The Intricate Web of Asymmetric Processing of Social Stimuli in Humans
Abstract
1. Introduction
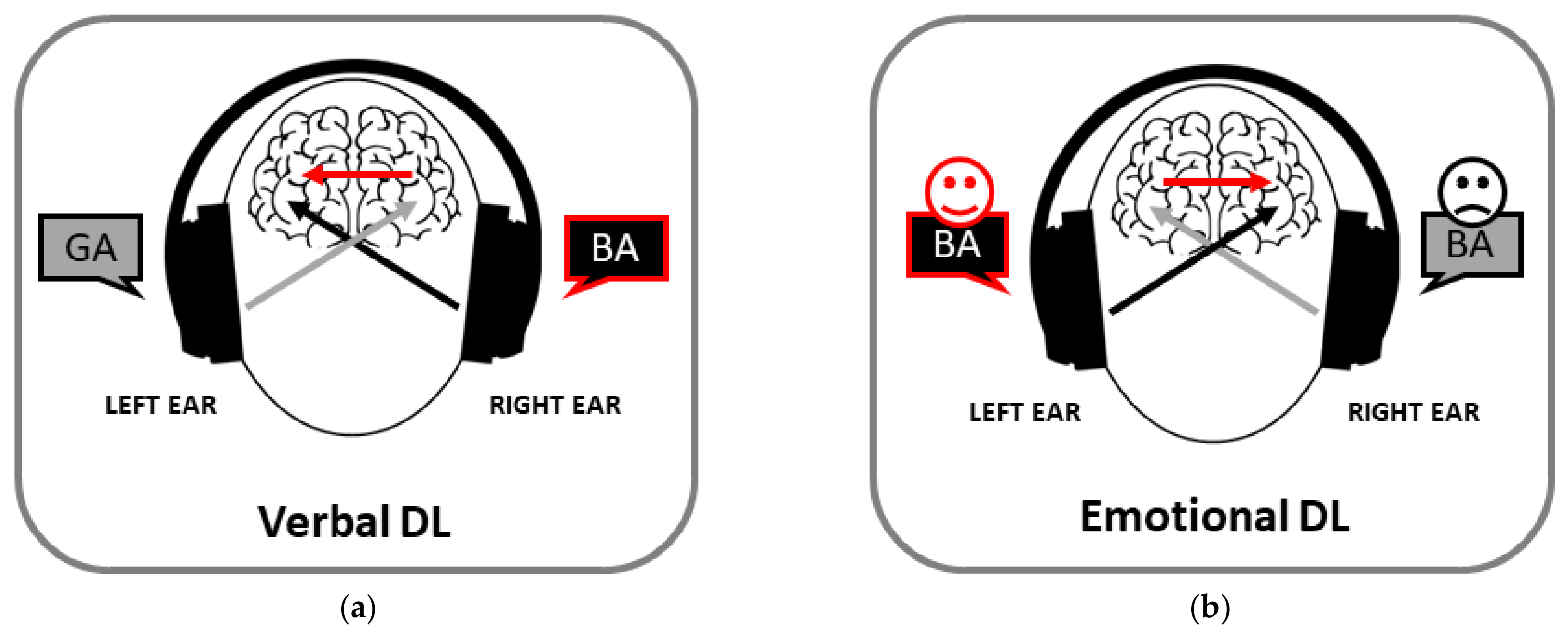
2. Auditory Asymmetries
3. Visual Asymmetries
4. Perceptual and Attentional Asymmetries for Human Bodies
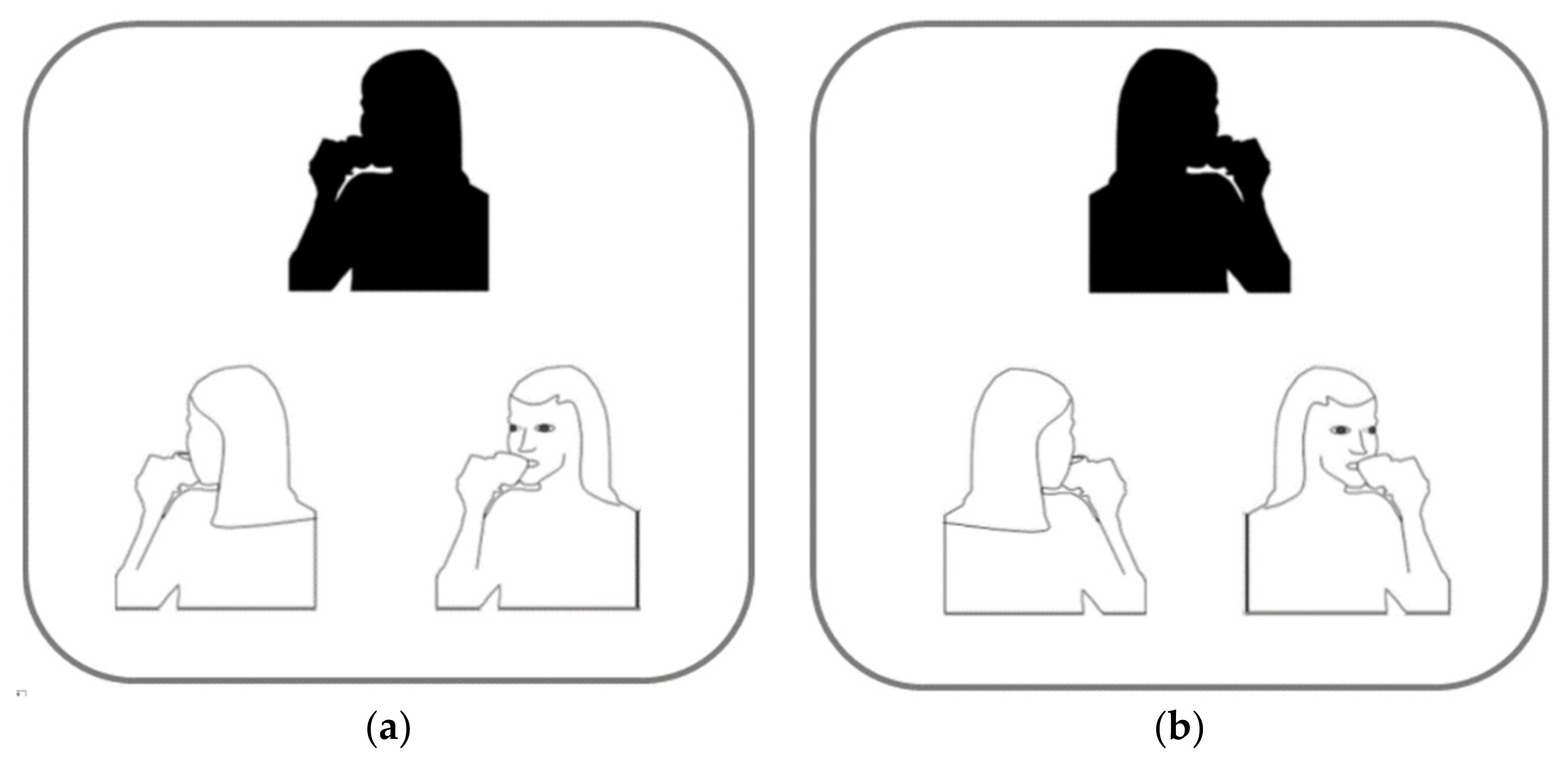
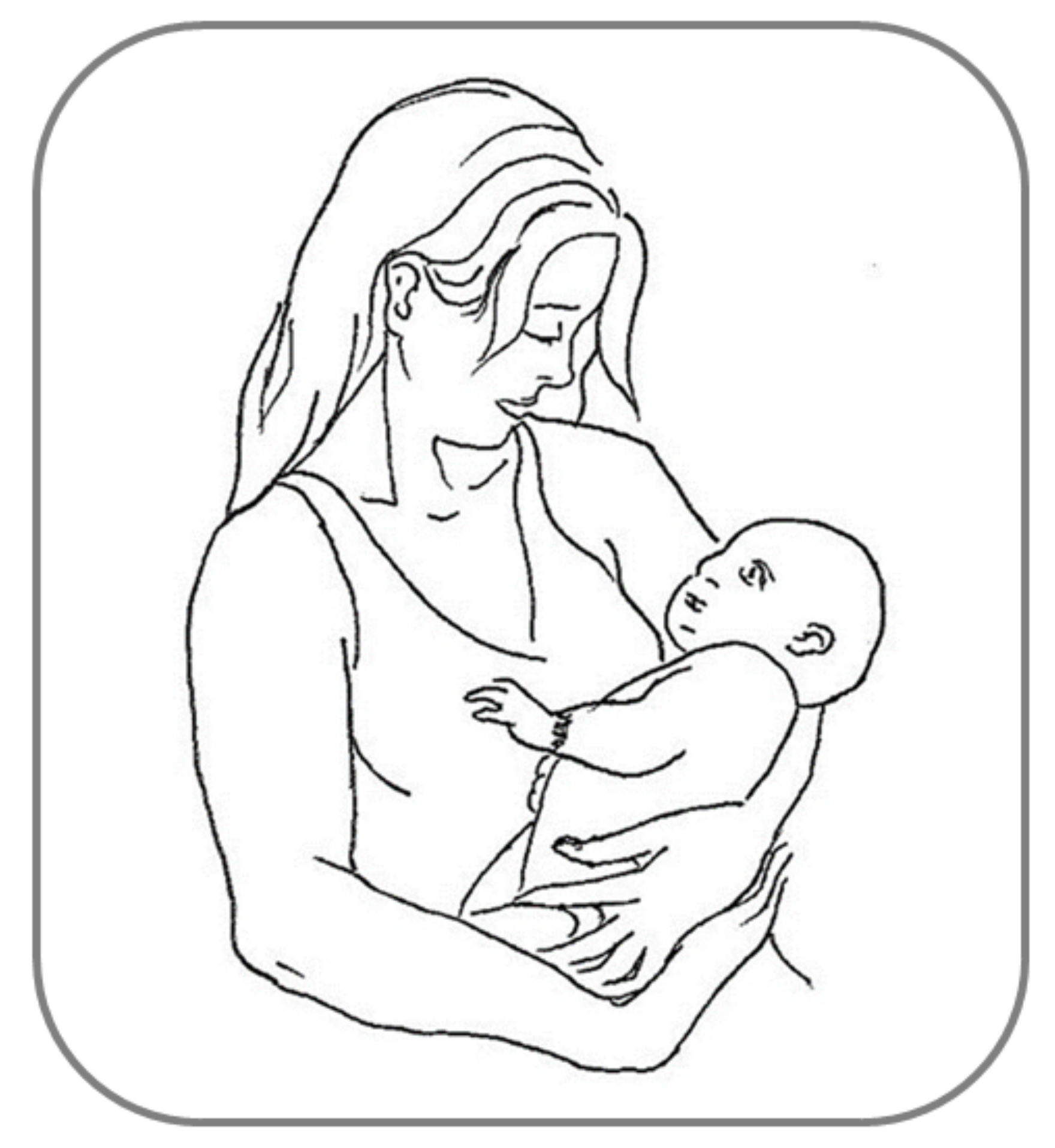
5. Asymmetries in Social Touch
6. A Possible Role for Social Interactions in the Link between Perceptual and Motor Asymmetries?
7. Potential Effects of Social Interactions on Functional Lateralization
8. Selectivity of Perceptual Asymmetries in Light of Face-to-Face Interactions with Right-Handed Individuals
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coren, S. The Lateral Preference Inventory for Measurement of Handedness, Footedness, Eyedness, and Earedness: Norms for Young Adults. Bull. Psychon. Soc. 1993, 31, 1–3. [Google Scholar] [CrossRef]
- Papadatou-Pastou, M.; Ntolka, E.; Schmitz, J.; Martin, M.; Munafò, M.R.; Ocklenburg, S.; Paracchini, S. Human Handedness: A Meta-Analysis. Psychol. Bull. 2020, 146, 481–524. [Google Scholar] [CrossRef]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A Review and Meta-Analysis of Performance Factors in Line Bisection Tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Kimura, D. Cerebral Dominance and the Perception of Verbal Stimuli. Can. J. Psychol./Rev. Can. Psychol. 1961, 15, 166–171. [Google Scholar] [CrossRef]
- Burt, D.M.; Perrett, D.I. Perceptual Asymmetries in Judgements of Facial Attractiveness, Age, Gender, Speech and Expression. Neuropsychologia 1997, 35, 685–693. [Google Scholar] [CrossRef]
- Packheiser, J.; Schmitz, J.; Metzen, D.; Reinke, P.; Radtke, F.; Friedrich, P.; Güntürkün, O.; Peterburs, J.; Ocklenburg, S. Asymmetries in Social Touch-Motor and Emotional Biases on Lateral Preferences in Embracing, Cradling and Kissing. Laterality 2020, 25, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Stochl, J.; Croudace, T. Predictors of Human Rotation. Laterality 2013, 18, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J. Survival with an Asymmetrical Brain: Advantages and Disadvantages of Cerebral Lateralization. Behav. Brain Sci. 2005, 28, 575–633. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. A Function for the Bicameral Mind. Cortex 2020, 124, 274–285. [Google Scholar] [CrossRef]
- Kimura, D. Some Effects of Temporal-Lobe Damage on Auditory Perception. Can. J. Psychol. 1961, 15, 156–165. [Google Scholar] [CrossRef]
- Broadbent, D.E. The Role of Auditory Localization in Attention and Memory Span. J. Exp. Psychol. 1954, 47, 191–196. [Google Scholar] [CrossRef]
- Bryden, M.P. An Overview of the Dichotic Listening Procedure and Its Relation to Cerebral Organization. In Handbook of Dichotic Listening: Theory, Methods and Research; John Wiley & Sons: Oxford, UK, 1988; pp. 1–43. ISBN 978-0-471-91267-5. [Google Scholar]
- Bryden, M.P. Order of Report in Dichotic Listening. Can. J. Psychol./Rev. Can. Psychol. 1962, 16, 291–299. [Google Scholar] [CrossRef]
- Shankweiler, D.; Studdert-Kennedy, M. Identification of Consonants and Vowels Presented to Left and Right Ears. Q. J. Exp. Psychol. 1967, 19, 59–63. [Google Scholar] [CrossRef]
- Brancucci, A.; D’Anselmo, A.; Martello, F.; Tommasi, L. Left Hemisphere Specialization for Duration Discrimination of Musical and Speech Sounds. Neuropsychologia 2008, 46, 2013–2019. [Google Scholar] [CrossRef]
- Kimura, D. Functional Asymmetry of the Brain in Dichotic Listening. Cortex 1967, 3, 163–178. [Google Scholar] [CrossRef]
- Sidtis, J.J. Dichotic Listening after Commissurotomy. In Handbook of Dichotic Listening: Theory, Methods and Research; John Wiley & Sons: Oxford, UK, 1988; pp. 161–184. ISBN 978-0-471-91267-5. [Google Scholar]
- Brancucci, A.; Babiloni, C.; Babiloni, F.; Galderisi, S.; Mucci, A.; Tecchio, F.; Zappasodi, F.; Pizzella, V.; Romani, G.L.; Rossini, P.M. Inhibition of Auditory Cortical Responses to Ipsilateral Stimuli during Dichotic Listening: Evidence from Magnetoencephalography. Eur. J. Neurosci. 2004, 19, 2329–2336. [Google Scholar] [CrossRef]
- Della Penna, S.; Brancucci, A.; Babiloni, C.; Franciotti, R.; Pizzella, V.; Rossi, D.; Torquati, K.; Rossini, P.M.; Romani, G.L. Lateralization of Dichotic Speech Stimuli Is Based on Specific Auditory Pathway Interactions: Neuromagnetic Evidence. Cereb. Cortex 2007, 17, 2303–2311. [Google Scholar] [CrossRef]
- Pollmann, S.; Maertens, M.; von Cramon, D.Y.; Lepsien, J.; Hugdahl, K. Dichotic Listening in Patients with Splenial and Nonsplenial Callosal Lesions. Neuropsychology 2002, 16, 56–64. [Google Scholar] [CrossRef]
- Kinsbourne, M. Dichotic Imbalance Due to Isolated Hemisphere Occlusion or Directional Rivalry? Brain Lang. 1980, 11, 221–224. [Google Scholar] [CrossRef]
- Kinsbourne, M. The Cerebral Basis of Lateral Asymmetries in Attention. Acta Psychol. 1970, 33, 193–201. [Google Scholar] [CrossRef]
- Binder, J.R.; Frost, J.A.; Hammeke, T.A.; Bellgowan, P.S.; Springer, J.A.; Kaufman, J.N.; Possing, E.T. Human Temporal Lobe Activation by Speech and Nonspeech Sounds. Cereb. Cortex 2000, 10, 512–528. [Google Scholar] [CrossRef]
- Brancucci, A.; Tommasi, L. “Binaural Rivalry”: Dichotic Listening as a Tool for the Investigation of the Neural Correlate of Consciousness. Brain Cogn. 2011, 76, 218–224. [Google Scholar] [CrossRef]
- Hugdahl, K.; Brønnick, K.; Kyllingsbaek, S.; Law, I.; Gade, A.; Paulson, O.B. Brain Activation during Dichotic Presentations of Consonant-Vowel and Musical Instrument Stimuli: A 15O-PET Study. Neuropsychologia 1999, 37, 431–440. [Google Scholar] [CrossRef]
- Brancucci, A.; Della Penna, S.; Babiloni, C.; Vecchio, F.; Capotosto, P.; Rossi, D.; Franciotti, R.; Torquati, K.; Pizzella, V.; Rossini, P.M.; et al. Neuromagnetic Functional Coupling during Dichotic Listening of Speech Sounds. Hum. Brain Mapp. 2008, 29, 253–264. [Google Scholar] [CrossRef]
- Van den Noort, M.; Specht, K.; Rimol, L.M.; Ersland, L.; Hugdahl, K. A New Verbal Reports FMRI Dichotic Listening Paradigm for Studies of Hemispheric Asymmetry. Neuroimage 2008, 40, 902–911. [Google Scholar] [CrossRef]
- Westerhausen, R.; Kompus, K.; Hugdahl, K. Mapping Hemispheric Symmetries, Relative Asymmetries, and Absolute Asymmetries Underlying the Auditory Laterality Effect. Neuroimage 2014, 84, 962–970. [Google Scholar] [CrossRef]
- Prete, G.; D’Anselmo, A.; Tommasi, L.; Brancucci, A. Modulation of the Dichotic Right Ear Advantage during Bilateral but Not Unilateral Transcranial Random Noise Stimulation. Brain Cogn. 2018, 123, 81–88. [Google Scholar] [CrossRef]
- D’Anselmo, A.; Prete, G.; Tommasi, L.; Brancucci, A. The Dichotic Right Ear Advantage Does Not Change with Transcranial Direct Current Stimulation (TDCS). Brain Stimul. 2015, 8, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.; Kusztrits, I.; Craven, A.R.; Hugdahl, K.; Specht, K.; Hirnstein, M. A Multimodal Study of the Effects of TDCS on Dorsolateral Prefrontal and Temporo-Parietal Areas during Dichotic Listening. Eur. J. Neurosci. 2021, 53, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K. Hemispheric Asymmetry: Contributions from Brain Imaging. Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Tervaniemi, M.; Hugdahl, K. Lateralization of Auditory-Cortex Functions. Brain Res. Rev. 2003, 43, 231–246. [Google Scholar] [CrossRef]
- Bless, J.J.; Westerhausen, R.; von Koss Torkildsen, J.; Gudmundsen, M.; Kompus, K.; Hugdahl, K. Laterality across Languages: Results from a Global Dichotic Listening Study Using a Smartphone Application. Laterality 2015, 20, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K. Fifty Years of Dichotic Listening Research—Still Going and Going and…. Brain Cogn. 2011, 76, 211–213. [Google Scholar] [CrossRef]
- Fennell, E.B.; Satz, P.; Morris, R. The Development of Handedness and Dichotic Ear Listening Asymmetries in Relation to School Achievement: A Longitudinal Study. J. Exp. Child Psychol. 1983, 35, 248–262. [Google Scholar] [CrossRef]
- Hirnstein, M.; Westerhausen, R.; Korsnes, M.S.; Hugdahl, K. Sex Differences in Language Asymmetry Are Age-Dependent and Small: A Large-Scale, Consonant-Vowel Dichotic Listening Study with Behavioral and FMRI Data. Cortex 2013, 49, 1910–1921. [Google Scholar] [CrossRef]
- Westerhausen, R.; Bless, J.; Kompus, K. Behavioral Laterality and Aging: The Free-Recall Dichotic-Listening Right-Ear Advantage Increases With Age. Dev. Neuropsychol. 2015, 40, 313–327. [Google Scholar] [CrossRef]
- Voyer, D. Sex Differences in Dichotic Listening. Brain Cogn. 2011, 76, 245–255. [Google Scholar] [CrossRef]
- Bryden, M.P. Correlates of the Dichotic Right-Ear Effect. Cortex 1988, 24, 313–319. [Google Scholar] [CrossRef]
- Dos Santos Sequeira, S.; Woerner, W.; Walter, C.; Kreuder, F.; Lueken, U.; Westerhausen, R.; Wittling, R.A.; Schweiger, E.; Wittling, W. Handedness, Dichotic-Listening Ear Advantage, and Gender Effects on Planum Temporale Asymmetry--a Volumetric Investigation Using Structural Magnetic Resonance Imaging. Neuropsychologia 2006, 44, 622–636. [Google Scholar] [CrossRef]
- Bedoin, N.; Ferragne, E.; Marsico, E. Hemispheric Asymmetries Depend on the Phonetic Feature: A Dichotic Study of Place of Articulation and Voicing in French Stops. Brain Lang. 2010, 115, 133–140. [Google Scholar] [CrossRef]
- D’Anselmo, A.; Reiterer, S.; Zuccarini, F.; Tommasi, L.; Brancucci, A. Hemispheric Asymmetries in Bilinguals: Tongue Similarity Affects Lateralization of Second Language. Neuropsychologia 2013, 51, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kanzaki, R.; Yoshibayashi, M.; Kamiya, T.; Sugishita, M. Dichotic Listening in Patients with Situs Inversus: Brain Asymmetry and Situs Asymmetry. Neuropsychologia 1999, 37, 869–874. [Google Scholar] [CrossRef]
- Prete, G.; D’Anselmo, A.; Brancucci, A.; Tommasi, L. Evidence of a Right Ear Advantage in the Absence of Auditory Targets. Sci. Rep. 2018, 8, 15569. [Google Scholar] [CrossRef] [PubMed]
- Altamura, M.; Prete, G.; Elia, A.; Angelini, E.; Padalino, F.A.; Bellomo, A.; Tommasi, L.; Fairfield, B. Do Patients with Hallucinations Imagine Speech Right? Neuropsychologia 2020, 146, 107567. [Google Scholar] [CrossRef] [PubMed]
- Prete, G.; Marzoli, D.; Brancucci, A.; Tommasi, L. Hearing It Right: Evidence of Hemispheric Lateralization in Auditory Imagery. Hear. Res. 2016, 332, 80–86. [Google Scholar] [CrossRef]
- Prete, G.; Tommasi, V.; Tommasi, L. Right News, Good News! The Valence Hypothesis and Hemispheric Asymmetries in Auditory Imagery. Lang. Cogn. Neurosci. 2020, 35, 409–419. [Google Scholar] [CrossRef]
- Marzoli, D.; Tommasi, L. Side Biases in Humans (Homo Sapiens): Three Ecological Studies on Hemispheric Asymmetries. Naturwissenschaften 2009, 96, 1099–1106. [Google Scholar] [CrossRef]
- Broca, M.P. Remarques Sur Le Siege de La Faculte Du Langage Articule Suivies Dune Observation Daphemie (Perte de La Parole). Bull. Mem. Soc. Anat. Paris 1861, 6, 330–357. [Google Scholar]
- Wernicke, C. Der Aphasische Symptomencomplex: Eine Psychologische Studie auf Anatomischer Basis; Max Cohn & Weigert: Breslau, Germany, 1874. [Google Scholar]
- Brancucci, A.; Babiloni, C.; Rossini, P.M.; Romani, G.L. Right Hemisphere Specialization for Intensity Discrimination of Musical and Speech Sounds. Neuropsychologia 2005, 43, 1916–1923. [Google Scholar] [CrossRef]
- Prete, G.; Fabri, M.; Foschi, N.; Tommasi, L. Voice Gender Categorization in the Connected and Disconnected Hemispheres. Soc. Neurosci. 2020, 15, 385–397. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Evans, A.C.; Meyer, E.; Gjedde, A. Lateralization of Phonetic and Pitch Discrimination in Speech Processing. Science 1992, 256, 846–849. [Google Scholar] [CrossRef]
- Erhan, H.; Borod, J.C.; Tenke, C.E.; Bruder, G.E. Identification of Emotion in a Dichotic Listening Task: Event-Related Brain Potential and Behavioral Findings. Brain Cogn. 1998, 37, 286–307. [Google Scholar] [CrossRef]
- Gadea, M.; Espert, R.; Salvador, A.; Martí-Bonmatí, L. The Sad, the Angry, and the Asymmetrical Brain: Dichotic Listening Studies of Negative Affect and Depression. Brain Cogn. 2011, 76, 294–299. [Google Scholar] [CrossRef]
- Musiek, F.E.; Weihing, J. Perspectives on Dichotic Listening and the Corpus Callosum. Brain Cogn. 2011, 76, 225–232. [Google Scholar] [CrossRef]
- Prete, G.; Marzoli, D.; Brancucci, A.; Fabri, M.; Foschi, N.; Tommasi, L. The Processing of Chimeric and Dichotic Emotional Stimuli by Connected and Disconnected Cerebral Hemispheres. Behav. Brain Res. 2014, 271, 354–364. [Google Scholar] [CrossRef]
- Hugdahl, K.; Law, I.; Kyllingsbaek, S.; Brønnick, K.; Gade, A.; Paulson, O.B. Effects of Attention on Dichotic Listening: An 15O-PET Study. Hum. Brain Mapp. 2000, 10, 87–97. [Google Scholar] [CrossRef]
- D’Anselmo, A.; Marzoli, D.; Brancucci, A. The Influence of Memory and Attention on the Ear Advantage in Dichotic Listening. Hear. Res. 2016, 342, 144–149. [Google Scholar] [CrossRef]
- Milovanov, R.; Tervaniemi, M.; Takio, F.; Hämäläinen, H. Modification of Dichotic Listening (DL) Performance by Musico-Linguistic Abilities and Age. Brain Res. 2007, 1156, 168–173. [Google Scholar] [CrossRef]
- Prete, G.; Fabri, M.; Foschi, N.; Brancucci, A.; Tommasi, L. The “Consonance Effect” and the Hemispheres: A Study on a Split-Brain Patient. Laterality 2015, 20, 257–269. [Google Scholar] [CrossRef]
- Beiter, R.C.; Sharf, D.J. Influence of Encoding and Acoustic Similarity on the Ear Advantage and Lag Effect in Dichotic Listening. J. Speech Hear. Res. 1976, 19, 78–92. [Google Scholar] [CrossRef]
- Freeman, B.A.; Beasley, D.S. Lead and Lag Effects Associated with the Staggered Spondaic Word Test. J. Speech Hear. Res. 1976, 19, 572–577. [Google Scholar] [CrossRef]
- Demareva, V.; Mukhina, E.; Bobro, T.; Abitov, I. Does Double Biofeedback Affect Functional Hemispheric Asymmetry and Activity? A Pilot Study. Symmetry 2021, 13, 937. [Google Scholar] [CrossRef]
- Schwartz, M.; Smith, M.L. Visual Asymmetries with Chimeric Faces. Neuropsychologia 1980, 18, 103–106. [Google Scholar] [CrossRef]
- Levy, J.; Heller, W.; Banich, M.T.; Burton, L.A. Asymmetry of Perception in Free Viewing of Chimeric Faces. Brain Cogn. 1983, 2, 404–419. [Google Scholar] [CrossRef]
- Bourne, V.J. Examining the Relationship between Degree of Handedness and Degree of Cerebral Lateralization for Processing Facial Emotion. Neuropsychology 2008, 22, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Voyer, D.; Voyer, S.D.; Tramonte, L. Free-Viewing Laterality Tasks: A Multilevel Meta-Analysis. Neuropsychology 2012, 26, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.E.R.; Smith, A.; Mattingley, J.B.; Bradshaw, J.L. The Effect of Body and Environment-Centred Coordinates on Free-Viewing Perceptual Asymmetries for Vertical and Horizontal Stimuli. Cortex 2006, 42, 336–346. [Google Scholar] [CrossRef]
- Tommasi, V.; Prete, G.; Tommasi, L. Assessing the Presence of Face Biases by Means of Anorthoscopic Perception. Atten. Percept. Psychophys. 2020, 82, 2673–2692. [Google Scholar] [CrossRef]
- Bourne, V.J. Chimeric Faces, Visual Field Bias, and Reaction Time Bias: Have We Been Missing a Trick? Laterality 2008, 13, 92–103. [Google Scholar] [CrossRef]
- Chiang, C.H.; Ballantyne, A.O.; Trauner, D.A. Development of Perceptual Asymmetry for Free Viewing of Chimeric Stimuli. Brain Cogn. 2000, 44, 415–424. [Google Scholar] [CrossRef]
- Prete, G.; Tommasi, L. The Own-Race Bias and the Cerebral Hemispheres. Soc. Neurosci. 2019, 14, 549–558. [Google Scholar] [CrossRef]
- Lundqvist, D.; Flykt, A.; Öhman, A. The Karolinska Directed Emotional Faces (KDEF); CD ROM from Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet: Solna, Stockholm County, Sweden, 1998; ISBN 91-630-7164-9. [Google Scholar]
- Bourne, V.J. The Divided Visual Field Paradigm: Methodological Considerations. Laterality 2006, 11, 373–393. [Google Scholar] [CrossRef]
- Prete, G.; Laeng, B.; Tommasi, L. Lateralized Hybrid Faces: Evidence of a Valence-Specific Bias in the Processing of Implicit Emotions. Laterality 2014, 19, 439–454. [Google Scholar] [CrossRef]
- Prete, G.; Marzoli, D.; Tommasi, L. Upright or Inverted, Entire or Exploded: Right-Hemispheric Superiority in Face Recognition Withstands Multiple Spatial Manipulations. PeerJ 2015, 3, e1456. [Google Scholar] [CrossRef]
- Prete, G.; Capotosto, P.; Zappasodi, F.; Tommasi, L. Contrasting Hemispheric Asymmetries for Emotional Processing from Event-Related Potentials and Behavioral Responses. Neuropsychology 2018, 32, 317–328. [Google Scholar] [CrossRef]
- Davidson, R.J.; Mednick, D.; Moss, E.; Saron, C.; Schaffer, C.E. Ratings of Emotion in Faces Are Influenced by the Visual Field to Which Stimuli Are Presented. Brain Cogn. 1987, 6, 403–411. [Google Scholar] [CrossRef]
- Wyczesany, M.; Capotosto, P.; Zappasodi, F.; Prete, G. Hemispheric Asymmetries and Emotions: Evidence from Effective Connectivity. Neuropsychologia 2018, 121, 98–105. [Google Scholar] [CrossRef]
- Prete, G.; Croce, P.; Zappasodi, F.; Tommasi, L.; Capotosto, P. Exploring Brain Activity for Positive and Negative Emotions by Means of EEG Microstates. Sci. Rep. 2022, 12, 3404. [Google Scholar] [CrossRef]
- Gainotti, G. Emotional Behavior and Hemispheric Side of the Lesion. Cortex 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious Processing of Emotions and the Right Hemisphere. Neuropsychologia 2012, 50, 205–218. [Google Scholar] [CrossRef]
- Stanković, M. A Conceptual Critique of Brain Lateralization Models in Emotional Face Perception: Toward a Hemispheric Functional-Equivalence (HFE) Model. Int. J. Psychophysiol. 2021, 160, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Prete, G.; Tommasi, L. Split-Brain Patients: Visual Biases for Faces. Prog. Brain Res. 2018, 238, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Prete, G.; Tommasi, L. Split-Brain Patients. In Encyclopedia of Evolutionary Psychological Science; Springer International Publishing AG: Cham, Switzerland, 2021; pp. 1–5. [Google Scholar]
- Prete, G.; Laeng, B.; Fabri, M.; Foschi, N.; Tommasi, L. Right Hemisphere or Valence Hypothesis, or Both? The Processing of Hybrid Faces in the Intact and Callosotomized Brain. Neuropsychologia 2015, 68, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Prete, G.; D’Ascenzo, S.; Laeng, B.; Fabri, M.; Foschi, N.; Tommasi, L. Conscious and Unconscious Processing of Facial Expressions: Evidence from Two Split-Brain Patients. J. Neuropsychol. 2015, 9, 45–63. [Google Scholar] [CrossRef]
- Prete, G.; Capotosto, P.; Zappasodi, F.; Laeng, B.; Tommasi, L. The Cerebral Correlates of Subliminal Emotions: An Eleoencephalographic Study with Emotional Hybrid Faces. Eur. J. Neurosci. 2015, 42, 2952–2962. [Google Scholar] [CrossRef]
- Prete, G.; Laeng, B.; Tommasi, L. Transcranial Random Noise Stimulation (TRNS) over Prefrontal Cortex Does Not Influence the Evaluation of Facial Emotions. Soc. Neurosci. 2019, 14, 676–680. [Google Scholar] [CrossRef]
- Prete, G.; Fabri, M.; Foschi, N.; Tommasi, L. Face Gender Categorization and Hemispheric Asymmetries: Contrasting Evidence from Connected and Disconnected Brains. Neuroscience 2016, 339, 210–218. [Google Scholar] [CrossRef]
- Prete, G.; Malatesta, G.; Tommasi, L. Facial Gender and Hemispheric Asymmetries: A Hf-TRNS Study. Brain Stimul. 2017, 10, 1145–1147. [Google Scholar] [CrossRef]
- Parente, R.; Tommasi, L. A Bias for the Female Face in the Right Hemisphere. Laterality 2008, 13, 374–386. [Google Scholar] [CrossRef]
- Luo, B.; Shan, C.; Zhu, R.; Weng, X.; He, S. Functional Foveal Splitting: Evidence from Neuropsychological and Multimodal MRI Investigations in a Chinese Patient with a Splenium Lesion. PLoS ONE 2011, 6, e23997. [Google Scholar] [CrossRef]
- Mason, M.F.; Macrae, C.N. Categorizing and Individuating Others: The Neural Substrates of Person Perception. J. Cogn. Neurosci. 2004, 16, 1785–1795. [Google Scholar] [CrossRef]
- Mattingley, J.B.; Bradshaw, J.L.; Nettleton, N.C.; Bradshaw, J.A. Can Task Specific Perceptual Bias Be Distinguished from Unilateral Neglect? Neuropsychologia 1994, 32, 805–817. [Google Scholar] [CrossRef]
- David, A.S. Spatial and Selective Attention in the Cerebral Hemispheres in Depression, Mania, and Schizophrenia. Brain Cogn. 1993, 23, 166–180. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Umiltà, C.; Berlucchi, G. Opposite Superiorities of the Right and Left Cerebral Hemispheres in Discriminative Reaction Time to Physiognomical and Alphabetical Material. Brain 1971, 94, 431–442. [Google Scholar] [CrossRef]
- McCarthy, G.; Puce, A.; Gore, J.C.; Allison, T. Face-Specific Processing in the Human Fusiform Gyrus. J. Cogn. Neurosci. 1997, 9, 605–610. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Barton, J.J.S.; Press, D.Z.; Keenan, J.P.; O’Connor, M. Lesions of the Fusiform Face Area Impair Perception of Facial Configuration in Prosopagnosia. Neurology 2002, 58, 71–78. [Google Scholar] [CrossRef]
- Barton, J.J.S. Structure and Function in Acquired Prosopagnosia: Lessons from a Series of 10 Patients with Brain Damage. J. Neuropsychol. 2008, 2, 197–225. [Google Scholar] [CrossRef]
- Frässle, S.; Krach, S.; Paulus, F.M.; Jansen, A. Handedness Is Related to Neural Mechanisms Underlying Hemispheric Lateralization of Face Processing. Sci. Rep. 2016, 6, 27153. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Iacoboni, M.; Zaidel, E. Hemispheric Sensitivity to Body Stimuli in Simple Reaction Time. Exp. Brain Res. 2006, 170, 116–121. [Google Scholar] [CrossRef]
- De Lussanet, M.H.E.; Fadiga, L.; Michels, L.; Seitz, R.J.; Kleiser, R.; Lappe, M. Interaction of Visual Hemifield and Body View in Biological Motion Perception. Eur. J. Neurosci. 2008, 27, 514–522. [Google Scholar] [CrossRef]
- Parsons, L.M.; Gabrieli, J.D.; Phelps, E.A.; Gazzaniga, M.S. Cerebrally Lateralized Mental Representations of Hand Shape and Movement. J. Neurosci. 1998, 18, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Marzoli, D.; Pagliara, A.; Prete, G.; Malatesta, G.; Lucafò, C.; Padulo, C.; Brancucci, A.; Tommasi, L. Hemispheric Asymmetries in the Processing of Body Sides: A Study with Ambiguous Human Silhouettes. Neurosci. Lett. 2017, 656, 114–119. [Google Scholar] [CrossRef]
- Marzoli, D.; Pagliara, A.; Prete, G.; Malatesta, G.; Lucafò, C.; Padulo, C.; Brancucci, A.; Tommasi, L. Lateralized Embodiment of Ambiguous Human Silhouettes: Data on Sex Differences. Data Brief 2019, 25, 104009. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, C.; Marzoli, D.; Prete, G.; Tommasi, L. Laterality Effects in the Spinning Dancer Illusion: The Viewing-from-above Bias Is Only Part of the Story. Br. J. Psychol. 2016, 107, 698–709. [Google Scholar] [CrossRef]
- Lucafò, C.; Marzoli, D.; Padulo, C.; Troiano, S.; Pelosi Zazzerini, L.; Malatesta, G.; Amodeo, I.; Tommasi, L. Hemifield-Specific Rotational Biases during the Observation of Ambiguous Human Silhouettes. Symmetry 2021, 13, 1349. [Google Scholar] [CrossRef]
- Hagemann, N. The Advantage of Being Left-Handed in Interactive Sports. Atten. Percept. Psychophys. 2009, 71, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Loffing, F.; Hagemann, N.; Schorer, J.; Baker, J. Skilled Players’ and Novices’ Difficulty Anticipating Left- vs. Right-Handed Opponents’ Action Intentions Varies across Different Points in Time. Hum. Mov. Sci. 2015, 40, 410–421. [Google Scholar] [CrossRef]
- Loffing, F.; Schorer, J.; Hagemann, N.; Baker, J. On the Advantage of Being Left-Handed in Volleyball: Further Evidence of the Specificity of Skilled Visual Perception. Atten. Percept. Psychophys. 2012, 74, 446–453. [Google Scholar] [CrossRef]
- Loffing, F.; Hagemann, N. Zum Einfluss Des Anlaufwinkels Und Der Füßigkeit Des Schützen Auf Die Antizipation von Elfmeterschüssen. Z. Sportpsychol. 2015, 21, 63–73. [Google Scholar] [CrossRef]
- Loffing, F.; Hagemann, N. Motor Competence Is Not Enough: Handedness Does Not Facilitate Visual Anticipation of Same-Handed Action Outcome. Cortex 2020, 130, 94–99. [Google Scholar] [CrossRef]
- McMorris, T.; Colenso, S. Anticipation of Professional Soccer Goalkeepers When Facing Right-and Left-Footed Penalty Kicks. Percept. Mot. Ski. 1996, 82, 931–934. [Google Scholar] [CrossRef]
- Schorer, J.; Loffing, F.; Hagemann, N.; Baker, J. Human Handedness in Interactive Situations: Negative Perceptual Frequency Effects Can Be Reversed! J. Sports Sci. 2012, 30, 507–513. [Google Scholar] [CrossRef]
- Richardson, T.; Gilman, R.T. Left-Handedness Is Associated with Greater Fighting Success in Humans. Sci. Rep. 2019, 9, 15402. [Google Scholar] [CrossRef]
- Bisiacchi, P.; Ripoll, H.; Stein, J.; Simonet, P.; Azemar, G. Left-Handedness in Fencers: An Attentional Advantage? Percept. Mot. Ski. 1985, 61, 507–513. [Google Scholar] [CrossRef]
- Breznik, K. On the Gender Effects of Handedness in Professional Tennis. J. Sports Sci. Med. 2013, 12, 346–353. [Google Scholar]
- Brooks, R.; Bussière, L.F.; Jennions, M.D.; Hunt, J. Sinister Strategies Succeed at the Cricket World Cup. Proc. Biol. Sci. 2004, 271 (Suppl. S3), S64–S66. [Google Scholar] [CrossRef]
- Cingoz, Y.E.; Gursoy, R.; Ozan, M.; Hazar, K.; Dalli, M. Research on the Relation between Hand Preference and Success in Karate and Taekwondo Sports with Regards to Gender. Adv. Phys. Educ. 2018, 8, 308. [Google Scholar] [CrossRef]
- Dochtermann, N.A.; Gienger, C.M.; Zappettini, S. Born to Win? Maybe, but Perhaps Only against Inferior Competition. Anim. Behav. 2014, 96, e1–e3. [Google Scholar] [CrossRef]
- Goldstein, S.R.; Young, C.A. “ Evolutionary” Stable Strategy of Handedness in Major League Baseball. J. Comp. Psychol. 1996, 110, 164. [Google Scholar] [CrossRef]
- Grouios, G. Motoric Dominance and Sporting Excellence: Training versus Heredity. Percept. Mot. Ski. 2004, 98, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Grouios, G.; Koidou, E.; Tsormpatzoudis, C.; Alexandris, K. Handedness in Sport. J. Hum. Mov. Stud. 2002, 43, 347–361. [Google Scholar]
- Gursoy, R. Effects of Left- or Right-Hand Preference on the Success of Boxers in Turkey. Br. J. Sports Med. 2009, 43, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J. In Fencing, What Gives Left-Handers the Edge? Views from the Present and the Distant Past. Laterality 2010, 15, 15–55. [Google Scholar] [CrossRef]
- Holtzen, D.W. Handedness and Professional Tennis. Int. J. Neurosci. 2000, 105, 101–119. [Google Scholar] [CrossRef]
- Lawler, T.P.; Lawler, F.H. Left-Handedness in Professional Basketball: Prevalence, Performance, and Survival. Percept. Mot. Ski. 2011, 113, 815–824. [Google Scholar] [CrossRef]
- Loffing, F.; Hagemann, N.; Strauss, B. Left-Handedness in Professional and Amateur Tennis. PLoS ONE 2012, 7, e49325. [Google Scholar] [CrossRef][Green Version]
- Loffing, F.; Hagemann, N. Pushing through Evolution? Incidence and Fight Records of Left-Oriented Fighters in Professional Boxing History. Laterality Asymmetries Body Brain Cogn. 2015, 20, 270–286. [Google Scholar] [CrossRef]
- Pollet, T.V.; Stulp, G.; Groothuis, T.G. Born to Win? Testing the Fighting Hypothesis in Realistic Fights: Left-Handedness in the Ultimate Fighting Championship. Anim. Behav. 2013, 86, 839–843. [Google Scholar] [CrossRef]
- Tran, U.S.; Voracek, M. Footedness Is Associated with Self-Reported Sporting Performance and Motor Abilities in the General Population. Front. Psychol. 2016, 7, 1199. [Google Scholar] [CrossRef]
- Ziyagil, M.A.; Gursoy, R.; Dane, S.; Yuksel, R. Left-Handed Wrestlers Are More Successful. Percept. Mot. Ski. 2010, 111, 65–70. [Google Scholar] [CrossRef]
- Marzoli, D.; Lucafò, C.; Pagliara, A.; Cappuccio, R.; Brancucci, A.; Tommasi, L. Both Right- and Left-Handers Show a Bias to Attend Others’ Right Arm. Exp. Brain Res. 2015, 233, 415–424. [Google Scholar] [CrossRef]
- Marzoli, D.; Lucafò, C.; Padulo, C.; Prete, G.; Giacinto, L.; Tommasi, L. Inversion Reveals Perceptual Asymmetries in the Configural Processing of Human Body. Front. Behav. Neurosci. 2017, 11, 126. [Google Scholar] [CrossRef]
- Lucafò, C.; Marzoli, D.; Zdybek, P.; Malatesta, G.; Smerilli, F.; Ferrara, C.; Tommasi, L. The Bias toward the Right Side of Others Is Stronger for Hands than for Feet. Symmetry 2021, 13, 146. [Google Scholar] [CrossRef]
- Faurie, C.; Raymond, M. Handedness, Homicide and Negative Frequency-Dependent Selection. Proc. Biol. Sci. 2005, 272, 25–28. [Google Scholar] [CrossRef]
- Marzoli, D.; Prete, G.; Tommasi, L. Perceptual Asymmetries and Handedness: A Neglected Link? Front. Psychol. 2014, 5, 163. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Packheiser, J.; Schmitz, J.; Rook, N.; Güntürkün, O.; Peterburs, J.; Grimshaw, G.M. Hugs and Kisses—The Role of Motor Preferences and Emotional Lateralization for Hemispheric Asymmetries in Human Social Touch. Neurosci. Biobehav. Rev. 2018, 95, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Packheiser, J.; Rook, N.; Dursun, Z.; Mesenhöller, J.; Wenglorz, A.; Güntürkün, O.; Ocklenburg, S. Embracing Your Emotions: Affective State Impacts Lateralisation of Human Embraces. Psychol. Res. 2019, 83, 26–36. [Google Scholar] [CrossRef]
- Turnbull, O.H.; Stein, L.; Lucas, M.D. Lateral Preferences in Adult Embracing: A Test of the “Hemispheric Asymmetry” Theory of Infant Cradling. J. Genet. Psychol. 1995, 156, 17–21. [Google Scholar] [CrossRef]
- Güntürkün, O. Adult Persistence of Head-Turning Asymmetry. Nature 2003, 421, 711. [Google Scholar] [CrossRef]
- Sedgewick, J.R.; Holtslander, A.; Elias, L.J. Kissing Right? Absence of Rightward Directional Turning Bias During First Kiss Encounters Among Strangers. J. Nonverbal. Behav. 2019, 43, 271–282. [Google Scholar] [CrossRef]
- Salk, L. The Effects of the Normal Heartbeat Sound on the Behaviour of the Newborn Infant: Implications for Mental Health. World Ment. Health 1960, 12, 168–175. [Google Scholar]
- Glocker, M.L.; Langleben, D.D.; Ruparel, K.; Loughead, J.W.; Valdez, J.N.; Griffin, M.D.; Sachser, N.; Gur, R.C. Baby Schema Modulates the Brain Reward System in Nulliparous Women. Proc. Natl. Acad. Sci. USA 2009, 106, 9115–9119. [Google Scholar] [CrossRef]
- Hahn, A.C.; Perrett, D.I. Neural and Behavioral Responses to Attractiveness in Adult and Infant Faces. Neurosci. Biobehav. Rev. 2014, 46, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M. Sex Differences in Social Cognition: The Case of Face Processing. J. Neurosci. Res. 2017, 95, 222–234. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Prete, G.; Tommasi, L. Human Lateralization, Maternal Effects and Neurodevelopmental Disorders. Front. Behav. Neurosci. 2021, 15, 668520. [Google Scholar] [CrossRef]
- Packheiser, J.; Schmitz, J.; Berretz, G.; Papadatou-Pastou, M.; Ocklenburg, S. Handedness and Sex Effects on Lateral Biases in Human Cradling: Three Meta-Analyses. Neurosci. Biobehav. Rev. 2019, 104, 30–42. [Google Scholar] [CrossRef]
- Donnot, J.; Vauclair, J. Biais de latéralité dans la façon de porter un très jeune enfant: Une revue de la question. Neuropsychiatr. Enfance Adolesc. 2005, 53, 413–425. [Google Scholar] [CrossRef]
- Todd, B.; Butterworth, G. Her Heart Is in the Right Place: An Investigation of the ‘Heartbeat Hypothesis’ as an Explanation of the Left Side Cradling Preference in a Mother with Dextrocardia. Early Dev. Parent. 1998, 7, 229–233. [Google Scholar] [CrossRef]
- Van der Meer, A.; Husby, Å. Handedness as a Major Determinant of Functional Cradling Bias. Laterality 2006, 11, 263–276. [Google Scholar] [CrossRef]
- Manning, J.T.; Chamberlain, A.T. Left-Side Cradling and Brain Lateralization. Ethol. Sociobiol. 1991, 12, 237–244. [Google Scholar] [CrossRef]
- Harris, L.J.; Almerigi, J.B.; Carbary, T.J.; Fogel, T.G. Left-Side Infant Holding: A Test of the Hemispheric Arousal-Attentional Hypothesis. Brain Cogn. 2001, 46, 159–165. [Google Scholar] [CrossRef]
- Bourne, V.J.; Todd, B.K. When Left Means Right: An Explanation of the Left Cradling Bias in Terms of Right Hemisphere Specializations. Dev. Sci. 2004, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Vauclair, J.; Donnot, J. Infant Holding Biases and Their Relations to Hemispheric Specializations for Perceiving Facial Emotions. Neuropsychologia 2005, 43, 564–571. [Google Scholar] [CrossRef]
- Harris, L.J.; Cárdenas, R.A.; Spradlin, M.P.; Almerigi, J.B. Why Are Infants Held on the Left? A Test of the Attention Hypothesis with a Doll, a Book, and a Bag. Laterality 2010, 15, 548–571. [Google Scholar] [CrossRef]
- Huggenberger, H.J.; Suter, S.E.; Reijnen, E.; Schachinger, H. Cradling Side Preference Is Associated with Lateralized Processing of Baby Facial Expressions in Females. Brain Cogn. 2009, 70, 67–72. [Google Scholar] [CrossRef]
- Borod, J.C.; Haywood, C.S.; Koff, E. Neuropsychological Aspects of Facial Asymmetry during Emotional Expression: A Review of the Normal Adult Literature. Neuropsychol. Rev. 1997, 7, 41–60. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Tommasi, L. Keep a Left Profile, Baby! The Left-Cradling Bias Is Associated with a Preference for Left-Facing Profiles of Human Babies. Symmetry 2020, 12, 911. [Google Scholar] [CrossRef]
- Donnot, J.; Vauclair, J. Infant Holding Preferences in Maternity Hospitals: Testing the Hypothesis of the Lateralized Perception of Emotions. Dev. Neuropsychol. 2007, 32, 881–890. [Google Scholar] [CrossRef]
- Harris, L.J.; Cárdenas, R.A.; Stewart, N.D.; Almerigi, J.B. Are Only Infants Held More Often on the Left? If so, Why? Testing the Attention-Emotion Hypothesis with an Infant, a Vase, and Two Chimeric Tests, One “Emotional,” One Not. Laterality 2019, 24, 65–97. [Google Scholar] [CrossRef]
- Lucas, M.D.; Turnbull, O.H.; Kaplan-Solms, K.L. Laterality of Cradling in Relation to Perception and Expression of Facial Affect. J. Genet. Psychol. 1993, 154, 347–352. [Google Scholar] [CrossRef]
- Vauclair, J.; Scola, C. Infant-Holding Biases in Mothers and Affective Symptoms during Pregnancy and after Delivery. Infant. Child Dev. 2009, 18, 106–121. [Google Scholar] [CrossRef]
- Sieratzki, J.S.; Woll, B. Why Do Mothers Cradle Babies on Their Left? Lancet 1996, 347, 1746–1748. [Google Scholar] [CrossRef]
- Turnbull, O.H.; Bryson, H.E. The Leftward Cradling Bias and Hemispheric Asymmetry for Speech Prosody. Laterality 2001, 6, 21–28. [Google Scholar] [CrossRef]
- Sieratzki, J.S.; Roy, P.; Woll, B. Left Cradling and Left Ear Advantage for Emotional Speech: Listen to the Other Side Too. Laterality 2002, 7, 351–353. [Google Scholar] [CrossRef]
- Turnbull, O.H. The Leftward Cradling Bias and Audition: Cross-Modal Confusion? A Response to Sieratzki, Roy, and Woll. Laterality 2002, 7, 355–357. [Google Scholar] [CrossRef]
- Donnot, J. Lateralisation of Emotion Predicts Infant-Holding Bias in Left-Handed Students, but Not in Left-Handed Mothers. Laterality 2007, 12, 216–226. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Rapino, M.; Tommasi, L. The Left-Cradling Bias and Its Relationship with Empathy and Depression. Sci. Rep. 2019, 9, 6141. [Google Scholar] [CrossRef]
- Pileggi, L.-A.; Storey, S.; Malcolm-Smith, S. Depressive Symptoms Disrupt Leftward Cradling. J. Child Adolesc. Ment. Health 2020, 32, 35–43. [Google Scholar] [CrossRef]
- Scola, C.; Arciszewski, T.; Measelle, J.; Vauclair, J. Infant-Holding Bias Variations in Mother-Child Relationships: A Longitudinal Study. Eur. J. Psychol. Educ. 2013, 10, 707–722. [Google Scholar] [CrossRef]
- Weatherill, R.P.; Almerigi, J.B.; Levendosky, A.A.; Bogat, G.A.; Von Eye, A.; Harris, L.J. Is Maternal Depression Related to Side of Infant Holding? Int. J. Behav. Dev. 2004, 28, 421–427. [Google Scholar] [CrossRef]
- Boulinguez-Ambroise, G.; Pouydebat, E.; Disarbois, É.; Meguerditchian, A. Human-like Maternal Left-Cradling Bias in Monkeys Is Altered by Social Pressure. Sci. Rep. 2020, 10, 11036. [Google Scholar] [CrossRef]
- Morgan, B.; Hunt, X.; Sieratzki, J.; Woll, B.; Tomlinson, M. Atypical Maternal Cradling Laterality in an Impoverished South African Population. Laterality 2019, 24, 320–341. [Google Scholar] [CrossRef]
- Reissland, N.; Hopkins, B.; Helms, P.; Williams, B. Maternal Stress and Depression and the Lateralisation of Infant Cradling. J. Child Psychol. Psychiatry 2009, 50, 263–269. [Google Scholar] [CrossRef]
- Suter, S.E.; Huggenberger, H.J.; Schächinger, H. Cold Pressor Stress Reduces Left Cradling Preference in Nulliparous Human Females. Stress 2007, 10, 45–51. [Google Scholar] [CrossRef]
- Suter, S.E.; Huggenberger, H.J.; Blumenthal, T.D.; Schachinger, H. Differential Effect of Ill-Being and Chronic Stress on Cradling Behavior of First and Multi-Time Parents. Infant Behav. Dev. 2011, 34, 170–178. [Google Scholar] [CrossRef]
- De Château, P.; Holmberg, H.; Winberg, J. Left-Side Preference in Holding and Carrying Newborn Infants. Acta Paediatr. 1978, 67, 169–175. [Google Scholar] [CrossRef]
- Bogren, L.Y. Side Preference in Women and Men When Holding Their Newborn Child: Psychological Background. Acta Psychiatr. Scand. 1984, 69, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, G.; Marzoli, D.; Piccioni, C.; Tommasi, L. The Relationship between the Left-Cradling Bias and Attachment to Parents and Partner. Evol. Psychol. 2019, 17, 147470491984811. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Davis, R.; Mareschal, D.; Malatesta, G.; Todd, B.K. The Left Cradling Bias: An Evolutionary Facilitator of Social Cognition? Cortex 2019, 118, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.S.; Davis, R.; Malatesta, G.; Todd, B.K. Evolutionary Motor Biases and Cognition in Children with and without Autism. Sci. Rep. 2020, 10, 17385. [Google Scholar] [CrossRef]
- Herdien, L.; Malcolm-Smith, S.; Pileggi, L.-A. Leftward Cradling Bias in Males and Its Relation to Autistic Traits and Lateralised Emotion Processing. Brain Cogn. 2020, 147, 105652. [Google Scholar] [CrossRef]
- Pileggi, L.-A.; Malcolm-Smith, S.; Hoogenhout, M.; Thomas, K.G.; Solms, M. Cradling Bias Is Absent in Children with Autism Spectrum Disorders. J. Child Adolesc. Ment. Health 2013, 25, 55–60. [Google Scholar] [CrossRef]
- Pileggi, L.-A.; Malcolm-Smith, S.; Solms, M. Investigating the Role of Social-Affective Attachment Processes in Cradling Bias: The Absence of Cradling Bias in Children with Autism Spectrum Disorders. Laterality 2015, 20, 154–170. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Morelli, L.; Pivetti, M.; Tommasi, L. The Role of Ethnic Prejudice in the Modulation of Cradling Lateralization. J. Nonverbal. Behav. 2021, 45, 187–205. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Apicella, F.; Abiuso, C.; Muratori, F.; Forrester, G.S.; Vallortigara, G.; Scattoni, M.L.; Tommasi, L. Received Cradling Bias During the First Year of Life: A Retrospective Study on Children With Typical and Atypical Development. Front. Psychiatry 2020, 11, 91. [Google Scholar] [CrossRef]
- Malatesta, G.; Marzoli, D.; Tommasi, L. The Association between Received Maternal Cradling and Neurodevelopment: Is Left Better? Med. Hypotheses 2020, 134, 109442. [Google Scholar] [CrossRef]
- Bryden, M.P. Perceptual Asymmetry in Vision: Relation to Handedness, Eyedness, and Speech Lateralization. Cortex 1973, 9, 419–435. [Google Scholar] [CrossRef]
- Lidzba, K.; Staudt, M.; Wilke, M.; Krägeloh-Mann, I. Visuospatial Deficits in Patients with Early Left-Hemispheric Lesions and Functional Reorganization of Language: Consequence of Lesion or Reorganization? Neuropsychologia 2006, 44, 1088–1094. [Google Scholar] [CrossRef]
- McManus, C. Right Hand, Left Hand: The Origins of Asymmetry in Brains, Bodies, Atoms and Cultures; Harvard University Press: Cambridge, MA, USA, 2002; ISBN 978-0-674-01613-2. [Google Scholar]
- Nicholls, M.E.R.; Chapman, H.L.; Loetscher, T.; Grimshaw, G.M. The Relationship between Hand Preference, Hand Performance, and General Cognitive Ability. J. Int. Neuropsychol. Soc. 2010, 16, 585–592. [Google Scholar] [CrossRef]
- Felisatti, A.; Aagten-Murphy, D.; Laubrock, J.; Shaki, S.; Fischer, M.H. The Brain’s Asymmetric Frequency Tuning: Asymmetric Behavior Originates from Asymmetric Perception. Symmetry 2020, 12, 2083. [Google Scholar] [CrossRef]
- Karim, A.K.M.R.; Proulx, M.J.; Likova, L.T. Anticlockwise or Clockwise? A Dynamic Perception-Action-Laterality Model for Directionality Bias in Visuospatial Functioning. Neurosci. Biobehav. Rev. 2016, 68, 669–693. [Google Scholar] [CrossRef]
- Rogers, L.J. Asymmetry of Motor Behavior and Sensory Perception: Which Comes First? Symmetry 2020, 12, 690. [Google Scholar] [CrossRef]
- McManus, I.C.; Davison, A.; Armour, J.A.L. Multilocus Genetic Models of Handedness Closely Resemble Single-Locus Models in Explaining Family Data and Are Compatible with Genome-Wide Association Studies. Ann. N. Y. Acad. Sci. 2013, 1288, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, M.; Plaut, D.C. A Vision of Graded Hemispheric Specialization. Ann. N. Y. Acad. Sci. 2015, 1359, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Coryell, J.F.; Michel, G.F. How Supine Postural Preferences of Infants Can Contribute toward the Development of Handedness. Infant Behav. Dev. 1978, 1, 245–257. [Google Scholar] [CrossRef]
- Hopkins, B.; Lems, W.; Janssen, B.; Butterworth, G. Postural and Motor Asymmetries in Newlyborns. Hum. Neurobiol. 1987, 6, 153–156. [Google Scholar] [PubMed]
- Konishi, Y.; Mikawa, H.; Suzuki, J. Asymmetrical Head-Turning of Preterm Infants: Some Effects on Later Postural and Functional Lateralities. Dev. Med. Child Neurol. 1986, 28, 450–457. [Google Scholar] [CrossRef]
- Michel, G.F. Right-Handedness: A Consequence of Infant Supine Head-Orientation Preference? Science 1981, 212, 685–687. [Google Scholar] [CrossRef]
- Michel, G.F.; Harkins, D.A. Postural and Lateral Asymmetries in the Ontogeny of Handedness during Infancy. Dev. Psychobiol. 1986, 19, 247–258. [Google Scholar] [CrossRef]
- Rönnqvist, L.; Hopkins, B.; van Emmerik, R.; de Groot, L. Lateral Biases in Head Turning and the Moro Response in the Human Newborn: Are They Both Vestibular in Origin? Dev. Psychobiol. 1998, 33, 339–349. [Google Scholar] [CrossRef]
- Fagard, J.; Lemoine, C. The Role of Imitation in the Stabilization of Handedness during Infancy. J. Integr. Neurosci. 2006, 5, 519–533. [Google Scholar] [CrossRef]
- Harkins, D.A.; Michel, G.F. Evidence for a Maternal Effect on Infant Hand-Use Preferences. Dev. Psychobiol. 1988, 21, 535–541. [Google Scholar] [CrossRef]
- Harkins, D.A.; Uẑgiris, I.Č. Hand-Use Matching between Mothers and Infants during the First Year. Infant Behav. Dev. 1991, 14, 289–298. [Google Scholar] [CrossRef]
- Michel, G.F. Maternal Influences on Infant Hand-Use during Play with Toys. Behav. Genet. 1992, 22, 163–176. [Google Scholar] [CrossRef][Green Version]
- Scola, C.; Vauclair, J. Is Infant Holding-Side Bias Related to Motor Asymmetries in Mother and Child? Dev. Psychobiol. 2010, 52, 475–486. [Google Scholar] [CrossRef]
- Dagenbach, D.; Harris, L.J.; Fitzgerald, H.E. A Longitudinal Study of Lateral Biases in Parents’ Cradling and Holding of Infants. Infant Ment. Health J. 1988, 9, 218–234. [Google Scholar] [CrossRef]
- Huheey, J.E. Concerning the Origin of Handedness in Humans. Behav Genet 1977, 7, 29–32. [Google Scholar] [CrossRef]
- Boulinguez-Ambroise, G.; Aychet, J.; Pouydebat, E. Limb Preference in Animals: New Insights into the Evolution of Manual Laterality in Hominids. Symmetry 2022, 14, 96. [Google Scholar] [CrossRef]
- Boulinguez-Ambroise, G.; Pouydebat, E.; Disarbois, É.; Meguerditchian, A. Maternal Cradling Bias in Baboons: The First Environmental Factor Affecting Early Infant Handedness Development? Dev. Sci. 2022, 25, e13179. [Google Scholar] [CrossRef]
- Hopkins, W.D. Laterality in Maternal Cradling and Infant Positional Biases: Implications for the Development and Evolution of Hand Preferences in Nonhuman Primates. Int. J. Primatol. 2004, 25, 1243–1265. [Google Scholar] [CrossRef]
- Watling, D.; Workman, L.; Bourne, V.J. Emotion Lateralisation: Developments throughout the Lifespan. Laterality 2012, 17, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Meints, K.; Hall, C.; Hall, S.; Mills, D. Left Gaze Bias in Humans, Rhesus Monkeys and Domestic Dogs. Anim. Cogn. 2009, 12, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.D.; Rasch, M.J.; Tomonaga, M.; Adachi, I. Laterality Effect for Faces in Chimpanzees (Pan Troglodytes). J. Neurosci. 2013, 33, 13344–13349. [Google Scholar] [CrossRef] [PubMed]
- Balas, B.; Moulson, M.C. Developing a Side Bias for Conspecific Faces during Childhood. Dev. Psychol. 2011, 47, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Cochet, H.; Byrne, R.W. Evolutionary Origins of Human Handedness: Evaluating Contrasting Hypotheses. Anim. Cogn. 2013, 16, 531–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the Origins of Human Handedness and Language: A Comparative Review of Hand Preferences for Bimanual Coordinated Actions and Gestural Communication in Nonhuman Primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar] [CrossRef]
- Lindell, A. Continuities in Emotion Lateralization in Human and Non-Human Primates. Front. Hum. Neurosci. 2013, 7, 464. [Google Scholar] [CrossRef]
- Greenough, A.; Lagercrantz, H.; Pool, J.; Dahlin, I. Plasma Catecholamine Levels in Preterm Infants. Acta Paediatr. 1987, 76, 54–59. [Google Scholar] [CrossRef]
- Vervloed, M.P.J.; Hendriks, A.W.; van den Eijnde, E. The Effects of Mothers’ Past Infant-Holding Preferences on Their Adult Children’s Face Processing Lateralisation. Brain Cogn. 2011, 75, 248–254. [Google Scholar] [CrossRef]
- Heath, R.; Rouhana, A.; Abi Ghanem, D. Asymmetric Bias in Perception of Facial Affect among Roman and Arabic Script Readers. Laterality 2005, 10, 51–64. [Google Scholar] [CrossRef]
- Megreya, A.M.; Havard, C. Left Face Matching Bias: Right Hemisphere Dominance or Scanning Habits? Laterality 2011, 16, 75–92. [Google Scholar] [CrossRef]
- Sakhuja, T.; Gupta, G.; Singh, M.; Vaid, J. Reading Habits Affect Asymmetries in Facial Affect Judgments: A Replication. Brain Cogn. 1996, 32, 162–165. [Google Scholar]
- Vaid, J.; Singh, M. Asymmetries in the Perception of Facial Affect: Is There an Influence of Reading Habits? Neuropsychologia 1989, 27, 1277–1287. [Google Scholar] [CrossRef]
- Dundas, E.M.; Best, C.A.; Minshew, N.J.; Strauss, M.S. A Lack of Left Visual Field Bias When Individuals with Autism Process Faces. J. Autism Dev. Disord. 2012, 6, 1104–1111. [Google Scholar] [CrossRef]
- Liu, S.; Quinn, P.C.; Wheeler, A.; Xiao, N.; Ge, L.; Lee, K. Similarity and Difference in the Processing of Same- and Other-Race Faces as Revealed by Eye Tracking in 4- to 9-Month-Olds. J. Exp. Child Psychol. 2011, 108, 180–189. [Google Scholar] [CrossRef]
- Anes, M.D.; Short, L.A. Adult-like Competence in Perceptual Encoding of Facial Configuration by the Right Hemisphere Emerges after 10 Years of Age. Perception 2009, 38, 333–342. [Google Scholar] [CrossRef]
- Maurer, D.; Grand, R.L.; Mondloch, C.J. The Many Faces of Configural Processing. Trends Cogn. Sci. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Reed, C.L.; Stone, V.E.; Bozova, S.; Tanaka, J. The Body-Inversion Effect. Psychol. Sci. 2003, 14, 302–308. [Google Scholar] [CrossRef]
- Reed, C.L.; Stone, V.E.; Grubb, J.D.; McGoldrick, J.E. Turning Configural Processing Upside down: Part and Whole Body Postures. J. Exp. Psychol. Hum. Percept. Perform. 2006, 32, 73–87. [Google Scholar] [CrossRef]
- Bourne, V.J. Examining the Effects of Inversion on Lateralisation for Processing Facial Emotion. Cortex 2011, 47, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Coolican, J.; Eskes, G.A.; McMullen, P.A.; Lecky, E. Perceptual Biases in Processing Facial Identity and Emotion. Brain Cogn. 2008, 66, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.D.; Shepherd, J.W. Recognition of Upright and Inverted Faces Presented in the Left and Right Visual Fields. Cortex 1975, 11, 3–7. [Google Scholar] [CrossRef]
- Leehey, S.; Carey, S.; Diamond, R.; Cahn, A. Upright and Inverted Faces: The Right Hemisphere Knows the Difference. Cortex 1978, 14, 411–419. [Google Scholar] [CrossRef]
- Luh, K.E. Effect of Inversion on Perceptual Biases for Chimeric Faces. Brain Cogn. 1998, 37, 105–108. [Google Scholar]
- Carey, S.; Diamond, R. From Piecemeal to Configurational Representation of Faces. Science 1977, 195, 312–314. [Google Scholar] [CrossRef]
- Diamond, R.; Carey, S. Developmental Changes in the Representation of Faces. J. Exp. Child Psychol. 1977, 23, 1–22. [Google Scholar] [CrossRef]
- Mondloch, C.J.; Le Grand, R.; Maurer, D. Configural Face Processing Develops More Slowly than Featural Face Processing. Perception 2002, 31, 553–566. [Google Scholar] [CrossRef]
- Hobson, R.P.; Ouston, J.; Lee, A. What’s in a Face? The Case of Autism. Br. J. Psychol. 1988, 79, 441–453. [Google Scholar] [CrossRef]
- Tantam, D.; Monaghan, L.; Nicholson, H.; Stirling, J. Autistic Children’s Ability to Interpret Faces: A Research Note. J. Child Psychol. Psychiatry 1989, 30, 623–630. [Google Scholar] [CrossRef]
- Taylor, S.; Workman, L.; Yeomans, H. Abnormal Patterns of Cerebral Lateralisation as Revealed by the Universal Chimeric Faces Task in Individuals with Autistic Disorder. Laterality 2012, 17, 428–437. [Google Scholar] [CrossRef]
- Dundas, E.; Gastgeb, H.; Strauss, M.S. Left Visual Field Biases When Infants Process Faces: A Comparison of Infants at High- and Low-Risk for Autism Spectrum Disorder. J. Autism Dev. Disord. 2012, 42, 2659–2668. [Google Scholar] [CrossRef]
- Behrmann, M.; Avidan, G.; Leonard, G.L.; Kimchi, R.; Luna, B.; Humphreys, K.; Minshew, N. Configural Processing in Autism and Its Relationship to Face Processing. Neuropsychologia 2006, 44, 110–129. [Google Scholar] [CrossRef]
- Leonards, U.; Scott-Samuel, N.E. Idiosyncratic Initiation of Saccadic Face Exploration in Humans. Vis. Res. 2005, 45, 2677–2684. [Google Scholar] [CrossRef]
- Mertens, I.; Siegmund, H.; Grüsser, O.-J. Gaze Motor Asymmetries in the Perception of Faces during a Memory Task. Neuropsychologia 1993, 31, 989–998. [Google Scholar] [CrossRef]
- Gallois, P.; Buquet, C.; Charlier, J.; Paris, V.; Hache, J.C.; Dereux, J.F. Asymmetry of visual perceptive activity of faces and emotional facial expressions. Rev. Neurol. 1989, 145, 661–664. [Google Scholar]
- Thompson, L.A.; Malloy, D.M.; LeBlanc, K.L. Lateralization of Visuospatial Attention across Face Regions Varies with Emotional Prosody. Brain Cogn. 2009, 69, 108–115. [Google Scholar] [CrossRef]
- Marotta, A.; Lupiáñez, J.; Casagrande, M. Investigating Hemispheric Lateralization of Reflexive Attention to Gaze and Arrow Cues. Brain Cogn. 2012, 80, 361–366. [Google Scholar] [CrossRef]
- Greene, D.J.; Zaidel, E. Hemispheric Differences in Attentional Orienting by Social Cues. Neuropsychologia 2011, 49, 61–68. [Google Scholar] [CrossRef]
- Mogg, K.; Bradley, B.P. Orienting of Attention to Threatening Facial Expressions Presented under Conditions of Restricted Awareness. Cogn. Emot. 1999, 13, 713–740. [Google Scholar] [CrossRef]
- Mogg, K.; Bradley, B.P. Selective Orienting of Attention to Masked Threat Faces in Social Anxiety. Behav. Res. Ther. 2002, 40, 1403–1414. [Google Scholar] [CrossRef]
- Field, A.P. Watch Out for the Beast: Fear Information and Attentional Bias in Children. J. Clin. Child Adolesc. Psychol. 2006, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.C.; Banich, M.T.; Koch-Weser, M. Variations in Patterns of Lateral Asymmetry among Dextrals. Brain Cogn. 1984, 3, 317–334. [Google Scholar] [CrossRef]
- Kim, H.; Levine, S.C.; Kertesz, S. Are Variations among Subjects in Lateral Asymmetry Real Individual Differences or Random Error in Measurement?: Putting Variability in Its Place. Brain Cogn. 1990, 14, 220–242. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Pierson-Savage, J.M.; Nettleton, N.C. Hemispace Asymmetries. In Contemporary Reviews in Neuropsychology; Whitaker, H.A., Ed.; Springer Series in Neuropsychology; Springer: New York, NY, USA, 1988; pp. 1–35. ISBN 978-1-4612-3780-8. [Google Scholar]
- Dellatolas, G.; Viguier, D.; Deloche, G.; De Agostini, M. Right-Left Orientation and Significance of Systematic Reversal in Children. Cortex 1998, 34, 659–676. [Google Scholar] [CrossRef]
- Failla, C.V.; Sheppard, D.M.; Bradshaw, J.L. Age and Responding-Hand Related Changes in Performance of Neurologically Normal Subjects on the Line-Bisection and Chimeric-Faces Tasks. Brain Cogn. 2003, 52, 353–363. [Google Scholar] [CrossRef]
- Workman, L.; Chilvers, L.; Yeomans, H.; Taylor, S. Development of Cerebral Lateralisation for Recognition of Emotions in Chimeric Faces in Children Aged 5 to 11. Laterality 2006, 11, 493–507. [Google Scholar] [CrossRef]
- Watling, D.; Bourne, V.J. Sex Differences in the Relationship Between Children’s Emotional Expression Discrimination and Their Developing Hemispheric Lateralization. Dev. Neuropsychol. 2013, 38, 496–506. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The Eye Direction Detector (EDD) and the Shared Attention Mechanism (SAM): Two Cases for Evolutionary Psychology. In Joint Attention: Its Origins and Role in Development; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1995; pp. 41–59. ISBN 978-0-8058-1437-8. [Google Scholar]
- Birmingham, E.; Ristic, J.; Kingstone, A. Investigating Social Attention: A Case for Increasing Stimulus Complexity in the Laboratory. In Cognitive Neuroscience, Development, and Psychopathology: Typical and Atypical Developmental Trajectories of Attention; Oxford University Press: New York, NY, USA, 2012; pp. 251–276. ISBN 978-0-19-531545-5. [Google Scholar]
- Tonks, J.; Williams, W.H.; Frampton, I.; Yates, P.; Slater, A. Assessing Emotion Recognition in 9–15-Years Olds: Preliminary Analysis of Abilities in Reading Emotion from Faces, Voices and Eyes. Brain Inj. 2007, 21, 623–629. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Gur, R.C. Lateral Asymmetry in Intensity of Emotional Expression. Neuropsychologia 1978, 16, 473–481. [Google Scholar] [CrossRef]
- Indersmitten, T.; Gur, R.C. Emotion Processing in Chimeric Faces: Hemispheric Asymmetries in Expression and Recognition of Emotions. J. Neurosci. 2003, 23, 3820–3825. [Google Scholar] [CrossRef]
- Workman, L.; Peters, S.; Taylor, S. Lateralisation of Perceptual Processing of Pro- and Anti-Social Emotions Displayed in Chimeric Faces. Laterality 2000, 5, 237–249. [Google Scholar] [CrossRef]
- Godard, O.; Fiori, N. Sex Differences in Face Processing: Are Women Less Lateralized and Faster than Men? Brain Cogn. 2010, 73, 167–175. [Google Scholar] [CrossRef]
- Raymond, M.; Pontier, D.; Dufour, A.B.; Møller, A.P. Frequency-Dependent Maintenance of Left Handedness in Humans. Proc. Biol. Sci. 1996, 263, 1627–1633. [Google Scholar] [CrossRef]
- Rahman, Q.; Anchassi, T. Men Appear More Lateralized When Noticing Emotion in Male Faces. Emotion 2012, 12, 174–179. [Google Scholar] [CrossRef]
- Bourne, V.J.; Vladeanu, M. Lateralisation for Processing Facial Emotion and Anxiety: Contrasting State, Trait and Social Anxiety. Neuropsychologia 2011, 49, 1343–1349. [Google Scholar] [CrossRef]
- Heller, W.; Etienne, M.A.; Miller, G.A. Patterns of Perceptual Asymmetry in Depression and Anxiety: Implications for Neuropsychological Models of Emotion and Psychopathology. J. Abnorm. Psychol. 1995, 104, 327–333. [Google Scholar] [CrossRef]
- Keller, J.; Nitschke, J.B.; Bhargava, T.; Deldin, P.J.; Gergen, J.A.; Miller, G.A.; Heller, W. Neuropsychological Differentiation of Depression and Anxiety. J. Abnorm. Psychol. 2000, 109, 3–10. [Google Scholar] [CrossRef]
- Voelz, Z.R.; Gencoz, F.; Gencoz, T.; Pettit, J.W.; Perez, M.; Joiner, T.E. Patterns of Hemispheric Perceptual Asymmetries: Left Hemispatial Biases Predict Changes in Anxiety and Positive Affect in Undergraduate Women. Emotion 2001, 1, 339–347. [Google Scholar] [CrossRef]
- McManus, I.C.; Humphrey, N.K. Turning the left cheek. Nature 1973, 243, 271–272. [Google Scholar] [CrossRef]
- Conesa, J. Preference for the Half-Left Profile Pose: Three Inclusive Models. Percept. Mot. Ski. 1996, 82, 1070. [Google Scholar] [CrossRef] [PubMed]
- Conesa, J.; Brunold-Conesa, C.; Miron, M. Incidence of the Half-Left Profile Pose in Single-Subject Portraits. Percept. Mot. Ski. 1995, 81, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, G. Phenotypes in Hemispheric Functional Segregation? Perspectives and Challenges. Phys. Life Rev. 2019, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Papadatou-Pastou, M.; Tomprou, D.-M. Intelligence and Handedness: Meta-Analyses of Studies on Intellectually Disabled, Typically Developing, and Gifted Individuals. Neurosci. Biobehav. Rev. 2015, 56, 151–165. [Google Scholar] [CrossRef]
- Giljov, A.; Karenina, K.; Malashichev, Y. Facing Each Other: Mammal Mothers and Infants Prefer the Position Favouring Right Hemisphere Processing. Biol. Lett. 2018, 14, 20170707. [Google Scholar] [CrossRef]
- Peirce, J.W.; Leigh, A.E.; Kendrick, K.M. Configurational Coding, Familiarity and the Right Hemisphere Advantage for Face Recognition in Sheep. Neuropsychologia 2000, 38, 475–483. [Google Scholar] [CrossRef]
- Peirce, J.W.; Leigh, A.E.; da Costa, A.P.C.; Kendrick, K.M. Human Face Recognition in Sheep: Lack of Configurational Coding and Right Hemisphere Advantage. Behav. Process. 2001, 55, 13–26. [Google Scholar] [CrossRef]
- Salva, O.R.; Regolin, L.; Vallortigara, G. Chicks Discriminate Human Gaze with Their Right Hemisphere. Behav. Brain Res. 2007, 177, 15–21. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzoli, D.; D’Anselmo, A.; Malatesta, G.; Lucafò, C.; Prete, G.; Tommasi, L. The Intricate Web of Asymmetric Processing of Social Stimuli in Humans. Symmetry 2022, 14, 1096. https://doi.org/10.3390/sym14061096
Marzoli D, D’Anselmo A, Malatesta G, Lucafò C, Prete G, Tommasi L. The Intricate Web of Asymmetric Processing of Social Stimuli in Humans. Symmetry. 2022; 14(6):1096. https://doi.org/10.3390/sym14061096
Chicago/Turabian StyleMarzoli, Daniele, Anita D’Anselmo, Gianluca Malatesta, Chiara Lucafò, Giulia Prete, and Luca Tommasi. 2022. "The Intricate Web of Asymmetric Processing of Social Stimuli in Humans" Symmetry 14, no. 6: 1096. https://doi.org/10.3390/sym14061096
APA StyleMarzoli, D., D’Anselmo, A., Malatesta, G., Lucafò, C., Prete, G., & Tommasi, L. (2022). The Intricate Web of Asymmetric Processing of Social Stimuli in Humans. Symmetry, 14(6), 1096. https://doi.org/10.3390/sym14061096






