Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a Series of Isophthalamides
Abstract
Highlights
- A complete series of structures differing from H-DIP to I-DIP.
- Rationalizing the Cl-DIP structure (Z’ = 3) with two distinct conformations.
- The great wall of bromines at the 2D sheet interfaces in Br-DIP.
- Comparison of organic halogenated materials Br-DIP and the inorganic PbBr2, CuBr2.
- The unusual synthon formed by the tightly bound hydrate in I-DIP•½(H2O).
Simple Summary
Abstract
1. Introduction
2. Experimental
2.1. Materials and Characterisation
2.2. Synthetic Procedures (1 and 2)
3. Methods
4. Results and Discussion
4.1. Crystal and Molecular Structural Analysis of H-DIP
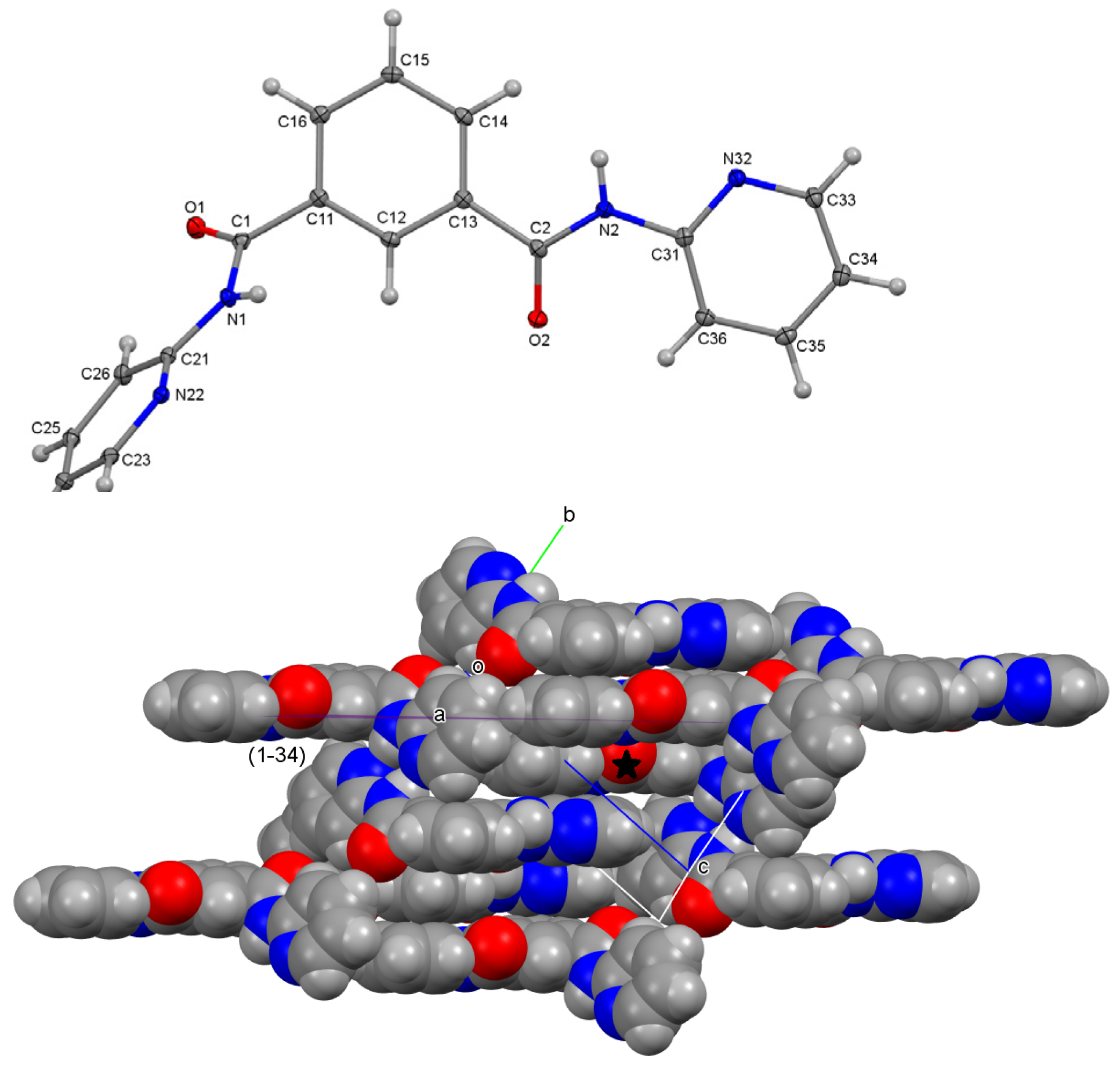
4.2. H-DIP: Short C-H⋯π(Arene) Contacts and Tight Ring⋯Ring Stacking
4.3. F-DIP

4.4. Cl-DIP: A Crystal Structure (Z’ = 3) with Two Different Conformations


4.5. Br-DIP: A Wall of Bromine Atoms at the 2D Sheet Interfaces


4.6. I-DIP•½(H2O): The N⋯I, I⋯I Halogen Bonding and Role of the Water Molecule

4.7. How Unusual Is the Water Environment in I-DIP•½(H2O)?
4.8. CSD Analyses with the 2021.3.0 Version (January 2023)
5. Comparisons with Related Structures
6. Crystallographic Structural Summary
7. Fingerprint and Contact Analysis
8. Contacts Statistics on the Hirshfeld Surface

9. Enriched Halogen···Halogen Contacts
10. Structure Optimization—Ab Initio Calculations
11. Conformational Analysis
12. Conclusions, Insights and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constable, E.; Parkin, G.; Que, L. (Eds.) Comprehensive Coordination Chemistry III, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780081026885. [Google Scholar]
- Stradiotto, M.; Lundgren, R.J. Ligand Design in Metal Chemistry; J. Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Storr, T. (Ed.) Ligand Design in Medicinal Inorganic Chemistry; J. Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Silverman, R.B.; Holladay, M.W. The Organic Chemistry of Drug Design and Drug Action, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Malone, J.F.; Murray, C.M.; Dolan, G.M.; Docherty, R.; Lavery, A.J. Intermolecular Interactions in the Crystal Chemistry of N,N‘-Diphenylisophthalamide, Pyridine-2,6-dicarboxylic Acid Bisphenylamide, and Related Compounds. Chem. Mater. 1997, 9, 2983–2989. [Google Scholar] [CrossRef]
- Gondi, S.R.; Son, D.R. Synthesis of N,N′-bis(2-Thiazolinyl)-, N,N′-bis(2-Thiazolyl)-, and N,N′-bis(2-Pyrimidinyl)-Benzene Dicarboxamides. Synth. Commun. 2004, 34, 3061–3072. [Google Scholar] [CrossRef]
- Odago, M.O.; Hoffman, A.E.; Carpenter, R.L.; Chi Tak Tse, D.; Sun, S.S.; Lees, A.J. Thioamide, urea and thiourea bridged rhenium(I) complexes as luminescent anion receptors. Inorg. Chim. Acta 2011, 374, 558–565. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, X.; Hu, J.L.; Li, Z.T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Ferrand, Y.; Huc, I. Designing Helical Molecular Capsules Based on Folded Aromatic Amide Oligomers. Acc. Chem. Res. 2018, 51, 970–977. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The use and misuse of van der Waals radii. Struct. Chem. 2021, 32, 623–629. [Google Scholar] [CrossRef]
- McMahon, J.; Gallagher, J.F.; Anderson, F.P.; Lough, A.J. A structural systematic study of four isomers of difluoro-N-(3-pyridyl)benzamide. Acta Crystallogr. 2009, C65, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Mocilac, P.; Tallon, M.; Lough, A.J.; Gallagher, J.F. Synthesis, structural and conformational analysis of a 3 × 3 isomer grid based on nine methyl-N-(pyridyl)benzamides. CrystEngComm 2010, 12, 3080–3090. [Google Scholar] [CrossRef][Green Version]
- Mocilac, P.; Donnelly, K.; Gallagher, J.F. Structural systematics and conformational analyses of a 3 × 3 isomer grid of fluoro-N-(pyridyl)benzamides: Physicochemical correlations, polymorphism and isomorphous relationships. Acta Crystallogr. 2012, B68, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.F.; Farrell, M.; Hehir, N.; Mocilac, P.; Aubert, E.; Espinosa, E.; Guillot, B.; Jelsch, C. At the Interface of Isomorphous Behavior in a 3 × 3 Isomer Grid of Monochlorobenzamides: Analyses of the Interaction Landscapes via Contact Enrichment Studies. Cryst. Growth Des. 2019, 19, 6141–6158. [Google Scholar] [CrossRef]
- Mocilac, P.; Gallagher, J.F. Monohalogenated carbamates where hydrogen bonding rules without halogen bonding: Is there a link between poor carbamate crystal growth and Z′ > 1? CrystEngComm 2019, 21, 4048–4062. [Google Scholar] [CrossRef]
- Khavasi, H.R.; Tehrani, A.A. Effect of halogen bonding interaction on supramolecular assembly of halogen-substituted phenylpyrazinamides. CrystEngComm 2013, 15, 3222–3235. [Google Scholar] [CrossRef]
- Abeysekera, A.M.; Day, V.W.; Sinha, A.S.; Aakeröy, C.B. Mapping out the Relative Influence of Hydrogen and Halogen Bonds in Crystal Structures of a Family of Amide-Substituted Pyridines. Cryst. Growth Des. 2020, 20, 7399–7410. [Google Scholar] [CrossRef]
- Dasgupta, M.; Nag, S.; Das, G.; Nethaji, M.; Bhattacharya, S. N,N′-Bis(aryl)pyridine-2,6-dicarboxamide complexes of ruthenium: Synthesis, structure and redox properties. Polyhedron 2008, 27, 139–150. [Google Scholar] [CrossRef]
- Møller, M.S.; Liljedahl, M.C.; McKee, V.; McKenzie, C.J. Solid phase nitrosylation of enantiomeric cobalt(II) complexes. Chemistry 2021, 3, 585–597. [Google Scholar] [CrossRef]
- Osman, I.A. Chapter 3. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2021. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. 3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. 20. Basis Set for Correlated Wave-Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Guillot, B.; Enrique, E.; Huder, L.; Jelsch, C. MoProViewer: A tool to study proteins from a charge density science perspective. Acta Crystallogr. 2014, C70, 279. [Google Scholar] [CrossRef]
- Czerny, F.; Döhlert, P.; Weidauer, M.; Irran, E.; Enthaler, S. Synthesis, characterization and application of nickel(II) complexes modified with N,N′,N″-pincer ligands. Inorg. Chim. Acta 2015, 425, 118–123. [Google Scholar] [CrossRef]
- Meyer, K.; Dalebrook, A.F.; Wright, L.J. Selective palladation of a large (32 ring atom) macrocyclic ligand at a bis(N-heterocyclic carbene) coordination pocket through transmetallation of the corresponding mercury(ii) derivative. Dalton Trans. 2012, 41, 14059–14067. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, S.S.; Okamura, T.A.; Su, Z.; Ueyama, N. Syntheses and crystal structures of two supramolecular isomers of manganese (II) with 3,5-bis(isonicotinamido) benzoate. J. Coord. Chem. 2009, 62, 2421–2428. [Google Scholar] [CrossRef]
- Ichii, T.; Arikawa, T.; Omoto, K.; Hosono, N.; Sato, H.; Kitagawa, S.; Tanaka, K. Observation of an exotic state of water in the hydrophilic nanospace of porous coordination polymers. Nat. Chem. Commun. 2020, 3, 16. [Google Scholar] [CrossRef]
- Waris, G.; Siddiqi, H.M.; Flörke, U.; Hussain, R.; Butt, M.S. N, N′-Bis (4-bromophenyl) pyridine-2, 6-dicarboxamide. Acta Crystallogr. 2013, E69, o416. [Google Scholar] [CrossRef]
- Gallagher, J.F.; Hehir, N.; Mocilac, P.; Violin, C.; O’Connor, B.F.; Aubert, E.; Espinosa, E.; Guillot, B.; Jelsch, C. Probing the Electronic Properties and Interaction Landscapes in a Series of N-(Chlorophenyl)pyridinecarboxamides. Cryst. Growth Des. 2022, 22, 3343–3358. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Karothu, D.P.; Pejov, L.; Commins, P.; Hu, Q.; Naumov, P. From Mechanical Effects to Mechanochemistry: Softening and Depression of the Melting Point of Deformed Plastic Crystals. J. Am. Chem. Soc. 2020, 142, 11219–11312. [Google Scholar] [CrossRef] [PubMed]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic rings in chemical and biological recognition: Energetics and structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef]
- Jelsch, C.; Soudani, S.; Ben Nasr, C. Likelihood of atom-atom contacts in crystal structures of halogenated organic compounds. IUCrJ 2015, 2, 327–340. [Google Scholar] [CrossRef]
- Jelsch, C.; Bibila Mayaya Bisseyou, Y. Atom interaction propensities of oxygenated chemical functions in crystal packings. IUCrJ 2017, 4, 158–174. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Type II halogen···halogen contacts are halogen bonds. IUCrJ 2014, 1, 5–7. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Parathasarathy, R.; Murray-Rust, P. Angular preferences of intermolecular forces around halogen centers: Preferred directions of approach of electrophiles and nucleophiles around carbon-halogen bond. J. Am. Chem. Soc. 1986, 108, 4308–4314. [Google Scholar] [CrossRef]
- Thalladi, V.R.; Weiss, H.C.; Bläser, D.; Boese, R.; Nangia, A.; Desiraju, G.R. C−H···F Interactions in the Crystal Structures of Some Fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar] [CrossRef]
- Britton, D. 4,4′-diiodobiphenyl. Acta Crystallogr. 2005, E61, o187–o188. [Google Scholar] [CrossRef]
- Lemee, M.H.; Toupet, L.; Delugeard, Y.; Messager, J.C.; Cailleau, H. Crystal structure and thermal-motion analysis of 4,4′-difluorobiphenyl. Acta Crystallogr. 1987, B43, 466–470. [Google Scholar] [CrossRef]
- Chopra, D.; Row, T.G. Evaluation of the interchangeability of C–H and C–F groups: Insights from crystal packing in a series of isomeric fluorinated benzanilides. CrystEngComm 2008, 10, 54–67. [Google Scholar] [CrossRef]
- Okamoto, T.; Reese, C.; Senatore, M.L.; Tang, M.L.; Jiang, Y.; Parkin, S.R.; Bao, Z. 2,9-Dibromopentacene: Synthesis and the role of substituent and symmetry on solid-state order. Synth. Met. 2010, 21, 2447–2451. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. The effect of temperature on interhalogen interactions in a series of isostructural organic systems. New J. Chem. 2014, 38, 897–901. [Google Scholar] [CrossRef]
- Ding, X.; Zahid, E.; Unruh, D.K.; Hutchins, K.M. Differences in thermal expansion and motion ability for herringbone and face-to-face π-stacked solids. IUCrJ 2022, 9, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. A DFT assessment of some physical properties of iodine-centered halogen bonding and other non-covalent interactions in some experimentally reported crystal geometries. Phys. Chem. Chem. Phys. 2018, 20, 15316–15329. [Google Scholar] [CrossRef]
- Shallangwa, G.A.; Uzairu, A.; Oltunji Ajibola, V.; Abba, H. Computational Study of the Mechanism of the Oxidation of Hydrazine/Hydrazinium Ion by Iodine in the Gas Phase. Int. J. Comp. Theor. Chem. 2015, 3, 6–18. [Google Scholar] [CrossRef][Green Version]
- Saccone, M.; Terraneo, G.; Pilati, T.; Cavallo, G.; Priimagi, A.; Metrangolo, P.; Resnati, G. Azobenzene-based difunctional halogen-bond donor: Towards the engineering of photoresponsive co-crystals. Acta Crystallogr. 2014, B70, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Danovich, D.; Mo, Y.; Shaik, S. On The Nature of the Chemical Bond. J. Chem. Theory Comput. 2014, 10, 3726–3737. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.L. Relationships between Interaction Energy and Electron Density Properties for Homo Halogen Bonds of the [(A)nY–X···X–Z(B)m] Type (X = Cl, Br, I). Molecules 2019, 24, 2733. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras, M.; Protas, J.; Jebbari, S.; Dirksen, G.J.; Schoonman, J. Structure and ionic conductivity of mixed lead halides PbCl2xBr2(1−x). II. Solid State Ion. 1986, 20, 295–304. [Google Scholar] [CrossRef]
- Altawarneh, M.; Marashdeh, A.; Dlugogorski, B.Z. Structures, electronic properties and stability phase diagrams for copper(I/II) bromide surfaces. Phys. Chem. Chem. Phys. 2015, 17, 9341–9351. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.R.; Zhang, J.; Szostak, M. Acyclic Twisted Amides. Chem. Rev. 2021, 21, 12746–12783. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]




| Crystal Structure | Crystal System: Space Group | Z’ | Volume (Å3) | R, wR2 R-Factors, GoF |
|---|---|---|---|---|
| H-DIP | Triclinic, P (No. 2) | 1 | 713.43 (4) | 0.031, 0.078, 1.030 |
| F-DIP | Monoclinic, P21/c (No. 14) | 1 | 1559.71 (8) | 0.045, 0.111, 1.026 |
| Cl-DIP | Triclinic, P (No. 2) | 3 | 2553.5 (3) | 0.062, 0.162, 1.075 |
| Br-DIP | Monoclinic, C2/c (No. 15) | 1 | 3509.3 (6) | 0.078, 0.205, 1.045 |
| I-DIP•½(H2O) | Monoclinic, P2/c (No. 13) | 1 | 1814.49 (5) | 0.023, 0.056, 1.060 |
| Structure | C6/C5N | C6/Amide | C5N/Amide | N⋯N/O # | Primary H-Bond |
|---|---|---|---|---|---|
| H-DIP | 68.17 (3) 5.65 (7) | 49.60 (4) 19.49 (6) | 15.04 (15) 14.10 (6) | 3.1252 (13) 3.3425 (13) | amide⋯amide amide⋯pyridine |
| F-DIP | 47.20 (4) 14.20 (6) | 45.69 (4) 10.47 (7) | 3.22 (7) 3.97 (7) | 3.3129 (19) 3.2605 (16) | amide⋯amide amide⋯pyridine |
| Cl-DIP (in sequence for molecules A, B, C) | 11.6 (3) 15.7 (3), 10.3 (3) 24.5 (2), 9.3 (3) 10.2 (3) | 30.3 (2) 31.6 (2), 29.3 (2) 21.8 (2), 33.5 (2) 19.4 (2) | 26.6 (2) 17.3 (3), 20.7 (3) 3.0 (3), 26.9 (2) 28.9 (2) | 2.924 (4) 3.029 (5), 2.973 (4) 3.289 (4), 2.865 (4) 3.078 (4) | amide⋯amide |
| Br-DIP | 36.4 (2) 46.6 (2) | 38.8 (2) 1.5 (4) | 2.6 (3) 47.8 (2) | 3.186 (8) 2.999 (8) | amide⋯amide amide⋯pyridine |
| I-DIP•½(H2O) | 4.6 (2) 19.21 (15) | 28.8 (3) 9.0 (6) | 24.2 (3) 10.2 (5) | 2.897 (4) 2.931 (3) 2.833 (3) | amide⋯H2O (pyridine)N⋯I-C |
| Dihedral Angle | O1–C1–C11–C12 (α1) | C1–N1–C21–C26 (β1) | O1–C1–N1–H1 (γ1) | O2–C2–C13–C12 (α2) | C2–N2–C31–C36 (β2) | O2–C2–N2–H2 (γ2) | Molecular Energy (103 kJ mol−1) |
|---|---|---|---|---|---|---|---|
| H-DIP | 152.38 | −4.12 | 171.73 | 152.38 | −4.12 | 171.73 | −2793.9 |
| F-DIP | 152.31 | −4.04 | 171.68 | 152.31 | −4.04 | 171.67 | −3315.1 |
| Cl-DIP | 152.53 | −3.98 | 171.68 | 152.53 | −3.98 | 171.69 | −5207.4 |
| Br-DIP | 152.43 | −3.98 | 171.63 | 152.43 | −3.97 | 171.63 | −16,307.6 |
| NO2-DIP | 152.48 | −4.00 | 171.18 | 152.48 | −3.98 | 171.20 | −3868.04 |
| I-DIP | O1–C1–C11–C12 (α1) | C1–N1–C21–C26 (β1) | O1–C1–N1–H1 (γ1) | O2–C2–C13–C12 (α2) | C2–N2–C31–C36 (β2) | O2–C2–N2–H2 (γ2) | Molecular Energy (103 kJ mol−1) |
|---|---|---|---|---|---|---|---|
| syn-syn | 170.73 | −0.41 | 175.15 | 170.72 | −0.40 | 175.51 | −38,982.94 |
| syn-anti | −13.20 | 164.03 | 163.25 | −178.75 | 0.04 | −179.41 | −38,982.73 |
| anti-anti | −17.65 | 161.91 | 161.16 | −17.65 | 161.91 | 161.16 | −38,982.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, I.A.; McKee, V.; Jelsch, C.; Gallagher, J.F. Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a Series of Isophthalamides. Symmetry 2023, 15, 738. https://doi.org/10.3390/sym15030738
Osman IA, McKee V, Jelsch C, Gallagher JF. Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a Series of Isophthalamides. Symmetry. 2023; 15(3):738. https://doi.org/10.3390/sym15030738
Chicago/Turabian StyleOsman, Islam Ali, Vickie McKee, Christian Jelsch, and John F. Gallagher. 2023. "Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a Series of Isophthalamides" Symmetry 15, no. 3: 738. https://doi.org/10.3390/sym15030738
APA StyleOsman, I. A., McKee, V., Jelsch, C., & Gallagher, J. F. (2023). Roles of Hydrogen, Halogen Bonding and Aromatic Stacking in a Series of Isophthalamides. Symmetry, 15(3), 738. https://doi.org/10.3390/sym15030738






