Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
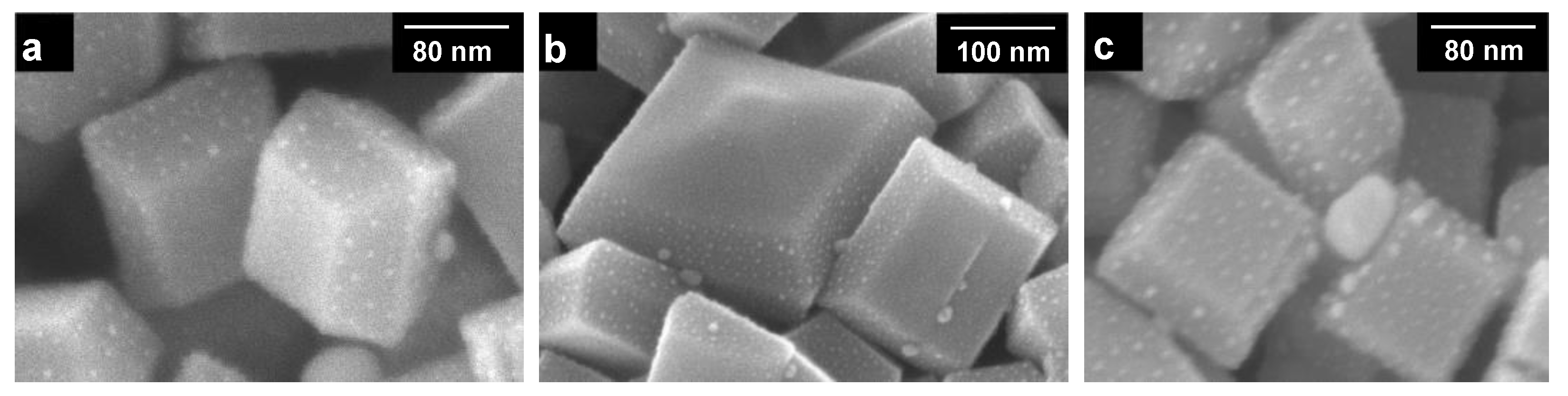
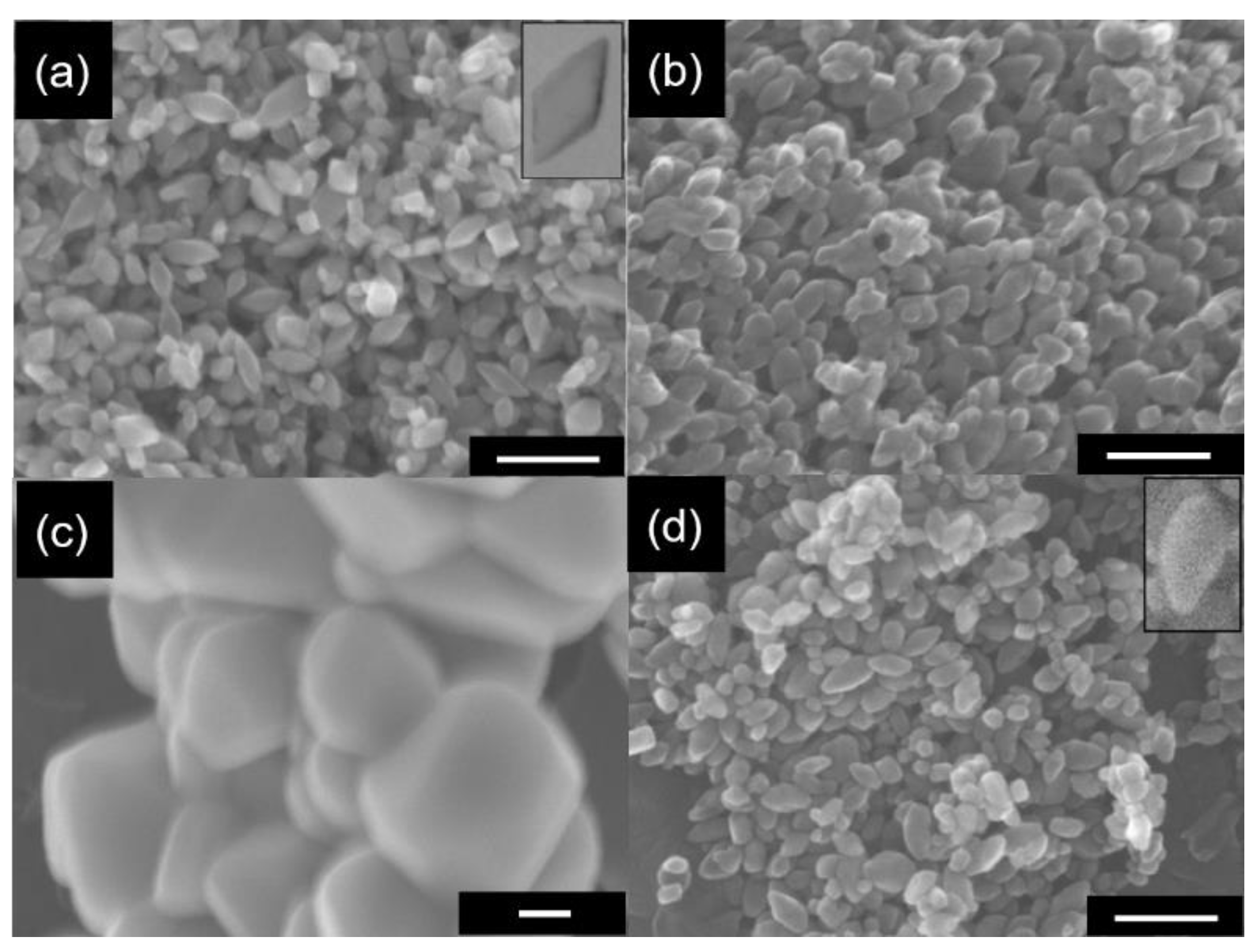
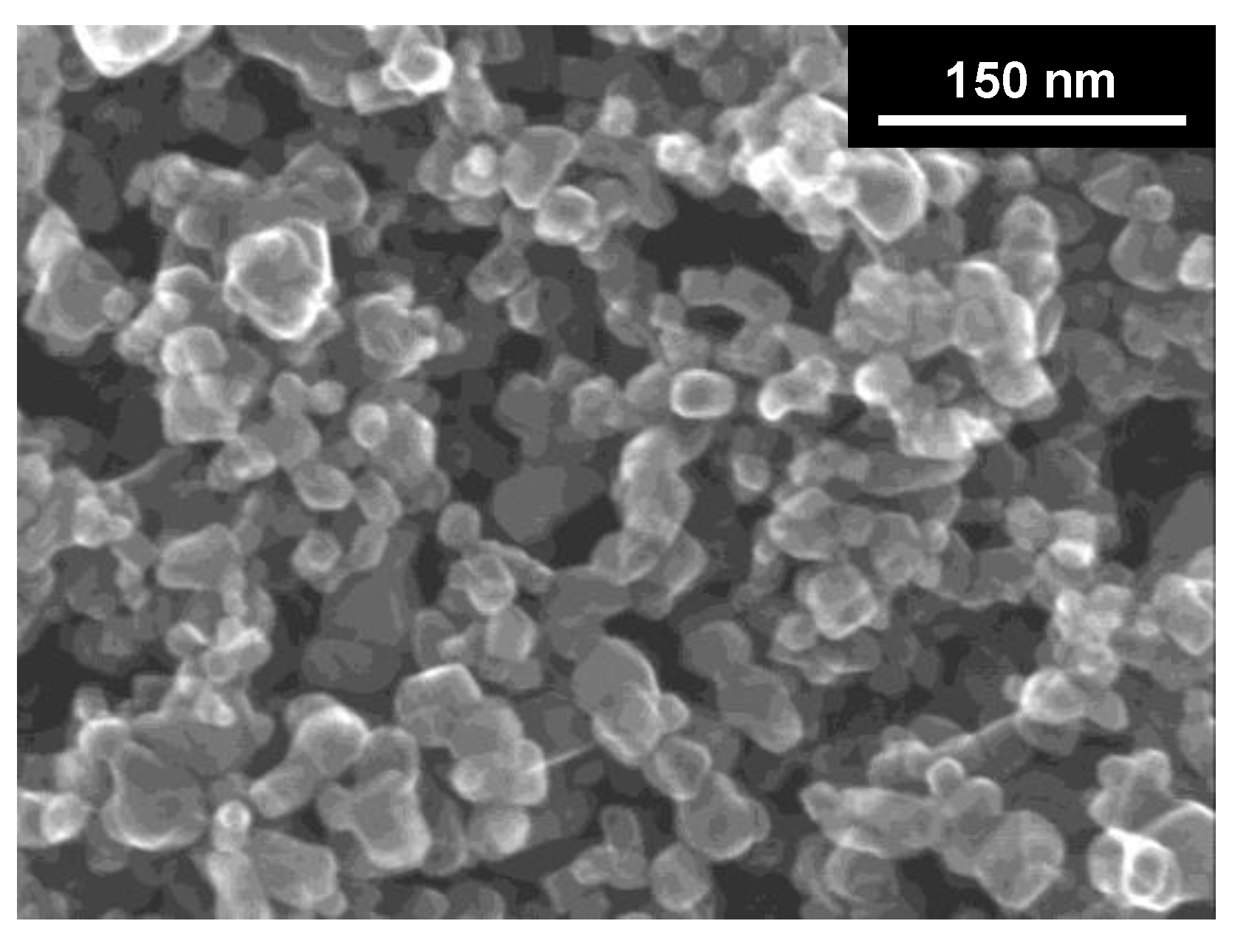
3.1. Preparation Conditions and Properties of DAPs
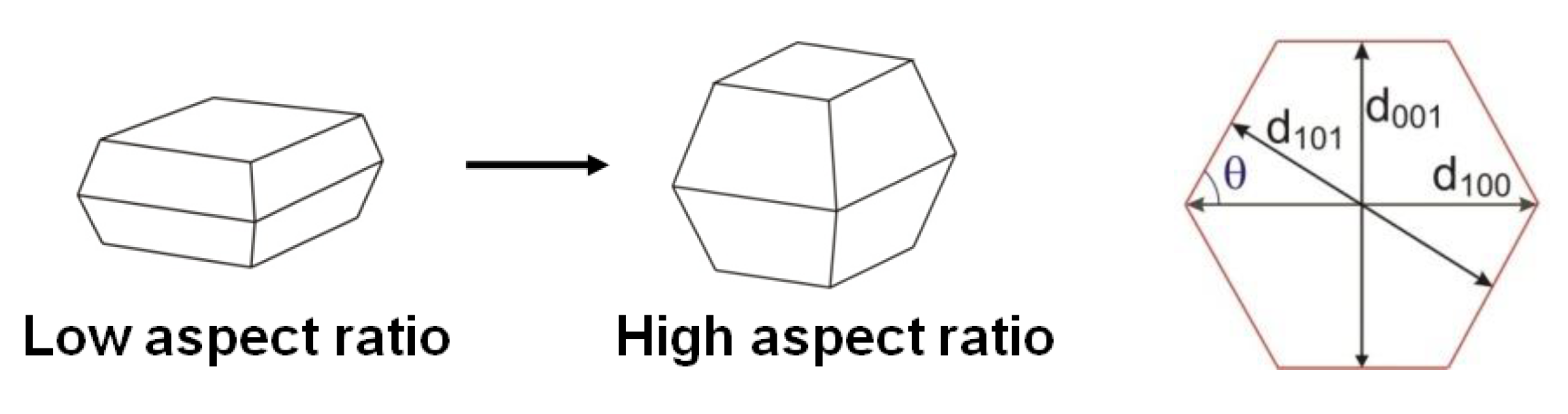
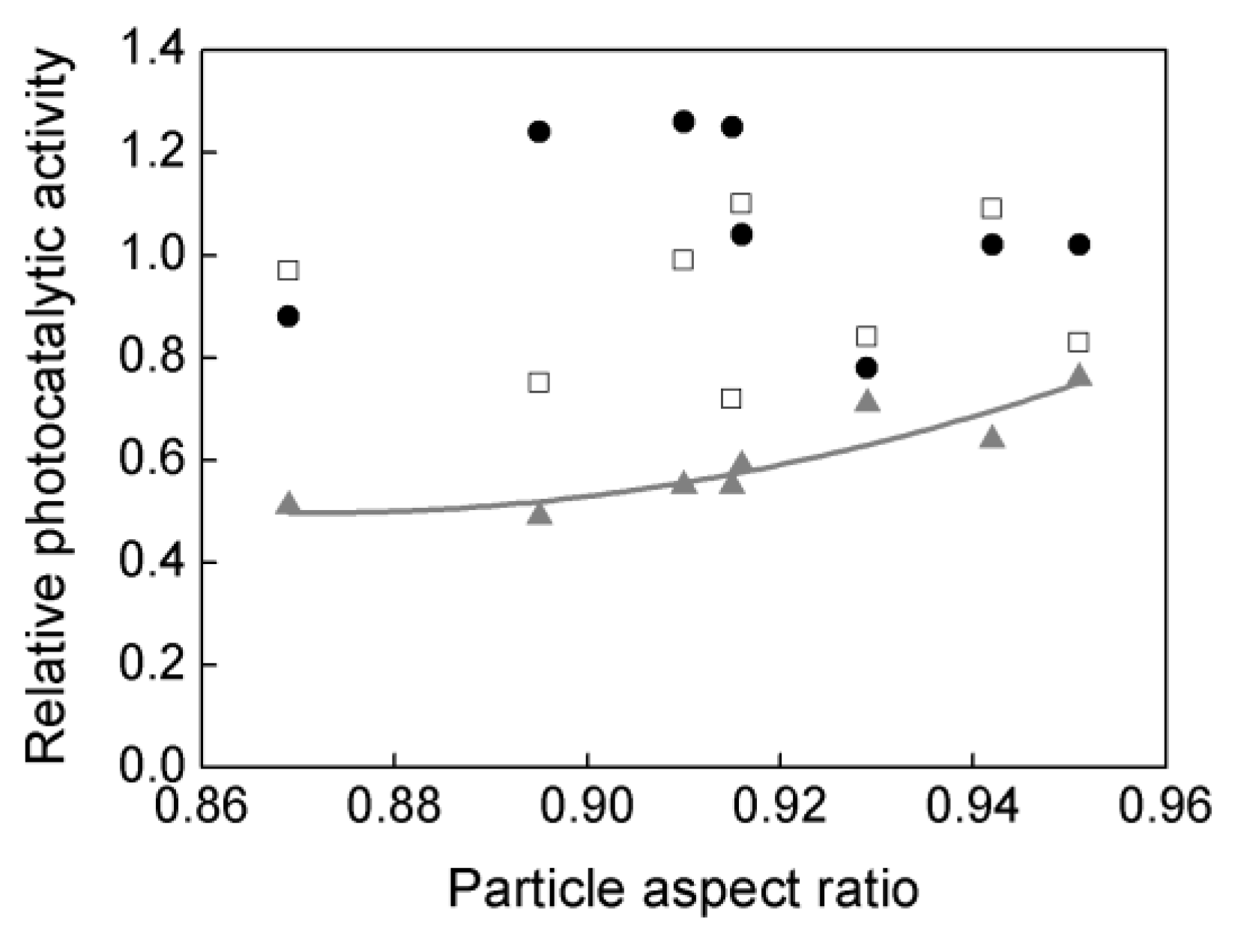
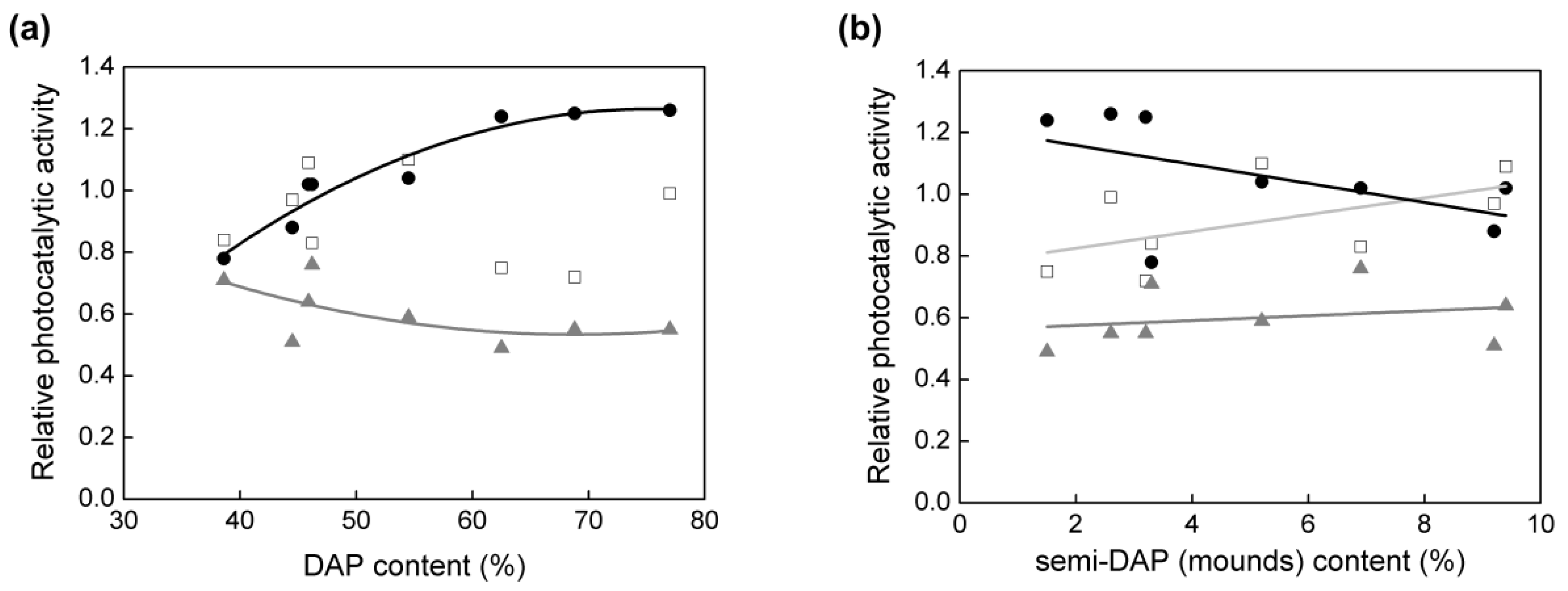
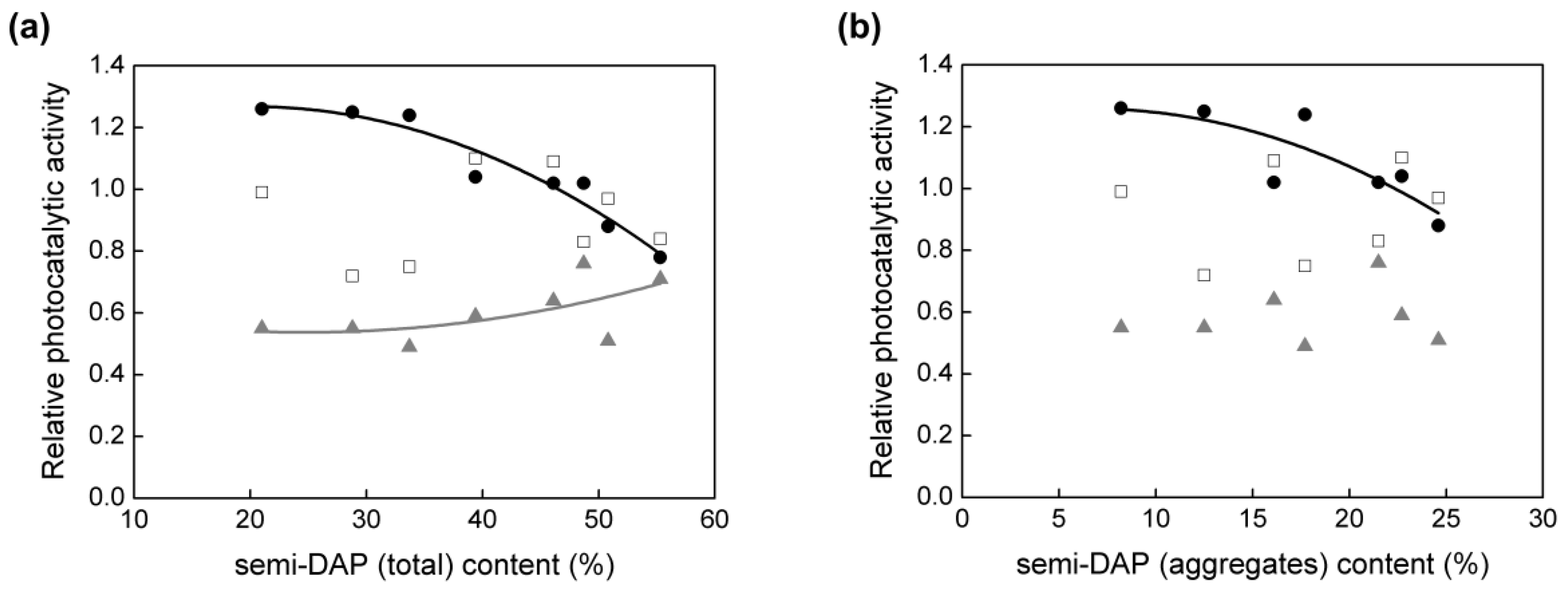
3.2. Particle Aspect Ratio (PAR) and Photocatalytic Activity
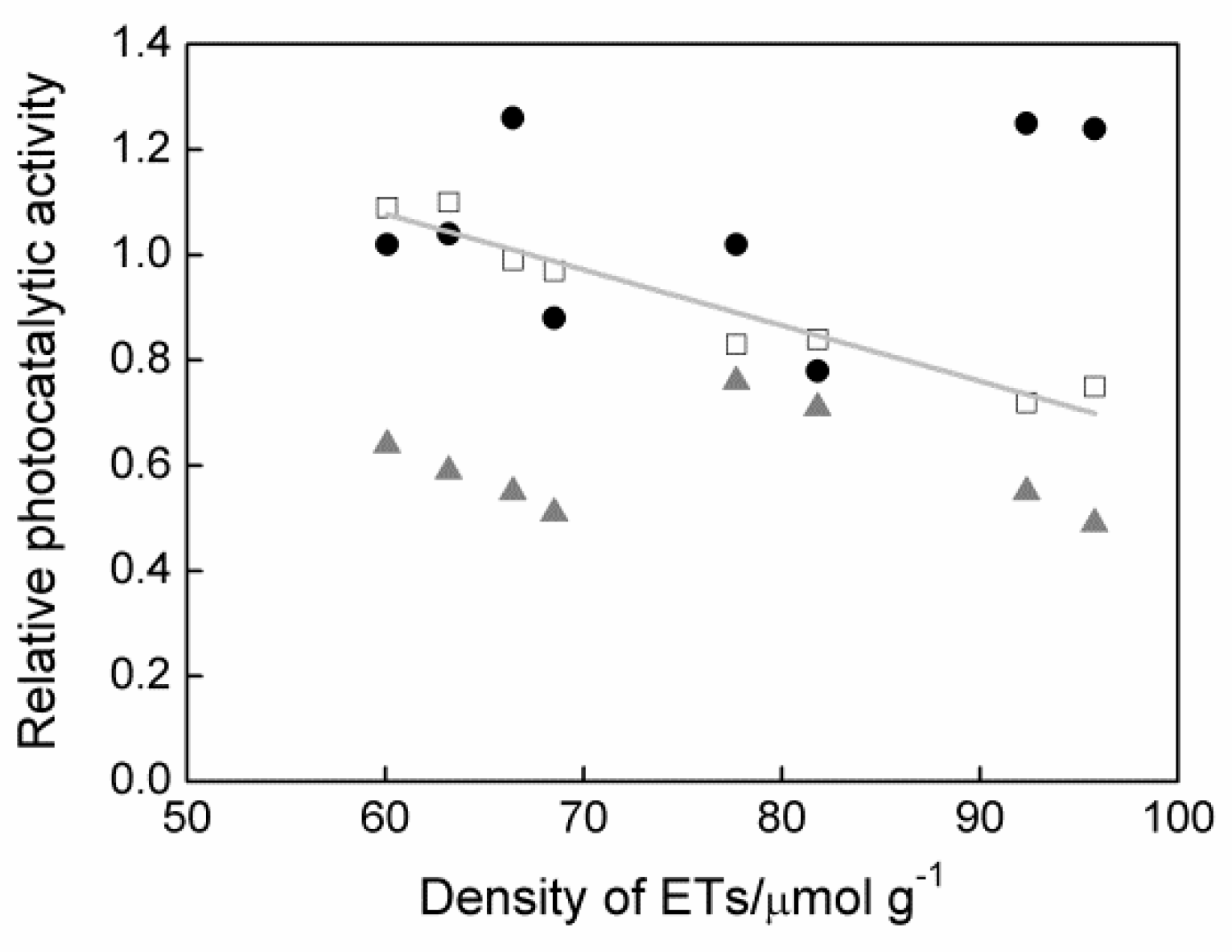
3.3. Density of Electron Traps (ETs) and Photocatalytic Activity
3.4. Morphology/Symmetry and Photocatalytic Activity
4. Discussion
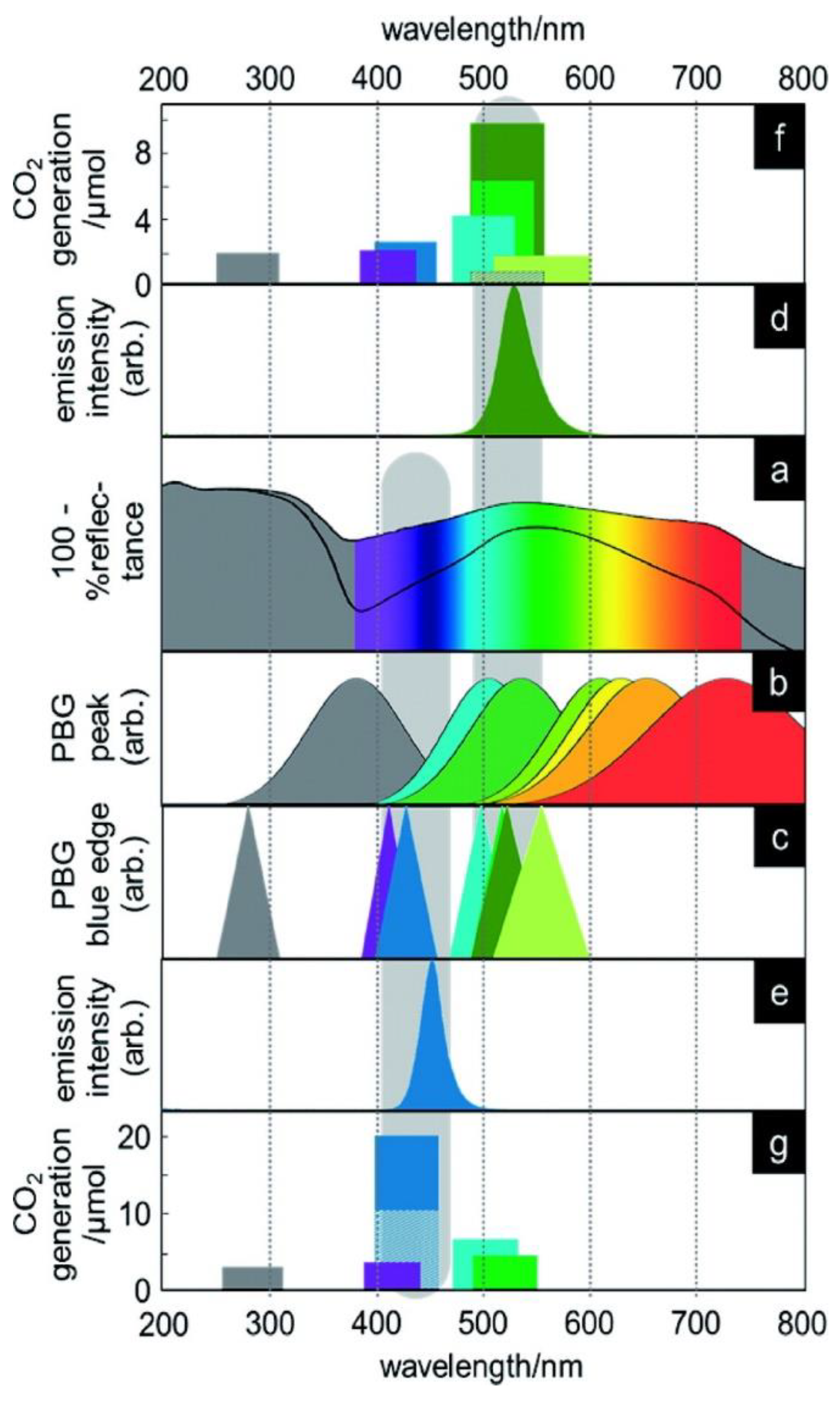
4.1. Pristine Titania
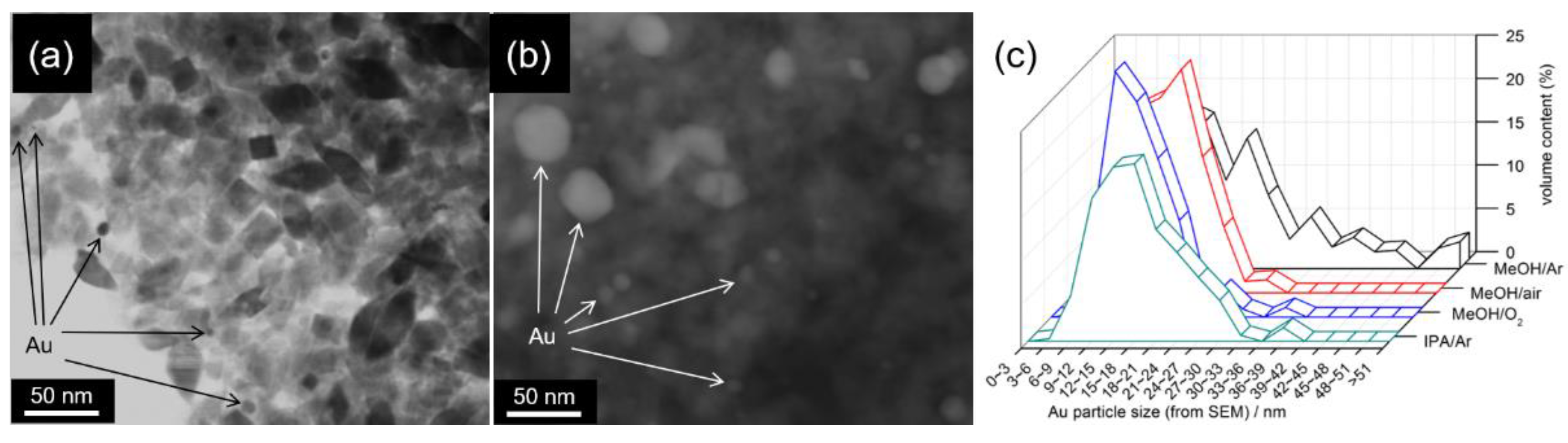
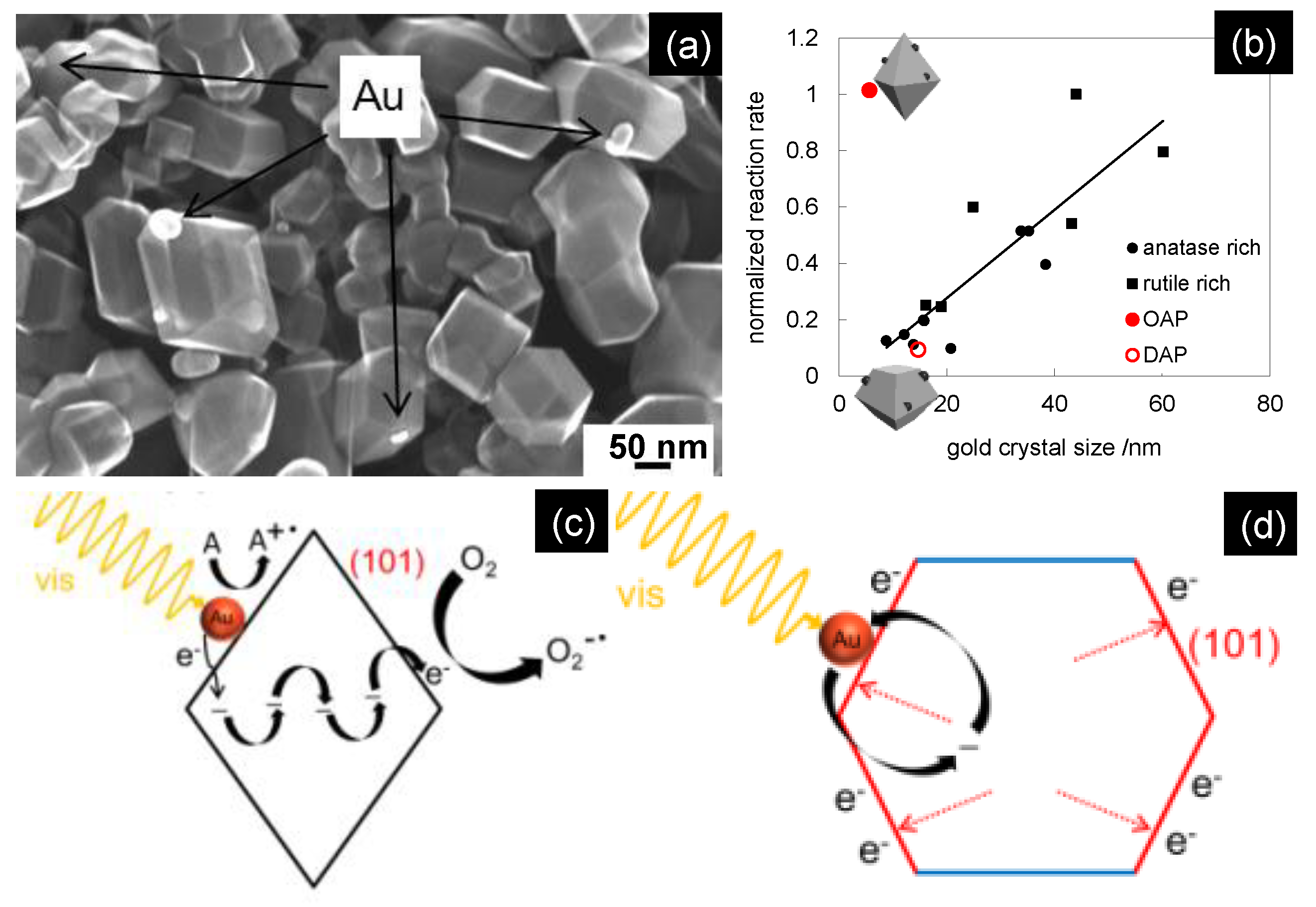
4.2. Plasmonic Photocatalysts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Chen, S.S.; Qi, Y.; Hisatomi, T.; Ding, Q.; Asai, T.; Li, Z.; Ma, S.S.K.; Zhang, F.X.; Domen, K.; Li, C. Efficient Visible-Light-Driven Z-Scheme Overall Water Splitting Using a MgTa2O6-xNy/TaON Heterostructure Photocatalyst for H2 Evolution. Angew. Chem. Int. Ed. 2015, 54, 8498–8501. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Takayama, T.; Sato, S.; Iwase, A.; Kudo, A.; Morikawa, T. Enhancement of CO2 reduction activity under visible light irradiation over Zn-based metal sulfides by combination with Ru-complex catalysts. Appl. Catal. B-Environ. 2018, 224, 572–578. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-Light-Induced Water Splitting Based on Two-Step Photoexcitation between Dye-Sensitized Layered Niobate and Tungsten Oxide Photocatalysts in the Presence of a Triiodide/Iodide Shuttle Redox Mediator. J. Am. Chem Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouame, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Yu, C.-C. Aligned TiO2 Nanorods and Nanowalls. J. Phys. Chem. B 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.; Janczarek, M.; Wei, Z.S.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology-and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Selloni, A. Crystal growth—Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef]
- Lovette, M.A.; Browning, A.R.; Griffin, D.W.; Sizemore, J.P.; Snyder, R.C.; Doherty, M.F. Crystal Shape Engineering. Ind. Eng. Chem. Res. 2008, 47, 9812–9833. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for Crystal-Face-Dependent TiO2 Photocatalysis from Single-Molecule Imaging and Kinetic Analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.S.; Kowalska, E.; Ohtani, B. Enhanced Photocatalytic Activity by Particle Morphology: Preparation, Characterization, and Photocatalytic Activities of Octahedral Anatase Titania Particles. Chem. Lett. 2014, 43, 346–348. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, M.; Pan, K.; Guo, L.; Wu, J.; Li, Z.; Yang, F.; Lin, K.; Zhou, W. Surface engineering of mesoporous anatase titanium dioxide nanotubes for rapid spatial charge separation on horizontal-vertical dimensions and efficient solar-driven photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 586, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, Y.; Jiang, G.; Zhao, Z.; Xu, C.; Wei, Y.; Duan, A.; Liu, J.; Gao, J. A photonic crystal-based CdS–Au–WO3 heterostructure for efficient visible-light photocatalytic hydrogen and oxygen evolution. RSC Adv. 2014, 4, 15689–15694. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic metamaterials: TiO2 inverse opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef]
- Araiedh, F.; Ducos, F.; Houas, A.; Chaoui, N. Photodegradation mode of stearic acid crystal on heterogeneous anatase/amorphous titania films observed by differential interference contrast microscopy. Appl. Catal. B-Environ. 2016, 187, 350–356. [Google Scholar] [CrossRef]
- Luo, Y.; Geng, S.H.; Dube, L.; Zhao, J. Tuning the Valency of Heterogeneous Au-Silica Nanostructure via Controlled Ostwald Ripening Process. J. Phys. Chem. C 2018, 122, 18077–18085. [Google Scholar] [CrossRef]
- Li, K.X.; Huang, Y.; Yan, L.S.; Dai, Y.H.; Xue, K.P.; Guo, H.Q.; Huang, Z.M.; Xiong, J.J. Simulated sunlight photodegradation of aqueous atrazine and rhodamine B catalyzed by the ordered mesoporous graphene-titania/silica composite material. Catal. Commun. 2012, 18, 16–20. [Google Scholar] [CrossRef]
- Barmparis, G.D.; Kopidakis, G.; Remediakis, I.N. Shape-Dependent Single-Electron Levels for Au Nanoparticles. Materials 2016, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.T.; Zhao, K.; Yu, J.G.; Jin, J.; Qi, Y.; Li, H.Q.; Hou, X.J.; Liu, G. Photocatalytic water splitting for hydrogen generation on cubic, orthorhombic, and tetragonal KNbO3 microcubes. Nanoscale 2013, 5, 8375–8383. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Boltersdorf, J.; Maggard, P.A.; Wong-Ng, W. Polymorphism and Structural Distortions of Mixed-Metal Oxide Photocatalysts Constructed with alpha-U3O8 Types of Layers. Crystals 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.Q.; Gong, J.L.; Nie, Z.H. Symmetry-Breaking Synthesis of Multicomponent Nanoparticles. Acc. Chem. Res. 2019, 52, 1125–1133. [Google Scholar] [CrossRef]
- Murakami, N.; Mahaney, O.O.P.; Torimoto, T.; Ohtani, B. Photoacoustic spectroscopic analysis of photoinduced change in absorption of titanium(IV) oxide photocatalyst powders: A novel feasible technique for measurement of defect density. Chem. Phys. Lett. 2006, 426, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Nitta, A.; Takase, M.; Takashima, M.; Murakamid, N.; Ohtani, B. A fingerprint of metal-oxide powders: Energy-resolved distribution of electron traps. Chem. Commun. 2016, 52, 12096–12099. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Terada, Y.; Ohtani, B. Highly active titania photocatalyst particles of controlled crystal phase, size, and polyhedral shapes. Top. Catal. 2010, 53, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-Controlled Anatase Titanium(IV) Oxide Particles Prepared by Hydrothermal Treatment of Peroxo Titanic Acid in the Presence of Polyvinyl Alcohol. J. Phys. Chem. C 2009, 113, 3062–3069. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, T.S.Y.; Sarukawa, K.; Ohno, T.; Matsumura, M. Formation of new crystal faces on TiO2 particles by treatment with aqueous HF solution or hot sulfuric acid. New J. Chem. 2003, 27, 1304–1306. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide. Chem. Commun. 2009, 2311–2313. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Takashima, M.; Takase, M.; Ohtani, B. Mechanistic Study on Facet-Dependent Deposition of Metal Nanoparticles on Decahedral-Shaped Anatase Titania Photocatalyst Particles. Catalysts 2018, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, A.; Wojtyla, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Ohtani, B. Influence of Post-Treatment Operations on Structural Properties and Photocatalytic Activity of Octahedral Anatase Titania Particles Prepared by an Ultrasonication-Hydrothermal Reaction. Molecules 2014, 19, 19573–19587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Wang, K.L.; Rokicka, P.; Endo, M.; Wei, Z.S.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobiol. B 2015, 151, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Kaewgun, S.; Nolph, C.A.; Lee, B.I.; Wang, L.-Q. Influence of hydroxyl contents on photocatalytic activities of polymorphic titania nanoparticles. Mater. Chem. Phys. 2009, 114, 439–445. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Deiana, C.; Fois, E.; Coluccia, S.; Martra, G. Surface Structure of TiO2 P25 Nanoparticles: Infrared Study of Hydroxy Groups on Coordinative Defect Sites. J. Phys. Chem. C 2010, 114, 21531–21538. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core–shell photocatalysts. J. Mater. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar] [CrossRef]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazi, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B-Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl Catal B-Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. Slow photon-induced enhancement of photocatalytic activity of gold nanoparticle-incorporated titania in-verse opal. Chem. Lett. 2021, 50, 711–713. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Kitaev, V.; Ozin, G.A. Effect of disorder on the optically amplified photocatalytic efficiency of titania inverse opals. J. Am. Chem. Soc. 2007, 129, 1196–1202. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Amplified photochemistry with slow photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Deng, Z.; Su, B.L. Three-Dimensionally Ordered Macroporous Titania with Structural and Photonic Effects for Enhanced Photocatalytic Efficiency. Chemsuschem 2011, 4, 1481–1488. [Google Scholar] [CrossRef] [PubMed]

| Sample | LSR (a.u.) | TiCl4(g) Flow Rate (mL h−1) | O2(g) Flow Rate (mL min−1) | Ar(g) Flow Rate (mL min−1) |
|---|---|---|---|---|
| DAP-1 | 0.049 | 1.5 | 1000 | 40 |
| DAP-2 | 0.059 | 2.2 | 1500 | 75 |
| DAP-3 | 0.074 | 1.5 | 1000 | 65 |
| DAP-4 | 0.084 | 1.5 | 1000 | 75 |
| DAP-5 | 0.096 | 1.5 | 1000 | 87 |
| DAP-6 | 0.109 | 1.5 | 1000 | 100 |
| DAP-7 | 0.125 | 1.0 | 650 | 75 |
| DAP-8 | 0.160 | 0.8 | 500 | 75 |
| Sample | SSA (m2 g−1) | PPS (nm) | Anatase Content (%) | Rutile Content (%) | Crystallinity (%) |
|---|---|---|---|---|---|
| DAP-1 | 14.6 | 64 | 96.3 | 3.7 | 93.8 |
| DAP-2 | 14.7 | 64 | 96.9 | 3.1 | 92.5 |
| DAP-3 | 15.2 | 67 | 97.7 | 2.3 | 93.3 |
| DAP-4 | 16.0 | 67 | 97.8 | 2.2 | 93.2 |
| DAP-5 | 16.3 | 66 | 97.6 | 2.4 | 94.1 |
| DAP-6 | 14.7 | 69 | 96.9 | 3.1 | 92.4 |
| DAP-7 | 16.0 | 64 | 97.8 | 2.2 | 92.9 |
| DAP-8 | 16.2 | 64 | 97.6 | 2.4 | 93.5 |
| Sample | Particle Shape Distribution * (%) | ||||
|---|---|---|---|---|---|
| DAP (A) Content | Semi-DAP Content | Other (E) | |||
| Total | Aggreg (C) | Mounds (B) | |||
| DAP-1 | 38.6 ± 5.5 | 55.3 ± 0.8 | 31.6 ± 13.4 | 3.3 ± 1.3 | 6.2 ± 5.0 |
| DAP-2 | 45.9 ± 11.0 | 46.1 ± 7.1 | 16.1 ± 1.7 | 9.4 ± 4.1 | 7.7 ± 4.4 |
| DAP-3 | 44.5 ± 6.3 | 50.8 ± 6.1 | 24.6 ± 2.6 | 9.2 ± 3.1 | 4.7 ± 1.8 |
| DAP-4 | 77.0 ± 4.2 | 21.0 ± 6.8 | 8.2 ± 1.7 | 2.6 ± 1.5 | 2.0 ± 2.7 |
| DAP-5 | 54.5 ± 9.9 | 39.4 ± 6.4 | 22.7 ± 5.9 | 5.2 ± 0.0 | 6.1 ± 3.1 |
| DAP-6 | 46.2 ± 9.7 | 48.7 ± 3.5 | 21.5 ± 2.9 | 6.9 ± 5.3 | 5.0 ± 7.1 |
| DAP-7 | 68.8 ± 4.4 | 28.8 ± 4.5 | 12.5 ± 1.8 | 3.2 ± 3.2 | 2.4 ± 1.4 |
| DAP-8 | 62.5 ± 5.6 | 33.7 ± 3.0 | 17.7 ± 2.8 | 1.5 ± 0.7 | 3.8 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Wei, Z.; Mogan, T.R.; Wang, L.; Wang, K.; Nitta, A.; Ohtani, B.; Kowalska, E. Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts? Symmetry 2021, 13, 1682. https://doi.org/10.3390/sym13091682
Janczarek M, Wei Z, Mogan TR, Wang L, Wang K, Nitta A, Ohtani B, Kowalska E. Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts? Symmetry. 2021; 13(9):1682. https://doi.org/10.3390/sym13091682
Chicago/Turabian StyleJanczarek, Marcin, Zhishun Wei, Tharishinny R. Mogan, Lei Wang, Kunlei Wang, Akio Nitta, Bunsho Ohtani, and Ewa Kowalska. 2021. "Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts?" Symmetry 13, no. 9: 1682. https://doi.org/10.3390/sym13091682
APA StyleJanczarek, M., Wei, Z., Mogan, T. R., Wang, L., Wang, K., Nitta, A., Ohtani, B., & Kowalska, E. (2021). Does Symmetry Control Photocatalytic Activity of Titania-Based Photocatalysts? Symmetry, 13(9), 1682. https://doi.org/10.3390/sym13091682











