Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
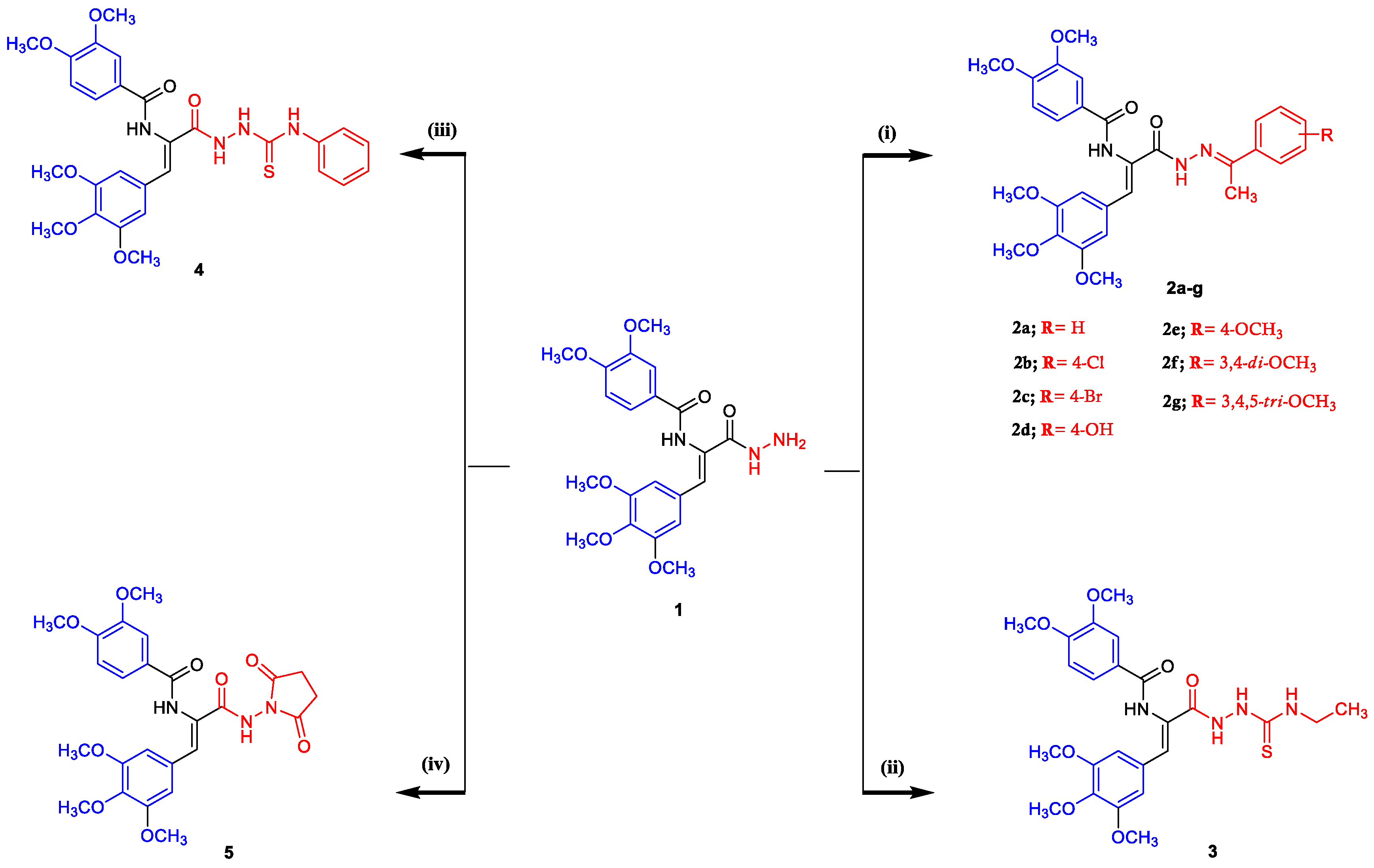
2.1. Chemistry
2.2. Biology
2.2.1. Cytotoxic Activity against MCF-7 Breast Cancer Cell Line
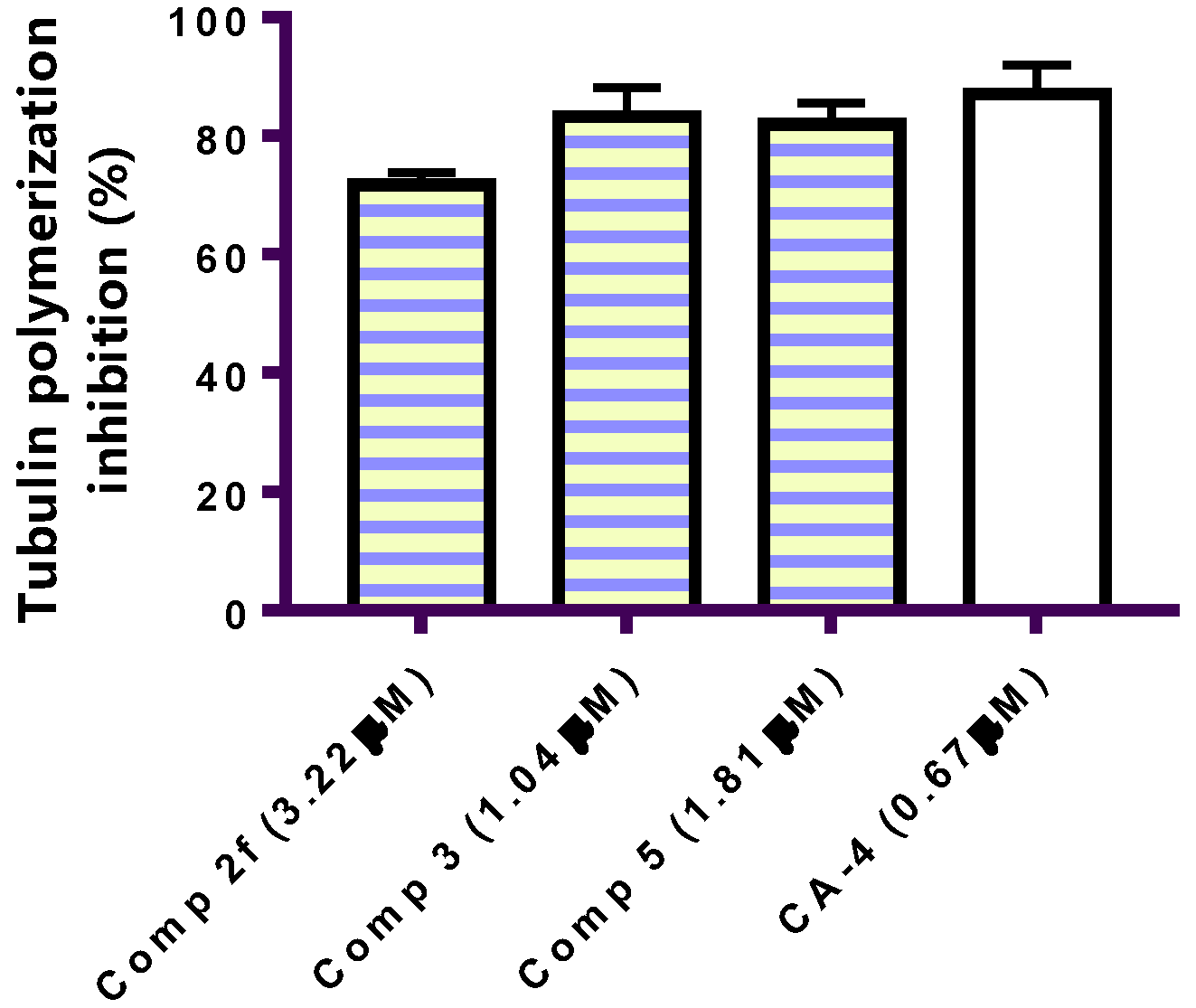
2.2.2. Tubulin Assay
2.2.3. Cell Cycle Analysis
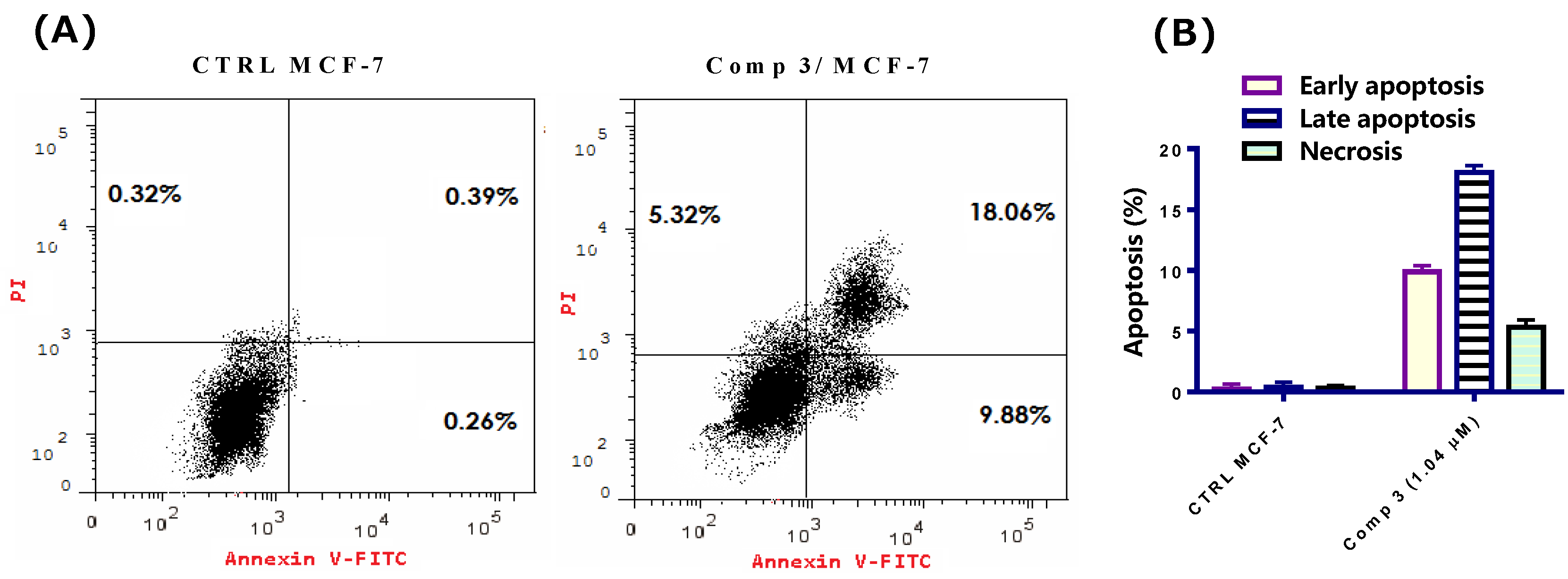
2.2.4. Apoptosis Assay
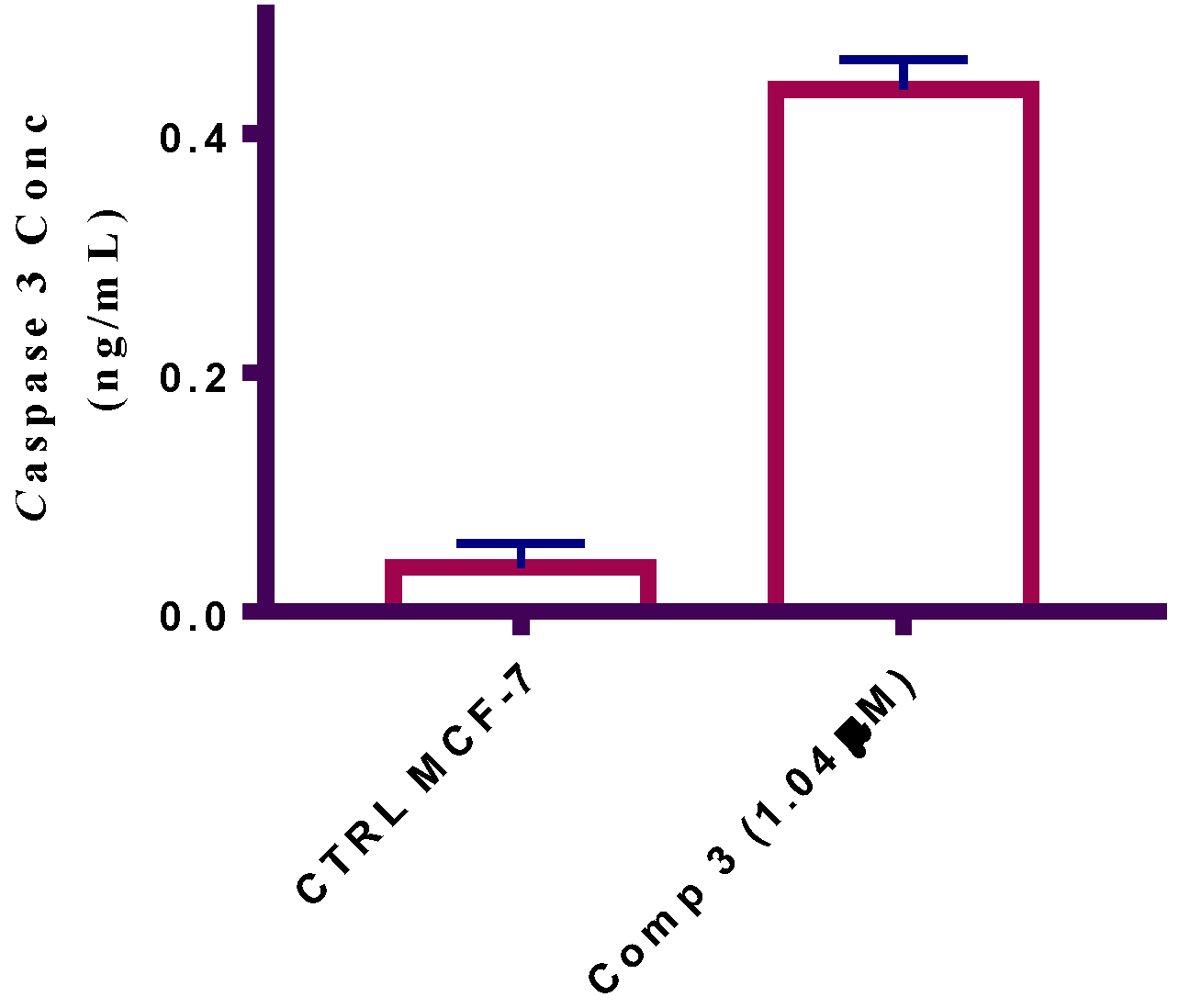
2.2.5. Caspase Assay
3. Conclusions
4. Experimental
4.1. Chemistry
4.1.1. General
4.1.2. General Procedure for the Synthesis of N-((Z)-3-((E)-2-(arylethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamides 2a–g
3,4-Dimethoxy-N-((Z)-3-oxo-3-((E)-2-(1-phenylethylidene)hydrazinyl)-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)benzamide (2a)
N-((Z)-3-((E)-2-(1-(4-Chlorophenyl)ethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (2b)
N-((Z)-3-((E)-2-(1-(4-Bromophenyl)ethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (2c)
N-((Z)-3-((E)-2-(1-(4-Hydroxyphenyl)ethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (2d)
3,4-Dimethoxy-N-((Z)-3-((E)-2-(1-(4-methoxyphenyl)ethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)benzamide (2e)
N-((Z)-3-((E)-2-(1-(3,4-Dimethoxyphenyl)ethylidene)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (2f)
3,4-Dimethoxy-N-((Z)-3-oxo-1-(3,4,5-trimethoxyphenyl)-3-((E)-2-(1-(3,4,5-trimethoxyphenyl)ethylidene)hydrazinyl)prop-1-en-2-yl)benzamide (2g)
4.1.3. General Procedure for the Synthesis of (Z)-N-(3-(2-(Ethylcarbamothioyl)hydrazinyl)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (3)
4.1.4. General Procedure for the Synthesis of (Z)-3,4-Dimethoxy-N-(3-oxo-3-(2-(phenylcarbamothioyl)hydrazinyl)-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)benzamide (4)
4.1.5. General Procedure for the Synthesis of (Z)-N-(3-((2,5-Dioxopyrrolidin-1-yl)amino)-3-oxo-1-(3,4,5-trimethoxyphenyl)prop-1-en-2-yl)-3,4-dimethoxybenzamide (5)
4.2. Biological Study
4.2.1. MTT Cytotoxicity Assay
4.2.2. Tubulin Assay
4.2.3. Cell Cycle Analysis
4.2.4. Annexin V/FITC Staining Assay
4.2.5. Caspase 3 Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
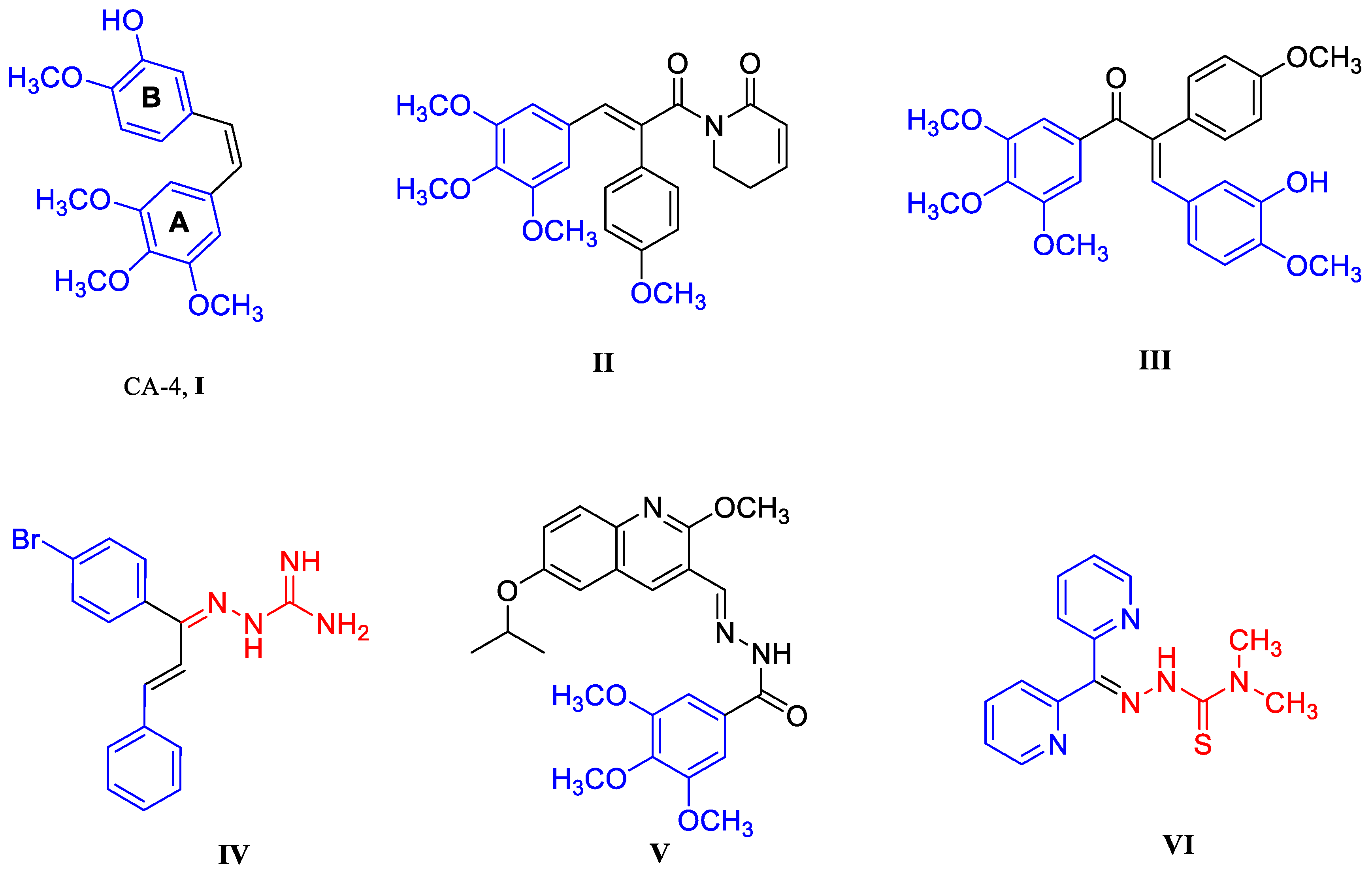
- Liu, Z.; Wang, P.; Wold, E.A.; Song, Q.; Zhao, C.; Wang, C.; Zhou, J. Small-Molecule Inhibitors Targeting the Canonical WNT Signaling Pathway for the Treatment of Cancer. J. Med. Chem. 2021, 64, 4257–4288. [Google Scholar] [CrossRef] [PubMed]
- Sereni, M.I.; Baldelli, E.; Gambara, G.; Deng, J.; Zanotti, L.; Bandiera, E.; Bignotti, E.; Ragnoli, M.; Tognon, G.; Ravaggi, A.; et al. Functional characterization of epithelial ovarian cancer histotypes by drug target based protein signaling activation mapping: Implications for personalized cancer therapy. Proteomics 2015, 15, 365–373. [Google Scholar] [CrossRef]
- Carotenuto, P.; Pecoraro, A.; Palma, G.; Russo, G.; Russo, A. Therapeutic Approaches Targeting Nucleolus in Cancer. Cells 2019, 8, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, V.; Singh, A.S.A.; Kaur, K.; Rath, G. Targeted based drug delivery system for colon cancer. J. Drug Delivery Ther. 2020, 10, 111–122. [Google Scholar] [CrossRef]
- Haider, K.; Rahaman, S.; Yar, M.S.; Kamal, A. Tubulin inhibitors as novel anticancer agents: An overview on patents (2013–2018). Expert Opin. Ther. Pat. 2019, 29, 623–641. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N. Microtubule Dynamics and Cancer. Cancers 2022, 14, 4368–4388. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, G.; Marougka, K.; Desquesnes, A.; Welti, N.; Sitterlin, D.; Gault, E.; Rameix-Welti, M.-A. Respiratory syncytial virus ribonucleoproteins hijack microtubule Rab11 dependent transport for intracellular trafficking. PLoS Pathog. 2022, 18, e1010619. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Duro, E.; Marston, A.L. From equator to pole: Splitting chromosomes in mitosis and meiosis. Genes Dev. 2015, 29, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef] [PubMed]
- GPettit, R.; Singh, S.B.; Boyd, M.R.; Hamel, E.; Pettit, R.K.; Schmidt, J.M.; Hogan, F. Antineoplastic Agents. 291. Isolation and Synthesis of Combretastatins A-4, A-5, and A-6. J. Med. Chem. 1995, 38, 1666–1672. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, B.; Xiao, Y.; Xue, M.; Liu, T.; Cao, H.; Chen, J. Discovery of novel CA-4 analogs as dual inhibitors of tubulin polymerization and PD-1/PD-L1 interaction for cancer treatment. Eur. J. Med. Chem. 2021, 213, 113058. [Google Scholar] [CrossRef]
- Wang, J.; Miller, D.D.; Li, W. Molecular interactions at the colchicine binding site in tubulin: An X-ray crystallography perspective. Drug Discov. Today 2022, 27, 759–776. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8–31. [Google Scholar] [CrossRef] [Green Version]
- Piekuś-Słomka, N.; Mikstacka, R.; Ronowicz, J.; Sobiak, S. Hybrid cis-stilbene Molecules: Novel Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNulty, J.; van den Berg, S.; Ma, D.; Tarade, D.; Joshi, S.; Church, J.; Pandey, S. Antimitotic activity of structurally simplified biaryl analogs of the anticancer agents colchicine and combretastatin A4. Bioorg. Med. Chem. Lett. 2015, 25, 117–121. [Google Scholar] [CrossRef]
- Jaroch, K.; Karolak, M.; Górski, P.; Jaroch, A.; Krajewski, A.; Ilnicka, A.; Sloderbach, A.; Stefański, T.; Sobiak, S. Combretastatins: In vitro structure-activity relationship, mode of action and current clinical status. Pharmacol. Rep. 2016, 68, 1266–1275. [Google Scholar] [CrossRef]
- Ibrahim, T.S.; Hawwas, M.M.; Malebari, A.M.; Taher, E.S.; Omar, A.M.; Neamatallah, T.; Abdel-Samii, Z.K.; Safo, M.K.; Elshaier, Y.A.M.M. Discovery of novel quinoline-based analogues of combretastatin A-4 as tubulin polymerisation inhibitors with apoptosis inducing activity and potent anticancer effect. J. Enzyme Inhib. Med. Chem. 2021, 36, 802–818. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Dunnigan, J.K.; Towne, A.C.; Zhao, Z.; Du, L.; Brittain, W.J. Photopharmacology of Azo-Combretastatin-A4: Utilizing Tubulin Polymerization Inhibitors and Green Chemistry as the Key Steps. Curr. Org. Chem. 2021, 25, 2457–2474. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Cruz-Lopez, O.; Cara, C.L.; Carrion, M.D.; Brancale, A.; Hamel, E.; Chen, L.; Bortolozzi, R.; Basso, G.; et al. Synthesis and Antitumor Activity of 1,5-Disubstituted 1,2,4-Triazoles as Cis-Restricted Combretastatin Analogues. J. Med. Chem. 2010, 53, 4248–4258. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef]
- Liu, W.; He, M.; Li, Y.; Peng, Z.; Wang, G. A review on synthetic chalcone derivatives as tubulin polymerisation inhibitors. J. Enzyme Inhib. Med. Chem. 2022, 37, 9–38. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, H.-J.; Lv, P.-C.; Zhu, H.-L. Synthesis, molecular modeling and biological evaluation of guanidine derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2010, 18, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, F.; Jafari, E.; Hakimelahi, G.; Khajouei, M.R.; Jalali, M.; Khodarahmi, G. Antibacterial, antifungal and cytotoxic evaluation of some new quinazolinone derivatives. Res. Pharm. Sci. 2012, 7, 87–94. [Google Scholar]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The new wave in cancer treatment. Future Med. Chem. 2009, 1, 1143–1151. [Google Scholar] [CrossRef]
- Gucký, T.; Fryšová, I.; Slouka, J.; Hajdúch, M.; Džubák, P. Cyclocondensation reaction of heterocyclic carbonyl compounds, Part XIII: Synthesis and cytotoxic activity of some 3,7-diaryl-5-(3,4,5-trimethoxyphenyl)pyrazolo[4,3-e][1,2,4]triazines. Eur. J. Med. Chem. 2009, 44, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Zaki, I.; Abou-Elkhair, R.A.I.; Almaaty, A.H.A.; Ali, O.A.A.; Fayad, E.; Gaafar, A.G.A.; Zakaria, M.Y. Design and Synthesis of Newly Synthesized Acrylamide Derivatives as Potential Chemotherapeutic Agents against MCF-7 Breast Cancer Cell Line Lodged on PEGylated Bilosomal Nano-Vesicles for Improving Cytotoxic Activity. Pharmaceuticals 2021, 14, 1021. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Abualnaja, M.; Ali, O.A.A.; Alyamani, N.M.; Elsaid, F.G.; Shati, A.A.; Albogami, S.; Fayad, E.; Almaaty, A.H.A.; Mohamed, K.O.; et al. Design, Synthesis and Cytotoxicity Screening of New Thiazole Derivatives as Potential Anticancer Agents through VEGFR-2 Inhibition. Symmetry 2022, 14, 1814. [Google Scholar] [CrossRef]
- Whitfield, M.L.; Sherlock, G.; Saldanha, A.J.; Murray, J.I.; Ball, C.A.; Alexander, K.E.; Matese, J.C.; Perou, C.M.; Hurt, M.M.; Brown, P.O.; et al. Identification of Genes Periodically Expressed in the Human Cell Cycle and Their Expression in Tumors. Mol. Biol. Cell 2002, 13, 1977–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaki, I.; Abdelhameid, M.K.; El-Deen, I.M.; Wahab, A.H.A.A.; Ashmawy, A.M.; Mohamed, K.O. Design, synthesis and screening of 1, 2, 4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line. Eur. J. Med. Chem. 2018, 156, 563–579. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Aldhahrani, A.; Althobaiti, F.; Fayad, E.; Ali, O.A.A.; Albogami, S.; Almaaty, A.H.A.; Khedr, A.I.M.; Bukhari, S.N.A.; Zaki, I. Design, Synthesis and Cytotoxic Activity Evaluation of Newly Synthesized Amides-Based TMP Moiety as Potential Anticancer Agents over HepG2 Cells. Molecules 2022, 27, 3960. [Google Scholar] [CrossRef]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Liu, C.; Qiu, M.; Ma, X.; Mao, Z.; Sun, Y.; Liu, Z. Recent advances in the development of activatable multifunctional probes for in vivo imaging of caspase-3. Chin. Chem. Lett. 2021, 32, 168–178. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]


| Comp No | IC50 Value (μM) |
|---|---|
| MCF-7 | |
| 2a | 19.43 ± 1.02 |
| 2b | 27.78 ± 1.43 |
| 2c | 25.41 ± 1.07 |
| 2d | 6.93 ± 0.33 |
| 2e | 8.45 ± 0.53 |
| 2f | 3.22 ± 0.40 |
| 2g | 4.82 ± 0.69 |
| 3 | 1.04 ± 0.13 |
| 4 | 4.11 ± 0.49 |
| 5 | 1.81 ± 0.15 |
| CA-4 | 0.67 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Warhi, T.; Alqahtani, L.S.; Alsharif, G.; Abualnaja, M.; Abu Ali, O.A.; Qahl, S.H.; Althagafi, H.A.E.; Alharthi, F.; Jafri, I.; Elsaid, F.G.; et al. Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents. Symmetry 2022, 14, 2088. https://doi.org/10.3390/sym14102088
Al-Warhi T, Alqahtani LS, Alsharif G, Abualnaja M, Abu Ali OA, Qahl SH, Althagafi HAE, Alharthi F, Jafri I, Elsaid FG, et al. Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents. Symmetry. 2022; 14(10):2088. https://doi.org/10.3390/sym14102088
Chicago/Turabian StyleAl-Warhi, Tarfah, Leena S. Alqahtani, Ghadi Alsharif, Matokah Abualnaja, Ola A. Abu Ali, Safa H. Qahl, Hussam Awwadh E. Althagafi, Fahad Alharthi, Ibrahim Jafri, Fahmy G. Elsaid, and et al. 2022. "Design, Synthesis, and Investigation of Cytotoxic Activity of cis-Vinylamide-Linked Combretastatin Analogues as Potential Anticancer Agents" Symmetry 14, no. 10: 2088. https://doi.org/10.3390/sym14102088






