Influence of Asymmetric Agglomerations Effects over the Photothermal Release of Liposome-Encapsulated Nanodiamonds Assisted by Opto-Mechanical Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. NDs Release
2.3. Nanovehicles Diffusion
3. Results and Discussion
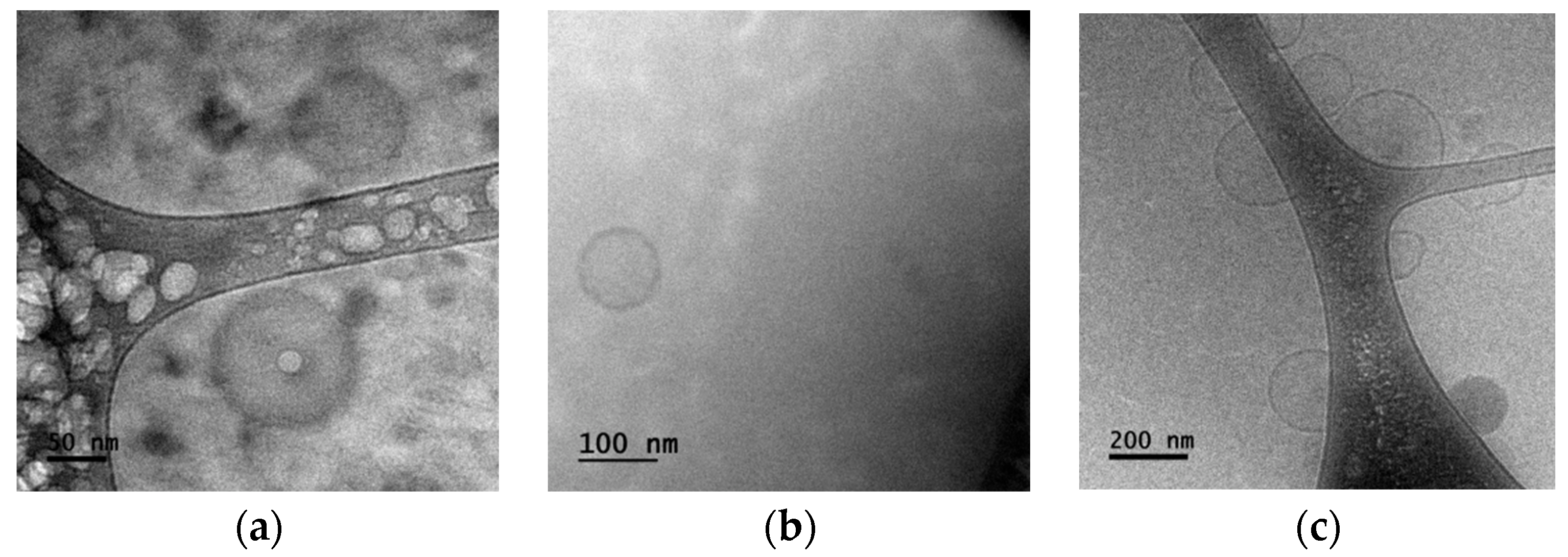
3.1. Sample Characterization
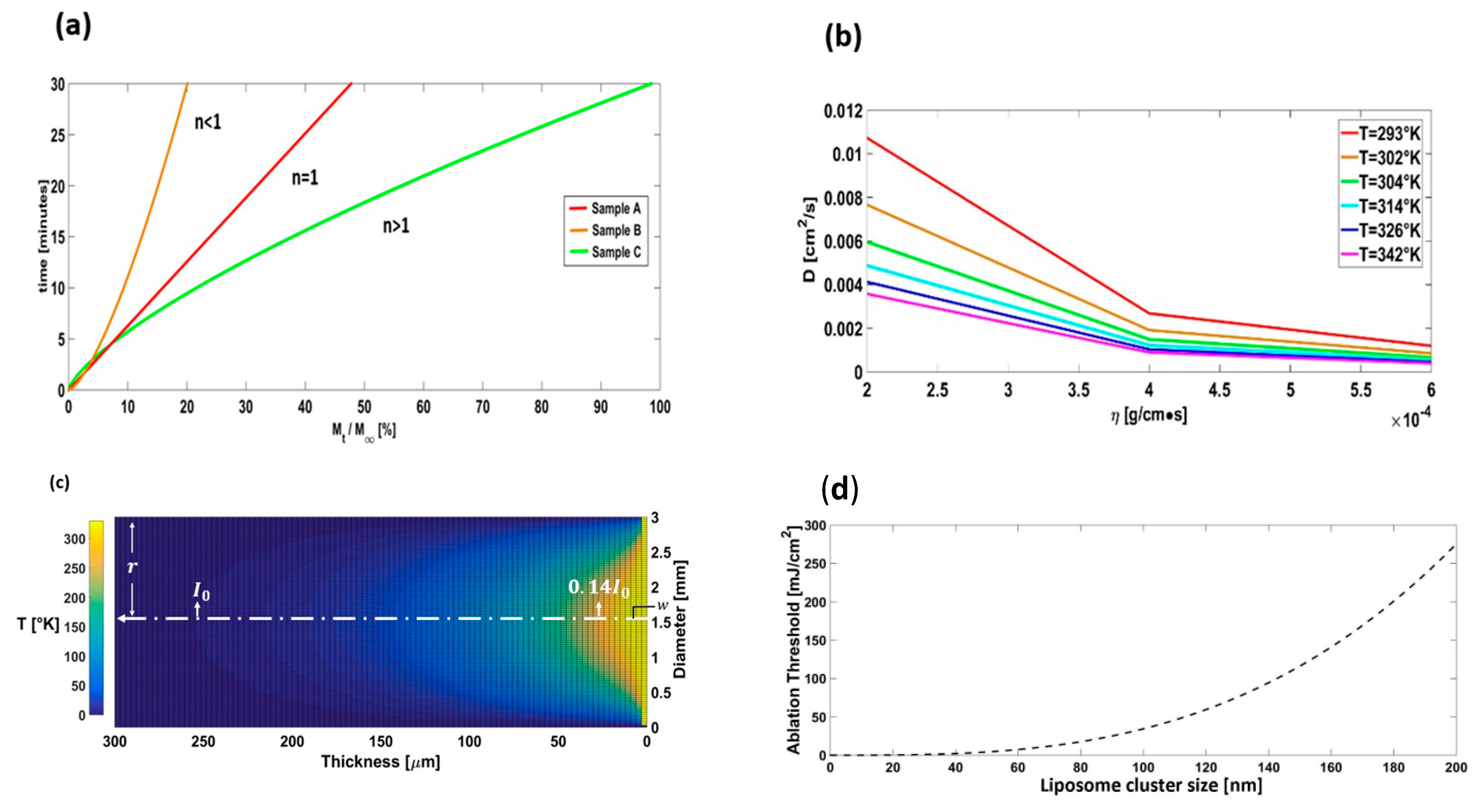
3.2. NDs Release
3.3. Nanovehicles Diffusion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shengchun, Q.; Haibin, Y.; Dawei, R.; Shihai, K.; Guangtian, Z.; Dongmei, L.; Minghui, L. Magnetite Nanoparticles Prepared by Precipitation from Partially Reduced Ferric Chloride Aqueous Solutions Author links open overlay panel. J. Colloid Interface Sci. 1999, 215, 190–192. [Google Scholar] [CrossRef]
- Widder, K.J.; Senyei, A.E.; Scarpelli, D.G. Magnetic microspheres: A model system for site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 1978, 58, 141–146. [Google Scholar] [CrossRef]
- Hatch, G.P.; Stelter, R.E. Magnetic design considerations for devices and particles used for biological high-gradient magnetic separation (HGMS) systems. J. Magn. Magn. Mater. 2001, 225, 262–276. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Lucassen, G.W.; Verkruysse, W.; Andersson-Engels, S.; van Gemert, M.J. Changes in optical properties of human whole blood in vitro due to slow heating. Photochem. Photobiol. 1997, 65, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.H.; Ikeda, R.M.; Stobbe, D.; Ogata, C.; Lui, H.; Mason, D.T.; Lee, G. Effects of laser irradiation on human erythrocytes: Considerations concerning clinical laser angioplasty. Clin. Cardiol. 1983, 6, 396–398. [Google Scholar] [CrossRef]
- Verkruysse, W.; Nilsson, A.M.; Milner, T.E.; Beek, J.F.; Lucassen, G.W.; van Gemert, M.J. Optical absorption of blood depends on temperature during a 0.5 ms laser pulse at 586 nm. Photochem. Photobiol. 1998, 67, 276–281. [Google Scholar] [CrossRef]
- Pfefer, T.J.; Chan, K.F.; Hammer, D.X.; Welch, A.J. Pulsed holmium: YAG-induced thermal damage in albumen. In Proceedings of the Proceedings SPIE, Laser-Tissue Interaction IX, San Jose, CA, USA, 26–28 January 1998; Volume 3254, pp. 192–202. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Rahim, N.A. Effect of Nanodiamonds on the Optoelectronic Properties of TiO2 Photoanode in Dye-Sensitized Solar Cell. Arab. J. Sci. Eng. 2018, 43, 3515–3519. [Google Scholar] [CrossRef]
- Lisik, K.; Krokosz, A. Application of carbon nanoparticles in oncology and regenerative medicine. Int. J. Mol. Sci. 2021, 22, 8341. [Google Scholar] [CrossRef]
- Kumar, V.B.; Perkas, N.; Porat, Z.E.; Gedanken, A. Solar-Light-Driven Photocatalytic Activity of Novel Sn@C-Dots-Modified TiO2 Catalyst. ChemistrySelect 2017, 2, 6683–6688. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Nguyen, P.L.; Jana, J.; Choi, W.M.; Chung, J.S.; Hur, S.H. Simple paper-based colorimetric and fluorescent glucose sensor using N-doped carbon dots and metal oxide hybrid structures. Anal. Chim. Acta 2021, 1147, 187–198. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Shadab; Nair, A.B.; Deb, P.K. Exploring the Potential of Carbon Dots to Combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef] [PubMed]
- Shenderova, O.A.; Gruen, D.M. Advances in synthesis and processing. In Book Ultrananocrystalline Diamond: Synthesis, Properties, and Applications, 2nd ed.; William Andrew Elsevier: New York, NY, USA, 2006; pp. 3–44. [Google Scholar]
- Kubo, T.; Sugita, T.; Shimose, S.; Nitta, Y.; Ikuta, Y.; Murakami, T. Targeted systemic chemotherapy using magnetic liposomes with incorporated adriamycin for osteosarcoma in hamsters. Int. J. Oncol. 2001, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov. Today 2014, 19, 1530–1546. [Google Scholar] [CrossRef]
- Sgorla, D.; Bunhak, É.J.; Cavalcanti, O.A.; Fonte, P.; Sarmento, B. Exploitation of lipid-polymeric matrices at nanoscale for drug delivery applications. Expert Opin. Drug Deliv. 2016, 13, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Ryman, B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971, 124, 58P. [Google Scholar] [CrossRef]
- Mayhew, E.; Papahadjopoulos, D.; Rustum, Y.M.; Dave, C. Inhibition of tumor cell growth in vitro and in vivo by 1-beta-D-arabinofuranosylcytosine entrapped within phospholipid vesicles. Cancer Res. 1976, 36, 4406–4411. [Google Scholar]
- Laginha, K.M.; Verwoert, S.; Charrois, G.J.; Allen, T.M. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin. Cancer Res. 2005, 11, 6944–6949. [Google Scholar] [CrossRef]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Edwards, K.; Eisenhardt, M.L.; Leng, E.C.; Karlsson, G.; Yanko, D.; Cullis, P.R. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim. Biophys. Acta 2006, 1758, 55–64. [Google Scholar] [CrossRef]
- Bothun, G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol. 2008, 6, 13. [Google Scholar] [CrossRef]
- Douda, J.; González-Vargas, C.R.; Mota-Díaz, I.I.; Basiuk, E.V.; Hernández-Contreras, X.A.; Fuentes-García, J.A.; Bornacelli, J.; Torres-Torres, C. Photoluminescent properties of liposome-encapsulated amine-functionalized nanodiamonds. Nano Express 2020, 1, 030009. [Google Scholar] [CrossRef]
- Eugene, H. Modern Optics: Lasers and Other Topics. In Book Optics, 5th ed.; Pearson, United States Edition: New York, NY, USA, 2016; pp. 625–627. [Google Scholar]
- Raciukaitis, G.; Brikas, M.; Gecys, P.; Gedvilas, M. Accumulation effects in laser ablation of metals with high-repetition-rate lasers. In Proceedings of the SPIE, High-Power Laser Ablation VII, Taos, NM, USA, 20–24 April 2008; Volume 7005. [Google Scholar] [CrossRef]
- Nelson, E. Chapter 4 Albert Einstein. In Book Dynamical Theories of Brownian Motion, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2020; pp. 13–17. [Google Scholar]
- Jain, A.; Jain, S.K. In Vitro release model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Paula, S.; Volkov, A.G.; Van Hoek, A.N.; Haines, T.H.; Deamer, D.W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of in vitro release methods in liposomal formulation development: Challenges and reg-ulatory perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Nothnagel, L.; Wacker, M.G. How to measure release from nanosized carriers? Eur. J. Pharm. Sci. 2018, 120, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bonilla, S.; Mota-Díaz, I.I.; Douda, J.; González-Vargas, C.R.; Villalpando, I.; Torres-Torres, C. Thermo-mechanical effects and photo-induced release of liposome-encapsulated nanodiamonds by polarization-resolved laser pulses. Optik 2021, 245, 167738. [Google Scholar] [CrossRef]
- Torres-Torres, R. Extracting characteristic impedance in low-loss substrates. Electron. Lett. 2011, 47, 191. [Google Scholar] [CrossRef]
- Mansfield, J.R.; Gossage, K.W.; Hoyt, C.C.; Levenson, R.M. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J. Biomed. Opt. 2005, 10, 41207. [Google Scholar] [CrossRef]
- Farkas, J.P.; Hoopman, J.E.; Kenkel, J.M. Five Parameters You Must Understand to Master Control of Your Laser/Light-Based Devices. Aesthet. Surg. J. 2013, 33, 1059–1064. [Google Scholar] [CrossRef]
- Aguiar, A.J.; Krc, J., Jr.; Kinkel, A.W.; Samyn, J.C. Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J. Pharm. Sci. 1967, 56, 847–853. [Google Scholar] [CrossRef]
- Wagner, J.G. Biopharmaceutics: Absorption aspects. J. Pharm. Sci. 1961, 50, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, H.C.; Lin, J.M.; Grover, W.H. Measuring dissolution profiles of single controlled-release drug pellets. Sci. Rep. 2020, 10, 19734. [Google Scholar] [CrossRef]
- Kautek, W.; Krüger, J. Femtosecond pulse laser ablation of metallic, semiconducting, ceramic, and biological materials. In Proceedings of the Laser Materials Processing: Industrial and Microelectronics Applications, Vienna, Austria, 7 September 1994. [Google Scholar]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burn. J. Int. Soc. Burn. Inj. 2017, 43, 909–932. [Google Scholar] [CrossRef]
- Abuin, E.; Lissi, E.; Ahumada, M. Diffusion of hydrogen peroxide across DPPC large unilamellar liposomes. Chem. Phys. Lipids 2012, 165, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, M.; Calderón, C.; Leon, L.; Lissi, E. Rate of solute incorporation to liposomes evaluated from encapsulated enzymes activities. Biophys. Rev. 2014, 6, 161–167. [Google Scholar] [PubMed]
- Ueno, M.; Yoshida, S.; Horikoshi, I. Characteristics of the membrane permeability of temperature-sensitive liposomes. Bull. Chem. Soc. Jpn. 1991, 64, 1588–1593. [Google Scholar]
- White, G.; Pencer, J.; Nickel, B.G.; Wood, J.M.; Hallet, F.R. Optical changes in unilamellar vesicles experiencing osmotic stress. Biophys. J. 1996, 71, 2701–2715. [Google Scholar]
- Fujiwara, K.; Yanagisawa, M. Generation of giant unilamellar liposomes containing biomacromolecules at physiological intracellular concentrations using hypertonic conditions. ACS Synth. Biol. 2014, 3, 870–874. [Google Scholar] [CrossRef]
- Disalvo, E.A.; Campos, A.M.; Abuin, E.; Lissi, E. Surface changes induced by osmotic shrinkage on large unilamellar vesicles. Chem. Phys. Lipids 1996, 84, 35–45. [Google Scholar]
- Sun, S.-T.; Milon, A.; Tanaka, T.; Ourisson, G.; Nakatani, Y. Osmotic swelling of unilamellar vesicles by the stopped-flow light scattering method. Elastic properties of vesicles. Biochim. Biophys. Acta Biomembr. 1986, 860, 525–530. [Google Scholar] [CrossRef]
- Chen, P.Y.; Pearce, D.; Verkman, A.S. Membrane water and solute permeability deter-mined quantitatively by self-quenching of an entrapped fluorophore. Biochemistry 1988, 27, 5713–5718. [Google Scholar]
- Ertel, A.; Marangoni, A.; Marsh, J.; Ross Hallett, F.; Wood, J. Mechanical properties of vesicles. 1. Coordinated analyses of osmotic swelling and lysis. Biophys. J. 1993, 64, 426–434. [Google Scholar]
- Lynch, I.; Dawson, K.A.; Linse, S. Detecting cryptic epitopes created by nanoparticles. Sci. STKE 2006, 2006, pe14. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.J. The interaction of proteins with solid surfaces. Curr. Opin. Struct. Biol. 2004, 14, 11011–110115. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Drotning, W.D. Optical properties of solar-absorbing oxide particles suspended in a molten salt heat transfer fluid. Sol. Energy 1978, 20, 313–319. [Google Scholar]
- Otanicar, T.P.; Phelan, P.E.; Golden, J.S. Optical properties of liquids for direct ab-sorption solar thermal energy systems. Sol. Energy 2009, 83, 969–977. [Google Scholar] [CrossRef]
- Grundfest, W.S.; Litvack, F.; Forrester, J.S.; Goldenberg, T.S.; Swan, H.J.; Morgenstern, L.; Fishbein, M.; McDermid, I.S.; Rider, D.M.; Pacala, T.J.; et al. Laser ablation of human atherosclerotic plaque without adjacent tissue injury. J. Am. Coll. Cardiol. 1985, 5, 929–933. [Google Scholar] [CrossRef]
- Miranda, D.; Carter, K.; Luo, D.; Shao, S.; Geng, J.; Li, C.; Chitgupi, U.; Turowski, S.G.; Li, N.; Atilla-Gokcumen, G.E.; et al. Multifunctional Liposomes for Image-Guided Intratumoral Chemo-Phototherapy. Adv. Healthc. Mater. 2017, 6, 1700253. [Google Scholar] [CrossRef]
- Rossmann, C.; McCrackin, M.A.; Armeson, K.E.; Haemmerich, D. Temperature sensitive liposomes combined with thermal ablation: Effects of duration and timing of heating in mathematical models and in vivo. PLoS ONE 2017, 12, e0179131. [Google Scholar] [CrossRef]
- Black, J.F.; Wade, N.; Barton, J.K. Mechanistic comparison of blood undergoing laser photocoagulation at 532 and 1064 nm. Laser Surg. Med. 2005, 36, 155–165. [Google Scholar] [CrossRef]
- Pfefer, T.J.; Choi, B.; Vargas, G.; McNally-Heintzelman, K.M.; Welch, A.J. Mechanisms of laser-induced thermal coagulation of whole blood in vitro. In Proceedings of the SPEI, Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems IX, San Jose, CA, USA, 23–29 January 1999; Volume 3590. [Google Scholar] [CrossRef]
- Pichot, V.; Muller, O.; Seve, A.; Yvon, A.; Merlat, L.; Spitzer, D. Optical properties of functionalized nanodiamonds. Sci. Rep. 2017, 7, 14086. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. Engl. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Adrian, R.; Prasher, R. Nanofluid optical property characterization: Towards efficient direct absorption solar collectors. Nanoscale Res. Lett. 2011, 6, 225. [Google Scholar] [CrossRef]
- Tesfai, W.; Singh, P.K.; Masharqa, S.J.S.; Souier, T.; Chiesa, M.; Shatlla, Y. Investigating the effect of suspensions nanostructure on the thermophysical properties of nanofluids. J. Appl. Phys. 2012, 112, 114315. [Google Scholar] [CrossRef]
- Frich, L.; Bjørnerud, A.; Fossheim, S.; Tillung, T.; Gladhaug, I. Experimental application of thermosensitive paramagnetic liposomes for monitoring magnetic resonance imaging guided thermal ablation. Magn. Reson. Med. 2004, 52, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Pai, S.H. Changes in hematologic parameters induced by thermal treatment of human blood. Ann. Clin. Lab. Sci. 2002, 32, 393–398. [Google Scholar] [PubMed]
- Kreuter, J. Drug targeting with nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 1994, 19, 253–256. [Google Scholar] [CrossRef]
- Ma, Q.M.; Zhang, M.; Xu, X.H.; Meng, K.; Yao, C.; Zhao, Y.F.; Sun, J.; Du, Y.P.; Yang, D.Y. Multiresponsive Supramolecular Luminescent Hydrogels Based on a Nucleoside/Lanthanide Complex. ACS Appl. Mater. Interfaces 2019, 11, 47404–47412. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bonilla, S.; Torres-Torres, C.; Trejo-Valdez, M.; Torres-Torres, D.; Urriolagoitia-Calderón, G. Mechano-optical transmittance and third order nonlinear optical properties exhibited by Au nanoparticles. Optik 2015, 126, 4093–4097. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Qu, Z.B.; Chen, Z.H.; Han, X.Y.; Deng, L.X.; Luo, Q.Y.; Jin, Z.W.; Shi, G.Y.; Zhang, M. The Marriage of Protein and Lanthanide: Unveiling a Time-Resolved Fluorescence Sensor Array Regulated by pH toward High-Throughput Assay of Metal Ions in Biofluids. Anal. Chem. 2019, 91, 11170. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Rémy, J.S.; Krötz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The magnetofection method: Using magnetic force to enhance gene delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bonilla, S.; Martines-Arano, H.; Torres-Torres, D.; Ochoa-Ortega, G.; Carrillo-Delgado, C.; Trejo-Valdez, M.; Torres-Torres, C. Dynamic and plasmonic response exhibited by Au nanoparticles suspended in blood plasma and cerebrospinal fluids. J. Mol. Liquids 2019, 281, 1–8. [Google Scholar] [CrossRef]
- Zimmet, P.Z. The pathogenesis and prevention of diabetes in adults. Genes, autoimmunity, and demography. Diabetes Care 1995, 18, 1050–1064. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a low-temperature nanofluid-based direct absorption solar collector. ASME J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]


| Material | Fluence [mJ/cm2] | I2 [MW/cm2] | T [°C] |
|---|---|---|---|
| Liposome-encapsulated nanodiamonds (ND size 4–15 nm) | 123 ± 30% (experiment) 250 (numerical) | 39.79 (numerical) | 51.85 (numerical) 50–70 [55] |
| Pure liposome | 181 ± 30% (experiment) | 58.56 (numerical) 0–300 [56] | 40–50 [57] |
| Pure blood plasma | 4000–5000 [58] | ---- | 80–100 [59] |
| Pure NDs | 1,000,000 [60] | ---- | ---- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Bonilla, S.; Mota-Díaz, I.I.; Douda, J.; Fuerte-Hernández, A.; Campos-López, J.P.; Torres-Torres, C. Influence of Asymmetric Agglomerations Effects over the Photothermal Release of Liposome-Encapsulated Nanodiamonds Assisted by Opto-Mechanical Changes. Symmetry 2023, 15, 775. https://doi.org/10.3390/sym15030775
Morales-Bonilla S, Mota-Díaz II, Douda J, Fuerte-Hernández A, Campos-López JP, Torres-Torres C. Influence of Asymmetric Agglomerations Effects over the Photothermal Release of Liposome-Encapsulated Nanodiamonds Assisted by Opto-Mechanical Changes. Symmetry. 2023; 15(3):775. https://doi.org/10.3390/sym15030775
Chicago/Turabian StyleMorales-Bonilla, Samuel, Isaac I. Mota-Díaz, Janna Douda, Ariel Fuerte-Hernández, Juan Pablo Campos-López, and Carlos Torres-Torres. 2023. "Influence of Asymmetric Agglomerations Effects over the Photothermal Release of Liposome-Encapsulated Nanodiamonds Assisted by Opto-Mechanical Changes" Symmetry 15, no. 3: 775. https://doi.org/10.3390/sym15030775
APA StyleMorales-Bonilla, S., Mota-Díaz, I. I., Douda, J., Fuerte-Hernández, A., Campos-López, J. P., & Torres-Torres, C. (2023). Influence of Asymmetric Agglomerations Effects over the Photothermal Release of Liposome-Encapsulated Nanodiamonds Assisted by Opto-Mechanical Changes. Symmetry, 15(3), 775. https://doi.org/10.3390/sym15030775







