Fluorescent Composites Prepared of Tb3+ and Sulfonated Sulfate Polymer Constructed through Post-Sulfonation Sulfur-Fluorine Exchange Polymerization by Symmetric Molecular
Abstract
:1. Introduction
2. Materials and Methods
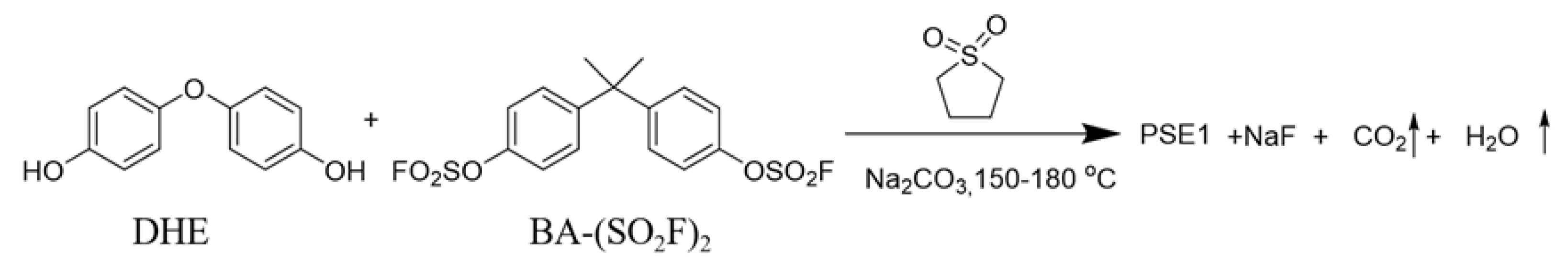
2.1. Preparation of PSE1 (Polysulfate-1)
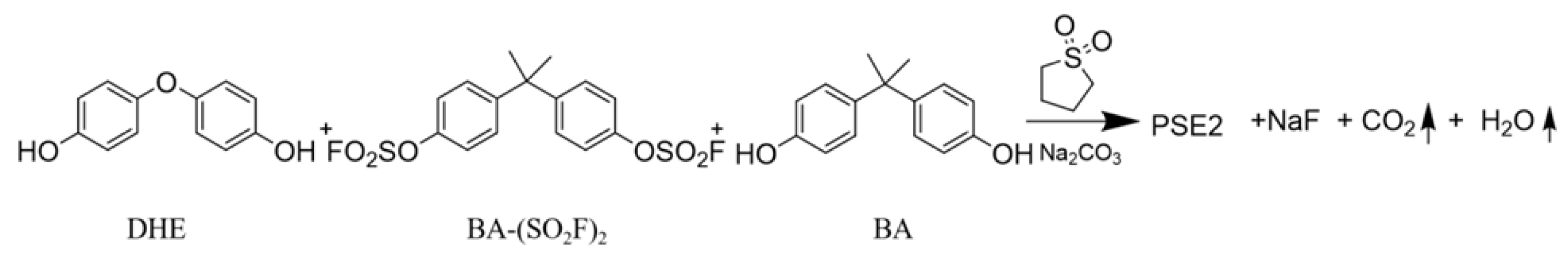
2.2. Preparation of (Polysulfate-2)
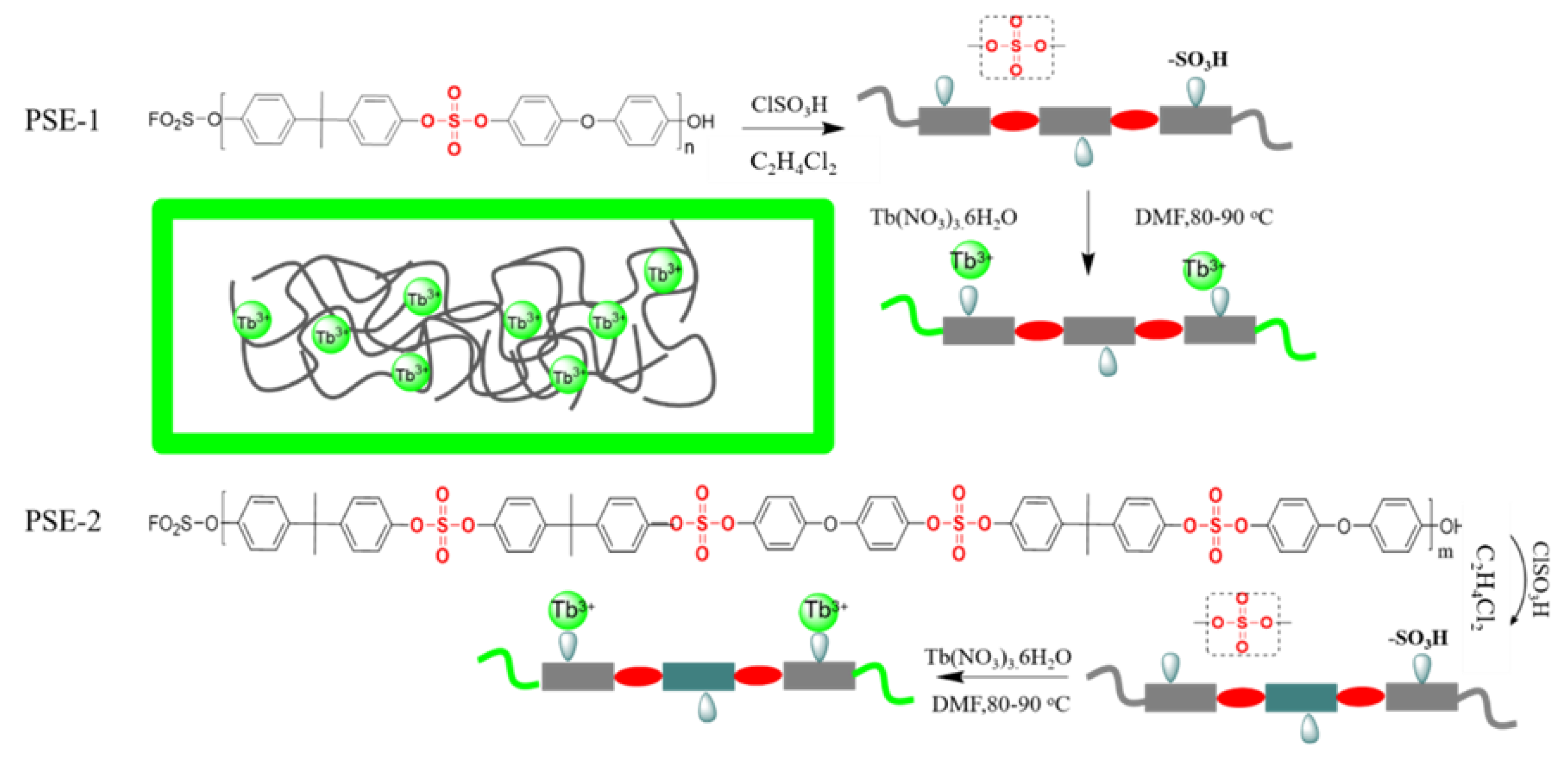
2.3. Preparation of PSE-SO3H
2.4. Device Fabrication Process of PSE−SO3H−Tb3+
3. Results and Discussion
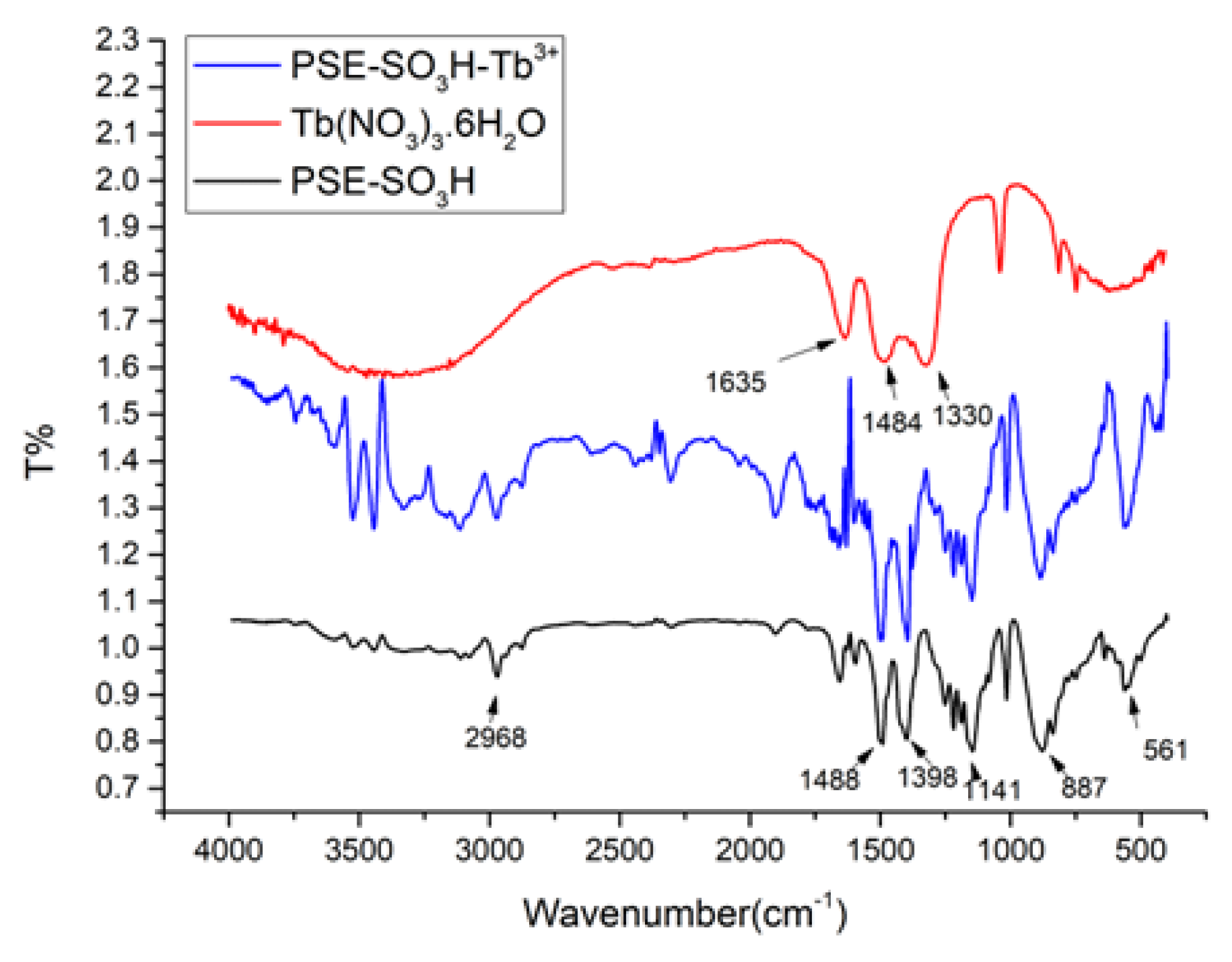
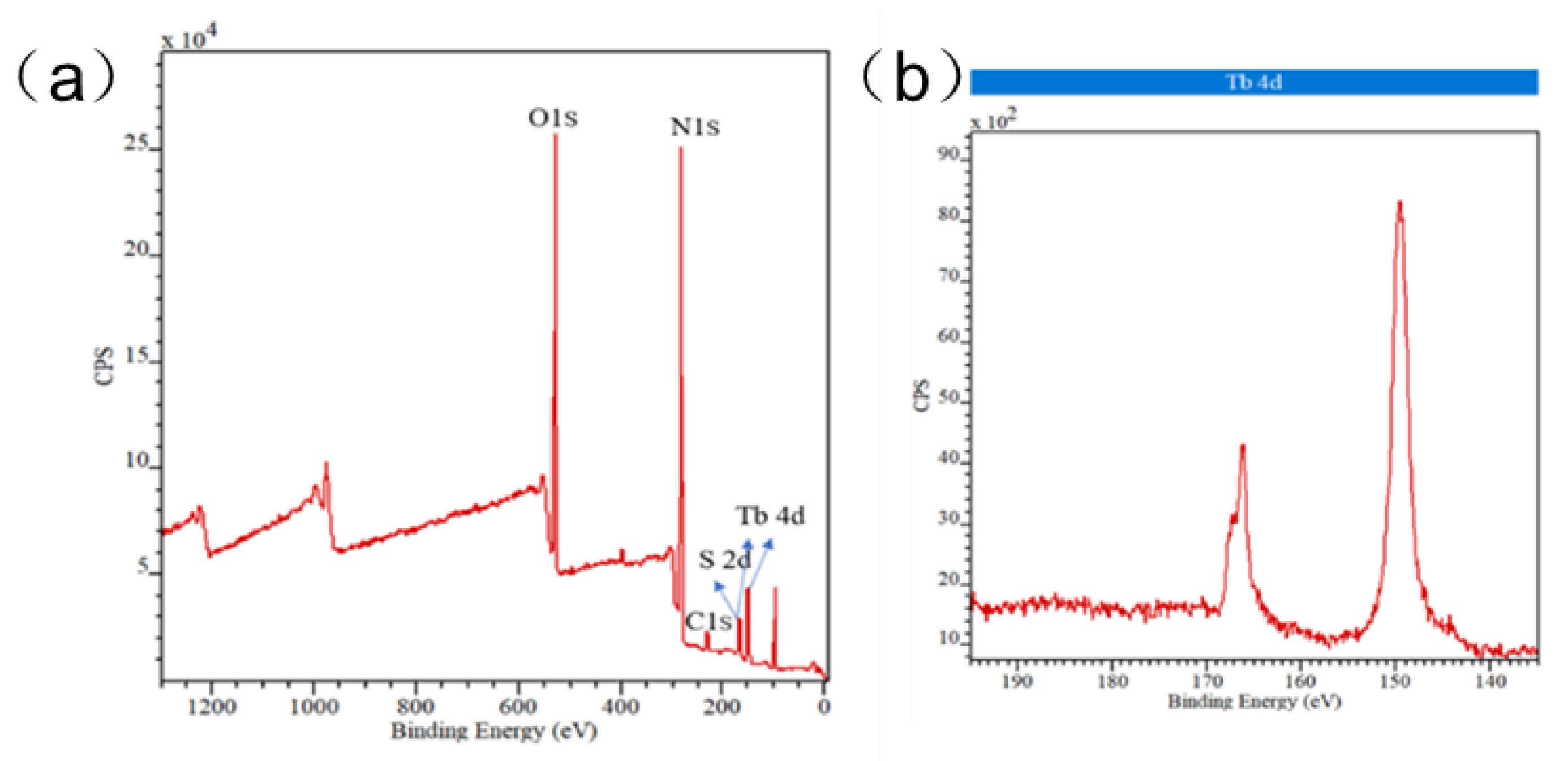
3.1. Structure Descripition
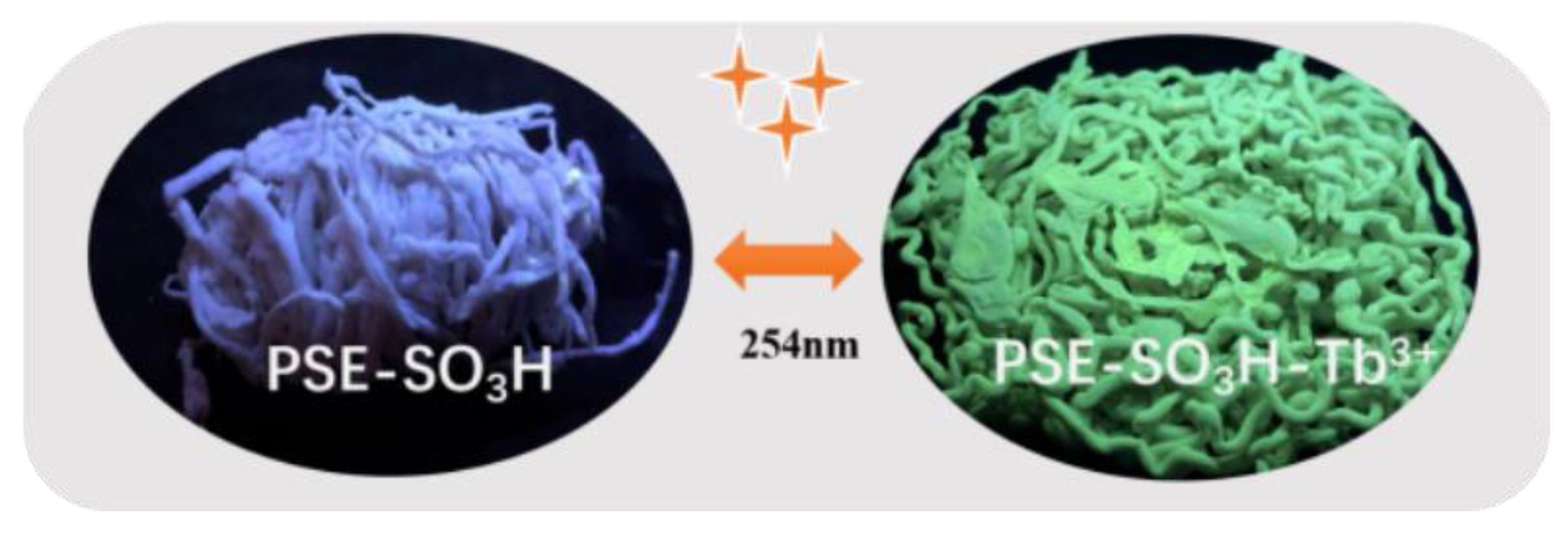
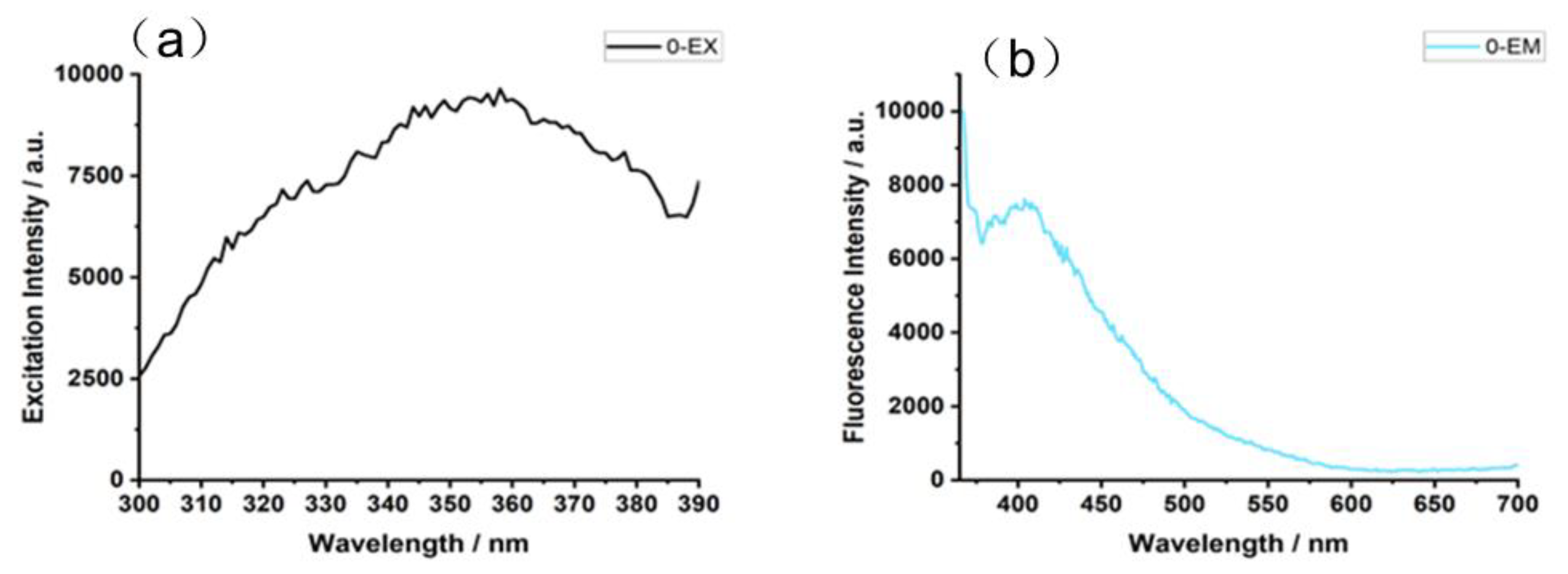
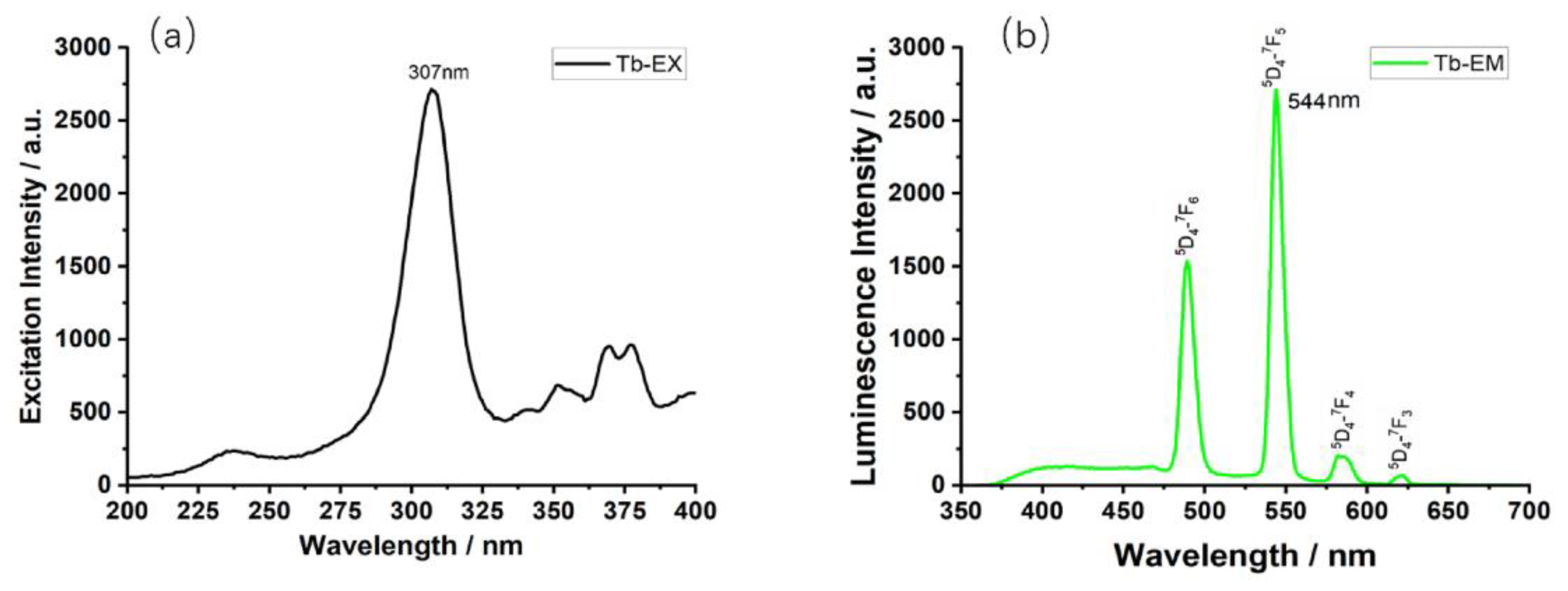
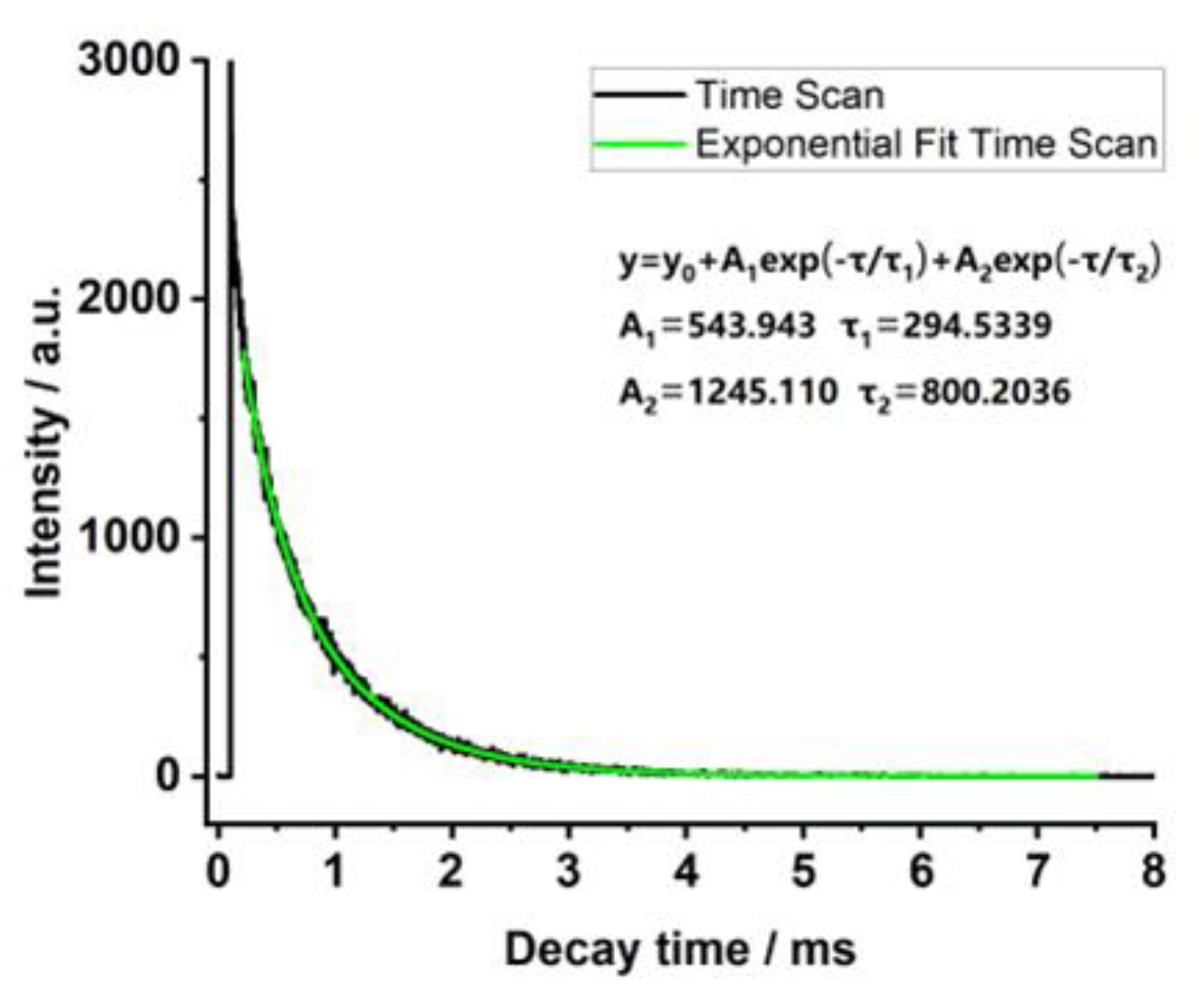
3.2. Luminescence
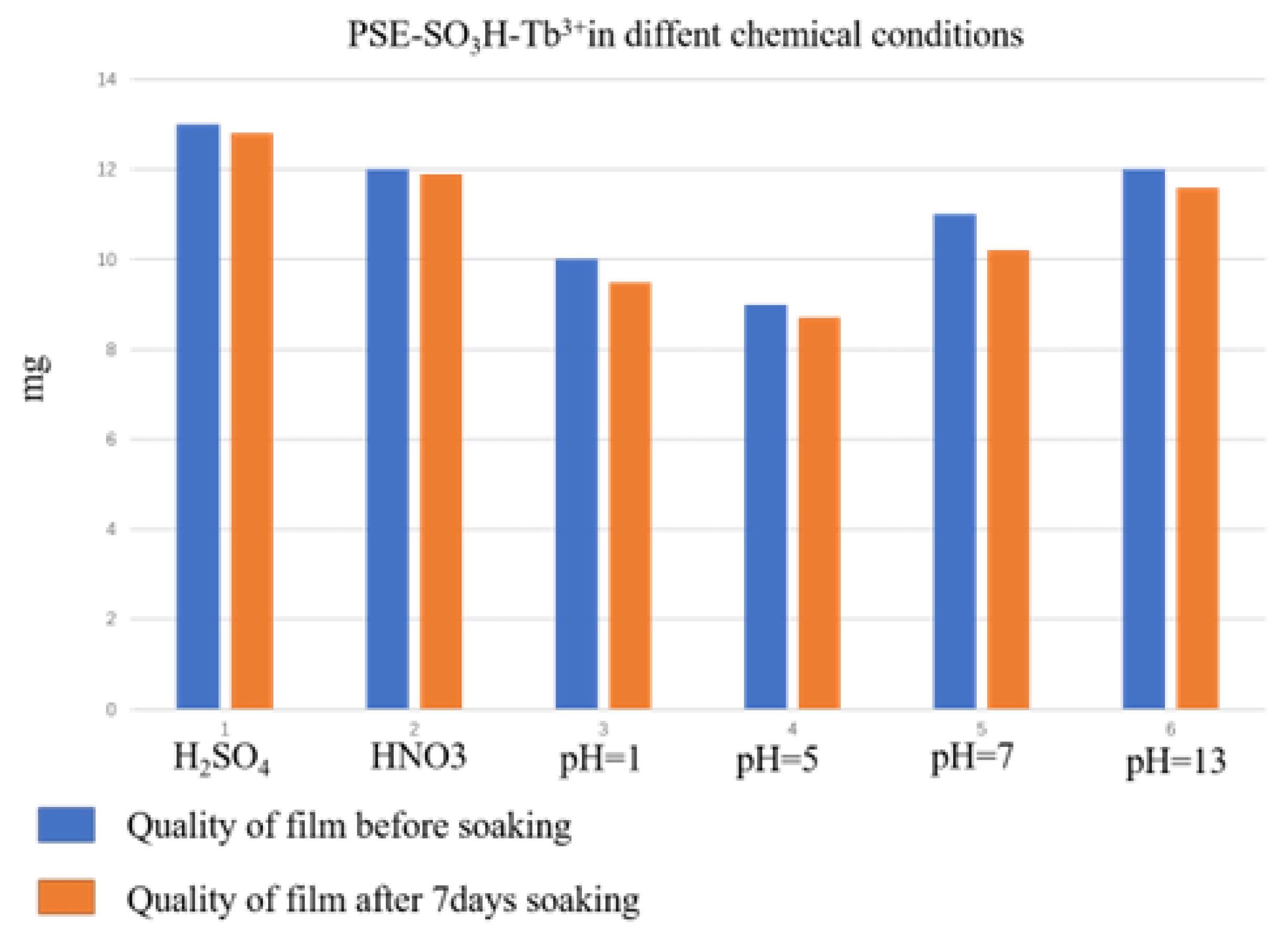
3.3. Chemical Stability
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmuth, C.K.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar]
- Patricia, L.G.; Matyjaszewski, K. Marrying click chemistry with polymerization: Expanding the scope of polymeric materials. Chem. Soc. Rev. 2010, 39, 1338–1354. [Google Scholar]
- Binder, W.H.; Sachsenhofer, R. Click Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54, Erratum in Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Tugce, N.G.; Aysun, D.; Rana, S.; Amitav, S. Multifunctional and Transformable ‘Clickable’ Hydrogel Coatings on Titanium Surfaces: From Protein Immobilization to Cellular Attachment. Polymers 2020, 12, 1211. [Google Scholar]
- Demarteau, J.; Winter, J.D.; Detrembleur, C.; Debuigne, A. Ethylene/vinyl acetate-based macrocycles via organometallic-mediated radical polymerization and CuAAC ‘click’ reaction. Polym. Chem. 2018, 9, 273–278. [Google Scholar] [CrossRef]
- Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Click Chemistry beyond Metal-Catalyzed Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 4900–4908. [Google Scholar] [CrossRef]
- Killops, K.L.; Campos, L.M.; Hawker, C.J. Robust, Efficient, and Orthogonal Synthesis of Dendrimers via Thiol-ene “Click” Chemistry. J. Am. Chem. Soc. 2008, 130, 5062–5064. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Lowe, A.B.; Hoyleb, C.E.; Bowman, C.N. Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 20, 4745–4750. [Google Scholar] [CrossRef]
- David, C.; Bernhard, G.; Kappe, C.O. Mechanistic Insights on Azide-Nitrile Cycloadditions: On the Dialkyltin Oxide-Trimethylsilyl Azide Route and a New Vilsmeier-Haack-Type Organocatalyst. J. Am. Chem. Soc. 2011, 133, 4465–4475. [Google Scholar]
- Penney, C.L.; Perlin, A.S. A method for the sulfation of sugars, employing a stable, aryl sulfate intermediate. Carbohydr. Res. 1981, 93, 241–246. [Google Scholar] [CrossRef]
- Dong, J.J.; Krasnova, L.; Finn, M.G.; Sharples, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Yatvin, J.; McNitt, C.D.; Reese, R.A.; Jung, C.; Popik, V.V.; Locklin, J. Multifunctional Surface Manipulation Using Orthogonal Click Chemistry. Langmuir 2006, 32, 6600–6605. [Google Scholar]
- Oakdale, J.S.; Kwisnek, L.; Fokin, V.V. Selective and Orthogonal Post-Polymerization Modification Using Sulfur(VI) Fluoride Exchange (SuFEx) and Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) Reactions. Macromolecules 2016, 49, 4473–4479. [Google Scholar] [CrossRef]
- Zhu, W.W.; Li, F.C.; Liu, J.K.; Ma, X.Y.; Jiang, X.X. Nucleophilic construction of sulfate bonds: Simplified access to polysulfates and polysulfonates. React. Chem. Eng. 2019, 4, 2074–2080. [Google Scholar] [CrossRef]
- Madhusudan, D.N.; Anup, P.; Svetlana, S. Recent Advancements in Developments of Novel Fluorescent Probes: In Cellulo Recognitions of Alkaline Phosphatases. Symmetry 2022, 14, 1634. [Google Scholar] [CrossRef]
- Qiao, W.G.; Li, Z.A. Recent Progress of Squaraine-Based Fluorescent Materials and Their Biomedical Applications. Symmetry 2022, 14, 966. [Google Scholar] [CrossRef]
- Mohamed, B.; Mustapha, C. Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment. Symmetry 2020, 12, 933. [Google Scholar] [CrossRef]
- Avetisov, R.; Lebedev, A.; Suslova, E.; Kazmina, K.; Runina, K.; Kovaleva, V.; Khomyakov, A.; Barkanov, A.; Zykova, M.; Petrova, O.; et al. Luminescent Hybrid Material Based on Boron Organic Phosphor and Silica Aerogel Matrix. Molecules 2022, 27, 5226. [Google Scholar] [CrossRef]
- Koen, B. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, W.; Gao, Y.; Tian, L.; Guo, J.; Cui, J.; Yang, B. Fluorescent Composites Prepared of Tb3+ and Sulfonated Sulfate Polymer Constructed through Post-Sulfonation Sulfur-Fluorine Exchange Polymerization by Symmetric Molecular. Symmetry 2022, 14, 2293. https://doi.org/10.3390/sym14112293
Li F, Liu W, Gao Y, Tian L, Guo J, Cui J, Yang B. Fluorescent Composites Prepared of Tb3+ and Sulfonated Sulfate Polymer Constructed through Post-Sulfonation Sulfur-Fluorine Exchange Polymerization by Symmetric Molecular. Symmetry. 2022; 14(11):2293. https://doi.org/10.3390/sym14112293
Chicago/Turabian StyleLi, Fuchong, Wei Liu, Yang Gao, Li Tian, Junhong Guo, Jinfeng Cui, and Baoping Yang. 2022. "Fluorescent Composites Prepared of Tb3+ and Sulfonated Sulfate Polymer Constructed through Post-Sulfonation Sulfur-Fluorine Exchange Polymerization by Symmetric Molecular" Symmetry 14, no. 11: 2293. https://doi.org/10.3390/sym14112293
APA StyleLi, F., Liu, W., Gao, Y., Tian, L., Guo, J., Cui, J., & Yang, B. (2022). Fluorescent Composites Prepared of Tb3+ and Sulfonated Sulfate Polymer Constructed through Post-Sulfonation Sulfur-Fluorine Exchange Polymerization by Symmetric Molecular. Symmetry, 14(11), 2293. https://doi.org/10.3390/sym14112293




