Differential Impact of Forest Fragmentation on Fluctuating Asymmetry in South Amazonian Small Mammals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Specimens
2.2. Fluctuating Asymmetry Estimates
2.3. Estimates of Landscape Metrics
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffé, R.; Nunes, S.; Dos Santos, J.F.; Gastauer, M.; Giannini, T.C.; Nascimento, W.; Sales, M.; Souza, C.M.; Souza-Filho, P.W.; Fletcher, R.J. Forecasting Deforestation in the Brazilian Amazon to Prioritize Conservation Efforts. Environ. Res. Lett. 2021, 16, 084034. [Google Scholar] [CrossRef]
- Umetsu, F.; Paul Metzger, J.; Pardini, R. Importance of Estimating Matrix Quality for Modeling Species Distribution in Complex Tropical Landscapes: A Test with Atlantic Forest Small Mammals. Ecography 2008, 31, 359–370. [Google Scholar] [CrossRef]
- Fialho, M.Y.G.; Cerboncini, R.A.S.; Passamani, M. Can Vegetation Corridors Support a Small Mammal Community Similar to That Found within Forest Fragments? A Case Study in Southeastern Brazil. Stud. Neotrop. Fauna Environ. 2017, 52, 64–67. [Google Scholar] [CrossRef]
- Rubio, A.V.; Ávila-Flores, R.; Suzán, G. Responses of Small Mammals to Habitat Fragmentation: Epidemiological Considerations for Rodent-Borne Hantaviruses in the Americas. Ecohealth 2014, 11, 526–533. [Google Scholar] [CrossRef]
- Cerboncini, R.A.S.; Roper, J.J.; Passos, F.C. Edge Effects without Habitat Fragmentation? Small Mammals and a Railway in the Atlantic Forest of Southern Brazil. Oryx 2016, 50, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.D.; Coda, J.; Simone, I.; Martínez, J.; Bonatto, F.; Steinmann, A.R.; Priotto, J. Agricultural Land-Use Intensity and Its Effects on Small Mammals in the Central Region of Argentina. Mammal Res. 2015, 60, 415–423. [Google Scholar] [CrossRef]
- Delciellos, A.C.; Vieira, M.V.; Grelle, C.E.V.; Cobra, P.; Cerqueira, R. Habitat Quality versus Spatial Variables as Determinants of Small Mammal Assemblages in Atlantic Forest Fragments. J. Mammal. 2016, 97, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Santos-Filho, M.; Bernardo, C.S.S.; Da Silva, D.J.; Ignácio, A.R.A.; Canale, G.R. The Importance of Considering Both Taxonomic and Habitat Guild Approaches in Small Mammal Research. Austral Ecol. 2016, 41, 854–863. [Google Scholar] [CrossRef]
- Manning, J.T.; Chamberlain, A.T. Fluctuating Asymmetry in Gorilla Canines: A Sensitive Indicator of Environmental Stress. Proc. R. Soc. B Biol. Sci. 1994, 255, 189–193. [Google Scholar] [CrossRef]
- Wauters, L.A.; Dhondt, A.A.; Knothe, H.; Parkin, D.T. Fluctuating Asymmetry and Body Size as Indicators of Stress in Red Squirrel Populations in Woodland Fragments. J. Appl. Ecol. 1996, 33, 735. [Google Scholar] [CrossRef]
- Teixeira, C.P.; Hirsch, A.; Perini, H.; Young, R.J. Marsupials from Space: Fluctuating Asymmetry, Geographical Information Systems and Animal Conservation. Proc. R. Soc. B Biol. Sci. 2006, 273, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccavo, A.; Lemos, H.; Maroja, L.S.; Gonçalves, P.R. Does Stress Mess with Rodents’ Heads? Influence of Habitat Amount and Genetic Factors in Mandible Fluctuating Asymmetry in South American Water Rats (Nectomys Squamipes, Sigmodontinae) from Brazilian Atlantic Rainforest Remnants. Ecol. Evol. 2021, 11, 7080–7092. [Google Scholar] [CrossRef]
- Dongen, S.V. Fluctuating Asymmetry and Developmental Instability in Evolutionary Biology: Past, Present and Future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Leamy, L.J.; Klingenberg, C.P. The Genetics and Evolution of Fluctuating Asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Nijhout HF, D.G. Developmental Perspectives on Phenotypic Variation: Canalization, and Fluctuating Asymmetry. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 3–13. [Google Scholar]
- Lens, L.; van Dongen, S.; Wilder, C.M.; Brooks, T.M.; Matthysen, E. Fluctuating Asymmetry Increases with Habitat Disturbance in Seven Bird Species of a Fragmented Afrotropical Forest. Proc. R. Soc. B Biol. Sci. 1999, 266, 1241–1246. [Google Scholar] [CrossRef]
- Anciães, M.; Marini, M. Ǎ The Effects of Fragmentation on Fluctuating Asymmetry in Passerine Birds of Brazilian Tropical Forests. J. Appl. Ecol. 2000, 37, 1013–1028. [Google Scholar] [CrossRef] [Green Version]
- Adler, G.H. Tropical Tree Diversity, Forest Structure and the Demography of a Frugivorous Rodent, the Spiny Rat (Proechimys Semispinosus). J. Zool. 2000, 250, 57–74. [Google Scholar] [CrossRef]
- Vieira, C.M.; Diniz-Filho, J.A.F. Macroecologia de Mamíferos Neotropicais Com Ocorrência No Cerrado. Rev. Bras. Zool. 2000, 17, 973–988. [Google Scholar] [CrossRef]
- Carvajal, A.; Adler, G.H. Seed Dispersal and Predation by Proechimys Semispinosus and Sciurus Granatensis in Gaps and Understorey in Central Panama. J. Trop. Ecol. 2008, 24, 485–492. [Google Scholar] [CrossRef]
- dos Santos Pires, A.; dos Santos Fernandez, F.A.; Feliciano, B.R.; de Freitas, D. Use of Space by Necromys Lasiurus (Rodentia, Sigmodontinae) in a Grassland among Atlantic Forest Fragments. Mamm. Biol. 2010, 75, 270–276. [Google Scholar] [CrossRef]
- De Lima Francisco, A.; Magnusson, W.E.; Sanaiotti, T.M. Variation in Growth and Reproduction of Bolomys Lasiurus (Rodentia: Muridae) in an Amazonian Savanna. J. Trop. Ecol. 1995, 11, 419–428. [Google Scholar] [CrossRef]
- Magnusson, W.E.; De Lima Francisco, A.; Sanaiotti, T.M. Home-Range Size and Territoriality in Bolomys Lasiurus (Rodentia: Muridae) in an Amazonian Savanna. J. Trop. Ecol. 1995, 11, 179–188. [Google Scholar] [CrossRef]
- Talamoni, S.A.; Couto, D.; Cordeiro Júnior, D.A.; Diniz, F.M. Diet of Some Species of Neotropical Small Mammals. Mamm. Biol. 2008, 73, 337–341. [Google Scholar] [CrossRef]
- Brito, D.; Fernandez, F.A.S. Patch Relative Importance to Metapopulation Viability: The Neotropical Marsupial Micoureus Demerarae as a Case Study. Anim. Conserv. 2002, 5, 45–51. [Google Scholar] [CrossRef]
- Palmeirim, A.F.; Santos-Filho, M.; Peres, C.A. Marked Decline in Forest-Dependent Small Mammals Following Habitat Loss and Fragmentation in an Amazonian Deforestation Frontier. PLoS ONE 2020, 15, e0230209. [Google Scholar] [CrossRef]
- Fernandes, M.E.B.; Andrade, F.A.G.; Silva, J.D.S., Jr. Diet of Micoreus Demerarae (Thomas) (Mammalia, Didelphidae) Associated with Contiguous Forests of Mangrove and Terra Firme in Bragança, Pará, Brazil. Rev. Bras. Zool. 2006, 23, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- dos Santos-Filho, M.; Da Silva, D.J.; Sanaiotti, T.M. Edge Effects and Landscape Matrix Use by a Small Mammal Community in Fragments of Semideciduous Submontane Forest in Mato Grosso, Brazil. Braz. J. Biol. Rev. Brasleira Biol. 2008, 68, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Brito, D.; Fernandez, S.F. Metapopulation Viability of the Marsupial Micoureus Demerarae in Small Atlantic Forest Fragments in South-Eastern Brazil. Anim. Conserv. 2000, 3, 201–209. [Google Scholar] [CrossRef]
- Dos Santos Pires, A.; Dos Santos Fernandez, F.A. Use of Space by the Marsupial Micoureus Demerarae in Small Atlantic Forest Fragments in South-Eastern Brazil. J. Trop. Ecol. 1999, 15, 279–290. [Google Scholar] [CrossRef]
- Quental, T.B.; Quental, T.F.; Dos Santos Fernandez, F.A.; Dias, A.T.C.; Rocha, F.S. Population Dynamics of the Marsupial Micoureus Demerarae in Small Fragments of Atlantic Coastal Forest in Brazil. J. Trop. Ecol. 2001, 17, 339–352. [Google Scholar] [CrossRef]
- Nowak RM, W.E. Walker’s Mammals of the World Volume I; JHU Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Castilheiro, W.F.F.; dos Santos Filho, M. Diet of Monodelphis Glirina (Mammalia: Didelphidae) in Forest Fragments in Southern Amazon. Zoologia 2013, 30, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Atchley, W.R.; Hall, B.K. A Model for Development and Evolution of Complex Morphological Structures. Biol. Rev. Camb. Philos. Soc. 1991, 66, 101–157. [Google Scholar] [CrossRef] [PubMed]
- Burgio, G.; Baylac, M.; Heyer, E.; Montagutelli, X. Exploration of the Genetic Organization of Morphological Modularity on the Mouse Mandible Using a Set of Interspecific Recombinant Congenic Strains between C57BL/6 and Mice of the Mus Spretus Species. G3 Genes Genomes Genet. 2012, 2, 1257–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Vargas, J.; Muñoz-Muñoz, F.; Martinez-Maza, C.; Molinero, A.; Ventura, J. Postnatal Mandible Growth in Wild and Laboratory Mice: Differences Revealed from Bone Remodeling Patterns and Geometric Morphometrics. J. Morphol. 2017, 278, 1058–1074. [Google Scholar] [CrossRef]
- Lovatt, F.M.; Hoelzel, A.R. The Impact of Population Bottlenecks on Fluctuating Asymmetry and Morphological Variance in Two Separate Populations of Reindeer on the Island of South Georgia. Biol. J. Linn. Soc. 2011, 102, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Askay, M.A.; Kostelnick, J.C.; Kerbis Peterhans, J.C.; Loew, S.S. Environmental Stress as an Indicator of Anthropogenic Impact across the African Albertine Rift: A Case Study Using Museum Specimens. Biodivers. Conserv. 2014, 23, 2221–2237. [Google Scholar] [CrossRef]
- Coda, J.; Gomez, D.; Martínez, J.J.; Steinmann, A.; Priotto, J. The Use of Fluctuating Asymmetry as a Measure of Farming Practice Effects in Rodents: A Species-Specific Response. Ecol. Indic. 2016, 70, 269–275. [Google Scholar] [CrossRef]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating Asymmetry: Methods, Theory, and Applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef] [Green Version]
- Hefti, E.; Trechsel, U.; Rüfenacht, H.; Fleisch, H. Use of Dermestid Beetles for Cleaning Bones. Calcif. Tissue Int. 1980, 31, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. The Tps Series of Software. Hystrix 2015, 26, 1–4. [Google Scholar] [CrossRef]
- Dryden IL, M.K. Statistical Shape Analysis; Wiley: Chichester, UK, 1998. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An Integrated Software Package for Geometric Morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P.; Mcintyre, G.S. Geometric Morphometrics of Developmental Instability: Analyzing Patterns of Fluctuating Asymmetry with Procrustes Methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape Analysis of Symmetric Structures: Quantifying Variation among Individuals and Asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Jung, M. LecoS—A Python Plugin for Automated Landscape Ecology Analysis. Ecol. Inform. 2016, 31, 18–21. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. In Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst; University of Massachusetts: Boston, MA, USA, 2012. [Google Scholar]
- Metzger, J.P. Landscape Structure Changes and Species Richness in Forest Fragments of Southeast Brazil. Comptes Rendus L’academie Sci. Ser. III Sci. Vie 1998, 321, 319–333. [Google Scholar]
- Palmer, A.R.; Strobeck, C. Fluctuating Asymmetry: Measurement, Analysis, Patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Analyzing Fluctuating Asymmetry with Geometric Morphometrics: Concepts, Methods, and Applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef] [Green Version]
- Anscombe, F.J.; Glynn, W.J. Distribution of the Kurtosis Statistic B2 for Normal Samples. Biometrika 1983, 70, 227–234. [Google Scholar] [CrossRef]
- Graham, J.H.; Freeman, D.C.; Emlen, J.M. Developmental stability: A sensitive indicator of populations under stress. ASTM Spec. Tech. Publ. 1993, 1179, 136–158. [Google Scholar]
- Lucky, N.S.; Ihara, R.; Yamaoka, K.; Hori, M. Behavioral Laterality and Morphological Asymmetry in the Cuttlefish, Sepia Lycidas. Zoolog. Sci. 2012, 29, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, B.A.; Murphy, D.D. Conservation Strategy: The Effects of Fragmentation on Extinction. Am. Nat. 1985, 125, 879–887. [Google Scholar] [CrossRef]
- Keinath, D.A.; Doak, D.F.; Hodges, K.E.; Prugh, L.R.; Fagan, W.; Sekercioglu, C.H.; Buchart, S.H.M.; Kauffman, M. A Global Analysis of Traits Predicting Species Sensitivity to Habitat Fragmentation. Glob. Ecol. Biogeogr. 2017, 26, 115–127. [Google Scholar] [CrossRef]
- da Rosa, C.A.; Secco, H.; Carvalho, N.; Maia, A.C.; Bager, A. Edge Effects on Small Mammals: Differences between Arboreal and Ground-Dwelling Species Living near Roads in Brazilian Fragmented Landscapes. Austral Ecol. 2018, 43, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Tocher, M.; Gascon, C.; Zimmerman, B.L. Fragmentation Effects on a Central Amazonian Frog Community: A Ten-Year Study. In Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities; Laurance, W.F., Bierregaard, R.O., Eds.; University of Chicago Press: Chicago, IL, USA, 1997; pp. 124–137. [Google Scholar]
- Kalko, E.K.V. Organisation and Diversity of Tropical Bat Communities through Space and Time. Zoology 1998, 101, 281–297. [Google Scholar]
- Santos-Filho, M.; Peres, C.A.; da Silva, D.J.; Sanaiotti, T.M. Habitat Patch and Matrix Effects on Small-Mammal Persistence in Amazonian Forest Fragments. Biodivers. Conserv. 2012, 21, 1127–1147. [Google Scholar] [CrossRef]
- Laurance, W.F.; Curran, T.J. Impacts of Wind Disturbance on Fragmented Tropical Forests: A Review and Synthesis. Austral Ecol. 2008, 33, 399–408. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G.W. Impacts of Roads and Linear Clearings on Tropical Forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef]
- Tinker, D.B.; Resor, C.A.C.; Beauvais, G.P.; Kipfmueller, K.F.; Fernandes, C.I.; Baker, W.L. Watershed Analysis of Forest Fragmentation by Clearcuts and Roads in a Wyoming Forest. Landsc. Ecol. 1998, 13, 149–165. [Google Scholar] [CrossRef]
- Mcgarigal, K.; Romme, W.H.; Crist, M.; Roworth, E. Cumulative Effects of Roads and Logging on Landscape Structure in the San Juan Mountains, Colorado (USA). Landsc. Ecol. 2001, 16, 327–349. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.A.; Hall, M.; Newton, A.C. Spatially Explicit Models to Analyze Forest Loss and Fragmentation between 1976 and 2020 in Southern Chile. Ecol. Modell. 2008, 212, 439–449. [Google Scholar] [CrossRef]
- Laurance, W.F.; Yensen, E. Predicting the Impacts of Edge Effects in Fragmented Habitats. Biol. Conserv. 1991, 55, 77–92. [Google Scholar] [CrossRef]
- Rocha, E.C.; Brito, D.; Silva, P.M.E.; Silva, J.; Bernardo, P.V.D.S.; Juen, L. Effects of Habitat Fragmentation on the Persistence of Medium and Large Mammal Species in the Brazilian Savanna of Goiás State. Biota Neotrop. 2018, 18, e20170483. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; González-Perez, I.M.; Garmendia, A.; Solà, M.; Estrada, A. The Relative Impact of Forest Patch and Landscape Attributes on Black Howler Monkey Populations in the Fragmented Lacandona Rainforest, Mexico. Landsc. Ecol. 2013, 28, 1717–1727. [Google Scholar] [CrossRef]
- Passamani, M.; Ribeiro, D. Small Mammals in a Fragment and Adjacent Matrix in Southeastern Brazil. Braz. J. Biol. 2009, 69, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-de-Jesús, H.A.; Arroyo-Rodríguez, V.; Andresen, E.; Escobar, F. Forest Loss and Matrix Composition Are the Major Drivers Shaping Dung Beetle Assemblages in a Fragmented Rainforest. Landsc. Ecol. 2016, 31, 843–854. [Google Scholar] [CrossRef]
- Brito, D. Genetic Consequences of Population Subdivision: The Marsupial Micoureus Paraguayanus (Mammalia: Didelphimorphia) as a Case Study. Zoologia 2009, 26, 684–693. [Google Scholar] [CrossRef]
- Eldridge, M.D.B.; King, J.M.; Loupis, A.K.; Spencer, P.B.S.; Taylor, A.C.; Pope, L.C.; Hall, G.P. Unprecedented Low Levels of Genetic Variation and Inbreeding Depression in an Island Population of the Black-Footed Rock-Wallaby. Conserv. Biol. 1999, 13, 531–541. [Google Scholar] [CrossRef]
- Pardini, R. Effects of Forest Fragmentation on Small Mammals in an Atlantic Forest Landscape. Biodivers. Conserv. 2004, 13, 2567–2586. [Google Scholar] [CrossRef]
- Michalski, F.; Peres, C.A. Disturbance-Mediated Mammal Persistence and Abundance-Area Relationships in Amazonian Forest Fragments. Conserv. Biol. 2007, 21, 1626–1640. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Olifiers, N.; Delciellos, A.C.; Antunes, V.Z.; Bernardo, L.R.; Grelle, C.E.V.; Cerqueira, R. Land Use vs. Fragment Size and Isolation as Determinants of Small Mammal Composition and Richness in Atlantic Forest Remnants. Biol. Conserv. 2009, 142, 1191–1200. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding Factors in the Detection of Species Responses to Habitat Fragmentation. Biol. Rev. Camb. Philos. Soc. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.; Knopper, L.; Mineau, P. A Critical Assessment of the Utility of Fluctuating Asymmetry as a Biomarker of Anthropogenic Stress. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 415–426. [Google Scholar]
- Lens, L.; Van Dongen, S.; Matthysen, E. Fluctuating Asymmetry as an Early Warning System in the Critically Endangered Taita Thrush. Conserv. Biol. 2002, 16, 479–487. [Google Scholar] [CrossRef] [Green Version]



| Rodentia-Mandibles (N) | |||||||
| Long-Tailed Spiny Rat | Hairy-Tailed Bolo Mouse | ||||||
| (Proechimys longicaudatus) | (Necromys lasiurus) | ||||||
| Sites | Area (ha) | Female | Male | Total | Female | Male | Total |
| S | 5–26 | 20 | 18 | 38 | 10 | 20 | 30 |
| L | 189–900 | 18 | 14 | 32 | 11 | 20 | 31 |
| Total | 38 | 32 | 70 | 21 | 40 | 61 | |
| Didelphimorphia-Mandibles (N) | |||||||
| Woolly mouse opossum | Amazonian red-sided opossum | ||||||
| (Marmosa demerarae) | (Monodelphis glirina) | ||||||
| Sites | Area (ha) | Female | Male | Total | Female | Male | Total |
| S | 5–26 | 45 | 22 | 67 | 24 | 26 | 50 |
| L | 189–900 | 13 | 11 | 24 | 11 | 21 | 32 |
| Total | 58 | 33 | 91 | 35 | 47 | 82 | |
| Rodentia | |
| Landmark | Description |
| 1 | Most cranio-dorsal point of the mandibular symphysis that meets the posterior part of the incisor’s alveolar margin |
| 2 | Point of maximum concavity between the incisor’s alveolus and the tooth row |
| 3 | Cranialmost point of the tooth row’s alveolar margin |
| 4 | Caudalmost point of the tooth row’s alveolar margin |
| 5 | Tip of the coronoid process |
| 6 | Cranialmost point of the edge of the condyle’s articular surface |
| 7 | Caudalmost point of the edge of the condyle’s articular surface |
| 8 | Point of maximum concavity between the condyloid and the angular process |
| 9 | Tip of the angular process |
| 10 | Point of maximum concavity of the mandible’s ventral margin |
| 11 | Point of maximum convexity of the dentary in the anterior-ventral part |
| 12 | Most cranio-ventral point of the mandibular symphysis that meets the anterior part of the incisor’s alveolar margin |
| Didelphimorphia | |
| Landmark | Description |
| 1 | Base of the lower first incisor |
| 2 | Base of the lower fourth incisor |
| 3 | Posterior base of the lower canine |
| 4 | Posterior base of the first molar |
| 5 | Posterior base of the fourth molar |
| 6 | Central point in the coronoid process |
| 7 | Endpoint of the caudal border of coronoid process |
| 8 | Point of inflection of the curve between the mandibular condyle and the caudal border of the coronoid process |
| 9 | Highest point at end of side of the mandibular condyle |
| 10 | Landmark 5 orthogonal projection on the ventral edge of the mandible |
| 11 | Landmark 4 orthogonal projection on the ventral edge of the mandible |
| 12 | Foramen’s edge |
| Rodentia | ||||||||||
| Proechimys longicaudatus | ||||||||||
| Centroid size | Shape | |||||||||
| Effect | SS | MS | df | F | p | SS | MS | df | F | p |
| Individual | 145 | 2.07 | 70 | 50.22 | 1 × 10−4 *** | 0.764 | 5.45 × 10−4 | 1400 | 4.63 | 1 × 10−4 *** |
| Side | 170 | 1.70 × 10−2 | 1 | 0.41 | 0.523 | 0.11 | 5.79 × 10−4 | 20 | 49.18 | 1 × 10−4 *** |
| Individual∗Side | 2.89 | 4.13 × 10−2 | 70 | 3.54 | 1 × 10−4 *** | 0.164 | 1.17 × 10−4 | 1400 | 7.28 | 1 × 10−4 *** |
| Measurement error | 3.31 | 1.16 × 10−2 | 284 | 0.0991 | 1.61 × 10−5 | 5680 | ||||
| Necromys lasiurus | ||||||||||
| Individual | 31.5 | 0.526 | 60 | 32.6 | 1 × 10−4 *** | 0.731 | 6.09 × 10−4 | 1200 | 3.53 | 1 × 10−4 *** |
| Side | 510 | 5.16 × 10−2 | 1 | 3.2 | 0.078 | 0.0459 | 2.29 × 10−3 | 20 | 13.30 | 1 × 10−4 *** |
| Individual∗Side | 0.985 | 1.61 × 10−2 | 61 | 3.10 | 1 × 10−4 *** | 0.210 | 1.72 × 10−4 | 1220 | 8.40 | 1 × 10−4 *** |
| Measurement error | 1.28 | 5.21 × 10−3 | 247 | 0.101 | 1.28 × 10−5 | 4940 | ||||
| Didelphimorphia | ||||||||||
| Marmosa demerarae | ||||||||||
| Individual | 416 | 4.63 | 90 | 78.00 | 1 × 10−4 *** | 0.792 | 4.40 × 10−4 | 1800 | 16.02 | 1 × 10−4 *** |
| Side | 2.37 | 2.37 | 1 | 65.06 | 1 × 10−4 *** | 1.10 × 10−2 | 5.22 × 10−4 | 20 | 19.02 | 1 × 10−4 *** |
| Individual∗Side | 4.19 | 4.65 × 10−2 | 90 | 2.88 | 1 × 10−4 *** | 4.94 × 10−2 | 2.74 × 10−5 | 1800 | 7.63 | 1 × 10−4 *** |
| Measurement error | 0.0370 | 1.85 × 10−2 | 2 | 8.49 × 10−5 | 2.12 × 10−6 | 40 | ||||
| Monodelphis glirina | ||||||||||
| Individual | 323 | 4.04 | 80 | 143.65 | 1 × 10−4 *** | 1.46 | 9.18 × 10−4 | 1600 | 15.83 | 1 × 10−4 *** |
| Side | 3.32 × 10−1 | 0.332 | 1 | 11.79 | 9 × 10−4 *** | 2.03 × 10−2 | 1.01 × 10−3 | 20 | 17.53 | 1 × 10−4 *** |
| Individual∗Side | 2.28 | 2.28 × 10−2 | 81 | 2.46 | 1 × 10−4 *** | 9.40 × 10−2 | 5.80 × 10−5 | 1620 | 10.32 | 1 × 10−4 *** |
| Measurement error | 3.75 | 1.14 × 10−2 | 328 | 3.69 × 10−2 | 5.62 × 10−6 | 6560 | ||||
| Species | Between Sexes | Between Fragment Groups | ||||
|---|---|---|---|---|---|---|
| Rodentia | Females | Males | p | Small | Large | p |
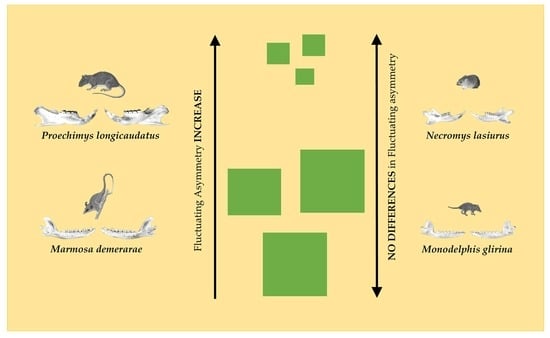
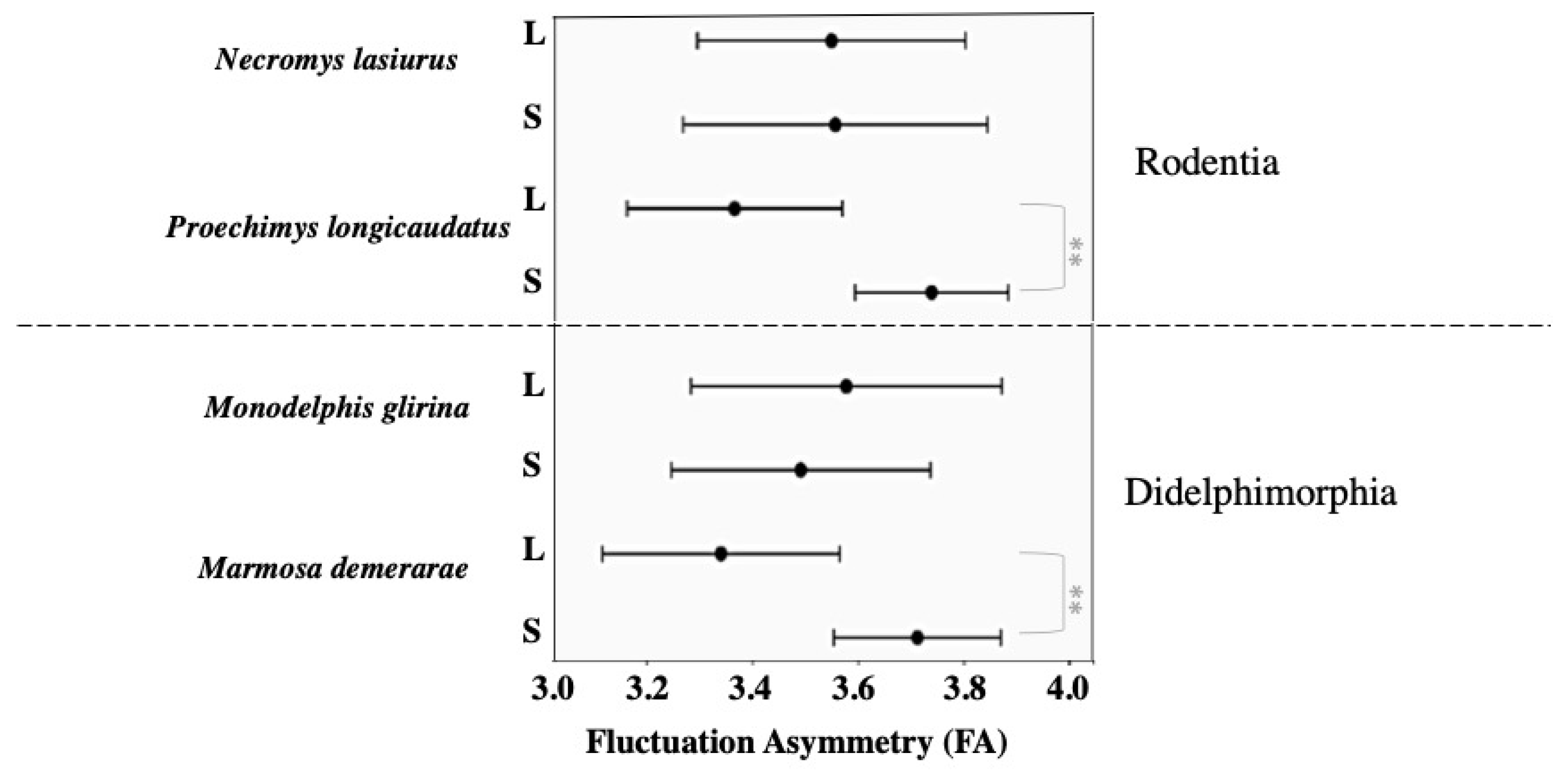
| Proechimys longicaudatus | 3.5 ± 0.7 | 3.6 ± 0.6 | 0.43 | 3.7 ± 0.4 | 3.3 ± 0.5 | 0.02 * |
| Necromys lasiurus | 3.4 ± 0.8 | 3.6 ± 0.7 | 0.28 | 3.5 ± 0.8 | 3.5 ± 0.7 | 0.96 |
| Didelphimorphia | Females | Males | p | Small | Large | p |
| Marmosa demerarae | 3.4 ± 0.6 | 3.6 ± 0.6 | 0.11 | 3.7 ± 0.6 | 3.3 ± 0.5 | 0.02 * |
| Monodelphis glirina | 3.5 ± 0.7 | 3.5 ± 0.9 | 0.68 | 3.4 ± 0.8 | 3.5 ± 0.8 | 0.43 |
| Landscape Metrics | Factor | Correlation Coefficient Matrix (Pearson) | ||||
|---|---|---|---|---|---|---|
| Loadings | FA-Rodentia | FA-Didelphimorphia | ||||
| F1 | F2 | P. longicaudatus | N. lasiurus | M. demerarae | M. glirina | |
| Area | 0.640 * | 0.110 | −0.614 * | 0.151 | −0.621 * | −0.056 |
| Edge length | −0.580 | 0.076 | −0.372 | 0.045 | −0.534 * | −0.012 |
| Isolation | 0.294 | 0.211 | 0.302 | 0.127 | 0.127 | 0.160 |
| Fragment shape | −0.060 | 0.193 | 0.110 | 0.057 | −0.338 | 0.079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilheiro, W.F.F.; Muñoz-Muñoz, F.; Ventura, J.; dos Santos-Filho, M.; Mathias, M.d.L.; Gabriel, S.I. Differential Impact of Forest Fragmentation on Fluctuating Asymmetry in South Amazonian Small Mammals. Symmetry 2022, 14, 981. https://doi.org/10.3390/sym14050981
Castilheiro WFF, Muñoz-Muñoz F, Ventura J, dos Santos-Filho M, Mathias MdL, Gabriel SI. Differential Impact of Forest Fragmentation on Fluctuating Asymmetry in South Amazonian Small Mammals. Symmetry. 2022; 14(5):981. https://doi.org/10.3390/sym14050981
Chicago/Turabian StyleCastilheiro, Welvis Felipe Fernandes, Francesc Muñoz-Muñoz, Jacint Ventura, Manoel dos Santos-Filho, Maria da Luz Mathias, and Sofia Isabel Gabriel. 2022. "Differential Impact of Forest Fragmentation on Fluctuating Asymmetry in South Amazonian Small Mammals" Symmetry 14, no. 5: 981. https://doi.org/10.3390/sym14050981
APA StyleCastilheiro, W. F. F., Muñoz-Muñoz, F., Ventura, J., dos Santos-Filho, M., Mathias, M. d. L., & Gabriel, S. I. (2022). Differential Impact of Forest Fragmentation on Fluctuating Asymmetry in South Amazonian Small Mammals. Symmetry, 14(5), 981. https://doi.org/10.3390/sym14050981