change and Its Biological Consequences. Proc. Natl. Sci. Acad. USA 2000, 97, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.D.; Macdonald, F.A.; Strauss, J.V.; Dudás, F.O.; Hallmann, C.; Selby, D. Re-Os Geochronology and Coupled Os-Sr Isotope Constraints on the Sturtian Snowball Earth. Proc. Natl. Sci. Acad. USA 2014, 111, 51–56. [Google Scholar] [CrossRef]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from Fluid Inclusions for Microbial Methanogenesis in the Early Archaean Era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef]
- Nojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R. Evidence for Life on Earth before 3800 Million Years Ago. Nature 1996, 384, 55–59. [Google Scholar]
- Hoffman, P.F.; Schrag, D.P. The Snowball Earth Hypothesis: Testing the Limits of Global Change. Terra Nova 2002, 14, 129–155. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kakegawa, T.; Ishida, A.; Nagase, T.; Rosing, M.T. Evidence for Biogenic Graphite in Early Archaean Isua Metasedimentary Rocks. Nat. Geosci. 2014, 7, 25–28. [Google Scholar]
- Tarduno, J.A.; Cottrell, R.D.; Davis, W.J.; Nimmo, F.; Bono, R.K. A Hadean to Paleoarchean Geodynamo Recorded by Single Zircon Crystals. Science 2015, 349, 521–524. [Google Scholar] [CrossRef]
- Tang, F.; Taylor, R.J.; Einsle, J.F.; Borlina, C.S.; Fu, R.R.; Weiss, B.P.; Williams, H.M.; Williams, W.; Nagy, L.; Midgley, P.A.; et al. Secondary Magnetite in Ancient Zircon Precludes Analysis of a Hadean Geodynamo. Proc. Natl Acad. Sci. USA 2019, 116, 407–412. [Google Scholar] [CrossRef]
- Witze, A. Greenland Rocks Suggest Earth’s Magnetic Field Is Older Than We Thought. Nature 2019, 576, 347. [Google Scholar] [CrossRef] [PubMed]
- Zawaski, M.J.; Kelly, N.M.; Orlandini, O.F.; Nichols, C.I.O.; Allwood, A.C.; Mojzsis, S.J. Reappraisal of Purported ca. 3.7 Ga Stromatolites from the Isua Supracrustal Belt (West Greenland) from Detailed Chemical and Structural Analysis. Earth Planet. Sci. Lett. 2020, 545, 116409. [Google Scholar] [CrossRef]
- Epstein, S.; Krishnamurthy, R.V.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Unusual Stable Isotope Ratios in Amino Acid and Carboxylic Acid Extracts from the Murchison Meteorite. Nature 1987, 326, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Macko, S.A. Isotopic Evidence for Extraterrestrial Non-racemic Amino Acids in the Murchison Meteorite. Nature 1987, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Frieden, B.R.; Plastino, A.; Plastino, A.R.; Soffer, B.H. Schrodinger Link Between Nonequilibrium Thermodynamics and Fisher Information. Phys. Rev. E 2002, 66, 046128. [Google Scholar] [CrossRef]
- Ornes, S. Core Concept: How Nonequilibrium Thermodynamics Speaks to The Mystery of Life. Proc. Natl. Acad. Sci. USA 2017, 114, 423–424. [Google Scholar] [CrossRef]
- Ribó, J.M.; Hochberg, D.; Crusats, J.; El-Hachemi, Z.; Moyano, A. Spontaneous Mirror Symmetry Breaking and Origin of Biological Homochirality. J. R. Soc. Interface 2017, 14, 20170699. [Google Scholar] [CrossRef]
- Goldanskiǐ, V.I.; Kuz’min, V.V. Spontaneous Breaking of Mirror Symmetry in Nature and the Origin of Life. Sov. Phys. Usp. 1989, 32, 1–29. [Google Scholar] [CrossRef]
- Avetisov, V.A.; Goldanskii, V.I.; Kuz’min, V.V. Handedness, Origin of Life and Evolution. Phys. Today 1991, 44, 33–41. [Google Scholar]
- Avetisov, V.; Goldanskii, V. Mirror Symmetry Breaking at the Molecular Level. Proc. Natl. Acad. Sci. USA 1996, 93, 11435–11442. [Google Scholar] [CrossRef]
- Frank, F.C. On Spontaneous Asymmetric Synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Kuhn, W.; Brown, E. Photochemische Erzeugung Optisch Aktiver Stoffe. Naturwissenschaften 1929, 17, 227–228. [Google Scholar] [CrossRef]
- Rau, H. Asymmetric Photochemistry in Solution. Chem. Rev. 1983, 83, 535–547. [Google Scholar] [CrossRef]
- Inoue, Y. Asymmetric Photochemical Reactions in Solution. Chem. Rev. 1992, 92, 741–770. [Google Scholar] [CrossRef]
- Rau, H. Direct Asymmetric Photochemistry with Circularly Polarized light. In Chiral Photochemistry: Molecular and Supramolecular Photochemistry; Inoue, Y., Ramamurthy, V., Eds.; Marcel Dekker: New York, NY, USA, 2004; Chapter 1; pp. 1–44. [Google Scholar]
- Meierhenrich, U.J.; Nahon, L.; Alcaraz, C.; Bredehöft, J.H.; Hoffmann, S.V.; Barbier, B.; Brack, A. Asymmetric Vacuum UV photolysis of the Amino Acid Leucine in the Solid State. Angew. Chem. Int. Ed. 2005, 44, 5630–5634. [Google Scholar] [CrossRef]
- INOUE Photo-Chirogenesis. Available online: https://www.jst.go.jp/erato/en/research_area/completed/ihh_P.html (accessed on 22 January 2021).
- Bernstein, W.J.; Calvin, M.; Buchardt, O. Absolute Asymmetric Synthesis. I. Mechanism of the Photochemical Synthesis of Nonracemic Helicenes with Circularly Polarized Light. Wavelength Dependence of the Optical Yield of Octahelicene. J. Am. Chem. Soc. 1972, 94, 494–498. [Google Scholar] [CrossRef]
- Meinert, C.; Hoffmann, S.V.; Cassam-Chenaï, P.; Evans, A.C.; Giri, C.; Nahon, L.; Meierhenrich, U.J. Photonenergy-Controlled Symmetry Breaking with Circularly Polarized Light. Angew. Chem. Int. Ed. 2014, 53, 210–214. [Google Scholar] [CrossRef]
- Fujiki, M.; Yoshida, K.; Suzuki, N.; Zhang, J.; Zhang, W.; Zhu, X. Mirror Symmetry Breaking and Restoration within μm-Sized Polymer Particles in Optofluidic Media by Pumping Circularly Polarised Light. RSC Adv. 2013, 3, 5213–5219. [Google Scholar] [CrossRef]
- Fujiki, M.; Donguri, Y.; Zhao, Y.; Nakao, A.; Suzuki, N.; Yoshida, K.; Zhang, W. Photon Magic: Chiroptical Polarisation, Depolarisation, Inversion, Retention and Switching of Non-Photochromic Light-Emitting Polymers in Optofluidic Medium. Polym. Chem. 2015, 6, 1627–1638. [Google Scholar] [CrossRef]
- Wang, L.; Yin, L.; Zhang, W.; Zhu, X.; Fujiki, M. Circularly Polarized Light with Sense and Wavelengths To Regulate Azobenzene Supramolecular Chirality in Optofluidic Medium. J. Am. Chem. Soc. 2017, 139, 13218–13226. [Google Scholar] [CrossRef]
- Auzinsh, M.; Blushs, K.; Ferber, R.; Gahbauer, F.; Jarmola, A.; Tamanis, M. Electric Field Induced Symmetry Breaking of Angular Momentum Distribution in Atoms. Phys. Rev. Lett. 2006, 97, 043002. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.; Surendran, A.; Chowdhury, C.; Datta, A. Steric and Electric Field Driven Distortions in Aromatic Molecules: Spontaneous and Non-Spontaneous Symmetry Breaking. Phys. Chem. Chem. Phys. 2016, 18, 31160–31167. [Google Scholar] [CrossRef] [PubMed]
- Tielens, A.G.G.M. Interstellar Polycyclic Aromatic Hydrocarbon Molecules. Annu. Rev. Astron. Astrophys. 2008, 46, 289–337. [Google Scholar] [CrossRef]
- Avalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C.; Barron, L.D. Absolute Asymmetric Synthesis under Physical Fields: Facts and Fictions. Chem. Rev. 1998, 98, 2391–2404. [Google Scholar] [CrossRef] [PubMed]
- Zadel, G.; Eisenbrdun, C.; Wolff, G.-J.; Breitmaier, E. Enantioselective Reactions in a Static Magnetic Field-A False Alarm! Angew. Chem. Int. Ed. Engl. 1994, 33, 454–456. [Google Scholar] [CrossRef]
- Kaupp, G.; Marquardt, T. Absolute Asymmetric Synthesis Solely under the Influence of a Static Homogeneous Magnetic Field? Angew. Chem. Int. Ed. 1994, 33, 1459–1461. [Google Scholar] [CrossRef]
- Feringa, B.L.; Kellogg, R.M.; Hulst, R.; Zondervan, C.; Kruizinga, W.H. Attempts to Carry Out Enantioselective Reactions in a Static Magnetic Field. Angew. Chem. Int. Ed. 1994, 33, 1458–1459. [Google Scholar] [CrossRef]
- Gölitz, P. Enantioselective Reactions in a Static Magnetic Field—False Alarm! Angew. Chem. Int. Ed. 1994, 33, 1457. [Google Scholar] [CrossRef]
- Barron, L.D. Can A Magnetic Field Induce Absolute Asymmetric Synthesis? Science 1994, 266, 1491–1492. [Google Scholar] [CrossRef]
- Naaman, R.; Wadeck, D.H. Chiral-Induced Spin Selectivity Effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. [Google Scholar] [CrossRef]
- Banerjee-Ghosh, K.; Dor, O.B.; Tassinari, F.; Capua, E.; Yochelis, S.; Capua, A.; Yang, S.-H.; Parkin, S.S.P.; Sarkar, S.; Kronik, L.; et al. Separation of Enantiomers by Their Enantiospecific Interaction with Achiral Magnetic Substrates. Science 2018, 360, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, F.; Steidel, J.; Paltiel, S.; Fontanesi, C.; Lahav, M.; Paltiel, Y.; Naaman, R. Enantioseparation by Crystallization Using Magnetic Substrates. Chem. Sci. 2019, 10, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Yang, C.N. Question of Parity Conservation in Weak Interactions. Phys. Rev. 1956, 104, 254–258. [Google Scholar] [CrossRef]
- Wu, C.S.; Ambler, E.; Hayword, R.W.; Hoppes, D.D.; Hudson, R.P. Experimental Test of Parity Conservation on Beta Decay. Phys. Rev. 1957, 105, 1413–1415. [Google Scholar] [CrossRef]
- Ambler, E.; Hayward, R.W.; Hoppes, D.D.; Hudson, R.P.; Wu, C.S. Further Experiments on β Decay of Polarized Nuclei. Phys. Rev. 1957, 106, 1361–1363. [Google Scholar] [CrossRef]
- Postma, H.; Huiskamp, W.J.; Miedema, A.R.; Steenland, M.J.; Tolhoek, H.A.; Gorter, C.J. Asymmetry of the Positron Emission by Polarized 58Co Nuclei. Physica 1957, 23, 259–260. [Google Scholar] [CrossRef]
- Rodberg, L.S.; Weisskopf, V.F. Fall of Parity—Recent Discoveries Related to Symmetry of Laws of Nature. Science 1957, 125, 627–633. [Google Scholar] [CrossRef]
- Goldhaber, M.; Grodzins, L.; Sunyar, A.W. Helicity of Neutrinos. Phys. Rev. 1958, 109, 1015–1017. [Google Scholar] [CrossRef]
- Wróblewski, A.K. The Downfall of Parity—The Revolution That Happened Fifty Years Ago. Acta. Phys. Pol. B 2008, 39, 251–264. [Google Scholar]
- Wu, C.S. The Discovery of the Parity Violation in Weak Interactions and Its Recent Developments. Lect. Notes Phys. 2008, 746, 43–49. [Google Scholar]
- Stevenson, C.D.; Davis, J.P. Magnetars and Magnetic Separation of Chiral Radicals in Interstellar Space: Homochirality. J. Phys. Chem. A 2019, 123, 9587–9593. [Google Scholar] [CrossRef] [PubMed]
- Kouveliotou, C.; Duncan, R.C.; Thompson, C. Magnetars. Sci. Am. 2003, 288, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J-aggregate Formation of a Water-Soluble Porphyrin in Acidic Aqueous Media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Ribó, J.M.; Crusats, J.; Sagués, F.; Claret, J.; Rubires, R. Chiral Sign Induction by Vortices During the Formation of Mesophases in Stirred Solutions. Science 2001, 292, 2063–2066. [Google Scholar] [CrossRef]
- Crusats, J.; El-Hachemi, Z.; Ribó, J.M. Hydrodynamic Effects on Chiral Induction. Chem. Soc. Rev. 2010, 39, 569–577. [Google Scholar] [CrossRef]
- Okano, K.; Taguchi, M.; Fujiki, M.; Yamashita, T. Circularly Polarized Luminescence of Rhodamine B in a Supramolecular Chiral Medium Formed by a Vortex Flow. Angew. Chem. Int. Ed. 2011, 50, 12474–12477. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Yan, F.; Liu, C.; Sang, Y.; Tian, F.; Feng, Q.; Duan, P.; Zhang, L.; Shi, X.; et al. Control over the Emerging Chirality in Supramolecular Gels and Solutions by Chiral Microvortices in Milliseconds. Nat. Commun. 2018, 9, 2599. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Stephens, P.J. Magnetic Optical Activity. Annu. Rev. Phys. Chem. 1966, 17, 399–432. [Google Scholar] [CrossRef]
- Schatz, P.N.; McCaffery, A.J. Faraday effect. Q. Rev. Chem. Soc. 1969, 23, 552–584. [Google Scholar] [CrossRef]
- Stephens, R.J. Magnetic Circular Dichroism. Annu. Rev. Phys. Chem. 1974, 25, 201–232. [Google Scholar] [CrossRef]
- Kobayashi, N.; Muranaka, A.; Mack, J. Circular Dichroism and Magnetic Circular Dichroism Spectroscopy for Organic Chemists; RSC Publishing: Cambridge, UK, 2012. [Google Scholar]
- Riehl, J.P.; Richardson, F.S. General Theory of Circularly Polarized Emission and Magnetic Circularly Polarized Emission from Molecular Systems. J. Chem. Phys. 1976, 65, 1011–1021. [Google Scholar] [CrossRef]
- Wu, T.; Kapitán, J.; Andrushchenko, V.; Bouř, P. Identification of Lanthanide (III) Luminophores in Magnetic Circularly Polarized Luminescence Using Raman Optical Activity Instrumentation. Anal. Chem. 2017, 89, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Hinterding, S.O.M.; Fainblat, R.; Creutz, S.E.; Li, X.; Gamelin, D.R. A Selective Cation Exchange Strategy for the Synthesis of Colloidal Yb3+-Doped Chalcogenide Nanocrystals with Strong Broadband Visible Absorption and Long-Lived Near-Infrared Emission. J. Am. Chem. Soc. 2017, 139, 6411–6421. [Google Scholar] [CrossRef] [PubMed]
- Ivchenko, E.L. Magnetic Circular Polarization of Exciton Photoluminescence. Phys. Solid State 2018, 60, 1514–1526. [Google Scholar] [CrossRef]
- Kaji, D.; Okada, H.; Hara, N.; Kondo, Y.; Suzuki, S.; Miyasaka, M.; Fujiki, M.; Imai, Y. Non-classically Controlled Sign in a 1.6 Tesla Magnetic Circularly Polarized Luminescence of Three Pyrenes in a Chloroform and a PMMA Film. Chem. Lett. 2020, 49, 674–676. [Google Scholar] [CrossRef]
- Okada, H.; Hara, N.; Kaji, D.; Shizuma, M.; Fujiki, M.; Imai, Y. Excimer-Origin CPL vs. Monomer-Origin Magnetic CPL in Photo-Excited Chiral Binaphthyl-Ester-Pyrenes: Critical Role of Ester Direction. Phys. Chem. Chem. Phys. 2020, 22, 13862–13866. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Nakajima, G.; Mimura, Y.; Kimoto, T.; Kondo, Y.; Suzuki, S.; Fujiki, M.; Imai, Y. Mirror-Image Magnetic Circularly Polarized Luminescence (MCPL) from Optically Inactive EuIII and TbIII Tris(β-Diketonate). Dalton Trans. 2020, 49, 9588–9594. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishna, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Raupach, E. Enantioselective Magnetochiral Photochemistry. Nature 2000, 405, 932–934. [Google Scholar] [CrossRef]
- Sharma, A. Enantiomeric Excess by Magnetic Circular Dichroism in Archaean Atmosphere. Sci. Rep. 2017, 7, 13295. [Google Scholar] [CrossRef]
- Vester, F.; Ulbricht, T.L.V. Asymmetry: The Non-Conservation of Parity and Optical Activity. Quart. Rev. 1959, 13, 48–60. [Google Scholar]
- Vester, F.; Ulbricht, T.L.V. Attempts to Induce Optically Activity with Polarized β-Radiation. Tetrahedron 1962, 18, 629–637. [Google Scholar]
- Bonner, W.A. Experimental Evidence for β-Decay as a Source of Chirality by Enantiomer Analysis. Orig. Life 1984, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gidley, D.W.; Rich, A.; Van House, J.; Zitzewitz, P.W. β Decay and the Origins of Biological Chirality: Experimental Results. Nature 1982, 297, 639–643. [Google Scholar] [CrossRef]
- Van House, J.; Rich, A.; Zitzewitz, P.W. Beta Decay and The Origin of Biological Chirality: New Experimental Results. Orig. Life 1984, 14, 413–420. [Google Scholar] [CrossRef][Green Version]
- Dreiling, J.; Gay, T. Chirally Sensitive Electron-Induced Molecular Breakup and the Vester-Ulbricht Hypothesis. Phys. Rev. Lett. 2014, 113, 118103. [Google Scholar] [CrossRef]
- Electroweak Interaction. Available online: https://en.wikipedia:wiki/Electroweak_interaction (accessed on 20 December 2020).
- Janoschek, R. Theories on the origin of biomolecular Homochirality. In Chirality—From Weak Bosons to the α-Helix; Janoschek, R., Ed.; Springer: Berlin, Germany, 1991; ISBN 978-3-642-76569-8. [Google Scholar]
- Close, E.E. Parity Violation in Atoms? Nature 1976, 264, 505–506. [Google Scholar] [CrossRef]
- Bucksbaum, P.H.; Commins, E.D.; Hunter, L.R. Observations of Parity Nonconservation in Atomic Thallium. Phys. Rev. D 1981, 24, 1134–1148. [Google Scholar] [CrossRef]
- Emmons, T.P.; Reeves, J.M.; Fortson, E.N. Parity-Nonconserving Optical Rotation in Atomic Lead. Phys. Rev. Lett. 1983, 51, 2089–2091. [Google Scholar] [CrossRef]
- Bouchiat, M.-A.; Pottier, L. Optical Experiments and Weak Interactions. Science 1986, 234, 1203–1210. [Google Scholar] [CrossRef]
- World Scientific. Parity Violation in Atoms and Polarized Electron Scattering; Bernard, F., Bouchiat, M.-A., Eds.; World Scientific: Singapore, 1999; ISBN 9810237316. [Google Scholar]
- Okun, L.B. Mirror Particles and Mirror Matter: 50 years of Speculation and Search. Phys. Usp. 2007, 50, 380–389. [Google Scholar] [CrossRef]
- Tsigutkin, K.; Dounas-Frazer, D.; Family, A.; Stalnaker, J.E.; Yashchuk, V.V.; Budker, D. Observation of a Large Atomic Parity Violation Effect in Ytterbium. Phys. Rev. Lett. 2009, 103, 071601. [Google Scholar] [CrossRef]
- Mason, A.; Tranter, G.E. Energy Inequivalence of Peptide Enantiomers from Parity Non-Conservation. J. Chem. Soc. Chem. Commun. 1983, 117–119. [Google Scholar] [CrossRef]
- Quack, M. Structure and Dynamics of Chiral Molecules. Angew. Chem. Int. Ed. 1989, 28, 571–586. [Google Scholar] [CrossRef]
- Quack, M.; Stohner, J.; Willeke, M. High-Resolution Spectroscopic Studies and Theory of Parity Violation in Chiral Molecules. Annu. Rev. Phys. Chem. 2008, 59, 741–769. [Google Scholar] [CrossRef]
- Quack, M. How Important is Parity Violation for Molecular and Biomolecular Chirality? Angew. Chem. Int. Ed. 2002, 41, 4618–4630. [Google Scholar] [CrossRef]
- Schwerdtfeger, P.; Gierlich, J.; Bollwein, T. Large Parity-Violation Effects in Heavy-Metal-Containing Chiral Compounds. Angew. Chem. Int. Ed. 2003, 42, 1293–1296. [Google Scholar] [CrossRef]
- Darquié, B.; Stoeffler, C.; Shelkovnikov, A.; Daussy, C.; Amy-Klein, A.; Chardonnet, C.; Zrig, S.; Guy, L.; Crassous, J.; Soulard, P.; et al. Progress Toward the First Observation of Parity Violation in Chiral Molecules by High-Resolution Laser Spectroscopy. Chirality 2010, 22, 870–884. [Google Scholar] [CrossRef]
- Berger, R. Molecular Parity Violation in Electronically Excited States. Phys. Chem. Chem. Phys. 2003, 5, 12–17. [Google Scholar] [CrossRef]
- Fujiki, M.; Koe, J.R.; Mori, T.; Kimura, Y. Questions of Mirror Symmetry at the Photoexcited and Ground States of Non-Rigid Luminophores Raised by Circularly Polarized Luminescence and Circular Dichroism Spectroscopy: Part 1. Oligofluorenes, Oligophenylenes, Binaphthyls and Fused Aromatics. Molecules 2018, 23, 2606. [Google Scholar] [CrossRef]
- Fujiki, M.; Koe, J.R.; Amazumi., S. Questions of Mirror Symmetry at the Photoexcited and Ground States of Non-Rigid Luminophores Raised by Circularly Polarized Luminescence and Circular Dichroism Spectroscopy. Part 2: Perylenes, BODIPYs, Molecular Scintillators, Coumarins, Rhodamine B, and DCM. Symmetry 2019, 11, 363. [Google Scholar] [CrossRef]
- Panspermia. Available online: https://en.wikipedia.org/wiki/Panspermia#cite_note-cometary_panspermia-3 (accessed on 2 November 2020).
- Wickramasinghe, N.C. Search for Our Cosmic Ancestry; World Scientific: Singapore, 2014. [Google Scholar]
- Kawaguchi, Y.; Yang, Y.; Kawashiri, N.; Shiraishi, K.; Takasu, M.; Narumi, I.; Satoh, K.; Hashimoto, H.; Nakagawa, K.; Tanigawa, Y.; et al. The Possible Interplanetary Transfer of Microbes: Assessing the Viability of Deinococcus spp. Under the ISS Environmental Conditions for Performing Exposure Experiments of Microbes in the Tanpopo Mission. Orig. Life. Evol. Biosph. 2013, 43, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Shibuya, M.; Kinoshita, I.; Yatabe, J.; Narumi, I.; Shibata, H.; Hayashi, R.; Fujiwara, D.; Murano, Y.; Hashimoto, H.; et al. DNA Damage and Survival Time Course of Deinococcal Cell Pellets During 3 Years of Exposure to Outer Space. Front. Microbiol. 2020, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Paganini, L.; Villanueva, G.L.; Roth, L.; Mandell, A.M.; Hurford, T.A.; Retherford, K.D.; Mumma, M.J. A Measurement of Water Vapour Amid a Largely Quiescent Environment on Europa. Nat. Astron. 2020, 4, 266–272. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Roy, L.L.; MacDermott, A.J.; et al. Organic Compounds on Comet 67P/Churyumov-Gerasimenko Revealed by COSAC Mass Spectrometry. Science 2015, 349, aab0689. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Langevin, Y.; Carter, J.; Eng, P.; Gondet, B.; Jorda, L.; Le Mouélic, S.; Mottola, S.; Pilorget, C.; Poulet, F.; et al. 67P/Churyumov-Gerasimenko Surface Properties as Derived from CIVA Panoramic Images. Science 2015, 349, aab0671. [Google Scholar] [CrossRef]
- Iess, L.; Stevenson, D.J.; Parisi, M.; Hemingway, D.; Jacobson, R.A.; Lunine, J.I.; Nimmo, F.; Armstrong, J.W.; Asmar, S.W.; Ducci, M.; et al. The Gravity Field and Interior Structure of Enceladus. Science 2014, 344, 78–80. [Google Scholar] [CrossRef]
- Lorenz, R.D.; Kirk, R.L.; Hayes, A.G.; Anderson, Y.Z.; Lunine, J.I.; Tokano, T.; Turtle, E.P.; Malaska, M.J.; Soderblom, J.M.; Lucas, A.; et al. A Radar Map of Titan Seas: Tidal Dissipation and Ocean Mixing through the Throat of Kraken. Icarus 2014, 237, 9–15. [Google Scholar] [CrossRef]
- Pepe, F.; Lovis, C.; Ségransan, D.; Benz, W.; Bouchy, F.; Dumusque, X.; Mayor, M.; Queloz, D.; Santos, N.C.; Udry, S. The HARPS Search for Earth-like Planets in the Habitable Zone. Astron. Astrophys. 2011, 534, A58. [Google Scholar] [CrossRef]
- Quintana, E.V.; Barclay, T.; Raymond, S.N.; Rowe, J.F.; Bolmont, E.; Caldwell, D.A.; Howell, S.B.; Kane, S.R.; Huber, D.; Crepp, J.R.; et al. An Earth-sized Planet in the Habitable Zone of a Cool Star. Science 2014, 344, 277–280. [Google Scholar] [CrossRef]
- McGuire, B.A.; Carroll, P.B.; Loomis, R.A.; Finneran, I.A.; Jewell, P.R.; Remijan, A.J.; Blake, G.A. Discovery of the Interstellar Chiral Molecule Propylene Oxide (CH3CHCH2O). Science 2016, 352, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Gavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial Ribose and Other Sugars in Primitive Meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef] [PubMed]
- Hayabusa-2 Project. Available online: http://www.hayabusa2.jaxa.jp/en/topics/20201209_capsulephotos/ (accessed on 9 December 2020).
- Miller, S.L. Production of Amino Acids under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic Compound Synthesis on the Primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. New Insights into Prebiotic Chemistry from Stanley Miller’s Spark Discharge Experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef]
- Enoto, T.; Wada, Y.; Furuta, Y.; Nakazawa, K.; Yuasa, T.; Okuda, K.; Makishima, K.; Sato, M.; Sato, Y.; Nakano, T.; et al. Photonuclear Reactions Triggered by Lightning Discharge. Nature 2017, 551, 481–484. [Google Scholar] [CrossRef]
- Rozanski, K.; Fröhlich, K. Radioactivity and Earth Sciences: Understanding the Natural Environment. IAEA Bull. 1996, 38, 9–15. [Google Scholar]
- Araki, T.; Enomoto, S.; Furuno, K.; Gando, Y.; Ichimura, K.; Ikeda, H.; Inoue, K.; Kishimoto, Y.; Koga, M.; Koseki, Y.; et al. Experimental Investigation of Geologically Produced Antineutrinos with KamLAND. Nature 2005, 436, 499–503. [Google Scholar] [CrossRef]
- Rosianna, I.; Nugraha, E.D.; Syaeful, H.; Putra, S.; Hosoda, M.; Akata, N.; Tokonami, S. Natural Radioactivity of Laterite and Volcanic Rock Sample for Radioactive Mineral Exploration in Mamuju, Indonesia. Geosciences 2020, 10, 376. [Google Scholar] [CrossRef]
- Córdova, A.; Engqvist, M.; Ibrahem, I.; Casa, J.; Sundén, H. Plausible Origins of Homochirality in the Amino Acid Catalyzed Neogenesis of Carbohydrates. Chem. Commun. 2005, 2047–2049. [Google Scholar] [CrossRef]
- Sato, I.; Urabe, H.; Ishiguro, S.; Shibata, T.; Soai, K. Amplification of Chirality from Extremely Low to Greater than 99.5 % ee by Asymmetric Autocatalysis. Angew. Chem. Int. Ed. 2003, 42, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. An Exact Test for Randomness of Mating. New Biol. 1954, 16, 12–27. [Google Scholar]
- Lu, T.; Spruijt, E. Multiphase Complex Coacervate Droplets. J. Am. Chem. Soc. 2020, 142, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Favorskiy, I.; Vu, D.; Peytavit, E.; Arscott, S.; Paget, D.; Rowe, A.C.H. Circularly Polarized Luminescence Microscopy for the Imaging of Charge and Spin Diffusion in Semiconductors. Rev. Sci. Instrum. 2010, 81, 103902. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W.; Claborn, K.; Kahr, B. Polarimetric Imaging of Crystals. Chem. Soc. Rev. 2004, 33, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Narushima, T.; Okamoto, H. Circular Dichroism Microscopy Free from Commingling Linear Dichroism via Discretely Modulated Circular Polarization. Sci. Rep. 2016, 6, 35731. [Google Scholar] [CrossRef]
- Le, K.Q.; Hashiyada, S.; Kondo, M.; Okamoto, H. Circularly Polarized Photoluminescence from Achiral Dye Molecules Induced by Plasmonic Two-Dimensional Chiral Nanostructures. J. Phys. Chem. C 2018, 122, 24924–24932. [Google Scholar] [CrossRef]
- Claborn, K.; Puklin-Faucher, E.; Kurimoto, M.; Kaminsky, W.; Kahr, B. Circular Dichroism Imaging Microscopy: Application to Enantiomorphous Twinning in Biaxial Crystals of 1,8-Dihydroxyanthraquinone. J. Am. Chem. Soc. 2003, 125, 14825–14831. [Google Scholar] [CrossRef]
- Narushima, T.; Okamoto, H. Circular Dichroism Nano-Imaging of Two-Dimensional Chiral Metal Nanostructures. Phys. Chem. Chem. Phys. 2013, 15, 13805–13809. [Google Scholar] [CrossRef]
- Hauser, E.A. The History of Colloid Science: In Memory of Wolfgang Ostwald. J. Chem. Educ. 1955, 32, 2–9. [Google Scholar] [CrossRef]
- Le Chatelier, H. Crystalloids Against Colloids in the Theory of Cements. Trans. Faraday Soc. 1919, 14, 8–11. [Google Scholar] [CrossRef]
- Hermann Staudinger. Available online: https://en.wikipedia.org/wiki/Hermann_Staudinger (accessed on 11 December 2020).
- Terayama, H. Method of colloid titration (a new titration between polymer ions). J. Polym. Sci. 1952, 8, 243–253. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral Symmetry Breaking Crystallization. Science 1990, 250, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Tharrington, A.; Wu, X.-I. Chiral Symmetry Breaking in Crystal Growth: Is Hydrodynamic Convection Relevant? Phys. Rev. Lett. 1996, 77, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C. Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C.; Ortiz, J.E.; Torres, T.D.; Izumi, T.; Blackmond, D.G. Evolution of Solid Phase Homochirality for a Proteinogenic Amino Acid. J. Am. Chem. Soc. 2008, 130, 15274–15275. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Vlieg, E.; Kellogg, R.M.; Kaptein, B. From Ostwald Ripening to Single Chirality. Angew. Chem. Int. Ed. 2009, 48, 9600–9606. [Google Scholar] [CrossRef]
- Viedma, C. Selective Chiral Symmetry Breaking during Crystallization: Parity Violation or Cryptochiral Environment in Control? Cryst. Growth Des. 2007, 7, 553–556. [Google Scholar] [CrossRef]
- Qian, S.X.; Snow, J.B.; Tzeng, H.M.; Chang, R.K. Lasing Droplets: Highlighting the Liquid-Air Interface by Laser Emission. Science 1986, 231, 486–488. [Google Scholar] [CrossRef]
- Psaltis, D.; Quack, S.R.; Yang, C. Developing Optofluidic Technology through the Fusion of Microfluidics and Optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic Microsystems for Chemical and Biological Analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H.; Hawkins, A.R. The Photonic Integration of Non-Solid Media using Optofluidics. Nat. Photon. 2011, 5, 598–604. [Google Scholar] [CrossRef]
- Fainman, Y.; Lee, L.P.; Psaltis, D. Optofluidics; Yang, C., Ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Tang, S.K.Y.; Li, Z.; Abate, A.R.; Agresti, J.J.; Weitz, D.A.; Psaltis, D.; Whitesides, G.M. A Multi-Color Fast-Switching Microfluidic Droplet Dye Laser. Lab Chip 2009, 9, 2767–2771. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Mondia, J.P.; Sharma, R.; Lu, Z.H.; Susha, A.S.; Rogach, A.L.; Wang, L.J. Quantum Dot Microdrop Laser. Nano Lett. 2008, 8, 1709–1712. [Google Scholar] [CrossRef]
- Domachuk, P.; Littler, I.C.M.; Cronin-Golomb, M.; Eggletona, B.J. Compact Resonant Integrated Microfluidic Refractometer. Appl. Phys. Lett. 2006, 88, 093513. [Google Scholar] [CrossRef]
- Steinberg, I.Z. Circular Polarization of Luminescence: Biochemical and Biophysical Applications. Annu. Rev. Biophys. Bioeng. 1978, 7, 113–137. [Google Scholar] [CrossRef]
- Barzda, V.; Musthrdy, L.; Garab, G. Size Dependency of Circular Dichroism in Macrocolloids of Photosynthetic Pigment-Protein Complexes. Biochemistry 1994, 33, 10837–10841. [Google Scholar] [CrossRef]
- Barzda, V.; Istokovics, A.; Simidjiev, I.; Garab, G. Structural Flexibility of Chiral Macrocolloids of Light-Harvesting Chlorophyll a/b Pigment-Protein Complexes. Light-Induced Reversible Structural Changes Associated with Energy Dissipation. Biochemistry 1996, 35, 8981–8985. [Google Scholar] [CrossRef]
- Gussakovsky, E.E.; Shahak, Y.; van Amerongen, H.; Barzda, V. Circularly Polarized Chlorophyll Luminescence Reflects the Macro-Organization of Grana in Pea Chloroplasts. Photosynth. Res. 2000, 65, 83–92. [Google Scholar] [CrossRef]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef]
- Nam, I.; Lee, J.K.; Nam, H.G.; Zare, R.N. Abiotic Production of Sugar Phosphates and Uridine Ribonucleoside in Aqueous Microdroplets. Proc. Natl. Acad. Sci. USA 2017, 114, 12396–12400. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Nam, H.G.; Zare, R.N. Abiotic Synthesis of Purine and Pyrimidine Ribonucleosides in Aqueous Microdroplets. Proc. Natl. Acad. Sci. USA 2018, 115, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Banerjee, S.; Nam, H.G.; Zare, R.N. Acceleration of Reaction in Charged Microdroplets. Q. Rev. Biophys. 2015, 48, 437–444. [Google Scholar] [CrossRef]
- Mortensen, D.N.; Williams, E.R. Microsecond and Nanosecond Polyproline II Helix Formation in Aqueous Nanodrops Measured by Mass Spectrometry. Chem. Commun. 2016, 52, 12218–12221. [Google Scholar] [CrossRef] [PubMed]
- Guardingo, M.; Busqué, F.; Ruiz-Molina, D. Reactions in Ultra-Small Droplets by Tip-Assisted Chemistry. Chem. Commun. 2016, 52, 11617–11626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Samanta, D.; Nam, H.G.; Zare, R.N. Micrometer-Sized Water Droplets Induce Spontaneous Reduction. J. Am. Chem. Soc. 2019, 141, 10585–10589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, X.; Lai, Y.H.; Zare, R.N. Fluorescence Polarization Anisotropy in Microdroplets. J. Phys. Chem. Lett. 2018, 9, 2928–2932. [Google Scholar] [CrossRef]
- Kageyama, Y. Robust Dynamics of Synthetic Molecular Systems as a Consequence of Broken Symmetry. Symmetry 2020, 12, 1688. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H. Chiroptical properties. In Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 1994; pp. 991–1118. [Google Scholar]
- Fujiki, M.; Wang, L.; Ogata, N.; Asanoma, F.; Okubo, A.; Okazaki, S.; Kamite, H.; Jalilah, A.J. Chirogenesis and Pfeiffer Effect in Optically Inactive EuIII and TbIII Tris (β-diketonate) Upon Intermolecular Chirality Transfer From Poly- and Monosaccharide Alkyl Esters and α-Pinene: Emerging Circularly Polarized Luminescence (CPL) and Circular Dichroism (CD). Front. Chem. 2020, 8, 685. [Google Scholar]
- Albano, G.; Pescitelli, G.; Di Bari, L. Chiroptical Properties in Thin Films of π-Conjugated Systems. Chem. Rev. 2020, 120, 10145–10243. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhao, Z.; Liu, Z.; Lam, J.W.; Tang, B.Z. Circularly Polarized Luminescence from AIEgens. J. Mater. Chem. C 2020, 8, 3284–3301. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Liu, J.; Su, H.; Meng, L.; Zhao, Y.; Deng, C.; Ng, J.C.Y.; Lu, P.; Faisal, M.; Lam, J.W.Y.; Huang, X.; et al. What Makes Efficient Circularly Polarised Luminescence in the Condensed Phase: Aggregation-induced Circular Dichroism and Light emission. Chem. Sci. 2012, 3, 2737–2747. [Google Scholar] [CrossRef]
- Li, H.; Zheng, X.; Su, H.; Lam, J.W.Y.; Wong, K.S.; Xue, S.; Huang, X.; Huang, X.; Li, B.S.; Tang, B.Z. Synthesis, Optical Properties, and Helical Self-Assembly of a Bivaline-Containing Tetraphenylethene. Sci. Rep. 2016, 6, 19277. [Google Scholar]
- Ghosh, A.; Fischer, P. Chiral Molecules Split Light: Reflection and Refraction in a Chiral Liquid. Phys. Rev. Lett. 2006, 97, 173002. [Google Scholar] [CrossRef]
- Silverman, M.P.; Badoz, J.; Briat, B. Chiral Reflection from a Naturally Optically Active Medium. Opt. Lett. 1992, 17, 886–888. [Google Scholar] [CrossRef]
- Pedersen, J.; Mortensen, N.A. Enhanced Circular Dichroism via Slow Light in Dispersive Structured Media. Appl. Phys. Lett. 2007, 91, 213501. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Xiao, S. Slow-Light Enhancement of Beer-Lambert-Bouguer Absorption. Appl. Phys. Lett. 2007, 90, 141108. [Google Scholar] [CrossRef]
- Uemura, N.; Toyoda, S.; Shimizu, W.; Yoshida, Y.; Mino, T.; Sakamoto, M. Absolute Asymmetric Synthesis Involving Chiral Symmetry Breaking in Diels-Alder Reaction. Symmetry 2020, 12, 910. [Google Scholar] [CrossRef]
- Kawagoe, K.; Fujiki, M.; Nakano, Y. Limonene Magic: Noncovalent Molecular Chirality Transfer Leading to Ambidextrous Circularly Polarised Luminescent π-Conjugated Polymers. New J. Chem. 2010, 34, 637–647. [Google Scholar] [CrossRef]
- Nakano, Y.; Fujiki, M. Circularly Polarized Light Enhancement by Helical Polysilane Aggregates Suspension in Organic Optofluids. Macromolecules 2011, 48, 7511–7919. [Google Scholar] [CrossRef]
- Nakano, Y.; Ichiyanagi, F.; Naito, M.; Yang, Y.-G.; Fujiki, M. Chiroptical Generation and Inversion during the Mirror-Symmetry-Breaking Aggregation of Dialkylpolysilanes due to Limonene Chirality. Chem. Commun. 2012, 48, 6636–6638. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Liu, Y.; Fujiki, M. Ambidextrous Circular Dichroism and Circularly Polarised Luminescence from Poly (9,9-di-n-decylfluorene) by Terpene Chirality Transfer. Polym. Chem. 2010, 1, 460–469. [Google Scholar] [CrossRef]
- Zhang, W.; Yoshida, K.; Fujiki, M.; Zhu, X. Unpolarized-Light-Driven Amplified Chiroptical Modulation Between Chiral Aggregation and Achiral Disaggregation of an Azobenzene-alt-Fluorene Copolymer in Limonene. Macromolecules 2011, 44, 5105–5111. [Google Scholar] [CrossRef]
- Fujiki, M.; Jalilah, A.J.; Suzuki, N.; Taguchi, M.; Zhang, W.; Abdellatif, M.M.; Nomura, K. Chiral Optofluidics: Gigantic Circularly Polarized Light Enhancement of all-trans-Poly (9,9-di-n-octylfluorene-2,7-vinylene) during Mirror-Symmetry-Breaking Aggregation by Optically Tuning Fluidic Media. RSC Adv. 2012, 2, 6663–6671. [Google Scholar] [CrossRef]
- Fujiki, M.; Kawagoe, Y.; Nakano, Y.; Nakao, A. Mirror-Symmetry-Breaking in Poly [(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-biphenyl] (PF8P2) is Susceptible to Terpene Chirality, Achiral Solvents, and Mechanical Stirring. Molecules 2013, 18, 7035–7057. [Google Scholar] [CrossRef]
- Wang, L.; Suzuki, N.; Liu, J.; Matsuda, T.; Rahim, N.A.A.; Zhang, W.; Fujiki, M.; Zhang, Z.; Zhou, N.; Zhu, X. Limonene Induced Chiroptical Generation and Inversion during Aggregation of Achiral Polyfluorene Analogs: Structure-Dependence and Mechanism. Polym. Chem. 2014, 5, 5920–5927. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhang, S.; Suzuki, N.; Fujiki, M.; Wang, L.; Li, L.; Zhang, W.; Zhou, N.; Zhu, X. Chiroptical Generation and Amplification of Hyperbranched π-Conjugated Polymers in Aggregation States Driven by Limonene Chirality. Polym. Chem. 2014, 5, 784–791. [Google Scholar] [CrossRef]
- Fujiki, M.; Yoshida, K.; Suzuki, N.; Rahim, N.A.A.; Jalil, J.A. Tempo-Spatial Chirogenesis. Limonene-Induced Mirror Symmetry Breaking of Si-Si Bond Polymers during Aggregation in Chiral Fluidic Media. J. Photochem. Photobiol. A 2016, 331, 120–129. [Google Scholar] [CrossRef]
- Rahim, N.A.A.; Fujiki, M. Aggregation-induced Scaffolding: Photoscissable Helical Polysilane Generates Circularly Polarized Luminescent Polyfluorene. Polym. Chem. 2016, 7, 4618–4629. [Google Scholar] [CrossRef]
- Duong, S.T.; Fujiki, M. The Origin of Bisignate Circularly Polarized Luminescence (CPL) Spectra from Chiral Polymer Aggregates and Molecular Camphor: Anti-Kasha’s Rule Revealed by CPL Excitation (CPLE) Spectra. Polym. Chem. 2017, 8, 4673–4679. [Google Scholar] [CrossRef]
- Fujiki, M.; Yoshimoto, S. Time-evolved, Far-red, Circularly Polarised Luminescent Polymer Aggregates Endowed with Sacrificial Helical Si–Si Bond Polymers. Mater. Chem. Front. 2017, 1, 1773–1785. [Google Scholar] [CrossRef]
- Chen, H.; Yin, L.; Liu, M.; Wang, L.; Fujiki, M.; Zhang, W.; Zhu, X. Aggregation-Induced Chiroptical Generation and Photoinduced Switching of Achiral Azobenzene-alt-Fluorene Copolymer Endowed with Left-and Right-Handed Helical Poly-silanes. RSC Adv. 2019, 9, 4849–4856. [Google Scholar] [CrossRef]
- Fujiki, M. Aggregation-Induced Chirogenesis of Luminescent Polymers. In Aggregation-Induced Emission: Materials and Applications; Fujiki, M., Liu, B., Tang, B.Z., Eds.; American Chemical Society: Columbus, OH, USA, 2016; Volume 2, pp. 63–92. [Google Scholar]
- Beth, R.A. Mechanical Detection and Measurement of the Angular Momentum of Light. Phys. Rev. 1936, 50, 115–125. [Google Scholar] [CrossRef]
- Saha, M.N.; Bhargava, Y. The Spin of the Photon. Nature 1931, 128, 870. [Google Scholar] [CrossRef]
- Simpson, N.B.; Dholakia, K.; Allen, L.; Padgett, M.J. Mechanical Equivalence of Spin and Orbital Angular Momentum of Light: An Optical Spanner. Opt. Lett. 1997, 22, 52–54. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, USA, 1991; ISBN 0935702717. [Google Scholar]
- Calvert, J.G.; Pitts, J.N. Photochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1973; ISBN 0471130907. [Google Scholar]
- Franck-Condon-Principle. Available online: https://en.wikipedia.org/wiki/Franck–Condon_principle (accessed on 23 December 2020).
- Nakashima, H.; Fujiki, M.; Koe, J.R.; Motonaga, M. Solvent and Temperature Effects on the Chiral Aggregation of Poly- (alkylarylsilane) s Bearing Remote Chiral Groups. J. Am. Chem. Soc. 2001, 123, 1963–1969. [Google Scholar] [CrossRef]
- Terao, K.; Mori, Y.; Dobashi, T.; Sato, T.; Teramoto, A.; Fujiki, M. Solvent and Temperature Effects on the Chiral Aggregation of Optically Active Poly (dialkylsilane)s Confined in Microcapsules. Langmuir 2004, 20, 306–308. [Google Scholar] [CrossRef]
- Fujiki, M. Ideal Exciton Spectra in Single- and Double-Screw-Sense Helical Polysilanes. J. Am. Chem. Soc. 1994, 116, 6017–6018. [Google Scholar] [CrossRef]
- Fujiki, M. Optically Active Polysilylenes: State-Of-The-Art Chiroptical Polymers. Macromol. Rapid Commun. 2001, 22, 539–563. [Google Scholar] [CrossRef]
- Fujiki, M.; Koe, J.R.; Terao, K.; Sato, T.; Teramoto, A.; Watanabe, J. Optically Active Polysilanes. Ten Years of Progress and New Polymer Twist for Nanoscience and Nanotechnology. Polym. J. 2003, 35, 297–344. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. The CD magnitude is normalized using the dimensionless Kuhn’s anisotropy factor in the ground state, defined as gCD = Δε/ε = (AbsL − AbsR)/[(AbsL + AbsR)/2] at extremum. In Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. The CPL magnitude is normalized by Kuhn’s anisotropy in the photoexcited state. gCPL = (IL − IR)/[(IL + IR)/2] at extremum, where IL and IR denote the emission intensities of the left- and right-CP light under excitation of unpolarized light, re-spectively. John Wiley & Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Nakashima, H.; Koe, J.R.; Torimitsu, K.; Fujiki, M. Transfer and Amplification of Chiral Molecular Information to Poly-silylene Aggregates. J. Am. Chem. Soc. 2001, 123, 4847–4848. [Google Scholar] [CrossRef] [PubMed]
- Righini, G.C.; Dumeige, Y.; Féron, P.; Ferrari, M.; Conti, G.N.; Ristic, D.; Soria, S. Whispering Gallery Mode Microresonators: Fundamentals and Applications. Riv. Nuovo Cimento 2011, 34, 435–488. [Google Scholar]
- Symes, R.; Sayer, R.M.; Reid, J.P. Cavity Enhanced Droplet Spectroscopy: Principles, Perspectives and Prospects. Phys. Chem. Chem. Phys. 2004, 6, 474–487. [Google Scholar] [CrossRef]
- Li, Z.; Psaltis, D. Optofluidic Dye Lasers. Microfluid Nanofluid. 2008, 4, 145–158. [Google Scholar] [CrossRef]
- Sun, Y.; Shopova, S.I.; Wu, C.S.; Arnold, S.; Fan, X. Bioinspired Optofluidic FRET Lasers via DNA Scaffolds. Proc. Nat. Acad. Sci. USA 2010, 107, 16039–16042. [Google Scholar] [CrossRef]
- He, C.; Yang, G.; Kuai, Y.; Shan, S.; Yang, L.; Hu, J.; Zhang, D.; Zhang, Q.; Zou, G. Dissymmetry Enhancement in Enantioselective Synthesis of Helical Polydiacetylene by Application of Superchiral Light. Nat. Commun. 2018, 9, 5117. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Hu, J.; Fujiki, M.; Zou, G. The Chirality Induction and Modulation of Polymers by Circularly Polarized Light. Symmetry 2019, 11, 474. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yeom, J.; Zhao, G.; Calcaterra, H.; Munn, J.; Zhang, P.; Kotov, N. Assembly of Gold Nanoparticles into Chiral Superstructures Driven by Circularly Polarized Light. J. Am. Chem. Soc. 2019, 141, 11739–11744. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Okada, T.; Shinkai, S.; Shigematsu, K.; Kusano, Y.; Manabe, O. Temperature and Pressure Dependences of Thermal Cis-to-Trans Isomerization of Azobenzenes which Evidence an Inversion Mechanism. J. Am. Chem. Soc. 1981, 103, 5161–5165. [Google Scholar] [CrossRef]
- Klimov, V.V.; Zabkov, I.V.; Pavlov, A.; Guzatov, D.V. Eigen Oscillations of a Chiral Sphere and Their Influence on Radiation of Chiral Molecules. Opt. Exp. 2014, 22, 18564–18578. [Google Scholar] [CrossRef] [PubMed]
- Ureña, F.P.; Moreno, J.R.A.; González, J.J.L. Conformational Study of (R)-(+)-Limonene in the Liquid Phase using Vibrational Spectroscopy (IR, Raman, and VCD) and DFT Calculations. Tetrahedron Asymmetry 2009, 20, 89–97. [Google Scholar] [CrossRef]
- Reinscheid, F.; Reinscheid, U.M. Stereochemical Analysis of (+)-Limonene using Theoretical and Experimental NMR and Chiroptical Data. J. Mol. Struct. 2016, 1106, 141–153. [Google Scholar] [CrossRef][Green Version]
- Gierschner, J.; Behera, S.K.; Park, S.Y. Dual Emission: Classes, Mechanisms and Conditions. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Röhrs, M.; Escudero, D. Multiple Anti-Kasha Emissions in Transition-Metal Complexes. J. Phys. Chem. Lett. 2019, 10, 5798–5804. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Catalán, J. Kasha’s rule: A reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Tomin, V.I.; Chou, P.T. Breaking the Kasha Rule for More Efficient Photochemistry. Chem. Rev. 2017, 117, 13353–13381. [Google Scholar] [CrossRef]
- Kunitake, T.; Okahata, Y. A Totally Synthetic Bilayer Membrane. J. Am. Chem. Soc. 1977, 99, 3860–3861. [Google Scholar] [CrossRef]
- Fujiki, M. Studies on Hydride Abstraction Reaction by Trityl Salts and Synthesis/Polymerization of Dialkylammonium Acrylate. Master’s Thesis, Kyushu University, Fukuoka, Japan, 1978. (In Japanese). [Google Scholar]
- Krishnamurthy, R.; Hud, N.V. Introduction: Chemical Evolution and the Origins of Life. Chem. Rev. 2020, 120, 4613–4615. [Google Scholar] [CrossRef] [PubMed]
- Sandford, S.A.; Nuevo, M.; Bera, P.P.; Lee, T.J. Prebiotic Astrochemistry and the Formation of Molecules of Astrobiological Interest in Interstellar Clouds and Protostellar Disks. Chem. Rev. 2020, 120, 4616–4659. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dworkin, J.P. The Search for Chiral Asymmetry as a Potential Biosignature in our Solar System. Chem. Rev. 2020, 120, 4660–4689. [Google Scholar] [CrossRef] [PubMed]
- Ribό, J.M. Chirality: The Backbone of Chemistry as a Natural Science. Symmetry 2020, 12, 1982. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Katsuhiro Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 3752–13990. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Hembury, G.A.; Inoue, Y. Origin, Control, and Application of Supramolecular Chirogenesis in Bisporphyrin-Based Systems. Acc. Chem. Res. 2004, 37, 449–459. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry II. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Brill, Z.G.; Condakes, M.L.; Ting, C.P.; Maimone, T.J. Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products. Chem. Rev. 2017, 117, 11753–11795. [Google Scholar] [CrossRef]
- Puneet, P.; Fujiki, M.; Nandan, B. Circularly Polarized Luminescent Polymers: Emerging Materials for Photophysical Applications. In Reactive and Functional Polymers; Gutierrez, T., Ed.; Springer: Cham, Switzerland, 2020; Volume 3, pp. 117–139. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiki, M. Resonance in Chirogenesis and Photochirogenesis: Colloidal Polymers Meet Chiral Optofluidics. Symmetry 2021, 13, 199. https://doi.org/10.3390/sym13020199
Fujiki M. Resonance in Chirogenesis and Photochirogenesis: Colloidal Polymers Meet Chiral Optofluidics. Symmetry. 2021; 13(2):199. https://doi.org/10.3390/sym13020199
Chicago/Turabian StyleFujiki, Michiya. 2021. "Resonance in Chirogenesis and Photochirogenesis: Colloidal Polymers Meet Chiral Optofluidics" Symmetry 13, no. 2: 199. https://doi.org/10.3390/sym13020199
APA StyleFujiki, M. (2021). Resonance in Chirogenesis and Photochirogenesis: Colloidal Polymers Meet Chiral Optofluidics. Symmetry, 13(2), 199. https://doi.org/10.3390/sym13020199






