Effect of Thermal Treatment of Symmetric TiO2 Nanotube Arrays in Argon on Photocatalytic CO2 Conversion
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
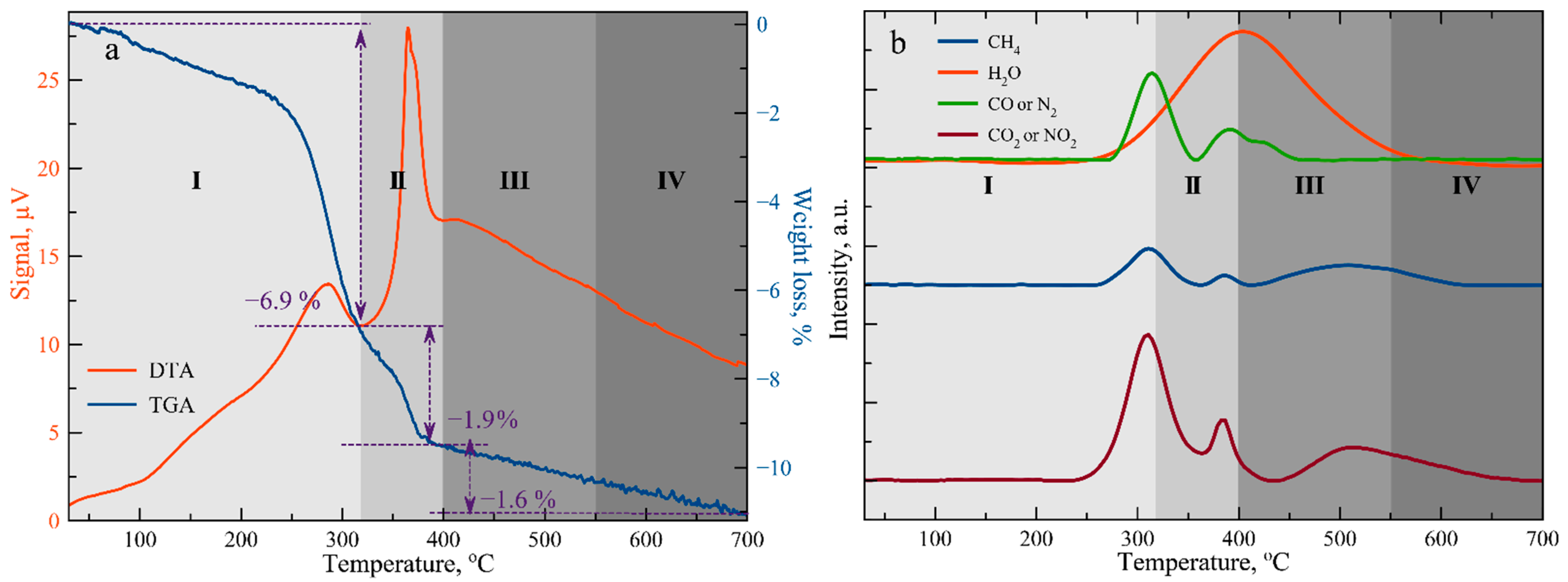
3.1. Thermal Treatment Condition Determination
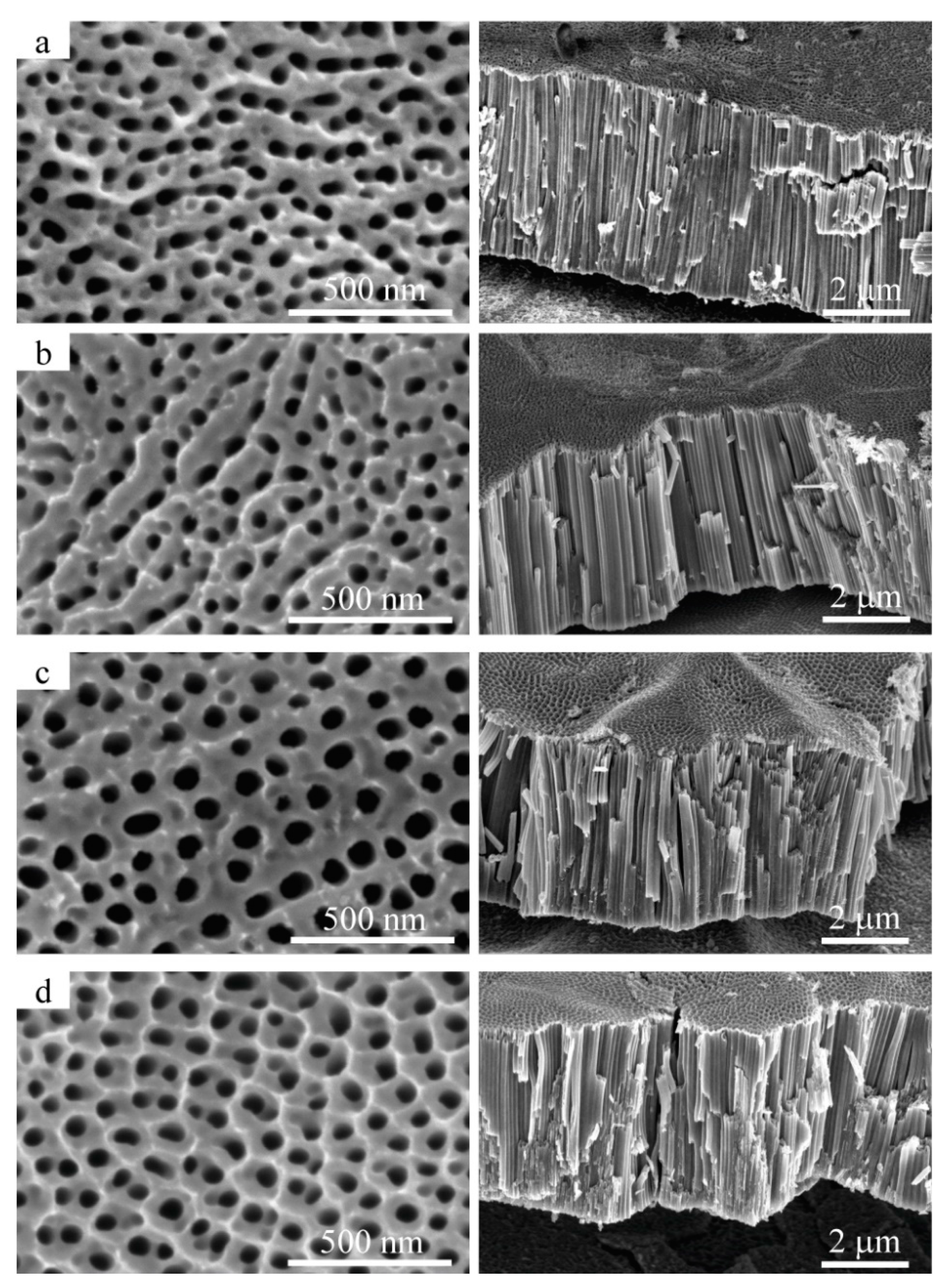
3.2. Morphology and Composition of Samples Characterization
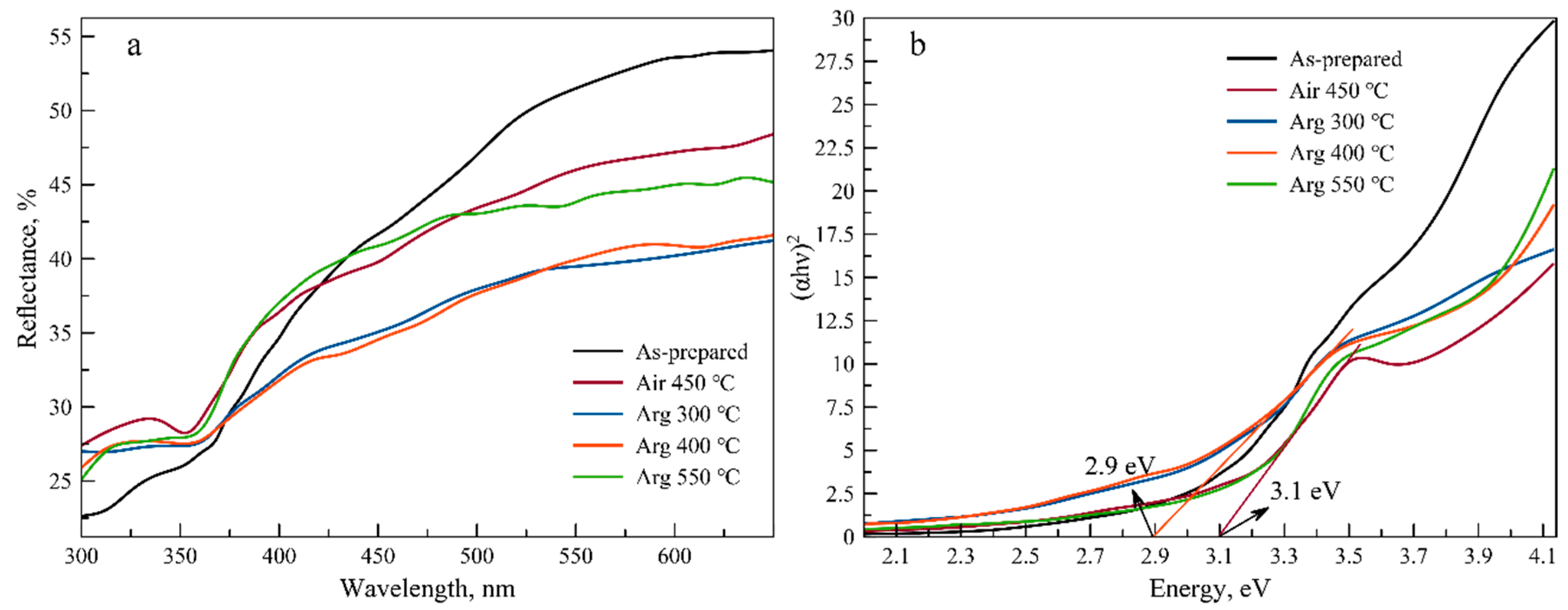
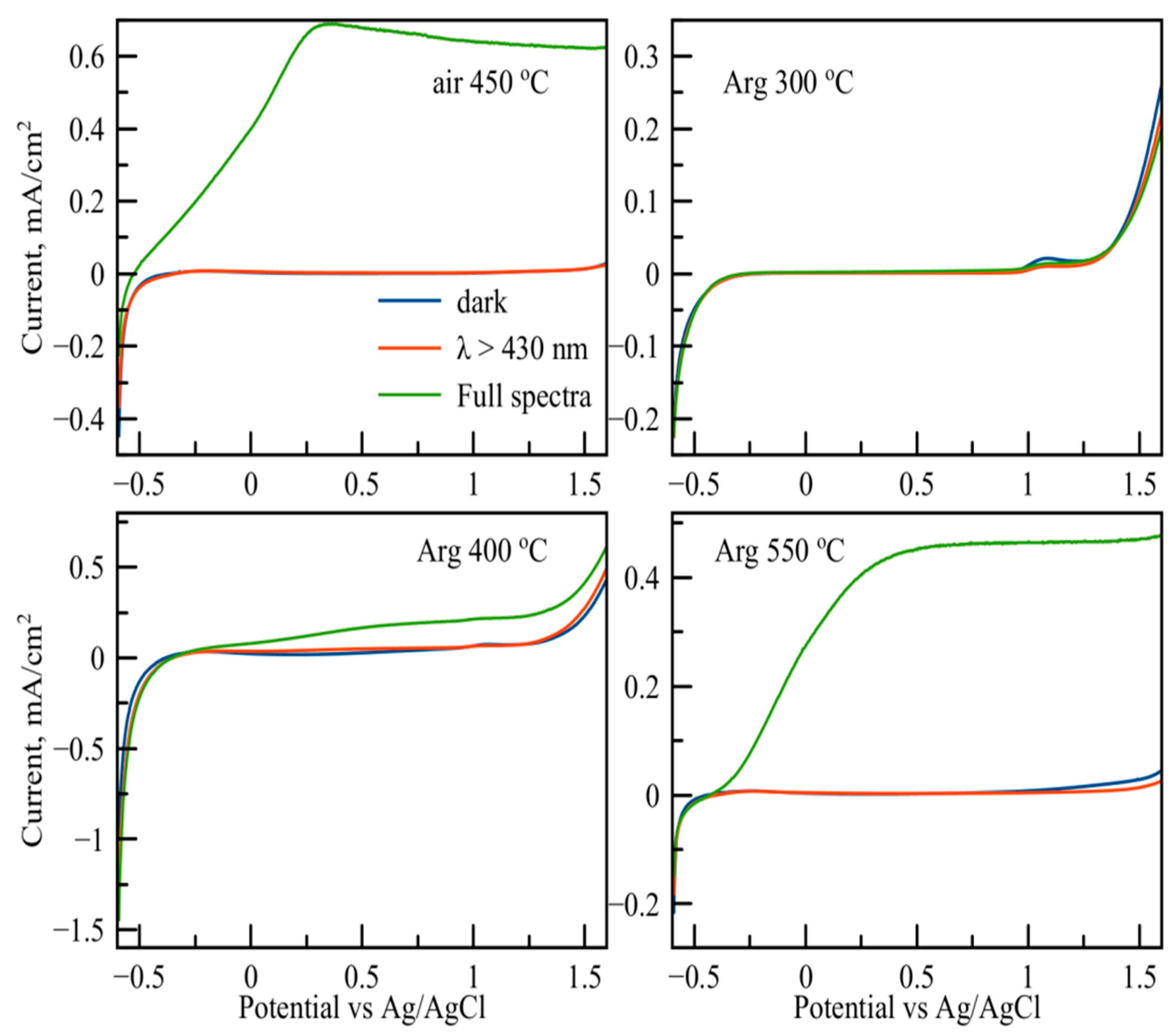
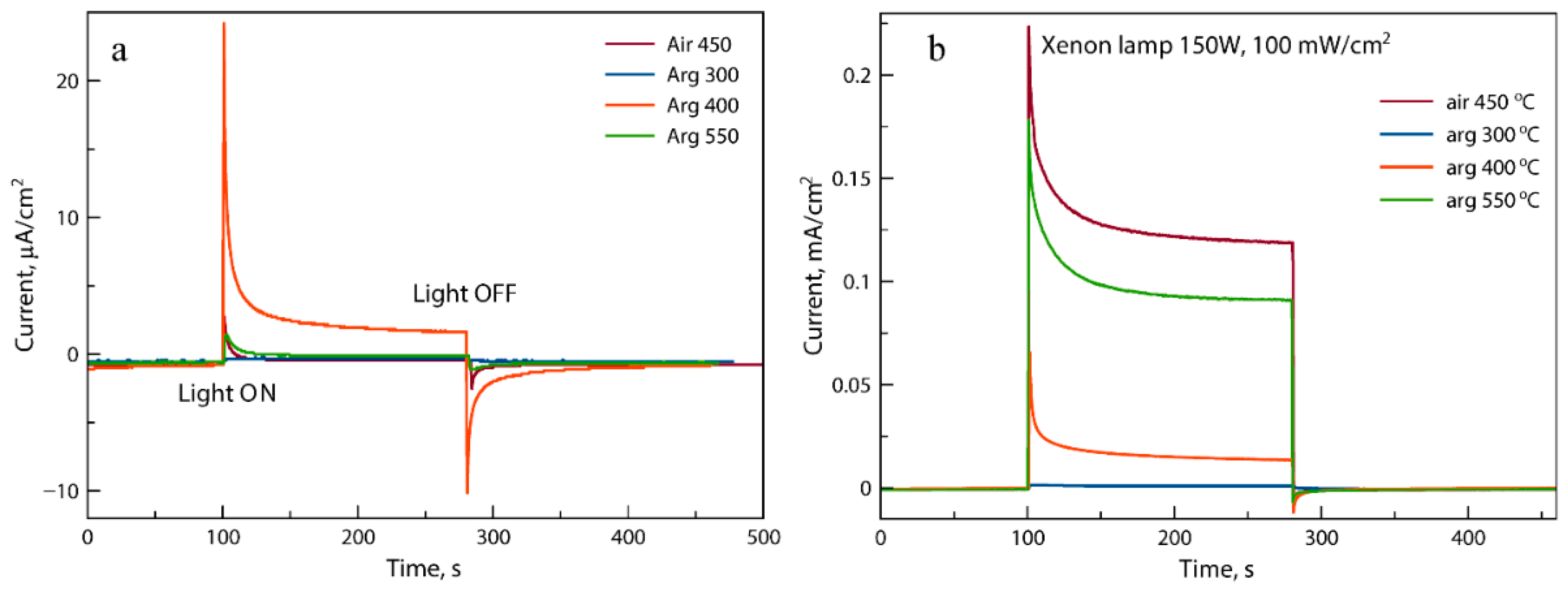
3.3. Functional Properties Investigation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic Conversions of CO2 to Help Mitigate Climate Change: Recent Process Developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product Selectivity of Photocatalytic CO2 Reduction Reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Zhang, Z.; Dong, Z.; Xu, J. 2D/2D CsPbBr3/BiOCl Heterojunction with an S-Scheme Charge Transfer for Boosting the Photocatalytic Conversion of CO2. Inorg. Chem. 2022, 61, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- Barrocas, B.T.; Ambrožová, N.; Kočí, K. Photocatalytic Reduction of Carbon Dioxide on TiO2 Heterojunction Photocatalysts—A Review. Materials 2022, 15, 967. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; Arias, D.M.; Rodríguez-González, C.; Sebastian, P.J. A Critical Review on Advances in TiO2-Based Photocatalytic Systems for CO2 Reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.T.; Bi, Z.X.; Chen, X.; Hu, X.; Pan, W.G. A Review on TiO2−x-Based Materials for Photocatalytic CO2 Reduction. Nanoscale 2022, 14, 11512–11528. [Google Scholar] [CrossRef]
- Palmas, S.; Mais, L.; Mascia, M.; Vacca, A. Trend in Using TiO2 Nanotubes as Photoelectrodes in PEC Processes for Wastewater Treatment. Curr. Opin. Electrochem. 2021, 28, 100699. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.M.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of Anodized TiO2 Nanotube Arrays on the Removal of Aqueous Contaminants of Emerging Concern: A Review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble Metal Modified TiO2 Microspheres: Surface Properties and Photocatalytic Activity under UV–Vis and Visible Light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Enachi, M.; Guix, M.; Braniste, T.; Postolache, V.; Ciobanu, V.; Ursaki, V.; Schmidt, O.G.; Tiginyanu, I. Photocatalytic Properties of TiO2 Nanotubes Doped with Ag, Au and Pt or Covered by Ag, Au and Pt Nanodots. Surf. Eng. Appl. Electrochem. 2015, 51, 3–8. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Murugananthan, M.; Zhang, Y.; Zhang, L. Electrochemically Self-Doped WO3/TiO2 Nanotubes for Photocatalytic Degradation of Volatile Organic Compounds. Appl. Catal. B Environ. 2020, 260, 118205. [Google Scholar] [CrossRef]
- Rimoldi, L.; Giordana, A.; Cerrato, G.; Falletta, E.; Meroni, D. Insights on the Photocatalytic Degradation Processes Supported by TiO2/WO3 Systems. The Case of Ethanol and Tetracycline. Catal. Today 2019, 328, 210–215. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, D.D.; Xu, L.S.; Chang, C.T. Photocatalytic Conversion of Terephthalic Acid Preparation Wastewater to Hydrogen by Graphene-Modified TiO2. Catal. Today 2016, 274, 8–14. [Google Scholar] [CrossRef]
- Masoud, M.; Nourbakhsh, A.; Hassanzadeh-Tabrizi, S.A. Influence of Modified CNT-Ag Nanocomposite Addition on Photocatalytic Degradation of Methyl Orange by Mesoporous TiO2. Inorg. Nano-Metal Chem. 2017, 47, 1168–1174. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Zhang, Z.; Jiang, Y.; Chu, Y.; Xu, J. Embedding CsPbBr3 Perovskite Quantum Dots into Mesoporous TiO2 Beads as an S-Scheme Heterojunction for CO2 Photoreduction. Chem. Eng. J. 2022, 433, 133762. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, J.; Zhang, Z.; Jiang, Y.; Zhou, R.; Yao, C. Construction of a P-n Type S-Scheme Heterojunction by Incorporating CsPbBr3Nanocrystals into Mesoporous Cu2O Microspheres for Efficient CO2Photoreduction. ACS Appl. Energy Mater. 2022, 5, 10076–10085. [Google Scholar] [CrossRef]
- Mohamed, A.E.R.; Barghi, S.; Rohani, S. N- and C-Modified TiO2 Nanotube Arrays: Enhanced Photoelectrochemical Properties and Effect of Nanotubes Length on Photoconversion Efficiency. Nanomaterials 2018, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.W.; Lee, J.Y.; Ahn, K.S.; Kang, S.H.; Kim, J.H. Visible Light Absorbing TiO2 Nanotube Arrays by Sulfur Treatment for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2015, 119, 13375–13383. [Google Scholar] [CrossRef]
- Saud, P.S.; Pant, B.; Alam, A.M.; Ghouri, Z.K.; Park, M.; Kim, H.Y. Carbon Quantum Dots Anchored TiO2 Nanofibers: Effective Photocatalyst for Waste Water Treatment. Ceram. Int. 2015, 41, 11953–11959. [Google Scholar] [CrossRef]
- Savchuk, T.; Yakubov, A.; Gavrilin, I.; Dronova, D.; Dronov, A. Influence of Thermal Post-Treatment on Electrophysical Properties of Carbon Modified Anodic TiO2 NTs. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Saint Petersburg and Moscow, Moscow, Russia, 28–31 January 2019; pp. 1970–1972. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Zhao, L.; Titirici, M.M. Solvothermal Carbon-Doped TiO2 Photocatalyst for the Enhanced Methylene Blue Degradation under Visible Light. Appl. Catal. A Gen. 2010, 390, 175–182. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In Situ Synthesis of Carbon-Doped TiO2 Single-Crystal Nanorods with a Remarkably Photocatalytic Efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Gao, Z.D.; Zhu, X.; Li, Y.H.; Zhou, X.; Song, Y.Y.; Schmuki, P. Carbon Cladded TiO2 Nanotubes: Fabrication and Use in 3D-RuO2 Based Supercapacitors. Chem. Commun. 2015, 51, 7614–7617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Huo, K.; Chen, R.; Gao, B.; Fu, J.; Chu, P.K. Recyclable and High-Sensitivity Electrochemical Biosensing Platform Composed of Carbon-Doped TiO2 Nanotube Arrays. Anal. Chem. 2011, 83, 8138–8144. [Google Scholar] [CrossRef]
- Song, Y.Y.; Li, Y.H.; Guo, J.; Gao, Z.D.; Li, Y. Facile Method to Synthesize a Carbon Layer Embedded into Titanium Dioxide Nanotubes with Metal Oxide Decoration for Electrochemical Applications. J. Mater. Chem. A 2015, 3, 23754–23759. [Google Scholar] [CrossRef]
- Chen, B.; Haring, A.J.; Beach, J.A.; Li, M.; Doucette, G.S.; Morris, A.J.; Moore, R.B.; Priya, S. Visible Light Induced Photocatalytic Activity of Fe3+/Ti3+ Co-Doped TiO2 Nanostructures. RSC Adv. 2014, 4, 18033–18037. [Google Scholar] [CrossRef]
- Gavrilin, I.; Dronov, A.; Volkov, R.; Savchuk, T.; Dronova, D.; Borgardt, N.; Pavlikov, A.; Gavrilov, S.; Gromov, D. Differences in the Local Structure and Composition of Anodic TiO2 Nanotubes Annealed in Vacuum and Air. Appl. Surf. Sci. 2020, 516, 146120. [Google Scholar] [CrossRef]
- Dronov, A.; Gavrilin, I.; Kirilenko, E.; Dronova, D.; Gavrilov, S. Investigation of Anodic TiO2 Nanotube Composition with High Spatial Resolution AES and ToF SIMS. Appl. Surf. Sci. 2018, 434, 148–154. [Google Scholar] [CrossRef]
- Savchuk, T.; Gavrilin, I.; Konstantinova, E.; Dronov, A.; Volkov, R.; Borgardt, N.; Maniecki, T.; Gavrilov, S.; Zaitsev, V. Anodic TiO2 nanotube Arrays for Photocatalytic CO2 conversion: Comparative Photocatalysis and EPR Study. Nanotechnology 2022, 33, 055706. [Google Scholar] [CrossRef]
- Varghese, O.K.; Grimes, C.A. Appropriate Strategies for Determining the Photoconversion Efficiency of Water Photoelectrolysis Cells: A Review with Examples Using Titania Nanotube Array Photoanodes. Sol. Energy Mater. Sol. Cells 2008, 92, 374–384. [Google Scholar] [CrossRef]
- Khan, T.T.; Rafiqul Bari, G.A.K.M.; Kang, H.J.; Lee, T.G.; Park, J.W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent Visible Light Responsive Photocatalytic Behavior of N-Doped TiO2 toward Decontamination of Organic Pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Tamarit, E.; Solsona, B.; Sánchez-Tovar, R.; García-García, D.; Fernández-Domene, R.M.; García-Antón, J. Influence of Annealing Atmosphere on Photoelectrochemical Response of TiO2 Nanotubes Anodized under Controlled Hydrodynamic Conditions. J. Electroanal. Chem. 2021, 897, 115579. [Google Scholar] [CrossRef]
- Pérez, E.; Torres, M.F.; Morales, G.; Murgia, V.; Sham, E. Synthesis of N-TiO2 Effect of the Concentration of Nitrogen in the Band Gap. Procedia Mater. Sci. 2015, 8, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Konstantinova, E.A.; Kytina, E.V.; Zaitsev, V.B.; Martyshov, M.N.; Savchuk, T.P.; Kamaleev, M.F. Photoelectron Properties of Multi-Walled and Single-Walled Titania a Nanotubes. Russ. J. Phys. Chem. B 2022, 16, 797–803. [Google Scholar] [CrossRef]
- Kwak, C.H.; Im, U.S.; Seo, S.W.; Kim, M., II; Huh, Y.S.; Im, J.S. Effects of Carbon Doping on TiO2 for Enhanced Visible Light-Driven NO Sensing Performance. Mater. Lett. 2021, 288, 129313. [Google Scholar] [CrossRef]




| Sample | Ti, at.% | O, at.% | C, at.% | N, at.% | F, at.% |
|---|---|---|---|---|---|
| Air 450 | 33.0 | 62.0 | 4.6 | 0 | 0 |
| Arg 300 | 24.6 | 55.4 | 11.2 | 2.0 | 6.2 |
| Arg 400 | 26.9 | 61.2 | 8.9 | 2.2 | 0.3 |
| Arg 550 | 30.0 | 64.0 | 5.0 | 0 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savchuk, T.; Gavrilin, I.; Savitskiy, A.; Dronov, A.; Dronova, D.; Pereverzeva, S.; Tarhanov, A.; Maniecki, T.; Gavrilov, S.; Konstantinova, E. Effect of Thermal Treatment of Symmetric TiO2 Nanotube Arrays in Argon on Photocatalytic CO2 Conversion. Symmetry 2022, 14, 2678. https://doi.org/10.3390/sym14122678
Savchuk T, Gavrilin I, Savitskiy A, Dronov A, Dronova D, Pereverzeva S, Tarhanov A, Maniecki T, Gavrilov S, Konstantinova E. Effect of Thermal Treatment of Symmetric TiO2 Nanotube Arrays in Argon on Photocatalytic CO2 Conversion. Symmetry. 2022; 14(12):2678. https://doi.org/10.3390/sym14122678
Chicago/Turabian StyleSavchuk, Timofey, Ilya Gavrilin, Andrey Savitskiy, Alexey Dronov, Daria Dronova, Svetlana Pereverzeva, Andrey Tarhanov, Tomasz Maniecki, Sergey Gavrilov, and Elizaveta Konstantinova. 2022. "Effect of Thermal Treatment of Symmetric TiO2 Nanotube Arrays in Argon on Photocatalytic CO2 Conversion" Symmetry 14, no. 12: 2678. https://doi.org/10.3390/sym14122678
APA StyleSavchuk, T., Gavrilin, I., Savitskiy, A., Dronov, A., Dronova, D., Pereverzeva, S., Tarhanov, A., Maniecki, T., Gavrilov, S., & Konstantinova, E. (2022). Effect of Thermal Treatment of Symmetric TiO2 Nanotube Arrays in Argon on Photocatalytic CO2 Conversion. Symmetry, 14(12), 2678. https://doi.org/10.3390/sym14122678







