Crystal Structure of Chiral Drug Prenalterol and Its Precursor Prone to Spontaneous Resolution
Abstract
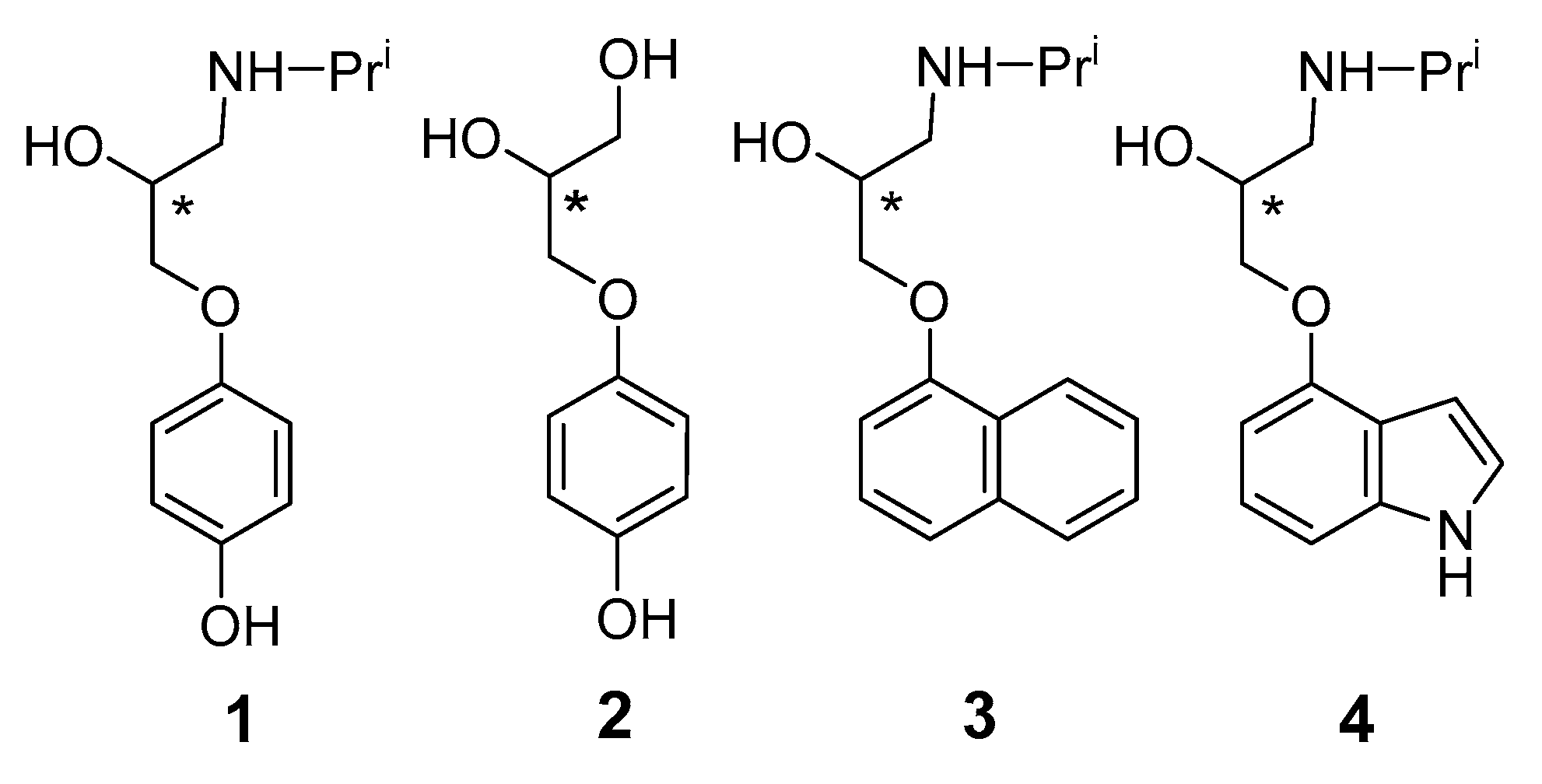
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
2.3. Single Crystal X-ray Diffraction
2.3.1. General Information
2.3.2. Crystallographic Data for (S)-1
2.3.3. Crystallographic Data for ras-1
2.3.4. Crystallographic Data for (R)-2
3. Results and Discussion
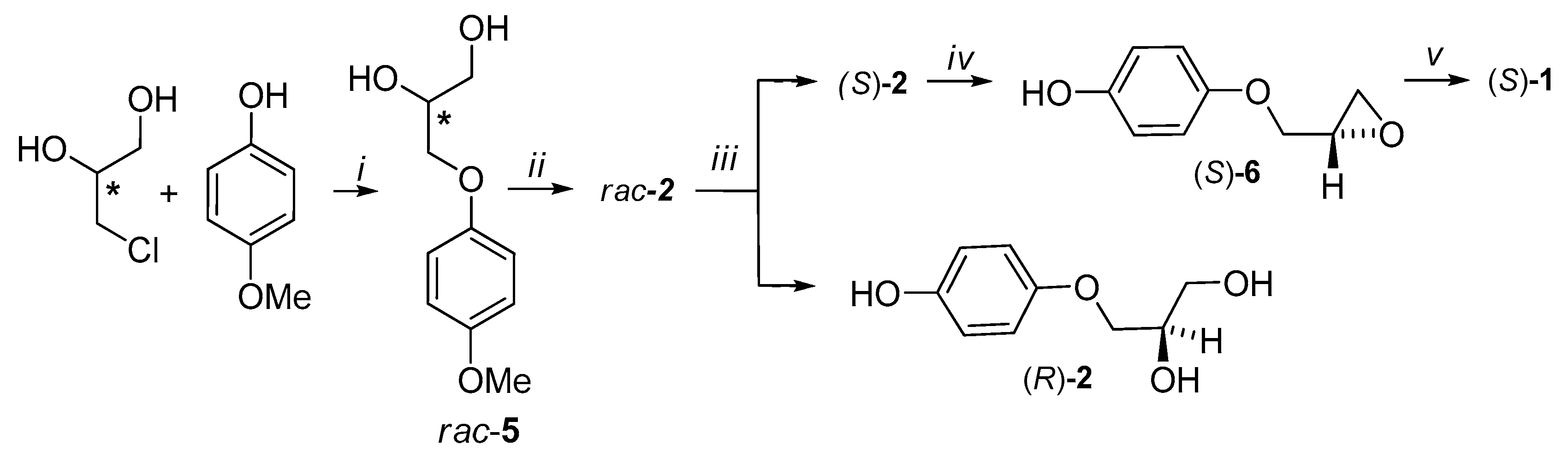
3.1. Synthesis of (S)-Prenalterol
3.2. SC-XRD Results
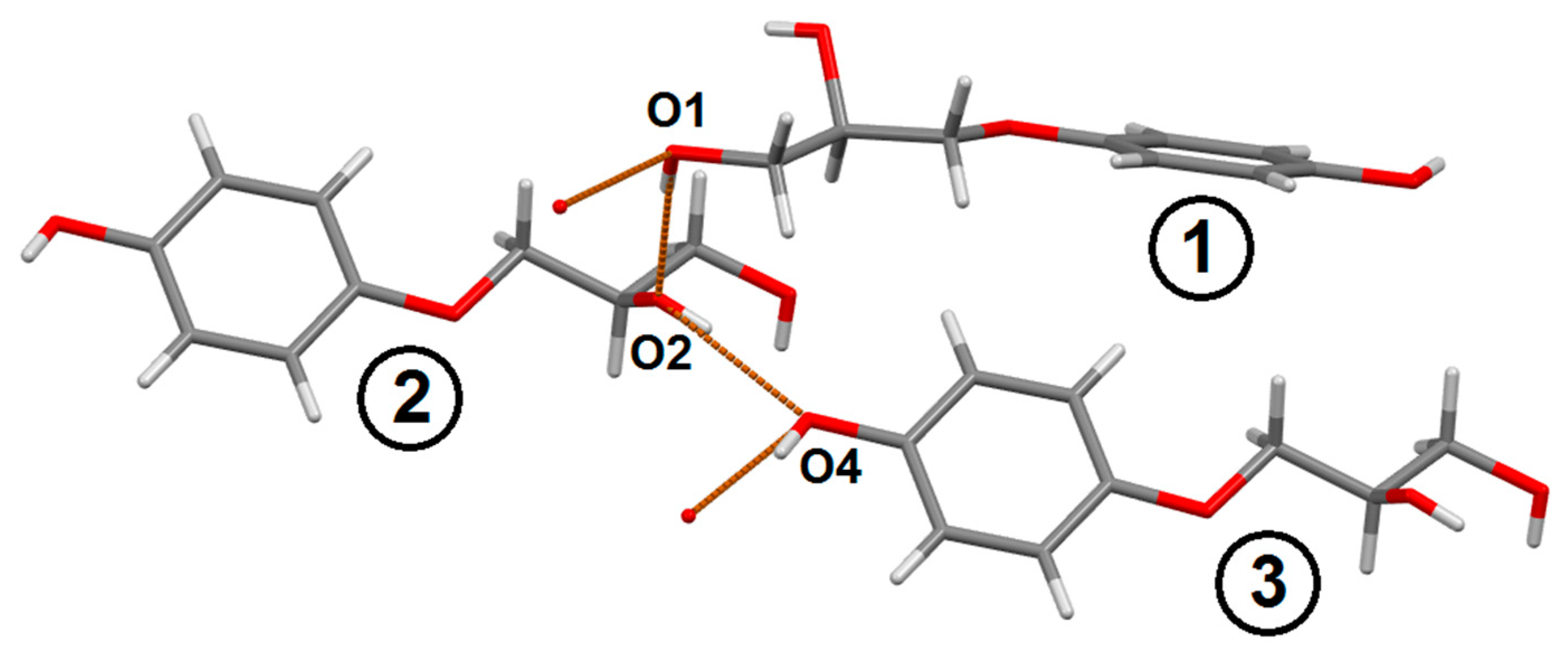
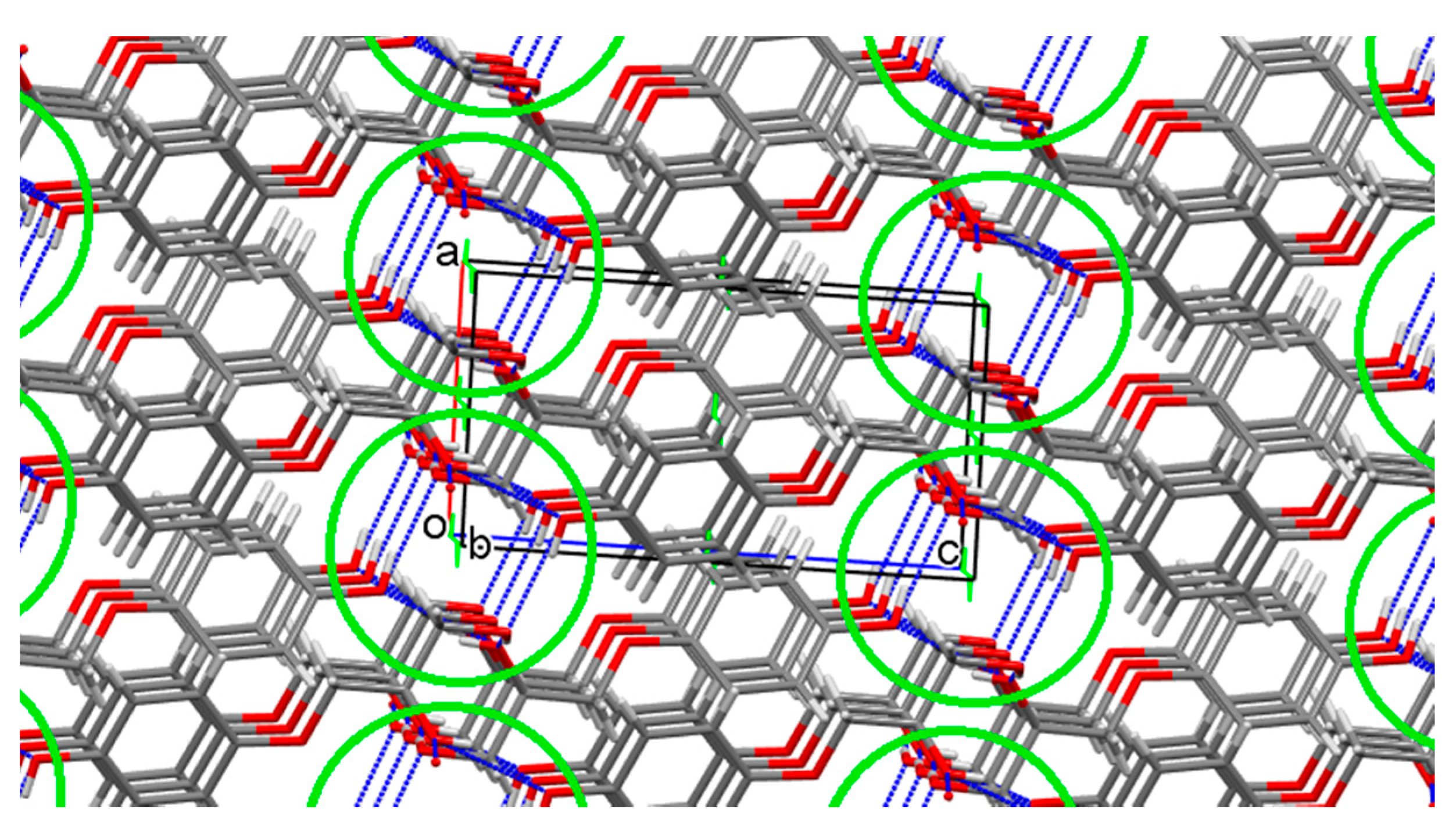
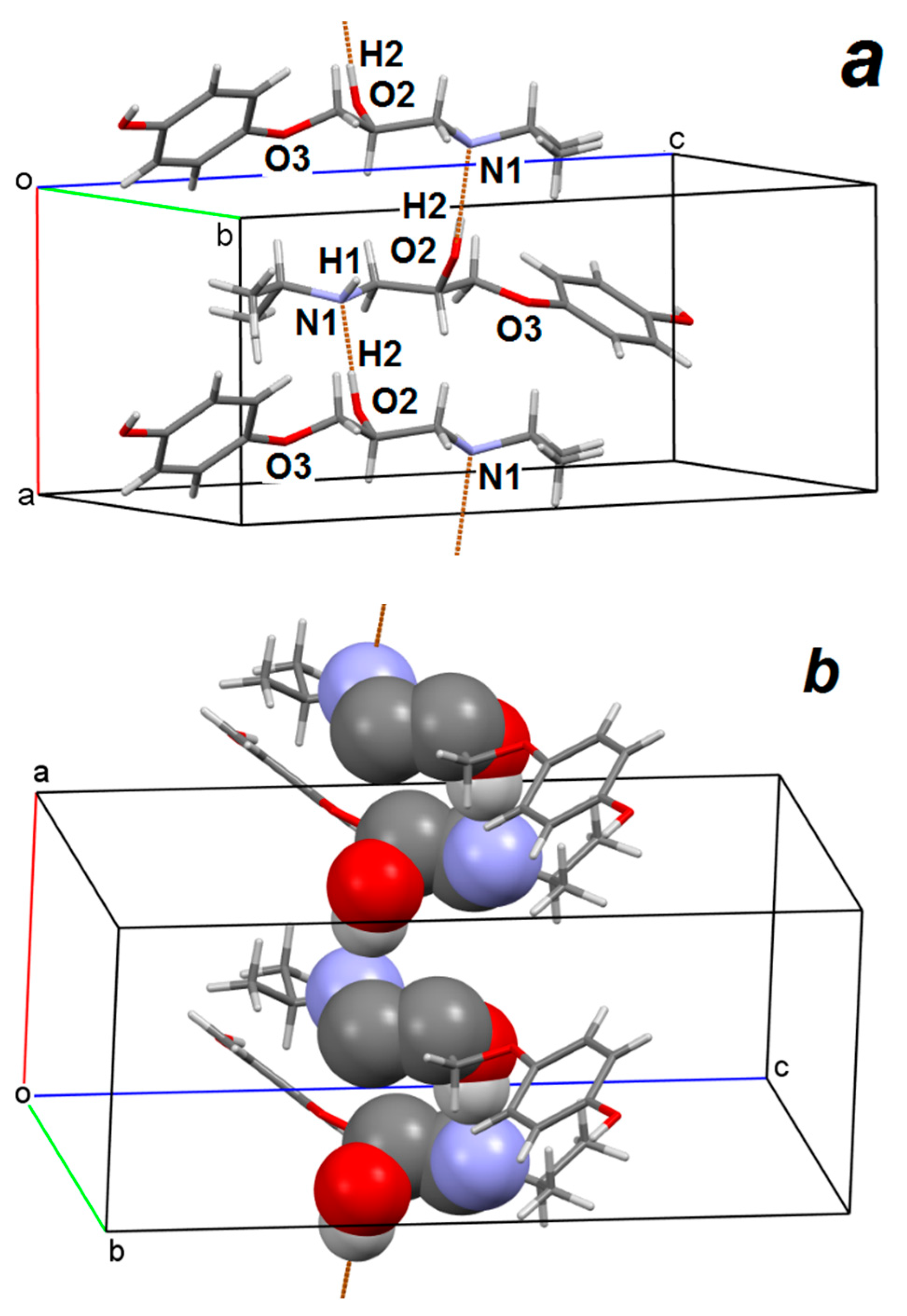
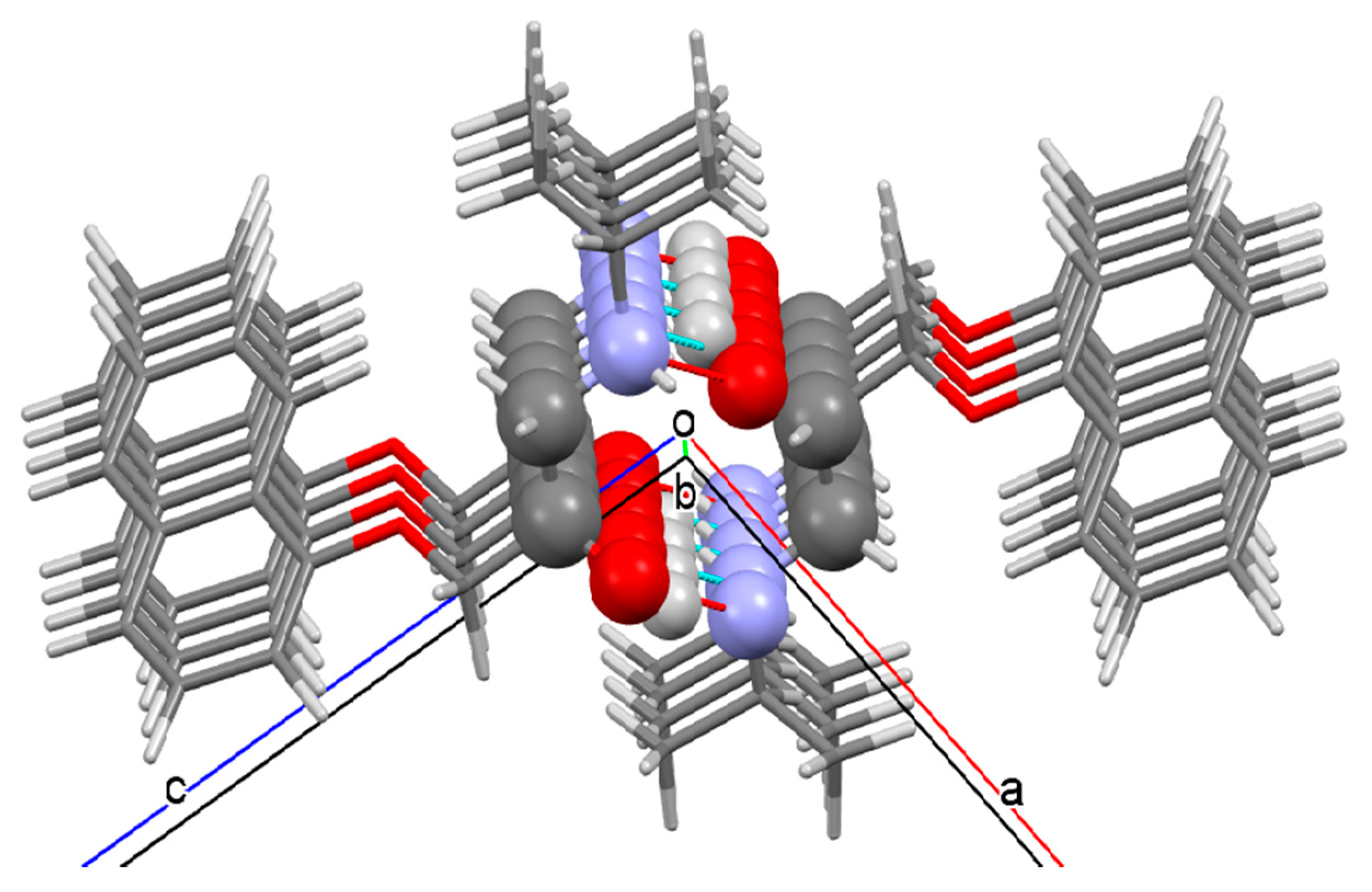
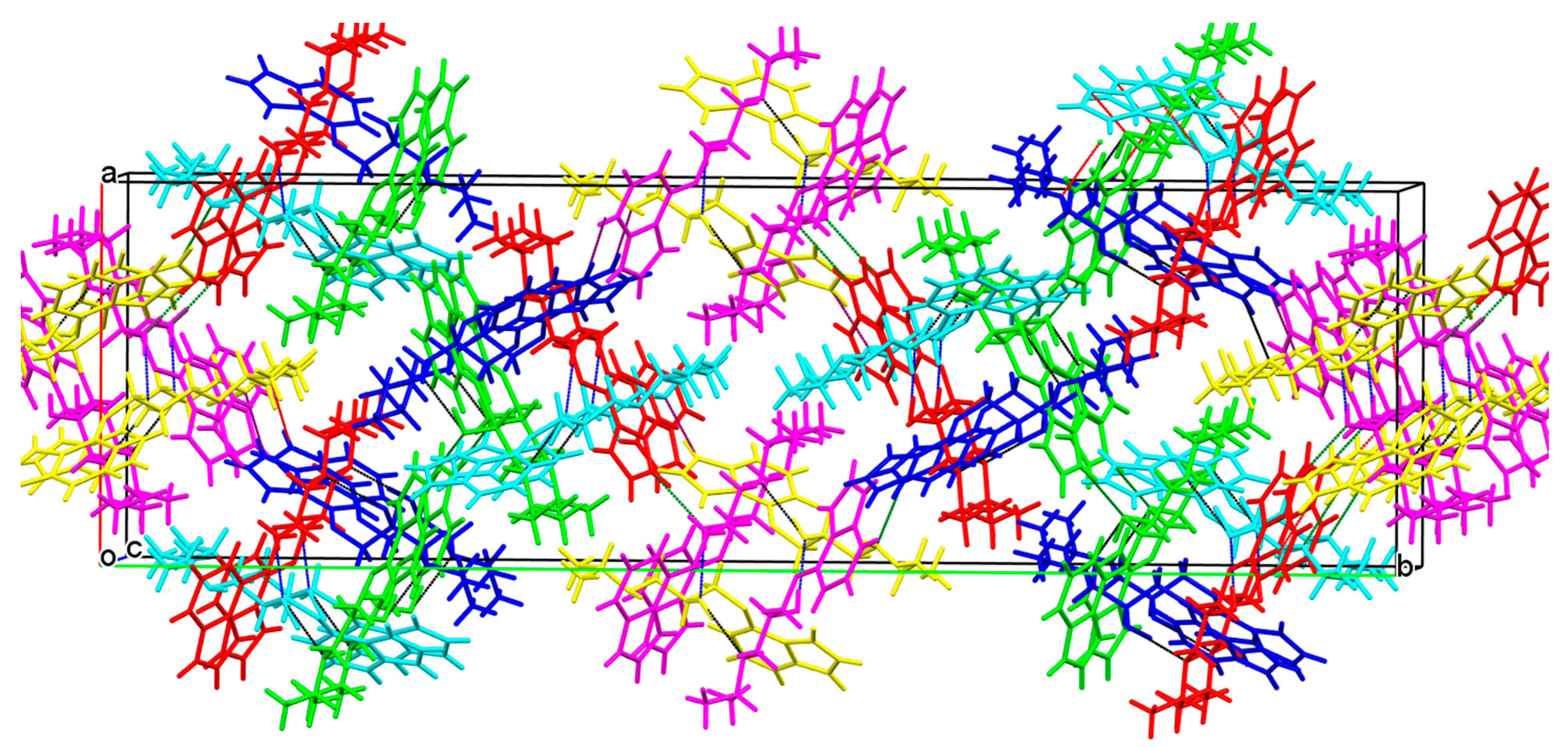
3.2.1. 3-(4-Hydroxyphenoxy)propane-1,2-diol 2
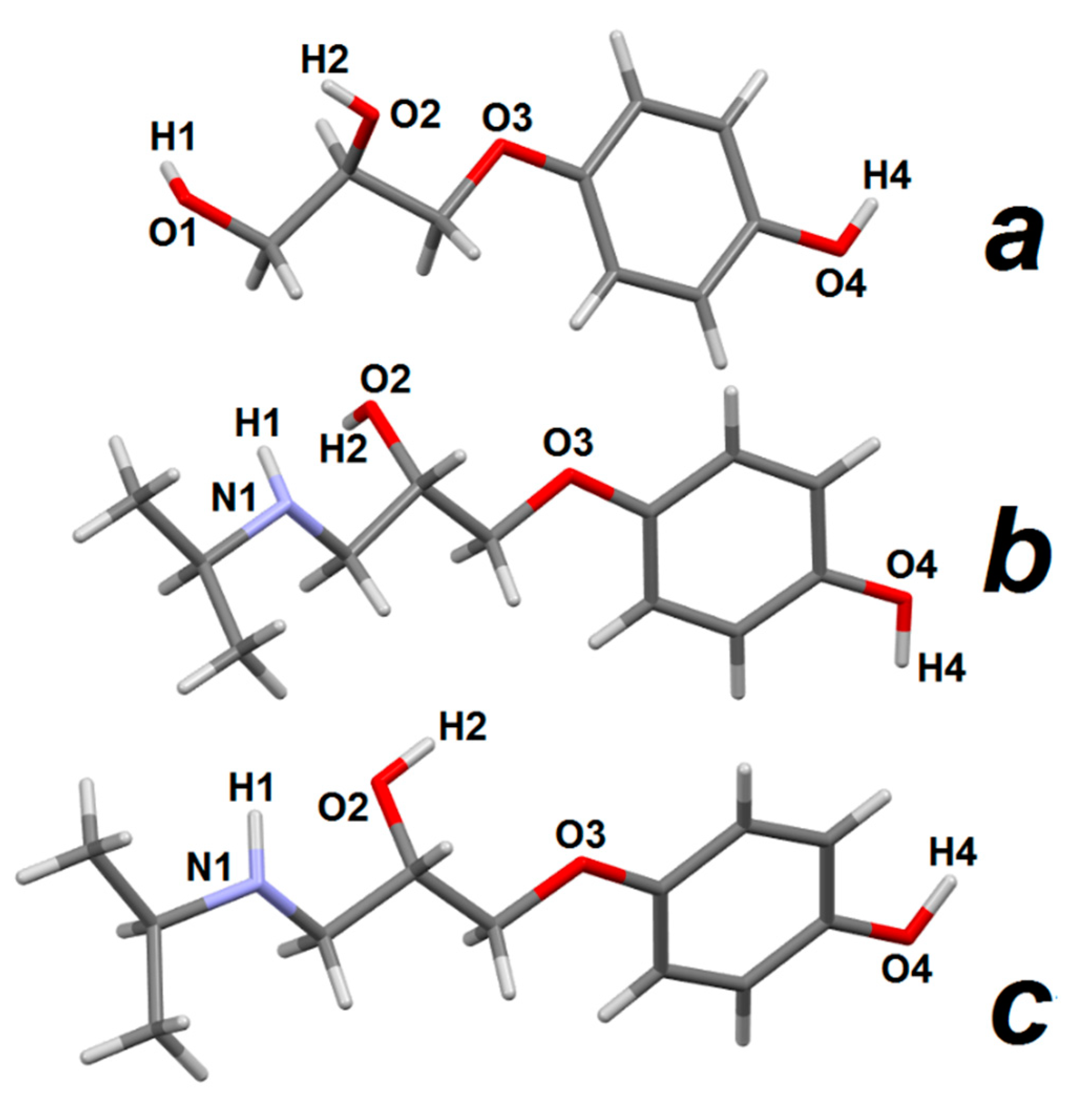
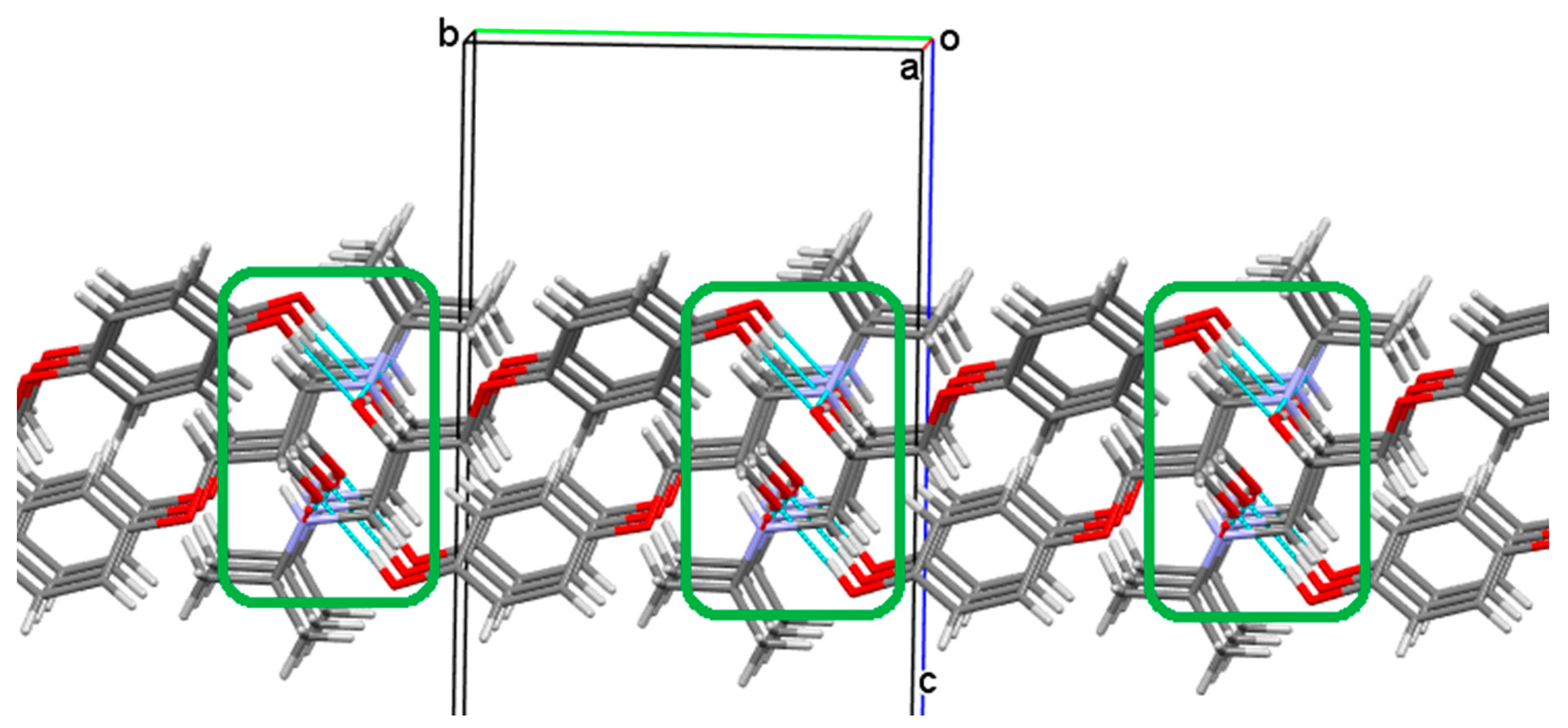
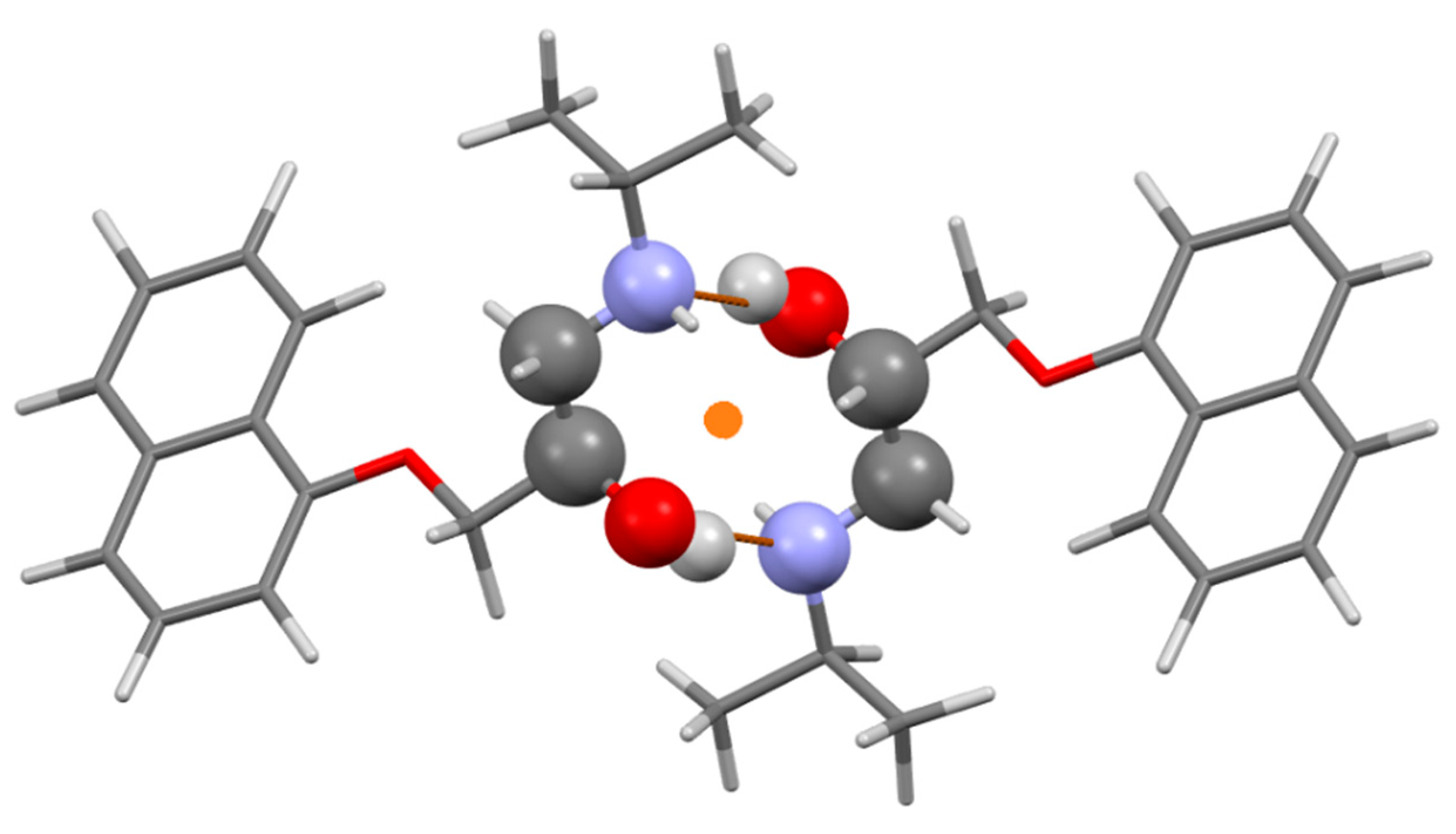
3.2.2. Prenalterol 1
3.2.3. Propranolol 3
3.2.4. Pindolol 4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kant, I. Prolegomena to Any Future Metaphysics that Will be Able to Come Forward as Science; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Kelvin, W.T. Molecular tactics of a crystal. J. Oxf. Univ. Jr. Sci. Club 1894, 18, 25, (DLC) 12034810. [Google Scholar]
- Gal, J.; Cintas, P. Early history of the recognition of molecular biochirality. Top. Curr. Chem. 2012, 333, 1–40. [Google Scholar] [CrossRef]
- Gal, J. Molecular chirality in chemistry and biology: Historical milestones. Helv. Chim. Acta 2013, 96, 1617–1657. [Google Scholar] [CrossRef]
- Wagniére, G.H. On Chirality and the Universal Asymmetry: Reflections on Image and Mirror Image; Wiley-VCH: Zürich, Switzerland, 2007; ISBN 978-3-906-39038-3. [Google Scholar]
- Devínsky, F. Chirality and the origin of life. Symmetry 2021, 13, 2277. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance Document, Development of New Stereoisomeric Drugs. May 1992. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-new-stereoisomeric-drugs (accessed on 15 May 2022).
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Mullin, R. Tufts study finds big rise in cost of drug development. Chem. Eng. News 2014, 92, Iss.47. Available online: https://cen.acs.org/articles/92/web/2014/11/Tufts-Study-Finds-Big-Rise.html (accessed on 15 May 2022).
- The Merck Index, 14th ed.; O’Neil, M.J. (Ed.) Merck and Co.: Whitehouse Station, NJ, USA, 2006; p. 1329, Entry 7735. [Google Scholar]
- Bredikhina, Z.A.; Kurenkov, A.V.; Bredikhin, A.A. Effective synthesis of non-racemic prenalterol based on spontaneous resolution of 3-(4-hydroxyphenoxy)propane-1,2-diol. Mendeleev Commun. 2019, 29, 198–199. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kenakin, T.P.; Beek, D. Is Prenalterol (H133/80) really a selective beta1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus-response relationships. J. Pharmacol. Exp. Ther. 1980, 213, 406–412. [Google Scholar]
- Mattsson, H.; Andersson, T.; Carlsson, E.; Hedberg, A.; Lundgren, B.; Olsson, T. Beta 1-and beta 2-adrenoceptor stimulatory effects of prenalterol. Naunyn Schmiedebergs Arch. Pharmacol. 1982, 321, 302–308. [Google Scholar] [CrossRef]
- Waller, D.G. Beta-adrenoceptor partial agonists: A renaissance in cardiovascular therapy? Br. J. Clin. Pharmacol. 1990, 30, 157–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisione, G.; Baroffio, M.; Crimi, E.; Brusasco, V. Beta-adrenergic agonists. Pharmaceuticals 2010, 3, 1016–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahrenholtz, K.E.; Guthrie, R.W.; Kierstead, R.W.; Tilley, J.W. Piperazine derivatives. Patent DE 2904799, 9 August 1979. [Google Scholar]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to separate enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Kellogg, R.M. Spontaneous deracemization. Isr. J. Chem. 2011, 51, 1034–1040. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Bredikhina, Z.A. Stereoselective crystallization as a basis for single-enantiomer drug production. Chem. Eng. Technol. 2017, 40, 1211–1220. [Google Scholar] [CrossRef] [Green Version]
- Bredikhin, A.A.; Bredikhina, Z.A.; Kurenkov, A.V.; Gubaidullin, A.T. Synthesis, crystal structure, and absolute configuration of the enantiomers of chiral drug xibenolol hydrochloride. Tetrahedron Asymmetry 2017, 28, 1359–1366. [Google Scholar] [CrossRef]
- Bredikhina, Z.A.; Kurenkov, A.V.; Krivolapov, D.B.; Bredikhin, A.A. Stereoselective crystallization of 3-(2,6-dimethylphenoxy)propane-1,2-diol; preparation of the single-enantiomer drug mexiletine. Tetrahedron Asymmetry 2015, 26, 577–583. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Bredikhina, Z.A.; Kurenkov, A.V.; Krivolapov, D.B. Synthesis and crystal structure of (S)-pindolol. Tetrahedron Asymmetry 2017, 28, 442–446. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Zakharychev, D.V.; Bredikhina, Z.A.; Kurenkov, A.V.; Samigullina, A.I.; Gubaidullin, A.T. Stereoselective crystallization of chiral 3,4-dimethylphenyl glycerol ether complicated by plurality of crystalline modifications. Crystals 2020, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Perez-Garcia, L.; Amabilino, D.B. Spontaneous resolution, whence and whither: From enantiomorphic solids to chiral liquid crystals, monolayers and macro- and supra-molecular polymers and assemblies. Chem. Soc. Rev. 2007, 36, 941–967. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic-compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding—functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Savel’ev, D.V.; Bredikhina, Z.A.; Gubaidullin, A.T.; Litvinov, I.A. Crystallization of chiral compounds. 2. Propranolol: Free base and hydrochloride. Russ. Chem. Bull. 2003, 52, 853–861. [Google Scholar] [CrossRef]
- Ammon, H.L.; Howe, D.-B.; Erhardt, W.D.; Balsamo, A.; Macchia, B.; Macchia, F.; Keefe, W.E. The crystal structures of dichloroisoproterenol, propranolol and propranolol hydrochloride. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 21–29. [Google Scholar] [CrossRef]
- Gadret, M.; Goursolle, M.; Leger, J.M.; Colleter, J.C. Crystal-structure of Pindolol, 4-(2-hydroxy-3-isopropylaminopropoxy)indole. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 17–20. [Google Scholar] [CrossRef]
- Chattopadhyay, T.K.; Palmer, R.A.; Mahadevan, D. Molecular and absolute crystal structure-of Pindolol-1-(1H-indol-4-yloxy)-3-[(1-methylethyl)amino]-2-propanol—A specific beta-adrenoceptor antagonist with partial agonist activity. J. Chem. Cryst. 1995, 25, 195–199. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre (CCDC). Available online: https://www.ccdc.cam.ac.uk/support-and-resoureces/ccdcresouces (accessed on 15 May 2022).
- Neau, S.H.; Shinwari, M.K.; Hellmuth, E.W. Melting-point phase-diagrams of free-base and hydrochloride salts of Bevantolol, Pindolol and Propranolol. Int. J. Pharm. 1993, 99, 303–310. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Bredikhina, Z.A.; Gubaidullin, A.T. Chirality-dependent supramolecular synthons based on the 1,3-oxazolidin-2-one framework: Chiral drugs mephenoxalone, metaxalone and 114 other examples. CrystEngComm 2020, 22, 7252–7261. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Gubaidullin, A.T.; Bredikhina, Z.A.; Fayzullin, R.R.; Lodochnikova, O.A. Chirality, gelation ability and crystal structure: Together or apart? Alkyl phenyl ethers of glycerol as simple LMWGs. Symmetry 2021, 13, 732. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredikhin, A.A.; Fayzullin, R.R.; Bredikhina, Z.A. Crystal Structure of Chiral Drug Prenalterol and Its Precursor Prone to Spontaneous Resolution. Symmetry 2022, 14, 1150. https://doi.org/10.3390/sym14061150
Bredikhin AA, Fayzullin RR, Bredikhina ZA. Crystal Structure of Chiral Drug Prenalterol and Its Precursor Prone to Spontaneous Resolution. Symmetry. 2022; 14(6):1150. https://doi.org/10.3390/sym14061150
Chicago/Turabian StyleBredikhin, Alexander A., Robert R. Fayzullin, and Zemfira A. Bredikhina. 2022. "Crystal Structure of Chiral Drug Prenalterol and Its Precursor Prone to Spontaneous Resolution" Symmetry 14, no. 6: 1150. https://doi.org/10.3390/sym14061150
APA StyleBredikhin, A. A., Fayzullin, R. R., & Bredikhina, Z. A. (2022). Crystal Structure of Chiral Drug Prenalterol and Its Precursor Prone to Spontaneous Resolution. Symmetry, 14(6), 1150. https://doi.org/10.3390/sym14061150






