Acquired Facial, Maxillofacial, and Oral Asymmetries—A Review Highlighting Diagnosis and Management
Abstract
:1. Introduction
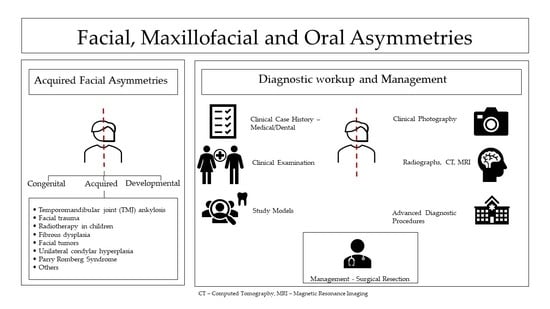
2. Acquired Facial, Maxillofacial, and Oral Asymmetries
- Temporomandibular joint (TMJ) ankylosis;
- Facial trauma;
- Radiotherapy in children;
- Fibrous dysplasia;
- Facial tumors;
- Unilateral condylar hyperplasia;
- Parry Romberg Syndrome;
- Others.
2.1. Diagnostic Evaluation for Acquired Facial, Maxillofacial, and Oral Asymmetries
2.1.1. Medical and Dental History
2.1.2. Clinical Examinations
- Extra oral evaluation
- Intraoral evaluation
2.1.3. Diagnostic Records
2.2. Conditions Presenting with Acquired Facial, Maxillofacial, and Oral Asymmetries
2.2.1. Temporomandibular Joint Ankylosis
2.2.2. Facial Trauma
2.2.3. Radiotherapy in Children
2.2.4. Fibrous Dysplasia
2.2.5. Facial Tumors
2.2.6. Unilateral Condylar Hyperplasia
2.2.7. Parry-Romberg Syndrome
2.2.8. Others
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandhya Maheshwari, S.K.V.; Aditi, G.; Sushma, D. Diagnosis and management of facial asymmetries. J. Orthod. Res. 2015, 3, 81–87. [Google Scholar] [CrossRef]
- Srivastava, D.; Singh, H.; Mishra, S.; Sharma, P.; Kapoor, P.; Chandra, L. Facial asymmetry revisited: Part I—Diagnosis and treatment planning. J. Oral Biol. Craniofac. Res. 2018, 8, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiesen, G.; Gribel, B.F.; Freitas, M.P. Facial asymmetry: A current review. Dental Press J. Orthod. 2015, 20, 110–125. [Google Scholar] [CrossRef]
- Peck, S.; Peck, L.; Kataja, M. Skeletal asymmetry in esthetically pleasing faces. Angle Orthod. 1991, 61, 43–48. [Google Scholar] [CrossRef]
- Maeda, M.; Katsumata, A.; Ariji, Y.; Muramatsu, A.; Yoshida, K.; Goto, S.; Kurita, K.; Ariji, E. 3D-CT evaluation of facial asymmetry in patients with maxillofacial deformities. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 382–390. [Google Scholar] [CrossRef]
- Wang, T.T.; Wessels, L.; Hussain, G.; Merten, S. Discriminative Thresholds in Facial Asymmetry: A Review of the Literature. Aesthet. Surg. J. 2017, 37, 375–385. [Google Scholar] [CrossRef]
- Gulsen, A.; Sibar, S.; Ozmen, S. Orthognathic treatment of facial asymmetry due to temporomandibular joint ankylosis. Arch. Plast. Surg. 2018, 45, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Severt, T.R.; Proffit, W.R. The prevalence of facial asymmetry in the dentofacial deformities population at the University of North Carolina. Int. J. Adult Orthodon. Orthognath. Surg. 1997, 12, 171–176. [Google Scholar]
- Cheong, Y.W.; Lo, L.J. Facial asymmetry: Etiology, evaluation, and management. Chang. Gung Med. J. 2011, 34, 341–351. [Google Scholar] [PubMed]
- Andrade, N.N.; Mathai, P.; Aggarwal, N. Facial Asymmetry. In Oral and Maxillofacial Surgery for the Clinician; Bonanthaya, K., Panneerselvam, E., Manuel, S., Kumar, V.V., Rai, A., Eds.; Springer: Singapore, 2021; pp. 1549–1576. [Google Scholar] [CrossRef]
- de Lima Lucas, B.; Júnior, R.B.; Gonçalves, L.C.; Gavião, M.B.; Gomes, V.L. Research and Clinical Applications of Facial Analysis in Dentistry, Oral Health Care—Prosthodontics, Periodontology, Biology, Research and Systemic Conditions; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, S.; Iguchi, Y.; Takada, K. Asymmetry of the face in orthodontic patients. Angle Orthod. 2008, 78, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milutinovic, J.; Zelic, K.; Nedeljkovic, N. Evaluation of facial beauty using anthropometric proportions. Sci. World J. 2014, 428250. [Google Scholar] [CrossRef] [Green Version]
- Hashim, P.W.; Nia, J.K.; Taliercio, M.; Goldenberg, G. Ideals of facial beauty. Cutis 2017, 100, 222–224. [Google Scholar] [PubMed]
- Porter, J.P. The average African American male face: An anthropometric analysis. Arch. Facial Plast. Surg. 2004, 6, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.P.; Olson, K.L. Anthropometric facial analysis of the African American woman. Arch. Facial Plast. Surg. 2001, 3, 191–197. [Google Scholar] [CrossRef]
- Dawei, W.; Guozheng, Q.; Mingli, Z.; Farkas, L.G. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast. Surg. 1997, 21, 265–269. [Google Scholar] [CrossRef]
- Farkas, L.G.; Forrest, C.R.; Litsas, L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast. Surg. 2000, 24, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chung, D.H.; Lee, J.W.; Cha, K.S. Assessing soft-tissue characteristics of facial asymmetry with photographs. Am. J. Orthod. Dentofacial. Orthop. 2010, 138, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, V.; Baserga, C.; Codari, M.; Beltramini, G.A.; Sforza, C.; Giannì, A.B. Three-Dimensional Stereophotogrammetric Evaluation of the Efficacy of Autologous Fat Grafting in the Treatment of Parry-Romberg Syndrome. J. Craniofac. Surg. 2018, 29, 2124–2127. [Google Scholar] [CrossRef]
- Baserga, C.; Cappella, A.; Gibelli, D.M.; Sacco, R.; Dolci, C.; Cullati, F.; Giannì, A.B.; Sforza, C. Efficacy of Autologous Fat Grafting in Restoring Facial Symmetry in Linear Morphea-Associated Lesions. Symmetry 2020, 12, 2098. [Google Scholar] [CrossRef]
- Primozic, J.; Perinetti, G.; Zhurov, A.; Richmond, S.; Ovsenik, M. Assessment of facial asymmetry in growing subjects with a three-dimensional laser scanning system. Orthod. Craniofac. Res. 2012, 15, 237–244. [Google Scholar] [CrossRef]
- Heike, C.L.; Upson, K.; Stuhaug, E.; Weinberg, S.M. 3D digital stereophotogrammetry: A practical guide to facial image acquisition. Head Face Med. 2010, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, A.; Fujishita, M.; Maeda, M.; Ariji, Y.; Ariji, E.; Langlais, R.P. 3D-CT evaluation of facial asymmetry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sales, S.; Silva, M.; Lehman, L.; Castro, W.; Campos, F. Management of temporomandibular joint ankylosis. Int. J. Oral Maxillofac. Surg. 2019, 48, 284. [Google Scholar] [CrossRef]
- Hegab, A.F. Outcome of Surgical Protocol for Treatment of Temporomandibular Joint Ankylosis Based on the Pathogenesis of Ankylosis and Re-Ankylosis. A Prospective Clinical Study of 14 Patients. Int. J. Oral Maxillofac. Surg. 2015, 73, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- De Roo, N.; Van Doorne, L.; Troch, A.; Vermeersch, H.; Brusselaers, N. Quantifying the outcome of surgical treatment of temporomandibular joint ankylosis: A systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2016, 44, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Babu, L.; Jain, M.K.; Ramesh, C.; Vinayaka, N. Is aggressive gap arthroplasty essential in the management of temporomandibular joint ankylosis?-a prospective clinical study of 15 cases. Br. Int. J. Oral Maxillofac. Surg. 2013, 51, 473–478. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D.; Yin, Q.; Hu, J. Treatment guidelines for temporomandibular joint ankylosis with secondary dentofacial deformities in adults. J. Craniomaxillofac. Surg. 2013, 41, e117–e127. [Google Scholar] [CrossRef]
- Vasconcelos, B.C.; Porto, G.G.; Bessa-Nogueira, R.V.; Nascimento, M.M. Surgical treatment of temporomandibular joint ankylosis: Follow-up of 15 cases and literature review. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, E34–E38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouinard, A.F.; Kaban, L.B.; Peacock, Z.S. Acquired Abnormalities of the Temporomandibular Joint. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 83–96. [Google Scholar] [CrossRef]
- McFadden, L.R.; Rishiraj, B. Treatment of temporomandibular joint ankylosis: A case report. J. Can. Dent. Assoc. 2001, 67, 659–663. [Google Scholar]
- Karamese, M.; Duymaz, A.; Seyhan, N.; Keskin, M.; Tosun, Z. Management of temporomandibular joint ankylosis with temporalis fascia flap and fat graft. J. Craniomaxillofac. Surg. 2013, 41, 789–793. [Google Scholar] [CrossRef]
- Swatantra Srivastava, G.S.N. Ramanpal Singh Makkad, Santosh R Patil, Mohammad Khursheed Alam. Evaluation of a Case of Temporomandibular Joint Ankylosis with Conventional and Advanced Imaging Modalities. Int. Med. J. 2020, 27, 639–640. [Google Scholar]
- Gundlach, K.K. Ankylosis of the temporomandibular joint. J. Craniomaxillofac. Surg. 2010, 38, 122–130. [Google Scholar] [CrossRef]
- Güven, O. Treatment of temporomandibular joint ankylosis by a modified fossa prosthesis. J. Craniomaxillofac. Surg. 2004, 32, 236–242. [Google Scholar] [CrossRef]
- Güven, O. A clinical study on temporomandibular joint ankylosis in children. J. Craniofac. Surg. 2008, 19, 1263–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaban, L.B.; Bouchard, C.; Troulis, M.J. A protocol for management of temporomandibular joint ankylosis in children. Int. J. Oral Maxillofac. Surg. 2009, 67, 1966–1978. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; He, D.; Yang, C.; Chen, M.; Zhang, S.; Li, H.; Ellis, E., III. Simultaneous treatment of temporomandibular joint ankylosis with severe mandibular deficiency by standard TMJ prosthesis. Sci. Rep. 2017, 7, 45271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fildes, M.F. The National Trauma Data Bank Annual Report. American College of Surgeons; NTDB: Washington, DC, USA, 2012. [Google Scholar]
- Morris, C.; Kushner, G.M.; Tiwana, P.S. Facial skeletal trauma in the growing patient. Oral Maxillofac. Surg. Clin. N. Am. 2012, 24, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, D.G. Systematic assessment of the patient with facial trauma. Oral Maxillofac. Surg. Clin. N. Am. 2013, 25, 537–544. [Google Scholar] [CrossRef]
- Mccormick, R.; Putnam, G. The management of facial trauma. Surgery 2018, 36, 587–594. [Google Scholar] [CrossRef]
- Koltai, P.J.; Rabkin, D. Management of facial trauma in children. Pediatr. Clin. N. Am. 1996, 43, 1253–1275. [Google Scholar] [CrossRef]
- Braun, T.L.; Xue, A.S.; Maricevich, R.S. Differences in the Management of Pediatric Facial Trauma. Semin. Plast. Surg. 2017, 31, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Lorenz, H.P.; Spain, D.A. Review of facial trauma management. J. Trauma Acute Care Surg. 2020, 88, e124–e130. [Google Scholar] [CrossRef] [PubMed]
- Laine, P.; Kontio, R.; Salo, A.; Mesimäki, K.; Lindqvist, C.; Suuronen, R. Secondary correction of malocclusion after treatment of maxillofacial trauma. Int. J. Oral Maxillofac. Surg. 2004, 62, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, D.S.; Bell, R.B.; Bagheri, S.C.; Dierks, E.J.; Potter, B.E. Management of laryngo-tracheal injuries associated with craniomaxillofacial trauma. Int. J. Oral Maxillofac. Surg. 2006, 64, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.N.; Kelly, A.; DeGiovanni, J.; Ong, A.A.; Carr, M.M. Maxillofacial trauma in children: Association between age and mandibular fracture site. Am. J. Otolaryngol. 2021, 42, 102874. [Google Scholar] [CrossRef]
- Mukherjee, C.G.; Mukherjee, U. Maxillofacial trauma in children. Int. J. Clin. Pediatr. Dent. 2012, 5, 231–236. [Google Scholar] [CrossRef]
- Huggare, G.D.a.J. Orthodontic Considerations in the Pediatric Cancer Patient: A Review. Semin. Orthod. 2004, 10, 266–276. [Google Scholar]
- Gevorgyan, A.; La Scala, G.C.; Neligan, P.C.; Pang, C.Y.; Forrest, C.R. Radiation-induced craniofacial bone growth disturbances. J. Craniofac. Surg. 2007, 18, 1001–1007. [Google Scholar] [CrossRef]
- O’Donovan, D.A.; Yeung, I.; Zeman, V.; Neligan, P.C.; Pang, C.Y.; Forrest, C.R. Radiation-induced craniofacial bone growth inhibition: Development of an animal model. J. Craniofac. Surg. 2001, 12, 533–543. [Google Scholar] [CrossRef]
- Estilo, C.L.; Huryn, J.M.; Kraus, D.H.; Sklar, C.A.; Wexler, L.H.; Wolden, S.L.; Zlotolow, I.M. Effects of therapy on dentofacial development in long-term survivors of head and neck rhabdomyosarcoma: The memorial sloan-kettering cancer center experience. J. Pediatr. Hematol. Oncol. 2003, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Miyawaki, D.; Mukumoto, N.; Ishihara, T.; Matsumura, M.; Hasegawa, T.; Akashi, M.; Kiyota, N.; Shinomiya, H.; Teshima, M.; et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Gao, X.J.; Deng, J.; Li, N.Y.; Lu, H.J. Progress of oral sequelae during head-neck radiotherapy. Chin. J. Dent. Res. 2010, 13, 51–55. [Google Scholar] [PubMed]
- Ahadian, H.; Yassaei, S.; Bouzarjomehri, F.; Ghaffari Targhi, M.; Kheirollahi, K. Oral Complications of The Oromaxillofacial Area Radiotherapy. Asian Pac. J. Cancer Prev. 2017, 18, 721–725. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen-Segarceanu, E.M.; Dorresteijn, L.D.; Pillen, S.; Biesma, D.H.; Vogels, O.J.; van Alfen, N. Progressive muscle atrophy and weakness after treatment by mantle field radiotherapy in Hodgkin lymphoma survivors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, E.S.; Kim, J.E.; Yoon, S.P.; Kim, Y.S. Neck muscle atrophy and soft-tissue fibrosis after neck dissection and postoperative radiotherapy for oral cancer. Radiat. Oncol. J. 2015, 33, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Furby, A.; Béhin, A.; Lefaucheur, J.P.; Beauvais, K.; Marcorelles, P.; Mussini, J.M.; Bassez, G.; Créange, A.; Eymard, B.; Pénisson-Besnier, I. Late-onset cervicoscapular muscle atrophy and weakness after radiotherapy for Hodgkin disease: A case series. J. Neurol. Neurosurg. Psychiatry 2010, 81, 101–104. [Google Scholar] [CrossRef]
- Zhang, L.L.; Mao, Y.P.; Zhou, G.Q.; Tang, L.L.; Qi, Z.Y.; Lin, L.; Yao, J.J.; Ma, J.; Lin, A.H.; Sun, Y. The Evolution of and Risk Factors for Neck Muscle Atrophy and Weakness in Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy: A Retrospective Study in an Endemic Area. Medicine 2015, 94, e1294. [Google Scholar] [CrossRef]
- Bianchi, A.; Crimi, S.; Cipriani, R.; De Ponte, F.S.; Cicciù, M.; Marchetti, C. Comprehensive Treatment of Facial Deformity Due to Radiotherapy in Rhabdomyosarcoma Patients: Distraction Osteogenesis and Free Flaps Surgical Technique. J. Craniofac. Surg. 2019, 30, 1275–1279. [Google Scholar] [CrossRef]
- Ihde, S.; Kopp, S.; Gundlach, K.; Konstantinović, V.S. Effects of radiation therapy on craniofacial and dental implants: A review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 56–65. [Google Scholar] [CrossRef]
- Palmer, J.D.; Tsang, D.S.; Tinkle, C.L.; Olch, A.J.; Kremer, L.C.M.; Ronckers, C.M.; Gibbs, I.C.; Constine, L.S. Late effects of radiation therapy in pediatric patients and survivorship. Pediatr. Blood Cancer 2021, 68 (Suppl. S2), e28349. [Google Scholar] [CrossRef]
- Martin, P.; Muller, E.; Paulus, C. Alteration of facial growth after radiotherapy: Orthodontic, surgical and prosthetic rehabilitation. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Maheshwari, S.; Khan, M.T.; Verma, S.K. Long term dento-facial effects of radiotherapy in a treated patient of retinoblastoma. J. Oral Biol. Craniofac. Res. 2014, 4, 214–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeffinger, K.C.; Hudson, M.M. Long-term complications following childhood and adolescent cancer: Foundations for providing risk-based health care for survivors. CA Cancer J. Clin. 2004, 54, 208–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, R.; Rodríguez-Campo, F.J.; Naval-Gías, L.; Sastre-Pérez, J.; Díaz-González, F.J. The effect of radiation in distraction osteogenesis for reconstruction of mandibular segmental defects. Br. Int. J. Oral Maxillofac. Surg. 2007, 45, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Jansma, J.; Vissink, A.; Roodenburg, J.L. Distraction osteogenesis in the irradiated mandible. A case report. J. Craniomaxillofac. Surg. 2005, 33, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Faghahati, S.; Delaporte, T.; Toussoun, G.; Gleizal, A.; Morel, F.; Delay, E. [Treatment by fat tissue transfer for radiation injury in childhood facial cancer]. Ann. Chir. Plast. Esthet. 2010, 55, 169–178. [Google Scholar] [CrossRef]
- Srivastava, D.; Singh, H.; Mishra, S.; Sharma, P.; Kapoor, P.; Chandra, L. Facial asymmetry revisited: Part II—Conceptualizing the management. J. Oral Biol. Craniofac. Res. 2018, 8, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, H.L.; Lichtenstein, L. Non-osteogenic fibroma of bone. Am. J. Pathol. 1942, 18, 205–221. [Google Scholar]
- Adetayo, O.A.; Salcedo, S.E.; Borad, V.; Richards, S.S.; Workman, A.D.; Ray, A.O. Fibrous dysplasia: An overview of disease process, indications for surgical management, and a case report. Eplasty 2015, 15, e6. [Google Scholar]
- Feller, L.; Wood, N.H.; Khammissa, R.A.; Lemmer, J.; Raubenheimer, E.J. The nature of fibrous dysplasia. Head Face Med. 2009, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Valentini, V.; Cassoni, A.; Marianetti, T.M.; Terenzi, V.; Fadda, M.T.; Iannetti, G. Craniomaxillofacial fibrous dysplasia: Conservative treatment or radical surgery? A retrospective study on 68 patients. Plast. Reconstr. Surg. 2009, 123, 653–660. [Google Scholar] [CrossRef]
- Parekh, S.G.; Donthineni-Rao, R.; Ricchetti, E.; Lackman, R.D. Fibrous dysplasia. J. Am. Acad. Orthop. Surg. 2004, 12, 305–313. [Google Scholar] [CrossRef]
- DiCaprio, M.R.; Enneking, W.F. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J. Bone Jt. Surg. Am. 2005, 87, 1848–1864. [Google Scholar] [CrossRef]
- Lee, J.S.; FitzGibbon, E.J.; Chen, Y.R.; Kim, H.J.; Lustig, L.R.; Akintoye, S.O.; Collins, M.T.; Kaban, L.B. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet. J. Rare Dis. 2012, 7 (Suppl. S1), S2. [Google Scholar] [CrossRef] [Green Version]
- Lung, H.; Hsiao, E.C.; Wentworth, K.L. Advances in Models of Fibrous Dysplasia/McCune-Albright Syndrome. Front. Endocrinol. 2020, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M.; Collins, M.T. Fibrous Dysplasia/McCune-Albright Syndrome: A Rare, Mosaic Disease of Gα s Activation. Endocr. Rev. 2019, 41, 345–370. [Google Scholar] [CrossRef]
- Murray, D.J.; Edwards, G.; Mainprize, J.G.; Antonyshyn, O. Advanced technology in the management of fibrous dysplasia. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Yu, C.C.; Chang, C.N.; Ma, L.; Chen, Y.R. Optic nerve compression in craniofacial fibrous dysplasia: The role and indications for decompression. Plast. Reconstr. Surg. 2007, 120, 1957–1962. [Google Scholar] [CrossRef]
- Stefanelli, S.; Mundada, P.; Rougemont, A.L.; Lenoir, V.; Scolozzi, P.; Merlini, L.; Becker, M. Masses of developmental and genetic origin affecting the paediatric craniofacial skeleton. Insights Imaging 2018, 9, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Frunza, A.; Slavescu, D.; Lascar, I. Aggressive tumor of the midface. Eplasty 2014, 14, ic26. [Google Scholar] [PubMed]
- Cohen, M.M., Jr. Perspectives on craniofacial asymmetry. III. Common and/or well-known causes of asymmetry. Int. Int. J. Oral Maxillofac. Surg. 1995, 24, 127–133. [Google Scholar] [CrossRef]
- Wright, J.M.; Vered, M. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors. Head Neck Pathol. 2017, 11, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldelaimi, A.A.; Enezei, H.H.; Aldelaimi, T.N.; Mohammed, K.A. Tumors of Craniofacial Region in Iraq (Clinicopathological Study). J. Res. Med. Dent. Sci. 2021, 9, 66–71. [Google Scholar]
- Riaz, N.; Warraich, R.A. Tumors and Tumor – Like Lesions of the Oro – Facial Region at Mayo Hospital, Lahore – A Five Year Study. Ann. King Edw. Med. Univ. 1970, 17, 123. [Google Scholar] [CrossRef]
- Natana, G.G.; Kalyanyama, B.M.; Simon, E.N. Orofacial tumours and tumour-like lesions in children treated at Muhimbili National Hospital, Tanzania. South. Sudan Med. J. 2019, 12, 5–8. [Google Scholar]
- Aregbesola, B.; Soyele, O.; Effiom, O.; Gbotolorun, O.; Taiwo, O.; Amole, I. Odontogenic tumours in Nigeria: A multicentre study of 582 cases and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e761–e766. [Google Scholar] [CrossRef]
- Pires, A.L.; Nascimento, I.S.; de Souza Assis, A.L.; Hassam, S.F.; de Farias, J.G. Prevalence of tumours of the maxillomandibular complex diagnosed in a reference center in Brazil. Braz. J. Oral Sci. 2021, 20, e211817. [Google Scholar] [CrossRef]
- da Silva, L.P.; de Paiva Macedo, R.A.; Serpa, M.S.; Sobral, A.P.; de Souza, L.B. Global frequency of benign and malignant odontogenic tumors according to the 2005 WHO classification. JORDI J. Oral Diagn. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours. Chapter 4: Tumors of the Oral Cavity and Oropharynx; IARC Press: Lyon, France, 2005. [Google Scholar]
- Westra, W.H.; Lewis, J.S., Jr. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Oropharynx. Head Neck Pathol. 2017, 11, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours. Chapter 1: Nasal Cavity and Paranasal Sinuses; IARC Press: Lyon, France, 2005. [Google Scholar]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. Pathology and Genetics of Head and Neck Tumours. Chapter 5: Salivary Glands; IARC Press: Lyon, France, 2005. [Google Scholar]
- Oniscu, A.; Salter, D. Pathology of soft tissue tumours. Surgery 2020, 38, 61–64. [Google Scholar]
- Duarte-Andrade, F.F.; Vitório, J.G.; Pereira, T.; Gomes, C.C.; Gomez, R.S. A review of the molecular profile of benign and malignant odontogenic lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 357–368. [Google Scholar] [CrossRef]
- Farshbaf, A.; Zare, R.; Mohajertehran, F.; Mohtasham, N. New diagnostic molecular markers and biomarkers in odontogenic tumors. Mol. Biol. Rep. 2021, 48, 3617–3628. [Google Scholar] [CrossRef]
- Woolgar, J.a.G.H. Cysts and Tumours of the Bony Facial Skeleton. Scott-Brown’s Otorhinolaryngology Head and Neck 769 Surgery, 8th ed.; CRC Press: Boca Raton, FL, USA, 2019; Volume 3. [Google Scholar]
- Flanagan, A.M.; Speight, P.M. Giant cell lesions of the craniofacial bones. Head Neck Pathol. 2014, 8, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Dahllöf, G. Craniofacial growth in children treated for malignant diseases. Acta Odontol. Scand. 1998, 56, 378–382. [Google Scholar] [CrossRef]
- Wollina, U. Reconstruction of Large Facial Defects after Delayed Mohs Surgery for Skin Cancer. Acta Dermatovenerol. Croat. 2015, 23, 265–269. [Google Scholar]
- Meaike, J.D.; Dickey, R.M.; Killion, E.; Bartlett, E.L.; Brown, R.H. Facial Skin Cancer Reconstruction. Semin. Plast. Surg. 2016, 30, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharathi, S.C.; Senthilnathan, S.; Kumar, L.D.; Mohan, A.C.; Taranath, M. Unilateral condylar hyperplasia: A case report and review of literature. J. Int. Soc. Prev. Community Dent. 2014, 4, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Ha, T.W.; Park, J.H.; Jung, H.D.; Jung, Y.S. Condylectomy as the treatment for active unilateral condylar hyperplasia of the mandible and severe facial asymmetry: Retrospective review over 18 years. Int. J. Oral Maxillofac. Surg. 2019, 48, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.E.; Zacharias, J.; Pierce, S. Condylar hyperplasia: An updated review of the literature. Korean J. Orthod. 2015, 45, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obwegeser, H.L.; Makek, M.S. Hemimandibular hyperplasia—Hemimandibular elongation. J. Maxillofac. Surg. 1986, 14, 183–208. [Google Scholar] [CrossRef]
- Arora, K.S.; Bansal, R.; Mohapatra, S.; Pareek, S. Review and Classification Update: Unilateral condylar hyperplasia. BMJ Case Rep. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Higginson, J.A.; Bartram, A.C.; Banks, R.J.; Keith, D.J.W. Condylar hyperplasia: Current thinking. Br. Int. J. Oral Maxillofac. Surg. 2018, 56, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Loli, D.; Coppotelli, E.; Toni, B.; Costantini, A.M.; Germanò, F. Condylar Hyperplasia: Classification over the years. WebMed Cent. Orthod. 2017, 8, 1–6. [Google Scholar]
- Chen, Y.; Ke, J.; Long, X.; Meng, Q.; Deng, M.; Fang, W.; Li, J.; Cai, H.; Chen, S. Insulin-like growth factor-1 boosts the developing process of condylar hyperplasia by stimulating chondrocytes proliferation. Osteoarthr. Cartil. 2012, 20, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olate, S.; Almeida, A.; Alister, J.P.; Navarro, P.; Netto, H.D.; de Moraes, M. Facial asymmetry and condylar hyperplasia: Considerations for diagnosis in 27 consecutives patients. Int. J. Clin. Exp. Med. 2013, 6, 937–941. [Google Scholar]
- Olate, S.; Netto, H.D.; Rodriguez-Chessa, J.; Alister, J.P.; de Albergaria-Barbosa, J.; de Moraes, M. Mandible condylar hyperplasia: A review of diagnosis and treatment protocol. Int. J. Clin. Exp. Med. 2013, 6, 727–737. [Google Scholar] [PubMed]
- Ferreira, S.; da Silva Fabris, A.L.; Ferreira, G.R.; Faverani, L.P.; Francisconi, G.B.; Souza, F.A.; Garcia, I.R., Jr. Unilateral condylar hyperplasia: A treatment strategy. J. Craniofac. Surg. 2014, 25, e256–e258. [Google Scholar] [CrossRef]
- Xavier, S.P.; Santos Tde, S.; Silva, E.R.; Faria, A.C.; de Mello Filho, F.V. Two-stage treatment of facial asymmetry caused by unilateral condylar hyperplasia. Braz. Dent. J. 2014, 25, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Maniskas, S.A.; Ly, C.L.; Pourtaheri, N.; Parsaei, Y.; Steinbacher, D.M. Concurrent High Condylectomy and Orthognathic Surgery for Treatment of Patients With Unilateral Condylar Hyperplasia. J. Craniofac. Surg. 2020, 31, 2217–2221. [Google Scholar] [CrossRef]
- Pinheiro, T.P.; Silva, C.C.; Silveira, C.S.; Botelho, P.C.; Pinheiro, M.; Pinheiro, J.D. Progressive Hemifacial Atrophy—Case report. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E112–E114. [Google Scholar] [PubMed]
- Wong, M.; Phillips, C.D.; Hagiwara, M.; Shatzkes, D.R. Parry Romberg Syndrome: 7 Cases and Literature Review. AJNR Am. J. Neuroradiol. 2015, 36, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, K.P.; Dong, E.; Truong, T.A.; Maricevich, R.S. Parry Romberg Syndrome. Clin. Plast. Surg. 2019, 46, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, A.; Riaz, N.; Shah, A.K. Parry romberg syndrome: A case report. J. Chitwan Med. Coll. 2020, 10, 75–77. [Google Scholar] [CrossRef]
- Rangare, A.L.; Babu, S.G.; Thomas, P.S.; Shetty, S.R. Parry-romberg syndrome: A rare case report. J. Oral Maxillofac. Res. 2011, 2, e5. [Google Scholar] [CrossRef]
- Arif, T.; Fatima, R.; Sami, M. Parry-Romberg syndrome: A mini review. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 193–199. [Google Scholar]
- Kumar, N.G.; Maurya, B.S.; Sudeep, C.S. Parry Romberg Syndrome: Literature Review and Report of Three Cases. J. Maxillofac. Oral Surg. 2019, 18, 210–216. [Google Scholar] [CrossRef]
- Dermarkarian, C.R.; Sweeney, A.R.; Chambers, C.B.; Chang, S.H. Ophthalmic changes in patients with hemifacial atrophy (Parry-Romberg syndrome). Int. Ophthalmol. 2021, 41, 599–604. [Google Scholar] [CrossRef]
- Liapakis, I.E.; Tzouganakis, A.C.; Paschalis, E.I.; Englander, M.; Christopoulos, A.; Gloustianou, G.; Kontoes, P. Parry-Romberg syndrome treatment with fat transfer and a new bleaching formula. J. Cosmet. Dermatol. 2019, 18, 1424–1429. [Google Scholar] [CrossRef]
- Alencar, J.C.; Andrade, S.H.; Pessoa, S.G.; Dias, I.S. Autologous fat transplantation for the treatment of progressive hemifacial atrophy (Parry-Romberg syndrome: Case report and review of medical literatute). An. Bras. Dermatol. 2011, 86, S85–S88. [Google Scholar] [CrossRef] [Green Version]
- Kasielska-Trojan, A.; Zieliński, T.; Antoszewski, B. Autologous fat transfer for facial recontouring in Parry-Romberg syndrome. J. Cosmet. Dermatol. 2020, 19, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Van der Cruyssen, F.; Meeus, J.; Schoenaers, J.; Politis, C. Parry Romberg syndrome: A long-term retrospective cohort study of 10 patients. Oral Maxillofac. Surg. Cases 2018, 4, 73–83. [Google Scholar] [CrossRef]
- Dhanvanth, M.; Ganapathy, D.; Jain, A. Choice of Antibiotics in the Management of Dentoalveolar Abscess among Dental 766 Practitioners. Drug Invent. Today 2018, 10, 2390–2394. [Google Scholar]
- Sidell, D.; Shapiro, N.L. Chapter 56—Salivary Glands. In Pediatric Surgery, 7th ed.; Coran, A.G., Ed.; Mosby: Philadelphia, PA, USA, 2012; pp. 729–735. [Google Scholar] [CrossRef]
- Kushchayeva, Y.S.; Kushchayev, S.V.; Glushko, T.Y.; Tella, S.H.; Teytelboym, O.M.; Collins, M.T.; Boyce, A.M. Fibrous dysplasia for radiologists: Beyond ground glass bone matrix. Insights Imaging 2018, 9, 1035–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Author | Based on | Details |
|---|---|---|
| Plint (1974) | Etiology |
|
| Obwegeser and Makek (1986)(Mandible only) | Morphology |
|
| Bishara (1994) | Involved structures |
|
| Cohen (1995) | Morphology |
|
| Chia (2008) | Etiology |
|
| Haraguchi (2008) | Etiology |
|
| Wolford (2009) | Etiology |
|
| Reyeneke (2010) | Etiology |
|
| Cheong (2011) | Etiology |
|
| Waite (2012) | Etiology |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, J.; Hariharan, A.; Cao, U.M.N.; Tran, S.D. Acquired Facial, Maxillofacial, and Oral Asymmetries—A Review Highlighting Diagnosis and Management. Symmetry 2021, 13, 1661. https://doi.org/10.3390/sym13091661
Iyer J, Hariharan A, Cao UMN, Tran SD. Acquired Facial, Maxillofacial, and Oral Asymmetries—A Review Highlighting Diagnosis and Management. Symmetry. 2021; 13(9):1661. https://doi.org/10.3390/sym13091661
Chicago/Turabian StyleIyer, Janaki, Arvind Hariharan, Uyen Minh Nha Cao, and Simon D. Tran. 2021. "Acquired Facial, Maxillofacial, and Oral Asymmetries—A Review Highlighting Diagnosis and Management" Symmetry 13, no. 9: 1661. https://doi.org/10.3390/sym13091661
APA StyleIyer, J., Hariharan, A., Cao, U. M. N., & Tran, S. D. (2021). Acquired Facial, Maxillofacial, and Oral Asymmetries—A Review Highlighting Diagnosis and Management. Symmetry, 13(9), 1661. https://doi.org/10.3390/sym13091661