Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring
Abstract
:1. Introduction
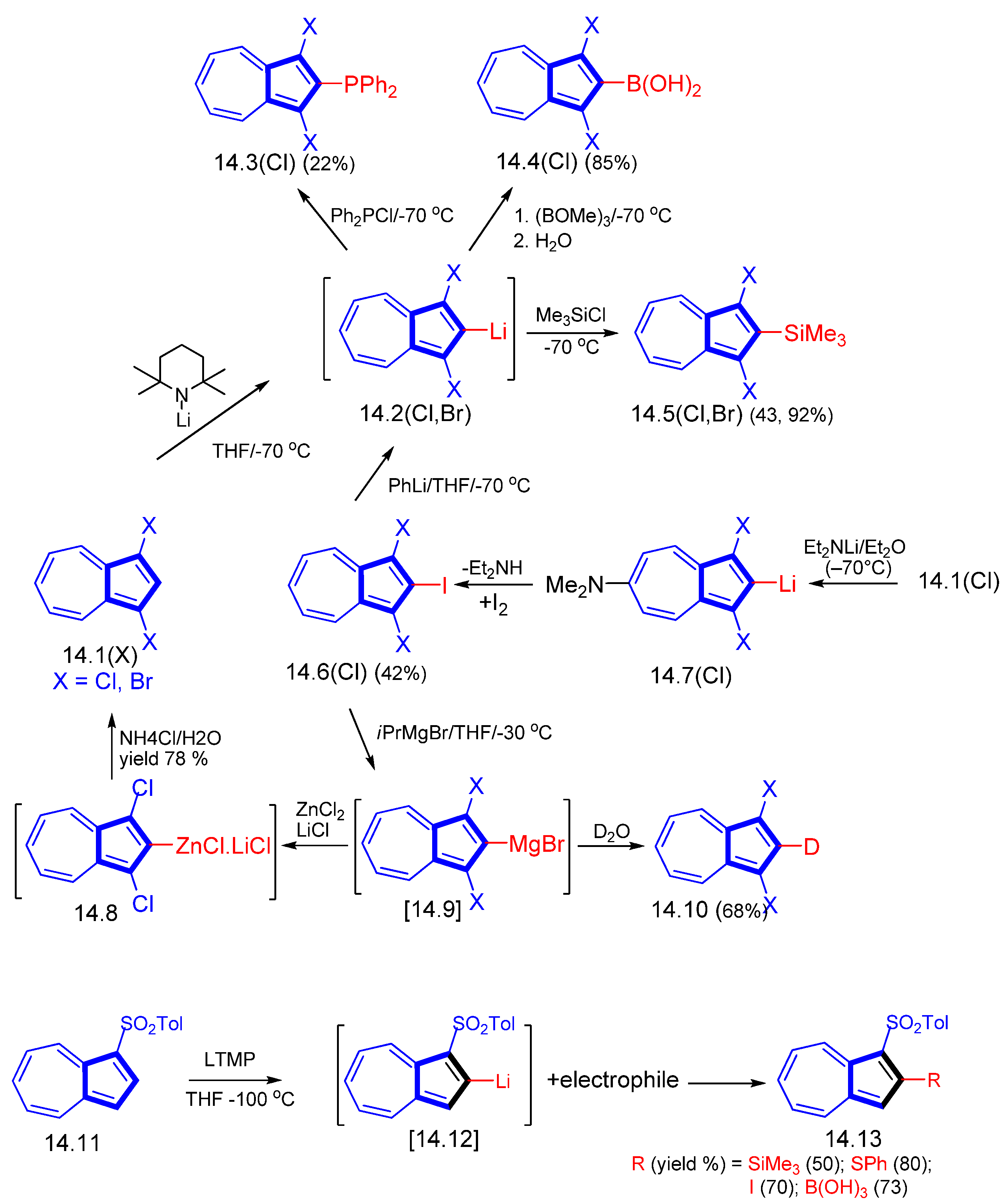
2. Starting Azulenyl Reagents
3. Reactions of Azulene Five Ring
3.1. Metal-Free Catalyst Reactions
3.1.1. Azulene Electrophile Substitution, SEAz
3.1.2. Azulene Nucleophilic Substitution, SNAz
3.1.3. Metalation of Position 2 and other Substitutions at This Position
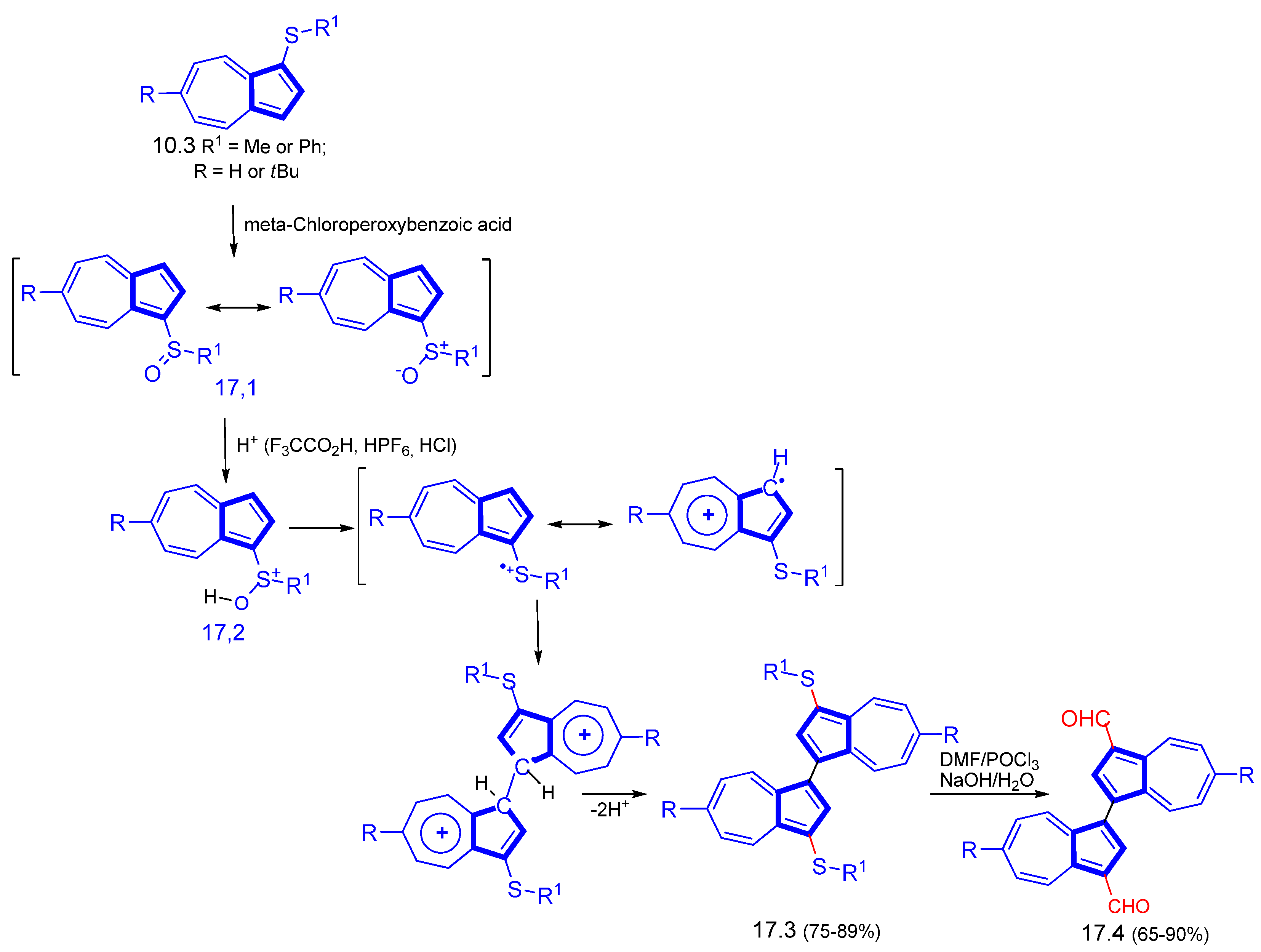
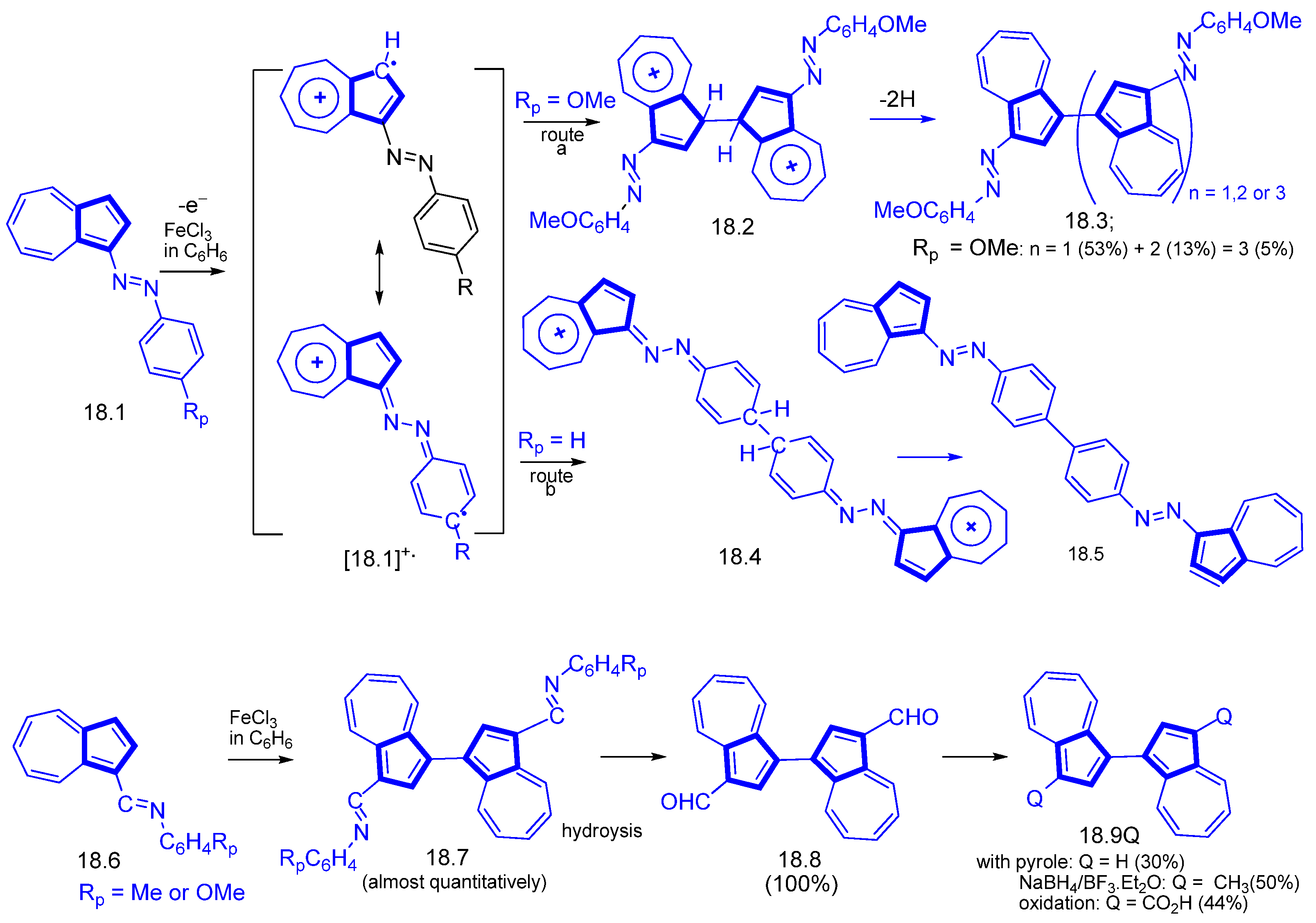
3.1.4. Radical Cation Route
3.2. Reactions Catalyzed by Metals
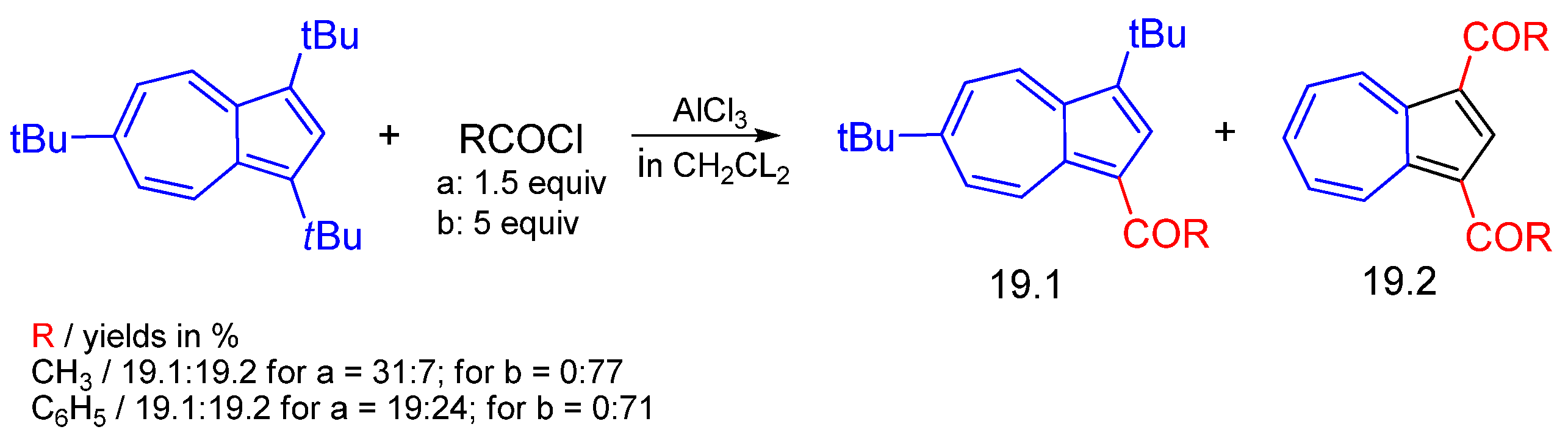
3.2.1. Aluminum Chloride as Catalyst
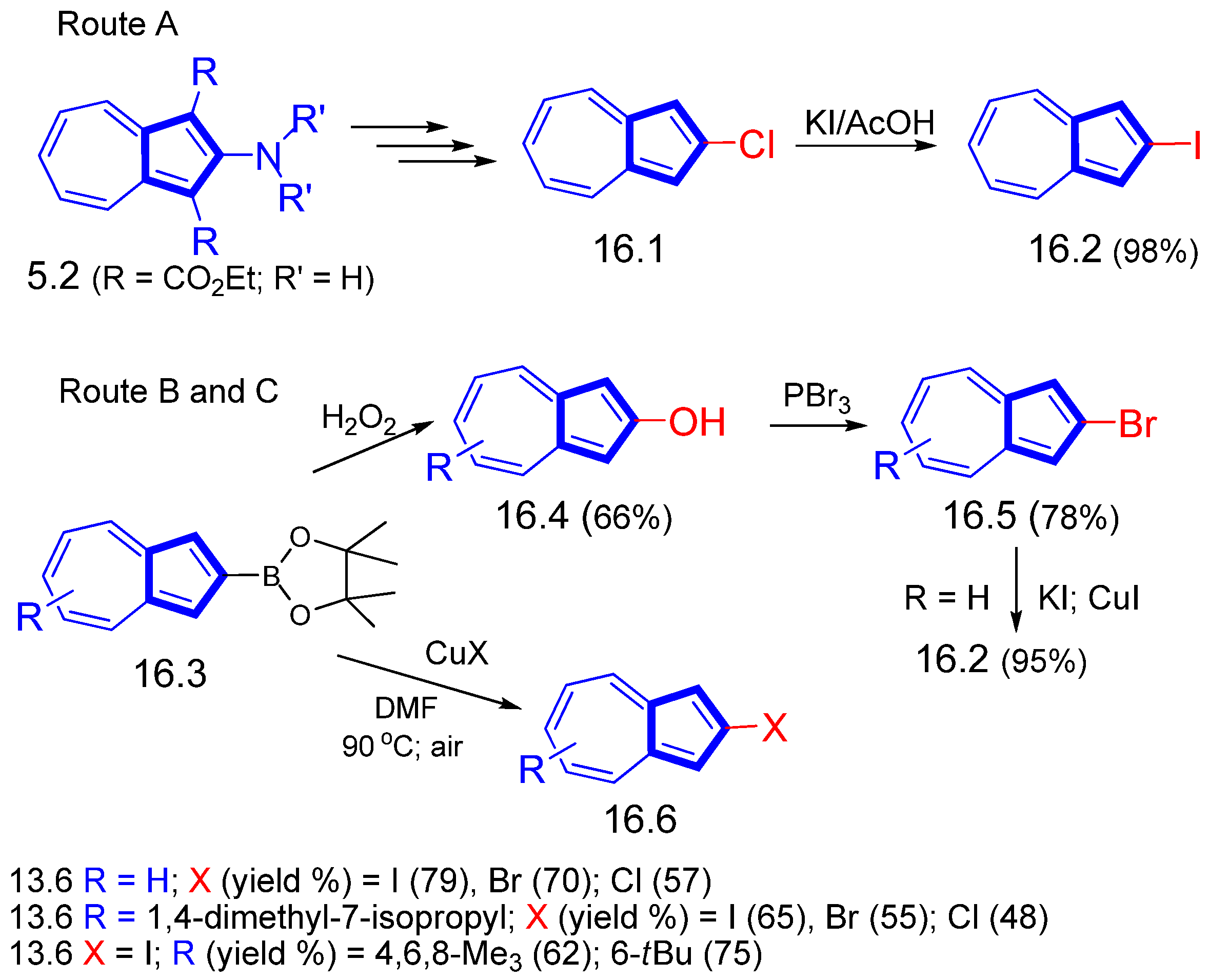
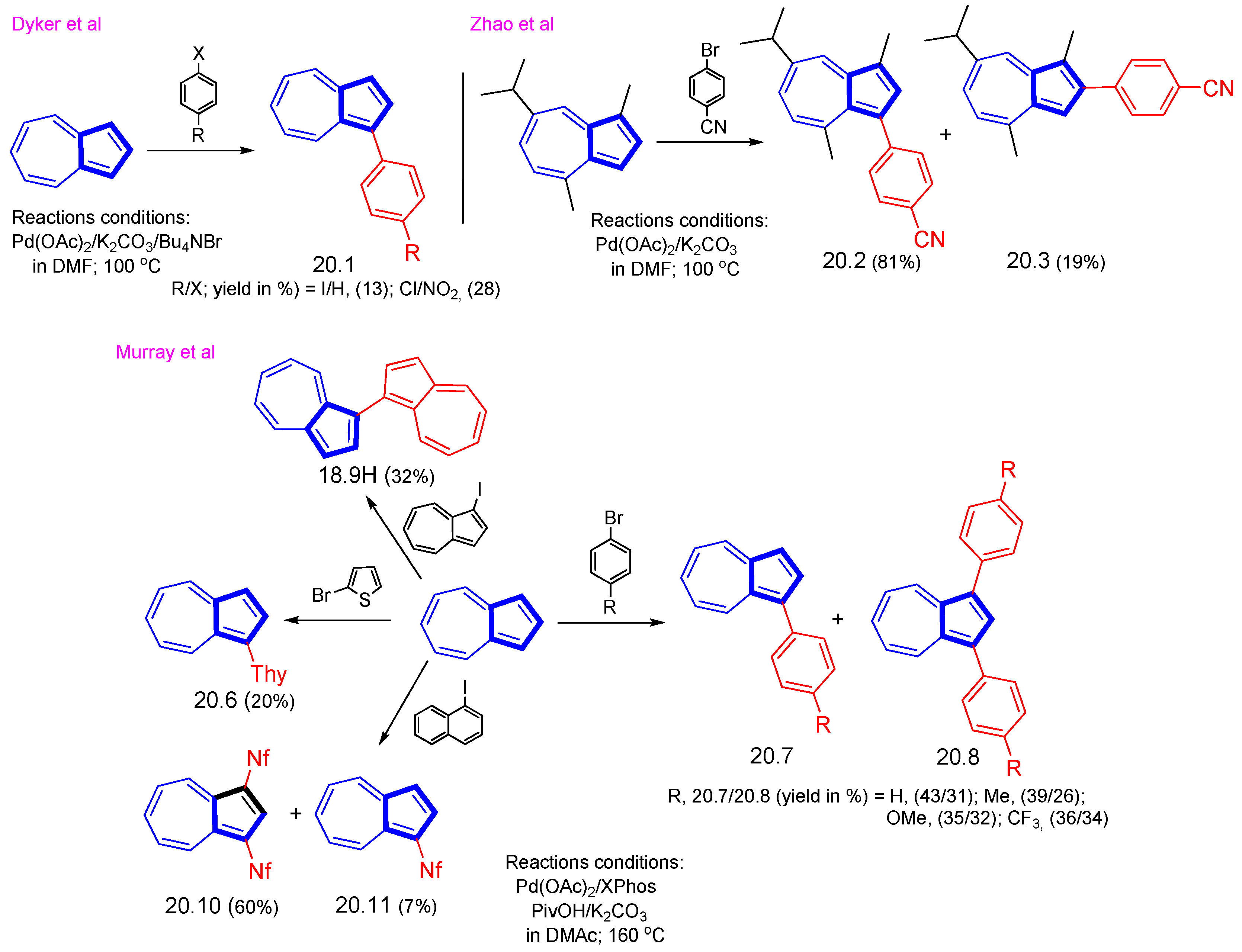
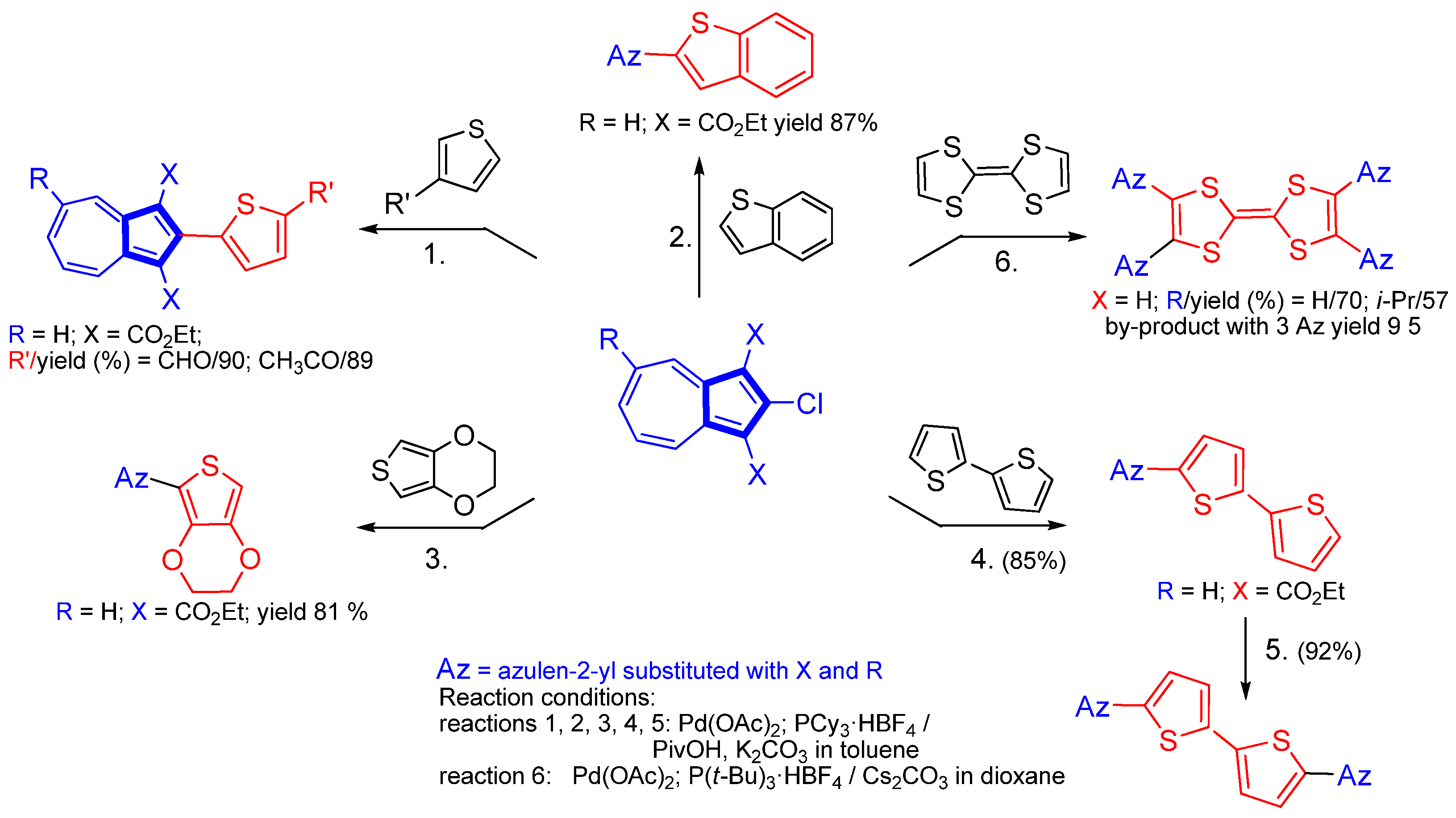
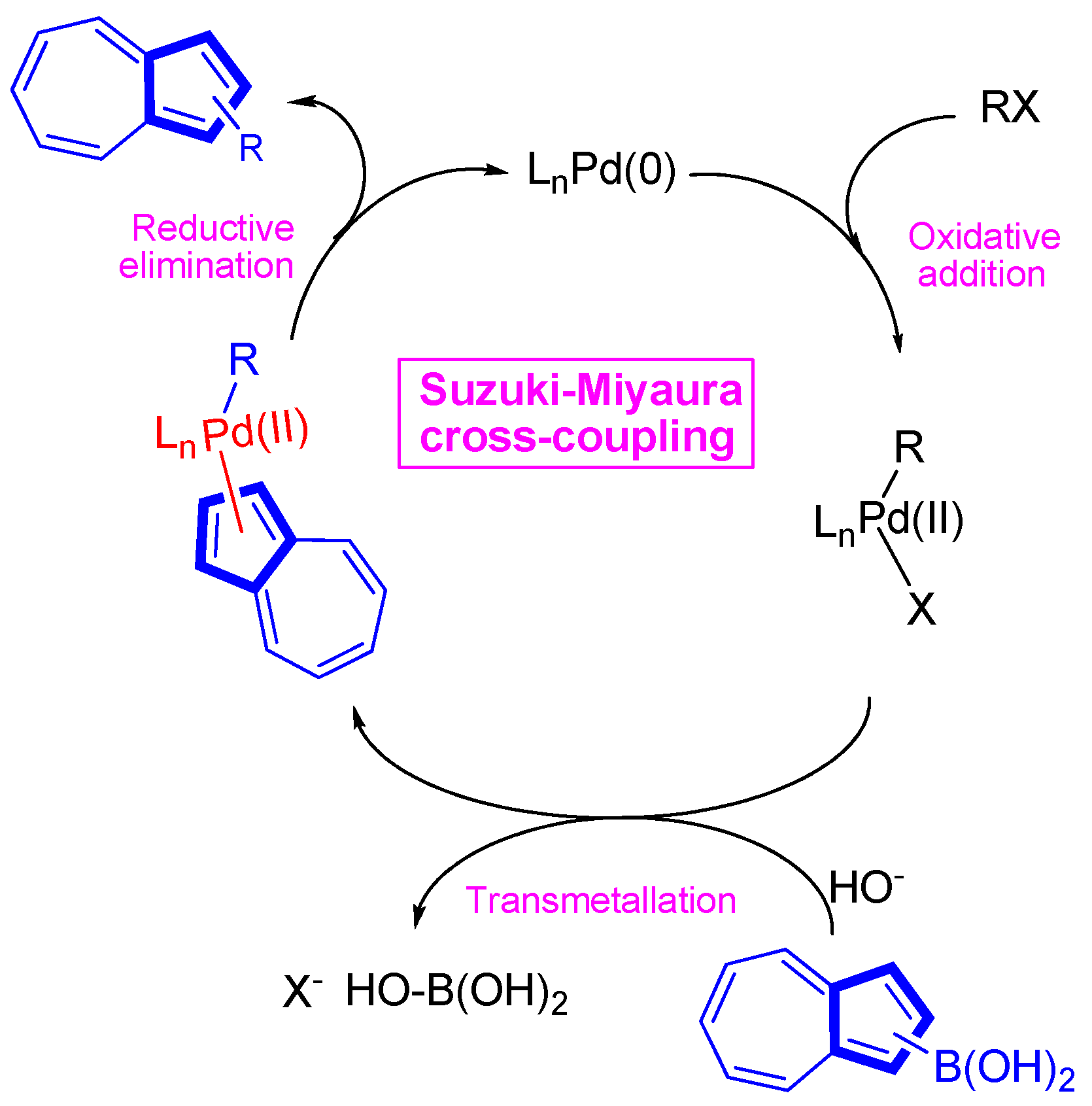
3.2.2. Cross-Coupling Reactions
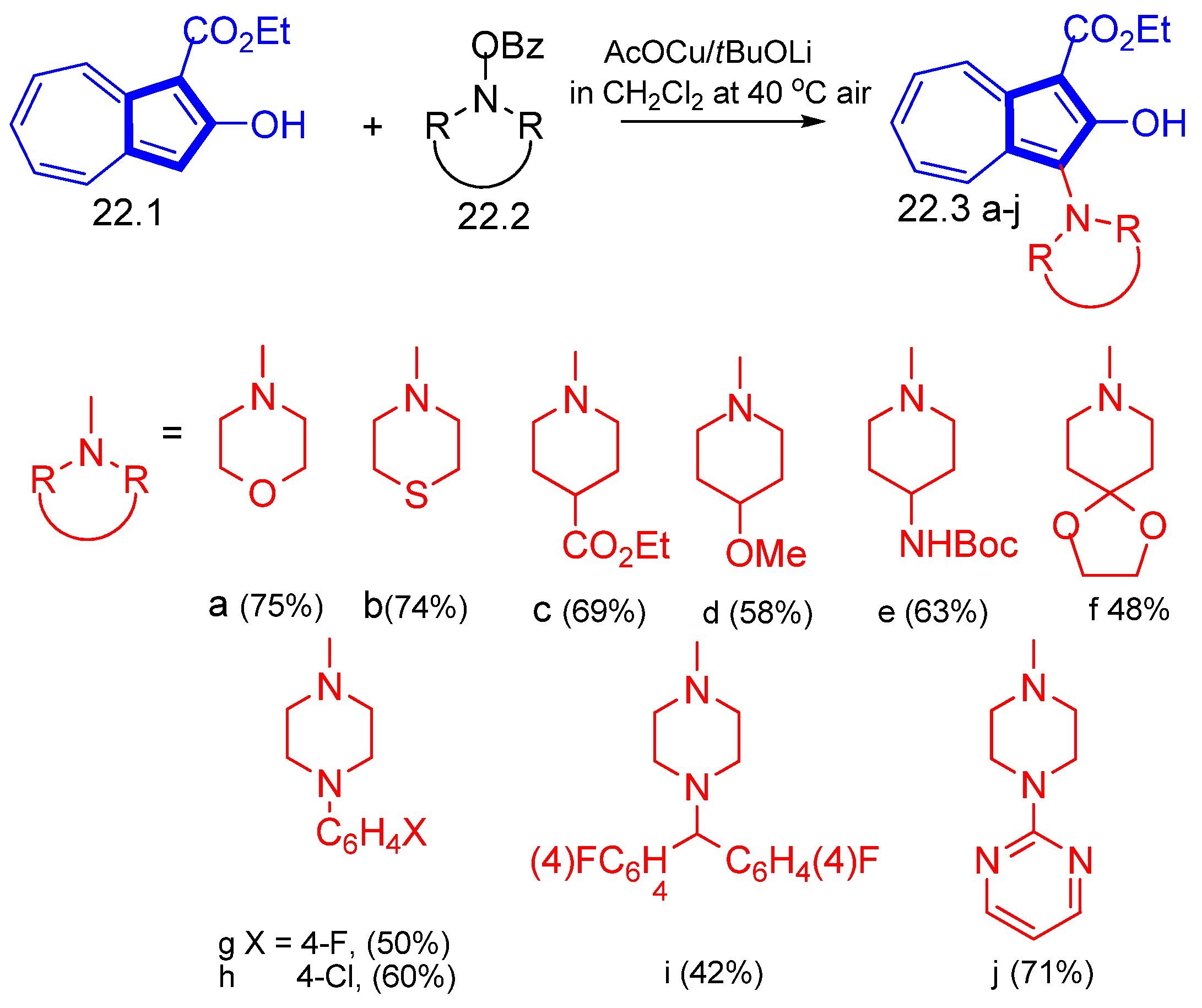
3.3. Various Azulene Five-Ring Substitutions
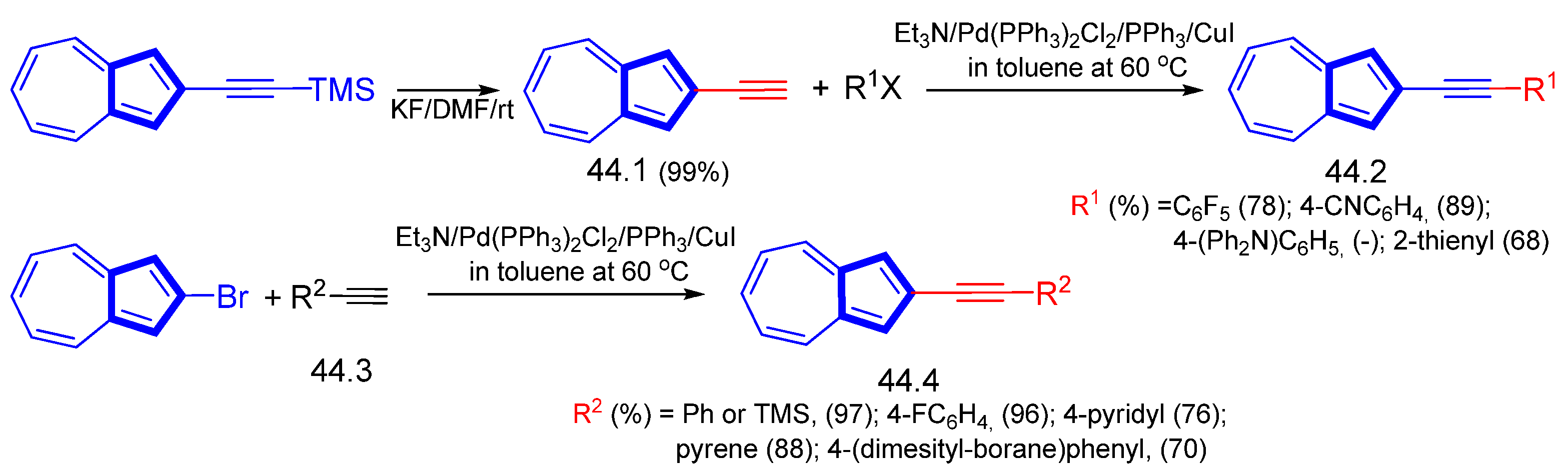
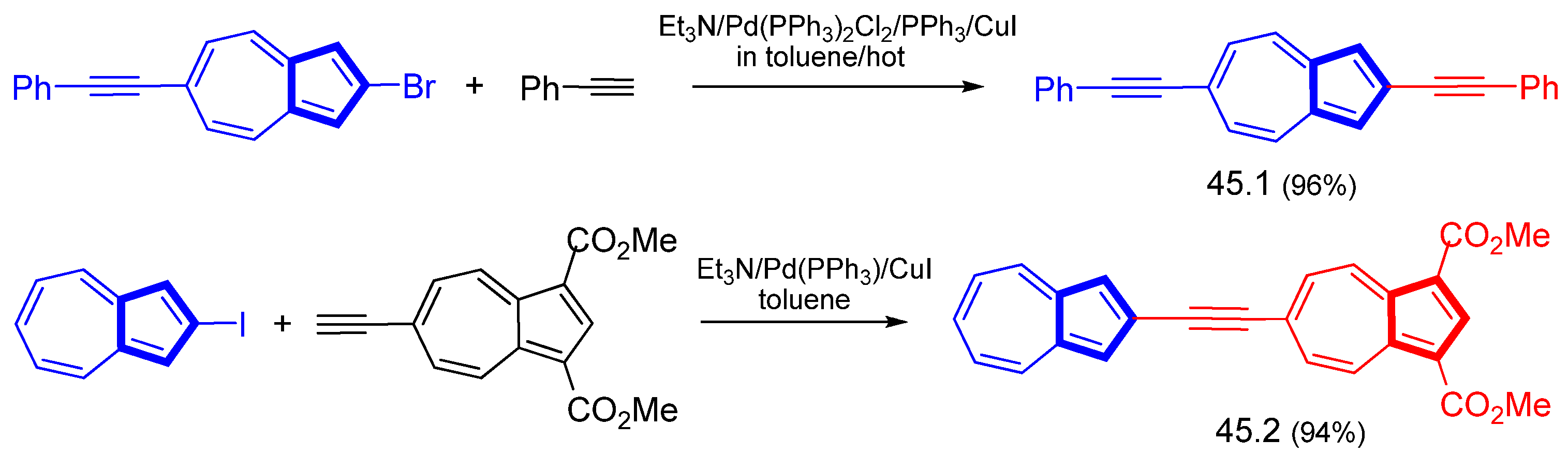
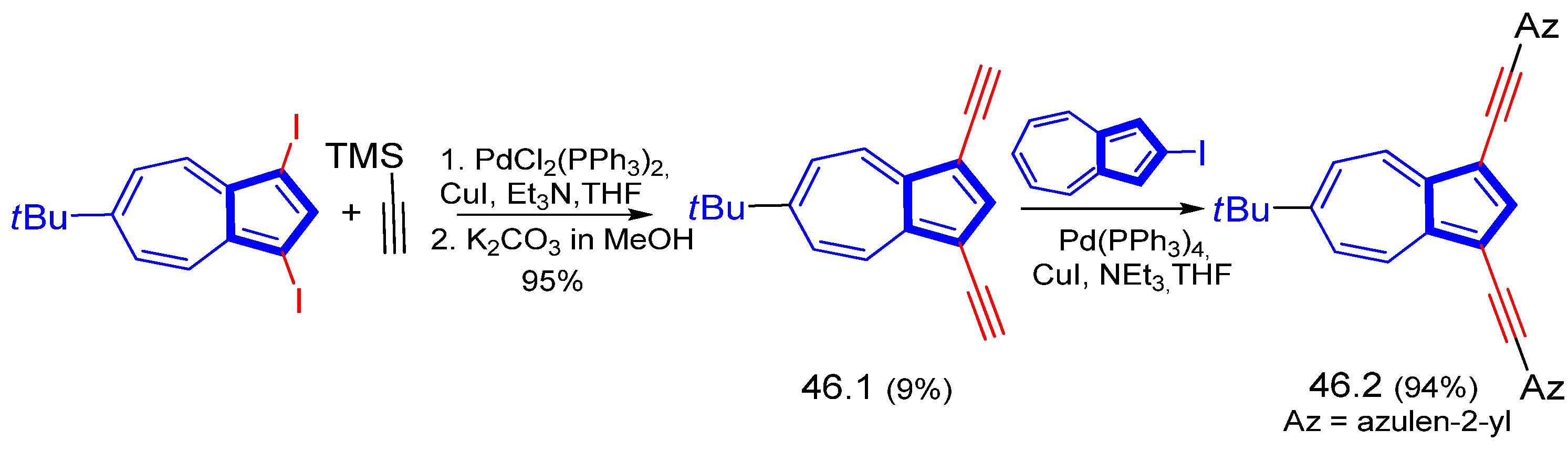
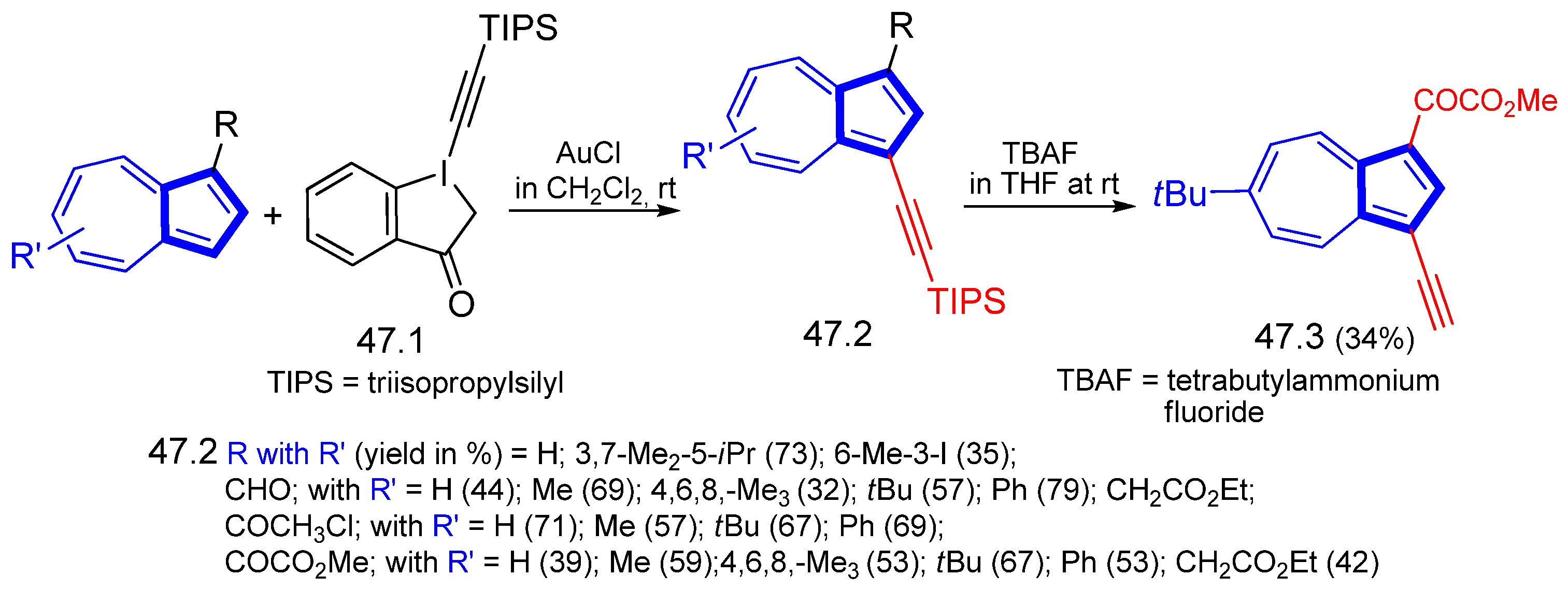
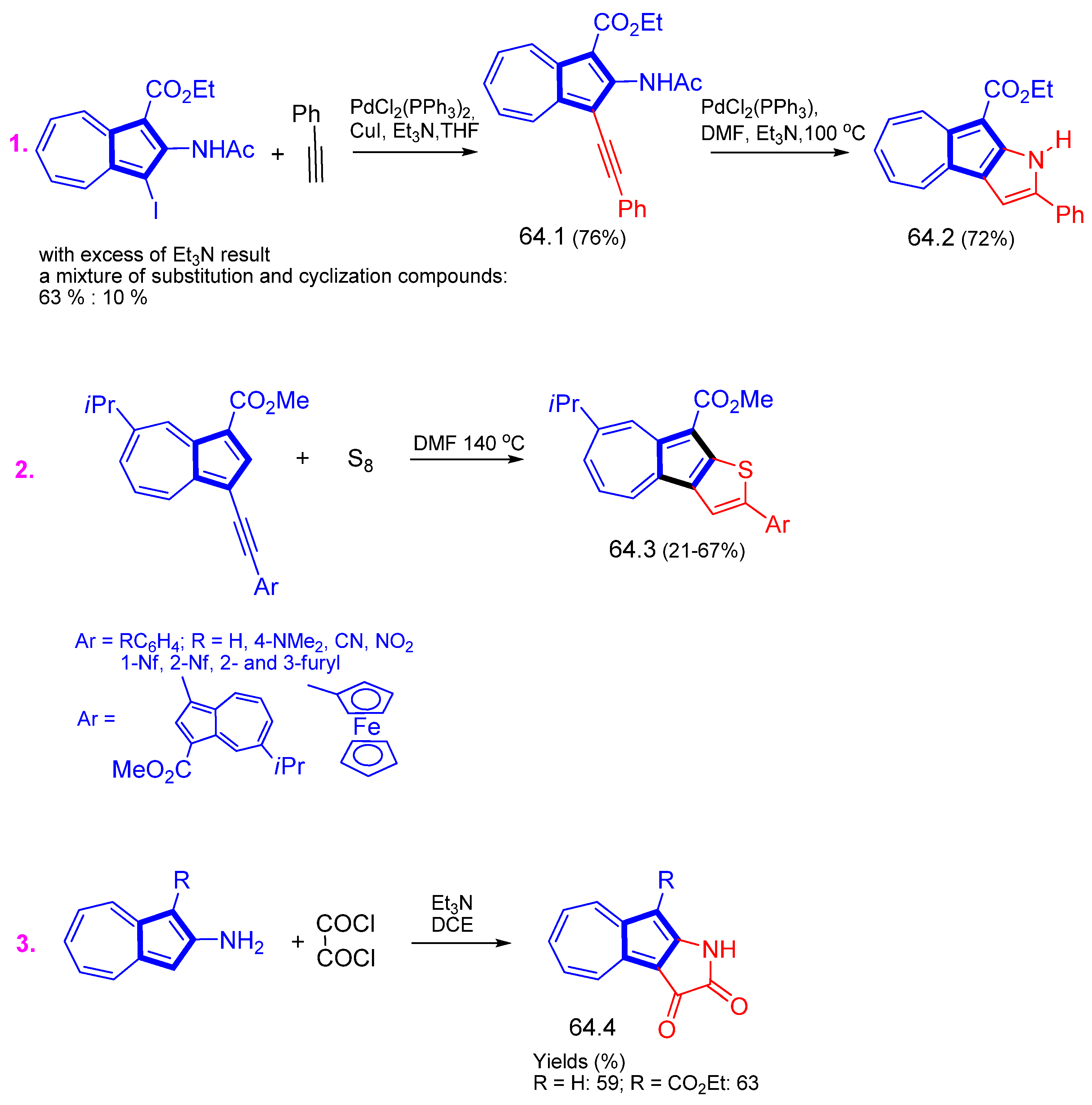
3.3.1. Azulene Alkynylation and Sonogashira–Hagihara Reaction of the 1,3-Diethynylazulene
3.3.2. Various Reactions in the Presence of Rhodium or Nickel Compounds
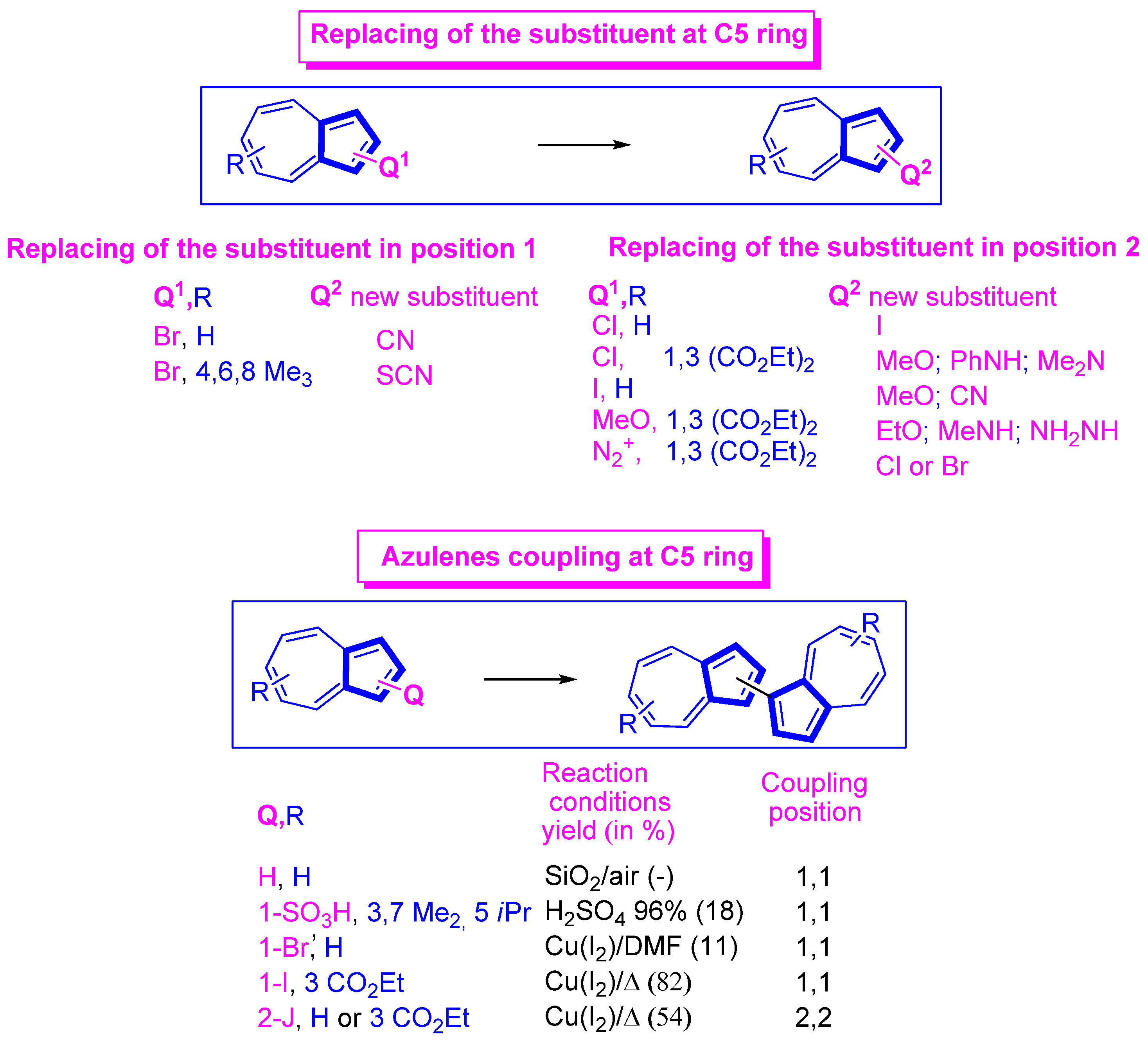
3.3.3. Some More Special Reactions at the 5th Ring
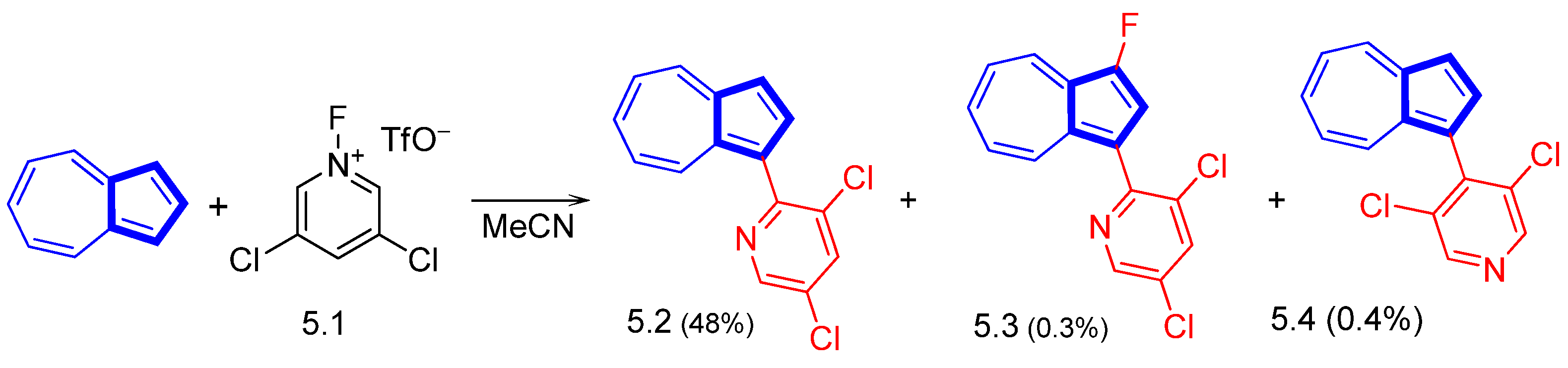
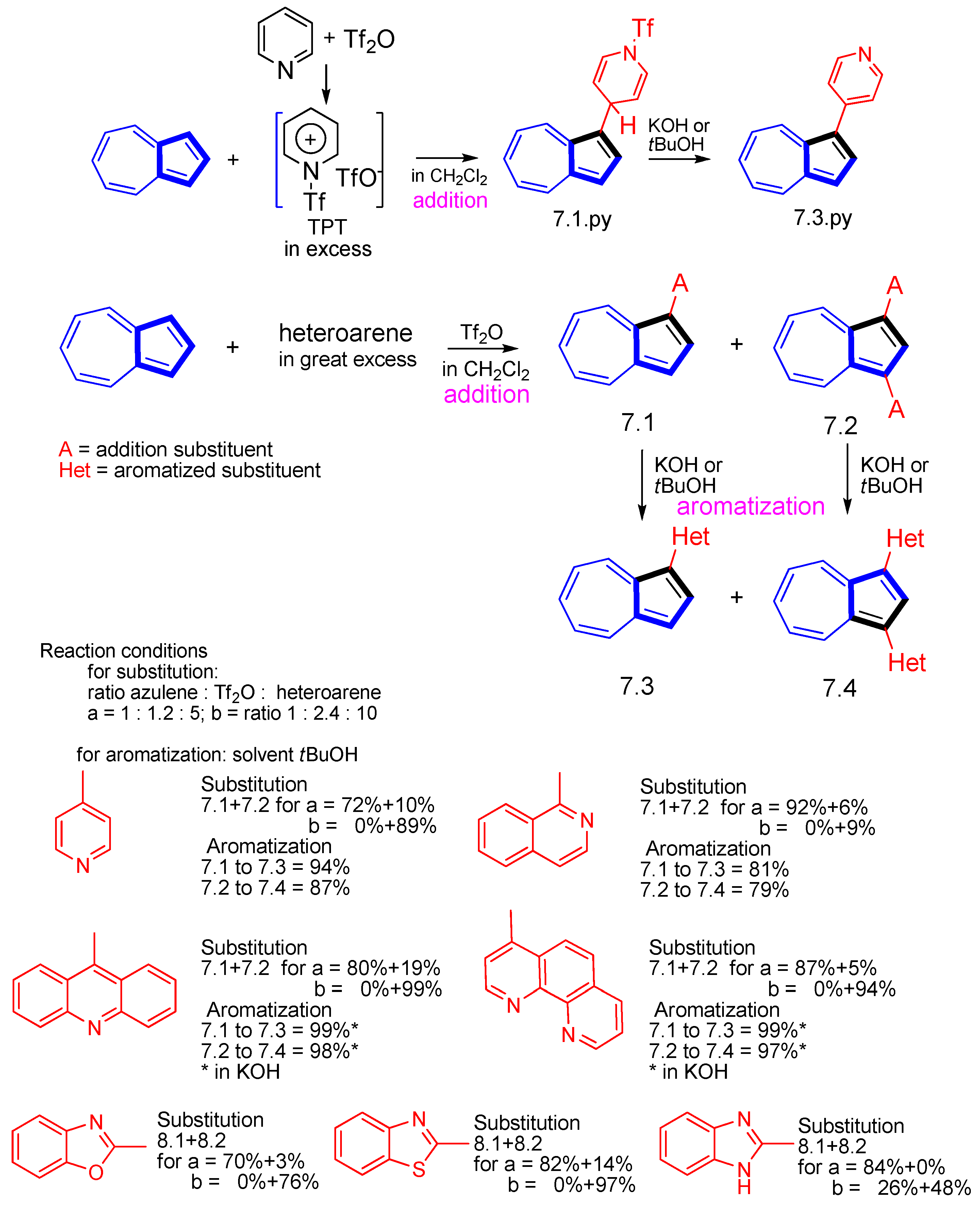
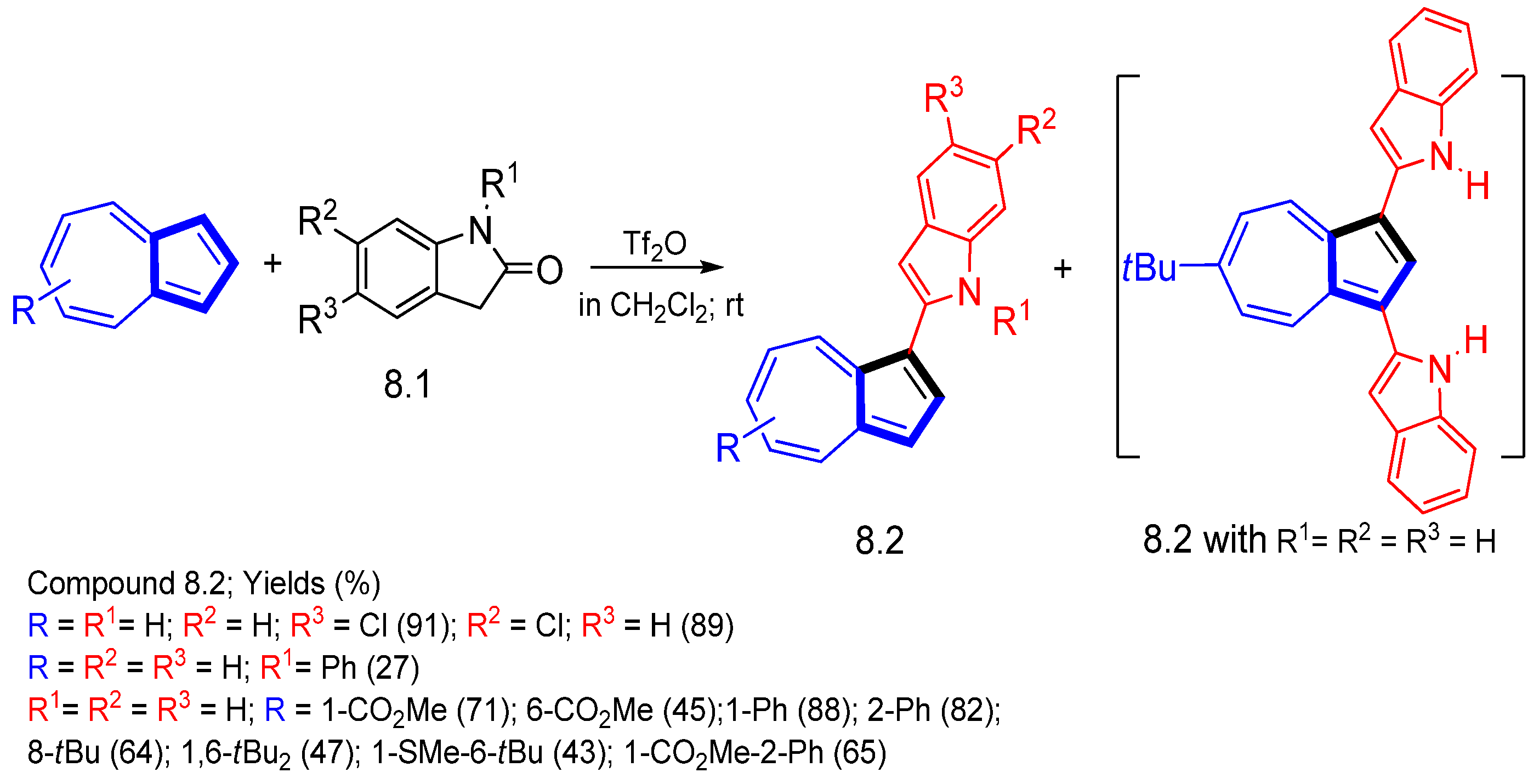
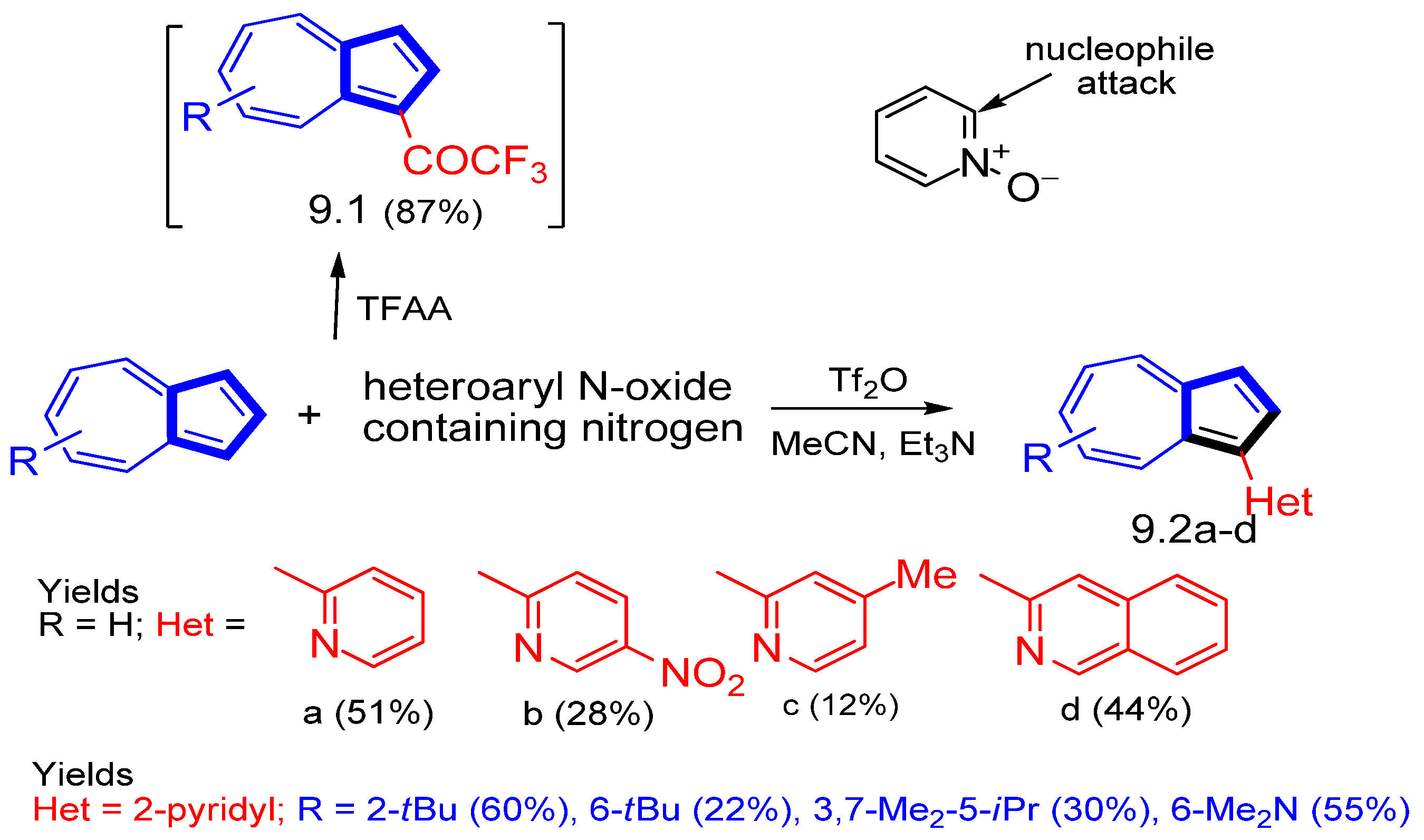
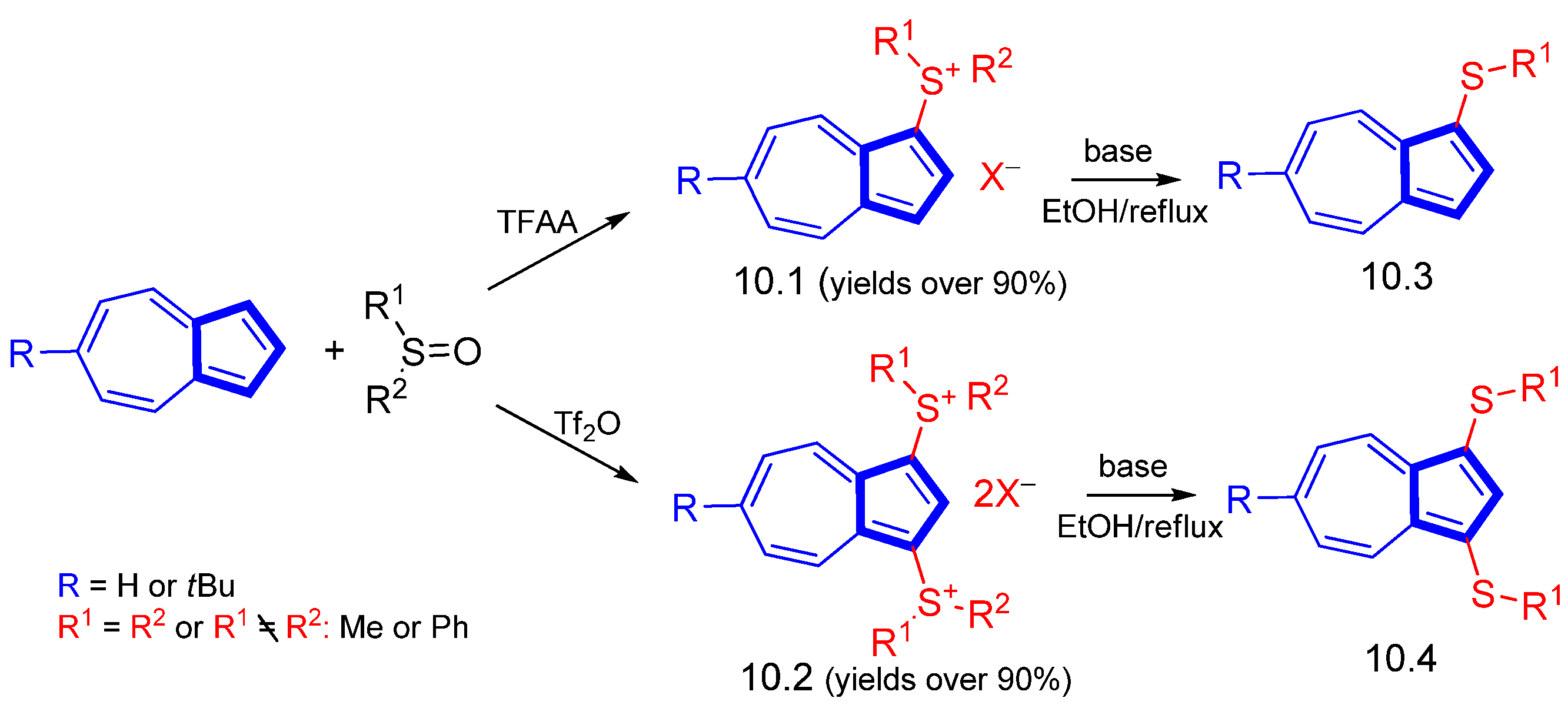
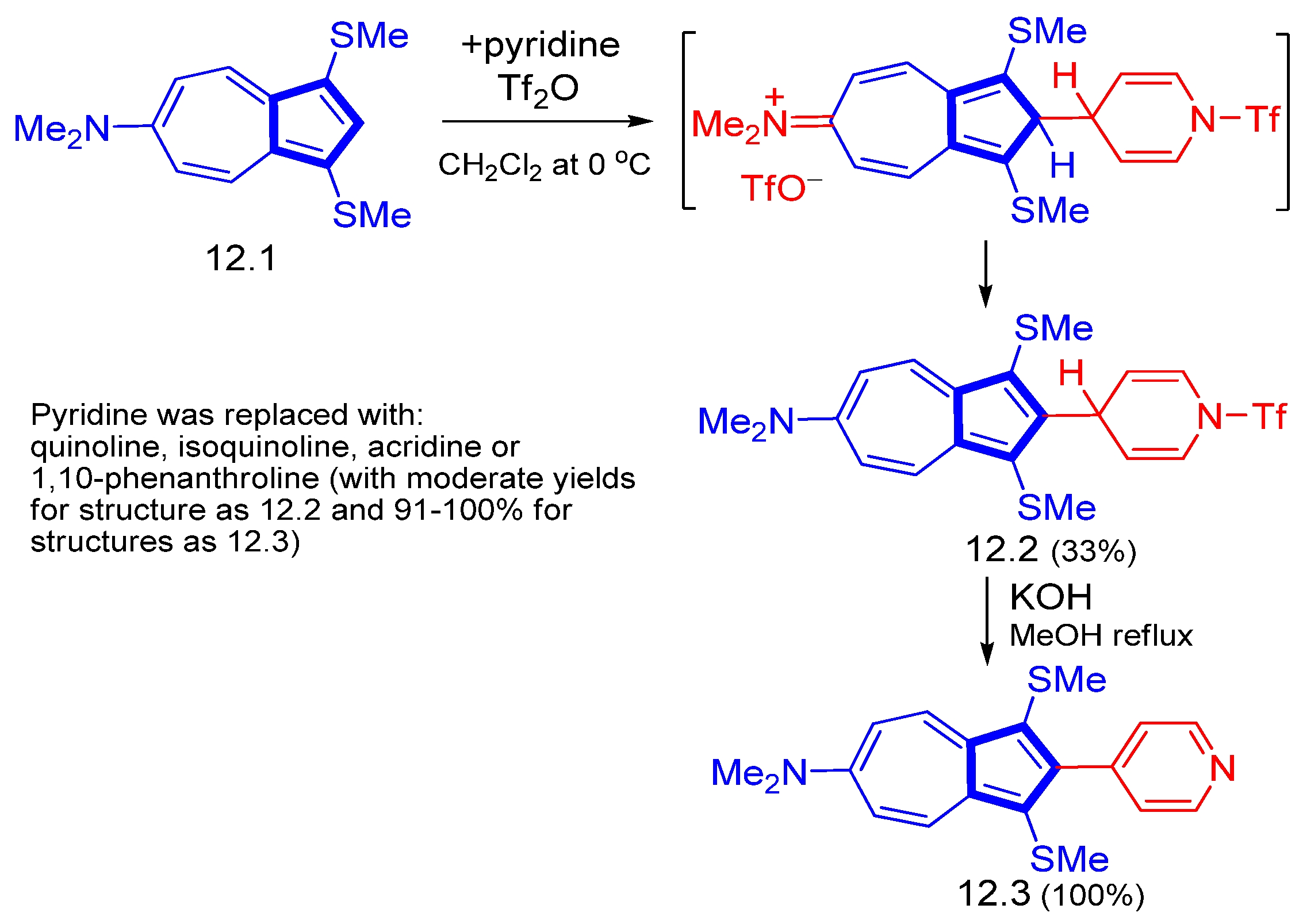
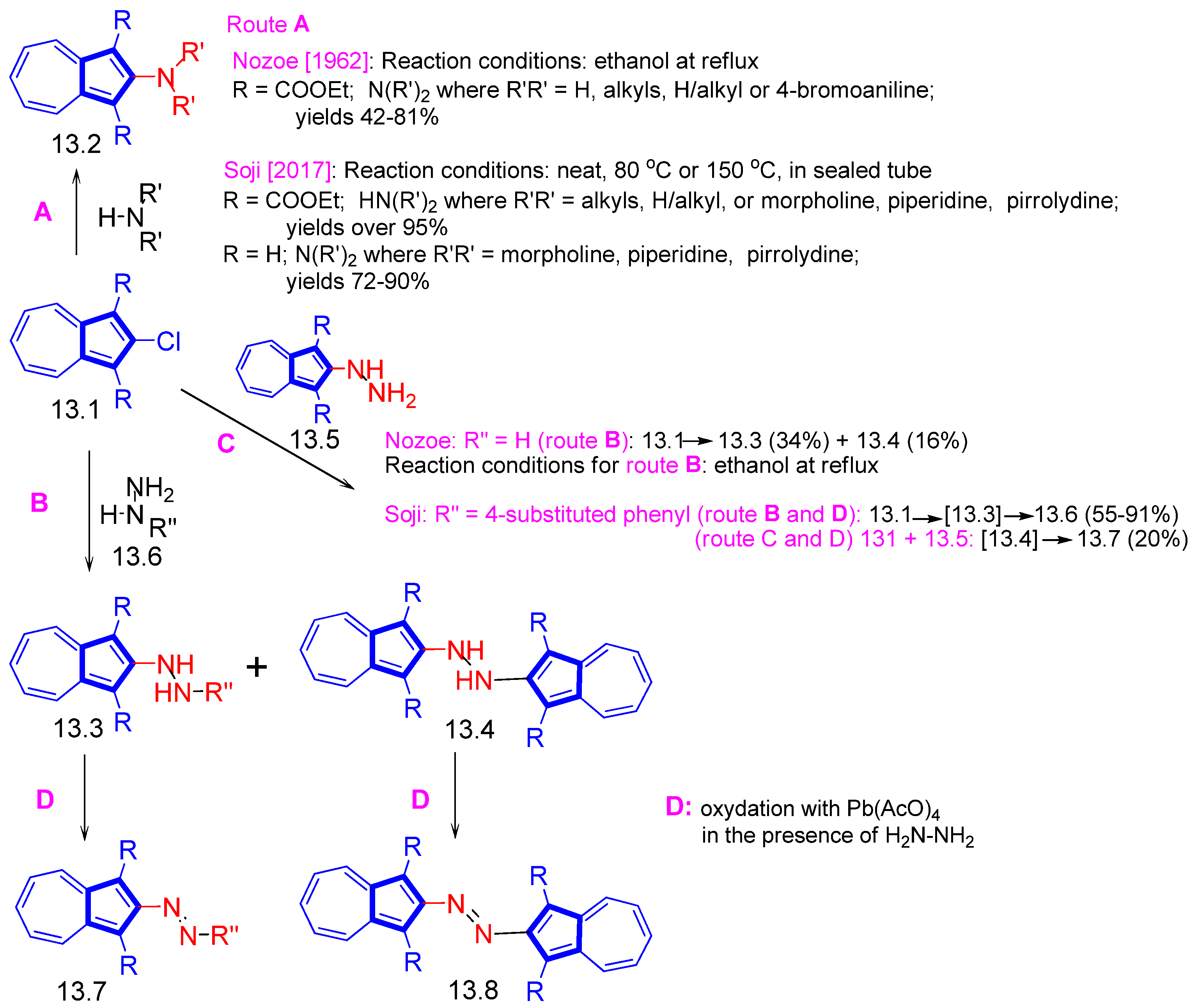
3.3.4. Heterocycle-Substituted and Fused Azulenes That Involve the Five-Membered Ring
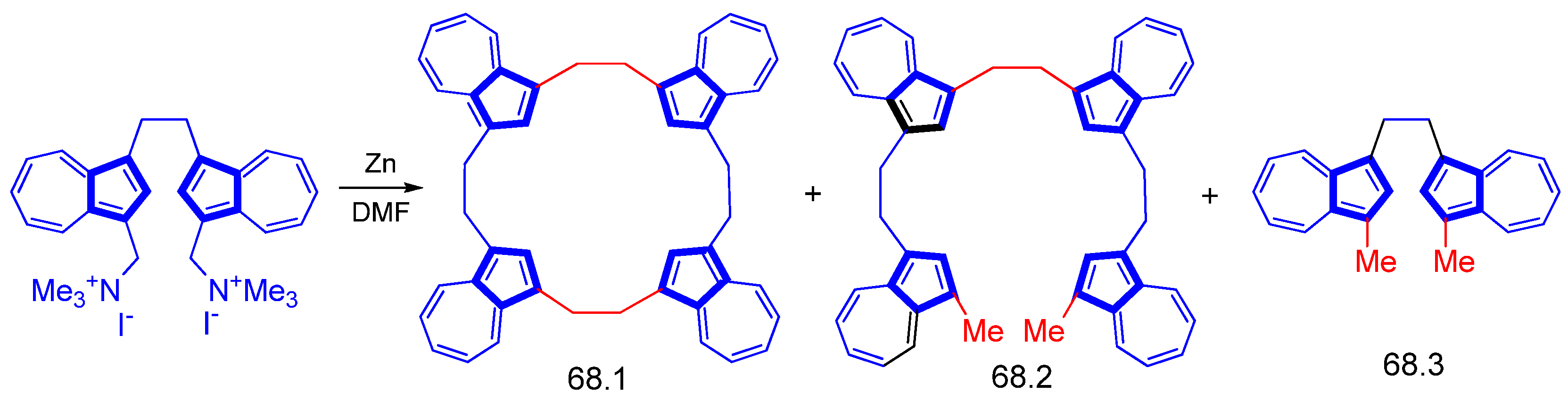
3.3.5. Calixarene, Azulenophane, and Azuliporphyrin
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanagisawa, T.; Kosakai, K.; Izawa, C.; Tomiyama, T.; Yasunami, M. Synthesis and Anti-peptic Activity ofCompounds Related to the Metabolites of Sodium 3-Ethyl-7-isopropyl-1-azulenesulfonate (KT1-32). Chem. Pharm. Bull. 1991, 39, 2429–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanari, K.; Hashimoto, M.; Wakabayashi, H.; Okudaira, N.; Bandow, K.; Nagai, J.; Tomomura, M.; Tomomura, A.; Uesawa, Y.; Sakagami, H. Quantitative Structure–Cytotoxicity Relationship of Azulene Amide Derivatives. Anticancer Res. 2019, 39, 3507–3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murfin, L.C.; Weber, M.; Park, S.J.; Kim, W.T.; Lopez-Alled, C.M.; McMullin, C.L.; Pradaux-Caggiano, F.; Lyall, C.L.; Kociok-Köhn, G.; Wenk, J.; et al. Azulene-Derived Fluorescent Probe for Bioimaging: Detection of Reactive Oxygen and Nitrogen Species by Two-Photon Microscopy. J. Am. Chem. Soc. 2019, 141, 19389–193896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razus, A.C.; Birzan, L.; Cristea, M.; Tecuceanu, V.; Enache, C. Extended π-electron conjugated dyes with three and four azo bonds and one or two azulen-1-yl moieties in molecule. Synthesis, electrochemical behavior and basicity. Dye. Pigment. 2012, 92, 1166–1176. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Razus, A.C.; Buica, G.O.; Ungureanu, E.-M. Vinylazulenes chromophores: Synthesis and characterization. Dye. Pigment. 2016, 131, 246–255. [Google Scholar] [CrossRef]
- Cowper, P.; Pockett, A.; Kociok-Köhn, G.; Cameron, P.J.; Lewis, S.E. Azulene–Thiophene–Cyanoacrylic acid dyes with donor-π-acceptor structures]. Synthesis, characterisation and evaluation in dye-sensitized solar cells. Tetrahedron 2018, 74, 2775–2786. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S. Azulene-Based Donor–Acceptor Systems: Synthesis, Optical, and Electrochemical Properties. Chem. Eur. J. 2017, 23, 16696–16709. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Zhou, Y.; Wu, B.; Zhu, L. The unusual physicochemical properties of azulene and azulene-based compounds. Chin. Chem. Lett. 2019, 30, 1903–1907. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Arnold, G.-L.; Ungureanu, E.-M.; Razus, A.C. 1-Vinylazulenes–potential host molecules in ligands for metal ion detectors. Tetrahedron 2016, 72, 2316–2326. [Google Scholar] [CrossRef]
- Zeller, K.-P. Houben Weyl, Methoden der Organischen Chemie; Thieme, G., Ed.; Verlag: Stuttgart, Germany; New York, NY, USA, 1985; Volume V/2c, pp. 258–263. [Google Scholar]
- Razus, A.C. Azulene Moiety as Electron Reservoir in Positive Charged Systems; Short Survey. Symmetry 2021, 13, 526. [Google Scholar] [CrossRef]
- Xin, H.; Gao, X. Application of Azulene in Constructing Organic Optoelectronic Materials: New Tricks for an Old Dog. ChemPlusChem 2017, 82, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Huang, S.; Zhao, Y.; Feng, B.; Jiang, K.; Sun, S.; Ke, C.; Kymakis, E.; Zhuang, X. Azulene-Based Molecules, Polymers, and Frameworks for Optoelectronic and Energy Applications. Small Methods 2020, 4, 2000628. [Google Scholar] [CrossRef]
- Shevyakov, S.V.; Li, H.; Muthyala, R.; Asato, A.E.; Croney, J.C.; Jameson, D.M.; Liu, R.S.H.J. Orbital Control of the Color and Excited State Properties of Formylated and Fluorinated Derivatives of Azulene. Phys. Chem. A 2003, 107, 3295–3299. [Google Scholar] [CrossRef]
- Ito, S.; Shoji, T.; Morita, N. Recent Advances in the Development of Methods for the Preparation of Functionalized Azulenes for Electrochromic Applications. Synlett 2011, 16, 2279–2298. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S. The Preparation and Properties of Heteroarylazulenes and Hetero-Fused Azulenes. Adv. Heterocycl. Chem. 2018, 126, 1–54. [Google Scholar] [CrossRef]
- Shoji, T.; Okujima, T.; Ito, S. Development of Heterocycle-Substituted and Fused Azulenes in the Last Decade (2010–2020). Int. J. Mol. Sci. 2020, 21, 7087. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sasmal, A.; Soulé, J.-F.; Doucet, H. Metal-Catalyzed C-H Bond Activation of 5-Membered Carbocyclic Rings: A Powerful Access to Azulene, Acenaphthylene and Fulvene Derivatives. Chem.-Asian, J. 2018, 13, 143–157. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L. Synthesis of azulenic compounds with a homo- or hetero-atomic double bond at position 1. Arkivoc 2018, IV, 1–56. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L. Synthesis of azulenic compounds substituted in the 1-position with heterocycles. Mon. Für Chem. 2018, 150, 139–161. [Google Scholar] [CrossRef]
- Razus, A.C. Dancing with Azulene. Symmetry 2022, 14, 297. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Abdelhamid, I.A.; Shaaban, M.R. Recent Advances in the Functionalization of Azulene Through Pd-Catalyzed Cross-Coupling Reactions. ChemistrySelect 2021, 6, 13664–13723. [Google Scholar] [CrossRef]
- Xin, H.; Hou, B.; Gao, X. Azulene-Based π-Functional Materials: Design, Synthesis, and Applications. Acc. Chem. Res. 2021, 54, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Toda, H.; Yasunami, M.; Yoshifuji, M. Synthesis and Properties of Fluoroazulenes. II. Electrophilic Fluorination of Azulenes with N-Fluoro Reagents. Bull. Chem. Soc. Jpn. 1996, 69, 1645–1656. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L.; Pavel, C.; Lehadus, O.; Corbu, A.C.; Enache, C. Azulene-substituted PyranyliumSalts. Syntheses and Products Characterization. J. Heterocycl. Chem. 2006, 43, 963–977. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L.; Pavel, C.; Lehadus, O.; Corbu, A.; Chiraleu, F.; Enache, C. Azulene-substituted Pyridines and Pyridinium Salts. Synthesis and Structure. 1. Azulene-substituted Pyridines. J. Heterocycl. Chem. 2007, 44, 245–250. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L.; Pavel, C.; Lehadus, O.; Corbu, A.; Chiraleu, F.; Enache, C. Azulene-substituted Pyridines and Pyridinium Salts. Synthesis and Structure. 2. Azulene-substituted Pyridinium salts. J. Heterocycl. Chem. 2007, 44, 251–260. [Google Scholar] [CrossRef]
- Ito, S.; Yokoyama, R.; Okujima, T.; Terazono, T.; Kubo, T.; Tajiri, A.; Morita, N. Reaction of azulenes with 1-trifluoromethanesulfonylpyridinium trifluoromethanesulfonate (TPT) and synthesis of the parent azulene. Org. Biomol. Chem. 2003, 1, 1947–1952. [Google Scholar] [CrossRef]
- Shoji, T.; Yokoyama, R.; Ito, S.; Watanabe, M.; Toyota, K.; Yasunami, M.; Morita, N. Synthesis of heteroarylazulenes: Transition metal free coupling strategy of azulene with heterocycles. Tetrahedron Lett. 2007, 48, 1099–1103. [Google Scholar] [CrossRef]
- Shoji, T.; Inoue, Y.; Ito, S. First synthesis of 1-(indol-2-yl)azulenes by the Vilsmeier–Haack type arylation with triflic anhydride as an activating reagent. Tetrahedron Lett. 2012, 53, 1493–1496. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Okada, K.; Ito, S.; Toyota, K.; Morita, N. Synthesis of 1-(pyridyl, quinolyl, and isoquinolyl)azulenes by Reissert–Henze type reaction. Tetrahedron Lett. 2010, 51, 5127–5130. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Higashi, J.; Ito, S.; Toyota, K.; Asao, T.; Yasunami, M.; Morita, N. Synthesis and Redox Behavior of 1-Azulenyl Sulfides and Efficient Synthesis of 1,1′-Biazulenes. Eur. J. Org. Chem. 2008, 1242–1252. [Google Scholar] [CrossRef]
- Cowper, P.; Jin, Y.; Turton, M.D.; Kociok-Köhn, G.; Lewis, S.E. Azulenesulfonium Salts: Accessible, Stable, and Versatile Reagents for Cross-Coupling. Angew. Chem. 2016, 55, 2564–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, T.; Inoue, Y.; Ito, S.; Okujima, T.; Morita, N. First synthesis of 2-heteroarylazulenes by the electrophilic substitution of azulene with triflate of n-containing heterocycles. Heterocycles 2012, 53, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Nozoe, T.; Seto, S.; Matsumura, S. Synthesis of 2-substituted azulenes by nucleophilic substitution reactions of 2-haloazulene derivatives. Bull. Chem. Soc. Jpn. 1962, 35, 1990–1998. [Google Scholar] [CrossRef]
- Shoji, T.; Sugiyama, S.; Araki, T.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T.; Yasunami, M. Synthesis of 2-amino- and 2-arylazoazulenesvianucleophilic aromatic substitution of 2-chloroazulenes with amines and arylhydrazines. Org. Biomol. Chem. 2017, 15, 3917–3923. [Google Scholar] [CrossRef] [Green Version]
- Kurotobi, K.; Tabata, H.; Miyauchi, M.; Mustafizur, R.; Migita, K.; Murafuji, T.; Sugihara, Y.; Shimoyama, H.; Fujimori, K. The First Generation of Azulenyl-Lithium and -Magnesium: A Novel, Versatile Method of Introducing a Substituent at the 2-Position of an Azulene Skeleton. Synthesis 2003, 1, 30–34. [Google Scholar] [CrossRef]
- Bredihhin, A.; Dubovik, J. The First Preparation of Azulenylzinc Reagents and Their Use in Negishi Cross-Coupling. Synthesis 2015, 47, 2663–2669. [Google Scholar] [CrossRef]
- Shibasaki, T.; Ooishi, T.; Yamanouchi, N.; Murafuji, T.; Kurotobi, K.; Sugihara, Y. A New Efficient Route to 2-Substituted Azulenes Based on Sulfonyl Group Directed Lithiation. J. Org. Chem. 2008, 73, 7971–7977. [Google Scholar] [CrossRef]
- Ito, S.; Terazono, T.; Kubo, T.; Okujima, T.; Morita, N.; Murafuji, T.; Sugihara, Y.; Tajiri, A. Efficient preparation of 2-azulenylboronate and Miyaura-Suzuki cross-coupling reaction with aryl bromides for easy access to poly(2-azulenyl)benzenes. Tetrahedron 2004, 60, 5357–5366. [Google Scholar] [CrossRef]
- Narita, M.; Murafuji, T.; Yamashita, S.; Fujinaga, M.; Hiyama, K.; Oka, Y.; Tani, F.; Kamijo, S.; Ishiguro, K. Synthesis of 2-Iodoazulenes by the Iododeboronation of Azulen-2-ylboronic Acid Pinacol Esters with Copper(I) Iodide. J. Org. Chem. 2018, 83, 1298–1303. [Google Scholar] [CrossRef]
- Morita, T.; Takase, K. Synthesis of 1,1′-, 2,2′-, 1,2′-, and 2,6′-Biazulenes and Their Derivatives by Ullmann Reaction. Bull. Chem. Soc. Jpn. 1982, 55, 1144–1152. [Google Scholar] [CrossRef] [Green Version]
- Iyoda, M.; Sato, K.; Oda, M. Nickel-catalyzed coupling of bromides of 1,6-methano[10]annulene and azulene. A facile synthesis of biannulene and bi-, ter-, quater-, and polyazulenes. Tetrahedron Lett. 1985, 26, 3829–3832. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. The novel transition metal free synthesis of 1,1′-biazulene. Tetrahedron Lett. 2007, 48, 4999–5002. [Google Scholar] [CrossRef]
- Razus, A.C. Syntheses of polycyclic compounds by oxidative coupling of azulene-1-azoarenes. J. Chem. Soc. Perkin Trans. 1 2000, 6, 981–988. [Google Scholar] [CrossRef]
- Razus, A.C.; Nitu, C.; Carvaci, S.; Birzan, L.; Razus, S.A.; Pop, M.; Tarko, L. Synthesis and reactions of N-(azulen-1-ylmethylene)arylamines. J. Chem. Soc. Perkin Trans. 1 2001, 1227–1233. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L.; Nae, S.; Tecuceanu, V.; Cimpeanu, V. 1,1′-Biazulene derivatives. Syntheses and reactions. Arkivoc 2002, 2, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Ito, S.; Okujima, T.; Higashi, J.; Yokoyama, R.; Toyota, K.; Morita, N. Electrophilicipso-Substitution and Some Unique Reaction Behavior of 1,3,6-Tri-tert-butylazulene. Eur. J. Org. Chem. 2009, 1554–1563. [Google Scholar] [CrossRef]
- Dyker, G.; Borowski, S.; Heiermann, J.; Körning, J.; Opwis, K.; Henkel, G.; Köckerling, M. First intermolecular palladium catalyzed arylation of an unfunctionalized aromatic hydrocarbon. J. Organomet. Chem. 2000, 606, 108–111. [Google Scholar] [CrossRef]
- Zhao, L.; Bruneau, C.; Doucet, H. Palladium-Catalyzed Intramolecular C-Arylation of Benzylic Carbon: Synthesis of 3-Benzoxazolylisoindolinones by a Sequence of Ugi-4CR/Postfunctionalization. Chem. Commun. 2013, 49, 5598–5600. [Google Scholar] [CrossRef]
- Murai, M.; Yanagawa, M.; Nakamura, M.; Takai, K. Palladium-Catalyzed Direct Arylation of Azulene Based on Regioselective C-H Bond Activation. Asian, J. Org. Chem. 2016, 5, 629–635. [Google Scholar] [CrossRef]
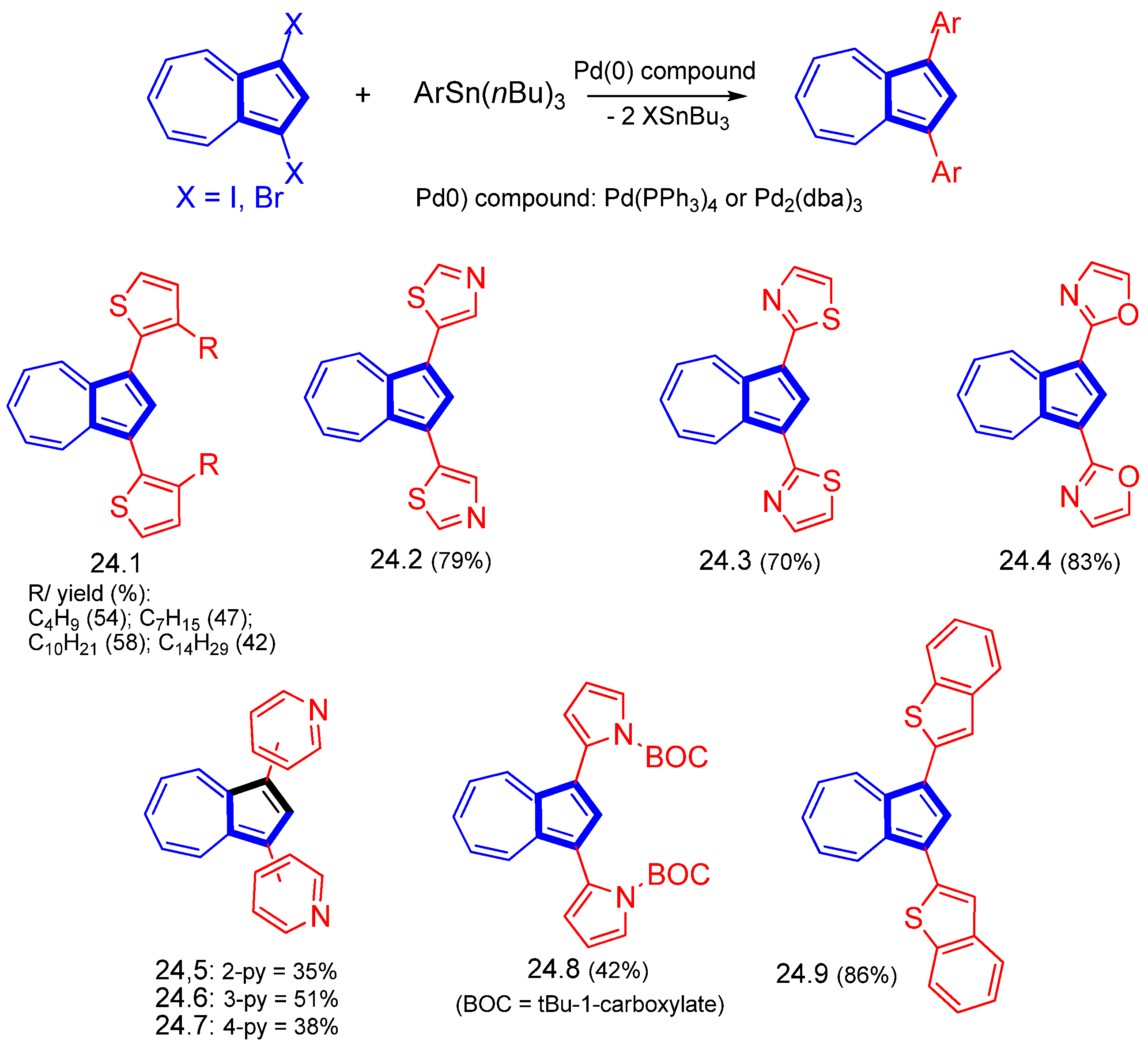
- Shoji, T.; Maruyama, A.; Araki, T.; Ito, S.; Okujima, T. Synthesis of 2- and 6-thienylazulenes by palladium-catalyzed direct arylation of 2- and 6-haloazulenes with thiophene derivatives. Org. Biomol. Chem. 2015, 13, 10191–10197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, T.; Araki, T.; Sugiyama, S.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T.; Toyota, K. Synthesis of 2-Azulenyltetrathiafulvalenes by Palladium-Catalyzed Direct Arylation of 2-Chloroazulenes with Tetrathiafulvalene and Their Optical and Electrochemical Properties. J. Org. Chem. 2017, 82, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Schipper, D.J.; Fagnou, K. Direct Arylation as a Synthetic Tool for the Synthesis of Thiophene-Based Organic Electronic Materials. Chem. Mater. 2011, 23, 1594–1600. [Google Scholar] [CrossRef]
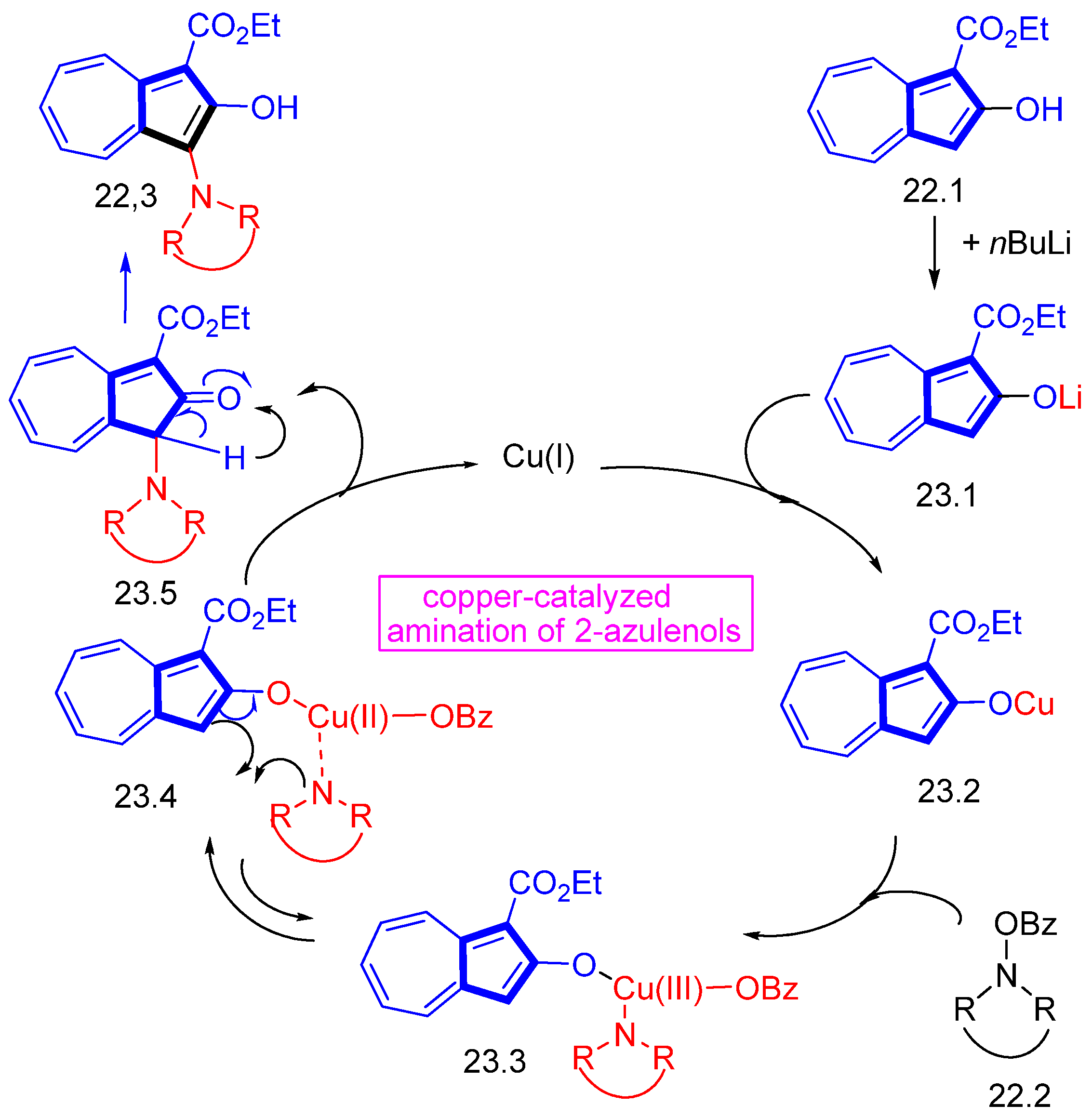
- Li, M.; Wang, D.-H. Copper-Catalyzed 3-Positional Amination of 2-Azulenols with O-Benzoylhydroxylamines. Org. Lett. 2021, 23, 6638–6641. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Thanh, N.C.; Ikai, M.; Fujikawa, H.; Nakajima, K.; Kuroda, S. Synthesis and properties of N,N,N’,N’-tetrasubstituted 1,3-bis(5-aminothien-2-yl)azulenes and their application as a hole-injecting material in organic light-emitting devices. Tetrahedron 2007, 63, 10608–10614. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Uriu, R.; Asakura, T.; Akamatsu, C.; Sugihara, Y. Synthesis of 1, 3-di (2-thiazolyl) azulene and its selective chromogenic response to mercury (II) ion. Heterocycles 2008, 75, 383–390. [Google Scholar] [CrossRef]
- Oda, M.; Kishi, S.; Thanh, N.C.; Kuroda, S. Synthetic methods for preparing 1, 3-di (2-pyridyl) azulene. Heterocycles 2007, 71, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, S.; Kato, Y.; Mochizuki, K.; Suzuki, R.; Matsumoto, M.; Sugihara, Y.; Shimizu, M. Pyridylazulenes: Synthesis, Color Changes, and Structure of the Colored Product. J. Org. Chem. 2007, 72, 744–749. [Google Scholar] [CrossRef]
- Salman, H.; Abraham, Y.; Tal, S.; Meltzman, S.; Kapon, M.; Tessler, N.; Speiser, S.; Eichen, Y. 1,3-Di(2-pyrrolyl)azulene: An Efficient Luminescent Probe for Fluoride. Eur. J. Org. Chem. 2005, 11, 2207–2212. [Google Scholar] [CrossRef]
- Amir, E.; Murai, M.; Amir, R.J.; Cowart, J.S.; Chabinyc, M.L.; Hawker, C.J. Conjugated oligomers incorporating azulene building blocks – seven- vs. five-membered ring connectivity. Chem. Sci. 2014, 5, 4483–4489. [Google Scholar] [CrossRef]
- Janusson, E.; Zijlstra, H.S.; Nguyen, P.P.T.; MacGillivray, L.; Martelino, J.; McIndoe, J.S. Real-Time Analysis of Pd2(dba)3 Activation by Phosphine Ligands. Chem. Commun. 2017, 53, 854–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinet, P.; Echavarren, A.M. The Mechanisms of the Stille Reaction. Angew. Chem. Int. Ed. 2004, 43, 4704–4734. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef]
- Mee, S.P.H.; Lee, V.; Baldwin, J.E. Stille Coupling Made Easier—The Synergic Effect of Copper (1) Salts and the Fluoride Ion. Angew. Chem. Int. Ed. 2004, 43, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
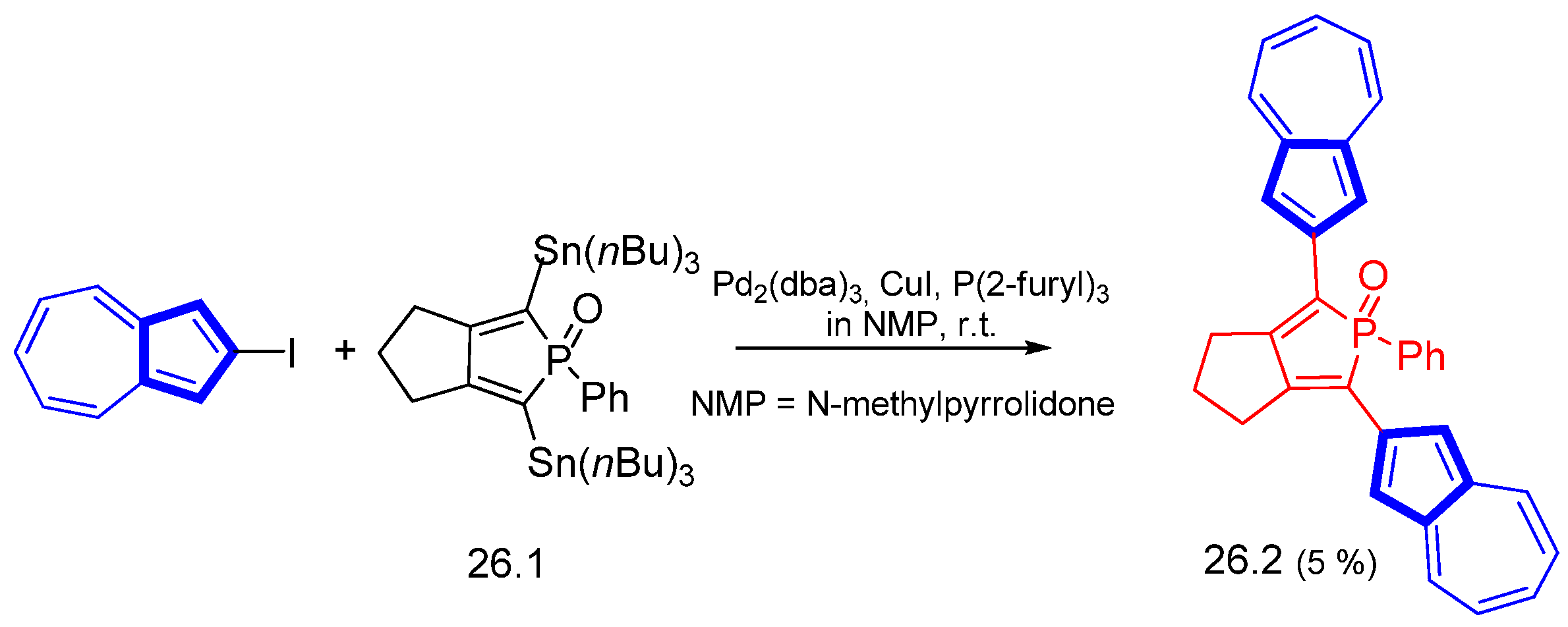
- Matano, Y.; Kon, Y.; Saito, A.; Kimura, Y.; Murafuji, T.; Imahori, H. Divergent Synthesis of 2,5-Diarylphospholes Based on Cross-coupling Reactions: Substituent Efects on the Optical and Redox Properties of Benzene–Phosphole–Benzene–π-Systems. Chem. Lett. 2011, 40, 919–921. [Google Scholar] [CrossRef]
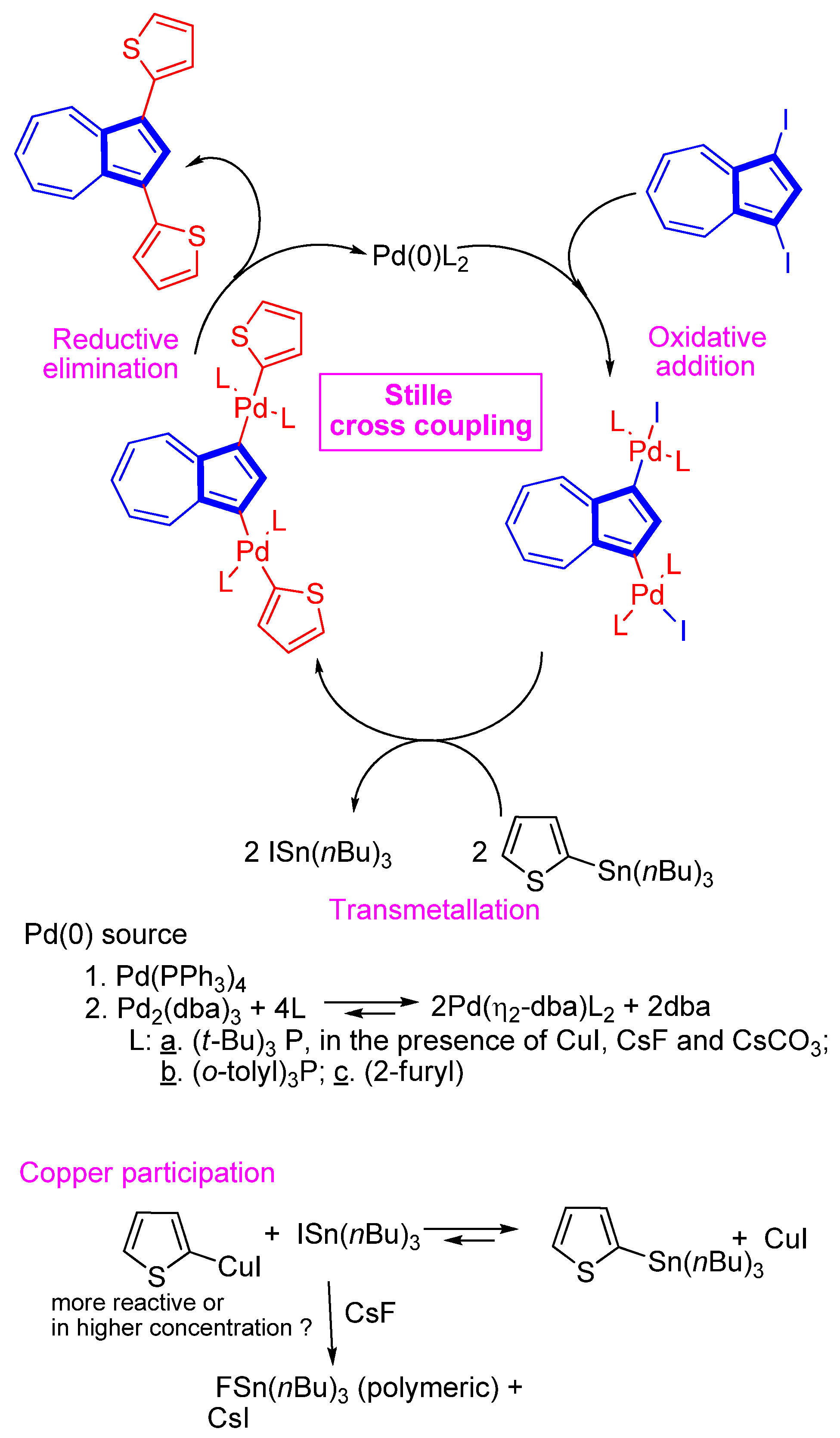
- Ito, S.; Okujima, T.; Morita, N. Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. Easy access to poly(azulen-6-yl)benzene derivatives. J. Chem. Soc. Perkin Trans. 1 2002, 16, 1896–1905. [Google Scholar] [CrossRef]
- Shetti, V.S. Chemical syntheses and salient features of azulene-containing homo- and copolymers. Beilstein, J. Org. Chem. 2021, 17, 2164–2185. [Google Scholar] [CrossRef]
- Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C.J.; Robb, M. Modulating the Properties of Azulene-Containing Polymers through Controlled Incorporation of Regioisomers. J. Adv. Funct. Mater. 2014, 24, 7338–7347. [Google Scholar] [CrossRef] [Green Version]
- Dubovik, J.; Bredihhin, A. A Convenient Synthesis of Functionalized Azulenes via Negishi Cross-Coupling. Synthesis 2015, 47, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Dubovik, J.; Bredihhin, A. A Convenient Synthesis of Functionalized Azulenes via Negishi Cross-Coupling. Synthesis 2015, 47, 2663–2669. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Hajiabbasi, P.; Hamidi, H. Advances in Kumada–Tamao–Corriu cross-coupling reaction: An update. Monatsh. Chem. 2019, 150, 535–591. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Y.-H. Conducting Azulene−Thiophene Copolymers with Intact Azulene Units in the Polymer Backbone. Macromolecules 2003, 36, 536–538. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Y.-H.; Han, M.-Y. Stimuli-Responsive Conjugated Copolymers Having Electro-Active Azulene and Bithiophene Units in the Polymer Skeleton: Effect of Protonation and p-Doping on Conducting Properties. Macromolecules 2004, 37, 3222–3230. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Dragu, E.A.; Ion, A.E.; Shova, S.; Bala, D.; Mihailciuc, C.; Voicescu, M.; Ionescu, S.; Nica, S. Visible-light triggered photoswitching systems based on fluorescent azulenyl-substituted dithienylcyclopentenes. RSC Adv. 2015, 5, 63282–63286. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-I.; Ohba, Y. Synthesis, Properties, and OFET Characteristics of 5,5′-Di(2-azulenyl)-2,2′-bithiophene (DAzBT) and 2,5-Di(2-azulenyl)-thieno[3,2-b]thiophene (DAzTT). Org. Lett. 2012, 14, 2316–2319. [Google Scholar] [CrossRef]
- Murai, M.; Ku, S.-Y.; Treat, N.D.; Robb, M.J.; Chabinyc, M.L.; Hawker, C.J. Modulating structure and properties in organic chromophores: Influence of azulene as a building block. Chem. Sci. 2014, 5, 3753–3760. [Google Scholar] [CrossRef] [Green Version]
- Murafuji, T.; Sugihara, Y.; Fujinaga, M.; Suetake, K.; Gyoji, K.; Kurotobi, K. An Easy Access to 2-Substituted Azulenes from Azulene-2-boronic Acid Pinacol Ester. Synthesis 2008, 23, 3745–3748. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, J.; Xiang, J.; Yang, X.; Gao, X. Azulene-Embedded [n]Helicenes (n = 5, 6 and 7). Angew. Chem. Int. Ed. 2022, 61, e202201494. [Google Scholar] [CrossRef]
- Murai, M.; Iba, S.; Ota, H.; Takai, K. Azulene-Fused Linear Polycyclic Aromatic Hydrocarbons with Small Bandgap, High Stability, and Reversible Stimuli Responsiveness. Org. Lett. 2017, 19, 5585–5588. [Google Scholar] [CrossRef]
- Kurotobi, K.; Osuka, A. Synthesis of meso-Azulenylporphyrins. Org. Lett. 2005, 7, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Blacque, O.; Venkatesan, K. Syntheses and Tunable Emission Properties of 2-Alkynyl Azulenes. Org. Lett. 2012, 14, 1580–1583. [Google Scholar] [CrossRef]
- Koch, M.; Blacque, O.; Venkatesan, K. Impact of 2,6-connectivity in azulene: Optical properties and stimuli responsive behavior. J. Mater. Chem. C 2013, 1, 7400–7408. [Google Scholar] [CrossRef] [Green Version]
- Ohtsu, K.; Hayami, R.; Sagawa, T.; Tsukada, S.; Yamamoto, K.; Gunji, T. Syntheses and properties of linear π-conjugated molecules composed of 1-azaazulene and azulene. Tetrahedron 2019, 74, 130658–130664. [Google Scholar] [CrossRef]
- Shoji, T.; Maruyama, M.; Maruyama, A.; Ito, S.; Okujima, T.; Toyota, K. Synthesis of 1,3-Bis(tetracyano-2-azulenyl-3-butadienyl)azulenes by the [2+2] Cycloaddition-Retroelectrocyclization of 1,3-Bis(azulenylethynyl)azulenes with Tetracyanoethylene. Chem. Eur. J. 2014, 20, 11903–11912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Székely, A.; Péter, Á.; Aradi, K.; Tolnai, G.L.; Novák, Z. Gold-Catalyzed Direct Alkynylation of Azulenes. Org. Lett. 2017, 19, 954–957. [Google Scholar] [CrossRef]
- Liu, J.; Muth, E.; Floerke, U.; Henkel, G.; Merz, K.; Sauvageau, J.; Schwake, E.; Dyker., G. Alkylation of Arenes with Benzylic and Propargylic Alcohols—Classical versus Fancy Catalysts. Adv. Synth. Catal. 2006, 348, 456–462. [Google Scholar] [CrossRef]
- Inada, Y.; Yoshikawa, M.; Milton, M.D.; Nishibayashi, Y.; Uemura, S. Ruthenium-Catalyzed Propargylation of Aromatic Compounds with Propargylic Alcohols. Eur. J. Org. Chem. 2006, 2006, 881–890. [Google Scholar] [CrossRef]
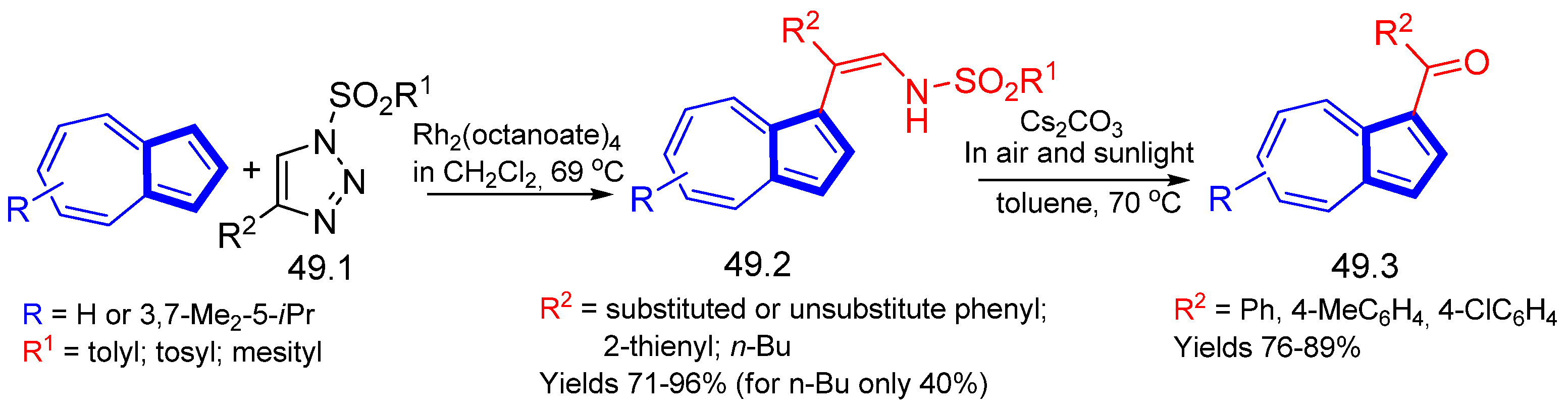
- Park, S.; Jeon, W.H.; Yong, W.-S.; Lee, P.H. Synthesis of Azulen-1-yl Ketones via Oxidative Cleavage of C–C Multiple Bonds in N-Sulfonyl Enamides and 1-Alkynes under Air and Natural Sunlight. Org. Lett. 2015, 17, 5060–5063. [Google Scholar] [CrossRef]
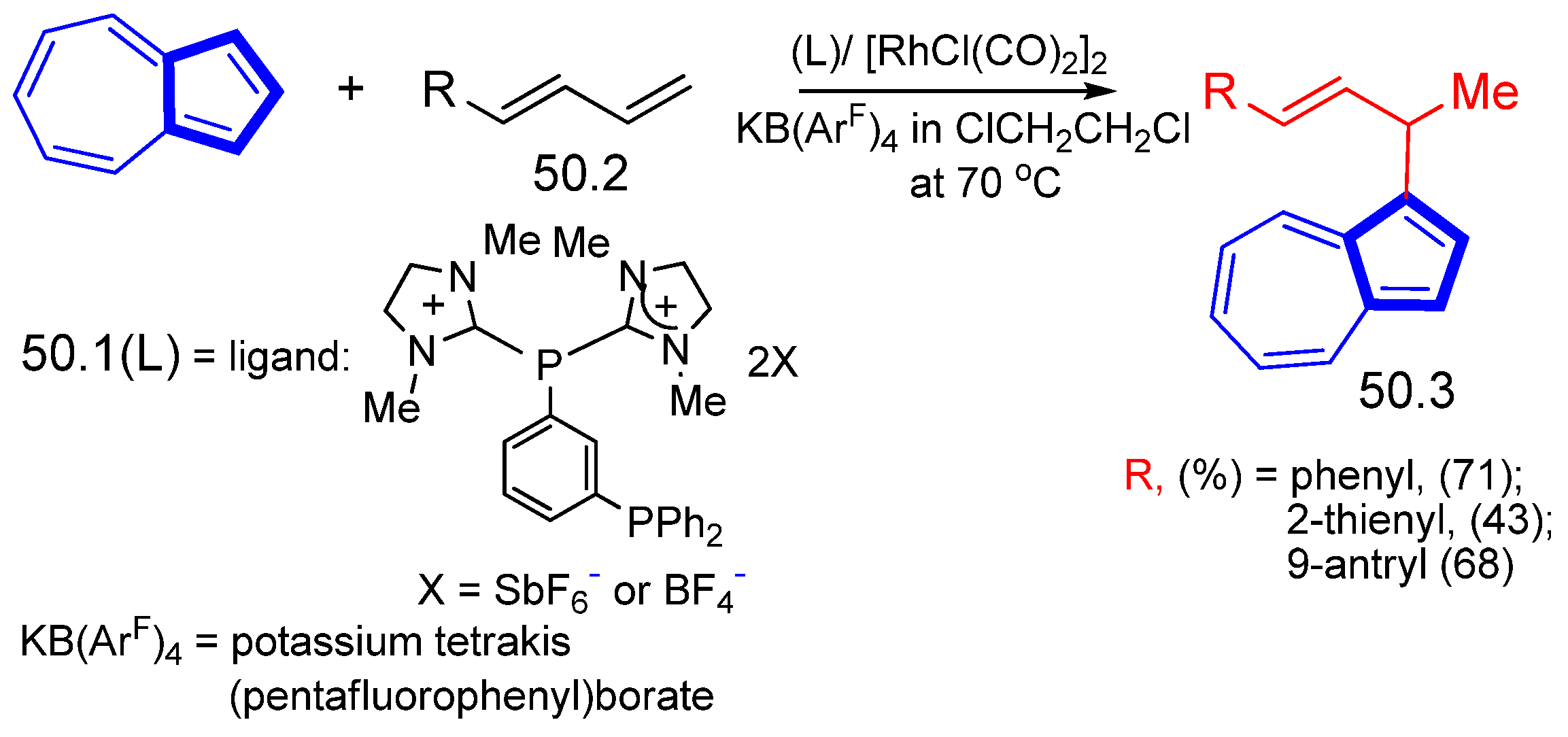
- Gu, L.; Wolf, L.M.; Zielinski, A.; Thiel, W.; Alcarazo, M. α-Dicationic Chelating Phosphines: Synthesis and Application to the Hydroarylation of Dienes. J. Am. Chem. Soc. 2017, 139, 4948–4953. [Google Scholar] [CrossRef]
- Nakatani, A.; Hirano, K.; Satoh, T.; Miura, M. Nickel-Catalyzed Direct Alkylation of Heterocycles with α-Bromo Carbonyl Compounds: C3-, Functionalization of 2-Pyridones. Chem. Eur. J. 2013, 19, 7691–7695. [Google Scholar] [CrossRef] [PubMed]
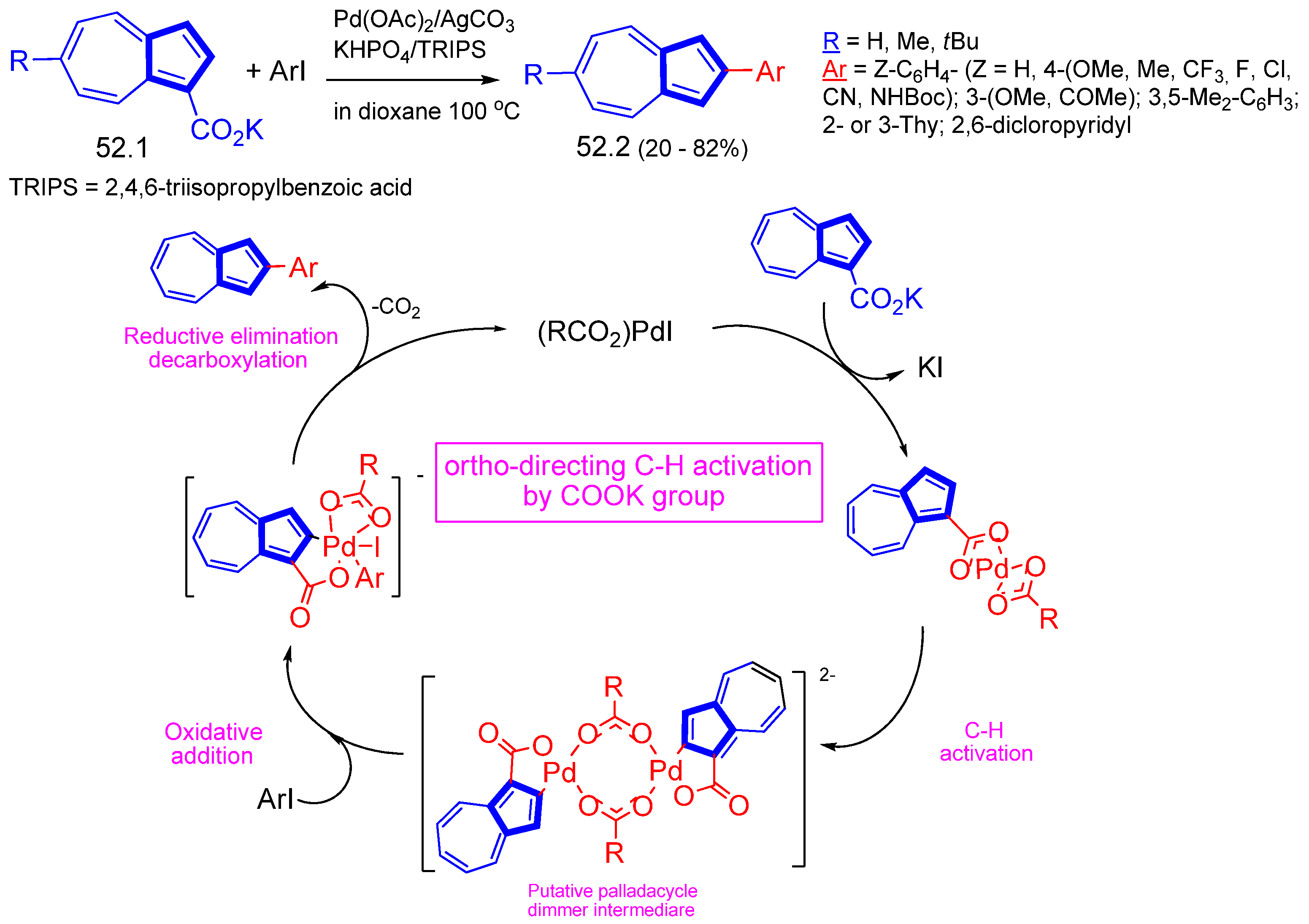
- Arroniz, C.; Ironmonger, A.; Rassias, G.; Larrosa, I. Direct ortho-Arylation of ortho-Substituted Benzoic Acids: Overriding Pd-Catalyzed Protodecarboxylation. I. Org. Lett. 2013, 15, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Png, Z.M.; Tam, T.L.D.; Xu, J. Carboxylic Acid Directed C–H Arylation of Azulene. Organic Lett. 2020, 22, 5009–5013. [Google Scholar] [CrossRef] [PubMed]
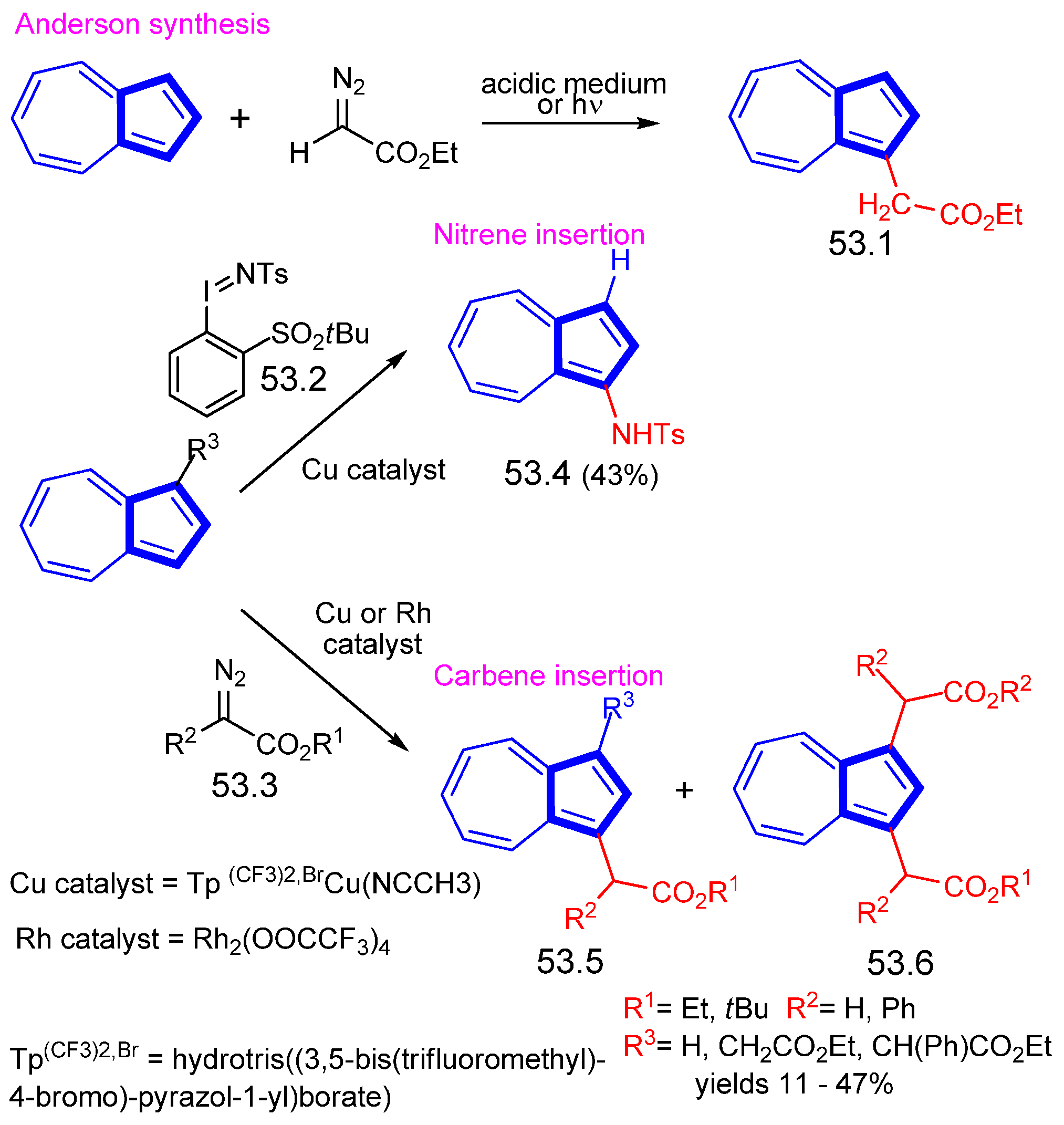
- Anderson, A.G.; Rhodes, R.C. The Reaction of Azulenes with Aliphatic Diazo Compounds. J. Org. Chem. 1965, 30, 1616–1619. [Google Scholar] [CrossRef]
- Carreras, J.; Popowski, Y.; Caballero, A.; Amir, E.; Pérez, P.J. Catalytic Functionalization of C–H Bonds of Azulene by Carbene/Nitrene Incorporation. J. Org. Chem. 2018, 83, 11125–11132. [Google Scholar] [CrossRef] [PubMed]
- Dunas, P.; Murfin, L.C.; Nilsson, O.J.; Jame, N.; Lewis, S.E.; Kann, N. Azulene Functionalization by Iron-Mediated Addition to a Cyclohexadiene Scaffold. J. Org. Chem. 2020, 85, 13453–13465. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Tanaka, M.; Takagaki, S.; Miura, K.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T. Synthesis of azulene-substituted benzofurans and isocoumarins via intramolecular cyclization of 1-ethynylazulenes, and their structural and optical properties. Org. Biomol. Chem. 2018, 16, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Ariga, Y.; Yamazaki, A.; Uda, M.; Nagasawa, T.; Ito., S. Synthesis, Photophysical and Electrochemical Properties of 1-, 2-, and 6-(2-Benzofuryl)azulenes. Bull. Chem. Soc. Jpn. 2021, 94, 1000–1009. [Google Scholar] [CrossRef]
- Sato, K.; Yokoo, E.; Takenaga, N. Facile Synthesis of Guaiazulene-Heterocycle Hybrids via Multicomponent Reactions Involving Formation of Zwitterionic Intermadiates. Heterocycles 2013, 87, 807–814. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.-L.; Xing, J.-J.; Liu, L. One-Pot Synthesis of 3-(Guaiazulen-3-yl)dihydro-1H-indol-4(5H)-ones via Domino Reaction. Heterocycles 2019, 98, 1547–1554. [Google Scholar] [CrossRef]
- Gong, J.; Peshkov, A.A.; Yu, J.; Amandykova, S.; Gimnkhan, A.; Huang, J.; Kashtanov, S.; Pereshivko, O.P.; Peshkov, V.A. Three-component reaction of azulene, aryl glyoxal and 1,3-dicarbonyl compound for the synthesis of various azulene derivatives. RSC Advances 2020, 10, 10113–10117. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-L.; Feng, S.-S.; Cui, Q.-T.; Yu, J.-Y. An Eficient One-Pot Synthesis of 1-Amino-3-cyano-4-aryl-10-ethoxycarbonylazuleno[2,1-b]pyrans. Heterocycles 2012, 85, 441–448. [Google Scholar] [CrossRef]
- Ito, S.; Yamazaki, S.; Kudo, S.; Sekiguchi, R.; Kawakami, J.; Takahashi, M.; Matsuhashi, T.; Toyota, K.; Morita, N. Synthesis and redox behavior of 1,2-dihydro-1-oxabenz[a]azulen-2-ones. Tetrahedron 2014, 70, 2796–2803. [Google Scholar] [CrossRef]
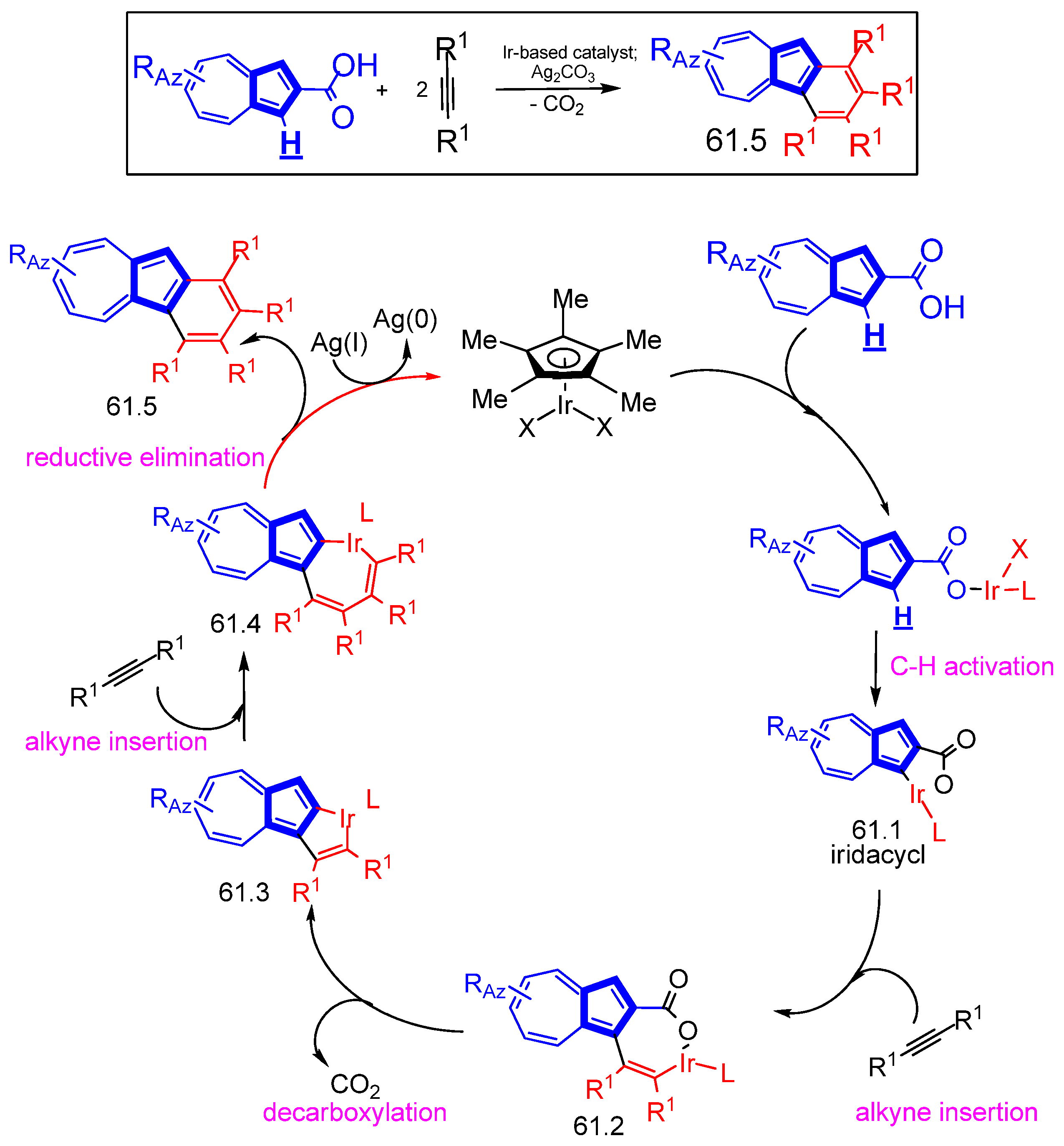
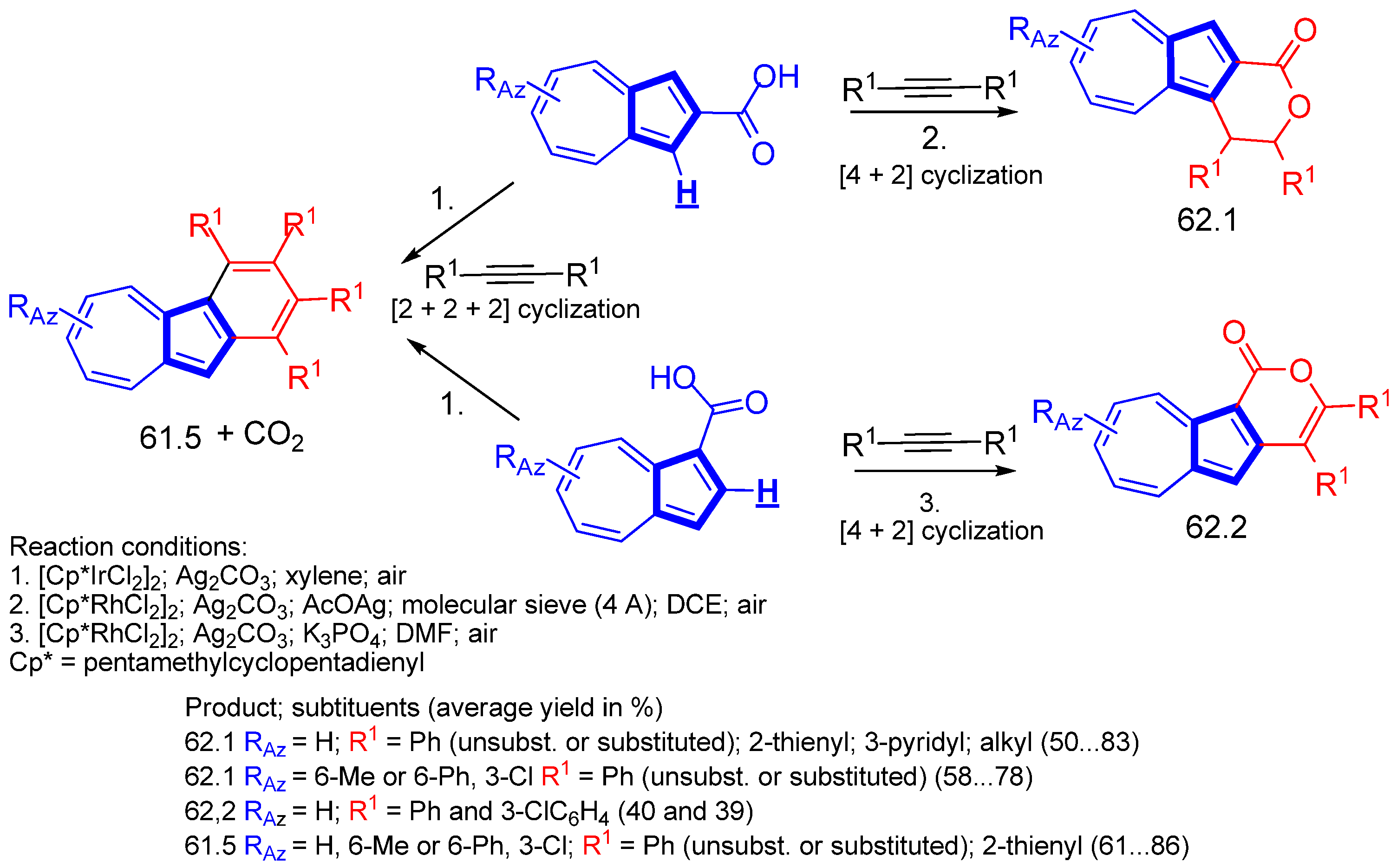
- Maeng, C.; Son, J.-Y.; Lee, S.C.; Baek, Y.; Um, K.; Han, S.H.; Lee, P.H. Expansion of Azulenes as Nonbenzenoid Aromatic Compounds for C–H Activation: Rhodium- and Iridium-Catalyzed Oxidative Cyclization of Azulene Carboxylic Acids with Alkynes for the Synthesis of Azulenolactones and Benzoazulenes. J. Org. Chem. 2020, 85, 3824–3837. [Google Scholar] [CrossRef] [PubMed]
- Maeng, C.; Seo, H.J.; Jeong, H.; Lee, K.; Noh, H.C.; Lee, P.H. Iridium(III)-Catalyzed Sequential C(2)-Arylation and Intramolecular C–O Bond Formation from Azulenecarboxylic Acids and Diaryliodonium Salts Access to Azulenofuranones. Org. Lett. 2020, 22, 7267–7272. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Nakagawa, H.; Gunji, T.; Ito, Y.; Kawai, Y.; Ikeda, R.; Hyoudou, M. Synthesis, Cyclization, and Evaluation of the Anticancer Activity against HeLa S-3 Cells of Ethyl 2-Acetylamino-3-ethynylazulene-1-carboxylates. Heterocycles 2012, 86, 233–244. [Google Scholar] [CrossRef]
- Shoji, T.; Miura, K.; Ohta, A.; Sekiguchi, R.; Ito, S.; Endo, Y.; Okujima, T. Synthesis of azuleno[2,1-b]thiophenes by cycloaddition of azulenylalkynes with elemental sulfur and their structural, optical and electrochemical properties. Org. Chem. Front. 2019, 6, 2801–2811. [Google Scholar] [CrossRef]
- Kogawa, C.; Fujiwara, A.; Sekiguchi, R.; Shoji, T.; Kawakami, J.; Okazaki, M.; Ito, S. Synthesis and photophysical properties of azuleno[10,20:4,5]pyrrolo[2,1-b]quinazoline-6,14-diones: Azulene analogs of tryptanthrin. Tetrahedron 2018, 74, 7018–7029. [Google Scholar] [CrossRef]
- Hou, B.; Li, J.; Yang, X.; Zhang, J.; Xin, H.; Ge, C.; Gao, X. Azulenoisoindigo: A building block for π-functional materials with reversible redox behavior and proton responsiveness. Chin. Chem. Lett. 2022, 23, 2147–2150. [Google Scholar] [CrossRef]
- Xin, H.; Li, J.; Yang, X.; Gao, X. Azulene-based BN-Heteroaromatics. J. Org. Chem. 2019, 85, 70–78. [Google Scholar] [CrossRef]
- Lash, T.D.; El-Beck, J.A.; Colby, D.A. Synthesis of a Tetraazulene Porphodimethene Analogue. J. Org. Chem. 2009, 74, 8830–8833. [Google Scholar] [CrossRef]
- Georghiou, P.E.; Rahman, S.; Alodhay, A.; Nishimura, H.; Lee, J.; Wakamiya, A.; Scott, L.T. Calixazulenes: Azulene-based calixarene analogues–an overview and recent supramolecular complexation studies. Beilstein, J. Org. Chem. 2018, 14, 2488–2494. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, M.; Nakazawa, T.; Murata, I. A novel coupling reaction of 1-azylylmethyltrimethylammonium iodide. Synthesis of 1,2-bis[1-azulyl)ethane and [2.2.2.2](1,3)azulenophane. Tetraherdon Lett. 1979, 20, 825–828. [Google Scholar] [CrossRef]
- Lash, T.D. Out of the Blue! Azuliporphyrins and Related Carbaporphyrinoid Systems. ACC Chem. Res. 2016, 49, 471–482. [Google Scholar] [CrossRef]
- Larsen, S.; McCormick-McPherson, L.J.; Teat, S.J.; Ghosh, A. Azulicorrole. ACS Omega 2019, 4, 6737–6745. [Google Scholar] [CrossRef] [Green Version]
- Sprutta, N.; Swiderska, M.; Latos-Grazùynski, L. Dithiadiazuliporphyrin: Facile Generation of Carbaporphyrinoid Cation Radical and Dication. J. Am. Chem. Soc. 2005, 127, 13108–13109. [Google Scholar] [CrossRef]
- Sprutta, N.; Siczek, M.; Latos-Grazùynski, L.; Pawlicki, M.; Szterenberg, L.; Lis, T. Dioxadiazuliporphyrin: A Near-IR Redox Switchable Chromophore. J. Org. Chem. 2007, 72, 9501–9509. [Google Scholar] [CrossRef]
- Zhang, Z.; Ferrence, G.M.; Lash, T.D. adj-Diazuliporphyrins, a New Family of Dicarbaporphyrinoids with Unprecedented Mesoionic Characteristics. Org. Lett. 2009, 11, 101–104. [Google Scholar] [CrossRef]






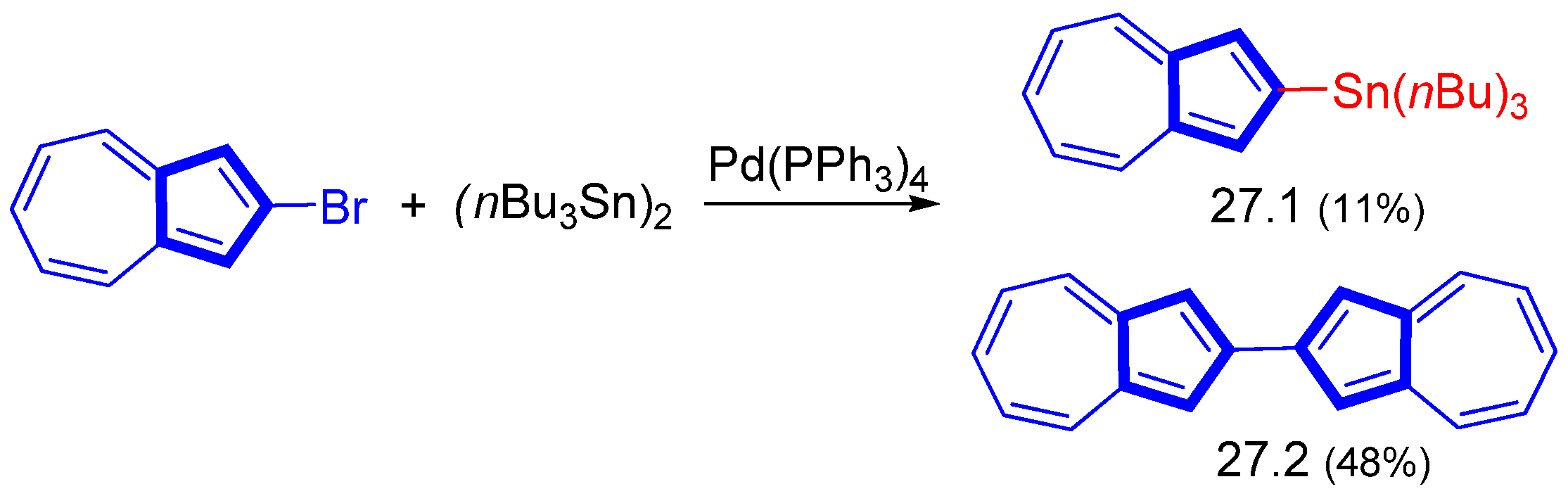
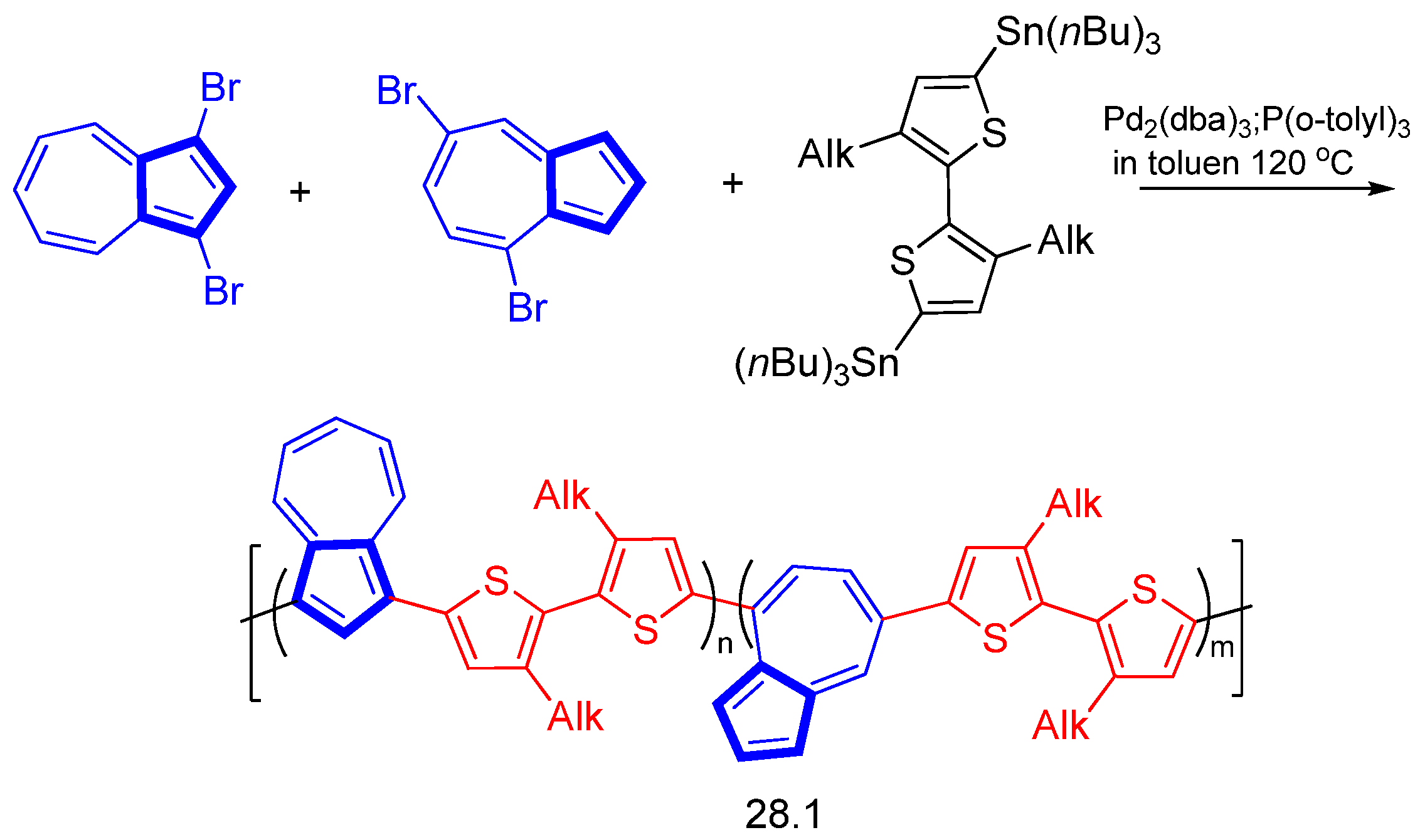
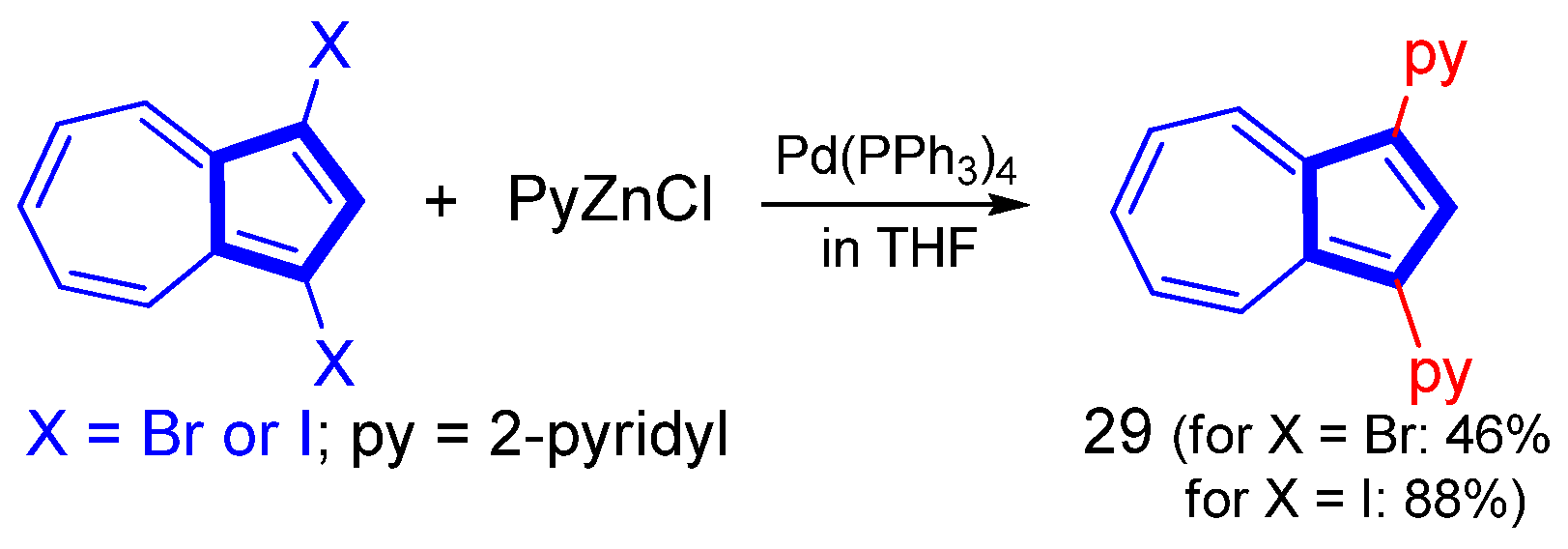


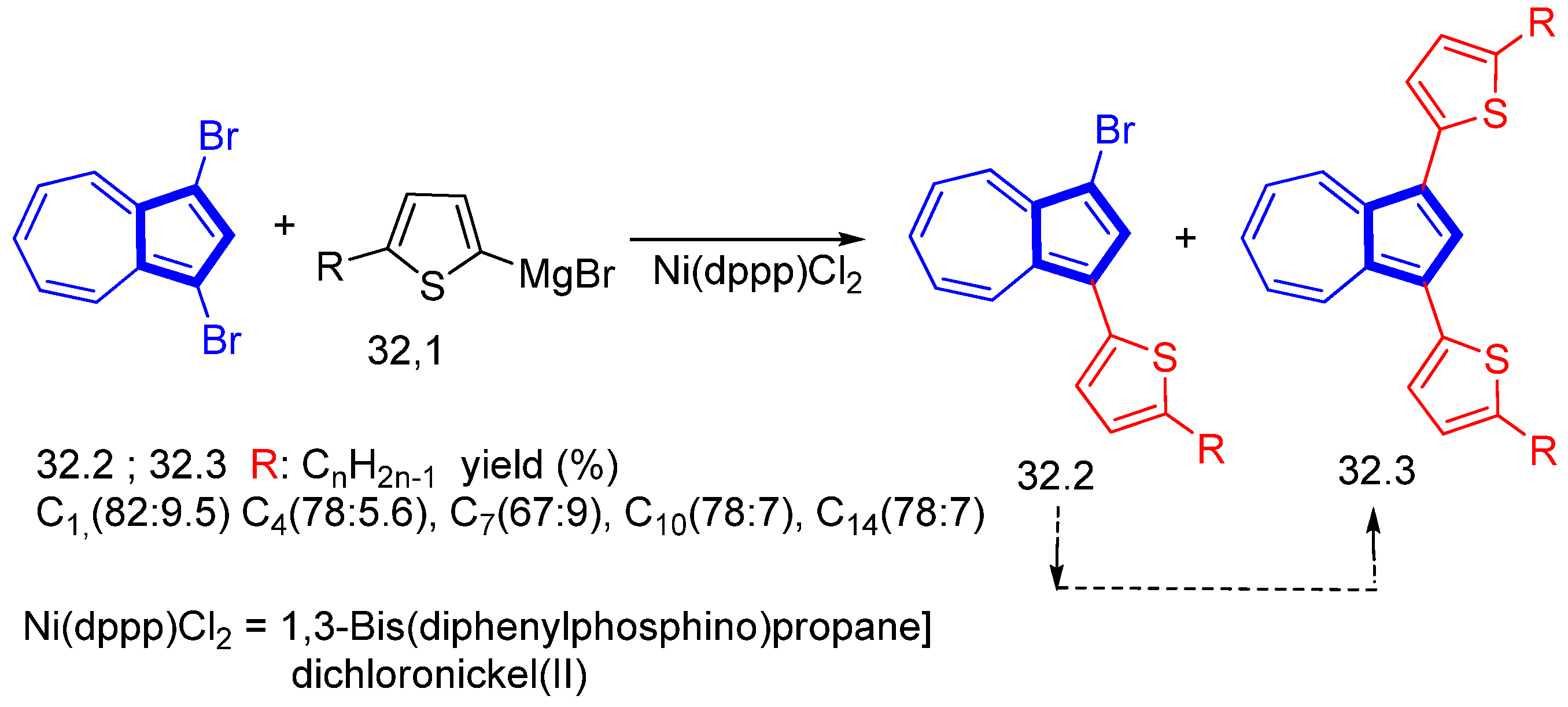
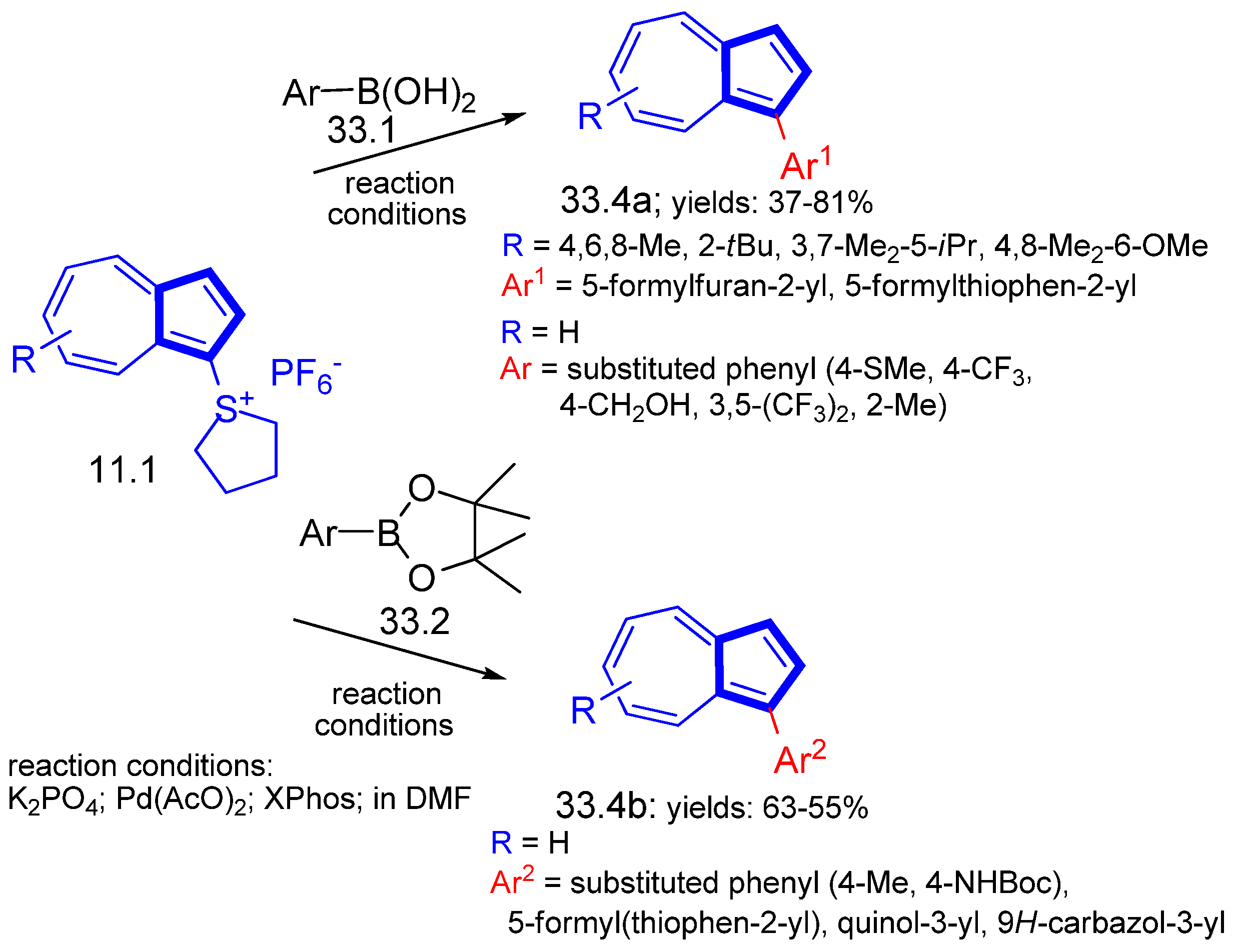
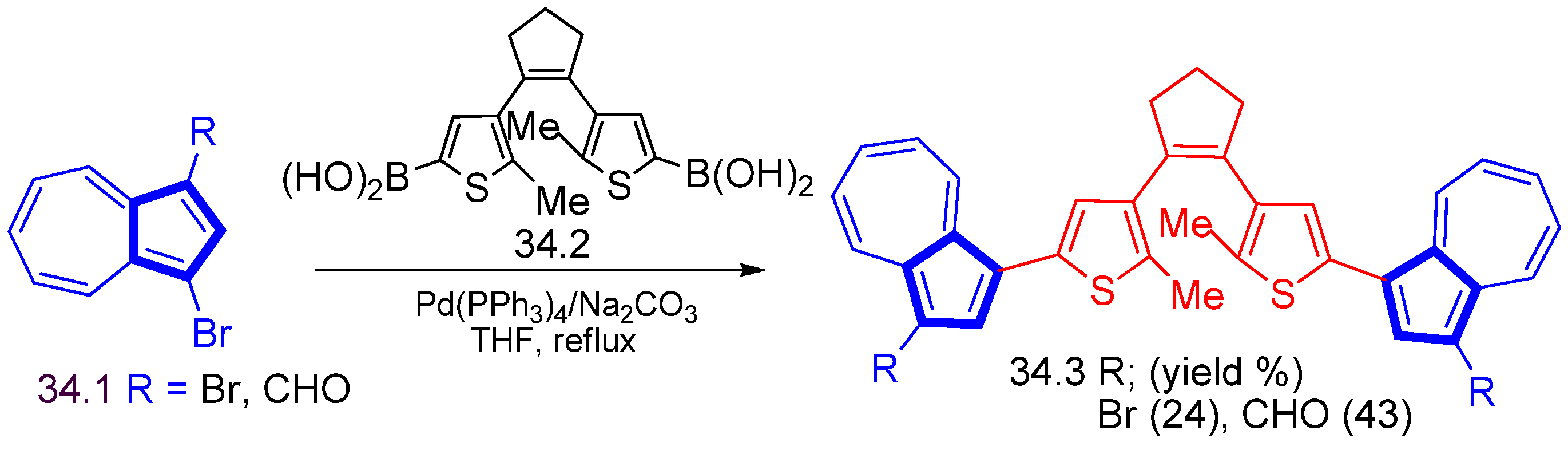
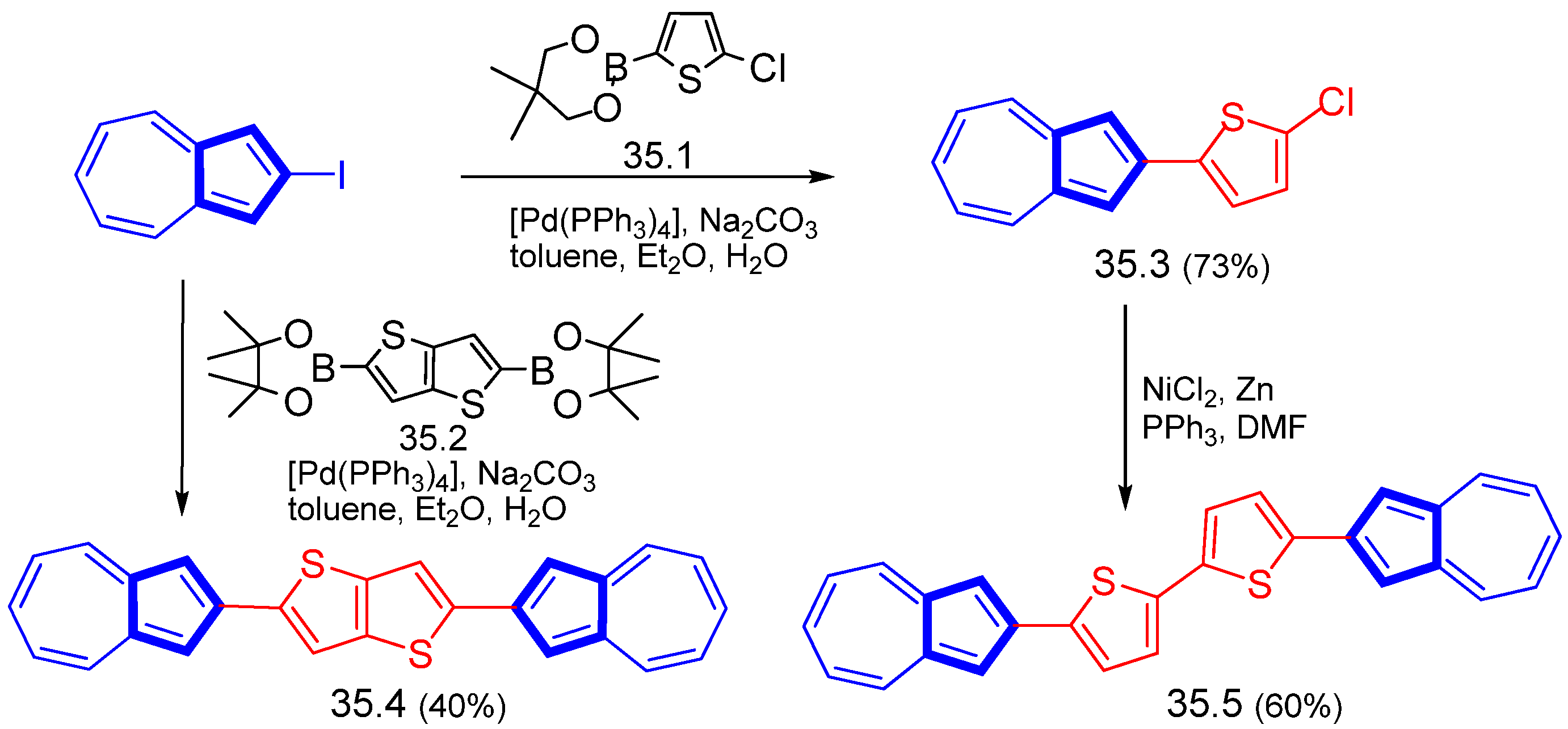

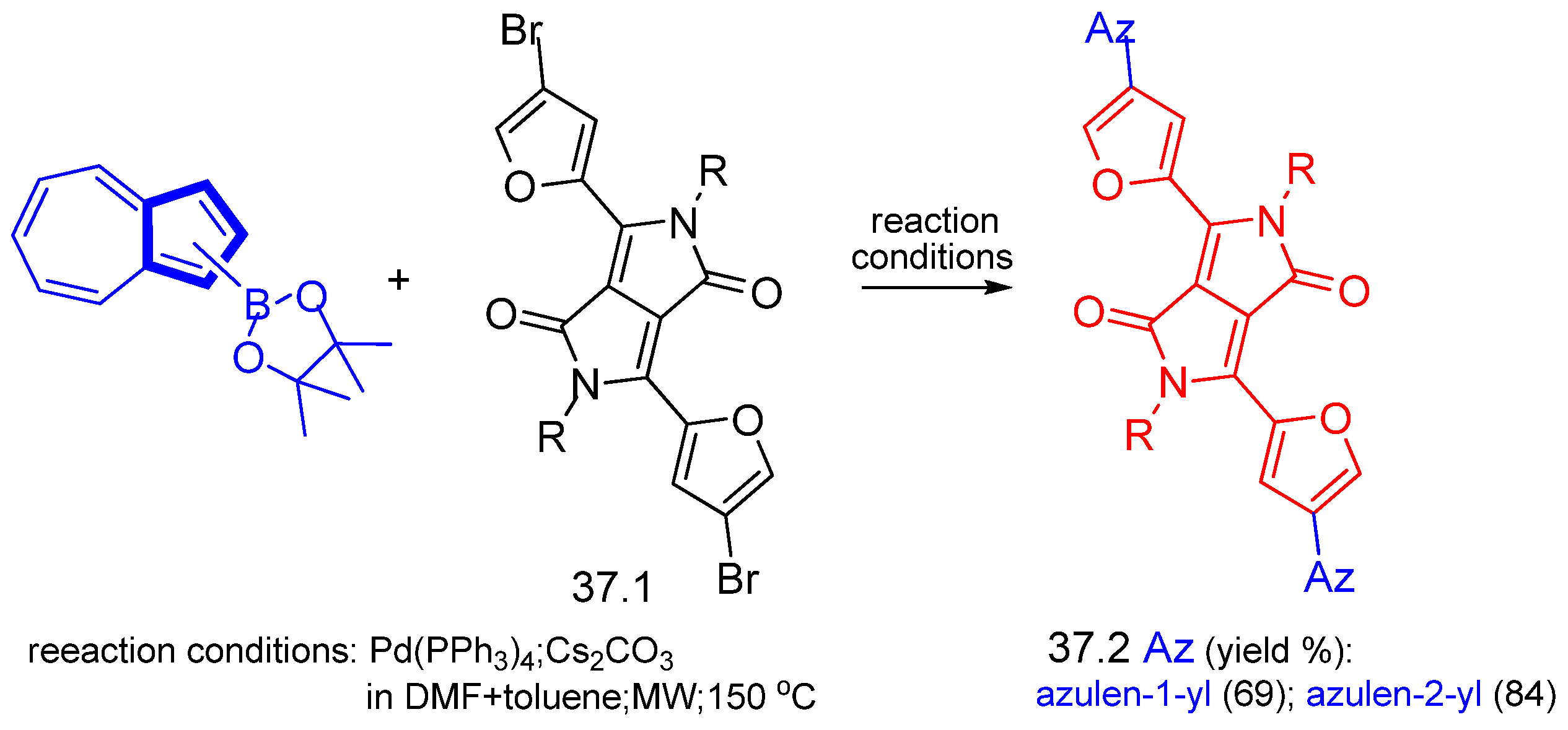

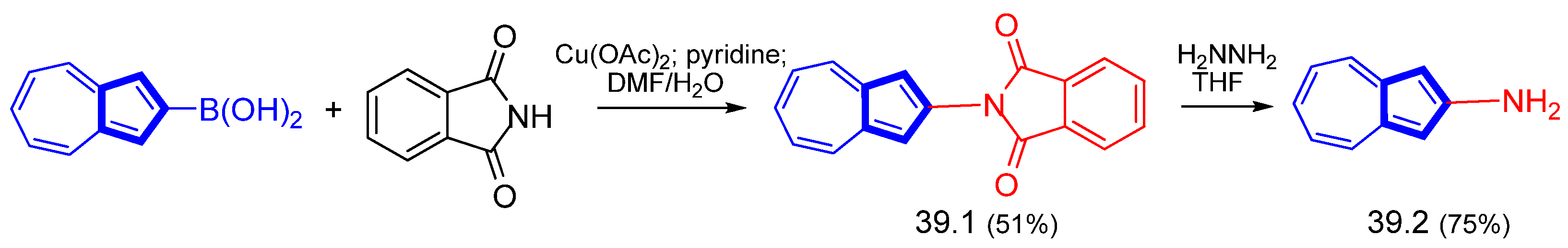

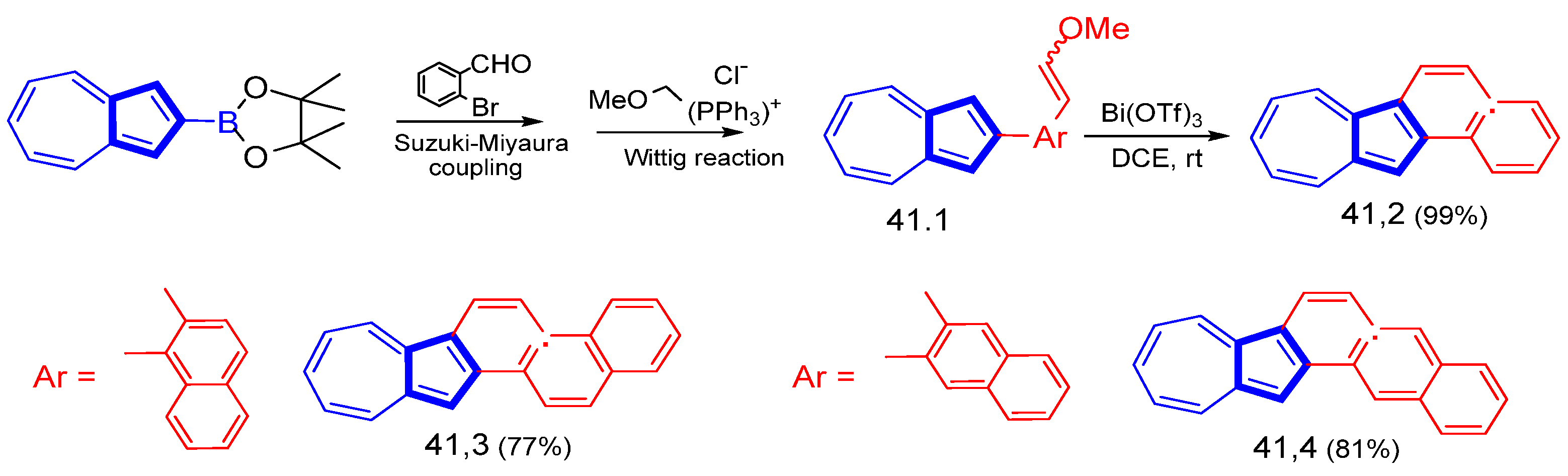
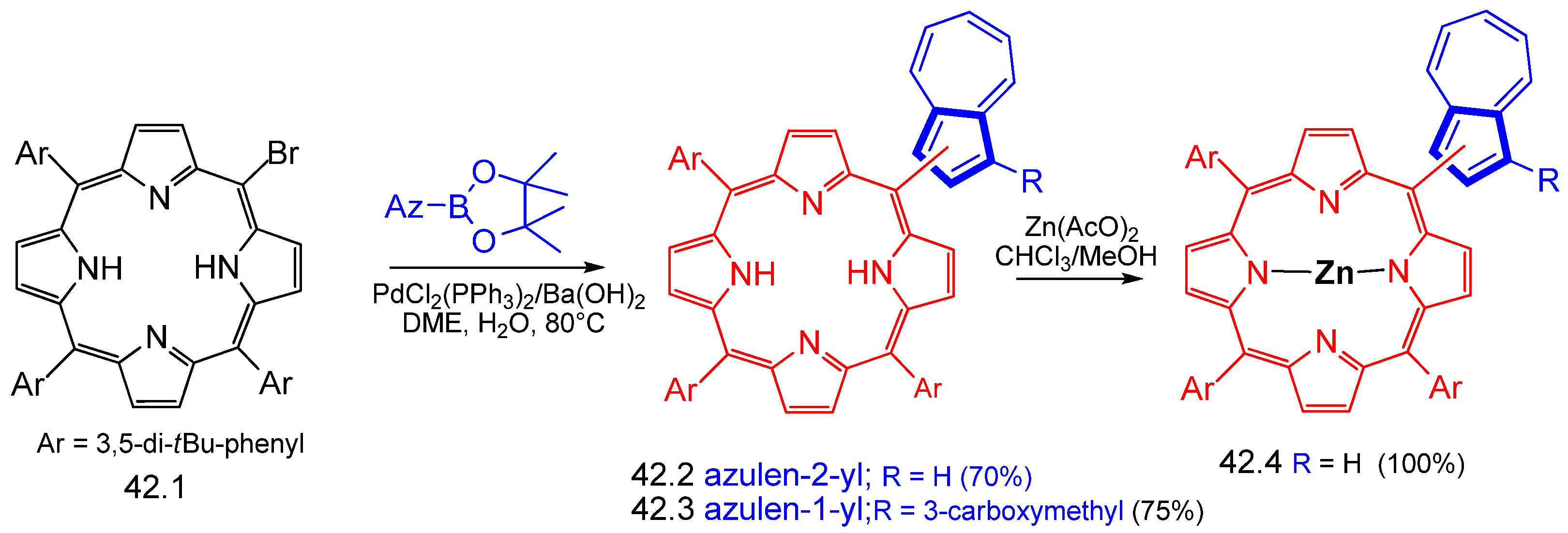



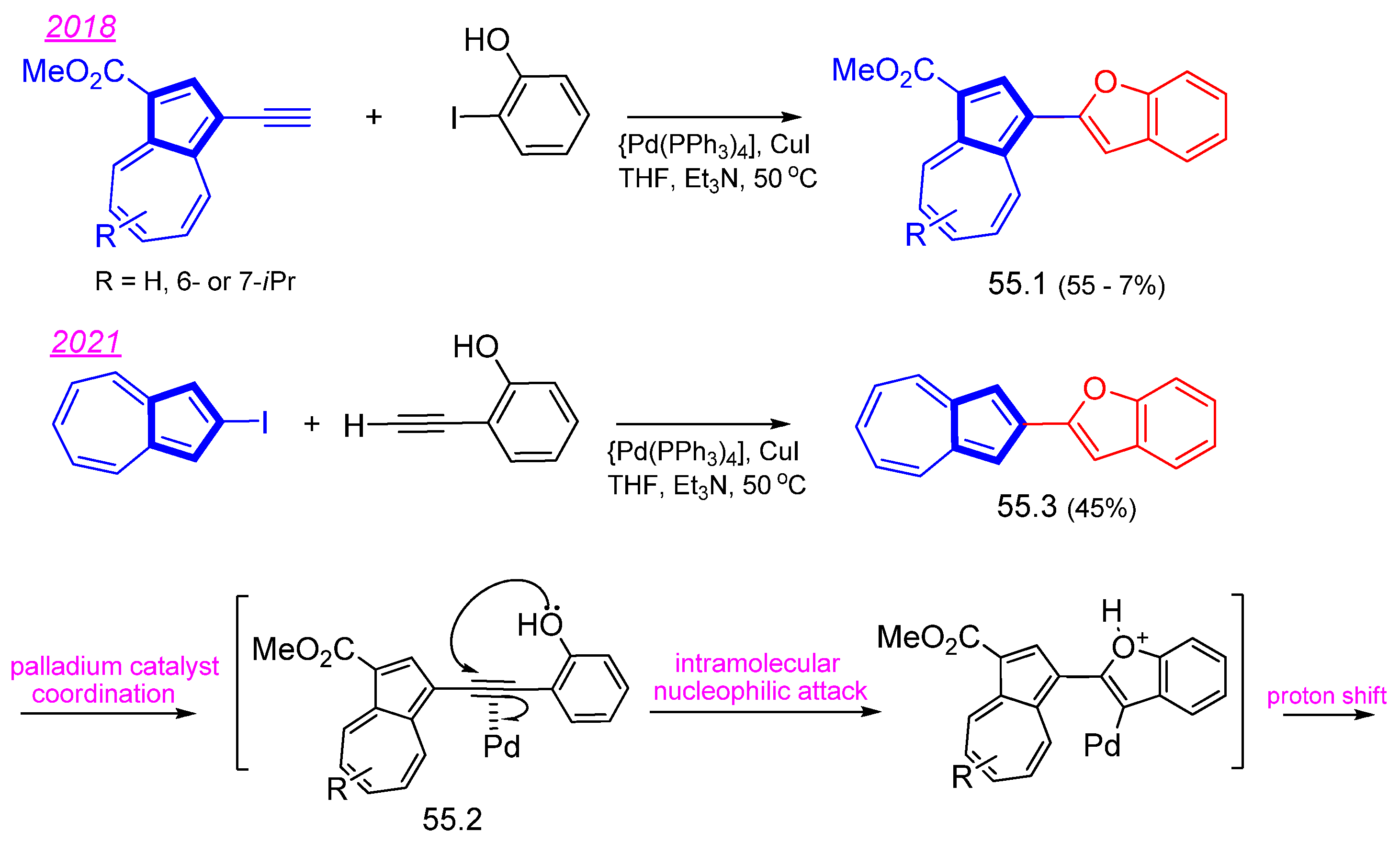
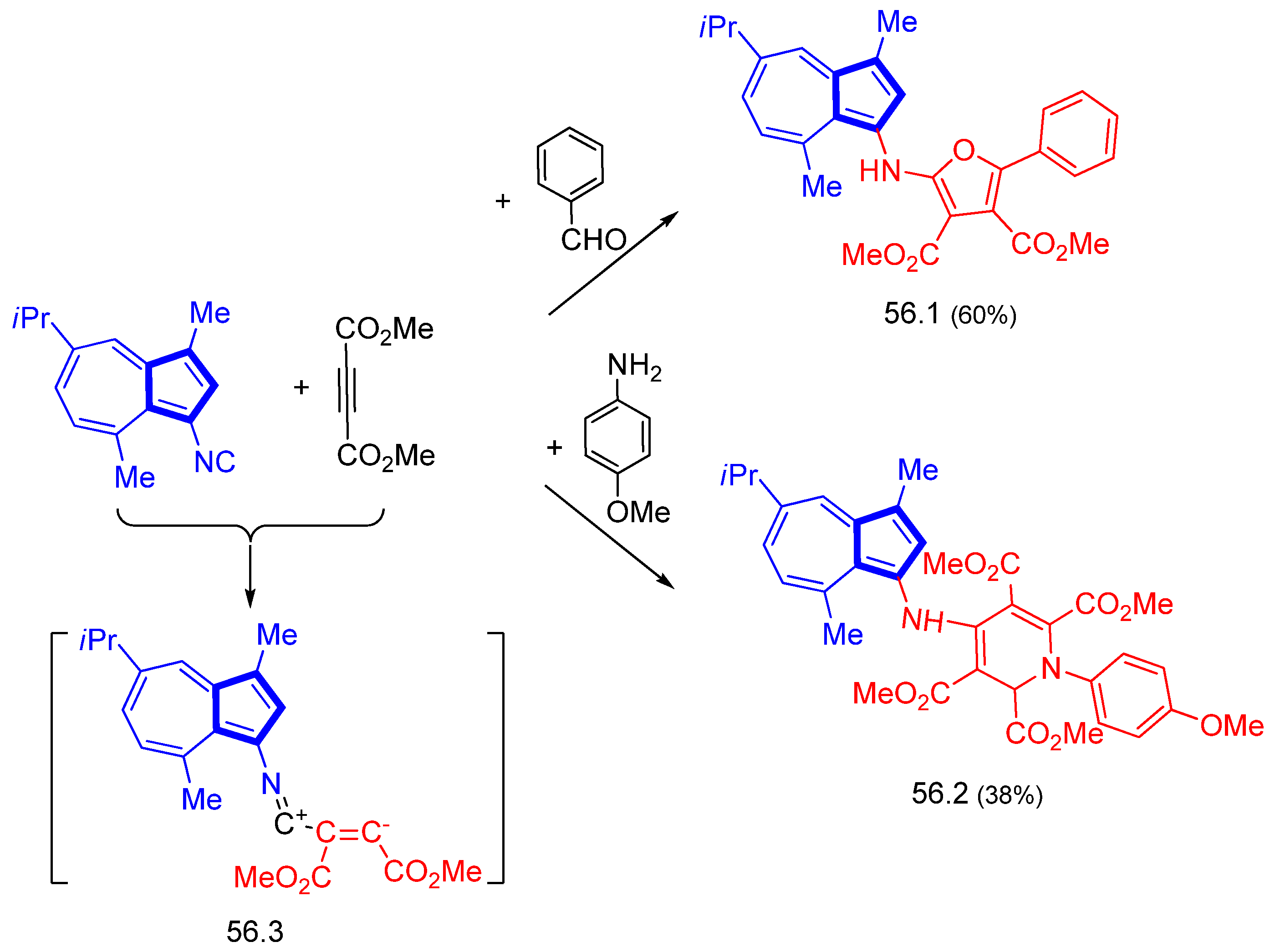
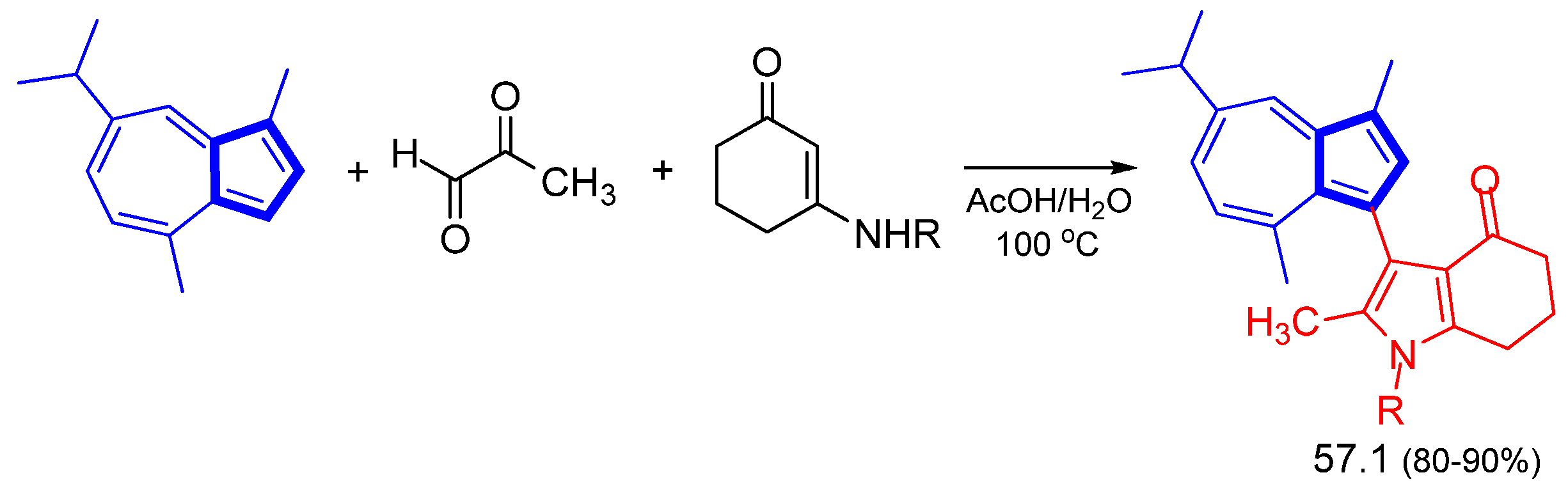

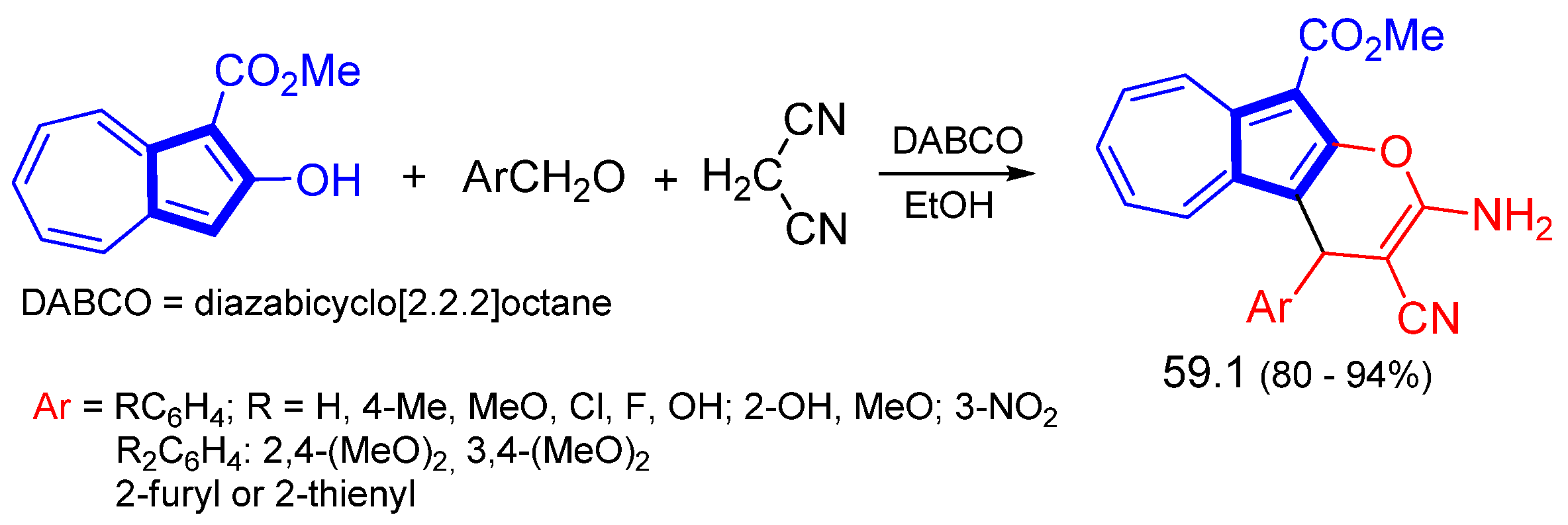
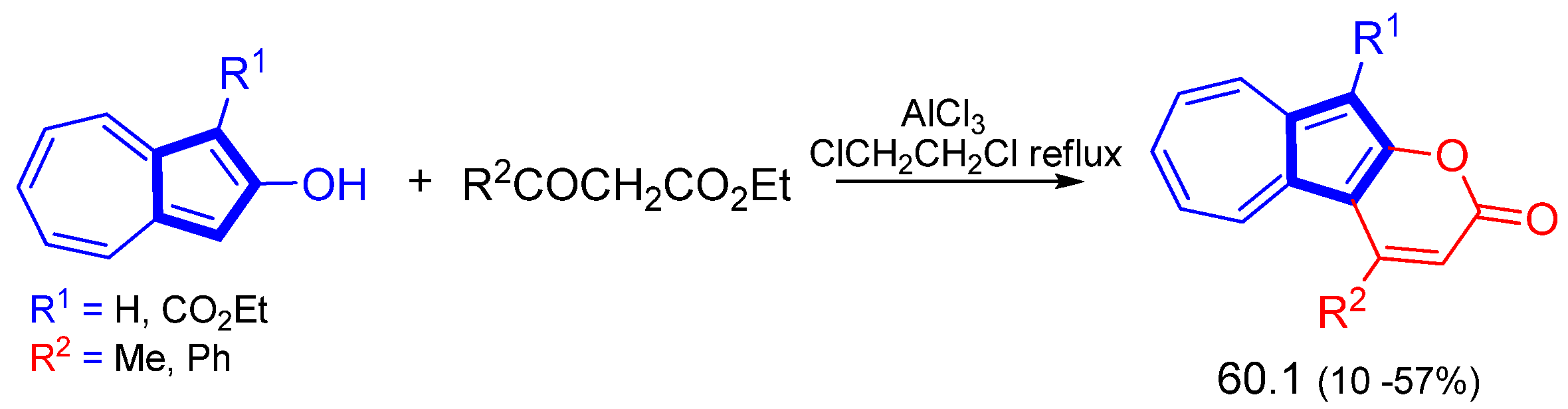





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razus, A.C. Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring. Symmetry 2023, 15, 310. https://doi.org/10.3390/sym15020310
Razus AC. Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring. Symmetry. 2023; 15(2):310. https://doi.org/10.3390/sym15020310
Chicago/Turabian StyleRazus, Alexandru C. 2023. "Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring" Symmetry 15, no. 2: 310. https://doi.org/10.3390/sym15020310
APA StyleRazus, A. C. (2023). Azulene, Reactivity, and Scientific Interest Inversely Proportional to Ring Size; Part 1: The Five-Membered Ring. Symmetry, 15(2), 310. https://doi.org/10.3390/sym15020310





