Assessment of the Orbital and Auricular Asymmetry in Italian and Sudanese Children: A Three-Dimensional Study
Abstract
:1. Introduction
2. Materials and Methods
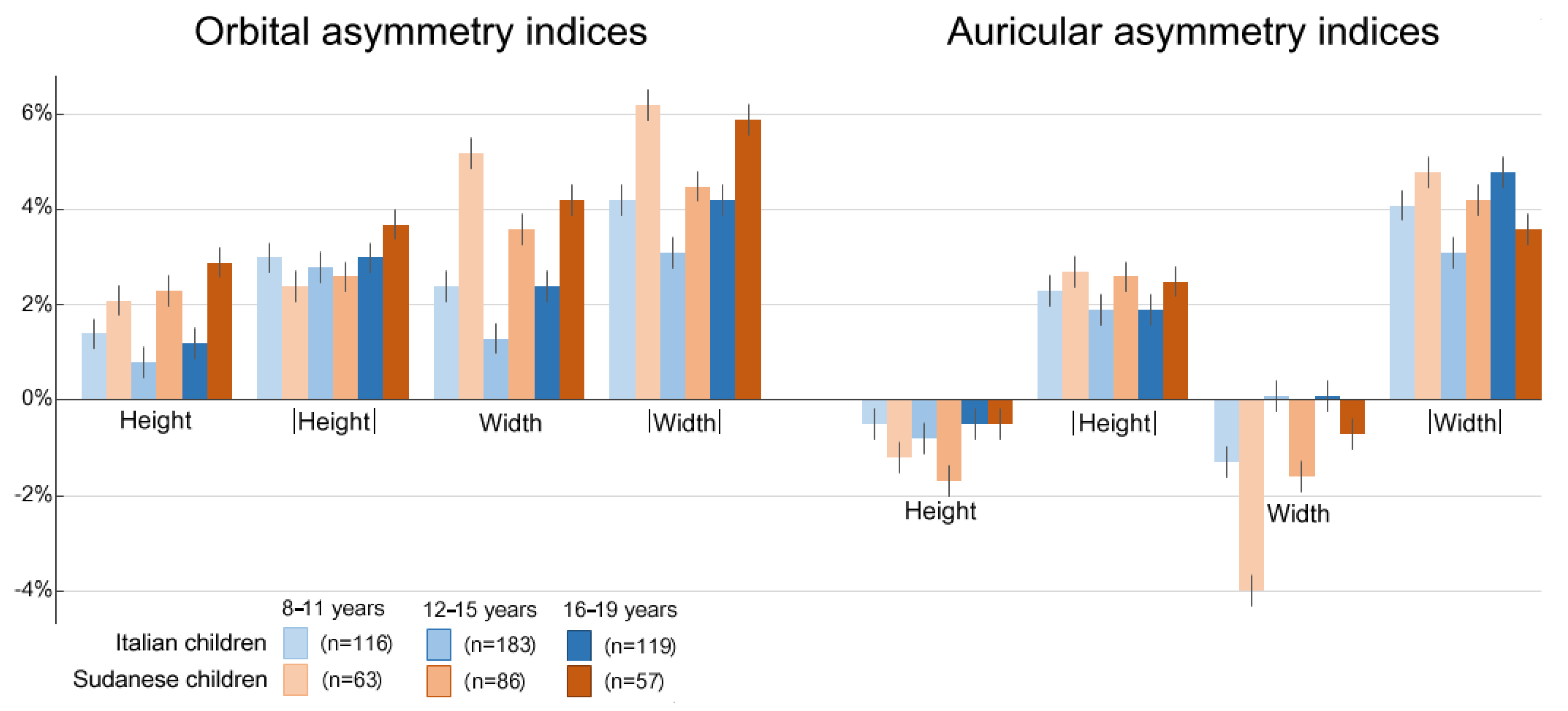
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, M.J.; Hallac, R.R.; Ramesh, J.; Seaward, J.R.; Hermann, N.V.; Darvann, T.A.; Lipira, A.; Kane, A.A. Quantifying Normal Craniofacial Form and Baseline Craniofacial Asymmetry in the Pediatric Population. Plast. Reconstr. Surg. 2018, 141, 380e–387e. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Denadai, R.; Chen, S.H.; Tseng, H.J.; Hsu, C.K.; Wang, S.W.; Hallac, R.; Chen, C.H.; Kane, A.A.; Lo, L.J. Identifying Three-Dimensional Facial Fluctuating Asymmetry in Normal Pediatric Individuals: A Panel Assessment Outcome Study of Clinicians and Observers. J. Clin. Med. 2019, 8, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coward, T.J.; Scott, B.J.J.; Watson, R.M.; Richards, R. Laser scanning of the ear identifying the shape and position in subjects with normal facial asymmetry. Int. J. Oral Maxillofac. Surg. 2000, 29, 18–23. [Google Scholar] [CrossRef]
- Coward, T.J.; Scott, B.J.J.; Watson, R.M.; Richards, R. Identifying the position of an ear from a laser scan: The significance for planning rehabilitation. Int. J. Oral Maxillofac. Surg. 2000, 31, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Kalcioglu, M.T.; Miman, M.C.; Toplu, Y.; Yakinci, C.; Ozturan, O. Anthropometric growth study of normal human auricle. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 1169–1177. [Google Scholar] [CrossRef]
- Sajid, M.; Shafique, T.; Riaz, I.; Imran, M.; Jabbar Aziz Baig, M.; Baig, S.; Manzoor, S. Facial asymmetry-based anthropometric differences between gender and ethnicity. Symmetry 2018, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Azaria, R.; Adler, N.; Silfen, R.; Regev, D.; Hauben, D.J. Morphometry of the adult human earlobe: A study of 547 subjects and clinical application. Plast. Reconstr. Surg. 2003, 111, 2398–2402. [Google Scholar] [CrossRef]
- Brucker, M.J.; Patel, J.; Sullivan, P.K. A morphometric study of the external ear: Age and sex-related differences. Plast. Reconstr. Surg. 2003, 112, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Pound, N.; Lawson, D.W.; Toma, A.M.; Richmond, S.; Zhurov, A.I.; Penton-Voak, I.S. Facial fluctuating asymmetry is not associated with childhood ill-health in a large British cohort study. Proc. R. Soc. B 2014, 281, 20141639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercan, I.; Ozdemir, S.T.; Etoz, A.; Sigirli, D.; Tubbs, R.S.; Loukas, M.; Guney, I. Facial asymmetry in young healthy subjects evaluated by statistical shape analysis. J. Anat. 2008, 213, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, D.W.; Aarde, S.M.; Baig, K. Appearance of symmetry, beauty, and health in human faces. Brain Cogn. 2005, 57, 261–263. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, S.; Ekrami, O.; Claes, P. Lack of Correlation between Facial Sexual Dimorphism, Fluctuating Asymmetry and Self-Perceived Attractiveness in Men and Women. Symmetry 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Ren, D.; Xie, C.; Pan, J.; Zhou, G. Normality mediates the effect of symmetry on facial attractiveness. Acta Psychol. 2021, 217, 103311. [Google Scholar] [CrossRef] [PubMed]
- Persing, S.; Timberlake, A.; Madari, S.; Steinbacher, D. Three-Dimensional Imaging in Rhinoplasty: A Comparison of the Simulated versus Actual Result. Aesthetic Plast. Surg. 2018, 42, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Weissler, J.M.; Stern, C.S.; Schreiber, J.E.; Amirlak, B.; Tepper, O.M. The Evolution of Photography and Three-Dimensional Imaging in Plastic Surgery. Plast. Reconstr. Surg. 2017, 139, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, O.; Claes, P.; White, J.D.; Weinberg, S.M.; Marazita, M.L.; Walsh, S.; Shriver, M.D.; van Dongen, S. A Multivariate Approach to Determine the Dimensionality of Human Facial Asymmetry. Symmetry 2020, 12, 348. [Google Scholar] [CrossRef] [Green Version]
- Ekrami, O.; Claes, P.; van Assche, E.; Shriver, M.D.; Weinberg, S.M.; Marazita, M.L.; Walsh, S.; van Dongen, S. Fluctuating Asymmetry and Sexual Dimorphism in Human Facial Morphology: A Multi-Variate Study. Symmetry 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Phenotypic Plasticity, Developmental Instability, and Robustness: The Concepts and How They Are Connected. Front. Ecol. Evol. 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Razzetti, E.; Faiman, R.; Werner, Y.L. Directional asymmetry and correlation of tail injury with left-side dominance occur in Serpentes (Sauropsida). Zoomorphology 2007, 126, 31–43. [Google Scholar] [CrossRef]
- Graham, J.H.; Özener, B. Fluctuating Asymmetry of Human Populations: A Review. Symmetry 2016, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Quinto-Sanchez, M.; Cintas, C.; Silva de Cerqueira, C.C.; Ramallo, V.; Acuña-Alonzo, V.; Adhikari, K.; Castillo, L.; Gomez-Valdès, J.; Everardo, P.; de Avila, F.; et al. Socioeconomic status is not related with facial fluctuating asymmetry: Evidence from Latin-American populations. PLoS ONE 2017, 12, e0169287. [Google Scholar]
- Werner, Y.L.; Seifan, T. Eye Size in Geckos: Asymmetry, Allometry, Sexual Dimorphism, and Behavioral Correlates. J. Morphol. 2006, 267, 1486–1500. [Google Scholar] [CrossRef]
- Seligmann, H. Evidence that minor directional asymmetry is functional in lizard hindlimbs. J. Zool. 1998, 245, 205–208. [Google Scholar] [CrossRef]
- Seligmann, H. Behavioral and morphological asymmetries in hindlimbs of Hoplodactylus duvaucelii (Lucertilia: Gekkon-omorpha: Gekkota: Diplodactylinae). Laterality 2002, 7, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Grandi, G.; Binelli, M.; Tommasi, D.G.; Rosati, R.; Ferrario, V.F. Age- and sex-related changes in the normal human ear. Forensic Sci. Int. 2009, 187, 110.e1–110.e7. [Google Scholar] [CrossRef]
- Sforza, C.; Grandi, G.; Catti, F.; Tommasi, D.G.; Ugolini, A.; Ferrario, V.F. Age- and sex-related changes in the soft tissues of the orbital region. Forensic Sci. Int. 2009, 185, 115.e1–115.e8. [Google Scholar] [CrossRef]
- Farkas, L.G.; Hreczko, T.A.; Katic, M.J. Craniofacial norms in North American Caucasians from birth (one year) to young adulthood. In Anthropometry of the Head and Face; Farkas, L.G., Ed.; Raven Press: New York, NY, USA, 1994; pp. 235–241. [Google Scholar]
- Ito, I.; Imada, M.; Ikeda, M.; Sueno, K.; Arikuni, T.; Kida, A. A morphological study of age changes in adult human auricular cartilage with special emphasis on elastic fibers. Laryngoscope 2001, 111, 881–886. [Google Scholar] [CrossRef]
- Claes, P.; Reijniers, J.; Shriver, M.D.; Snyders, J.; Suetens, P.; Nielandt, J.; de Tré, G.; Vandermeulen, D. An investigation of matching symmetry in the human pinnae with possible implications for 3D ear recognition and sound localization. J. Anat. 2015, 226, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launonen, A.M.; Vuollo, V.; Aarnivale, H.; Heikkinen, T.; Pirttiniemi, P.; Valkama, A.M.; Harila, V. Craniofacial asymmetry from one to three years of age: A prospective cohort study with 3D imaging. J. Clin. Med. 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, M.R. What Is the Role of Plastic Surgery in Global Health? A Review. World J. Plast. Surg. 2018, 7, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, S.; Iguchi, Y.; Takada, K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008, 78, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Gibelli, D.; Dolci, C.; Cappella, A.; Sforza, C. Reliability of optical devices for three-dimensional facial anatomy description: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 1092–1106. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Elamin, F.; Tommasi, D.G.; Dolci, C.; Ferrario, V.F. Morphometry of the soft tissues of the orbital region in Northern Sudanese persons. Forensic Sci. Int. 2013, 228, 180.e1-11. [Google Scholar] [CrossRef]
- De Menezes, M.; Rosati, R.; Ferrario, V.F.; Sforza, C. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J. Oral Maxillofac Surg. 2010, 68, 2129–2135. [Google Scholar] [CrossRef]
- Gibelli, D.; Pucciarelli, V.; Cappella, A.; Dolci, C.; Sforza, C. Are Portable Stereophotogrammetric Devices Reliable in Facial Imaging? A Validation Study of VECTRA H1 Device. J. Oral Maxillofac Surg. 2018, 76, 1772–1784. [Google Scholar] [CrossRef] [Green Version]
- Sforza, C.; Elamin, F.; Rosati, R.; Lucchini, M.A.; Tommasi, D.G.; Ferrario, V.F. Three-dimensional assessment of nose and lip morphology in north Sudanese subjects with Down’s syndrome. Angle Orthod. 2011, 81, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Aung, S.C.; Ngim, R.C.; Lee, S.T. Evaluation of the laser scanner as a surface measuring tool and its accuracy compared with direct facial anthropometric measurements. Br. J. Plast. Surg. 1995, 48, 551Y558. [Google Scholar] [CrossRef]
- Hennessy, R.J.; Kinsella, A.; Waddington, J.L. 3D laser surface scanning and geometric morphometric analysis of craniofacial shape as an index of cerebro-craniofacial morphogenesis: Initial application to sexual dimorphism. Biol. Psychiatry 2002, 51, 507Y514. [Google Scholar] [CrossRef]
- Othman, S.A.; Saffai, L.; Wan Hassan, W.N. Validity and reproducibility of the 3D VECTRA photogrammetric surface imaging system for the maxillofacial anthropometric measurement on cleft patients. Clin. Oral Investig. 2020, 24, 2853–2866. [Google Scholar] [CrossRef]
- Farkas, L.G. (Ed.) Anthropometry of the Head and Face; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Guo, Y.; Schaub, F.; Mor, J.M.; Jia, R.; Koch, K.R.; Heindl, L.M. A Simple Standardized Three-Dimensional Anthropometry for the Periocular Region in a European Population. Plast. Reconstr. Surg. 2020, 145, 514e–523e. [Google Scholar] [CrossRef]
- Tomkinson, G.R.; Olds, T.S. Physiological correlates of bilateral symmetry in humans. Int. J. Sports Med. 2000, 21, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effect size: Estimation of effect size indices and standardized parameters. J. Open Source Softw 2020, 5, 2815. [Google Scholar] [CrossRef]
- Hews, D.K.; Castellano, M.; Hara, E. Aggression in females is also lateralized: Left-eye bias during aggressive courtship rejection in lizards. Anim. Behav. 2004, 68, 1201–1207. [Google Scholar] [CrossRef]
- Lusting, A.; Ketter-Katz, H.; Katzir, G. Lateralization of visually guided detour behavior in the common chameleon, (Chamaeleo chameleon): A reptile with highly independent eye movements. Behav. Process. 2013, 100, 110–115. [Google Scholar] [CrossRef]
- Sion, G.; Tal, R.; Meiri, S. Asymmetric behavior in Ptyodactylus guttatus: Can a digit ratio reflect brain laterality? Symmetry 2020, 12, 1490. [Google Scholar] [CrossRef]
- Sforza, C.; Dellavia, C.; Colombo, A.; Serrao, G.; Ferrario, V.F. Nasal dimensions in normal subjects: Conventional anthropometry versus computerized anthropometry. Am. J. Med. Genet. A 2004, 130A, 228–233. [Google Scholar] [CrossRef]
- Gibelli, D.; Pucciarelli, V.; Poppa, P.; Cummaudo, M.; Dolci, C.; Cattaneo, C.; Sforza, C. Three-dimensional facial anatomy evaluation: Reliability of laser scanner consecutive scans procedure in comparison with stereophotogrammetry. J. Craniomaxillofac. Surg. 2018, 46, 1807–1813. [Google Scholar] [CrossRef]

| Landmark | Abbreviation | Definition | |
|---|---|---|---|
| Orbital region | Sopraorbitale | Os | Point of the superior orbital edge corresponding to the supraorbital notch |
| Orbitale | Or | Medial point of the inferior orbital edge | |
| Endocanthion | En | Point at which the inner end of the upper and lower eyelid meet | |
| Exocanthion | Ex | Point at which the outer end of the upper and lower eyelid meet | |
| Auricular region | Superaurale | Sa | Most superior point of the auricle |
| Subaurale | Sba | Most inferior point of the auricle | |
| Preaurale | Pra | Most anterior point of the auricle | |
| Postaurale | Pa | Most posterior point of the auricle |
| Orbital Measurements | Auricular Measurements | |||||
|---|---|---|---|---|---|---|
| Height (mm) | Width (mm) | Height (mm) | Width (mm) | |||
| Italian | 8–11 years (N = 116) | Right side | 36.3 (7.9) | 36.9 (7.8) | 53.3 (7.3) | 32.5 (4.9) |
| Left side | 35.2 (7.2) | 35.0 (6.4) | 53.8 (6.9) | 33.2 (4.4) | ||
| 12–15 years (N = 183) | Right side | 36.2 (7.8) | 37.5 (6.8) | 56.2 (5.9) | 34.4 (4.0) | |
| Left side | 35.5 (6.8) | 36.4 (5.7) | 57.1 (5.7) | 34.3 (3.7) | ||
| 16–19 years (N = 119) | Right side | 40.6 (7.5) | 40.8 (8.4) | 55.6 (5.3) | 33.2 (3.8) | |
| Left side | 39.5 (6.5) | 38.6 (6.9) | 56.2 (5.5) | 33.1 (3.2) | ||
| Sudanese | 8–11 years (N = 63) | Right side | 43.0 (3.0) | 43.2 (4.5) | 49.2 (3.9) | 30.2 (3.1) |
| Left side | 41.2 (2.4) | 39.2 (4.6) | 50.4 (3.9) | 32.8 (3.6) | ||
| 12–15 years (N = 86) | Right side | 44.8 (3.9) | 45.1 (5.0) | 50.3 (3.4) | 31.2 (2.9) | |
| Left side | 42.8 (4.1) | 41.6 (5.2) | 52.0 (3.7) | 32.2 (3.3) | ||
| 16–19 years (N = 57) | Right side | 46.4 (4.2) | 47.0 (5.6) | 53.3 (4.2) | 32.1 (3.4) | |
| Left side | 43.8 (4.3) | 43.3 (5.6) | 53.9 (4.9) | 32.5 (2.7) | ||
| Ethnicity | Age Group | Ethnicity × Age Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | Eta Squared | F | P | Eta Squared | F | p | Eta Squared | ||
| Orbital asymmetry indices | Height | 19.076 | <0.001 | 0.030 | 1.611 | 0.201 | 0.005 | 1.901 | 0.336 | 0.004 |
| |Height| | 0.636 | 0.426 | 0.001 | 3.959 | 0.020 | 0.013 | 2.615 | 0.074 | 0.008 | |
| Width | 28.093 | <0.001 | 0.043 | 1.882 | 0.153 | 0.006 | 0.476 | 0.621 | 0.002 | |
| |Width| | 24.218 | <0.001 | 0.038 | 3.500 | 0.031 | 0.011 | 2.850 | 0.204 | 0.001 | |
| Auricular asymmetry indices | Height | 7.113 | 0.008 | 0.010 | 1.582 | 0.206 | 0.005 | 0.632 | 0.532 | 0.002 |
| |Height| | 13.045 | <0.001 | 0.020 | 1.367 | 0.256 | 0.004 | 0.052 | 0.950 | 0.000 | |
| Width | 24.477 | <0.001 | 0.036 | 10.971 | <0.001 # | 0.033 | 4.379 | 0.013 | 0.013 | |
| |Width| | 10.260 | 0.001 | 0.015 | 4.158 | 0.016 | 0.013 | 0.210 | 0.810 | 0.001 | |
| Eye Asymmetry Indices | Ear Asymmetry Indices | ||||
|---|---|---|---|---|---|
| Height | Width | Height | Width | ||
| Italian (%) | Right | 67.0 (p < 0.001) | 64.0 (p < 0.001) | 43.9 (p: 0.0714) | 52.0 (p: 0.5484) |
| Left | 33.0 (p < 0.001) | 36.0 (p < 0.001) | 56.1 (p: 0.0714) | 48.0 (p: 0.5484) | |
| Sudanese (%) | Right | 86.8 (p < 0.001) | 83.4 (p < 0.001) | 36.3 (p: 0.0053) | 33.8 (p < 0.001) |
| Left | 13.2 (p < 0.001) | 16.6 (p < 0.001) | 63.7 (p: 0.0053) | 66.2 (p < 0.001) | |
| Differences according to ethnicity | <0.001 | <0.001 | 0.0718 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolci, C.; Elamin, F.; Cappella, A.; Barni, L.; Gibelli, D.M.; Sforza, C. Assessment of the Orbital and Auricular Asymmetry in Italian and Sudanese Children: A Three-Dimensional Study. Symmetry 2021, 13, 1657. https://doi.org/10.3390/sym13091657
Dolci C, Elamin F, Cappella A, Barni L, Gibelli DM, Sforza C. Assessment of the Orbital and Auricular Asymmetry in Italian and Sudanese Children: A Three-Dimensional Study. Symmetry. 2021; 13(9):1657. https://doi.org/10.3390/sym13091657
Chicago/Turabian StyleDolci, Claudia, Fadil Elamin, Annalisa Cappella, Luisa Barni, Daniele M. Gibelli, and Chiarella Sforza. 2021. "Assessment of the Orbital and Auricular Asymmetry in Italian and Sudanese Children: A Three-Dimensional Study" Symmetry 13, no. 9: 1657. https://doi.org/10.3390/sym13091657








