Organ Patterning at the Shoot Apical Meristem (SAM): The Potential Role of the Vascular System
Abstract
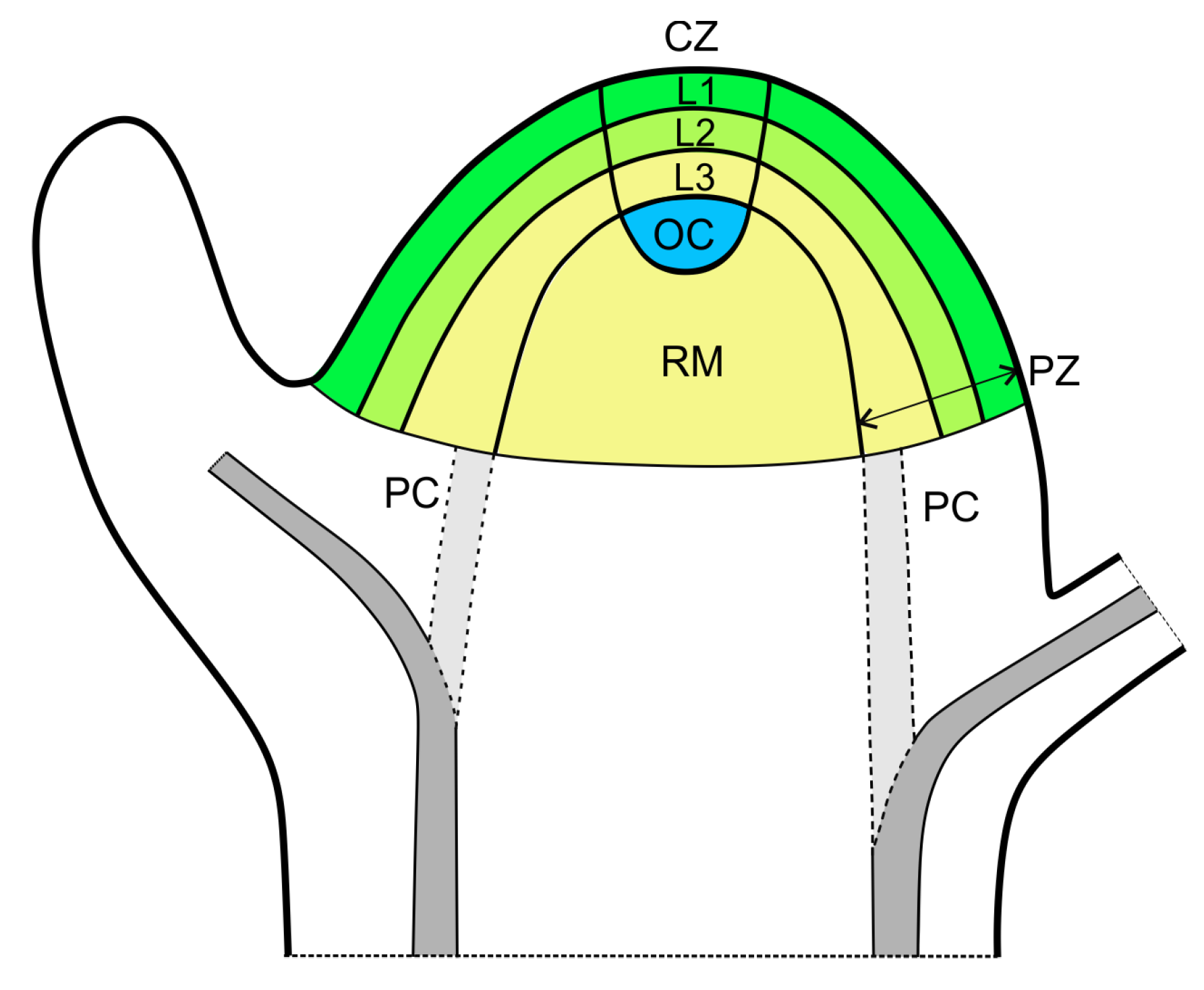
1. Introduction
2. Two Main Historical Views on the Regulation of Phyllotactic Pattern Formation
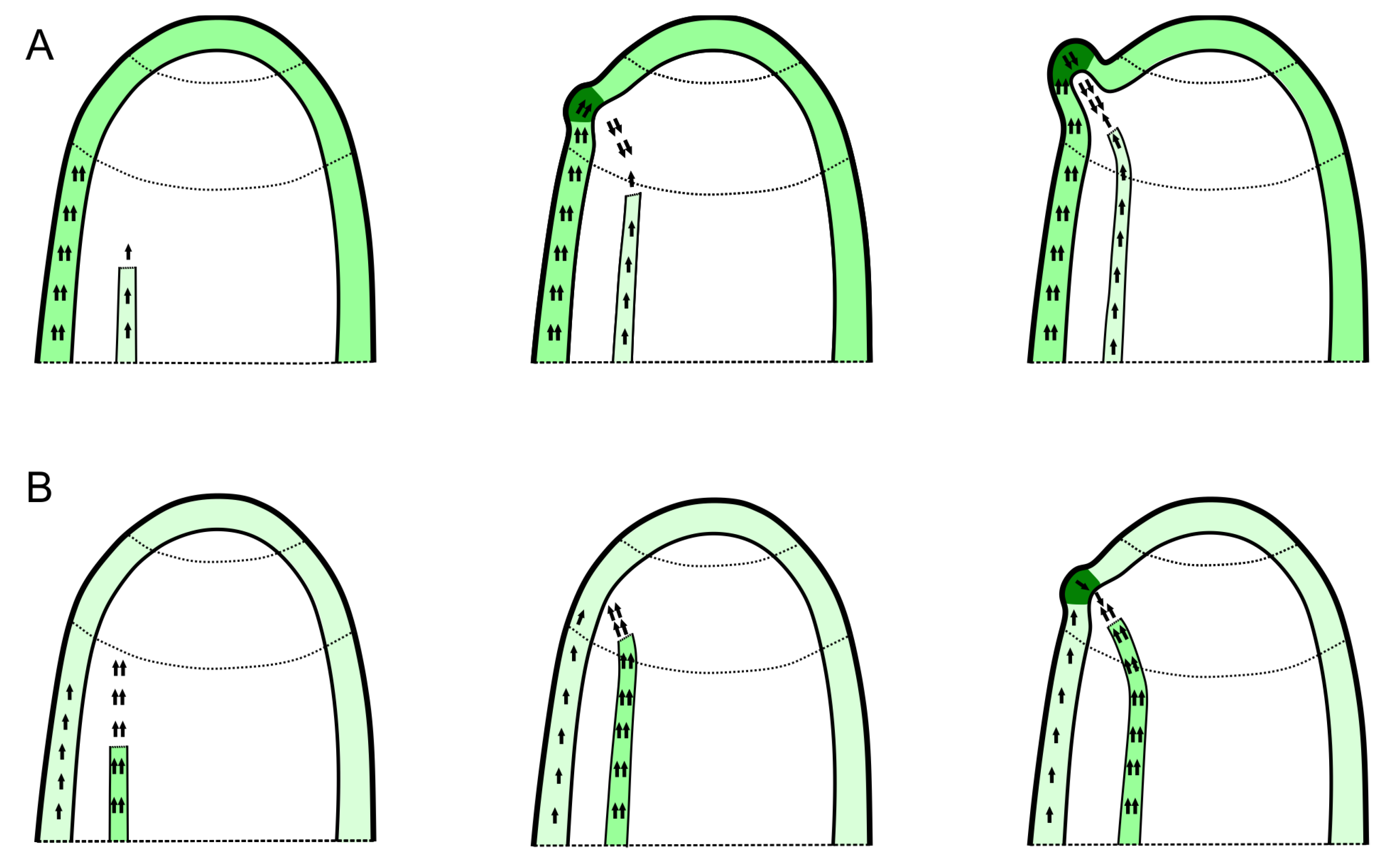
3. Potential Role of the Interactions between Organ Primordia in Phyllotactic Pattern Formation
4. Possibility of Vascular System as an Additional Source of the Signals Interacting with the Superficial PAT
5. Relationship between Vascular System and Organ Positioning in Other Major Groups of Land Plants
5.1. Gymnosperms
5.2. Ferns
5.3. Lycophytes
5.4. Bryophytes
6. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soyars, C.L.; James, S.R.; Nimchuk, Z.L. Ready, aim, shoot: Stem cell regulation of the shoot apical meristem. Curr. Opin. Plant Biol. 2016, 29, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Vernoux, T. Patterning at the shoot apical meristem and phyllotaxis. Curr. Top. Dev. Biol. 2019, 131, 81–107. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Reinhardt, D. Phyllotaxis—A new chapter in an old tale about beauty and magic numbers. Curr. Opin. Plant Biol. 2005, 8, 487–493. [Google Scholar] [CrossRef]
- Kuhlemeier, C. Phyllotaxis. Trends Plant Sci. 2007, 12, 143–150. [Google Scholar] [CrossRef]
- Snow, M.; Snow, R. Experiments on phyllotaxis. I. The effect of isolating a primordium. Philos. Trans. R. Soc. Lond. Ser. B 1931, 222, 353–400. [Google Scholar]
- Snow, M.; Snow, R. Experiments on phyllotaxis. II. The effect of displacing a primordium. Philos. Trans. R. Soc. Lond. Ser. B 1933, 220, 1–43. [Google Scholar]
- Schwabe, W.W. Phyllotaxis. In Positional Controls in Plant Development; Barlow, P.W., Carr, D.J., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 403–440. [Google Scholar]
- Jean, R.V. Phyllotaxis: A Systemic Study in Plants Morphogenesis; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Adler, I.; Barabé, D.; Jean, R.V. A history of the study of phyllotaxis. Ann. Bot. 1997, 80, 231–244. [Google Scholar] [CrossRef]
- Reinhardt, D.; Gola, E.M. Law and order in plants—The origin and functional relevance of phyllotaxis. Trends Plant Sci. 2022, 27, 1017–1032. [Google Scholar] [CrossRef]
- Yin, X. Phyllotaxis: From classical knowledge to molecular genetics. J. Plant Res. 2021, 134, 373–401. [Google Scholar] [CrossRef] [PubMed]
- Dengler, N.G. The shoot apical meristem and development of vascular architecture. Can. J. Bot. 2006, 84, 1660–1671. [Google Scholar] [CrossRef]
- Hofmeister, W. Allgemeine Morphologie der Gewachse. In Handbuch der Physiologischen Botanik; Bary, A., Irmisch, T.H., Sachs, J., Eds.; Engelmann: Leipzig, Germany, 1868; pp. 405–664. [Google Scholar]
- Snow, M.; Snow, R. Experiments on phyllotaxis. III. Diagonal split through decussate apices. Philos. Trans. R. Soc. Lond. Ser. B 1935, 225, 63–94. [Google Scholar]
- Snow, M.; Snow, R. Auxin and leaf formation. New Phytol. 1937, 36, 1–18. [Google Scholar] [CrossRef]
- Snow, M.; Snow, R. On the determination of leaves. New Phytol. 1947, 46, 5–19. [Google Scholar] [CrossRef]
- Wardlaw, C.W. Further experimental observations on the shoot apex of Dryopteris aristata Druce. Philos. Trans. R. Soc. Lond. Ser. B 1949, 233, 415–451. [Google Scholar]
- Mitchison, G.J. Phyllotaxis and the Fibonacci series. Science 1977, 196, 270–275. [Google Scholar] [CrossRef]
- Williams, R.F.; Brittain, E.G. A geometrical model of phyllotaxis. Austr. J. Bot. 1984, 32, 43–72. [Google Scholar] [CrossRef]
- Green, P.B.; Steele, C.; Rennich, S.C. Phyllotactic patterns: A biophysical mechanism for their origin. Ann. Bot. 1996, 77, 515–527. [Google Scholar] [CrossRef]
- Young, D.A. On the diffusion theory of phyllotaxis. J. Theor. Biol. 1978, 71, 421–432. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley: New York, NY, USA, 1965. [Google Scholar]
- Esau, K. Vascular Differentiation in Plants; Holt, Rinehart, Winston: New York, NY, USA, 1965. [Google Scholar]
- Girolami, G. Relation between phyllotaxis and primary vascular organization in Linum. Amer. J. Bot. 1953, 40, 618–625. [Google Scholar] [CrossRef]
- Larson, P.R. Development and organization of the primary vascular system in Populus deltoides according to phyllotaxy. Am. J. Bot. 1975, 62, 1084–1099. [Google Scholar] [CrossRef]
- Larson, P.R. Phyllotactic transitions in the vascular system of Populus deltoides Bartr. as determined by 14C labeling. Planta 1977, 134, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.R. Establishment of the vascular system in seedlings of Populus deltoides Bartr. Am. J. Bot. 1979, 66, 452–462. [Google Scholar] [CrossRef]
- Larson, P.R. Interrelations between phyllotaxis, leaf development and the primary-secondary vascular transition in Populus deltoides. Ann. Bot. 1980, 46, 757–769. [Google Scholar] [CrossRef]
- Namboodiri, K.K.; Beck, C.B. A comparative study of the primary vascular system of conifers. I. Genera with helical phyllotaxis. Am. J. Bot. 1968, 55, 447–457. [Google Scholar] [CrossRef]
- Namboodiri, K.K.; Beck, C.B. A comparative study of the primary vascular system of conifers. II. Genera with opposite and whorled phyllotaxis. Am. J. Bot. 1968, 55, 458–463. [Google Scholar] [CrossRef]
- Wardlaw, C.W. The organization of the shoot apex. In Encyclopedia of Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1965; Volume 15, pp. 966–1076. [Google Scholar]
- Steeves, T.A.; Sussex, I.A. Patterns in Plant Development; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Ball, E. The effects of synthetic growth substances on the shoot apex of Tropaeolum majus L. Am. J. Bot. 1944, 31, 316–327. [Google Scholar] [CrossRef]
- Meicenheimer, R.D. Changes in Epilobium phyllotaxy induced by N-1-naphthylphthalamic acid and α-4-chlorophenoxyisobutyric acid. Am. J. Bot. 1981, 68, 1139–1154. [Google Scholar] [CrossRef]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef]
- Mattsson, J.; Sung, Z.R.; Berleth, T. Responses of plant vascular systems to auxin transport inhibition. Development 1999, 126, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Besnard, F.; Refahi, Y.; Morin, V.; Marteaux, B.; Brunoud, G.; Chambrier, P.; Rozier, F.; Mirabet, V.; Legrand, J.; Lainé, S.; et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2014, 505, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Heisler, M.G.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Guyomarch, S.; Mandel, T.; Reinhardt, D.; Kuhlemeier, C.; Prusinkiewicz, P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA 2006, 103, 1301–1306. [Google Scholar] [CrossRef]
- Jönsson, H.; Heisler, M.G.; Shapiro, B.E.; Meyerowitz, E.M.; Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis Proc. Natl. Acad. Sci. USA 2006, 103, 1633–1638. [Google Scholar] [CrossRef]
- Galvan-Ampudia, C.S.; Cerutti, G.; Legrand, J.; Brunoud, G.; Martin-Arevalillo, R.; Azais, R.; Bayle, V.; Moussu, S.; Wenzl, C.; Jaillais, Y.; et al. Temporal integration of auxin information for the regulation of patterning. eLife 2020, 9, e55832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cieslak, M.; Owens, A.; Wang, F.; Broholm, S.K.; Teeri, T.H.; Elomaa, P.; Prusinkiewicz, P. Phyllotactic patterning of gerbera flower heads. Proc. Natl. Acad. Sci. USA 2021, 118, e2016304118. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Zhang, T.; Owens, A.; Cieslak, M.; Elomaa, P. Phyllotaxis without symmetry: What can we learn from flower heads? J. Exp. Bot. 2022, 73, 3319–3329. [Google Scholar] [CrossRef]
- Rutishauser, R. Acacia (wattle) and Cananga (ylang-ylang): From spiral to whorled and irregular (chaotic) phyllotactic patterns—A pictorial report. Acta Soc. Bot. Pol. 2016, 85, 3531. [Google Scholar] [CrossRef]
- Bayer, E.M.; Smith, R.S.; Mandel, T.; Nakayama, N.; Sauer, M.; Prusinkiewicz, P.; Kuhlemeier, C. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 2009, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.P.; Barbier de Reuille, P.; Kuhlemeier, C. Toward a 3D model of phyllotaxis based on a biochemically plausible auxin transport mechanism. PLoS Comput. Biol. 2019, 15, e1006896. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.; Sasayama, D.; Cho, H.T. The M3 phosphorylation site is required for trafficking and biological roles of PIN-FORMED1, 2, and 7 in Arabidopsis. Front. Plant Sci. 2016, 7, 1479. [Google Scholar] [CrossRef]
- Jesuthasan, S.; Green, P.B. On the mechanism of decussate phyllotaxis: Biophysical studies on the tunica layer of Vinca major. Amer. J. Bot. 1989, 76, 1152–1166. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jönsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Heisler, M.G.; Hamant, O.; Krupinski, P.; Uyttewaal, M.; Ohno, C.; Jönsson, H.; Traas, J.; Meyerowitz, E.M. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010, 8, e1000516. [Google Scholar] [CrossRef]
- Stoma, S.; Lucas, M.; Chopard, J.; Schaedel, M.; Traas, J.; Godin, C. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput. Biol. 2008, 4, e1000207. [Google Scholar] [CrossRef]
- Merks, R.M.; Van de Peer, Y.; Inzé, D.; Beemster, G.T. Canalization without flux sensors: A traveling-wave hypothesis. Trends Plant Sci. 2007, 12, 384–390. [Google Scholar] [CrossRef]
- Wabnik, K.; Kleine-Vehn, J.; Balla, J.; Sauer, M.; Naramoto, S.; Reinöhl, V.; Merks, R.M.; Govaerts, W.; Friml, J. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol Syst. Biol. 2010, 6, 447. [Google Scholar] [CrossRef]
- Cieslak, M.; Runions, A.; Prusinkiewicz, P. Auxin-driven patterning with unidirectional fluxes. J. Exp. Bot. 2015, 66, 5083–5102. [Google Scholar] [CrossRef]
- Cieslak, M.; Owens, A.; Prusinkiewicz, P. Computational Models of Auxin-Driven Patterning in Shoots. Cold Spring Harb. Perspect. Biol. 2021, 14, a040097. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Bozorg, B.; Larsson, A.; Ohno, C.; Jönsson, H.; Heisler, M.G. Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Curr. Biol. 2016, 26, 3202–3208. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Lenhard, M.; Smith, R.; Kuhlemeier, C. Interaction between meristem tissue layers controls phyllotaxis. Dev. Cell 2013, 26, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Deb, Y.; Marti, D.; Frenz, M.; Kuhlemeier, C.; Reinhardt, D. Phyllotaxis involves auxin drainage through leaf primordia. Development 2015, 142, 1992–2001. [Google Scholar] [CrossRef]
- Banasiak, A. Putative dual pathway of auxin transport in organogenesis of Arabidopsis. Planta 2011, 233, 49–61. [Google Scholar] [CrossRef]
- Banasiak, A.; Biedroń, M.; Dolzblasz, A.; Berezowski, M.A. Ontogenetic changes in auxin biosynthesis and distribution determine the organogenic activity of the shoot apical meristem in pin1 mutants. Int. J. Mol. Sci. 2019, 20, 180. [Google Scholar] [CrossRef]
- Avsian-Kretchmer, O.; Cheng, J.C.; Chen, L.; Moctezuma, E.; Renee Sung, Z. Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol. 2002, 130, 199–209. [Google Scholar] [CrossRef]
- Kwiatkowska, D. The relationships between the primary vascular system and phyllotactic patterns of Anagallis arvensis (Primulaceae). Amer. J. Bot. 1992, 79, 904–913. [Google Scholar] [CrossRef]
- Kwiatkowska, D. Ontogenetic changes in the shoot primary vasculature of Anagallis arvensis L. Acta Soc. Bot. Pol. 1995, 64, 213–222. [Google Scholar] [CrossRef]
- Kang, J.; Tang, J.; Donnelly, P.; Dengler, N. Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytol. 2003, 158, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Lavania, D.; Linh, N.M.; Scarpella, E. Of Cells, Strands, and Networks: Auxin and the Patterned Formation of the Vascular System. Cold Spring Harb. Perspect. Biol. 2021, 13, a039958. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Bayer, E.M. Auxin transport-feedback models of patterning in plants. Plant Cell Environ. 2009, 32, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Runions, A.; Sluis, A.; Bragg, J.; Vogel, J.P.; Prusinkiewicz, P.; Hake, S. A division in PIN-mediated auxin patterning during organ initiation in grasses. PLoS Comput. Biol. 2014, 10, e1003447. [Google Scholar] [CrossRef]
- Verna, C.; Ravichandran, S.J.; Sawchuk, M.G.; Linh, N.M.; Scarpella, E. Coordination of tissue cell polarity by auxin transport and signaling. eLife 2019, 8, e51061. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, X.; De Storme, N.; Jensen, K.H.; Xu, Q.; Yang, J.; Liu, X.; Chen, S.; Martens, H.J.; Schulz, A.; et al. Directionality of plasmodesmata-mediated transport in Arabidopsis leaves supports auxin channeling. Curr. Biol. 2020, 30, 1970–1977.e4. [Google Scholar] [CrossRef]
- Band, L.R. Auxin fluxes through plasmodesmata. New Phytol. 2021, 231, 1686–1692. [Google Scholar] [CrossRef]
- Han, X.; Hyun, T.K.; Zhang, M.; Kumar, R.; Koh, E.J.; Kang, B.H.; Lucas, W.J.; Kim, J.Y. Auxin-callose-mediated plasmodesmalgating is essential for tropic auxin gradient formation and signaling. Dev. Cell 2014, 28, 132–146. [Google Scholar] [CrossRef]
- Sager, R.; Wang, X.; Hill, K.; Yoo, B.C.; Caplan, J.; Nedo, A.; Tran, T.; Bennett, M.J.; Lee, J.Y. Auxin-dependent control of a plasmodesmal regulator creates a negative feedback loop modulating lateral root emergence. Nat. Commun. 2020, 11, 364. [Google Scholar] [CrossRef]
- Rutishauser, R. Polymerous leaf whorls in vascular plants: Developmental morphology and fuzziness or organ identities. Int. J. Plant Sci. 1999, 160 (Suppl. S6), S81–S103. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Elton, S.; Ticchiarelli, F.; Hsia, M.M.; Vogel, J.P.; Leyser, O. Cross-species functional diversity within the PIN auxin efflux protein family. eLife 2017, 6, e31804. [Google Scholar] [CrossRef] [PubMed]
- Palovaara, J.; Hallberg, H.; Stasolla, C.; Luit, B.; Hakman, I. Expression of a gymnosperm PIN homologous gene correlates with auxin immunolocalization pattern at cotyledon formation and in demarcation of the procambium during Picea abies somatic embryo development and in seedling tissues. Tree Phys. 2010, 30, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Hakman, I.; Hallberg, H.; Palovaraa, J. The polar auxin transport inhibitor NPA impairs embryo morphology and increases expression of an auxin efflux facilitator protein PIN during Picea abies somatic embryo development. Tree Phys. 2009, 29, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Holloway, D.M.; Rozada, I.; Bray, J.J.H. Two-stage patterning dynamics in conifer cotyledon whorl morphogenesis. Ann. Bot. 2018, 121, 525–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodriguez, L.; Li, L.; Zhang, X.; Friml, J. Functional innovations of PIN auxin transporters mark crucial evolutionary transitions during rise of flowering plants. Sci. Adv. 2020, 6, eabc8895. [Google Scholar] [CrossRef]
- Parke, R.V. Growth periodicity and the shoot tip of Abies concolor. Am. J. Bot. 1959, 46, 110–119. [Google Scholar] [CrossRef]
- Zagórska-Marek, B. Phyllotactic patterns and transitions in Abies balsamea. Can. J. Bot. 1985, 63, 1844–1854. [Google Scholar] [CrossRef]
- Zagórska-Marek, B.; Banasiak, A. Related to phyllotaxis interlocked systems of vascular sympodia and cortical resin canals in Abies and Picea shoots. Acta Soc. Bot. Pol. 2000, 69, 165–172. [Google Scholar] [CrossRef]
- Banasiak, A.; Zagórska-Marek, B. Signals flowing from mature tissues to SAM determine the phyllotactic continuity in successive annual increments of the conifer shoot. Acta Soc. Bot. Pol. 2006, 75, 113–121. [Google Scholar] [CrossRef]
- Bierhorst, D.W. On the stem apex, leaf initiation and early leaf ontogeny in filicalean ferns. Amer. J. Bot. 1977, 64, 125–152. [Google Scholar] [CrossRef]
- Gola, E.; Banasiak, A. Diversity of phyllotaxis in land plants in reference to the shoot apical meristem structure. Acta Soc. Bot. Pol. 2016, 85, 3529. [Google Scholar] [CrossRef]
- Wetmore, R.H.; Pratt, C. The growth and auxin relations of leaves of the maidenhair fern, Adiantum pedatum L. Am. J. Bot. 1949, 36, 806. [Google Scholar]
- Steeves, T.A.; Briggs, W.R. Morphogenetic studies on Osmunda cinnamomea L.—The origin and early development of vegetative fronds. Phytomorphology 1958, 8, 60–72. [Google Scholar]
- Steeves, T.A.; Briggs, W.R. Morphogenetic studies on Osmunda cinnamomea L. The auxin relationships of expanding fronds. J. Exp. Bot. 1960, 11, 45–67. [Google Scholar] [CrossRef]
- Steeves, T.A. On the determination of leaf primordia in ferns. In Trends in Plant Morphogenesis; Cutter, E.G., Ed.; Longmans, Green and Co. Ltd.: London, UK, 1967; pp. 200–219. [Google Scholar]
- Steeves, T.; Hicks, G.; Steeves, M.; Retalla, K.B. Leaf determination in the fern Osmunda cinnamomea—A reinvestigation. Ann. Bot. 1993, 71, 511–517. [Google Scholar] [CrossRef]
- Wardlaw, C.W. The comparative investigation of apices of vascular plants by experimental methods. Philos. Trans. R. Soc. Lond. Ser. B 1950, 234, 583–604. [Google Scholar]
- Voeller, B.R. Regulation of “fiddlehead” uncoiling in ferns. Naturwissenschaften 1960, 47, 70–71. [Google Scholar] [CrossRef]
- Bennett, T.A.; Liu, M.M.; Aoyama, T.; Bierfreund, N.M.; Braun, M.; Coudert, Y.; Dennis, R.J.; O’Connor, D.; Wang, X.Y.; White, C.D.; et al. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 2014, 24, 2776–2785. [Google Scholar] [CrossRef]
- Bennett, T.A. PIN proteins and the evolution of plant development. Trends Plant Sci. 2015, 20, 498–507. [Google Scholar] [CrossRef]
- Wardlaw, C.W.; Cutter, E.G. Experimental and analytical studies of Pteridophytes. XXXI. The effect of shallow incisions on organogenesis in Dryopteris aristata Druce. Ann. Bot. 1948, 20, 39–56. [Google Scholar] [CrossRef]
- Ma, Y.; Steeves, T.A. Auxin effects on vascular differentiation in Ostrich fern. Ann. Bot. 1992, 70, 277–282. [Google Scholar] [CrossRef]
- Ma, Y.; Steeves, T.A. Vascular differentiation in the shoot apex of Matteuccia struthiopteris. Ann. Bot. 1994, 74, 573–585. [Google Scholar] [CrossRef]
- Imaichi, R. Meristem organization and organ diversity. In Biology and Evolution of Ferns and Lycophytes; Ranker, T.A., Haufler, C.H., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 75–106. [Google Scholar]
- Harrison, C.J.; Rezvani, M.; Langdale, J.A. Growth from two transient apical initials in the meristem of Selaginella kraussiana. Development 2007, 134, 881–889. [Google Scholar] [CrossRef]
- Frank, M.H.; Edwards, M.B.; Schultz, E.R.; McKain, M.R.; Fei, Z.; Sørensen, I.; Rose, J.K.C.; Scanlon, M.J. Dissecting the molecular signatures of apical cell-type shoot meristems from two ancient land plant lineages. New Phytol. 2015, 207, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Sanders, H.L.; Langdale, J.A. Conserved transport mechanisms but distinct auxin responses govern shoot patterning in Selaginella kraussiana. New Phytol. 2013, 198, 419–428. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, L.; Zhang, C.; Hao, P.; Jing, X.; Li, X. Global transcriptome analysis reveals extensive gene remodeling, alternative splicing and differential transcription profiles in non-seed vascular plant Selaginella moellendorffii. BMC Genom. 2017, 18 (Suppl. 1), e1042. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.; Efroni, I.; Rahni, R.; Birnbaum, K.D. The Selaginella rhizophore has a unique transcriptional identity compared with root and shoot meristems. New Phytol. 2019, 222, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Gifford, E.M.; Foster, A.S. Morphology and Evolution of Vascular Plants, 3rd ed.; Freeman: New York, NY, USA, 1989. [Google Scholar]
- Jacobs, W.P. Development of procambium, xylem, and phloem in the shoot apex of Selaginella. Bot. Gaz. 1988, 149, 64–70. [Google Scholar] [CrossRef]
- Gola, E.M.; Jernstedt, J.A.; Zagórska-Marek, B. Vascular architecture in shoots of early divergent vascular plants, Lycopodium clavatum L. and Lycopodium annotinum L. New Phytol. 2007, 107, 774–786. [Google Scholar] [CrossRef]
- Tomescu, A.M.F. The stele—A developmental perspective on the diversity and evolution of primary vascular architecture. Biol. Rev. 2021, 96, 1263–1283. [Google Scholar] [CrossRef]
- Glime, J. Bryophyte Ecology. 2021. Available online: https://digitalcommons.mtu.edu/oabooks/4 (accessed on 5 December 2022).
- Crum, H. Structural Diversity of Bryophytes; University of Michigan Herbarium: Ann Arbor, MI, USA, 2001. [Google Scholar]
- Lin, W.; Wang, Y.; Coudert, Y.; Kierzkowski, D. Leaf morphogenesis: Insights from the moss Physcomitrium patens. Front. Plant Sci. 2021, 12, 736212. [Google Scholar] [CrossRef]
- Véron, E.; Vernoux, T.; Coudert, Y. Phyllotaxis from a single apical cell. Trends Plant Sci. 2021, 26, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Marek, B.; Sokołowska, K.; Turzańska, M. Chiral events in developing gametophores of Physcomitrella patens and other moss species are driven by an unknown, universal direction-sensing mechanism. Am. J. Bot. 2018, 105, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Kamamoto, N.; Tano, T.; Fujimoto, K.; Shimamura, M. Rotation angle of stem cell division plane controls spiral phyllotaxis in mosses. J. Plant Res. 2021, 134, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Kozgunova, E.; Yoshida, M.W.; Reski, R.; Goshima, G. Spindle motility skews division site determination during asymmetric cell division in Physcomitrella. Nat. Commun. 2022, 13, 2488. [Google Scholar] [CrossRef] [PubMed]
- Sztein, A.E.; Cohen, J.D.; de la Fuente, I.G.; Cooke, T.J. Auxin metabolism in mosses and liverworts. Am. J. Bot. 1999, 86, 1544–1555. [Google Scholar] [CrossRef]
- Sztein, A.E.; Cohen, J.D.; Cooke, T.J. Evolutionary patterns in the auxin metabolism in green plants. Int. J. Plant Sci. 2000, 161, 849–885. [Google Scholar] [CrossRef]
- Harrison, C.J.; Roeder, A.H.K.; Meyerowitz, E.M.; Langdale, J.A. Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr. Biol. 2009, 19, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Coudert, Y.; Palubicki, W.; Ljung, K.; Novak, O.; Leyser, O.; Harrison, C.J. Three ancient hormonal cues co-ordinate shoot branching in a moss. eLife 2015, 4, e06808. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.D.; Thelander, M.; Landberg, K.; Staldal, V.; Nilsson, A.; Valsecchi, J.M.; Pederson, E.R.; Kowalczyk, M.; Ljung, K.; Ronne, H.; et al. Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development 2010, 137, 1275–1284. [Google Scholar] [CrossRef]
- Landberg, K.; Simura, J.; Ljung, K.; Sundberg, E.; Thelander, M. Studies of moss reproductive development indicate that auxin biosynthesis in apical stem cells may constitute an ancestral function for focal growth control. New Phytol. 2021, 229, 845–860. [Google Scholar] [CrossRef]
- Viaene, T.; Delwiche, C.F.; Rensing, S.A.; Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Viaene, T.; Landberg, K.; Thelander, M.; Medvecka, E.; Pederson, E.; Feraru, E.; Cooper, E.D.; Karimi, M.; Delwiche, C.F.; Ljung, K.; et al. Directional auxin transport mechanisms in early diverging land plant. Curr. Biol. 2014, 24, 2786–2791. [Google Scholar] [CrossRef]
- Mohanasundaram, B.; Bhide, A.J.; Palit, S.; Chaturvedi, G.; Lingwan, M.; Masakapalli, S.K.; Banerjee, A.K. The unique bryophyte-specific repeat-containing protein SHORT-LEAF regulates gametophore development in moss. Plant Physiol. 2021, 187, 203–217. [Google Scholar] [CrossRef]
- Sokołowska, K.; Turzańska, M.; Nilsson, M.-C. Symplasmic and apoplasmic transport inside feather moss stems of Pleurozium schreberi and Hylocomium splendens. Ann. Bot. 2017, 120, 805–817. [Google Scholar] [CrossRef]
- Regmi, K.C.; Li, L.; Gaxiola, R.A. Alternate modes of photosynthate transport in the alternating generations of Physcomitrella patens. Front. Plant Sci. 2017, 8, 1956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasiak, A.; Gola, E.M. Organ Patterning at the Shoot Apical Meristem (SAM): The Potential Role of the Vascular System. Symmetry 2023, 15, 364. https://doi.org/10.3390/sym15020364
Banasiak A, Gola EM. Organ Patterning at the Shoot Apical Meristem (SAM): The Potential Role of the Vascular System. Symmetry. 2023; 15(2):364. https://doi.org/10.3390/sym15020364
Chicago/Turabian StyleBanasiak, Alicja, and Edyta M. Gola. 2023. "Organ Patterning at the Shoot Apical Meristem (SAM): The Potential Role of the Vascular System" Symmetry 15, no. 2: 364. https://doi.org/10.3390/sym15020364
APA StyleBanasiak, A., & Gola, E. M. (2023). Organ Patterning at the Shoot Apical Meristem (SAM): The Potential Role of the Vascular System. Symmetry, 15(2), 364. https://doi.org/10.3390/sym15020364






