Absolute Configuration of Aliphatic Hydrocarbon Enantiomers Identified by Gas Chromatography: Theorized Application for Isoprenoid Alkanes and the Search of Molecular Biosignatures on Mars
Abstract
:1. Introduction
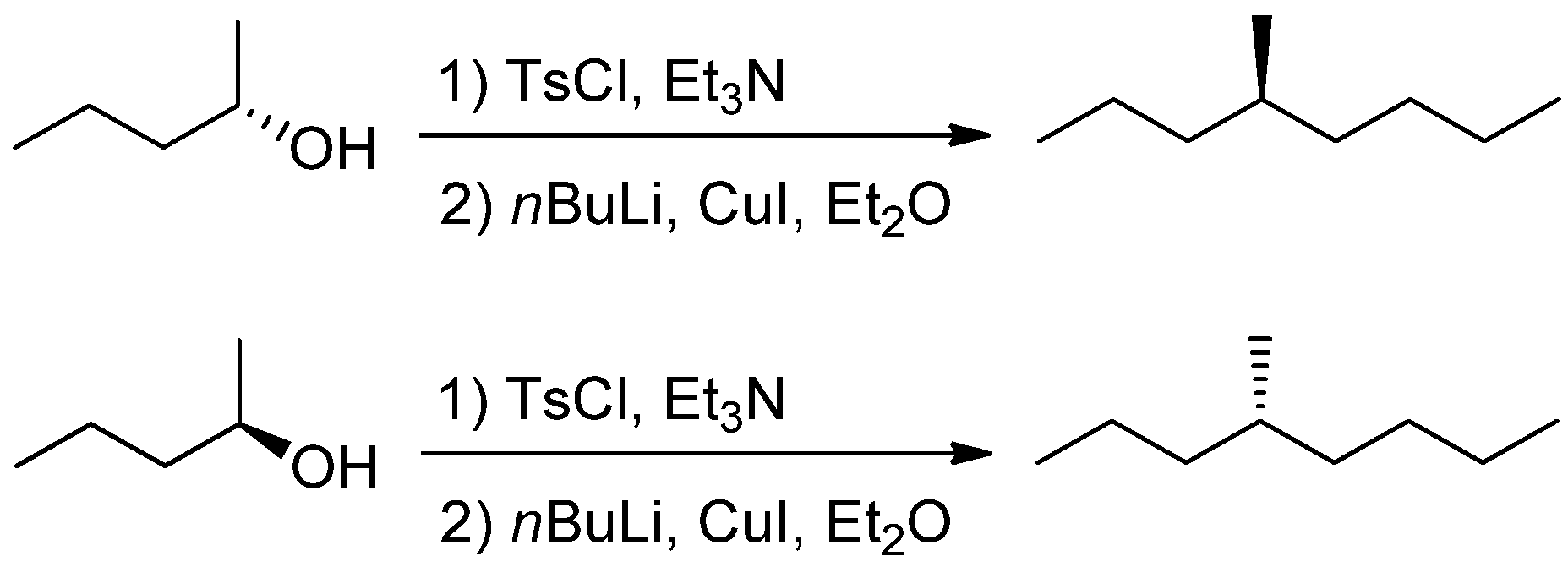
2. Materials and Methods
3. Results
4. Discussion
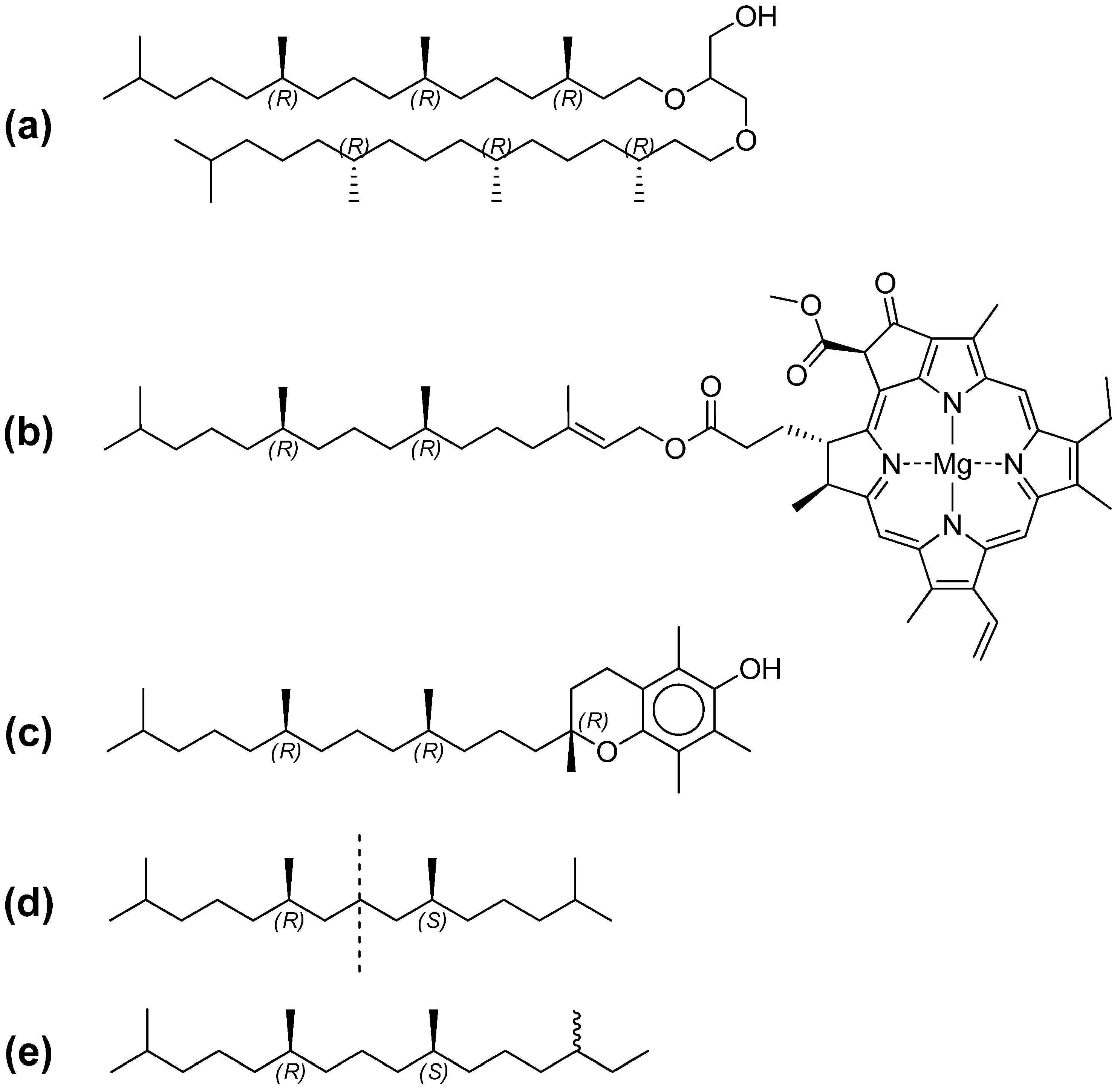
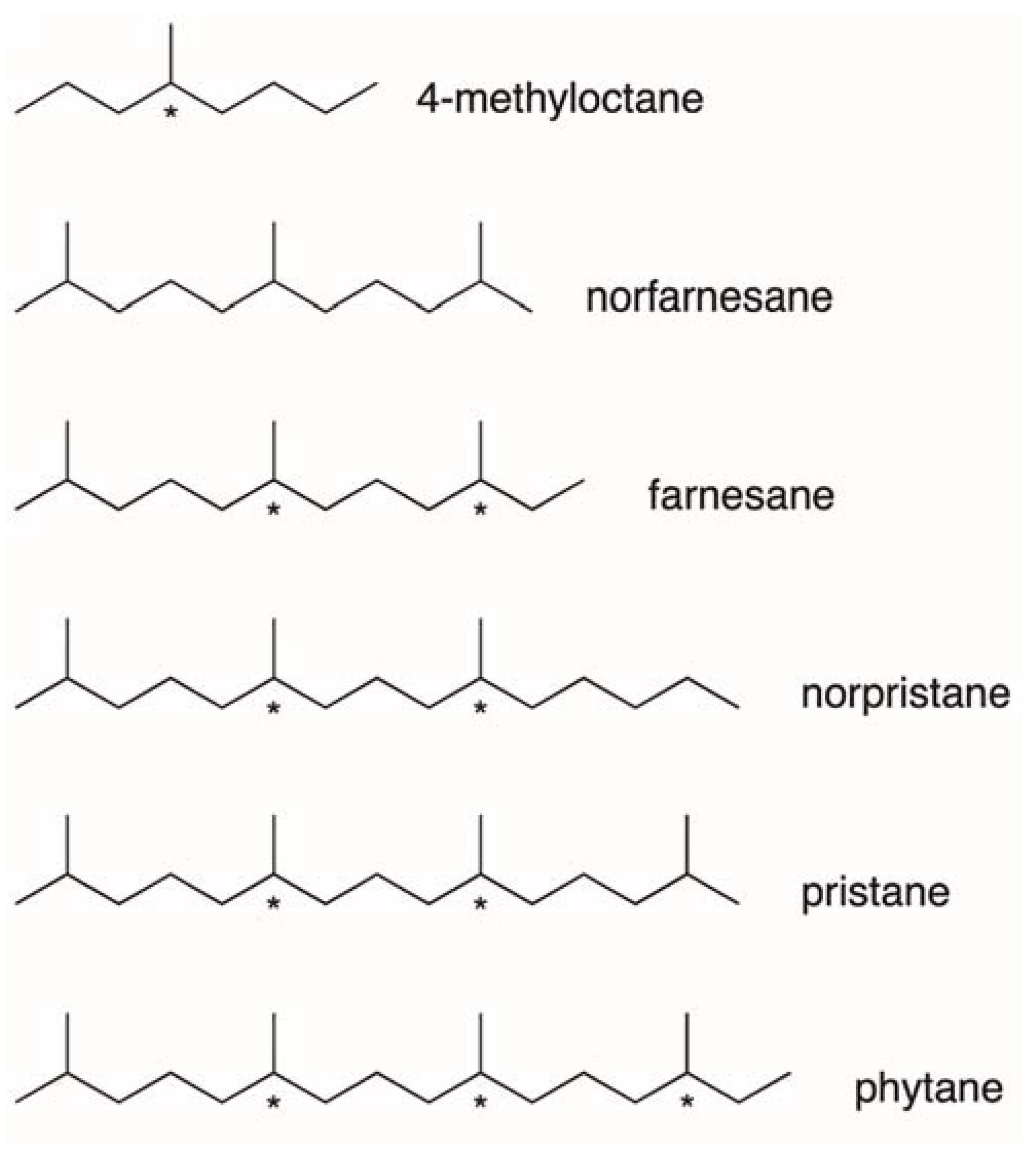
4.1. Hydrocarbon Enantiomers in Biology
4.2. Lifetime of “Molecular Fossiles”
4.3. Extraterrestrial Biology
4.4. The Chirality Part of ExoMars
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meierhenrich, U.J.; Nguyen, M.-J.; Barbier, B.; Brack, A.; Thiemann, W.H.-P. Gas chromatographic separation of saturated aliphatic hydrocarbon enantiomers on permethylated cyclodextrin. Chirality 2003, 15, S13–S16. [Google Scholar] [CrossRef]
- Sicoli, G.; Kreidler, D.; Czesla, H.; Hopf, H.; Schurig, V. Gas chromatographic enantioseparation of unfunctionalized chiral alkanes: A challenge in separation science (overview, state of the art, and perspectives). Chirality 2009, 21, 183–198. [Google Scholar] [CrossRef]
- Saito, F.; Schreiner, P.R. Determination of the Absolute Configurations of Chiral Alkanes–An Analysis of the Available Tools. Eur. J. Org. Chem. 2020, 2020, 6328–6339. [Google Scholar] [CrossRef]
- Nissenbaum, A.; Baedecker, M.J.; Kaplan, I.R. Organic geochemistry of Dead Sea sediments. Geochim. Cosmochim. Acta 1972, 36, 709–727. [Google Scholar] [CrossRef]
- Rowland, S.J. Production of acyclic isoprenoid hydrocarbons by laboratory maturation of methanogenic bacteria. Org. Geochem. 1990, 15, 9–16. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Bonin, P. Production of pristane and phytane in the marine environment: Role of prokaryotes. Res. Microbiol. 2011, 162, 923–933. [Google Scholar] [CrossRef]
- Dawson, D.; Grice, K.; Alexander, R.; Edwards, D. The effect of source and maturity on the stable isotopic compositions of individual hydrocarbons in sediments and crude oils from the Vulcan Sub-basin, Timor Sea, Northern Australia. Org. Geochem. 2007, 38, 1015–1038. [Google Scholar] [CrossRef]
- Goossens, H.; de Leeuw, J.W.; Schenck, P.A.; Brassell, S.C. Tocopherols as likely precursors of pristane in ancient sediments and crude oils. Nature 1984, 312, 440–442. [Google Scholar] [CrossRef]
- Kates, M.; Joo, C.N.; Palameta, B.; Shier, T. Absolute Stereochemical Configuration of Phytanyl (Dihydrophytyl) Groups in Lipids of Halobacterium cutirubrum. Biochemistry 1967, 6, 3329–3338. [Google Scholar] [CrossRef]
- Meierhenrich, U.J. Amino Acids and the Asymmetry of Life: Caught in the Act of Formation; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Bada, J.L.; McDonald, G.D. Amino Acid Racemization on Mars: Implications for the Preservation of Biomolecules from an Extinct Martian Biota. Icarus 1995, 114, 139–143. [Google Scholar] [CrossRef]
- Bada, J.L.; Wang, X.S.; Hamilton, H. Preservation of key biomolecules in the fossil record: Current knowledge and future challenges. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1999, 354, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Vago, J.L.; Westall, F.; Pasteur Instrument Teams, L.S.; Coates, A.J.; Jaumann, R.; Korablev, O.; Ciarletti, V.; Mitrofanov, I.; Josset, J.-L.; De Sanctis, M.C.; et al. Habitability on Early Mars and the Search for Biosignatures with the ExoMars Rover. Astrobiology 2017, 17, 471–510. [Google Scholar] [CrossRef]
- Calvin, M. Chemical evolution. Chem. Br. 1969, 5, 22–28. [Google Scholar]
- Harold, A. Illich Pristane, Phytane, and Lower Molecular Weight Isoprenoid Distributions in Oils. Am. Assoc. Pet. Geol. Bull. 1983, 67, 385–393. [Google Scholar] [CrossRef]
- Mackenzie, A.S. Applications of Biological Markers in Petroleum Geochemistry. In Advances in Petroleum Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 1, pp. 115–214. ISBN 0120320010. [Google Scholar]
- Patience, R.L.; Rowland, S.J.; Maxwell, J.R. The effect of maturation on the configuration of pristane in sediments and petroleum. Geochim. Cosmochim. Acta 1978, 42, 1871–1875. [Google Scholar] [CrossRef]
- Gassmann, G. Chromatographic separation of diasteriomeric isoprenoids for the identification of fossil oil contamination. Mar. Pollut. Bull. 1981, 12, 78–84. [Google Scholar] [CrossRef]
- van Graas, G.; de Leeuw, J.W.; Schenck, P.A.; Haverkamp, J. Kerogen of Toarcian shales of the Paris Basin. A study of its maturation by flash pyrolysis techniques. Geochim. Cosmochim. Acta 1981, 45, 2465–2474. [Google Scholar] [CrossRef]
- Summons, R.E. The Exceptional Preservation of Interesting and Informative Biomolecules. Paleontol. Soc. Pap. 2014, 20, 217–236. [Google Scholar] [CrossRef]
- Goesmann, F.; Brinckerhoff, W.B.; Raulin, F.; Goetz, W.; Danell, R.M.; Getty, S.A.; Siljeström, S.; Mißbach, H.; Steininger, H.; Arevalo, R.D.; et al. The Mars Organic Molecule Analyzer (MOMA) Instrument: Characterization of Organic Material in Martian Sediments. Astrobiology 2017, 17, 655–685. [Google Scholar] [CrossRef]
- Guzman, M.; Szopa, C.; Freissinet, C.; Buch, A.; Stalport, F.; Kaplan, D.; Raulin, F. Testing the capabilities of the Mars Organic Molecule Analyser (MOMA) chromatographic columns for the separation of organic compounds on Mars. Planet. Space Sci. 2020, 186, 104903. [Google Scholar] [CrossRef]
- Reinhardt, M.; Goetz, W.; Thiel, V. Testing Flight-like Pyrolysis Gas Chromatography–Mass Spectrometry as Performed by the Mars Organic Molecule Analyzer Onboard the ExoMars 2020 Rover on Oxia Planum Analog Samples. Astrobiology 2020, 20, 415–428. [Google Scholar] [CrossRef]
- Summons, R.E.; Albrecht, P.; McDonald, G.D.; Moldowan, J.M. Molecular Biosignatures. Space Sci. Rev. 2008, 135, 133–159. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Y.; Wang, Y.; Lei, T.; Liu, Y.; Liu, Y.; Hou, X.; Wang, Y. The Geochemical Characteristics of Coals from the Junggar Basin in Northwest China and the Relation of the Configuration of Pristane with Maturity in Highly Mature and Over-Mature Samples. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2016, 71, 35. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.R.; Cox, R.E.; Eglinton, G.; Pillinger, C.T.; Ackman, R.G.; Hooper, S.N. Stereochemical studies of acyclic isoprenoid compounds—II The role of chlorophyll in the derivation of isoprenoid-type acids in a lacustrine sediment. Geochim. Cosmochim. Acta 1973, 37, 297–313. [Google Scholar] [CrossRef]
- Püttmann, W.; Eckardt, C.B. Influence of an intrusion on the extent of isomerism in acyclic isoprenoids in the Permian Kupferschiefer of the Lower Rhine Basin, N.W. Germany. Org. Geochem. 1989, 14, 651–658. [Google Scholar] [CrossRef]
- Patience, R.L.; Yon, D.A.; Ryback, G.; Maxwell, J.R. Acyclic isoprenoid alkanes and geochemical maturation. Phys. Chem. Earth 1980, 12, 287–293. [Google Scholar] [CrossRef]
- McIntyre, C.P.; Harvey, P.M.; Ferguson, S.H.; Wressnig, A.M.; Volk, H.; George, S.C.; Snape, I. Determining the Extent of Biodegradation of Fuels Using the Diastereomers of Acyclic Isoprenoids. Environ. Sci. Technol. 2007, 41, 2452–2458. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Tang, Y.; Zukowski, J. Resolution of enantiomeric hydrocarbon biomarkers of geochemical importance. Anal. Chem. 1991, 63, 2858–2861. [Google Scholar] [CrossRef]
- Schurig, V.; Kreidler, D. Gas-Chromatographic Enantioseparation of Unfunctionalized Chiral Hydrocarbons: An Overview. Methods Mol. Biol. 2013, 970, 45–67, ISBN 9781627032629. [Google Scholar]
- Meierhenrich, U.J.; Thiemann, W.H.-P.; Rosenbauer, H. Molecular parity violation via comets? Chirality 1999, 11, 575–582. [Google Scholar] [CrossRef]
- Meierhenrich, U.J. Comets and Their Origin; Meierhenrich, U., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; ISBN 9783527412778. [Google Scholar]
- Meierhenrich, U.J.; Thiemann, W.H.-P.; Goesmann, F.; Roll, R.; Rosenbauer, H. Enantiomer separation of hydrocarbons in preparation for ROSETTA’s chirality-experiment. Chirality 2001, 13, 454–457. [Google Scholar] [CrossRef]
- König, W.A.; Icheln, D.; Runge, T.; Pforr, I.; Krebs, A. Cyclodextrins as chiral stationary phases in capillary gas chromatography. Part VII: Cyclodextrins with an inverse substitution pattern-synthesis and enantioselectivity. J. High Resolut. Chromatogr. 1990, 13, 702–707. [Google Scholar] [CrossRef]
- Freissinet, C.; Buch, A.; Szopa, C.; Sternberg, R. Enantiomeric separation of volatile organics by gas chromatography for the in situ analysis of extraterrestrial materials: Kinetics and thermodynamics investigation of various chiral stationary phases. J. Chromatogr. A 2013, 1306, 59–71. [Google Scholar] [CrossRef]
- Johnson, C.R.; Dutra, G.A. Reactions of lithium diorganocuprates(I) with tosylates. II. Stereochemical, kinetic, and mechanistic aspects. J. Am. Chem. Soc. 1973, 95, 7783–7788. [Google Scholar] [CrossRef]
- Schurig, V.; Nowotny, H.-P. Gas Chromatographic Separation of Enantiomers on Cyclodextrin Derivatives. Angew. Chem. Int. Ed. Engl. 1990, 29, 939–957. [Google Scholar] [CrossRef]
- Sicoli, G.; Jiang, Z.; Jicsinsky, L.; Schurig, V. Modified Linear Dextrins (“Acyclodextrins”) as New Chiral Selectors for the Gas-Chromatographic Separation of Enantiomers. Angew. Chem. Int. Ed. 2005, 44, 4092–4095. [Google Scholar] [CrossRef]
- Huang, K.; Armstrong, D.W. GC–MS analysis of crocetane, phytane and some of their stereoisomers using cyclodextrin-based stationary phases. Org. Geochem. 2009, 40, 283–286. [Google Scholar] [CrossRef]
- Cox, R.E.; Maxwell, J.R.; Ackman, R.G.; Hooper, S.N. Stereochemical Studies of Acyclic Isoprenoid Compounds. III. The Stereochemistry of Naturally Occurring (Marine) 2,6,10,14-Tetramethylpentadecane. Can. J. Biochem. 1972, 50, 1238–1241. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, MA, USA, 2004; ISBN 9780521781589. [Google Scholar]
- Koga, Y. Thermal Adaptation of the Archaeal and Bacterial Lipid Membranes. Archaea 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Matsumi, R.; Atomi, H.; Driessen, A.J.M.; van der Oost, J. Isoprenoid biosynthesis in Archaea–Biochemical and evolutionary implications. Res. Microbiol. 2011, 162, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, Y. Early Evolution of Membrane Lipids: How did the Lipid Divide Occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef]
- Wächtershäuser, G. From pre-cells to Eukarya—A tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woese, C.R.; Magrum, L.J.; Fox, G.E. Archaebacteria. J. Mol. Evol. 1978, 11, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rontani, J.-F.; Volkman, J.K. Phytol degradation products as biogeochemical tracers in aquatic environments. Org. Geochem. 2003, 34, 1–35. [Google Scholar] [CrossRef]
- Bucke, C.; Leech, R.M.; Hallaway, M.; Morton, R.A. The taxonomic distribution of plastoquinone and tocopherolquinone and their intracellular distribution in leaves of Vicia faba L. Biochim. Biophys. Acta Biophys. Incl. Photosynth. 1966, 112, 19–34. [Google Scholar] [CrossRef]
- Hughes, P.E.; Tove, S.B. Occurrence of alpha-tocopherolquinone and alpha-tocopherolquinol in microorganisms. J. Bacteriol. 1982, 151, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nip, M. Chemical Characterization of Coals, Coal Macerals and their Precursors: A Study by Analytical Pyrolysis; Technische Universiteit Delft: Delft, The Netherlands, 1987. [Google Scholar]
- Meierhenrich, U.J.; Thiemann, W.H.-P.; Barbier, B.; Schubert, C.J.; Brack, A. Isoprenoid enantiomers as molecular biomarkers in ancient sediments. In Proceedings of the Geochemistry and the Origin of Life; Nakashima, S., Maruyama, S., Brack, A., Windley, B.F., Eds.; Universal Academy Press: Tokyo, Japan, 2001; pp. 269–284. [Google Scholar]
- Kates, M. Biology of halophilic bacteria, Part II. Experientia 1993, 49, 1027–1036. [Google Scholar] [CrossRef]
- Jordan, S.F.; Nee, E.; Lane, N. Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide. Interface Focus 2019, 9, 20190067. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Irwin, L.N.; Lipps, J.H.; LeMone, D.; Dohm, J.M.; Fairén, A.G. Scenarios for the evolution of life on Mars. J. Geophys. Res. 2005, 110, E12S23. [Google Scholar] [CrossRef] [Green Version]
- Cronin, J.R.; Pizzarello, S. Aliphatic hydrocarbons of the Murchison meteorite. Geochim. Cosmochim. Acta 1990, 54, 2859–2868. [Google Scholar] [CrossRef]
- Kissin, Y.V. Hydrocarbon components in carbonaceous meteorites. Geochim. Cosmochim. Acta 2003, 67, 1723–1735. [Google Scholar] [CrossRef]
- Freissinet, C.; Glavin, D.P.; Mahaffy, P.R.; Miller, K.E.; Eigenbrode, J.L.; Summons, R.E.; Brunner, A.E.; Buch, A.; Szopa, C.; Archer, P.D.; et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J. Geophys. Res. Planets 2015, 120, 495–514. [Google Scholar] [CrossRef] [Green Version]
- Risatti, J.B.; Rowland, S.J.; Yon, D.A.; Maxwell, J.R. Stereochemical studies of acyclic isoprenoids—XII. Lipids of methanogenic bacteria and possible contributions to sediments. Org. Geochem. 1984, 6, 93–104. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leseigneur, G.; Filippi, J.-J.; Baldovini, N.; Meierhenrich, U. Absolute Configuration of Aliphatic Hydrocarbon Enantiomers Identified by Gas Chromatography: Theorized Application for Isoprenoid Alkanes and the Search of Molecular Biosignatures on Mars. Symmetry 2022, 14, 326. https://doi.org/10.3390/sym14020326
Leseigneur G, Filippi J-J, Baldovini N, Meierhenrich U. Absolute Configuration of Aliphatic Hydrocarbon Enantiomers Identified by Gas Chromatography: Theorized Application for Isoprenoid Alkanes and the Search of Molecular Biosignatures on Mars. Symmetry. 2022; 14(2):326. https://doi.org/10.3390/sym14020326
Chicago/Turabian StyleLeseigneur, Guillaume, Jean-Jacques Filippi, Nicolas Baldovini, and Uwe Meierhenrich. 2022. "Absolute Configuration of Aliphatic Hydrocarbon Enantiomers Identified by Gas Chromatography: Theorized Application for Isoprenoid Alkanes and the Search of Molecular Biosignatures on Mars" Symmetry 14, no. 2: 326. https://doi.org/10.3390/sym14020326




