Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions
Abstract
:1. Review Summary
2. Prebiotic Chemistry, Chirality, and the Origins of Life
2.1. Chirality, Homochirality, and Enantiomeric Excess
2.2. Chirality in a Prebiotic Context
2.3. Enantiomeric Excess as a Biosignature
3. Organic Chirality
3.1. Point/Molecular Chirality
3.2. Heteroatom Chirality
3.3. Additional Forms of Chirality
3.4. Asymmetric Organic Synthesis Reactions
4. Organometallic Systems Related to Chirality
5. Minerals Relevant to Prebiotic Chemistry
6. Mineral Chirality
6.1. Enantiomorphic Minerals
6.2. Achiral Minerals with Chiral Faces
6.3. Local Chiral Sites
6.4. Factors That Influence a Mineral’s Degree of Enantioselectivity
6.5. Mineral–Organic Interactions for Driving Enantiomeric Excess
6.6. Future Directions
7. Alteration during Geochemical Processes
7.1. Terrestrial Geochemical Alteration and the Preservation of Organics
7.2. Formation of Insoluble Macromolecular Organic Matter
7.3. Stereochemistry of Lipids in Kerogen
7.4. Compositional Alteration on Other Planetary Bodies
8. Laboratory Analysis and Reactions
8.1. Analytical Instrumentation
8.2. Solution Phase Reactions
8.2.1. Reductive Amination
8.2.2. Strecker Synthesis
8.2.3. Formose Reaction
8.3. Solid-State Reactions
9. Recommendations for Future Research
9.1. Capabilities of Flight-Ready Technology
9.1.1. COSAC on Rosetta’s Philae Lander
9.1.2. SAM on MSL’s Curiosity Rover
9.1.3. MOMA on ExoMars’ Rosalind Franklin Rover
9.2. Next Generation Instrumentation
9.3. Contamination Control
9.4. Future Directions
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cafferty, B.J.; Gállego, I.; Chen, M.C.; Farley, K.I.; Eritja, R.; Hud, N.V. Efficient self-assembly in water of long noncovalent polymers by nucleobase analogues. J. Am. Chem. Soc. 2013, 135, 2447–2450. [Google Scholar] [CrossRef]
- Novikov, Y.; Copley, S.D. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 13283–13288. [Google Scholar] [CrossRef] [Green Version]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 2019, 569, 104–107. [Google Scholar] [CrossRef]
- Barge, L.M.; Flores, E.; Baum, M.M.; VanderVelde, D.G.; Russell, M.J. Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl. Acad. Sci. USA 2019, 116, 4828–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barge, L.M.; Flores, E.; VanderVelde, D.G.; Weber, J.M.; Baum, M.M.; Castonguay, A. Effects of geochemical and environmental parameters on abiotic organic chemistry driven by iron hydroxide minerals. JGR Planets 2020, 125, e2020JE006423. [Google Scholar] [CrossRef]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Takai, K.; Yoshida, N.; Oono, Y. Metals likely promoted protometabolism in early ocean alkaline hydrothermal systems. Sci. Adv. 2019, 5, eaav7848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fani, R. The origin and evolution of metabolic pathways: Why and how did primordial cells construct metabolic routes? Evol. Educ. Outreach 2012, 5, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Preiner, M.; Xavier, J.C.; do Nascimento Vieria, A.; Kleinermanns, K.; Allen, J.F.; Martin, W.F. Catalysts, autocatalysis and the origin of metabolism. Interface Focus 2019, 9, 20190027. [Google Scholar] [CrossRef]
- Vogt, P.F.; Miller, M.J. Development and applications of amino acid-derived chiral acylnitroso hetero Diels-Alder reactions. Tetrahedron 1998, 54, 1317–1348. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Y. The development and application of chiral trisoxazolines in asymmetric catalysis and molecular recognition. Chem. Soc. Rev. 2005, 34, 664–676. [Google Scholar] [CrossRef]
- Smith, D.K. Lost in translation? Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem. Soc. Rev. 2009, 38, 684–694. [Google Scholar] [CrossRef]
- Li, Y.; Pan, B.; He, X.; Xia, W.; Zhang, Y.; Liang, H.; Subba Reddy, C.V.; Cao, R.; Qiu, L. Pd-catalyzed asymmetric Suzuki–Miyaura coupling reactions for the synthesis of chiral biaryl compounds with a large steric substituent at the 2-position. Beilstein J. Org. Chem. 2020, 16, 966–973. [Google Scholar] [CrossRef]
- Burton, A.S.; Berger, E.L. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Blackmond, D.G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 2019, 11, a032540. [Google Scholar] [CrossRef] [Green Version]
- Sczepanski, J.T.; Joyce, G.F. A cross-chiral RNA polymerase ribozyme. Nature 2014, 515, 440–442. [Google Scholar] [CrossRef] [Green Version]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dworkin, J.P. The search for chiral asymmetry as a potential biosignature in our Solar System. Chem. Rev. 2020, 120, 4660–4689. [Google Scholar] [CrossRef] [Green Version]
- Ehrenfreund, P.; Irvine, W.; Becker, L.; Blank, J.; Brucato, J.R.; Colangeli, L.; Derenne, S.; Despois, D.; Dutrey, A.; Fraaije, H. Astrophysical and astrochemical insights into the origin of life. Rep. Prog. Phys. 2002, 65, 1427. [Google Scholar] [CrossRef] [Green Version]
- Kwok, S. The synthesis of organic and inorganic compounds in evolved stars. Nature 2004, 430, 985–991. [Google Scholar] [CrossRef]
- Kwok, S. Organic matter in space: From star dust to the Solar System. Astrophys. Space Sci. 2008, 319, 5–21. [Google Scholar] [CrossRef]
- Kwok, S. Complex organics in space from Solar System to distant galaxies. Astron. Astrophys. Rev. 2016, 24, 8. [Google Scholar] [CrossRef] [Green Version]
- Ehrenfreund, P.; Cami, J. Cosmic carbon chemistry: From the interstellar medium to the early Earth. Cold Spring Harb. Perspect. Biol. 2010, 2, a002097. [Google Scholar] [CrossRef] [Green Version]
- Ehrenfreund, P.; Charnley, S.B. Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the Early Earth. Annu. Rev. Astron. Astrophys. 2000, 38, 427–483. [Google Scholar] [CrossRef]
- Brandenburg, A. Homochirality: A prerequisite or consequence of life? In Prebiotic Chemistry and the Origin of Life. Advances in Astrobiology and Biogeophysics; Neubeck, A., McMahon, S., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Frank, F.C. 1953. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Soai, K.; Osanai, S.; Kadowaki, K.; Yonekubo, S.; Shibata, T.; Sato, I. d- and l-quartz-promoted highly enantioselective synthesis of a chiral organic compound. J. Am. Chem. Soc. 1999, 121, 11235–11236. [Google Scholar] [CrossRef]
- Orme, C.A.; Noy, A.; Wierzbicki, A.; McBride, M.T.; Grantham, M.; Teng, H.H.; Dove, P.M.; DeYoreo, J.J. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 2001, 411, 775–779. [Google Scholar] [CrossRef]
- Wagner, A.J.; Zubarev, D.Y.; Aspuru-Guzik, A.; Blackmond, D.G. Chiral sugars drive enantioenrichment in prebiotic amino acid synthesis. ACS Cent. Sci. 2017, 3, 322–328. [Google Scholar] [CrossRef]
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef]
- Takano, Y.; Takahashi, J.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett. 2007, 254, 106–114. [Google Scholar] [CrossRef]
- Modica, P.; Meinert, C.; de Marcellus, P.; Nahon, L.; Meierhenrich, U.J.; Le Sargent d’Hendecourt, L. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys. J. 2014, 788, 79. [Google Scholar] [CrossRef]
- Pizzarello, S.; Wang, Y.; Chaban, G.M. A comparative study of the hydroxy acids from the Murchison, GRA 95229 and LAP 02342 meteorites. Geochim. Cosmochim. Acta 2010, 74, 6206–6217. [Google Scholar] [CrossRef]
- Aponte, J.C.; Elsila, J.E.; Hein, J.E.; Dworkin, J.P.; Glavin, D.P.; McLain, H.L.; Parker, E.T.; Cao, T.; Berger, E.L.; Burton, A.S. Analysis of amino acids, hydroxy acids, and amines in CR chondrites. Meteorit Planet Sci. 2020, 55, 2422–2439. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–959. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non- racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Alanine enantiomers in the Murchison meteorite. Nature 1998, 394, 236. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Pizzarello, S.; Zolensky, M.; Turk, K.A. Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid l-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [Green Version]
- Herd, C.D.K.; Blinova, A.; Simkus, D.N.; Huang, Y.; Tarozo, R.; O’D Alexander, C.M.; Gyngard, F.; Nittler, L.R.; Cody, G.D.; Fogel, M.L.; et al. Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 2011, 332, 1304–1307. [Google Scholar] [CrossRef] [Green Version]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 1948–1972. [Google Scholar] [CrossRef] [Green Version]
- Glavin, D.P.; Elsila, J.E.; Burton, A.S.; Callahan, M.P.; Dworkin, J.P.; Hilts, R.W.; Herd, C.K. Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2012, 47, 1347–1364. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11945–11954. [Google Scholar] [CrossRef] [Green Version]
- Burton, A.S.; Elsila, J.E.; Hein, J.E.; Glavin, D.P.; Dworkin, J.P. Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit. Planet. Sci. 2013, 48, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.; Rios, A.C. Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl. Acad. Sci. USA 2016, 113, E3322–E3331. [Google Scholar] [CrossRef] [Green Version]
- Thiemann, W. Life and chirality beyond the earth. Orig. Life Evol. Biosph. 1975, 6, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Neveu, M.; Hays, L.E.; Voytek, M.A.; New, M.H.; Schulte, M.D. The ladder of life detection. Astrobiology 2018, 18, 1375–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avnir, D. Critical review of chirality indicators of extraterrestrial life. New Astron. Rev. 2021, 92, 101596. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New insights into the mechanisms and biological roles of D-amino acids in complex ecosystems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasabe, J.; Suzuki, M. Emerging role of D-amino acid metabolism in the innate defense. Front. Microbiol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zheng, Q.; Zhang, S.; Noll, L.; Wanek, W. Significant release and microbial utilization of amino sugars and D-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol. Biochem. 2018, 23, 115–125. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Klyce, B.; Davies, P.C.W.; Davies, P. Bacterial utilization of L-sugars and D-amino acids. Proc. SPIE 2006, 6309, 63090A. [Google Scholar] [CrossRef]
- Clayden, J.; Geeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Cross, L.C.; Klyne, W. Rules for the nomenclature of organic chemistry. Section E: Stereochemistry. Pure Appl. Chem. 1974, 45, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Gualtieri, G.; Geib, S.J.; Curran, D.P. A new class of chiral organogermanes derived from C2-symmetric dithiols: Synthesis, characterization and stereoselective free radical reactions. J. Org. Chem. 2003, 68, 5013–5019. [Google Scholar] [CrossRef]
- Böhme, U.; Wiesner, S.; Günther, B. Easy access to chiral penta- and hexacoordinate silicon compounds. Inorg. Chem. Commun. 2006, 9, 806–809. [Google Scholar] [CrossRef]
- Koga, S.; Ueki, S.; Shimada, M.; Ishii, R.; Kurihara, Y.; Yamanoi, Y.; Yuasa, J.; Kawai, T.; Uchida, T.; Iwamura, M.; et al. Access to chiral silicon centers for application to circularly polarized luminescence materials. J. Org. Chem 2017, 82, 6108–6117. [Google Scholar] [CrossRef]
- Xu, L.-W. Chapter 4—Chiral organosilicon compounds. Organosilicon Compd. Theory Exp. 2017, 145–194. [Google Scholar] [CrossRef]
- Montgomery, C.D. Factors affecting energy barriers for pyramidal inversion in amines and phosphines: A computational chemistry lab exercise. J. Chem. Educ. 2013, 90, 661–664. [Google Scholar] [CrossRef]
- Mandal, N.; Pal, A.K.; Gain, P.; Zohaib, A.; Datta, A. Transition-state-like planar structures for amine inversion with ultralong C–C bonds in diamino-o-carborane and diamino-o-dodecahedron. J. Am. Chem. Soc 2020, 142, 5331–5337. [Google Scholar] [CrossRef]
- Kölmel, C.; Ochsenfeld, C.; Ahlrichs, R. An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines. Theor. Chim. Acta 1992, 82, 271–284. [Google Scholar] [CrossRef]
- Marom, H.; Biedermann, U.; Agranat, I. Pyramidal inversion mechanism of simple chiral and achiral sulfoxides: A theoretical study. Chirality 2007, 19, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. Chiral phosphines in nucleophilic organocatalysis. Beilstein J. Org. Chem. 2014, 10, 2089–2121. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiang, S.; Zhou, X.; Ji, Y.; Xiang, B. Enantiomeric separation and determination of the enantiomeric impurity of armodafinil by capillary electrophoresis with sulfobutyl ether-β-]cyclodextrin as chiral selector. Molecules 2012, 17, 303–314. [Google Scholar] [CrossRef]
- Lemouzy, S.; Giordano, L.; Hérault, D.; Buono, G. Introducing chirality at phosphorus atoms: An update on the recent synthetic strategies for the preparation of optically pure P-stereogenic molecules. Eur. J. Org. Chem. 2020, 2020, 3351–3366. [Google Scholar] [CrossRef]
- Feng, H.-X.; Tan, R.; Liu, Y.-K. An efficient one-pot approach to the construction of chiral nitrogen-containing heterocycles under mild conditions. Org. Lett. 2015, 17, 3794–3797. [Google Scholar] [CrossRef]
- Walsh, M.P.; Phelps, J.M.; Lennon, M.E.; Yufit, D.S.; Kitching, M.O. Enantioselective synthesis of ammonium cations. Nature 2021, 597, 70–76. [Google Scholar] [CrossRef]
- Rickhaus, M.; Mayor, M.; Juríček, M. Strain-induced helical chirality in polyaromatic systems. Chem. Soc. Rev. 2016, 45, 1542–1556. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Z.; Spuling, E.; Knoll, D.M.; Lahann, J.; Bräse, S. Planar chiral [2.2]paracyclophanes: From synthetic curiosity to applications in asymmetric synthesis and materials. Chem. Soc. Rev. 2018, 47, 6947–6963. [Google Scholar] [CrossRef]
- Noyori, R.; Takaya, H. BINAP: An efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 1990, 23, 345–350. [Google Scholar] [CrossRef]
- Welch, C.J.; Biba, M.; Sajonz, P. Fast methods of enantiopurity determination for the Soai reaction: Towards a general enantioenrichment detector? Chirality 2007, 19, 34–43. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Feng, X. Asymmetric Strecker reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef]
- Ma, D.; Tian, H.; Zou, G. Asymmetric Strecker-type reaction of α-aryl ketones. Synthesis of (S)-αM4CPG, (S)-MPPG, (S)-AIDA, and (S)-APICA, the antagonists of metabotropic glutamate receptors. J. Org. Chem. 1999, 64, 120–125. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, J. Asymmetric reductive amination. Top. Curr. Chem. 2014, 343, 261–282. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, L.; Wang, Y.-Z.; Zhang, X.; Yin, Q. Recent advances on transition-metal-catalysed asymmetric reductive amination. Org. Chem. Front. 2021, 8, 2328–2342. [Google Scholar] [CrossRef]
- Ritson, D.; Sutherland, J. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Stick, R.V.; Williams, S.J. The reactions of monosaccharides. In Carbohydrates: The Essential Molecules of Life, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 75–131. [Google Scholar] [CrossRef]
- Katsuki, T.; Sharpless, K.B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 1980, 102, 5974–5976. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Marko, I.; Mungall, W.S.; Schroeder, G.; Sharpless, K.B. Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 1988, 110, 1968–1970. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Patrick, D.W.; Truesdale, L.K.; Biller, S.A. New reaction. Stereospecific vicinal oxyamination of olefins by alkyl imido osmium compounds. J. Am. Chem. Soc. 1975, 97, 2305–2307. [Google Scholar] [CrossRef]
- Midland, M.M.; Lee, P.E. Efficient asymmetric reduction of acyl cyanides with B-3-pinanyl 9-BBN (Alpine-borane). J. Org. Chem. 1985, 50, 3237–3239. [Google Scholar] [CrossRef]
- Noyori, R.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. Asymmetric hydrogenation of β-keto carboxylic esters. A practical, purely chemical access to β-hydroxy esters in high enantiomeric purity. J. Am. Chem. Soc. 1987, 109, 5856–5858. [Google Scholar] [CrossRef]
- Hirao, A.; Itsuno, S.; Nakahama, S.; Yamazaki, N. Asymmetric reduction of aromatic ketones with chiral alkoxy-amineborane complexes. J. Chem.Soc. Chem. Commun. 1981, 7, 315–317. [Google Scholar] [CrossRef]
- Corey, E.J.; Bakshi, R.K.; Shibata, S. Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987, 109, 5551–5553. [Google Scholar] [CrossRef]
- Corey, E.J. Catalytic enantioselective Diels–Alder reactions: Methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. 2002, 41, 1650–1667. [Google Scholar] [CrossRef]
- Evans, D.A.; Chapman, K.T.; Bisaha, J. New asymmetric Diels-Alder cycloaddition reactions. Chiral.alpha.,.beta.-unsaturated carboximides as practical chiral acrylate and crotonate dienophile synthons. J. Am. Chem. Soc. 1984, 106, 4261–4263. [Google Scholar] [CrossRef]
- Kozmin, S.A.; Rawal, V.H. Chiral amino siloxy dienes in the Diels−Alder reaction: Applications to the asymmetric synthesis of 4-substituted and 4,5-disubstituted cyclohexenones and the total synthesis of (−)-α-elemene. J. Am. Chem. Soc. 1999, 121, 9562–9573. [Google Scholar] [CrossRef]
- Hashimoto, S.; Komeshima, N.; Koga, K. Asymmetric Diels–Alder reaction catalysed by chiral alkoxyaluminium dichloride. J. Chem. Soc., Chem. Commun. 1979, 10, 437–438. [Google Scholar] [CrossRef]
- Corey, E.J.; Imwinkelried, R.; Pikul, S.; Xiang, Y. Practical enantioselective Diels-Alder and aldol reactions using a new chiral controller system. J. Am. Chem. Soc. 1989, 111, 5493–5495. [Google Scholar] [CrossRef]
- Kagan, H.B.; Riant, O. Catalytic asymmetric Diels Alder reactions. Chem. Rev. 1992, 92, 1007–1019. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Crépy, K.V.L. The first asymmetric Suzuki cross-coupling reaction. Chem. Commun. 2000, 18, 1723–1724. [Google Scholar] [CrossRef]
- Schäfer, P.; Palacin, T.; Sidera, M.; Fletcher, S.P. Asymmetric Suzuki-Miyaura coupling of heterocycles via rhodium-catalysed allylic arylation of racemates. Nat. Commun. 2017, 8, 15762. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Lei, A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 2021, 50, 10058–10086. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, P.; Wang, D.; Xu, W.; Wang, H.; Xu, T. Dual Ni/photoredox-catalyzed asymmetric cross-coupling to access chiral benzylic boronic esters. Nat. Commun. 2021, 12, 1646. [Google Scholar] [CrossRef]
- Lautens, M.; Loup, J. Asymmetric reductive cross-coupling of aryl iodides with α-chloroboranes by nickel/photoredox catalysis. Synfacts 2021, 17, 0657. [Google Scholar] [CrossRef]
- Yuan, M.; Gutierrez, O. Mechanisms, challenges, and opportunities of dual Ni/photoredox-catalyzed C (sp2)–C (sp3) cross-couplings. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, e1573. [Google Scholar] [CrossRef]
- Cosgrove, S.C.; Thompson, M.P.; Ahmed, S.T.; Parmeggiani, F.; Turner, N.J. One-pot synthesis of chiral N-arylamines by combining biocatalytic aminations with Buchwald–Hartwig N-arylation. Angew. Chem. Int. Ed. 2020, 59, 18156–18160. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.-M.; Xu, Q.; Guo, C.-Q.; Wang, P.; Lu, C.-J. Enantioselective synthesis of atropisomeric biaryls by Pd-catalyzed asymmetric Buchwald–Hartwig amination. Angew. Chem. Int. Ed. 2021, 60, 21718–21722. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Wang, Z. Advances in the asymmetric total synthesis of natural products ssing chiral secondary amine catalyzed reactions of α,β-unsaturated aldehydes. Molecules 2019, 24, 3412. [Google Scholar] [CrossRef] [Green Version]
- MacMillan, D.W.C. Facts—2021. NobelPrize.org. Nobel Prize Outreach AB. 2021. Available online: https://www.nobelprize.org/prizes/chemistry/2021/macmillan/facts/ (accessed on 30 November 2021).
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels−Alder reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-catalyzed direct asymmetric Aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; MacMillan, D.W.C. Organocatalysis. Proc. Natl. Acad. Sci. USA 2010, 107, 20618–20619. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.-L. Privileged Chiral Ligands and Catalysts; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sell, T.; Meiswinkel, A.; Mehler, G. A new principle in combinatorial asymmetric transition-metal catalysis: Mixtures of chiral monodentate P ligands. Angew. Chem. Int. Ed. 2003, 42, 790–793. [Google Scholar] [CrossRef]
- Yang, H.; Tang, W. Efficient enantioselective syntheses of chiral natural products facilitated by ligand design. Chem. Rec. 2020, 20, 23–40. [Google Scholar] [CrossRef]
- Hamilton, G.L.; Kang, E.J.; Mba, M.; Toste, F.D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 2017, 317, 496–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herdeis, C.; Hubmann, H.P.; Lotter, H. Chiral pool synthesis of trans-(2S3S)-3-hydroxyproline and castanodiol from S-pyroglutamic acid. Tetrahedron Asymmetry 1994, 5, 119–128. [Google Scholar] [CrossRef]
- Brill, Z.G.; Condakes, M.L.; Ting, C.P.; Maimone, T.J. Navigating the chiral pool in the total synthesis of complex terpene natural products. Chem. Rev. 2017, 117, 11753–11795. [Google Scholar] [CrossRef] [PubMed]
- Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 2015, 115, 9587–9652. [Google Scholar] [CrossRef]
- Toste, F.D.; You, S.-L. Asymmetric synthesis enabled by organometallic complexes. Organometallics 2019, 38, 3899–3901. [Google Scholar] [CrossRef] [Green Version]
- Parshall, G.W. Trends and opportunities for organometallic chemistry in industry. Organometallics 1987, 6, 687–692. [Google Scholar] [CrossRef]
- Tang, J.; Redl, F.; Zhu, Y.; Siegrist, T.; Brus, L.E.; Steigerwald, M.L. An organometallic synthesis of TiO2 nanoparticles. Nano Lett. 2005, 5, 543–548. [Google Scholar] [CrossRef]
- Amiens, C.; Chaudret, B.; Ciuculescu-Pradines, D.; Collière, V.; Fajerwerg, K.; Fau, P.; Kahn, M.; Maisonnat, A.; Soulantica, K.; Phillipot, K. Organometallic approach for the synthesis of nanostructures. New J. Chem. 2013, 37, 3374–3401. [Google Scholar] [CrossRef]
- Martins, P.; Marques, M.; Coito, L.; Pombeiro, A.J.L.; Baptista, P.V.; Fernandes, A.R. Organometallic compounds in cancer therapy: Past lessons and future directions. Anticancer Agents Med. Chem. 2014, 14, 1199–1212. [Google Scholar] [CrossRef]
- Campeau, L.-C.; Fogg, D.E. The roles of organometallic chemistry in pharmaceutical research and development. Organometallics 2019, 38, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sharma, R.; Kamaluddin, M.R. Formamide-based synthesis of nucleobases by metal(II) octacyanomolybdate(IV): Implication in prebiotic chemistry. Astrobiology 2014, 14, 769–779. [Google Scholar] [CrossRef]
- Fioroni, M. Transition metal organometallic/metallorganic chemistry: Its role in prebiotic chemistry and life’s origin. In Prebiotic Chemistry and the Origin of Life. Advances in Astrobiology and Biogeophysics; Neubeck, A., McMahon, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–41. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Sargon, A.B.; Glass, J.B.; Hud, N.V.; Williams, L.D. Transition metals enhance prebiotic depsipeptide oligomerization reactions involving histidine. RSC Adv. 2021, 11, 3534–3538. [Google Scholar] [CrossRef]
- Barge, L.M.; Rodriguez, L.E.; Weber, J.M.; Theiling, B. Determining the “biosignature threshold” for life detection on biotic, abiotic, or prebiotic worlds. Astrobiology 2021. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Reynolds, W.B. Factors determining the course and mechanisms of Grignard reactions. X. The oxidation of Grignard reagents—Effect of metallic catalysts. J. Am. Chem. Soc. 1943, 65, 501–504. [Google Scholar] [CrossRef]
- Bäckvall, J.E.; Sellen, M.; Grant, B. Regiocontrol in copper-catalyzed Grignard reactions with allylic substrates. J. Am. Chem. Soc. 1990, 112, 6615–6621. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Brossmer, C.; Reisinger, C.-P.; Riermeier, T.H.; Öfele, K.; Beller, M. Palladacycles: Efficient new catalysts for the Heck vinylation of aryl halides. Chem. A Eur. J. 1997, 3, 1357–1364. [Google Scholar] [CrossRef]
- Martin, W.B.; Kateley, L.J. The Heck reaction: A microscale synthesis using a palladium catalyst. J. Chem. Educ. 2000, 77, 757. [Google Scholar] [CrossRef]
- Bhakta, S.; Ghosh, T. Emerging nickel catalysis in Heck reactions: Recent developments. Adv. Catal. Synth. 2020, 362, 5257–5274. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings–homogeneous or heterogeneous catalysis, a critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Percival, W.C.; Wagner, R.B.; Cook, N.C. Grignard reactions. XXI. The synthesis of aliphatic ketones. J. Am. Chem. Soc. 1953, 75, 3731–3734. [Google Scholar] [CrossRef]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 2017, 1, 0025. [Google Scholar] [CrossRef]
- Weber, J.M.; Longstreet, A.R.; Jamison, T.F. Bench-stable nickel precatalysts with Heck-type activation. Organometallics 2018, 37, 2716–2722. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Bartnikas, T.B.; Gitlin, J.D. How to make a metalloprotein. Nat. Struct. Mol. Biol. 2001, 8, 733–734. [Google Scholar] [CrossRef]
- Messina, M.S.; Stauber, J.M.; Waddington, M.A.; Rheingold, A.L.; Maynard, H.D.; Spokoyny, A.M. Organometallic gold(III) reagents for cysteine arylation. J. Am. Chem. Soc. 2018, 140, 7065–7069. [Google Scholar] [CrossRef]
- Kamo, N.; Kujirai, T.; Kurumizaka, H.; Murakami, H.; Hayashi, G.; Okamoto, A. Organoruthenium-catalyzed chemical protein synthesis to elucidate the functions of epigenetic modifications on heterochromatin factors. Chem Sci. 2021, 12, 5926–5937. [Google Scholar] [CrossRef]
- Isied, S.; Lyon, J.; Vassilian, A. Peptide formation in the presence of metal ion protecting groups. II. Determination of the optical purity of amino acids and peptides bound to pentaamine cobalt (III). Int. J. Pept. Protein Res. 1982, 19, 354–360. [Google Scholar] [CrossRef]
- Arbo, B.E.; Isied, S. Solid-phase synthesis of protected peptides using new cobalt(III) ammine linkers. Int. J. Pept. Protein Res. 1993, 42, 138–154. [Google Scholar] [CrossRef]
- Ruf, A.; Kanawati, B.; Hertkorn, N.; Yin, Q.-Z.; Moritz, F.; Harir, M.; Lucio, M.; Michalke, B.; Wimpenny, J.; Shilobreeva, S.; et al. Previously unknown class of metalorganic compounds revealed in meteorites. Proc. Natl. Acad. Sci. USA 2017, 114, 2819–2824. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.E.; House, C.H.; Arevalo, R.D., Jr.; Dworking, J.P.; Callahan, M.P. Organometallic compounds as carriers of extraterrestrial cyanide in primitive meteorites. Nat. Commun. 2019, 10, 2777. [Google Scholar] [CrossRef]
- Matzka, M.; Lucio, M.; Kanawati, B.; Quirico, E.; Bonal, L.; Loehle, S.; Schmitt-Kopplin, P. Thermal history of asteroid parent bodies is reflected in their metalorganic chemistry. ApJL 2021, 915, L7. [Google Scholar] [CrossRef]
- Lunine, J.I. Origin of water ice in the Solar System. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 309–319. [Google Scholar]
- Slade, M.A.; Butler, B.J.; Muhleman, D.O. Mercury radar imaging: Evidence for polar ice. Science 1992, 258, 635–640. [Google Scholar] [CrossRef]
- Ingersoll, A.P.; Svitek, T.; Murray, B.C. Stability of polar frosts in spherical bowl-shaped craters on the Moon, Mercury, and Mars. Icarus 1992, 100, 40–47. [Google Scholar] [CrossRef]
- Paige, D.A.; Wood, S.E.; Vasavada, A.R. The thermal stability of water ice at the poles of Mercury. Science 1992, 258, 643–646. [Google Scholar] [CrossRef]
- Namur, O.; Collinet, M.; Charlier, B.; Grove, T.L.; Holtz, F.; McCammon, C. Melting processes and mantle sources of lavas on Mercury. Earth Planet. Sci. Lett. 2016, 439, 117–128. [Google Scholar] [CrossRef]
- Namur, O.; Charlier, B. Silicate mineralogy at the surface of Mercury. Nat. Geosci. 2017, 10, 9–13. [Google Scholar] [CrossRef]
- Khodakovsky, I.L.; Volkov, V.P.; Sidorov, Y.I.; Borisov, M.V. Venus: Preliminary prediction of the mineral composition of surface rocks. Icarus 1979, 39, 352–363. [Google Scholar] [CrossRef]
- Fegley, B.; Treiman, A.H.; Sharpton, V.L. Venus surface mineralogy: Observational and theoretical constraints. Proc. Lunar Planet. Sci. 1992, 22, 3–19. [Google Scholar]
- Buffet, B.; Archer, D. Global inventory of methane clathrate: Sensitivity to changes in the deep ocean. Earth Planet Sci. Lett. 2004, 227, 185–199. [Google Scholar] [CrossRef]
- Prockter, L.M. Ices in the Solar System. Johns Hopkins APL Tech. Dig. 2005, 26, 175–188. [Google Scholar]
- Reimold, W.U.; Gibson, R.L. Processes on the early Earth. Geol. Soc. Am. 2006, 405. [Google Scholar] [CrossRef]
- Westall, F.; Brack, A. The importance of water for life. Space Sci. Rev. 2018, 214, 50. [Google Scholar] [CrossRef]
- Taylor, S.R.; Jakes, P. The geochemical evolution of the Moon. Proc. Lunar Sci. Conf. 1974, 5, 1287–1305. [Google Scholar]
- Li, S.; Lucey, P.G.; Milliken, R.E.; Hayne, P.O.; Fisher, E.; Williams, J.-P.; Hurley, D.M.; Elphic, R.C. Direct evidence of surface exposed water ice in the lunar polar regions. Proc. Natl. Acad. Sci. USA 2018, 115, 8907–8912. [Google Scholar] [CrossRef] [Green Version]
- Gendrin, A.; Mangold, N.; Bibring, J.-P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in martian layered terrains: The OMEGA/Mars Express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef] [Green Version]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; et al. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Lasue, J.; Mangold, N.; Hauber, E.; Clifford, S.; Feldmann, W.; Gasnault, O.; Grima, C.; Maurice, S.; Mousis, O. Quantitative assessments of the Martian hydrosphere. Space Sci. Rev. 2013, 174, 155–212. [Google Scholar] [CrossRef]
- Mousis, O.; Chassefière, E.; Holm, N.G.; Bouquet, A.; Hunter Waite, J.; Geppert, W.D.; Picaud, S.; Aikawa, Y.; Ali-Dib, M.; Charlou, J.-L.; et al. Methane clathrates in the Solar System. Astrobiology 2015, 15, 308–326. [Google Scholar] [CrossRef] [Green Version]
- Owen, T.C.; Roush, T.L.; Cruikshank, D.P.; Elliot, J.L.; Young, L.A.; de Bergh, C.; Schmitt, B.; Geballe, T.R.; Brown, R.H.; Bartholomew, M.J. Surface ices and the atmospheric composition of Pluto. Science 1993, 261, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Mousis, O.; Gautier, D.; Coustenis, A. The D/H ratio in methane in Titan: Origin and history. Icarus 2002, 159, 156–165. [Google Scholar] [CrossRef]
- Rivkin, A.S.; Howell, E.S.; Vilas, F.; Lebofsky, L.A. Hydrated minerals on asteroids: The astronomical record. In Asteroids III; Bottke, W.F., Jr., Cellino, A., Paolicchi, P., Binzel, P., Eds.; University of Arizona Press: Tucson, AZ, USA, 2002; pp. 235–253. [Google Scholar]
- Fujiwara, A.; Kawaguchi, J.; Yeomans, D.K.; Abe, M.; Okada, T.; Saito, J.; Yano, H.; Yoshikawa, M.; Scheeres, D.J.; Barnouin-Jha, O.; et al. The rubble-pile asteroid Itokawa as observed by Hayabusa. Science 2006, 312, 1330–1334. [Google Scholar] [CrossRef]
- Waite, J.H., Jr.; Lewis, W.S.; Magee, B.A.; Lunine, J.I.; McKinnon, W.B.; Glein, C.R.; Mousis, O.; Young, D.T.; Brockwell, T.; Westlake, J.; et al. Liquid water on Enceladus from observations of ammonia and 40Ar in the plume. Nature 2009, 460, 487–490. [Google Scholar] [CrossRef]
- Schwartz, A.W. Did minerals perform prebiotic combinatorial chemistry? Chem. Biol. 1996, 3, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth-Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Bass, M.N. Montmorillonite and serpentine in Orgueil meteorite. Geochim. Cosmochim. Acta 1971, 35, 139–147. [Google Scholar] [CrossRef]
- Tomeoka, K.; Buseck, P.R. Intergrown mica and montmorillonite in the Allende carbonaceous chondrite. Nature 1982, 299, 326–327. [Google Scholar] [CrossRef]
- Pinnavaia, T.J. Intercalated clay catalysts. Science 1983, 220, 365–371. [Google Scholar] [CrossRef]
- Herschy, B.; Whicher, A.; Camprubí, E.; Watson, C.; Dartnell, L.; Ward, J.; Evans, J.R.G.; Lane, N. An origin-of-life reactor to simulate alkaline hydrothermal vents. J. Mol. Evol. 2014, 79, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubí, E.; Lane, N. The origin of life in alkaline hydrothermal vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Mompeán, C.; Marín-Yaseli, M.R.; Espigares, P.; González-Toril, E.; Zorzano, M.-P.; Ruiz-Bermejo, M. Prebiotic chemistry in neutral/reduced-alkaline gas-liquid interfaces. Sci. Rep. 2019, 9, 1916. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, S.; Krissansen-Totton, J.; Catling, D.C. Probable cold and alkaline surface environment of the Hadean Earth caused by impact ejecta weathering. Geochem. Geophys. Geosyst. 2020, 21, e2019GC008734. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb. Perspect. Biol. 2010, 2, A002162. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J.D. The physical basis of life. Proc. Phys. Soc. Sect. B 1951, 62, 597. [Google Scholar] [CrossRef]
- Paecht-Horowitz, M.; Eirich, F.R. The polymerization of amino-acid adenylates on sodium montmorillonite with preadsorbed polypeptides. Orig. Life Evol. Biosph. 1988, 18, 359–387. [Google Scholar] [CrossRef]
- Ferris, J.P.; Ertem, G. Oligomerization of ribonucleosides on montmorillonite: Reaction of the 5′-phosphoimidazolide of adenosine. Science 1992, 257, 1387–1389. [Google Scholar] [CrossRef]
- Pitsch, S.; Eschenmoser, A.; Gedulin, B.; Hui, S.; Arrhenius, G. Mineral-induced formation of sugar phosphates. Orig. Life Evol. Biosph. 1995, 25, 297–334. [Google Scholar] [CrossRef]
- Jiang, W.; Pacella, M.S.; Athanasiadou, D.; Nelea, V.; Vali, H.; Hazen, R.M.; Gray, J.J.; McKee, M.D. Chiral acidic amino acids induce chiral hierarchical structure in calcium carbonate. Nat. Commun. 2017, 8, 15066. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Crystals, asymmetry and life. Nature 2001, 411, 753–755. [Google Scholar] [CrossRef]
- Grande, C.; Patel, N.H. Nodal signalling is involved in left-right asymmetry in snails. Nature 2009, 457, 1007–1011. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M. Chiral crystal faces of common rock-forming minerals. In Progress in Biological Chirality; Palyi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier: Oxford, UK, 2004; pp. 137–151. [Google Scholar]
- Hazen, R.M.; Filley, T.R.; Goodfriend, G.A. Selective adsorption of L- and D-amino acids on calcite: Implications for biochemical homochirality. Proc. Natl. Acad. Sci. USA 2001, 98, 5487–5490. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M.; Sholl, D.S. Chiral selection on inorganic crystalline surfaces. Nat. Mater. 2003, 2, 367–374. [Google Scholar] [CrossRef]
- Hazen, R.M. Mineral surfaces and the prebiotic selection and organization of biomolecules. Am. Mineral. 2006, 91, 1715–1729. [Google Scholar] [CrossRef]
- Cody, A.M.; Cody, R.D. Chiral habit modifications of gypsum from epitaxial-like adsorption of stereospecific growth inhibitors. J. Cryst. Growth 1991, 113, 508–519. [Google Scholar] [CrossRef]
- Bortnovsky, O.; Sobafik, Z.; Wichterlová, B.; Bastl, Z. Structure of Al-Lewis site in beta zeolite active in the Meerwein-Ponndorf-Verley reduction of ketone to alcohol. J. Catal. 2002, 210, 171–182. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Shimoyama, A.; Friebele, E. Clay and the origin of life. Orig. Life 1982, 12, 9–40. [Google Scholar] [CrossRef]
- Siffert, B.; Naidja, A. Stereoselectivity of montmorillonite in the adsorption and deamination of some amino acids. Clay Miner. 1992, 27, 109–118. [Google Scholar] [CrossRef]
- Ikeda, T.; Amoh, H.; Yasunaga, T. Stereoselective exchange kinetics of L- and D-histidines for chloride in the interlayer of a hydrotalcite-like compound by the chemical relaxation method. J. Am. Chem. Soc. 1984, 106, 5772–5775. [Google Scholar] [CrossRef]
- Fraser, D.G.; Fitz, D.; Jakschitz, T.; Rode, B.M. Selective adsorption and chiral amplification of amino acids in vermiculite clay-implications for the origin of biochirality. Phys. Chem. Chem. Phys. 2011, 13, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D.G.; Greenwell, H.C.; Skipper, N.T.; Smalley, M.V.; Wilkinson, M.A.; Deme, B.; Heenan, R.K. Chiral interactions of histidine in a hydrated vermiculite clay. Phys. Chem. Chem. Phys. 2011, 13, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, R.T.; Hazen, R.M. Chiral indices of crystalline surfaces as a measure of enantioselective potential. J. Mol. Catal. A Chem. 2004, 216, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.D.; Meinert, C.; Finger, F.; Meierheinrich, U.J.; Hejl, E. Racemate resolution of alanine and leucine on homochiral quartz, and its alteration by strong radiation damage. Life 2021, 11, 1222. [Google Scholar] [CrossRef]
- Thompson, R.M.; Downs, R.T. Quantifying distortion from ideal closest-packing in a crystal structure with analysis and application. Acta Crystallogr. Sect. B 2001, 57, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amariglio, A.; Amariglio, H.; Duval, X. Asymmetric reactions on optically active quartz. Helv. Chim. Acta 1968, 51, 2110–2115. [Google Scholar] [CrossRef]
- Bonner, W.A.; Kavasmaneck, P.R.; Martin, F.S.; Flores, J.J. Asymmetric adsorption of alanine by quartz. Science 1974, 186, 143–144. [Google Scholar] [CrossRef]
- Bonner, W.A.; Kavasmaneck, P.R.; Martin, F.S.; Flores, J.J. Asymmetric adsorption by quartz: A model for the prebiotic origin of optical activity. Orig. Life 1975, 6, 367–376. [Google Scholar] [CrossRef]
- Kahr, B.; Chittenden, B.; Rohl, A. Robert Boyle’s chiral crystal chemistry: Computational re-evaluation of enantioselective adsorption on quartz. Chirality 2006, 18, 127–133. [Google Scholar] [CrossRef]
- Easson, L.H.; Stedman, E. Molecular dissymmetry and physiological activity. J. Chem. Soc. 1933, 261, 1094–1098. [Google Scholar] [CrossRef]
- Ogston, A.G. Interpretation of experiments on metabolic processes, using isotopic tracer elements. Nature 1984, 162, 963. [Google Scholar] [CrossRef]
- Davankov, V.A. The nature of chiral recognition: Is it a three-point interaction? Chirality 1997, 9, 99–102. [Google Scholar] [CrossRef]
- Asthagiri, A.; Hazen, R.M. An ab initio study of adsorption of alanine on the chiral calcite (213) surface. Mol. Simul. 2007, 33, 343–351. [Google Scholar] [CrossRef]
- Booth, T.D.; Wahnon, D.; Wainer, I.W. Is chiral recognition a three-point process? Chirality 1997, 9, 96–98. [Google Scholar] [CrossRef]
- Mesecar, A.D.; Koshland, D.E., Jr. A new model for protein stereospecificity. Nature 2000, 403, 614–615. [Google Scholar] [CrossRef]
- Berthod, A. Chiral recognition mechanisms. Anal. Chem. 2006, 78, 2093–2099. [Google Scholar] [CrossRef]
- Goldberg, S.I. Enantiomeric enrichment on the prebiotic earth. Orig. Life Evol. Biosph. 2007, 37, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. Racemization of amino acids. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 399–414. [Google Scholar] [CrossRef]
- Soai, K.; Matsumoto, A.; Kawasaki, T. Asymmetric autocatalysis as a link between crystal chirality and highly enantioenriched organic compounds. Isr. J. Chem. 2021, 61, 507–516. [Google Scholar] [CrossRef]
- Sato, I.; Kadowaki, K.; Soai, K. Asymmetric synthesis of an organic compound with high enantiomeric excess induced by inorganic ionic sodium chlorate. Angew. Chem. Int. Ed. Engl. 2000, 39, 1510–1512. [Google Scholar] [CrossRef]
- Sato, I.; Kadowaki, K.; Ohgo, Y.; Soai, K. Highly enantioselective asymmetric autocatalysis induced by chiral ionic crystals of sodium chlorate and sodium bromate. J. Mol. Catal. A Chem. 2004, 216, 209–214. [Google Scholar] [CrossRef]
- Shindo, H.; Shirota, Y.; Niki, K.; Kawasaki, T.; Suzuki, K.; Araki, Y.; Matsumoto, A.; Soai, K. Asymmetric autocatalysis induced by cinnabar: Observation of the enantioselective adsorption of a 5-pyrimidyl alkanol on the crystal surface. Angew. Chem. Int. Ed. 2013, 52, 9135–9138. [Google Scholar] [CrossRef]
- Matsumoto, A.; Ozawa, H.; Inumaru, A.; Soai, K. Asymmetric induction by retgersite, nickel sulfate hexahydrate, in conjunction with asymmetric autocatalysis. New J. Chem. 2015, 39, 6742–6745. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Kaimori, Y.; Uchida, M.; Omori, H.; Kawasaki, T.; Soai, K. Achiral inorganic gypsum acts as an origin of chirality through Its enantiotopic surface in conjunction with asymmetric autocatalysis. Angew. Chem. Int. Ed. 2017, 56, 545–548. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral symmetry breaking in sodium chlorate crystallization. Science 1990, 16, 975–976. [Google Scholar] [CrossRef]
- McBride, J.M.; Carter, R.L. Spontaneous resolution by stirred crystallization. Angew. Chem. Intl. Ed. 1991, 30, 293–295. [Google Scholar] [CrossRef]
- Shinitzky, M.; Nudelman, F.; Barda, Y.; Haimovitz, R.; Chen, E.; Deamer, D.W. Unexpected differences between D- and L-tyrosine lead to chiral enhancement in racemic mixtures. Orig. Life Evol. Biosph. 2002, 32, 285–297. [Google Scholar] [CrossRef]
- Lahav, M.; Weissbuch, I.; Shavit, E.; Reiner, C.; Nicholson, G.J.; Schurig, V. Parity violating energetic difference and enantiomorphous crystals—Caveats; reinvestigation of tyrosine crystallization. Orig. Life Evol. Biosph. 2006, 36, 151–170. [Google Scholar] [CrossRef]
- Goldberg, S.I. Experimental evidence leading to an alternative explanation of why D-tyrosine sometimes crystallizes faster than its L-enantiomer. Orig Life Evol. Biosph. 2008, 38, 149–153. [Google Scholar] [CrossRef]
- Shinitzky, M.; Deamer, D. Comments in a discussion: Differential rates of D- and L-tyrosine crystallization. Orig. Life Evol. Biosph. 2008, 38, 271–275. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhao, K.; Cao, M.; Xu, H.; Xia, D.; Lu, J.R. Molecular modulation of calcite dissolution by organic acids. Cryst. Growth Des. 2011, 11, 3153–3162. [Google Scholar] [CrossRef]
- Frondel, C. Characters of quartz fibers. Am. Miner. 1978, 63, 17–27. [Google Scholar]
- Evgenii, K.; Wolfram, T. The role of quartz in the origin of optical activity on Earth. Orig. Life Evol. Biosph. 2000, 30, 431–434. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V. Asymmetry: The non-conservation of parity and optical activity. Q. Rev. Chem. Soc. 1959, 13, 48–60. [Google Scholar] [CrossRef]
- Ulbricht, T.; Vester, F. Attempts to induce optical activity with polarized β-radiation. Tetrahedron. 1962, 18, 629–637. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Hoffmann, S.; Jones, N.; Nahon, L.; Meierhenrich, U. Light on chirality: Absolute asymmetric formation of chiral molecules relevant in prebiotic evolution. ChemPlusChem 2017, 82, 74–87. [Google Scholar] [CrossRef]
- Nair, N.N.; Schreiner, E.; Marx, D. Glycine at the pyrite-water interface: The role of surface defects. J. Am. Chem. Soc. 2006, 128, 13815–13826. [Google Scholar] [CrossRef]
- Xue, N.; Chen, X.; Nie, L.; Guo, X.; Ding, W.; Chen, Y.; Gu, M.; Xie, Z. Understanding the enhancement of catalytic performance for olefin cracking: Hydrothermally stable acids in P/HZSM-5. J. Catal. 2007, 248, 20–28. [Google Scholar] [CrossRef]
- Fleming, G.J.; Adib, K.; Rodriguez, J.A.; Barteau, M.; White, J.; Idriss, H. The adsorption and reactions of the amino acid proline on rutile TiO2(1 1 0) surfaces. Surf. Sci. 2008, 602, 2029–2038. [Google Scholar] [CrossRef]
- Wu, J.; Buseck, P. Carbon storage at defect sites in mantle mineral analogues. Nat. Geosci. 2013, 6, 875–878. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, L. Zwitterionic versus canonical amino acids over the various defects in zeolites: A two-layer ONIOM calculation. Sci. Rep. 2014, 4, 6594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, H.; Yang, G. Promoting the adsorption of metal ions on kaolinite by defect sites: A molecular dynamics study. Sci. Rep. 2015, 5, 14377. [Google Scholar] [CrossRef] [Green Version]
- Zaia, D.A.M.; Zaia, C.T.B.V. A few experimental suggestions using minerals to obtain peptides with a high concentration of L-amino acids and protein amino acids. Symmetry 2020, 12, 2046. [Google Scholar] [CrossRef]
- Tosca, N.J.; McLennan, S.M. Chemical divides and evaporite assemblages on Mars. Earth Planet. Sci. Lett. 2006, 241, 21–31. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Martinez, G.M.; Rampe, E.B.; Bristow, T.F.; Blake, D.F.; Yen, A.S.; Ming, D.W.; Rapin, W.; Meslin, P.-Y.; Morookian, J.M.; et al. Gypsum, bassanite, and anhydrite at Gale crater, Mars. Am. Miner. 2018, 103, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Samotoin, N.D. Enantiomorphism of kaolinite: Manifestation at the levels of elementary layer and microcrystals. Crystallogr. Rep. 2011, 56, 327–334. [Google Scholar] [CrossRef]
- Wray, J.J.; Milliken, R.E.; Dundas, C.M.; Swayze, G.A.; Andrews-Hanna, J.C.; Baldridge, A.M.; Chojnacki, M.; Bishop, J.L.; Ehlmann, B.L.; Murchie, S.L.; et al. Columbus crater and other possible groundwater-fed paleolakes of Terra Sirenum, Mars. JGR Planets 2011, 116, E01001. [Google Scholar] [CrossRef] [Green Version]
- Cuadros, J.; Michalski, J.R. Investigation of Al-rich clays on Mars: Evidence for kaolinite–smectite mixed-layer versus mixture of end-member phases. Icarus 2013, 222, 296–306. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G.; Hall, A.J.; Russell, M.J. Mineral theories of the origin of life and an iron sulfide example. In Marine Hydrothermal Systems and the Origin of Life; Holm, N.G., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 161–180. [Google Scholar] [CrossRef]
- Zamaraev, K.I.; Romannikov, V.N.; Salganik, R.I.; Wlassoff, W.A.; Khramtsov, V.V. Modelling of the prebiotic synthesis of oligopeptides: Silicate catalysts help to overcome the critical stage. Orig. Life Evol. Biosph. 1997, 27, 325–337. [Google Scholar] [CrossRef]
- Ruff, S.W. Spectral evidence for zeolite in the dust on Mars. Icarus 2004, 168, 131–143. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Christensen, P.R.; Wyatt, M.B.; Glotch, T.D.; Rogers, A.D.; Anwar, S.; Arvidson, R.E.; Banfield, J.L.; Blaney, D.L.; Budney, C.; Calvin, W.M.; et al. Mineralogy at Meridiani Planum from the mini-TES experiment on the Opportunity rover. Science 2004, 306, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Stromberg, J.M.; Applin, D.M.; Cloutis, E.A.; Rice, M.; Berard, G.; Mann, P. The persistence of a chlorophyll spectral biosignature from Martian evaporite and spring analogues under Mars-like conditions. Int. J. Astrobiol. 2013, 13, 203–223. [Google Scholar] [CrossRef] [Green Version]
- Cockell, C.S.; Wilhelm, M.B.; Perl, S.; Wadsworth, J.; Payler, S.; McMahon, S.; Paling, S.; Edwards, T. 0.25 Ga salt deposits preserve signatures of habitable conditions and ancient lipids. Astrobiology 2020, 20, 864–877. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Raponi, A.; Ammannito, E.; Ciarniello, M.; Toplis, M.J.; McSween, H.Y.; Castillo-Rogez, J.C.; Ehlmann, B.L.; Carrozzo, F.G.; Marchi, S.; et al. Bright carbonate deposits as evidence of aqueous alteration on (1) Ceres. Nature 2016, 536, 54–57. [Google Scholar] [CrossRef]
- Raponi, A.; De Sanctis, M.C.; Carrozzo, F.G.; Ciarniello, M.; Castillo-Rogez, J.C.; Ammannito, E.; Frigeri, A.; Longobardo, A.; Palomba, E.; Tosi, F.; et al. Mineralogy of Occator crater on Ceres and insight into its evolution from the properties of carbonates, phyllosilicates, and chlorides. Icarus 2019, 320, 83–96. [Google Scholar] [CrossRef]
- Banin, A.; Ben-Shlomo, T.; Margulies, L.; Blake, D.F.; Mancinelli, R.L.; Gehring, A.U. The nanophase iron mineral(s) in Mars soil. J. Geophys. Res. 1993, 98, 20831–20853. [Google Scholar] [CrossRef]
- Madsen, M.B.; Hviid, S.F.; Gunnlaugsson, H.P.; Knudsen, J.M.; Goetz, W.; Pedersen, C.T.; Dinesen, A.R.; Mogensen, C.T.; Olsen, M.; Hargraves, R.B. The magnetic properties experiments on Mars Pathfinder. J. Geophys. Res. 1999, 104, 8761–8779. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.V.; Golden, D.C.; Bell, J.F., III; Shelfer, T.D.; Scheinost, A.C.; Hinman, N.W.; Furniss, G.; Mertzman, S.A.; Bishop, J.L.; Ming, D.W.; et al. Mineralogy, composition, and alteration of Mars Pathfinder rocks and soils: Evidence from multispectral, elemental, and magnetic data on terrestrial analogue, SNC meteorite, and Pathfinder samples. J. Geophys. Res. 2000, 105, 1757–1817. [Google Scholar] [CrossRef]
- Barrn, V.; Torrent, J. Evidence for a simple pathway to maghemite in Earth and Mars soils. Geochim. Cosmochim. Acta 2002, 66, 2801–2806. [Google Scholar] [CrossRef]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef] [Green Version]
- Scorei, R.; Cimpoiaşu, V.M. Boron enhances the thermostability of carbohydrates. Orig. Life Evol. Biosph. 2006, 36, 1–11. [Google Scholar] [CrossRef]
- Furukawa, Y.; Horiuchi, M.; Kakegawa, T. Selective stabilization of ribose by borate. Orig. Life Evol. Biosph. 2013, 43, 353–361. [Google Scholar] [CrossRef]
- Gasda, P.J.; Haldeman, E.B.; Wiens, R.C.; Rapin, W.; Bristow, T.F.; Bridges, J.C.; Schwenzer, S.P.; Clark, B.; Herkenhoff, K.; Frydenvang, J.; et al. In situ detection of boron by ChemCam on Mars. Geophys. Res. Lett. 2017, 44, 8739–9748. [Google Scholar] [CrossRef]
- Stephenson, J.D.; Hallis, L.J.; Nagashima, K.; Freeland, S.J. Boron enrichment in Martian clay. PLoS ONE 2013, 8, e64624. [Google Scholar] [CrossRef]
- Das, D.; Gasda, P.J.; Wiens, R.C.; Berlo, K.; Leveille, R.J.; Frydenvang, J.; Mangold, N.; Kronyak, R.E.; Schwenzer, S.P.; Forni, O.; et al. Boron and lithium in calcium sulfate veins: Tracking precipitation of diagenetic materials in Vera Rubin ridge, Gale crater. JGR Planets 2020, 125, e2019JE006301. [Google Scholar] [CrossRef]
- Eglinton, G.; Logan, G.A.; Ambler, R.P.; Boon, J.J.; Perizonius, W.R.K. Molecular preservation [and discussion]. Philos. Trans. Biol. Sci. 1991, 333, 315–328. [Google Scholar]
- Castañeda, I.S.; Schouten, S. A review of molecular organic proxies for examining modern and ancient lacustrine environments. Quat. Sci. Rev. 2011, 30, 2851–2891. [Google Scholar] [CrossRef]
- Sollai, M.; Villanueva, L.; Hopmans, E.C.; Keil, R.G.; Sinninghe Damsté, J.S. Archaeal sources of intact membrane lipid biomarkers in the oxygen deficient zone of the Eastern Tropical South Pacific. Front. Microbiol. 2019, 10, 765. [Google Scholar] [CrossRef]
- Lee, C.; Brocks, J.J. Identification of carotane breakdown products in the 1.64 billion year old Barney Creek Formation, McArthur Basin, northern Australia. Org. Geochem. 2011, 42, 425–430. [Google Scholar] [CrossRef]
- Summons, R.E.; Welander, P.V.; Gold, D.A. Lipid biomarkers: Molecular tools for illuminating the history of microbial life. Nat. Rev. Microbiol. 2021, 20, 174–185. [Google Scholar] [CrossRef]
- Burgos, C.; Ayer, D.; Johnson, R.A. A new, asymmetric synthesis of lipids and phospholipids. J. Org. Chem. 1987, 52, 4973–4977. [Google Scholar] [CrossRef]
- Listunov, D.; Fabing, I.; Saffon-Merceron, N.; Gaspard, H.; Volovenko, Y.; Maraval, V.; Chauvin, R.; Génnison, Y. Asymmetric synthesis and biological evaluation of natural or bioinspired cytotoxic C2-symmetrical lipids with two terminal chiral alkynylcarbinol pharmacophores. J. Org. Chem. 2015, 80, 5386–5394. [Google Scholar] [CrossRef]
- Mountanea, O.G.; Limnios, D.; Kokotou, M.G.; Bourboula, A.; Kokotos, G. Asymmetric synthesis of saturated hydroxy fatty acids and fatty acid esters of hydroxy fatty acids. Eur. J. Org. Chem. 2019, 2019, 2010–2019. [Google Scholar] [CrossRef]
- Altamura, E.; Comte, A.; D’Onofrio, A.D.; Roussillon, C.; Fayolle, D.; Buchet, R.; Mavelli, F.; Stano, P.; Fiore, M.; Strazewski, P. Racemic phospholipids for origin of life studies. Symmetry 2020, 12, 1108. [Google Scholar] [CrossRef]
- Des Marais, D.J. Isotopic evolution of the biogeochemical carbon cycle during the Precambrian. Rev. Mineral. Geochem. 2001, 43, 555–578. [Google Scholar] [CrossRef]
- Tegelaar, E.W.; Derenne, S.; Largeau, C.; de Leeuw, J.W. A reappraisal of kerogen formation. Geochim. Cosmochim. Acta 1989, 3, 3103–3107. [Google Scholar] [CrossRef]
- Burdige, D. Preservation of organic matter in marine sediments: Controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem. Rev. 2007, 107, 467–485. [Google Scholar] [CrossRef]
- Brocks, J.J.; Summons, R.E. Sedimentary hydrocarbons, biomarkers for early life. In Treatise on Geochemistry, 2nd ed.; Holland, D.H., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 8, pp. 61–103. [Google Scholar]
- Lee, C.; Love, G.D.; Jahnke, L.L.; Kubo, M.D.; Des Marais, D.J. Early diagenetic sequestration of microbial mat lipid biomarkers through covalent binding into insoluble macromolecular organic matter (IMOM) as revealed by sequential chemolysis and catalytic hydropyrolysis. Org. Geochem. 2019, 132, 11–22. [Google Scholar] [CrossRef]
- Lee, C.; Love, G.D.; Jahnke, L.L.; Kubo, M.D.; Des Marais, D.J. Diagenetic transformations and preservation of free and bound lipids in a hypersaline microbial mat from Guerrero Negro, Baja California Sur, Mexico. Org. Geochem. 2021, 153, 104196. [Google Scholar] [CrossRef]
- Bishop, A.N.; Love, G.D.; McAulay, A.D.; Snape, C.E.; Farrimond, P. Release of kerogen bound hopanoids by hydropyrolysis. Org. Geochem. 1998, 29, 989–1001. [Google Scholar] [CrossRef]
- Farrimond, P.; Love, G.D.; Bishop, A.N.; Innes, H.E.; Watson, D.F.; Snape, C.E. Evidence for rapid incorporation of hopanoids into kerogen. Geochim. Cosmochim. Acta 2003, 67, 1383–1394. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide: Biomarkers and Isotopes in Petroleum Exploration and Earth History, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005; Volume 2. [Google Scholar]
- Horsfield, B.; Rullkötter, J. Diagenesis, catagenesis, and metagenesis of organic matter. In The Petroleum System—From Source to Trap; Magoon, L.B., Dow, W.G., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; Volume 60. [Google Scholar] [CrossRef]
- Durand, B. Sedimentary organic matter and kerogen: Definition and quantitative importance of kerogen. In Kerogen: Insoluble Organic Matter from Sedimentary Rocks; Durand, B., Ed.; Editions Technip: Paris, France, 1980; pp. 13–33. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Keil, R.G.; Montluçon, D.B.; Prahl, F.G.; Hedges, J.I. Sorptive preservation of labile organic matter in marine sediments. Nature 1994, 370, 549–552. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M. Mineral matrices and organic matter. In Treatise on Geochemistry, 2nd ed.; Holland, D.H., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 12, pp. 337–359. [Google Scholar] [CrossRef]
- Fox, A.C.; Eigenbrode, J.L.; Freeman, K.H. Radiolysis of macromolecular organic material in Mars-relevant mineral matrices. JGR Planets 2019, 124, 3257–3266. [Google Scholar] [CrossRef] [Green Version]
- Salmon, V.; Derenne, S.; Lallier-Vergès, E.; Largeau, C.; Beaudoin, B. Protection of organic matter by mineral matrix in a Cenomanian black shale. Org. Geochem. 2000, 31, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Love, G.D.; Stalvies, C.; Grosjean, E.; Meredith, W.; Snape, C.E. Analysis of molecular biomarkers covalently bound within Neoproterozoic sedimentary kerogen. In From Evolution to Geobiology: Research Questions Driving Paleontology at the Start of a New Century, Paleontological Society Short Course, Paleontological Society Papers; Kelley, P.H., Bambach, R.K., Eds.; Cambridge University Press: Cambridge, UK, 2008; Volume 14, pp. 67–83. [Google Scholar] [CrossRef] [Green Version]
- Kerogen, P.S. Encyclopedia of Geochemistry. Encyclopedia of Earth Sciences Series; White, W.M., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kerridge, J.F. Formation and processing of organics in the early Solar System. Space Sci. Rev. 1999, 290, 275–288. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. Discrimination of stereoisomers by their enantioselective interactions with chiral cholesterol-containing membranes. Molecules 2018, 23, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerst, N.; Ruan, B.; Pang, J.; Wilson, W.K.; Schroepfer, G.J., Jr. An updated look at the analysis of unsaturated C27 sterols by gas chromatography and mass spectrometry. J. Lipid Res. 1997, 38, 1685–1701. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Mackenzie, A.S.; Volkman, J.K. Configuration at C-24 in steranes and sterols. Nature 1980, 286, 694–697. [Google Scholar] [CrossRef]
- vaan Kaam-Peters, H.M.E.; Köster, J.; van der Gaast, S.J.; Dekker, M.; de Leeuw, J.W.; Sinninghe Damsté, J.S. The effect of clay minerals on diasterane/sterane ratios. Geochim. Cosmochim. Acta 1998, 62, 2923–2929. [Google Scholar] [CrossRef]
- Elie, M.; Nogueira, A.C.R.; Nedlec, A.; Trindale, R.I.F.; Kenig, F. A red algal bloom in the aftermath of the Marinoan Snowball Earth. Terra Nova 2007, 19, 303–308. [Google Scholar] [CrossRef]
- Tannenbaum, E.; Huizinga, B.J.; Kaplan, I.R. Role of minerals in thermal alteration of organic matter--II: A material balance. Amer. Assoc. Petrol. Geol. Bull. 1986, 70, 1156–1165. [Google Scholar] [CrossRef]
- Soldan, A.L.; Cerqueira, J.R. Effects of thermal maturation on geochemical parameters obtained by simulated generation of hydrocarbons. Org. Geochem. 1986, 10, 339–345. [Google Scholar] [CrossRef]
- Love, G.D.; Snape, C.E.; Carr, A.D.; Houghton, R.C. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis. Org. Geochem. 1995, 23, 981–986. [Google Scholar] [CrossRef]
- Love, G.D.; Snape, C.E.; Carr, A.D.; Houghton, R.C. Changes in molecular biomarker and bulk carbon skeletal parameters of vitrinite concentrates as a function of rank. Energy Fuels 1996, 10, 149–157. [Google Scholar] [CrossRef]
- Love, G.D.; McAulay, A.; Snape, C.E.; Bishop, A.N. Effect of process variables in catalytic hydropyrolysis on the release of Covalently bound aliphatic hydrocarbons from sedimentary organic matter. Energy Fuels 1997, 11, 522–531. [Google Scholar] [CrossRef]
- Love, G.D.; Snape, C.E.; Fallick, A.E. Differences in the mode of incorporation and biogenicity of the principal aliphatic constituents of a Type I oil shale. Org. Geochem. 1998, 28, 797–811. [Google Scholar] [CrossRef]
- Love, G.D.; Bowden, S.A.; Summons, R.E.; Jahnke, L.L.; Snape, C.E.; Campbell, C.N.; Day, J.G. An optimised catalytic hydropyrolysis method for the rapid screening of microbial cultures for lipid biomarkers. Org. Geochem. 2005, 36, 63–82. [Google Scholar] [CrossRef]
- Sudgen, M.A.; Abbott, G.D. The stereochemistry of bound and extractable pentacyclic triterpenoids during closed system pyrolysis. Org. Geochem. 2002, 33, 1515–1521. [Google Scholar] [CrossRef]
- Krot, A.N.; Amelin, Y.; Bland, P.; Ciesla, F.J.; Connelly, J.; Davis, A.M.; Huss, G.R.; Hutcheon, I.D.; Makide, K.; Nagashima, K.; et al. Origin and chronology of chondritic components: A review. Geochim. Cosmochim. Acta 2009, 73, 4963–4997. [Google Scholar] [CrossRef]
- Cloutis, E.A.; Izawa, M.R.M.; Beck, P. Chapter 4—Reflectance Spectroscopy of Chondrites. In Primitive Meteorites and Asteroids Physical, Chemical and Spectroscopic Observations Paving the Way to Exploration; Abreu, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 273–343. [Google Scholar] [CrossRef]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry; Holland, D.H., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 1–63. [Google Scholar]
- Maurel, C.; Bryson, J.F.J.; Lyons, R.J.; Ball, M.R.; Chopdekar, R.V.; Scholl, A.; Ciesla, F.J.; Bottke, W.F.; Wiess, B.P. Meteorite evidence for partial differentiation and protracted accretion of planetesimals. Sci. Adv. 2014, 6, eaba1303. [Google Scholar] [CrossRef]
- Weisberg, M.K.; McCoy, T.J.; Krot, A.N. Systematics and evaluation of meteorite classification. In Meteorites and the early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 19–52. [Google Scholar]
- Wood, J.A. Chondritic meteorites and the solar nebula. Ann. Rev. Earth Planet. Sci. 1988, 16, 53–72. [Google Scholar] [CrossRef]
- Braukmüller, N.; Wombacher, F.; Hezel, D.C.; Escoube, R.; Münker, C. The chemical composition of carbonaceous chondrites: Implications for volatile element depletion, complementarity and alteration. Geochim. Cosmochim. Acte 2018, 239, 17–48. [Google Scholar] [CrossRef]
- Van Schmus, W.R.; Wood, J.A. A chemical-petrologic classification for the chondritic meteorites. Geochim. Cosmochim. Acta 1967, 31, 747–754. [Google Scholar] [CrossRef]
- Grimm, R.E.; McSween, H.Y., Jr. Water and the thermal evolution of carbonaceous chondrite parent bodies. Icarus 1989, 82, 244–280. [Google Scholar] [CrossRef]
- Brearley, A.J.; Jones, R.H. Chondritic meteorites. In Planetary Materials; Papike, J.J., Ed.; De Gruyter: Berlin, Germany, 1998; Volume 36, pp. 3.1–3.398. [Google Scholar] [CrossRef]
- Scott, E.R.D.; Krot, A.N. Chondrites and their components. In Meteorites, Comets and Planets: Treatise on Geochemistry; Davis, A.M., Holland, H.D., Turekian, K.K., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2005; Volume 1, p. 143. [Google Scholar]
- Scott, E.R. Chondrites and the protoplanetary disk. Annu. Rev. Earth Planet. Sci. 2007, 35, 577–620. [Google Scholar] [CrossRef] [Green Version]
- Hiroi, T.; Pieters, C.M.; Zolensky, M.; Lipschutz, M.E. Evidence of thermal metamorphism on the C, G, B, and F asteroids. Science 1993, 261, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Huss, G.R.; Rubin, A.E.; Grossman, J.N. Thermal metamorphism in chondrites. In Meteorites and the early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 567–586. [Google Scholar]
- Gyollai, I.; Bérczi, S.; Fintor, K.; Nagy, S.; Gucsik, A. Thermal metamorphism of the Mócs meteorite (L6) revealed by optical microscopy and BSE imaging. Cent. Eur. Geol. 2015, 58, 321–333. [Google Scholar] [CrossRef] [Green Version]
- King, A.J.; Schofield, P.F.; Russell, S.S. Thermal alteration of CM carbonaceous chondrites: Mineralogical changes and metamorphic temperatures. Geochim. Cosmochim. Acta 2021, 298, 167–190. [Google Scholar] [CrossRef]
- Yesiltas, M.; Young, J.; Glotch, T.D. Thermal metamorphic history of Antarctic CV3 and CO3 chondrites inferred from the first- and second-order Raman peaks of polyaromatic organic carbon. Am. Miner. 2021, 106, 506–517. [Google Scholar] [CrossRef]
- Kallemeyn, G.W.; Rubin, A.E.; Wasson, J.T. The compositional classification of chondrites: VI. The CR carbonaceous chondrite group. Geochim. Cosmochim. Acta 1994, 58, 2873–2888. [Google Scholar] [CrossRef]
- Amelin, Y.; Ghosh, A.; Rotenberg, E. Unraveling the evolution of chondrite parent asteroids by precise U-Pb dating and thermal modeling. Geochim. Cosmochim. Acta 2005, 69, 505–518. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Scott, E.R.D.; Chabot, N.L. Iron meteorites: Crystallization, thermal history, parent bodies, and origin. Chem. Der Erde 2009, 69, 293–325. [Google Scholar] [CrossRef]
- Yang, J.; Goldstein, J.I.; Michael, J.R.; Kotula, P.G.; Scott, E.R.D. Thermal history and origin of the IVB iron meteorites and their parent body. Geochim. Cosmochim. Acta 2010, 74, 4493–4506. [Google Scholar] [CrossRef]
- Henke, S.; Gail, H.-P.; Trieloff, M.; Schwarz, W.H.; Kleine, T. Thermal history modelling of the H chondrite parent body. Astron. Astrophys. 2012, 545, A135. [Google Scholar] [CrossRef] [Green Version]
- Gail, H.-P.; Trieloff, M. Thermal history modelling of the L chondrite parent body. Astron. Astrophys. 2019, 628, 1–21. [Google Scholar] [CrossRef]
- Brearley, A.J. The action of water. In Meteorites and the early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 587–624. [Google Scholar]
- Doyle, P.M.; Jogo, K.; Nagashima, K.; Krot, A.N.; Wakita, S.; Ciesla, F.J. Early aqueous activity on the ordinary and carbonaceous chondrite parent bodies recorded by fayalite. Nat. Commun. 2015, 6, 7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endress, M.; Bischoff, A. Carbonates in CI chondrites: Clues to parent body evolution. Geochim. Cosmochim. Acta 1996, 60, 489–507. [Google Scholar] [CrossRef]
- Endress, M.; Zinner, E.; Bischoff, A. Early aqueous activity on primitive meteorite parent bodies. Nature 1996, 379, 701–703. [Google Scholar] [CrossRef]
- Berger, E.L.; Zega, T.L.; Keller, L.P.; Lauretta, D.S. Evidence for aqueous activity on comet 81P/Wild 2 from sulfide mineral assemblages in Stardust samples and CI chondrites. Geochim. Cosmochim. Acta 2011, 75, 3501–3513. [Google Scholar] [CrossRef]
- Berger, E.L.; Keller, L.P.; Lauretta, D.S. An experimental study of the formation of cubanite (CuFe2S3) in primitive meteorites. Meteorit. Planet. Sci. 2015, 50, 1–14. [Google Scholar] [CrossRef]
- Sarafian, A.R.; Nielsen, S.G.; Marshall, H.R.; Gaetani, G.A.; Righter, K.; Berger, E.L. The water and fluorine content of 4 Vesta. Geochim. Cosmochim. Acta 2019, 266, 568–581. [Google Scholar] [CrossRef]
- Kojima, T.; Tomeoka, K. Indicators of aqueous alteration and thermal metamorphism on the CV parent body: Microtextures of a dark inclusion from Allende. Geochim. Cosmochim. Acta 1996, 60, 2651–2666. [Google Scholar] [CrossRef]
- Kikuchi, S.; Shibuya, T.; Abe, M.; Uematsu, K. Experimental chondrite–water reactions under reducing and low-temperature hydrothermal conditions: Implications for incipient aqueous alteration in planetesimals. Geochim. Comsochim. Acta, 2021; in press. [Google Scholar] [CrossRef]
- Dyl, K.A.; Bischoff, A.; Ziegler, K.; Young, E.D.; Wimmer, K.; Bland, P.A. Early Solar System hydrothermal activity in chondritic asteroids on 1–10-year timescales. Proc. Natl. Acad. Sci. USA 2012, 109, 18306–18311. [Google Scholar] [CrossRef] [Green Version]
- Putnis, A.; Austrheim, H. Fluid-induced processes: Metasomatism and metamorphism. Geofluids 2010, 10, 245–269. [Google Scholar] [CrossRef]
- Yasui, M.; Tazawa, T.; Hashimoto, R.; Arakawa, M.; Ogawa, K. Impacts may provide heat for aqueous alteration and organic solid formation on asteroid parent bodies. Commun. Earth Environ. 2021, 2, 95. [Google Scholar] [CrossRef]
- Hirakawa, N.; Kebukawa, Y.; Furukawa, Y.; Kondo, M.; Nakano, H.; Kobayashi, K. Aqueous alteration without initial water: Possibility of organic-induced hydration of anhydrous silicates in meteorite parent bodies. Earth Planets Space 2021, 73, 16. [Google Scholar] [CrossRef]
- Scott, E.R.D.; Keil, K.; Stoffler, D. Shock metamorphism of carbonaceous chondrites. Geochim. Cosmochim. Acta 1992, 56, 4281–4293. [Google Scholar] [CrossRef]
- Sharp, T.G.; DeCarli, P.S. Shock effects in meteorites. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 653–677. [Google Scholar]
- Bischoff, A.; Scott, E.R.D.; Metzler, K.; Goodrich, C.A. Nature and origins of meteoritic breccias. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 679–712. [Google Scholar]
- Glavin, D.P.; Alexander, C.M.O.D.; Aponte, J.C.; Dworkin, J.P.; Elsila, J.E.; Yabuta, H. Chapter 3: The origin and evolution of organic matter in carbonaceous chondrites and links to their parent bodies. In Primitive Meteorites and Asteroids: Physical, Chemical and Spectroscopic Observations Paving the Way to Exploration; Abreu, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–271. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, J., II; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Elsila, J.E.; Aponte, J.C.; Blackmond, D.G.; Burton, A.S.; Dworkin, J.P.; Glavin, D.P. Meteoritic amino acids: Diversity in compositions reflects parent body histories. ACS Cent. Sci. 2016, 2, 370–379. [Google Scholar] [CrossRef]
- Glavin, D.P.; McLain, H.L.; Dworkin, J.P.; Parker, E.T.; Elsila, J.E.; Aponte, J.C.; Simkus, D.N.; Pozarycki, C.I.; Graham, H.V.; Nittler, L.R.; et al. Abundant extraterrestrial amino acids in the primitive CM carbonaceous chondrite Asuka 12236. Meteorit. Planet. Sci. 2020, 55, 1979–2006. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous chondrites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of CI type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef] [Green Version]
- Wächtershäuser, G. Pyrite formation, the first energy source for life: A hypothesis. Syst. Appl. Microbiol. 1988, 10, 207–210. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 1992, 58, 85–201. [Google Scholar] [CrossRef]
- Huber, C.; Wächtershäuser, G. α-hyydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science 2006, 314, 630–632. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of abiotic nucleotide synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef]
- Weber, J.M.; Henderson, B.L.; LaRowe, D.E.; Goldman, A.D.; Perl, S.M.; Billings, K.; Barge, L.M. Testing abiotic reduction of NAD+ directly mediated by iron/sulfur minerals. Astrobiology 2021, 22, 25–34. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Bruylants, G.; Wouters, J.; Michaux, C. Differential scanning calorimetry in life science: Thermodynamics, stability, molecular recognition and application in drug design. Curr. Med. Chem. 2005, 12, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Gil-Av, E.; Feibush, B.; Charles-Sigler, R. Separation of enantiomers by gas liquid chromatography with an optically active stationary phase. Tetrahedron Lett. 1966, 7, 1009–1015. [Google Scholar] [CrossRef]
- Grybinik, S.; Bosakova, Z. An overview of chiral separations of pharmaceutically active substances by HPLC (2018–2020). Mon. Chem. Chem. Mon. 2021, 152, 1033–1043. [Google Scholar] [CrossRef]
- Labuta, J.; Ishihara, S.; Šikorský, T.; Futera, Z.; Shundo, A.; Hanyková, L.; Burda, J.V.; Ariga, K.; Hill, J.P. NMR spectroscopic detection of chirality and enantiopurity in referenced systems without formation of diastereomers. Nat. Commun. 2013, 4, 2188. [Google Scholar] [CrossRef]
- Gottarelli, G.; Osipov, M.A.; Spada, G.P. A study of solvent effect on the optical rotation of chiral biaryls. J. Phys. Chem. 1991, 95, 3879–3884. [Google Scholar] [CrossRef]
- Langeveld-Voss, B.M.W.; Christiaans, M.P.T.; Janssen, R.A.J.; Meijer, E.W. Inversion of optical activity of chiral polythiophene aggregates by a change of solvent. Macromolecules 1998, 31, 6702–6704. [Google Scholar] [CrossRef]
- Thiemann, W.H.-P.; Rosenbauer, H.; Meierhenrich, U.J. Conception of the ’chirality-experiment’ on ESA’s mission ROSETTA to comet P46/Wirtanen. Adv. Space Res. 2001, 27, 323–328. [Google Scholar] [CrossRef]
- Freissinet, C.; Buch, A.; Sternberg, R.; Szopa, C.; Geffroy-Rodier, C.; Jelinek, C.; Stambouli, M. Search for evidence of life in space: Analysis of enantiomeric organic molecules by N,N-dimethylformamide dimethylacetal derivative dependant gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 731–740. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Conrad, P.G.; Coll, P.; Atreya, S.K.; Arvey, R.; Barciniak, M.; Benna, M.; Bleacher, L.; et al. The Sample Analysis at Mars Investigation and Instrument Suite. Space Sci. Rev. 2012, 170, 401–478. [Google Scholar] [CrossRef]
- Goesmann, F.; Brinckerhoff, W.B.; Raulin, F.; Goetz, W.; Danell, R.M.; Getty, S.A.; Siljeström, S.; Mißbach, H.; Steininger, H.; Arevalo, R.D., Jr.; et al. The Mars Organic Molecule Analyzer (MOMA) instrument: Characterization of organic material in Martian sediments. Astrobiology 2017, 17, 655–685. [Google Scholar] [CrossRef] [PubMed]
- Ulamec, S.; Goesmann, F.; Meierhenrich, U.J. Philae landing on comet 67P/Churyumov-Gerasimenko—Planned chirality measurements and ideas for the future. J. Interdiscip. Methodol. Issues Sci. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Sousa, E.P.; Tiritan, M.E.; Oliveira, R.; Afonso, C.M.M.; Cass, Q.B.; Pinto, M.M.M. Enantiomeric resolution of kielcorin derivatives by HPLC on polysaccharide stationary phases using multimodal elution. Chirality 2004, 16, 279–285. [Google Scholar] [CrossRef]
- Dossou, K.S.S.; Chiap, P.; Servais, A.C.; Fillet, M.; Crommen, J. Evaluation of chlorine containing cellulose-based chiral stationary phases for the LC enantioseparation of basic pharmaceuticals using polar non-aqueous mobile phases. J. Sep. Sci. 2011, 34, 617–622. [Google Scholar] [CrossRef]
- Ianni, F.; Saluti, G.; Galarini, R.; Fiorito, S.; Sardella, R.; Natalini, B. Enantioselective high-performance liquid chromatography analysis of oxygenated polyunsaturated fatty acids. Free Radic. Biol. Med. 2019, 144, 35–54. [Google Scholar] [CrossRef]
- Mangelings, D.; Vander Heyden, Y. Chiral separations in sub- and supercritical fluid chromatography. J. Sep. Sci. 2008, 31, 1252–1273. [Google Scholar] [CrossRef]
- West, C. Enantioselective separations with supercritical fluid—Review. Curr. Anal. Chem. 2014, 10, 99–120. [Google Scholar] [CrossRef]
- Gübitz, G.; Schmid, M.G. Chiral separation principles in capillary electrophoresis. J. Chromatogr. A 1997, 792, 179–225. [Google Scholar] [CrossRef]
- Mathies, R.A.; Razu, M.E.; Kim, J.; Stockton, A.M.; Turin, P.; Butterworth, A. Feasibility of detecting bioorganic compounds in Enceladus plumes with the Enceladus Organic Analyzer. Astrobiology 2017, 17, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Zamuruyev, K.; Santos, M.S.F.; Mora, M.F.; Kurfman, E.A.; Noell, A.C.; Willis, P.A. Automated capillary electrophoresis system compatible with multiple detectors for potential in situ spaceflight missions. Anal. Chem. 2021, 93, 9647–9655. [Google Scholar] [CrossRef]
- Gübitz, G.; Schmid, M.G. Chiral separation by capillary electrochromatography. Enantiomer 2000, 5, 5–11. [Google Scholar]
- Wistuba, D.; Schurig, V. Recent progress in enantiomer separation by capillary electrochromatography. Electrophoresis 2000, 21, 4136–4158. [Google Scholar] [CrossRef]
- Fanali, C.; Della Posta, S.; Fanali, S. Capillary electrochromatography applied to drug analysis. J. Chromatogr. Open 2021, 1, 100015. [Google Scholar] [CrossRef]
- Kodama, S.; Yamamoto, A.; Iio, R.; Sakamoto, K.; Matsunaga, A.; Hayakawa, K. Chiral ligand exchange capillary electrophoresis using borate anion as a central ion. Analyst 2004, 129, 1238–1242. [Google Scholar] [CrossRef]
- Mu, X.; Qi, L.; Shen, Y.; Zhang, H.; Qiao, J.; Ma, H. A novel chiral ligand exchange capillary electrophoresis system with amino acid ionic liquid as ligand and its application in screening d-amino-acid oxidase inhibitors. Analyst 2012, 137, 4235–4240. [Google Scholar] [CrossRef]
- Liu, L.; Bao, P.; Qiao, J.; Zhang, H.; Qi, L. Chiral ligand exchange capillary electrophoresis with L-dipeptides as chiral ligands for separation of Dns-D.,L-amino acids. Talanta 2020, 217, 121069. [Google Scholar] [CrossRef]
- Wang, F.; Khaledi, M.G. Chiral separations by nonaqueous capillary electrophoresis. Anal. Chem. 1996, 68, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Limero, T.; Reese, E.; Trowbridge, J.; Hohman, R.; James, J.T. The Volatile Organic Analyzer (VOA) aboard the International Space Station; SAE Technical Paper 2002-01-2407; SAE International: Warrendale, PA, USA, 2002. [Google Scholar] [CrossRef]
- Dwivedi, P.; Wu, C.; Matz, L.M.; Clowers, B.H.; Siems, W.F.; Hill, H.H., Jr. Gas-phase chiral separations by ion mobility spectrometry. Anal. Chem. 2006, 78, 8200–8206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mie, A.; Jörntén-Karlsson, M.; Axelsson, B.-O.; Ray, A.; Reimann, C.T. Enantiomer separation of amino acids by complexation with chiral reference compounds and high-field asymmetric waveform ion mobility spectrometry: Preliminary results and possible limitations. Anal. Chem. 2007, 79, 2850–2858. [Google Scholar] [CrossRef]
- Will, J.M.L.; Behrens, A.; Macke, M.; Derrick Quales, C., Jr.; Karst, U. Automated chiral analysis of amino acids based on chiral derivatization and trapped ion mobility–mass spectrometry. Anal. Chem. 2021, 93, 878–885. [Google Scholar] [CrossRef]
- Brodbelt, J.S. Photodissociation mass spectrometry: New tools for characterization of biological molecules. Chem. Soc. Rev. 2014, 43, 2757–2783. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N.; Doan, T.N.; Hayakawa, S. Enantiomeric excess determination for monosaccharides using chiral transmission to cold gas-phase tryptophan in ultraviolet photodissociation. J. Am. Soc. Mass Spectrom. 2017, 28, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, M.; Zhang, K.; Ma, L.; Kong, X. Chiral differentiation of non-covalent diastereomers based on multichannel dissociation induced by 213-nm ultraviolet photodissociation. J. Am. Soc. Mass Spectrom. 2019, 30, 2297–2305. [Google Scholar] [CrossRef]
- Koehbach, J.; Gruber, C.W.; Becker, C.; Kreil, D.P.; Jilek, A. MALDI TOF/TOF-based approach for the identification of D-amino acids in biologically active peptides and proteins. J. Proteome Res. 2016, 15, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Casy, A.F.; Mercer, A.D. Application of cyclodextrins to chiral analysis by 1H NMR spectroscopy. Magn. Reson. Chem. 1988, 26, 765–774. [Google Scholar] [CrossRef]
- Kubo, Y.; Maeda, S.; Tokita, S.; Kubo, M. Colorimetric chiral recognition by a molecular sensor. Nature 1996, 382, 522–524. [Google Scholar] [CrossRef]
- Tsubaki, K.; Nuruzzaman, M.; Kusumoto, T.; Hayashi, N.; Bin-Gui, W.; Fuji, K. Visual enantiomeric recognition using chiral phenolphthalein derivatives. Org. Lett. 2001, 3, 4071–4073. [Google Scholar] [CrossRef]
- Wirz, R.; Bürgi, T.; Baiker, A. Probing enantiospecific interactions at chiral solid−liquid interfaces by absolute configuration modulation infrared spectroscopy. Langmuir 2003, 19, 785–792. [Google Scholar] [CrossRef]
- Hinsmann, P.; Arce, L.; Svasek, P.; Lämmerhofer, M.; Lendl, B. Separation and on-line distinction of enantiomers: A non-aqueous capillary electrophoresis Fourier transform infrared spectroscopy study. Appl Spectrosc. 2004, 58, 662–666. [Google Scholar] [CrossRef]
- Giorgio, E.; Viglione, R.G.; Zanasi, R.; Rosini, C. Ab initio calculation of optical rotatory dispersion (ORD) curves: A simple and reliable approach to the assignment of the molecular absolute configuration. J. Am. Chem. Soc. 2004, 126, 12968–12976. [Google Scholar] [CrossRef]
- Qiu, S.; De Gussem, E.; Tehrani, K.A.; Sergeyev, S.; Bultinck, P.; Herrebout, W. Stereochemistry of the Tadalafil diastereoisomers: A critical assessment of vibrational circular dichroism, electronic circular dichroism, and optical rotatory dispersion. J. Med. Chem. 2013, 56, 8903–8914. [Google Scholar] [CrossRef]
- Beaulieu, S.; Comby, A.; Descamps, D.; Fabre, B.; Garcia, G.A.; Géneaux, R.; Harvey, A.G.; Légaré, F.; Mašín, Z.; Nahon, L.; et al. Photoexcitation circular dichroism in chiral molecules. Nat. Phys. 2018, 14, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Kneer, L.M.; Roller, E.-M.; Besteiro, L.V.; Schreiber, R.; Govorov, A.O.; Liedl, T. Circular dichroism of chiral molecules in DNA-assembled plasmonic hotspots. ACS Nano 2018, 12, 9110–9115. [Google Scholar] [CrossRef] [Green Version]
- Vestler, D.; Ben-Moshe, A.; Markovich, G. Enhancement of circular dichroism of a chiral Material by dielectric nanospheres. J. Phys. Chem. C 2019, 123, 5017–5022. [Google Scholar] [CrossRef]
- Bégin, J.-L.; Alsaawy, M.; Bhardwaj, R. Chiral discrimination by recollision enhanced femtosecond laser mass spectrometry. Sci. Rep. 2020, 10, 14074. [Google Scholar] [CrossRef]
- Sparks, W.; Hough, J.H.; Germer, T.; Robb, F.; Kolokolova, L. Remote sensing of chiral signatures on Mars. Planet. Space Sci. 2012, 72, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Sofikitis, D.; Bougas, L.; Katsoprinakis, G.E.; Spiliotis, A.K.; Loppinet, B.; Rakitzis, T.P. Evanescent-wave and ambient chiral sensing by signal-reversing cavity ringdown polarimetry. Nature 2014, 514, 76–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wang, J.; Kang, L.; Liu, W.; Yu, L.; Zheng, B.; Brongersma, M.L.; Werner, D.H.; Lan, S.; Shi, Y.; et al. Monolithic full-Stokes near-infrared polarimetry with chiral plasmonic metasurface integrated graphene–silicon photodetector. ACS Nano 2020, 14, 16634–16642. [Google Scholar] [CrossRef] [PubMed]
- Risley, D.S.; Strege, M.A. Chiral separations of polar compounds by hydrophilic interaction chromatography with evaporative light scattering detection. Anal. Chem. 2000, 72, 1736–1739. [Google Scholar] [CrossRef]
- Zhang, T.; Nguyen, D.; Franco, P. Use of evaporative light scattering detector in the detection and quantification of enantiomeric mixtures by HPLC. J. Sep. Sci. 2006, 29, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.S.; Andrews, D.L. Laser optical separation of chiral molecules. Opt. Lett. 2015, 40, 677–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, M.L.; Saleh, A.A.E.; Poulikakos, L.V.; Abendroth, J.M.; Tadesse, L.F.; Dionne, J.A. Nanophotonic platforms for chiral sensing and separation. Acc. Chem. Res. 2020, 53, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Bryne, S.; Drouet d’Aubigny, C.; Viola, D.; Mikucki, J.; Ellis, W. Detection limits for chiral amino acids using a polarization camera. Planet. Sci. J. 2020, 1, 46. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef]
- Marcellos, C.F.C.; Durand, H.; Kwon, J.S.; Barreto, A.G., Jr.; da Cunha Lage, P.L.; de Souza, M.B., Jr.; Secchi, A.R.; Christofides, P.D. Optimal enantiomer crystallization operation using ternary diagram information. Comput. Aided Chem. Eng. 2018, 44, 499–504. [Google Scholar] [CrossRef]
- Lam, W.H.; Ng, K.M. Diastereomeric salt crystallization synthesis for chiral resolution of ibuprofen. AIChE J. 2007, 53, 429–437. [Google Scholar] [CrossRef]
- Pham, X.-H.; Kim, J.-M.; Chang, S.-M.; Kim, I.; Sim, W.K. Enantioseparation of D/L-mandelic acid with L-phenylalanine in diastereomeric crystallization. J. Mol. Catal. B Enzym. 2009, 60, 87–92. [Google Scholar] [CrossRef]
- Simon, M.; Wood, B.; Ferguson, S.; Glennon, B.; Jones, R.C. Diastereomeric salt crystallization of chiral molecules via sequential coupled-Batch operation. AIChE J. 2018, 65, 604–616. [Google Scholar] [CrossRef]
- Robinson, D.E.J.E.; Bull, S.D. Kinetic resolution strategies using non-enzymatic catalysts. Tetrahedron Asymmetry 2003, 14, 1407–1446. [Google Scholar] [CrossRef]
- Mousaw, P.; Saranteas, K.; Prytko, B. Crystallization improvements of a diastereomeric kinetic resolution through understanding of secondary nucleation. Org. Process Res. Dev. 2008, 12, 243–248. [Google Scholar] [CrossRef]
- Imayoshi, A.; Lakshmi, B.V.; Ueda, Y.; Yoshimura, T.; Matayoshi, A.; Furuta, T.; Kawabata, T. Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy. Nat. Commun. 2021, 12, 404. [Google Scholar] [CrossRef]
- Skelley, A.M.; Mathies, R.A. Chiral separation of fluorescamine-labeled amino acids using microfabricated capillary electrophoresis devices for extraterrestrial exploration. J. Chromatogr A 2003, 1021, 191–199. [Google Scholar] [CrossRef]
- Pu, L. Fluorescence of organic molecules in chiral recognition. Chem. Rev. 2004, 104, 1687–1716. [Google Scholar] [CrossRef]
- Ohrui, H.; Kato, R.; Kodaira, T.; Shimizu, H.; Akasaka, K.; Kitahara, T. Development of highly potent D-glucosamine-based chiral fluorescent labeling reagents and a microwave-assisted beta-selective glycosidation of a methyl glycoside reagent. Biosci. Biotechnol. Biochem. 2005, 69, 1054–1057. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Qu, K.; Ren, J.; Qu, X. Chiral detection using reusable fluorescent amylose-functionalized graphene. Chem. Sci. 2011, 2, 2050–2056. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Noell, A.C.; Willis, P.A. Long-term thermal stability of fluorescent dye used for chiral amino acid analysis on future spaceflight missions. Electrophoresis 2019, 40, 3117–3122. [Google Scholar] [CrossRef]
- Miller, S.L.; Van Trump, J.E. The Strecker synthesis in the primitive ocean. In Origin of Life; Wolman, Y., Ed.; Springer: Dordrecht, The Netherlands, 1981; pp. 135–141. [Google Scholar] [CrossRef]
- Rodriguez, L.E.; Altari, T.; Hermis, N.Y.; Jia, T.Z.; Roche, T.P.; Steller, L.H.; Weber, J.M. Astrobiology Primer 3.0 Chapter 4: A geological and chemical context for the origins of life on early Earth. Astrobiology, 2022; in review. [Google Scholar]
- Butlerov, A. Bildung einer zuckerartigen Substanz durch Synthese. Liebigs Ann. Chem. 1861, 120, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Mangas-Sanchez, J.; Turner, N.J.; Grogan, G. NAD(P)H-dependent dehydrogenases for the asymmetric reductive amination of ketones: Structure, mechanism, evolution and application. Adv. Synth. Catal. 2017, 359, 2011–2025. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, R.T.; Yadav, M.; Krishnamurthy, R.; Springsteen, G. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nat. Chem. 2020, 12, 1016–1022. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Maurya, R.A. Single nucleotide-catalyzed biomimetic reductive amination. Adv. Synth. Catal. 2010, 352, 2227–2232. [Google Scholar] [CrossRef]
- Storer, R.I.; Carrera, D.E.; Ni, Y.; MacMillan, D.W.C. Enantioselective organocatalytic reductive amination. J. Am. Chem. Soc. 2006, 128, 84–86. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.P.; Verma, S.S.; Pandey, J.; Tiwari, V.K. Recent development on catalytic reductive amination and applications. Curr. Org. Chem. 2008, 12, 1092–1115. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef]
- Strecker, A. Ueber einen neuen aus Aldehyd—Ammoniak und Blausäure entstehenden Körper. Justus Liebigs Ann. Der Chem. 1854, 91, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Harada, K. Asymmetric synthesis of α-amino-acids by the Strecker synthesis. Nature 1963, 200, 1201. [Google Scholar] [CrossRef]
- Gröger, H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem. Rev. 2003, 103, 2795–2828. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Pulletikurti, S.; Yadav, M.; Springsteen, G. Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways. Res. Sq. 2021; in review. [Google Scholar] [CrossRef]
- Davis, F.A.; Reddy, R.E.; Portonovo, P.S. Asymmetric strecker synthesis using enantiopure sulfinimines: A convenient synthesis of α-amino acids. Tetrahedron Lett. 1994, 35, 9351–9354. [Google Scholar] [CrossRef]
- Iyer, M.S.; Gigstad, K.M.; Namdev, N.D.; Lipton, M. Asymmetric catalysis of the Strecker amino acid synthesis by a cyclic dipeptide. Amino Acids 1996, 11, 259–268. [Google Scholar] [CrossRef]
- Aiba, S.; Takamatsu, N.; Sasai, T.; Tokunaga, Y.; Kawasaki, T. Replication of α-amino acids via Strecker synthesis with amplification and multiplication of chiral intermediate aminonitriles. Chem. Commun. 2016, 52, 10834–10837. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, S.; Yoshimura, K.; Yamazaki, Y.; Takamatsu, N.; Kuraish, T.; Aiba, S.; Tokunaga, Y.; Kawasaki, T. Asymmetric Strecker reaction arising from the molecular orientation of an achiral imine at the single-crystal face: Enantioenriched L- and D-amino acids. Angew. Chem. Int. Ed. 2016, 56, 1055–1058. [Google Scholar] [CrossRef]
- Legnani, L.; Darù, A.; Jones, A.X.; Blackmond, D.G. Mechanistic insight into the origin of stereoselectivity in the ribose-mediated Strecker synthesis of alanine. J. Am. Chem. Soc. 2021, 143, 7852–7858. [Google Scholar] [CrossRef]
- Jeilani, Y.A.; Nguyen, M.T. Autocatalysis in formose reaction and formation of RNA nucleosides. J. Phys. Chem. B 2020, 124, 11324–11336. [Google Scholar] [CrossRef]
- Cleaves, H.J. Formose reaction. In Encyclopedia of Astrobiology; Gargaud, M., Irvine, W.M., Amils, R., Cleaves, H.J., II, Pinti, D.L., Quintanilla, J.C., Rouan, D., Spohn, T., Tirard, S., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Fialho, D.M.; Clarke, K.C.; Moore, M.K.; Schuster, G.B.; Krishnamurthy, R.; Hud, N.V. Glycosylation of a model proto-RNA nucleobase with non-ribose sugars: Implications for the prebiotic synthesis of nucleosides. Org. Biomol. Chem. 2018, 16, 1263–1271. [Google Scholar] [CrossRef]
- Omran, A.; Menor-Salvan, C.; Springsteen, G.; Pasek, M. The messy alkaline formose reaction and its link to metabolism. Life 2020, 10, 125. [Google Scholar] [CrossRef]
- Mizuno, T.; Mori, T.; Shiomi, N.; Nakatsuji, H. Studies on synthesis and utilization of formose Part I. J. Agric. Chem. Soc. Jpn. 1970, 44, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Shigemasa, Y.; Nagae, O.; Sakazawa, C.; Nakashima, R.; Matsuura, T. Formose reactions. 5. A selective formose reaction. J. Am. Chem. Soc. 1978, 100, 1309–1310. [Google Scholar] [CrossRef]
- SMatsumoto, T.; Inoue, S. Formose reactions. Part 3. Selective formose reaction catalyzed by organic bases. J. Chem. Soc. Perkin Trans. 1982, 1, 1975–1979. [Google Scholar] [CrossRef]
- Kopetzki, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef] [Green Version]
- Eschenmoser, A. On a hypothetical generational relationship between HCN and constituents of the reductive citric acid cycle. Chem. Biodivers. 2007, 4, 554–573. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Skoblin, A.A.; Zanin, A.M.; Tverdislov, V.A.; Taran, O.P.; Parmon, V.N. Formation of chiral structures in UV-initiated formose reaction. Phys. Chem. 2018, 479, 57–60. [Google Scholar] [CrossRef]
- Breslow, R.; Cheng, Z.-L. L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 5723–5725. [Google Scholar] [CrossRef] [Green Version]
- Burroughs, L.; Clarke, P.A.; Forintos, H.; Gilks, J.A.R.; Hayes, C.J.; Vale, M.E.; Wade, W.; Zbytniewski, M. Asymmetric organocatalytic formation of protected and unprotected tetroses under potentially prebiotic conditions. Org. Biomol. Chem. 2012, 10, 1565–1570. [Google Scholar] [CrossRef]
- Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernández, J.G. Mechanochemical activation of iron cyano complexes: A prebiotic impact scenario for the synthesis of α-amino acid derivatives. Angew. Chem. 2018, 130, 2447–2450. [Google Scholar] [CrossRef]
- Eguaogie, O.; Vyle, J.S.; Conlon, P.F.; Gîlea, M.A.; Liang, Y. Mechanochemistry of nucleosides, nucleotides and related materials. Beilstein J. Org. Chem. 2018, 14, 955–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamour, S.; Pallmann, S.; Haas, M.; Trapp, O. Prebiotic sugar formation under nonaqueous conditions and mechanochemical acceleration. Life 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolar, T.; Grubešić, S.; Cindro, N.; Meštrović, E.; Užarević, K.; Hernández, J.G. Mechanochemical prebiotic peptide bond formation. Angew. Chem. Int. Ed. 2021, 60, 12727–12731. [Google Scholar] [CrossRef]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-mediated and metal-catalyzed reactions under mechanochemical conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Goldanskii, V.I. Nontraditional pathways of extraterrestrial formation of prebiotic matter. J. Phys. Chem. A 1997, 101, 3424–3432. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Bolm, C. A highly efficient asymmetric organocatalytic Aldol reaction in a ball mill. Chem. A Eur. J. 2007, 13, 4710–4722. [Google Scholar] [CrossRef]
- Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A.M.; Yang, W.; Chen, Y.I. Mechanochemistry: A force in disguise and conditional effects towards chemical reactions. Chem. Commun. 2021, 57, 1080–1092. [Google Scholar] [CrossRef]
- Fiss, B.G.; Richard, A.J.; Friščić, T.; Moores, A. Mechanochemistry for sustainable and efficient dehydrogenation/hydrogenation. Can. J. Chem. 2020, 99, 93–112. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Constable, D.J.C.; Ponder, C.S. Evaluating the “Greenness” of chemical processes and products in the pharmaceutical industry—a green metrics primer. Chem. Soc. Rev. 2012, 41, 1485–1498. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical synthesis of small organic molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Chyba, C.F.; Thomas, P.J.; Brookshaw, L.; Sagan, C. Cometary delivery of organic molecules to the early Earth. Science 1990, 249, 366–373. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef]
- Bernstein, M. Prebiotic materials from on and off the early Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1689–1702. [Google Scholar] [CrossRef] [Green Version]
- Pierazzo, E.; Chyba, C. Impact delivery of prebiotic organic matter to planetary surfaces. In Comets and the Origin and Evolution of Life. Advances in Astrobiology and Biogeophysics; Thomas, P.J., Hicks, R.D., Chyba, C.F., McKay, C.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 137–168. [Google Scholar] [CrossRef]
- Hörz, F.; Cintala, M.J. Impact experiments related to the evolution of planetary regoliths. Meteorit. Planet. Sci. 1997, 32, 179–209. [Google Scholar] [CrossRef]
- Hörz, F.; Basilevsky, A.T.; Head, J.W.; Cintala, M.J. Erosion of lunar surface rocks by impact processes: A synthesis. Planet Space Sci. 2020, 194, 105105. [Google Scholar] [CrossRef]
- Peterson, E.; Hörz, F.; Chang, S. Modification of amino acids at shock pressures of 3.5 to 32 GPa. Geochim. Cosmochim. Acta 1997, 61, 3937–3950. [Google Scholar] [CrossRef]
- Martins, Z.; Price, M.C.; Goldman, N.; Sephton, M.A.; Burchell, M.J. Shock synthesis of amino acids from impacting cometary and icy planet surface analogues. Nat. Geosci. 2013, 6, 1045–1049. [Google Scholar] [CrossRef]
- McCaffrey, V.P.; Zellner, N.E.B.; Waun, C.; Bennett, E.R.; Karl, E.K. Reactivity and survivability of glycolaldehyde in simulated meteorite impact experiments. Orig. Life Evol. Biosph. 2014, 44, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, H.; Mimura, K. Shock-induced pyrolysis of amino acids at ultra high pressures ranged from 3.2 to 35.3 GPa. J. Anal. Appl. Pyrolysis 2014, 108, 170–175. [Google Scholar] [CrossRef]
- Sugahara, H.; Mimura, K. Glycine oligomerization up to triglycine by shock experiments simulating comet impacts. Geochem. J. 2014, 48, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, H.; Mimura, K. Peptide synthesis triggered by comet impacts: A possible method for peptide delivery to the early Earth and icy satellites. Icarus 2015, 257, 103–112. [Google Scholar] [CrossRef]
- Frantseva, K.; Mueller, M.; ten Kate, I.L.; van der Tak, F.F.S.; Greenstreet, S. Delivery of organics to Mars through asteroid and comet impacts. Icarus 2018, 309, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Botero, A.; Cable, M.L.; Hofmann, A.E.; Malaska, M.; Hodyss, R.; Lunine, J. Understanding hypervelocity sampling of biosignatures in space missions. Astrobiology 2020, 21, 421–442. [Google Scholar] [CrossRef]
- Klenner, F.; Postberg, F.; Hillier, J.; Khawaja, N.; Cable, M.L.; Abel, B.; Kempf, S.; Glein, C.R.; Lunine, J.I.; Hodyss, R.; et al. Discriminating abiotic and biotic fingerprints of amino acids and fatty acids in ice grains relevant to ocean worlds. Astrobiology 2020, 20, 1168–1184. [Google Scholar] [CrossRef]
- Des Marais, D.J.; Nuth, J.A., III; Allamandola, L.J.; Boss, A.P.; Farmer, J.D.; Hoehler, T.M.; Jakosky, B.M.; Meadows, V.S.; Pohorille, A.; Runnegar, B.; et al. The NASA Astrobiology Roadmap. Astrobiology 2008, 8, 715–730. [Google Scholar] [CrossRef]
- Beegle, L.W.; Bhartia, R.; White, M.; DeFlores, L.; Abbey, W.; Wu, Y.-H.; Cameron, B.; Moore, J.; Fries, M.; Burton, A.; et al. SHERLOC: Scanning Habitable Environments with Raman & Luminescence for Organics & Chemicals. In Proceedings of the IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2015. [Google Scholar] [CrossRef]
- Bhartia, R.; Beegle, L.W.; DeFlores, L.; Abbey, W.; Razzell Hollis, J.; Uckert, K.; Monacelli, B.; Edgett, K.S.; Kennedy, M.R.; Sylvia, M.; et al. Perseverance’s Scanning Habitable Environments with Raman and Luminescence for Organics and Chemicals (SHERLOC) Investigation. Space Sci. Rev. 2021, 217, 58. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Thiemann, W.H.; Goesmann, F.; Roll, R.; Rosenbauer, H. Enantiomer separation of hydrocarbons in preparation for ROSETTA’s “chirality-experiment”. Chirality 2001, 13, 454–457. [Google Scholar] [CrossRef]
- Szopa, C.; Sternberg, R.; Coscia, D.; Raulin, F.; Vidal-Madjar, C.; Rosenbauer, H. Gas chromatography for in situ analysis of a cometary nucleus III. Multi-capillary column system for the cometary sampling and composition experiment of the Rosetta lander probe. J. Chromatogr. A 2002, 953, 165–173. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Rosenbauer, H.; Boehnhardt, H.; Ulamec, S.; Biele, H.; Espinasees, S.; Feuerbacher, B.; Gaudon, P.; Hemmerich, P.; Kletzkine, P.; et al. The Rosetta lander (“Philae”) investigations. Space Sci. Rev. 2007, 128, 205–220. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Le Roy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, aab0689. [Google Scholar] [CrossRef]
- Sephton, M.A.; Carter, J.N. The chances of detecting life on Mars. Planet. Space Sci. 2015, 112, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Fairén, A.G.; Parro, V.; Schulze-Makuch, D.; Whyte, L. Is searching for Martian life a priority for the Mars community? Astrobiology 2018, 18, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Carrier, B.L.; Beaty, D.W.; Meyer, M.A.; Blank, J.G.; Chou, L.; DasSharma, S.; Des Marais, D.J.; Eigenbrode, J.L.; Grefenstette, N.; Lanza, N.L.; et al. Mars extant life: What’s next? Conference Report. Astrobiology 2020, 20, 785–814. [Google Scholar] [CrossRef]
- Steele, A.; McCubbin, F.M.; Fries, M.; Kater, L.; Boctor, Z.; Fogel, M.L.; Conrad, P.G.; Glamocija, M.; Spencer, M.; Morrow, A.L.; et al. A reduced organic carbon component in Martian basalts. Science 2012, 337, 212–215. [Google Scholar] [CrossRef]
- Steele, A.; McCubbin, F.M.; Fries, M.D. The provenance, formation, and implications of reduced carbon phases in Martian meteorites. Meteorit. Planet. Sci. 2016, 51, 2203–2225. [Google Scholar] [CrossRef]
- Steele, A.; Benning, L.G.; Wirth, R.; Schreiber, A.; Araki, T.; McCubbin, F.M.; Fries, M.D.; Nittler, L.R.; Wang, J.; Hallis, L.J.; et al. Organic synthesis associated with serpentinization and carbonation on early Mars. Science 2022, 375, 172–177. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Crisp, J.; Vasavada, A.R.; Anderson, R.C.; Baker, C.J.; Barry, R.; Blake, D.F.; Conrad, P.; Edgett, K.S.; Ferdowski, B.; et al. Mars Science Laboratory mission and science investigation. Space Sci. Rev. 2012, 170, 5–56. [Google Scholar] [CrossRef] [Green Version]
- Farley, K.A.; Williford, K.H.; Stack, K.M.; Bhartia, R.; Chen, A.; de la Torre, M.; Hand, K.; Goreva, Y.; Herd, C.D.K.; Hueso, R.; et al. Mars 2020 mission overview. Space Sci. Rev. 2020, 216, 142. [Google Scholar] [CrossRef]
- Millan, M.; Szopa, C.; Buch, A.; Cabane, M.; Teinturier, S.; Mahaffy, P.; Johnson, S.S. Performance of the SAM gas chromatographic columns under simulated flight operating conditions for the analysis of chlorohydrocarbons on Mars. J. Chromatogr. A 2019, 1598, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Quantin-Nataf, C.; Carter, J.; Mandon, L.; Thollot, P.; Balme, M.; Volat, M.; Pan, L.; Loizeau, D.; Millot, C.; Breton, S.; et al. Oxia Planum: The landing site for the ExoMars “Rosalind Franklin” rover mission: Geological context and prelanding interpretation. Astrobiology 2021, 21, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.L.; Westall, F.; Pasteur Instrument Teams; Landing Site Selection Working Group and Other Contributors; Coates, A.J.; Jaumann, R.; Korablev, O.; Ciarletti, V.; Mitrofanov, I.; Josset, J.-L.; et al. Habitability on early Mars and the search for biosignatures with the ExoMars rover. Astrobiology 2017, 17, 471–510. [Google Scholar] [CrossRef]
- Razzell Hollis, J.; Ireland, S.; Abbey, W.; Bhartia, R.; Beegle, L.W. Deep-ultraviolet Raman spectra of Mars-relevant evaporite minerals under 248.6 nm excitation. Icarus 2021, 357, 114067. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Henderson, B.L.; Herman, J.; Zhong, F.; Lin, Y.; Kanik, I.; Nixon, C.A. Extraction and separation of chiral amino acids for life detection on ocean worlds without using organic solvents or derivatization. Astrobiology 2021, 21, 575–586. [Google Scholar] [CrossRef]
- Foroughbakhshfasaei, M.; Dobó, M.; Boda, F.; Szabó, Z.; Tóth, G. Comparative chiral separation of thalidomide class of drugs using polysaccharide-type stationary phases with emphasis on elution order and hysteresis in polar organic mode. Molecules 2022, 27, 111. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, X.; Qin, S.; Dong, Q.; Hu, X.; Chu, H. A covalent organic framework for chiral capillary electrochromatography using a cyclodextrin mobile phase additive. Chirality 2022, 24, 537–549. [Google Scholar] [CrossRef]
- Li, X.-L.; Han, Y.; Huang, Y.; Sun, X.; Xiao, S.; Min, J.Z. Highly sensitive novel fluorescent chiral probe possessing (S)-2-methylproline structures for the determination of chiral amino compounds by ultra-performance liquid chromatography with fluorescence: An application in the saliva of healthy volunteer. J. Chromatogr. A 2022, 1661, 462672. [Google Scholar] [CrossRef]
- Niu, X.; Yan, S.; Chen, J.; Li, H.; Wang, K. Enantioselective recognition of L/D-amino acids in the chiral nanochannels of a metal-organic framework. Electrochim. Acta 2022, 405, 139809. [Google Scholar] [CrossRef]
- Arevalo, R., Jr.; Ni, Z.; Danell, R.M. Mass spectrometry and planetary exploration: A brief review and future projection. J. Mass Spectrom. 2020, 55, e4454. [Google Scholar] [CrossRef]
- Chou, L.; Mahaffy, P.; Trainer, M.; Eigenbrode, J.; Arevalo, R.; Brinckerhoff, W.; Getty, S.; Grefenstette, N.; Da Poian, V.; Fricke, G.M.; et al. Planetary mass spectrometry for agnostic life detection in the Solar System. Front. Astron. Space Sci. 2021, 8, 755100. [Google Scholar] [CrossRef]
- Willis, P.A.; Creamer, J.S.; Mora, M.F. Implementation of microchip electrophoresis instrumentation for future spaceflight missions. Anal. Bioanal. Chem. 2015, 407, 6939–6963. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Enhanced resolution of chiral amino acids with capillary electrophoresis for biosignature detection in extraterrestrial samples. Anal. Chem. 2017, 89, 1329–1337. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Stability of reagents used for chiral amino acid analysis during spaceflight missions in high-radiation environments. Electrophoresis 2018, 39, 2864–2871. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, Y. Atomic-level handedness determination of chiral crystals using aberration-corrected scanning transmission electron microscopy. Nat. Commun. 2020, 11, 1588. [Google Scholar] [CrossRef]
- Eigenbrode, J.L.; Gold, R.; Canham, J.S.; Schulze, E.; Davila, A.F.; Seas, A.; Errigo, T.; Kujawa, F.; Kusnierkiewicz, D.; Lorentson, C.; et al. Contamination control for ultra-sensitive life-detection missions. Front. Space Technol. 2021, 2, 734423. [Google Scholar] [CrossRef]
- Spiers, E.M.; Weber, J.M.; Venigalla, C.; Annex, A.M.; Chen, C.P.; Lee, C.; Gray, P.C.; McIntyre, K.J.; Berdis, J.R.; Carberry Mogan, S.R.; et al. Tiger: Concept study for a New Frontiers Enceladus habitability mission. Planet. Sci. J. 2021, 2, 195. [Google Scholar] [CrossRef]
- Hendrickson, R.; Urbaniak, C.; Minich, J.J.; Aronson, H.S.; Martino, C.; Stepanauskas, R.; Knight, R.; Venkateswaran, K. Clean room microbiome complexity impacts planetary protection bioburden. Microbiome 2021, 9, 238. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P.; Lupisella, M.; Kminek, G.; Rummel, J.D. Biological contamination studies of lunar landing sites: Implications for future planetary protection and life detection on the Moon and Mars. Int. J. Astrobiol. 2005, 3, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. JGR Planets 2013, 118, 1955–1973. [Google Scholar] [CrossRef]
- Miller, K.E.; Kotrc, B.; Summons, R.E.; Belmahdi, I.; Buch, A.; Eigenbrode, J.L.; Freissinet, C.; Glavin, D.P.; Szopa, C. Evaluation of the Tenax trap in the Sample Analysis at Mars instrument suite on the Curiosity rover as a potential hydrocarbon source for chlorinated organics detected in Gale Crater. JGR Planets 2015, 120, 1446–1459. [Google Scholar] [CrossRef] [Green Version]
- Craven, E.; Winters, M.; Smith, A.L.; Lalime, E.; Mancinelli, R.; Shirey, B.; Schubert, W.; Schuerger, A.; Burgin, M.; Seto, E.P.; et al. Biological safety in the context of backward planetary protection and Mars Sample Return: Conclusions from the Sterilization Working Group. Int. J. Astrobiol. 2021, 20, 1–28. [Google Scholar] [CrossRef]
- Longo, A.; Damer, B. Factoring origin of life hypotheses into the search for life in the Solar System and beyond. Life 2020, 10, 52. [Google Scholar] [CrossRef]
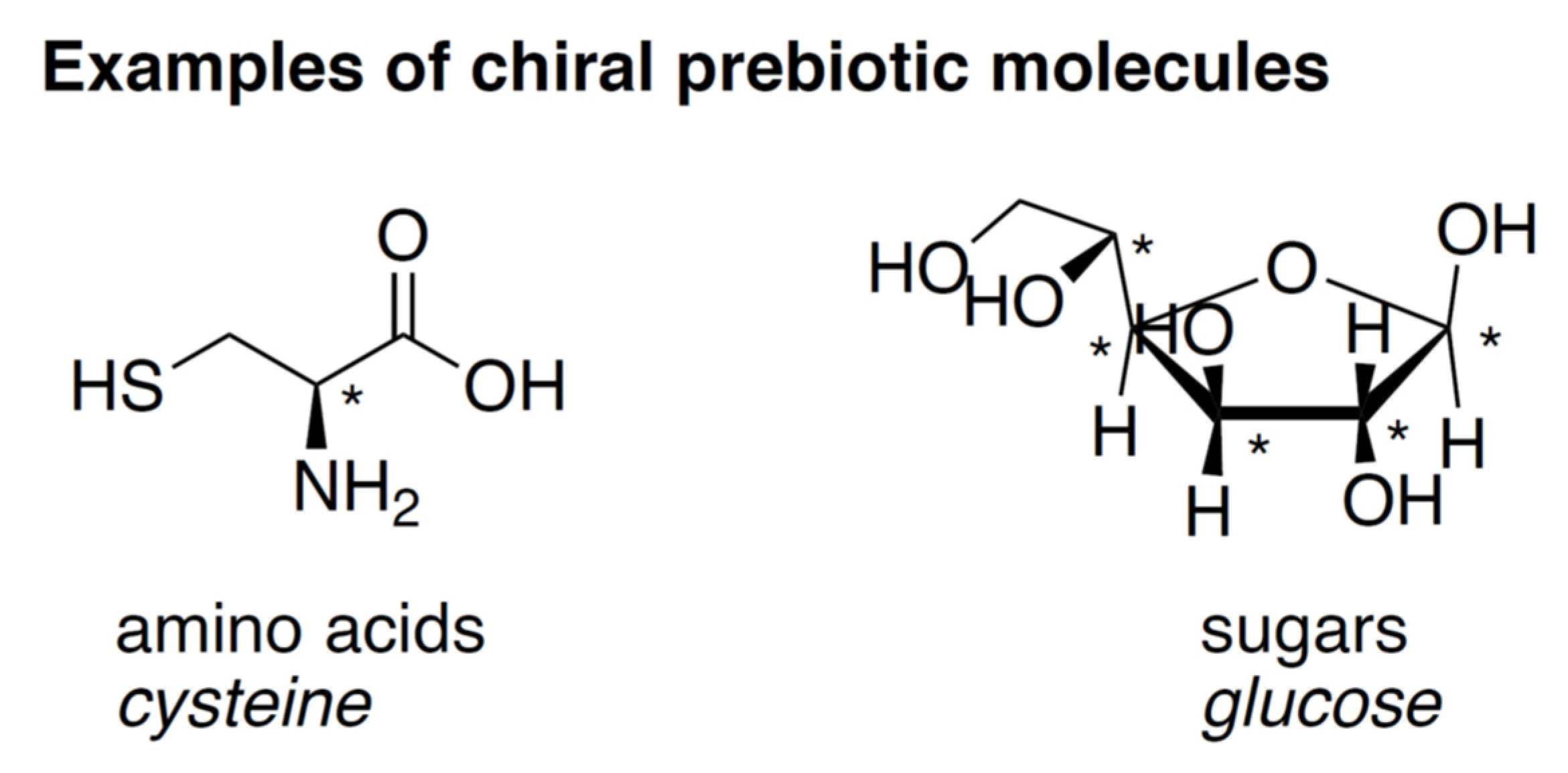






| Reaction | Starting Material | Product | Relevant Field(s) | References |
|---|---|---|---|---|
| Soai | pyrimidine-5-carbaldehyde | pyrimidyl alcohol | Origins of life; autocatalysis | [26,74] |
| Aldehyde | Strecker | Amino acid | Origins of life, pharmaceutical and natural product synthesis | [75] |
| Strecker | Ketone | ɑ,ɑ-disubstituted amino acids | Origins of life, pharmaceutical and natural product synthesis | [76] |
| Reductive amination | Alpha keto acid | Amino acid | Origins of life, pharmaceutical and natural product synthesis | [77,78] |
| Kiliani–Fischer synthesis | Sugar | Monosaccharide | Origins of life | [79,80] |
| Sharpless epoxidation | Allylic alcohols | 2,3-epoxyalcohols | Pharmaceutical and natural product synthesis | [81] |
| Sharpless bishydroxylation | Alkene | Vicinal diol | Pharmaceutical and natural product synthesis | [82] |
| Sharpless oxyamination | Alkene | Vicinal amino diol | Pharmaceutical and natural product synthesis | [83] |
| Midland reduction | Carbonyl (ketone) | Alcohol | Pharmaceutical and natural product synthesis | [84] |
| Noyori asymmetric hydrogenation | Keto ester | Hydroxy ester | Pharmaceutical and natural product synthesis | [85] |
| Corey-Itsuno reduction | Ketone (achiral) | Alcohol (chiral, non-racemic) | Pharmaceutical and natural product synthesis; industrial synthesis | [86,87] |
| Asymmetric Diels-Alder | Diene and alkene | Cyclohexene | Pharmaceutical and natural product synthesis; industrial synthesis | [88,89,90,91,92,93] |
| Examples of asymmetric cross-coupling reactions | ||||
| Suzuki-Miyaura | Alkyl- or arylhalides + organoborates | Alkyl or aryl compounds | Pharmaceutical and natural product synthesis; industrial synthesis; catalysis | [13,94,95] |
| Ni/Photoredox dual catalysis | Varied | Varied | Pharmaceutical and natural product synthesis; catalysis | [96,97,98,99] |
| Buckwald-Hartwig amination | Varied | Amine | Pharmaceutical and natural product synthesis; industrial synthesis; catalysis | [100,101] |
| Planetary Body | Major Surface Minerals | Major Ices | References |
|---|---|---|---|
| Mercury | Plagioclase, olivine, pyroxene, sulfide, graphite | Water | [145,146,147,148,149] |
| Venus | Theorized: Olivine, pyroxene, sulfide, Fe oxides, carbonates, ilmenite, sulfate | None identified to date | [150,151] |
| Earth | Olivine, pyroxene, plagioclase, anorthite, quartz, Ca carbonate, phyllosilicates, Fe oxides | Water, lesser methane | [152,153,154,155] |
| Moon | Anorthite, plagioclase, pyroxene, olivine, ilmenite | Water | [146,156,157] |
| Mars | Olivine, pyroxene, phyllosilicates, sulfates, Fe oxides | Water, CO2, possibly methane | [146,158,159,160,161] |
| Asteroids, moons, and dwarf planets | Olivine, pyroxene, phyllosilicates, carbonates, Fe oxides | Water, methane, nitrogen, CO2, CO | [162,163,164,165,166] |
| Crystal Family | Crystal Class Number | Crystal Class | Space Group | Example Mineral | Formula | Category |
|---|---|---|---|---|---|---|
| Triclinic | 1 | Pedial | P1 | Kaolinite Amesite Nordstrandite | Al2(Si2O5)(OH)4 Mg2Al2SiO5(OH)4 Al(OH)3 | Phyllosilicate Phyllosilicate Metal oxide |
| Monoclinic | 2 | Sphenoidal | P2, P21, C2 | Buddingtonite Bassanite | NH4AlSi3O8 Ca(SO4)·0.5H2O | Tectosilicate Sulfate |
| Orthorhombic | 222 | Rhombic-disphenoidal | P222, P2221, P21212, P212121, C222, C2221, F222, I222, I212121 | Wülfingite Epsomite Sanderite Lecontite Abuite | Zn(OH)2 MgSO4·7H2O MgSO4·2H2O (NH4,K)NaSO4·2H2O CaAl2(PO4)2F2 | Metal oxide Sulfate Sulfate Sulfate Phosphate |
| Tetragonal | 4 | Tetragonal-pyramidal | P4, P41, P42, P43, I4, I41 | |||
| 422 | Tetragonal-trapezoidal | P422, P4212, P4122, P41212, P4222, P42212, P4322, P43212, I4122, I4212 | Cristobalite | SiO2 | Tectosilicate | |
| Wardite | NaAl3(PO4)2(OH)4·2(H2O) | Phosphate | ||||
| Hexagonal | 3 | Trigonal-pyramidal | P3, P31, P32, R3 | Monohydrocalcite | CaCO3·H2O | Carbonate |
| 32 | Trigonal-trapezohedral | P312, P3112, P3212c, P3212, P3121, P3221, R32 | Berlinite α-D-quartz α-L-quartz Antarcticite Huntite | AlPO4 SiO2 SiO2 CaCl2 ·6H2O Mg3Ca(CO3)4 | Phosphate Tectosilicate Tectosilicate Chloride Carbonate | |
| 6 | Hexagonal-pyramidal | P6, P61, P62, P63, P64, P65 | Trinepheline Kellyite Nagelschmidtite | SiO2 SiO2 KAlSiO4 Mg4Al2(OH)12(CO3)·3 H2O | Silicate Phyllosilicate Neosilicate | |
| 622 | Hexagonal-trapezohedral | P622, P6122, P6222, P6322, P6422, P6522 | β-D-quartz β-L-quartz Kalsilite Quintinite | (Ni,Fe)4P MnSi FeSi K2Mg2(SO4)3 | Tectosilicate Tectosilicate Kalsilite Carbonate | |
| Cubic | 23 | Tetaroidal | P23, P213, F23, I23, I213 | Melliniite Brownleeite Naquite Langbeinite | NH4Clγ-Fe2O3 | Phosphide Silicide Silicide Sulfate |
| 432 | Gyroidal | P423, P4232, P4332, P4132, F432, F432, I432, I4132 | Salammoniac Maghemite | Chloride Metal oxide |
| Mineral | Formula | Face {Miller Index} | Category |
|---|---|---|---|
| Calcite | CaCO3 | (214) | Carbonate |
| Gypsum | CaSO4·2H2O | (110), (111) | Sulfate |
| Olivine | (Mg2+, Fe2+)2SiO4 | (111) | Silicate |
| Clinopyroxene | (Ca,Mg,Fe,Na)(Mg,Fe,Al)(Si,Al)2O6 | (110), (111) | Oxide |
| Clinoamphibole: e.g., hornblende | (Ca,Na)2–3(Mg,Fe,Al)5(Al,Si)8O22(OH,F)2 | (110), (011) | Inosilicate |
| Petrologic Type | CI | CM | CK | CV | CO | CR | CH | CB |
|---|---|---|---|---|---|---|---|---|
| Petrologic type | 1 | 1–2 | 3–6 | 2–3 | 3 | 1–2 | 3 | 3 |
| Chondrule abundance (vol.%) | ≪1 † | 20 ‡ | 15 | 45 | 40–48 | 50–60 | ~70 | 20–40 |
| Matrix abundance (vol.%) | >99 † | 70 ‡ | 75 | 40 | 30–34 | 30–50 | 5 | <5 |
| Refractory abundance ⧺ (vol.%) | ≪1 | 5 | 4 | 10 | 13 | 0.5 | 0.1 | <0.1 |
| Metal (Fe,Ni) abundance (vol.%) | ≪1 | 0.1 | ≪1 | 0–5 | 1–5 | 5–8 | 20 | 60–80 |
| Average chondrule diameter (mm) | n.a. | 0.3 | 0.7–0.8 | 1.0 | 0.15 | 0.7 | 0.02–0.09 | 0.2–10 |
| Olivine composition | ||||||||
| (mol% Fe2SiO4; range) | * | * | <1–47 | * | * | <1–36 | 2–3 | |
| (mol% Fe2SiO4; mode) | 29–33 | 1–3 | 2 | 3 | ||||
| Refractory lithophiles ∦ | 1.00 | 1.15 | 1.21 | 1.35 | 1.13 | 1.03 | 1.00 | 1.0–1.4 |
| Instrument | Separation | Detector | Application | Mission Relevance | References |
|---|---|---|---|---|---|
| Chromatography and Spectrometry | |||||
| Gas chromatography– mass spectrometry | GC with a chiral column | MS | Organic chemistry; origins of life | Cometary Sampling and Composition (COSAC)-Rosetta: launched March 2004 but sampling unsuccessful Sample analysis at Mars-Mars Science Laboratory: in progress, landed August 2012 Mars Organic Molecule Analyzer (MOMA)-ExoMars: planned September 2022 launch | [361,362,363,364,365] |
| Liquid chromatography– mass spectrometry; high performance LC-MS | (HP)LC with a chiral column | MS (various) | Organic chemistry; origins of life | No | [366,367,368] |
| Sub- and supercritical fluid chromatography (SFC) | SF (CO2 plus polar co-solvent) | Various: UV-Vis, diode-array, evaporative light scattering (ELS) detector, charged-aerosol detection, MS (atmospheric pressure chemical ionization, electrospray ionization) | Organic chemistry; forensics | No | [369,370] |
| Capillary electrophoresis (CE) | CE | Laser-induced fluorescence (LIF) | Origins of life; organic chemistry; instrument development | Proposed | [371,372,373] |
| Capillary electrochromatography (CEC) | CE/HPLC | Various; UV detectors | Organic chemistry | No | [374,375,376] |
| Ligand exchange CE | CE | Various; UV detectors | Organic chemistry | No | [377,378,379] |
| Non-aqueous CE (NACE) | CE | Various detectors; UV, conductivity, MS, LIF | Organic chemistry; medicine | No | [380] |
| Ion-mobility mass spectrometry (IM-MS) | Derivatization, chiral neutral gases | IM-MS | Organic chemistry; origins of life | Volatile Organic Analyzer (VOA) on the International Space Station (ISS); for air quality control not enantiomeric separation—deployed August 2001 | [381,382,383,384] |
| Photodissociation | Photodissociation in cold gas phase | Various MS; e.g., ESI | Biochemistry | No | [385,386,387] |
| Matrix-assisted laser desorption ionization (MALDI)—time of flight (TOF) MS | Stereosensitive fragmentation (SF) | MALDI-TOF/TOF MS | Biochemistry | No | [388] |
| Spectroscopy | |||||
| Nuclear Magnetic Resonance (NMR) | Various, derivatization (typically to form diastereomers) | NMR | Organic chemistry | No | [358,389] |
| Ultraviolet (UV)-visible (Vis) spectrophotometry | Various | UV-Vis | Organic chemistry | No | [390,391] |
| Infrared (IR) spectroscopy | Various, e.g., CE, NACE | FT-IR | Organic chemistry | No | [392,393] |
| Optical rotatory dispersion (ORD) | Polarized light | Detector | Organic chemistry | No | [394,395] |
| Circular dichroism (CD) | Circularly polarized light | CD detector (various) | Organic chemistry; biochemistry | No | [396,397,398] |
| Femto-second (fs) laser mass spectrometry | fs-laser | MS | Organic chemistry | No | [399] |
| Polarimetry | Various; cavity ringdown, near IR | Detector, photodetector | Materials science; origins of life | Proposed | [400,401,402] |
| Optical techniques | |||||
| Evaporative light scattering (ELS) | Hydrophilic interaction chromatography (HILIC) | Light scattering detector (LSD) | Organic chemistry | No | [403] |
| ELS | High performance liquid chromatography (HPLC) | LSD | Organic chemistry | No | [404] |
| Laser | Off-resonant laser beam | Detector | Nanotechnology | No | [405] |
| Atomic force microscopy (AFM) | Optical tweezers | Optical and AFM | Nanotechnology; materials science | No | [406] |
| Polarization camera | Micropolarizer array | Detector | Origins of life | Proposed | [407] |
| Calorimetry | |||||
| Differential scanning calorimetry (DSC) | Thermal | Calorimeter | Organic chemistry; macromolecules | No | [254,408] |
| Separation | |||||
| Batch crystallization | Various; e.g., chromatography | Model that calculates the optimal conditions for separation | Organic chemistry | No | [409] |
| Diastereoisomeric recrystallization | Crystallization | Various, e.g., MS, DSC, X-ray diffraction (XRD) | Organic chemistry | No | [410,411,412] |
| Kinetic resolution | Various, e.g., chiral catalysts | Various, e.g., HPLC-MS, ESI-MS | Organic chemistry | No | [413,414,415] |
| Labeling | |||||
| Fluorescent sensors/dyes | Various dyes, e.g., 5-carboxyfluorescein succinimidyl ester, fluorescamine | Various fluorescence detectors (e.g., confocal fluorescence microscope) | Origins of life; organic chemistry | Proposed | [416,417,418,419,420] |
| Agency | Mission | Status | Instrument | Total GC Columns | Chiral Column(s) |
|---|---|---|---|---|---|
| ESA | Rosetta | Flown but unsuccessful | COSAC | 8 | Chirasil Dex CB Chirasil L Val Cyclodextrin G-TA |
| NASA | MSL | In progress | SAM | 6 | Chirasil-β Dex CB |
| ESA/Roscosmos | ExoMars | Planned | MOMA | 4 | CP Chirasil Dex |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Weber, J.M.; Rodriguez, L.E.; Sheppard, R.Y.; Barge, L.M.; Berger, E.L.; Burton, A.S. Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions. Symmetry 2022, 14, 460. https://doi.org/10.3390/sym14030460
Lee C, Weber JM, Rodriguez LE, Sheppard RY, Barge LM, Berger EL, Burton AS. Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions. Symmetry. 2022; 14(3):460. https://doi.org/10.3390/sym14030460
Chicago/Turabian StyleLee, Carina, Jessica M. Weber, Laura E. Rodriguez, Rachel Y. Sheppard, Laura M. Barge, Eve L. Berger, and Aaron S. Burton. 2022. "Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions" Symmetry 14, no. 3: 460. https://doi.org/10.3390/sym14030460
APA StyleLee, C., Weber, J. M., Rodriguez, L. E., Sheppard, R. Y., Barge, L. M., Berger, E. L., & Burton, A. S. (2022). Chirality in Organic and Mineral Systems: A Review of Reactivity and Alteration Processes Relevant to Prebiotic Chemistry and Life Detection Missions. Symmetry, 14(3), 460. https://doi.org/10.3390/sym14030460







