Abstract
Asymmetric gait is associated with pain, injury, and reduced stability in patient populations. Data from side by side walking suggest that unintentional synchronization with an external cue may reduce gait asymmetry. Two types of asymmetric gait were examined here: (1) mass imbalance between limbs to simulate single limb amputation and (2) restriction of plantarflexion during toe-off to simulate reduced propulsion from neurological impairment. Twenty-five healthy participants walked normally and with simulated gait asymmetry on a custom-designed treadmill that oscillated in the vertical direction via pneumatic actuation (amplitude: 2 cm, frequency: participant’s preferred step frequency). Swing Time Asymmetry (STA) and Phase Coordination Index (PCI) both increased significantly with the application of unilateral mass and plantarflexion restriction (p < 0.001). However, walking with simulated asymmetry did not alter unintentional synchronization with the treadmill motion. Further, oscillation of the treadmill did not improve STA or PCI while walking with simulated asymmetry. Analysis of synchronized step clusters using the Weibull survival function revealed that synchronization with the platform persisted for longer durations when compared with data from side by side walking. These results suggest that walking on a vertically oscillating surface may not be an effective approach for improving gait asymmetry.
1. Introduction
Asymmetric gait is a common problem in many patient populations [1,2,3,4,5,6,7,8,9,10]. Asymmetry during gait can result from imbalances in physical properties of the extremities such as disproportionate leg lengths or masses [10], imbalances in muscle strength, resting length, and flexibility [11,12,13], or neurological deficits that can occur at several levels of the nervous system [14,15,16]. Gait asymmetry is a concern for rehabilitation professionals because it can lead to increased risk of fall by hindering appropriate reactions to perturbation [4,5,6,7,17,18,19,20,21]. Asymmetric loading during gait can also result in pain and degenerative changes in the spine, knee, and hip [13,22,23].
Several interventions for improving gait asymmetry have been proposed. These include muscle strength training [24,25], split-belt treadmill training [16], wearable devices [26], robotic training [27], and intentional synchronization of gait to an auditory cue [28,29,30]. Unintentional synchronization of gait between individuals walking on side by side treadmills also appears to improve symmetry, but the coupling strength between walkers has been shown to be relatively weak [31,32]. Coupling strength refers to how closely paired oscillators maintain a consistent relative phase [32,33]. This is an important consideration for gait rehabilitation because the extent to which a walking human adheres to a reference signal (or another walker) may impact the efficacy of their therapy. Recently, we developed a vertically oscillating platform and treadmill that results in a greater coupling strength when healthy individuals walk and the platform oscillates at a frequency that is close to their preferred stepping frequency [34,35]. This approach is based upon the “wobbly bridge” phenomenon, which suggests that humans will subconsciously entrain their gait to sinusoidal movement of the walking surface if the frequency of motion is near their normal step frequency [36,37,38]. Unintentional synchronization with the motion of this platform may affect gait asymmetry in a manner similar to that of side by side treadmill walking, but this has not been evaluated.
Asymmetric gait can be simulated by introducing a detuning factor in healthy subjects as they walk on a treadmill. This has been accomplished by selectively adding mass to one leg [31,39,40]. For example, unilateral, transtibial amputees often demonstrate increased swing time and decreased stance time on their prosthetic, due in part to differences in the inertial properties of each limb [1,2,10]. Detuning of limb oscillation can also be accomplished with a unilateral decrease in ankle propulsive force. At its peak, approximately 2.5 W·kg−1 is produced by the ankle during push-off, which is more than the maximum power generated at the knee or hip [41]. Removal or impairment of plantar flexors can compromise one’s ability to generate the propulsive forces needed for symmetrical locomotion [1,14,15,42]. Parkinson’s disease patients, cerebral palsy patients, and poststroke patients often exhibit asymmetrical gait patterns due in part to low ankle joint force production [9,14,15,16,43,44].
The purpose of this study was to examine the behavior of healthy individuals with simulated gait asymmetry as they walked on the vertically oscillating treadmill. Symmetry was evaluated using a measure of Swing Time Asymmetry (STA) and Phase Coordination Index (PCI) [8,45,46,47]. Synchronization was evaluated by examining phase and frequency locking, as well as application of the Weibull survival function to characterize clusters of synchronization behavior [34]. Two types of gait impairment were simulated: (1) mass imbalance between the limbs, as generally occurs in the case of a single limb amputee [10,31,40], and (2) restriction of plantarflexion during toe-off, leading to a reduction in propulsion, which often occurs with certain neurological diseases [14,15,16,43,44,48]. We hypothesized that walking on the oscillating platform under these conditions would result in less synchronization when compared with a control condition and with data previously reported for healthy walkers [49,50,51]. We also hypothesized that walking with the oscillating platform would reduce the gait asymmetry induced by unilateral ankle weight and restriction to plantarflexion, based upon results reported for side by side walking [31].
2. Materials and Methods
2.1. Participants
A convenience sample of 25 healthy, recreationally active young adults (>18 years) (12 males and 13 females) with no known neurological or musculoskeletal impairments that may affect gait or balance were recruited from the local student population (Table 1). This sample size was estimated using effect sizes from a previous study of a similar nature [31]. Participants were restricted to a body weight of 82 kg due to mechanical limitations of the oscillating treadmill. All procedures were approved by the institutional review board at California State University, San Marcos, and all the participants were given an informed consent document to read and sign prior to collecting any data.

Table 1.
Participant characteristics (n = 25).
2.2. Apparatus/Equipment
An 8-camera motion capture system (Qualisys Track Manager 2019.1, Göteborg, Sweden) was used to acquire kinematic data by tracking reflective markers placed over the participants’ lower extremities and hips. These included the left and right great toes, heel, half shank midway between the lateral condyle of the tibia and lateral malleolus, lateral knee, half thigh midway between the greater trochanter and lateral epicondyle of the femur, and greater trochanter, as well as the sacrum for reference. The cameras captured data at 180 Hz.
Participants walked on a motorized treadmill that was raised approximately 90 cm above the ground. The platform was designed to oscillate in the vertical direction, and this motion was driven by 8 pneumatic cylinders (TRD Manufacturing, Machesney Park, IL, USA): one under each corner and four grouped directly under the center of the platform. The endpoint of each cylinder was individually controlled by an electronic position control valve (Enfield Technologies, Shelton, CT). Custom software created in MATLAB’s Simulink RealTime environment (R2017, Natick, MA, USA) precisely controlled the vertical position of the treadmill surface. The maximum possible range of motion (amplitude) of the platform was 15 cm and the maximum oscillation frequency was approximately 3 Hz. An overhead gantry and harness (Maine Anti-Gravity Systems, Inc., Portland, ME, USA) ensured participant safety while walking on the treadmill. Participants were not instructed to intentionally synchronize with the treadmill but were told to walk as normally as possible. All participants wore the same make and model of athletic shoe (Athletic Works, Betonville, AR, USA) for all trials.
An ankle weight of 3% of the participant’s mass (mean = 1.93 ± 0.3 kg) was placed around the left ankle during the ankle weight trials. A previous study determined that a mass between 1 and 5% of the participants’ body mass was effective at inducing gait asymmetry without discomfort [31].
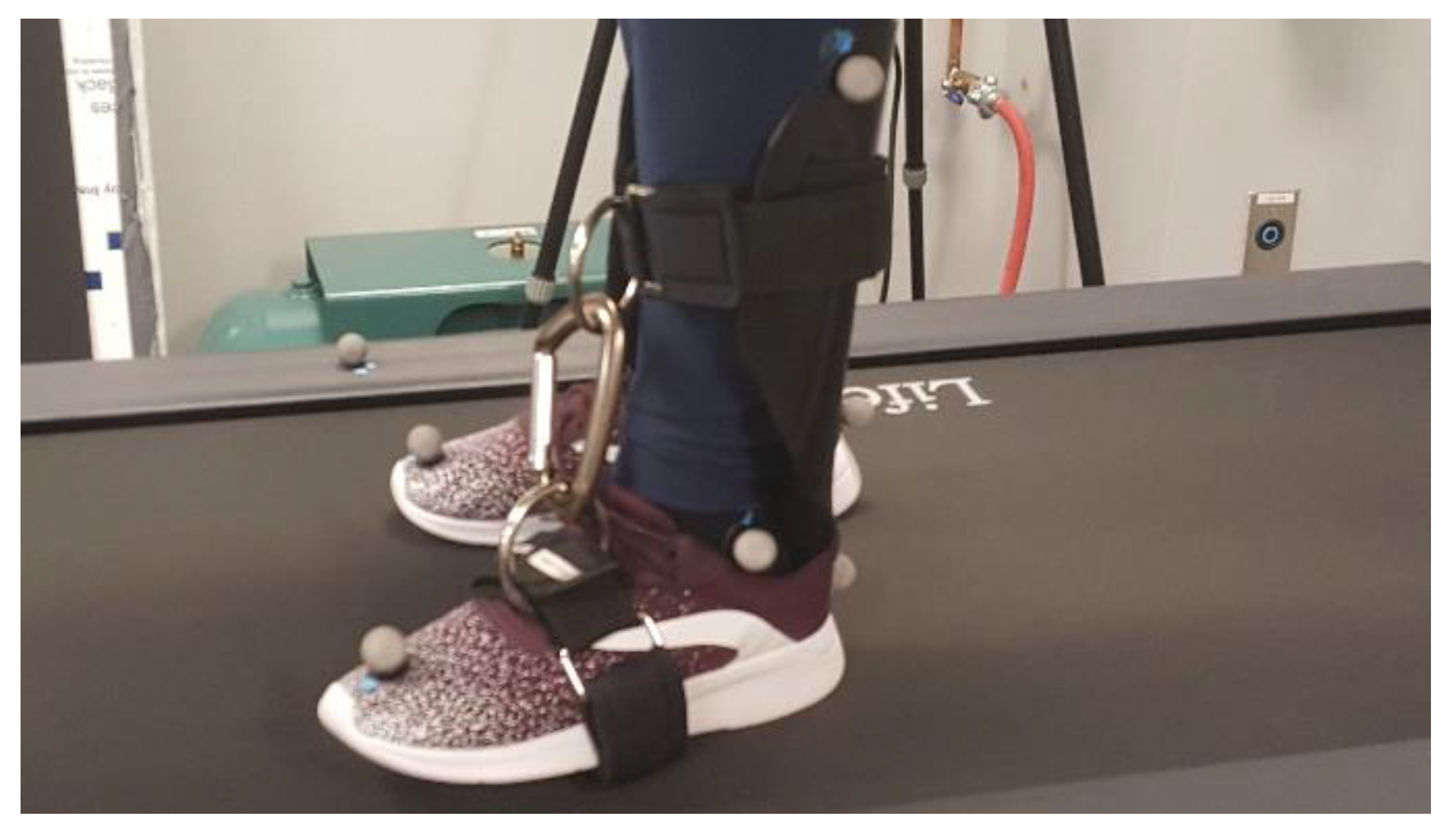
An ankle brace that restricted ankle joint range of motion was custom-made in the Biomechanics Laboratory at CSU, San Marcos. The brace was made from a single piece of moldable thermoplastic which included (1) a foot region that was designed to be placed inside the shoe, (2) rigid straps wrapped about the forefoot on the outside of the shoe, (3) a region surrounding the upper shank, and (4) rigid straps connecting the forefoot and shank region while opposing ankle plantarflexion (Figure 1). The brace was placed on the left ankle during the ankle brace trials. Restricting ankle range of motion to a total of 17° or less will significantly decrease the ability of the ankle joint to produce adequate propulsion force [40,52,53,54]. In the current study, the ankle brace reduced participants’ ankle joint range of motion by 39%, from an average of 26.45 ± 3.82° to an average of 16.05 ± 2.66° while walking on the nonoscillating treadmill. The dominant limb of each participant was not determined; therefore, it is possible that the ankle weight and brace were placed on the dominant limb for some participants, and the nondominant limb for others.
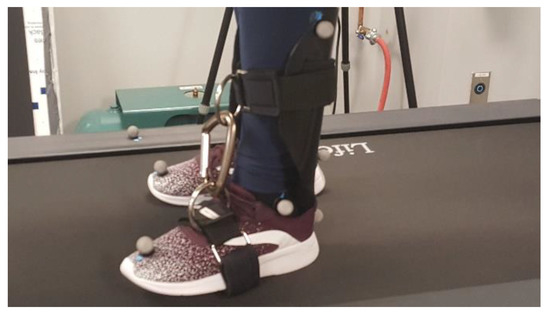
Figure 1.
Brace designed to restrict ankle range of motion by opposing plantarflexion.
2.3. Procedure
Participants performed a total of six walking trials on the treadmill at their individual preferred walking speed (mean = 1.12 ± 0.10 m·s−1). Preferred speed was identified during a familiarization trial in which the treadmill speed began at 0.9 m·s−1 and was slowly ramped up until each participant indicated that they had reached their comfortable walking pace. Participants walked under three asymmetry conditions: control (no modification to gait), while wearing the unilateral ankle brace, and while wearing a unilateral ankle weight. Each of the three conditions was performed once while the treadmill was stationary, and once while it oscillated vertically at their individual preferred step frequency (mean = 11.21 ± 0.62 rad·s−1) with an amplitude of approximately 2 cm. Previous analysis indicated that amplitudes greater than 2 cm can lead to phase lag in the oscillation frequency of the platform due to mechanical limitations, and did not appreciably increase the level of synchronization [34]. The normal condition was always performed first to allow for the assessment of preferred step frequency to be used in subsequent oscillation trials. Within asymmetry conditions, the nonoscillation trial was always performed before the oscillation trial. Trials were 2 min in duration with 2 min washout periods between each asymmetry condition. Previous studies with similar interventions have shown that a duration of 2 min is sufficient to allow temporary adaptations (i.e., after-effects) to abate [31,34,39]. Participants were allowed to sit and rest between trials when the unilateral ankle weight or brace was applied to or removed from their left ankle by one of the researchers.
2.4. Data Analysis
The level of unintentional synchronization with the oscillating treadmill was examined to determine differences among walking trials. Synchronization was evaluated by examining both frequency locking and phase locking. Frequency locking refers to points in time where the frequency of the limb matches the frequency of the moving platform, calculated by comparing the averages of a moving five second window (described in [51]). Phase locking was calculated by comparing the phase of the foot with respect to the phase of the platform at each heel strike. A relative phase at this instance that was within ± 10° (0.17 rad) was considered to be “phase locked.” Frequency locking and phase locking of the “impaired” limb with the oscillation of the treadmill were expressed as percentages of the entire two minute trial.
Swing Time Asymmetry was quantified by comparing the swing times of one leg to the other according to the formula [8]:
where SSWT and LSWT are the mean swing time of the short and long swing times, respectively.
Bilateral coordination was determined by calculating the Phase Coordination Index (PCI) [8,45,46,47]. The Phase Coordination Index measures variations in phase relationship between the left and right limbs throughout the entire trial. An assumption of walking symmetry would hold that the phase difference between limbs should converge to 180 degrees. During asymmetrical gait, this phase difference can exhibit both an average (absolute) deviation from 180 degrees across all strides (represented below as ) and it can exhibit variability in this deviation from stride to stride (represented below as ). Both of these aspects of phase coordination are accounted for in the calculation of PCI. The calculation began by first normalizing step time and stride time using the equation:
where are the time of the ith heel strike of the legs with the short and long swing times, respectively. Next, the accuracy of phase coordination was calculated by finding the mean absolute deviation of φ (the phase difference between limbs) and converting it to its percentile value using:
where
Finally, PCI was calculated as the sum of the coefficient of variance of the mean phase of each participant (φCV) and the mean absolute deviation of phase coordination in its percentile value (φABS):
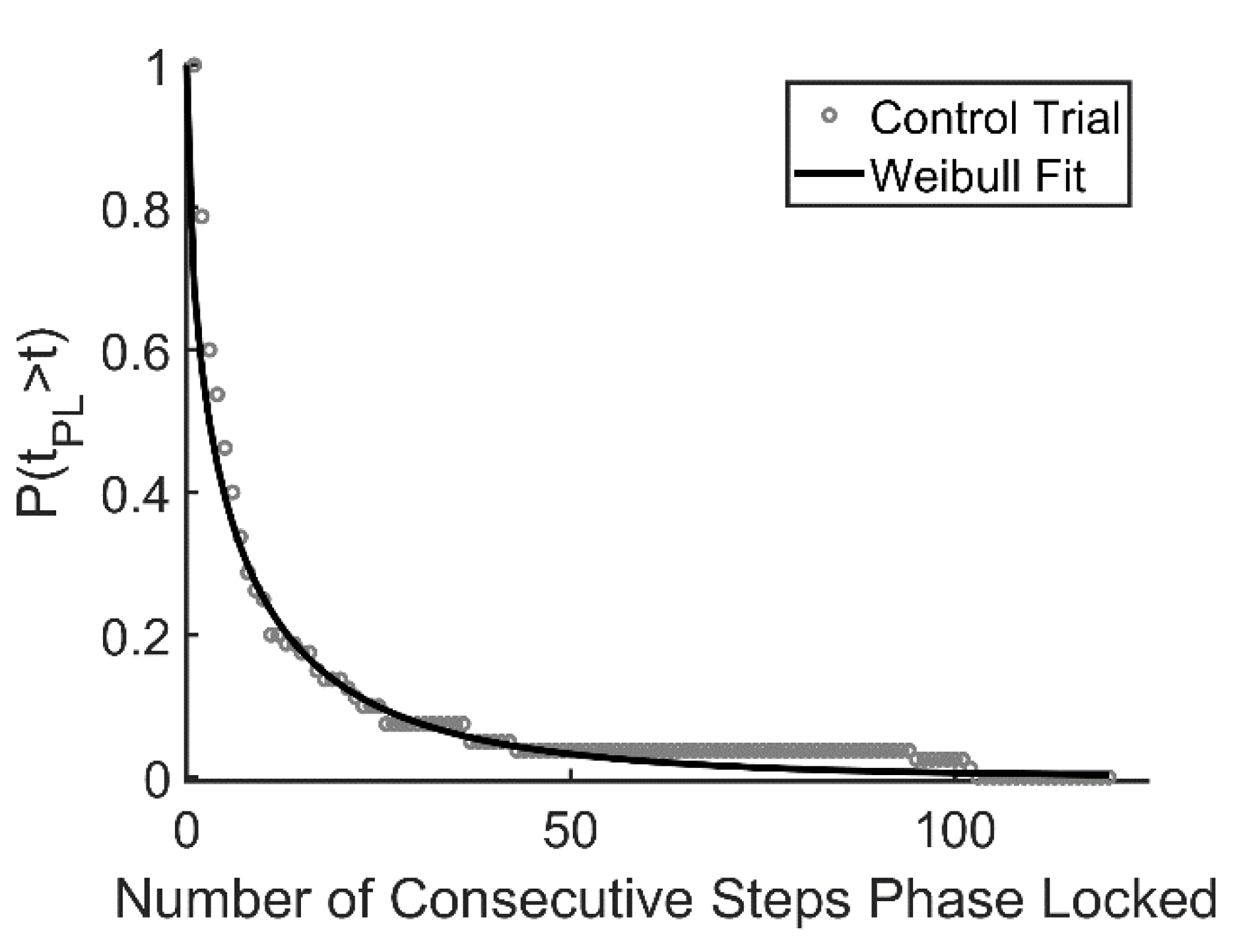
Unintentional synchronization of gait between an individual and an external cue or between two individuals walking side by side is typically transient in nature [34,51]. Recently, we described several different patterns of synchronized behavior observed as a person walked on the oscillating platform, referring to periods of synchronization as laminar phases [34]. In the current analysis, laminar phase lengths (i.e., the duration of each period of phase locked synchronization) were used to construct a survival function P(tpl > t), in which the proportion of laminar phases (tpl) that persisted for longer than each unit of time (t, expressed in terms of steps) were plotted and fit to the Weibull survival function:
where λ and β refer to positive constants, optimized through an iterative process in MATLAB (Figure 2). Once these constants are determined, the mean length of each laminar phase can be estimated as λ−1/βΓ(1 + 1/β), where Γ refers to the gamma function. In addition, the arithmetic means of the laminar phases for each walking trial were calculated directly from the data for comparison.
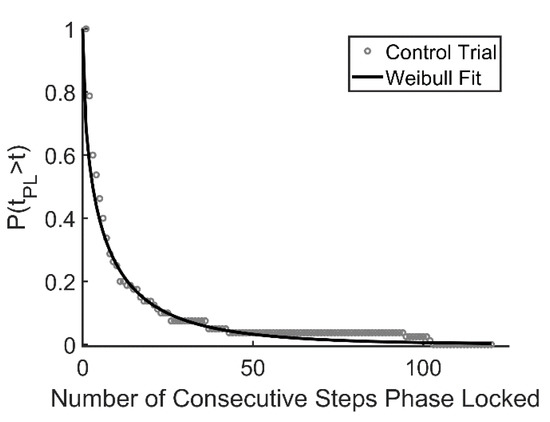
Figure 2.
Example of data fit to Weibull survival function. Data are aggregated across 25 subjects. Open circles represent the percentage of trials that survive at each time point; the solid line represents the Weibull curve with λ = 0.375 and β = 0.565 (control condition).
PCI and swing time asymmetry were compared using repeated measures ANOVA (3 asymmetry conditions × 2 oscillation conditions). Significant results were followed up with individual paired t-tests to determine where significant differences lay. Separate repeated measures ANOVA (3 asymmetry conditions) were also conducted to examine differences in phase and frequency locking with the oscillation of the treadmill, also followed up with paired t-tests where appropriate. All tests were conducted using a 95% confidence interval; outcomes of p < 0.05 were accepted as significantly different. The Benjamini–Hochberg procedure was utilized to control for false discovery rate among post hoc comparisons [55].
3. Results
3.1. Intrapersonal Coordination
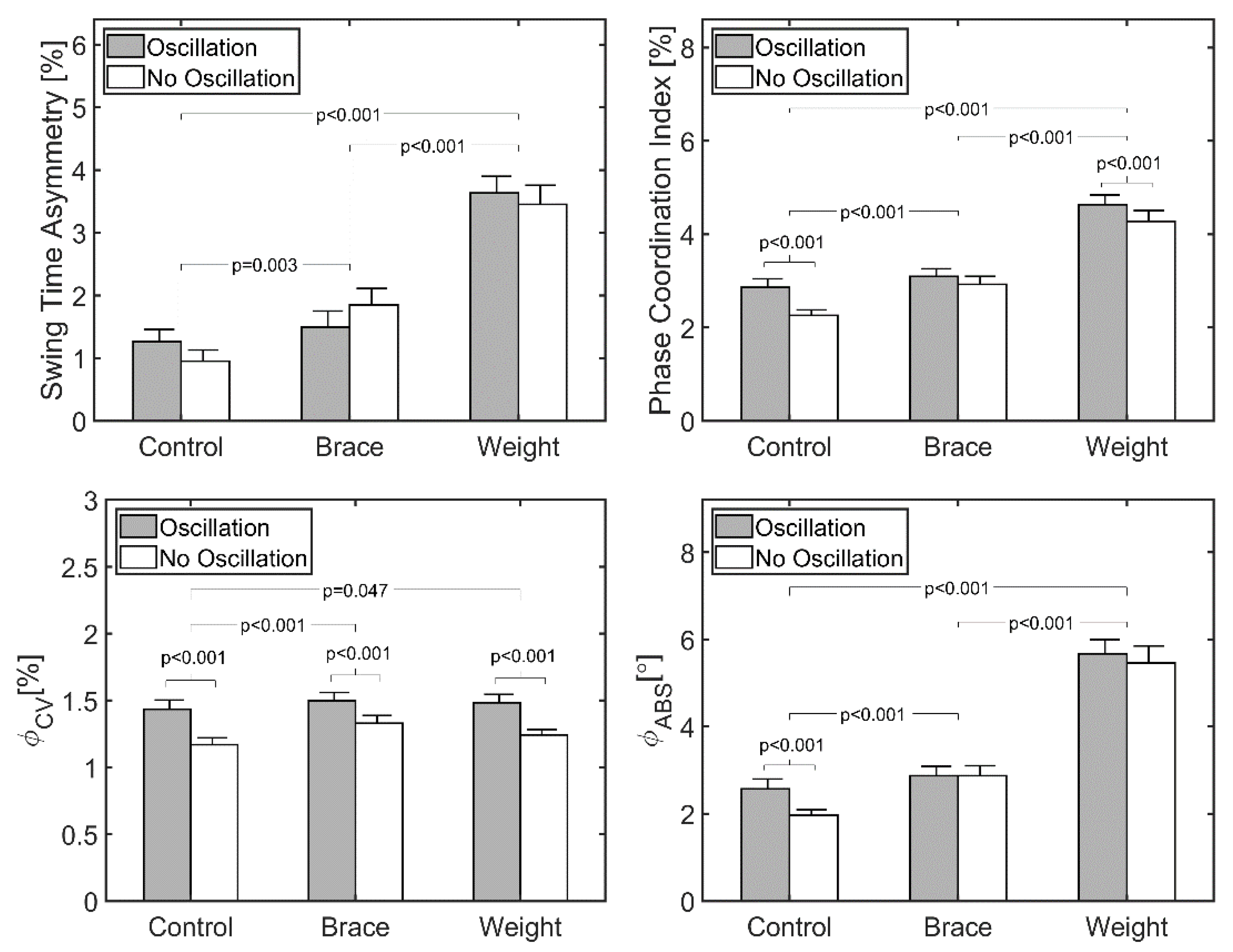
Factorial ANOVA revealed no statistically significant difference in swing time asymmetry due to the oscillation of the treadmill in any of the conditions (p = 0.81) (Table 2, Figure 3). However, STA was significantly different across the three perturbed walking conditions (p < 0.001). Post hoc analysis revealed a significant increase of 0.53 ± 0.21% between the control and brace conditions (p = 0.003), a significant increase of 1.90 ± 0.31% between the brace and weight conditions (p < 0.001), and a significant increase of 2.43 ± 0.23% between the control and weight conditions (p < 0.001). No interaction was found between treadmill oscillation and three walking conditions (p = 0.316).

Table 2.
Summary data for all walking conditions.
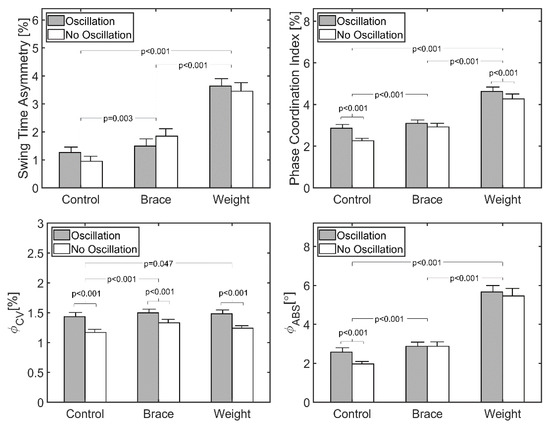
Figure 3.
Measures of asymmetric gait for all walking trials (n = 25). Participants walked for 2 min at their preferred walking speed (mean = 1.12 ± 0.10 m·s−1). Bars represent standard error of the mean.
Factorial ANOVA revealed a statistically significant increase in PCI due to the oscillation of the treadmill (p = 0.001). Post hoc analysis revealed that oscillation generated a significant increase of 0.27 ± 0.23% in the control condition (p < 0.001) and a significant increase of 0.12 ± 0.22% for the weight condition (p = 0.047), but no significant difference for the brace condition (0.11 ± 0.27% difference, p = 0.209). There was also a significant difference across the three perturbed walking conditions (p < 0.001). The post hoc analysis showed a significant increase of 0.38 ± 0.17% between the control and brace conditions (p < 0.001), a significant increase of 1.89 ± 0.18% between the control and weight conditions (p < 0.001), and a significant increase of 1.51 ± 0.18% between the brace and weight conditions (p < 0.001). No significant interaction was found between the treadmill oscillation and three walking conditions (p = 0.28).
Oscillation of the treadmill did not result in a significant change in (p = 0.140). However, was significantly different across the three perturbed walking conditions (p < 0.001). The post hoc analysis showed a significant increase of 0.55 ± 0.22° between the control and brace conditions (p = 0.001), a significant increase of 3.30 ± 0.30° between the control and the weight conditions (p < 0.001), and a significant increase of 2.75 ± 0.27° between the brace and weight conditions (p < 0.001). No significant interaction was found between the treadmill oscillation and three walking conditions (p = 0.35).
Oscillation of the treadmill resulted in a significant increase in (p < 0.001). There was also a significant difference across the three perturbed walking conditions (p = 0.016) and the post hoc analysis showed a significant increase of 0.08 ± 0.06% between the control and the brace conditions (p = 0.027). No significant interaction was found between the treadmill oscillation and three walking conditions (p = 0.440).
3.2. Interpersonal Coordination
Repeated measures ANOVA showed no statistically significant difference in phase locking between any of the three perturbed walking conditions during the oscillation of the treadmill (p = 0.38), with means of 35.49 ± 33.27%, 44.47 ± 31.28%, and 43.09 ± 33.21% for the control, brace, and weight conditions, respectively (Table 3). Additionally, frequency locking was not significantly different (p = 0.23) between the three conditions during the oscillation of the treadmill, with means of 94.98 ± 17.79%, 88.94 ± 25.75%, and 93.04 ± 19.20% for the control, brace, and weight conditions, respectively (Table 3). Analysis of survival curves indicated the greatest laminar phase length occurred during the control trials, while the shortest average laminar phase occurred during the brace trials. The predicted laminar phase length underestimated the measured lengths by approximately two steps in all three walking conditions (Table 3). The greatest disparity between the predicted and actual laminar phase length occurred during the brace trials (2.17 steps), and the lowest disparity occurred during the weight trials (1.96 steps).

Table 3.
Laminar phase statistics.
4. Discussion
The purpose of this study was to examine the effect of a vertically oscillating treadmill on walking asymmetry in healthy individuals induced by: (1) adding a unilateral ankle weight and (2) restricting ankle joint range of motion to restrict ankle joint plantarflexion propulsive power using a custom-made brace. There were four primary outcomes and three secondary outcomes to this analysis. First, the analysis of limb coordination revealed an increase in swing time asymmetry (STA) with the addition of both the unilateral ankle weight and the unilateral ankle brace relative to the control condition. However, there was no change in STA due to the oscillation of the treadmill across all three walking conditions (Figure 3). This finding did not support the hypothesis that STA would decrease with the oscillation of the treadmill. Second, PCI increased with the addition of both the unilateral ankle weight and the unilateral ankle brace relative to the control condition. This was expected because a similar result was observed in a previous analysis of side by side walking [31]. However, PCI was further increased in all three walking conditions with the oscillation of the treadmill, a finding that did not support the hypothesis that synchronization with vertical oscillation of the treadmill would improve (decrease) phase coordination (Figure 3). Third, phase locking and frequency locking with the oscillation of the treadmill were unchanged across all three walking conditions (Table 3). Finally, the addition of a unilateral ankle weight and brace resulted in laminar phases that were shorter, on average, compared to normal walking, but these phases were still longer and decayed more slowly than those previously reported between partners while walking side by side [34,49].
The secondary outcomes of this study center around the result that both (the limb’s deviation from 180 degrees) and (variability in the deviation from stride to stride) exhibited uniquely different patterns of behavior across the conditions tested. The addition of the unilateral ankle brace increased both and , while the addition of the unilateral ankle weight increased only. Further, was unchanged and was increased with the oscillation of the treadmill. Taken together, these results do not support the hypothesis that introducing the vertical oscillation improves phase coordination. However, these results suggest that different aspects of phase coordination might be selectively altered by each type of perturbation (mass imbalance versus plantarflexion restriction), and that they may each be affected disproportionately by synchronization.
4.1. Swing Time Asymmetry and PCI
Swing time asymmetry (STA) was unchanged with the oscillation of the treadmill (Figure 3). This result is different from that of a similar analysis of symmetry during side by side walking, which reported that gait asymmetry caused by a unilateral ankle weight was significantly decreased when participants synchronized with the stepping of a partner on an adjacent treadmill [31]. One explanation for this difference may be found in an analysis of limb dominance. A previous study of asymmetry of ground reaction forces during gait reported differences between dominant and nondominant limbs [56]. This study suggests that the limb affected by perturbation may shape the degree of influence of synchronization on gait asymmetry. If a greater number of participants in the current study had their dominant limb perturbed, they may have experienced a greater degree of asymmetry compared to the earlier study in side by side walking. Coupling strength may have also contributed to this difference. The coupling strength between side by side walkers was shown to be lower than that of an individual walking on an oscillating treadmill [34]. Coupling strength is often evaluated by examining the standard deviation of the relative phase between oscillators [32,33], suggesting that weaker coupling allows for more variability in behavior while stronger coupling enforces a particular movement pattern more strongly [32,34,57]. Therefore, for the case of a more strongly coupled oscillator system, an asymmetric walker may have less freedom to correct for imbalances in cycle time between the left and right limbs. This may explain the reduction in STA exhibited during side by side walking which did not occur during synchronization with the oscillating platform.
PCI significantly increased with the addition of the unilateral ankle weight and ankle brace (Table 2, Figure 1, Figure 2 and Figure 3). This result is consistent with a previous analysis of treadmill walking with a unilateral ankle weight [31]. However, rather than decrease during trials with the oscillation of the treadmill, the phase coordination index increased with the oscillation across all conditions. This increase in PCI during oscillation of the treadmill differed from previous behavior described during side by side walking [31] and did not support the hypothesis that PCI would be decreased with synchronization to the oscillating treadmill. Analysis of and indicates that these increases in PCI are primarily driven by increases in (Table 2, Figure 1, Figure 2 and Figure 3). As describes the variability in phase coordination between limbs, this suggests that there is an increased variability of movement when the treadmill oscillates vertically. While additional research is needed to fully understand this behavior, we might speculate that an increased drive to synchronize with the treadmill oscillation during each stride may have led to the observed increased variability. It is possible that this drive to synchronize was the result of intentional cognitive effort. However, data from side by side walking conditions indicates that unintentional synchronization occurs often, particularly when participants are reminded to avoid purposeful synchronization [51,57]. Whether intentional or unintentional, this behavioral attractor toward synchronization may have led to participants to make small corrections throughout the swing phase to ensure that the foot landed on the treadmill surface at the appropriate time, leading to greater variability in phase coordination. In short, the increase in PCI and with the oscillation of the treadmill when other measures of gait were unchanged may indicate an effort to explore a greater range of stride durations while attempting to match the oscillation of the treadmill. This assertion is supported by other studies that have noted changes in the patterns of stride duration when walkers attempt to synchronize to an external signal [57,58]. Further, recent data suggest that the addition of small amounts of variability to the cycle period of the oscillating treadmill can actually enhance unintentional synchronization [35].
An alternative explanation for an increase in PCI and might be derived from studies of rhythmic auditory stimulation (RAS) and its effect on temporal gait asymmetry (TGA) in patients following stroke [28,29,30]. RAS has been shown to have a relatively large impact on several aspects of gait in poststroke patients [29], but to date most studies have reported only a modest impact on TGA [30]. This may be related to a limitation in beat perception and production (i.e., rhythm ability) for certain patients, and individuals with weak perception or production often exhibit an increase in “beat-to-step” temporal variability [28,59]. While the participants included here were healthy, variations in rhythmic ability exist within the nonimpaired population [60,61], and this may affect movement variability in response to an external cue. In addition, rhythmic ability is related to a cyclical interaction between the perception of one’s environment, generation of action, and the impact of that action on the environment [62,63]. Any novel change to the environment or lower limb requires an adjustment of this perception–action coupling [64]. It is possible that participants did not have sufficient practice to adapt this perception–action coupling for each of the novel situations they experienced (oscillation, unilateral weight and brace), and this led to a reduction in rhythmic ability.
4.2. Phase and Frequency Locking
The phase and frequency locking in the present analysis were higher than those reported previously during side by side walking [31]. This is consistent with previous comparisons of unintentional synchronization during side by side walking versus vertical oscillation of the walking surface [34]. Interestingly, walking side by side with a unilateral ankle weight resulted in a reduction in STA and PCI when compared to walking alone with a unilateral ankle weight [31]. During treadmill oscillation in the current study, these variables either remained unchanged (STA) or increased (PCI). Other investigators [48,65] have suggested that the coupling strength of interpersonal synchronization may be a factor in how gait asymmetry is altered. While coupling strength cannot be measured directly, phase locking, frequency locking, and laminar phase length provide indirect measures of coupling strength. Taken together, these data suggest that coupling strength is greater while walking on the oscillating treadmill and may therefore lead to walking behavior that is different from the side by side walking condition.
4.3. Brace and Weight Asymmetry
In this study, a unilateral ankle weight and unilateral ankle brace were placed on the participants’ left limbs to induce gait asymmetry. Similar studies have added a mass to one ankle to simulate the gait of individuals who suffer from neurological diseases [31,39,40]. In addition to the ankle weight, the ankle brace was also used to restrict ankle ROM in order to simulate the gait of individuals who suffer from gait impairments that present with reduced propulsive forces [1,42,44]. It is unclear how closely these conditions mimic physical and neurological impairments, but they were successful at inducing gait asymmetry. When the ankle weight was compared to the brace, the ankle weight resulted in a greater increase in asymmetry (3.54 ± 1.42% versus 1.68 ± 1.29%) and PCI (4.45 ± 1.10% versus 3.01 ± 0.84%, Table 3). These results suggest that the weight imbalance applied here (approximately 3% of body weight) presented a greater challenge to bilateral coordination and symmetry when compared to the brace, which resulted in a decrease of 10.41° of ankle range of motion.
4.4. Laminar Phase Lengths
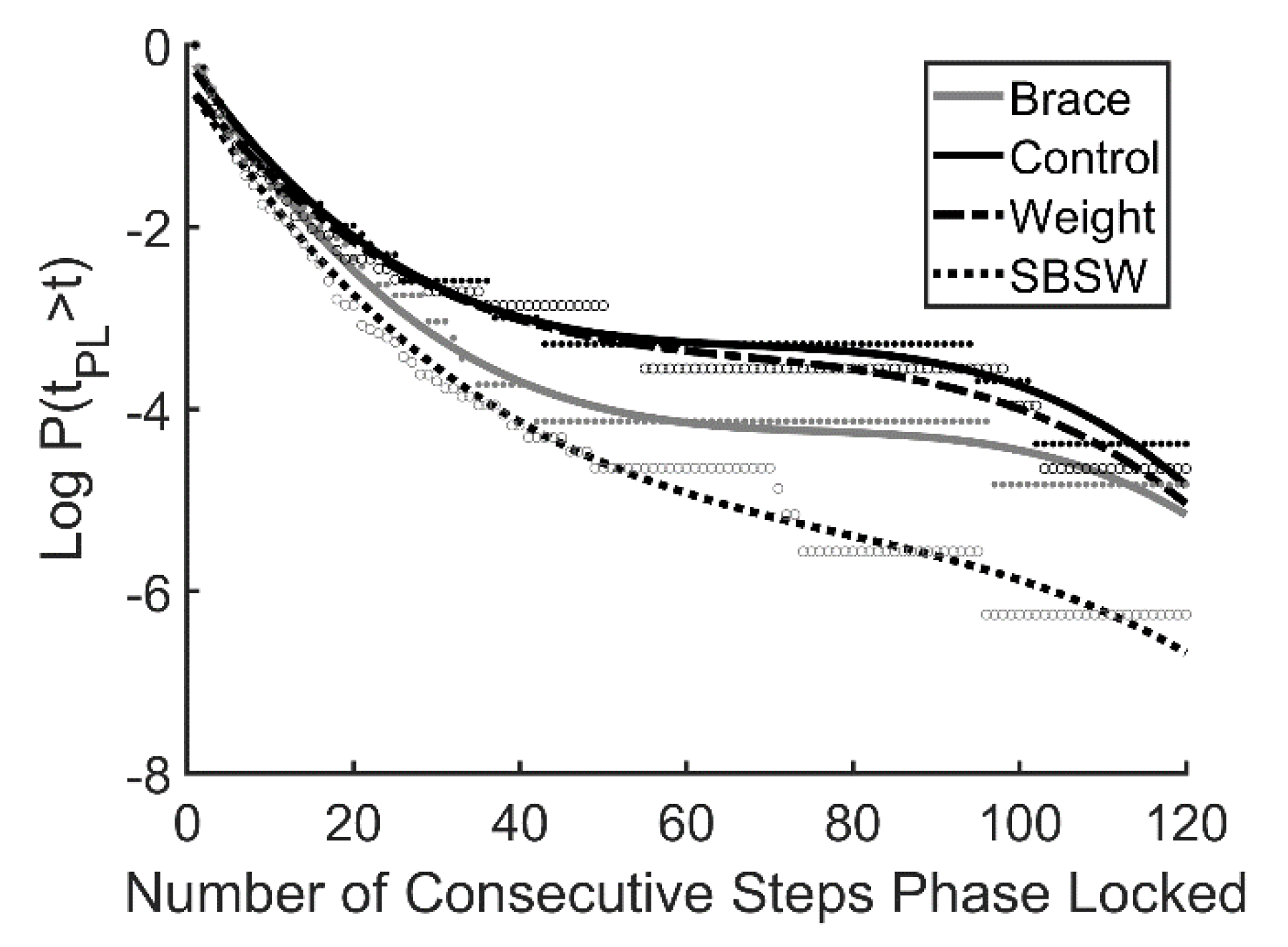
The ankle brace had a greater impact on laminar phase length and β than the ankle weight (Table 3). This suggests that even though the ankle weight presented a great challenge to symmetrical walking, the ankle brace presented a greater challenge to synchronization with the oscillating platform, as periods of transient synchronization were shorter on average during the brace trials. A comparison with previous data obtained during side by side walking [49,50,51] suggests that laminar phases were longer and decayed more slowly on the oscillating treadmill, even with unilateral ankle weight and plantarflexion restriction (Figure 4). Though participants synchronized with the treadmill for longer periods of time when compared with side by side walking, this behavior did not appear to impact gait asymmetry.
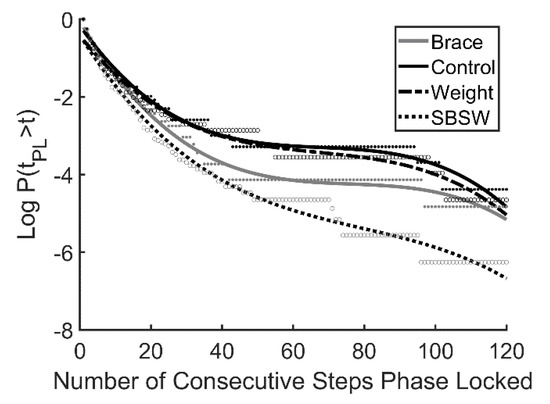
Figure 4.
Survival curves plotted on a log scale for the 3 walking conditions examined here and data from a previous study that examined side by side walking (SBSW, Nessler et al. 2011). Participants walked for 2 min at their preferred walking speed (mean = 1.12 ± 0.10 m·s−1). Curves represent 3rd order polynomial best fit.
4.5. Limitations
Participants walked at their preferred speed and the treadmill oscillated at their preferred step frequency for all trials analyzed here. The variables analyzed in this study may be dependent on stepping speed and duration, which may have affected the results of the study [34,65,66]. For example, Kao et al. (2003) found that variability of intralimb coordination increased at higher walking speeds [33] and Plotnik et al. (2013) found that coordination and stability increased with lower walking speeds [48]. Further research is needed to explore the effects of different speeds on intralimb coordination.
Additionally, the unilateral ankle mass and unilateral ankle brace utilized here were designed to induce gait asymmetry through external perturbation. This is different from the case where gait asymmetry arises from underlying neuromuscular disease or amputation because these conditions may have additional effects on the neuromuscular and central nervous systems. For example, research on split-belt treadmill walking suggests that Parkinson’s patients adapt differently to external perturbation when compared to healthy controls [16].
Finally, all participants were instructed to walk as normally as possible. It was assumed that they did not purposefully synchronize with the platform motion, but this was not verified.
5. Conclusions
Overall, the results of this study demonstrate that swing time asymmetry (STA), synchronization, and bilateral coordination (PCI) are altered with the addition of a perturbation (ankle weight and ankle brace). However, STA was unchanged and PCI increased with the vertical oscillation of the treadmill, which differed from previous research in side by side walking. These results, combined with the fact that phase locking and frequency locking were not different during the asymmetrical walking trials, suggest that coupling strength may have an effect on how gait asymmetry is affected by synchronization with an external cue. Therefore, more research is needed to further understand the effect of the vertical oscillation on measurements of gait and how it may extend to special populations.
Author Contributions
Both authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work supported by NIH SC3GM096900.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of California State University, San Marcos (protocol ID: 1302456-1, date of approval: 08/06/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data acquired and analyzed in support of the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanderson, D.J.; Martin, P.E. Lower extremity kinematic and kinetic adaptations in unilateral below-knee amputees during walking. Gait Posture 1997, 6, 126–136. [Google Scholar] [CrossRef]
- Kovac, I.; Medved, V.; Ostojic, L. Spatial, temporal and kinematic characteristics of traumatic transtibial amputees’ gait. Coll. Antropol. 2010, 34, 205–213. [Google Scholar]
- Rodgers, M.; Forrester, L.; Mizelle, C.; Harris-Love, M.L. Effects of gait velocity on COP symmetry measures in individuals with stroke. In Proceedings of the 28th Annual Meeting of the American Society of Biomechanics, Portland, OR, USA, 8–11 September 2004. [Google Scholar]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Bradley, C.E.; Wutzke, C.J.; Zinder, S.M.; Lewek, M.D. Spatiotemporal gait asymmetry is related to balance/fall risk in individuals with chronic stroke. In Proceedings of the 36th Annual Meeting of the American Society of Biomechanics, Gainesville, FL, USA, 15–18 August 2012. [Google Scholar]
- Mahon, C.E.; Farris, D.J.; Sawicki, G.S.; Lewek, M.D. Individual limb mechanical analysis of gait following stroke. J. Biomech. 2015, 48, 984–989. [Google Scholar] [CrossRef]
- Hendrickson, J.; Patterson, K.K.; Inness, E.L.; McIlroy, W.E.; Mansfield, A. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture 2014, 39, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 2007, 181, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, A.; Salarian, A.; Wickstrom, N. A new measure of movement symmetry in early Parkinson’s disease patients using symbolic processign of inertial sensor data. IEEE Trans. Biomed. Eng. 2011, 58, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Sturk, J.A.; Lemaire, E.D.; Sinitski, E.; Dudek, N.L.; Besemann, M.; Hebert, J.S.; Baddour, N. Gait differences between K3 and K4 persons with transfemoral amputation across level and non-level walking conditions. Prosthet. Orthot. Int. 2018, 42, 626–635. [Google Scholar] [CrossRef]
- Leijendekkers, R.A.; Marra, M.A.; Kolk, S.; van Bon, G.; Schreurs, B.W.; Weerdesteyn, V.; Verdonschot, N. Gait symmetry and hip strength in women with developmental dysplasia following hip arthroplasty compared to healthy subjects: A cross-sectional study. PLoS ONE 2018, 13, e0193487. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, C.; Agostini, V.; Rosati, S.; Balestra, G.; Knaflitz, M. Asymmetry index in muscle activations. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 772–779. [Google Scholar] [CrossRef]
- Shi, H.; Huang, H.; Ren, S.; Yu, Y.; Liang, Z.; Wang, Q.; Hu, Z.; Ao, Y. The relationship between quadriceps strength asymmetry and knee biomechanics asymmetry during walking in individuals with anterior cruciate ligament reconstruction. Gait Posture 2019, 73, 74–79. [Google Scholar] [CrossRef]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and function motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin. Biomech. 2014, 29, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Seithe, J.; D’Cruz, N.; Ginis, P.; Weisser, B.; Berg, D.; Gunther, D.; Nieuwboer, A.; Schlenstedt, C. Split-belt treadmill walking in patients with Parkinson’s disease: A systematic review. Gait Posture 2019, 69, 187–194. [Google Scholar] [CrossRef]
- Rogers, M.W.; Johnson, M.E.; Martinez, K.M.; Mille, M.L.; Hedman, L.D. Step training improves the speed of voluntary step initiation in aging. Gerontol. Ser. A 2003, 58, 46–51. [Google Scholar] [CrossRef]
- Raibert, M.H. Symmetry in running. Science 1986, 231, 1292–1294. [Google Scholar] [CrossRef]
- Nasirzade, A.; Sadeghi, H.; Mokhtarinia, H.R.; Rahimi, A. A review of selected factors affecting gait symmetry. Phys. Treat. Specif. Phys. Ther. J. 2017, 7, 3–12. [Google Scholar] [CrossRef]
- Ellis, R.G.; Howard, K.C.; Kram, R. The metabolic and mechanical costs of step time asymmetry in walking. Proc. R. Soc. Biol. Sci. 2013, 280, 20122784. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.S.; Liu, P.T.; Chang, L.W.; Liu, S.Y. Gait asymmetry, ankle spasticity, and depression as independent predictors of falls in ambulatory stroke patients. PLoS ONE 2017, 12, e0177136. [Google Scholar]
- Nolan, L.; Dudzinski, K.; Lees, A.; Lake, M.; Wychowanski, M. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 2003, 17, 142–151. [Google Scholar] [CrossRef]
- Macfarlane, P.A.; Nielsen, D.H.; Shurr, D.G.; Meier, K. Gait comparisons for below-knee amputees using a Flex-Foot versus a conventional prosthetic foot. J. Prosthet. Orthot. 1991, 3, 150–161. [Google Scholar] [CrossRef]
- Wist, S.; Clivaz, J.; Sattelmayer, M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, L.R.; Petersen, A.K.; Soballe, K.; Mikkelsen, S.S.; Mechlenburg, I. Asymmetry and pelvic movements 6 months after total hip replacement; secondary analyses from a randomized controlled trial. Acta Orthop. Belg. 2019, 85, 338–345. [Google Scholar]
- Huizenga, D.; Rashford, L.; Darcy, B.; Lundin, E.; Medas, R.; Shultz, S.T.; DuBose, E.; Reed, K.B. Wearable gait device for stroke gait rehabilitation at home. Top. Stroke Rehabil. 2020. [Google Scholar] [CrossRef]
- Seo, J.S.; Yang, H.S.; Jung, S.; Chang, S.K.; Jang, S.; Kim, D.H. Effect of reducing assistance during robot-assisted gait training on step length asymmetry in patients with hemiplegic stroke: A randomized controlled. Medicine 2018, 97, e11792. [Google Scholar] [CrossRef]
- Crosby, L.D.; Wong, J.S.; Chen, J.L.; Grahn, J.; Patterson, K.K. An initial investigation of the responsiveness of temporal gait asymmetry to rhythmic auditory stimulation and the relationship to rhythm ability following stroke. Front. Neurol. 2020, 11, 1214. [Google Scholar] [CrossRef]
- Thaut, M.H.; Leins, A.K.; Rice, R.R.; Argstatter, H.; Kenyon, G.P.; McIntosh, G.C.; Bolay, H.V.; Fetter, M. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: A single-blind, randomized trial. Neurorehab. Neural Repair 2007, 21, 455–459. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, S. Rhythmic auditory cueing in motor rehabilitation for stroke patients: Systematic review and meta-analysis. J. Music Ther. 2016, 53, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Nessler, J.A.; Gutierrez, V.; Werner, J.; Punsalan, A. Side by side treadmill walking reduces gait asymmetry induced by unilateral ankle weight. Hum. Mov. Sci. 2015, 41, 32–45. [Google Scholar] [CrossRef]
- van Ulzen, N.R.; Lamoth, C.J.; Daffertshofer, A.; Semin, G.R.; Beek, P.J. Characteristics of instructed and uninstructed interpersonal coordination while walking side by side. Neurosci. Lett. 2008, 432, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.C.; Ringenbach, S.D.; Martin, P.E. Gait transitions are not dependent on changes in intralimb coordination variability. J. Mot. Behav. 2003, 35, 211–214. [Google Scholar] [CrossRef]
- Nessler, J.A.; Heredia, S.; Belair, J.; Milton, J.G. Walking on a vertically oscillating treadmill: Phase synchronization and gait kinematics. PLoS ONE 2017, 12, e0169924. [Google Scholar] [CrossRef] [PubMed]
- Tackett, E.; Nessler, J.A. Sensorimotor synchronization during gait is altered by the addition of variability to an external cue. Hum. Mov. Sci. 2020, 71, 102626. [Google Scholar] [CrossRef]
- Dallard, P.; Fitzpatrick, T.; Flint, A.; Low, A.; Smith, R.R.; Willford, M.; Roche, M. London Millennium Bridge: Pedestrian-Induced Lateral Vibration. J. Bridge Eng. 2001, 6, 412–417. [Google Scholar] [CrossRef]
- Strogatz, S.H.; Abrams, D.M.; McRobie, A.; Eckhardt, B.; Ott, E. Theoretical mechanics: Crowd synchrony on the Millenium Bridge. Nature 2005, 438, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Srinivasan, M. Walking crowds on a shaky surface: Stable walkers discover Millennium Bridge oscillations with and without pedestrian synchrony. Biol. Lett. 2018, 14, 20180564. [Google Scholar] [CrossRef]
- Noble, J.W.; Prentice, S.D. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp. Brain Res. 2006, 169, 482–495. [Google Scholar] [CrossRef]
- Romkes, J.; Schweizer, K. Immediate effects of unilateral restricted ankle motion on gait kinematics in healthy subjects. Gait Posture 2015, 41, 835–840. [Google Scholar] [CrossRef]
- Zelik, K.E.; Kuo, A.D. Human walking isn’t all hard work: Evidence of soft tissue contributions to energy dissipation and return. J. Exp. Biol. 2010, 213, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.K.; Fey, N.P.; Portillo, A.; Walden, J.G.; Bosker, G.; Neptune, R.R. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture 2008, 28, 602–609. [Google Scholar] [CrossRef]
- Volpe, R. Alterations of gait in neuromuscular disease. Clin. Podiatr. Med. Surg. 1988, 5, 627–638. [Google Scholar] [PubMed]
- Morris, M.E.; Huxham, F.; McGinley, J.; Dodd, K.; Iansek, R. The biomechanics and motor control of gait in Parkinson’s disease. Clin. Biomech. 2001, 16, 459–470. [Google Scholar] [CrossRef]
- Plotnik, M.; Wagner, J.M.; Adusumilli, G.; Gottlieb, A.; Naismith, R.T. Gait asymmetry, and bilateral coordination of gait during a six-minute walk test in persons with multiple sclerosis. Sci. Rep. 2020, 10, 12382. [Google Scholar] [CrossRef] [PubMed]
- Kribus-Shmiel, L.; Zeilig, G.; Sokolovski, B.; Plotnik, M. How many strides are required for a reliable estimation of temporal gait parameters? Implementation of a new algorithm on the phase coordination index. PLoS ONE 2018, 13, e0192049. [Google Scholar] [CrossRef]
- Gimmon, Y.; Rashad, H.; Kurz, I.; Plotnik, M.; Riemer, R.; Ronen, D.; Shapiro, A.; Melzer, I. Gait coordination deteriorates in independent old-old adults. J. Aging Phys. Act. 2018, 26, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Bartsch, R.; Zeev, A.; Giladi, N.; Hausdorff, J.M. Effects of walking speed on asymmetry and bilateral coordination of gait. Gait Posture 2013, 38, 864–869. [Google Scholar] [CrossRef]
- Nessler, J.A.; Kephart, G.; Cowell, J.; De Leone, C.J. Varying treadmill speed and inclination affects spontaneous synchronization during side by side walking. J. Appl. Biomech. 2011, 27, 322–329. [Google Scholar] [CrossRef][Green Version]
- Nessler, J.A.; De Leone, C.J.; McMillan, D.; Schoulten, M.; Shallow, T.; Stewart, B. Side by side treadmill walking with intentionally desynchronized gait. Ann. Biomed. Eng. 2012, 41, 1680–1691. [Google Scholar] [CrossRef]
- Nessler, J.A.; Gillilland, S. Interpersonal synchonization during side by side treadmill walking is influenced by leg length differential and altered sensory feedback. Hum. Mov. Sci. 2009, 28, 772–785. [Google Scholar] [CrossRef]
- Cho, K.H.; Jeon, Y.; Lee, H. Range of motion of the ankle according to pusing force, gender, and knee position. Ann. Rehabil. Med. 2016, 40, 271–278. [Google Scholar] [CrossRef]
- Hunsaker, F.G.; Cioffi, D.A.; Amadio, P.C.; Wright, J.; Caughlin, B. The American Academy of Orthopaedic Surgeons Outcomes Instruments: NOrmative Values from the General Population. J. Bone Jt. Surg. 2002, 84, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.C.; Lewis, C.L.; Ferris, D.P. Joint kinetic response during unexpectedly reduced plantar flexor torque provided by a robotic ankle exoskeleton during walking. J. Biomech. 2010, 43, 1401–1407. [Google Scholar] [CrossRef][Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Polk, J.D.; Stumpf, R.M.; Rosengren, K.S. Limb dominance, foot orientation and functional asymmetry during walking gait. Gait Posture 2017, 52, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Nessler, J.A.; Gillilland, S. Kinematic analysis of side by side stepping with intentional and unintentional synchronization. Gait Posture 2010, 31, 527–529. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Purdon, P.L.; Peng, C.-K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Fractal dynamics of human gait: Stability of long-range correlations in stride interval fluctuations. J. Appl. Physiol. 1996, 80, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Leow, L.A.; Parrott, T.; Grahn, J.A. Individual differences in beat perception affect gait responses to low- and high-groove music. Front. Hum. Neurosci. 2014, 8, 811. [Google Scholar] [CrossRef]
- Igaga, J.M.; Versey, J. Cultural differences in rhythmic perception. Psychol. Music 1977, 5, 23–27. [Google Scholar] [CrossRef]
- Igaga, J.M.; Versey, J. Cultural differences in rhythmic performance. Psychol. Music 1978, 6, 61–64. [Google Scholar] [CrossRef]
- Gibson, J.J. The Ecological Approach to Visual Perception; Houghton Mifflin: Boston, MA, USA, 1979. [Google Scholar]
- Warren, W.H. The Perception-Action Coupling; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Schoner, G.; Dijkstra, T.M.H.; Jeka, J.J. Action-perception patterns emerge from coupling and adaptation. Ecol. Psychol. 2011, 10, 323–346. [Google Scholar] [CrossRef]
- Goble, D.J.; Marino, G.W.; Potvin, J.R. The influence of horizontal velocity on interlimb symmetry in normal walking. Hum. Mov. Sci. 2003, 22, 271–283. [Google Scholar] [CrossRef]
- Beek, P.J.; Peper, C.E.; Stegeman, D.F. Dynamical models of movement coordination. Hum. Mov. Sci. 1995, 14, 573–608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).