Ultrafast Synthesis of Mo2C-Based Catalyst by Joule Heating towards Electrocatalytic Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
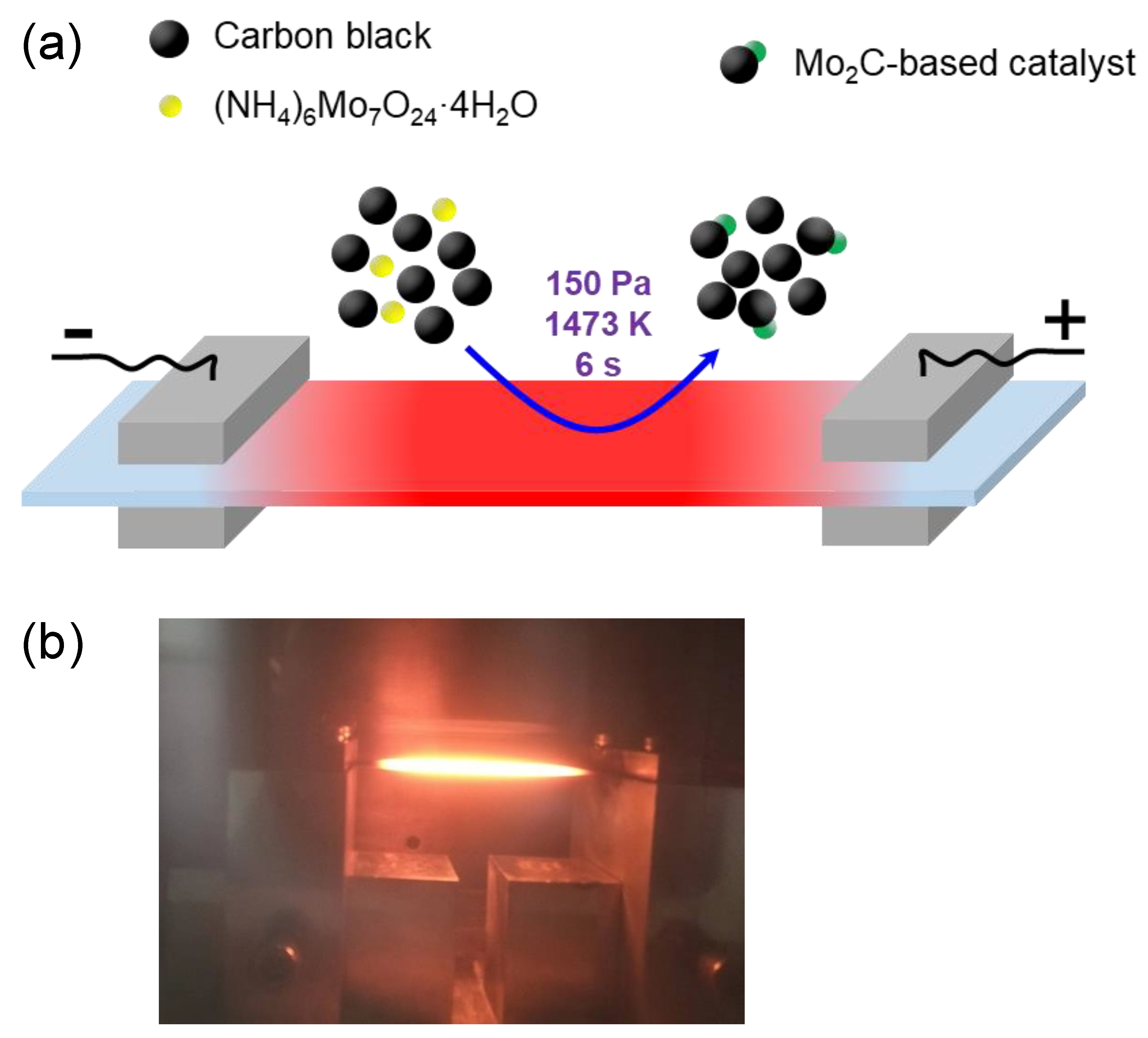
2.2. Preparation of the Mo2C-Based Electrocatalyst
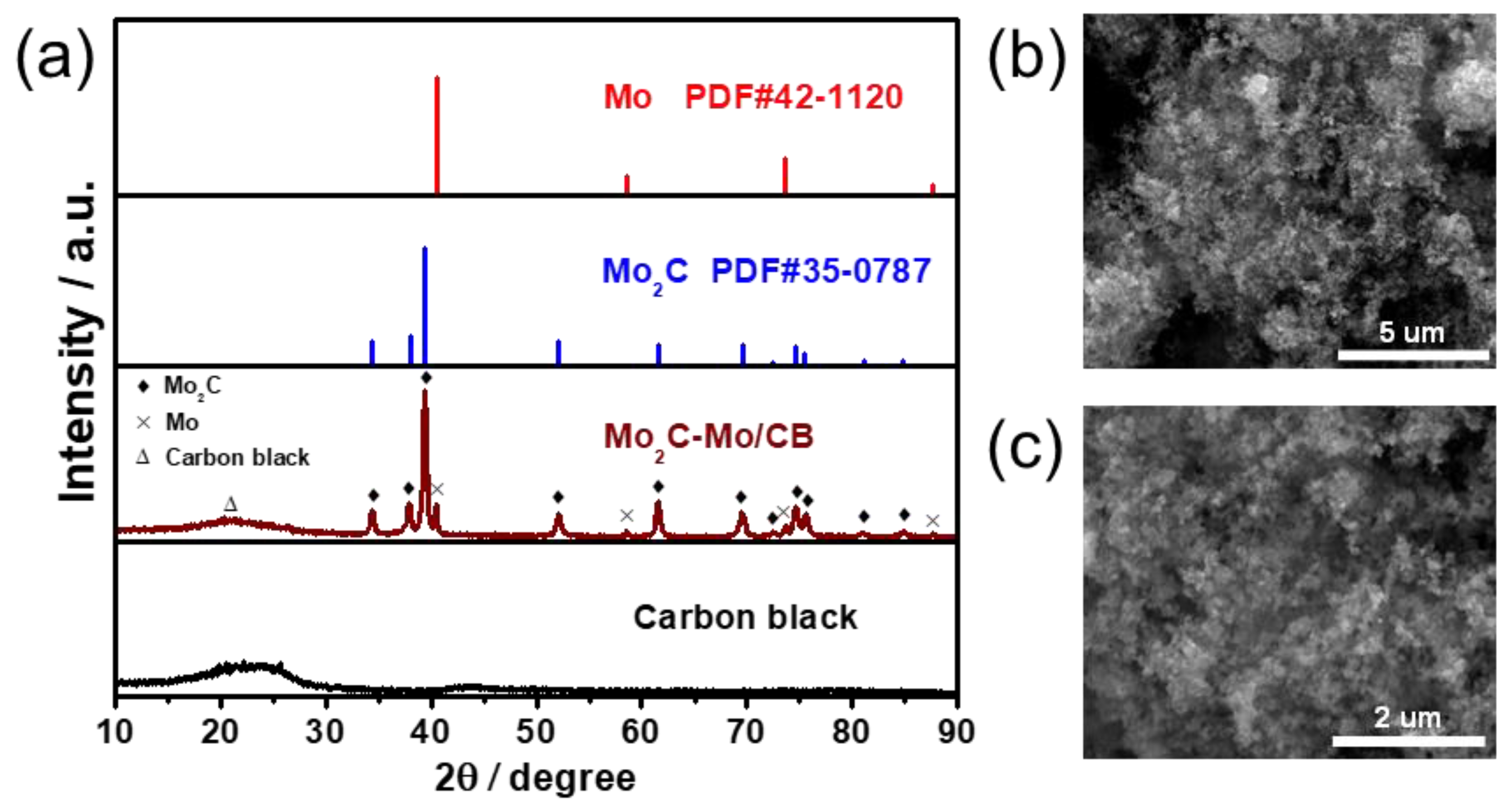
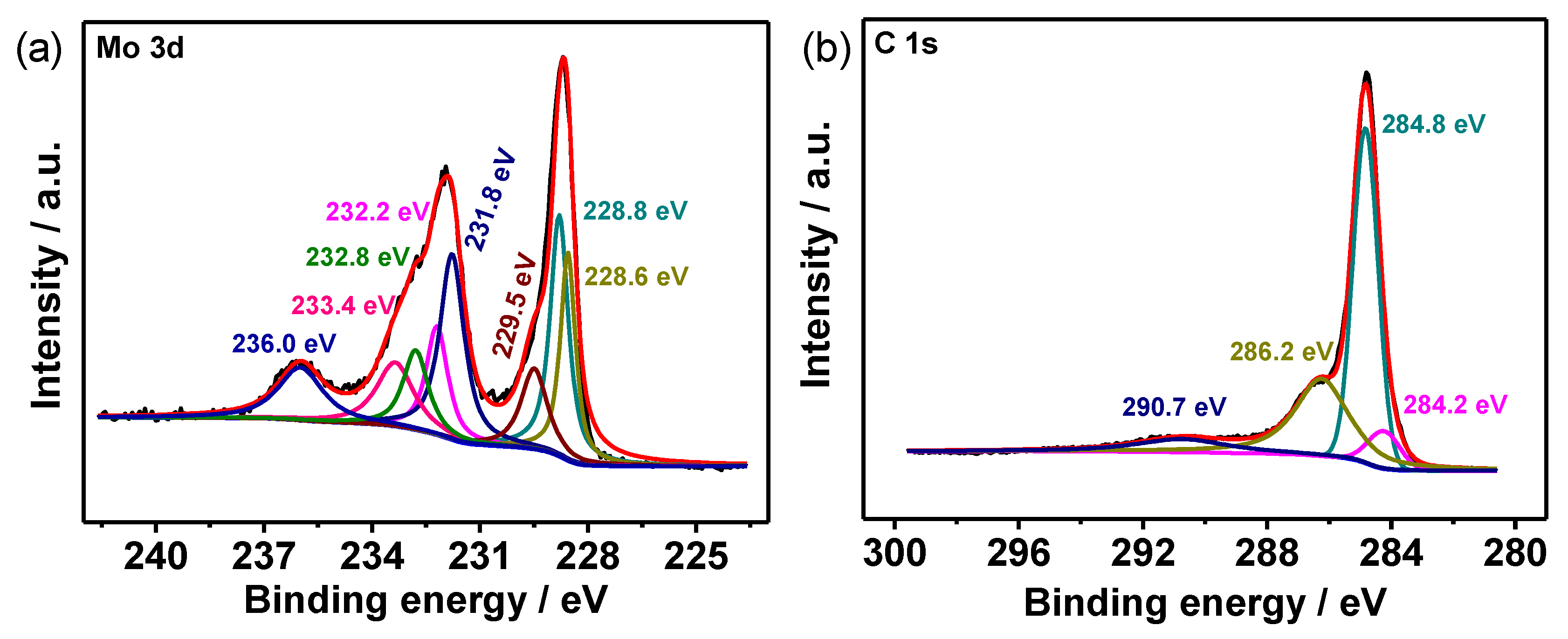
2.3. Material Characterizations
2.4. Electrode Preparation
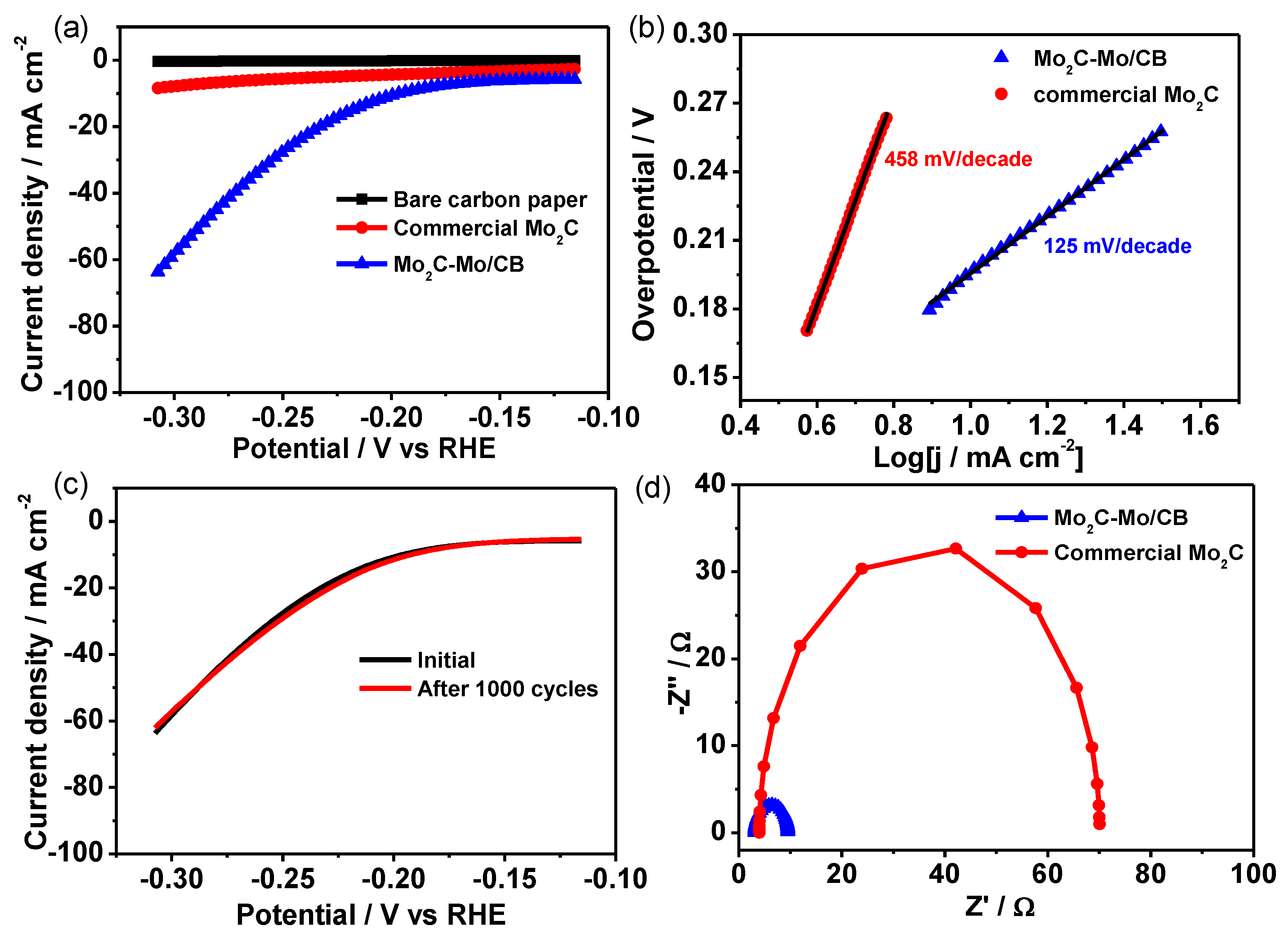
2.5. Electrochemical Measurements
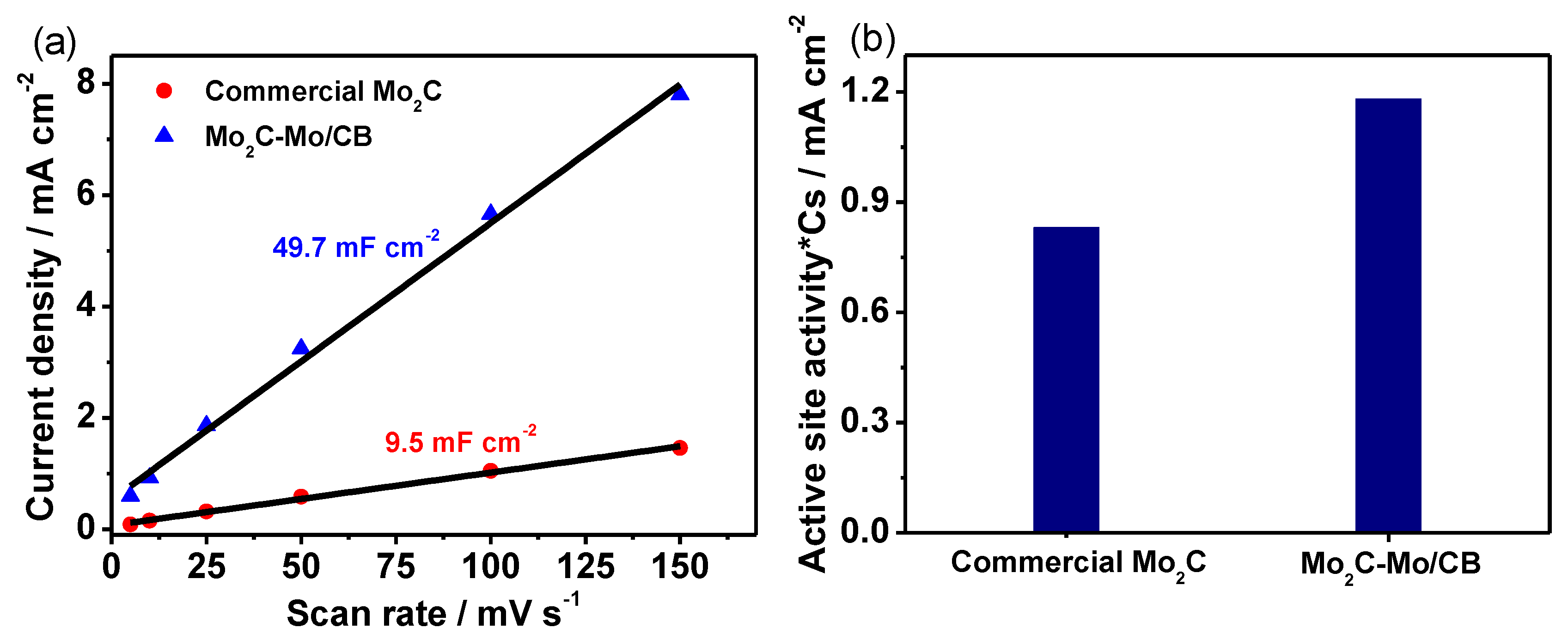
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.-M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H.B.; Liu, B. Recent Advances in Carbon-Supported Noble-Metal Electrocatalysts for Hydrogen Evolution Reaction: Syntheses, Structures, and Properties. Adv. Energy Mater. 2022, 12, 2200928. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Alam, M.A.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Response surface optimization of syngas production from greenhouse gases via DRM over high performance Ni–W catalyst. Int. J. Hydrogen Energy 2022, 47, 31058–31071. [Google Scholar] [CrossRef]
- Iqbal, F.; Abdullah, B.; Oladipo, H.; Yusuf, M.; Alenazey, F.; Nguyen, T.D.; Ayoub, M. Nanostructured Photocatalysts; Nguyen, V.-H., Vo, D.-V.N., Nanda, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 519–540. [Google Scholar]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Mondal, A.; Vomiero, A. 2D Transition Metal Dichalcogenides-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2208994. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H.; et al. Ultrahigh Pt-Mass-Activity Hydrogen Evolution Catalyst Electrodeposited from Bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Wan, X.-K.; Wu, H.B.; Guan, B.Y.; Luan, D.; Lou, X.W. Confining Sub-Nanometer Pt Clusters in Hollow Mesoporous Carbon Spheres for Boosting Hydrogen Evolution Activity. Adv. Mater. 2020, 32, 1901349. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhang, Z.; Zhang, Z.; Tan, Y.; Yu, L.; Wu, W.; Wang, Z.; Jiang, T.; Gao, S.; Cheng, N. Electronic modulation of Pt nanoclusters through tuning the interface of Pt-SnO2 clusters for enhanced hydrogen evolution catalysis. Chem. Eng. J. 2022, 435, 135102. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, T.H.; Lee, M.H.; Kawashima, K.; Mullins, C.B.; Lim, H.-K.; Youn, D.H. Highly active and stable nickel–molybdenum nitride (Ni2Mo3N) electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2021, 9, 4945–4951. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, H.; Zhou, W.; Zhou, Y.; Ni, Z.; Chen, J.; Zhao, S.; Zhao, Y.; Yu, F.; Chen, A.; et al. Interface engineering of bimetallic nitrides nanowires as a highly efficient bifunctional electrocatalyst for water splitting. J. Alloys Compd. 2022, 919, 165862. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and Direction toward High-Performance MoS2 Hydrogen Evolution Catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, F.; Song, S. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2021, 405, 127013. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.; Yang, H.; Wang, H.; Xia, B.Y. Rational Design of Transition Metal Phosphide-Based Electrocatalysts for Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2208358. [Google Scholar] [CrossRef]
- Wang, Z.; Heng, N.; Wang, X.; He, J.; Zhao, Y. Surface and morphology structure evolution of metal phosphide for designing overall water splitting electrocatalyst. J. Catal. 2019, 374, 51–59. [Google Scholar] [CrossRef]
- Humagain, G.; MacDougal, K.; MacInnis, J.; Lowe, J.M.; Coridan, R.H.; MacQuarrie, S.; Dasog, M. Highly Efficient, Biochar-Derived Molybdenum Carbide Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2018, 8, 1801461. [Google Scholar] [CrossRef]
- Hu, R.; Jiang, H.; Xian, J.; Mi, S.; Wei, L.; Fang, G.; Guo, J.; Xu, S.; Liu, Z.; Jin, H.; et al. Microwave-pulse sugar-blowing assisted synthesis of 2D transition metal carbides for sustainable hydrogen evolution. Appl. Catal. B Environ. 2022, 317, 121728. [Google Scholar] [CrossRef]
- Fan, J.; Cui, X.; Yu, S.; Gu, L.; Zhang, Q.; Meng, F.; Peng, Z.; Ma, L.; Ma, J.-Y.; Qi, K.; et al. Interstitial Hydrogen Atom Modulation to Boost Hydrogen Evolution in Pd-Based Alloy Nanoparticles. ACS Nano 2019, 13, 12987–12995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, K.; Fan, L.; Liu, H.; Zhu, H.; Yan, S. High-valence metal doped Co2FeAl alloy as efficient noble-metal-free electrocatalyst for alkaline hydrogen evolution reaction. J. Alloys Compd. 2023, 933, 167613. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Y.; Zheng, J.; Cui, M.; Wang, K.; Li, N. Activating molybdenum carbide via a surface sulfur modification to enhance hydrogen evolution activity. J. Alloys Compd. 2023, 933, 167664. [Google Scholar] [CrossRef]
- Lin, F.; Lv, B.; Gao, H.; Feng, J.; Chen, D.; Zheng, C.; Li, D.; Chen, Y.; Sun, C. Graphite Nanoflake-Modified Mo2C with Ameliorated Interfacial Interaction as an Electrocatalyst for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 56407–56415. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine Molybdenum Carbide Nanoparticles Composited with Carbon as a Highly Active Hydrogen-Evolution Electrocatalyst. Angew. Chem. Int. Ed. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Cheng, Z.; Fu, Q.; Han, Q.; Xiao, Y.; Liang, Y.; Zhao, Y.; Qu, L. A Type of 1 nm Molybdenum Carbide Confined within Carbon Nanomesh as Highly Efficient Bifunctional Electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705967. [Google Scholar] [CrossRef]
- Wan, J.; Huang, L.; Wu, J.; Xiong, L.; Gao, X.; Hu, Z.; Jin, H.; Zhang, G.; Zhou, J. Rapid synthesis of size-tunable transition metal carbide nanodots under ambient conditions. J. Mater. Chem. A 2019, 7, 14489–14495. [Google Scholar] [CrossRef]
- Wu, K.-H.; Jiang, Y.; Jiao, S.; Chou, K.-C.; Zhang, G.-H. Synthesis of high purity nano-sized transition-metal carbides. J. Mater. Res. Technol. 2020, 9, 11778–11790. [Google Scholar] [CrossRef]
- Reynard, D.; Nagar, B.; Girault, H. Photonic Flash Synthesis of Mo2C/Graphene Electrocatalyst for the Hydrogen Evolution Reaction. ACS Catal. 2021, 11, 5865–5872. [Google Scholar] [CrossRef]
- Joo, S.H.; Lee, J.S. Metal carbides as alternative electrocatalysts for energy conversion reactions. J. Catal. 2021, 404, 911–924. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, L.; Luo, L.; Lu, J.; Yin, S.; Shen, P.K.; Tsiakaras, P. N-Doped Porous Molybdenum Carbide Nanobelts as Efficient Catalysts for Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2018, 224, 533–540. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, H.; Wang, Z.; Xiong, Y.; Yuan, S.; Zeng, X.; He, D.; Mu, S. Metal-organic frameworks derived reverse-encapsulation Co-NC@Mo2C complex for efficient overall water splitting. Nano Energy 2019, 57, 746–752. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, P.; Wang, F.; Fang, W.; Xu, Z.; Gao, W.; Gao, C. Rapid roll-to-roll production of graphene films using intensive Joule heating. Carbon 2019, 155, 462–468. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Hwang, S.; Yang, M.; Overa, S.; Dong, Q.; Yao, Y.; Brozena, A.H.; Cullen, D.A.; Chi, M.; et al. Multi-principal elemental intermetallic nanoparticles synthesized via a disorder-to-order transition. Sci. Adv. 2022, 8, eabm4322. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Liu, R.; Gong, Z.; Liu, J.; Liu, J.; Gong, H.; Huang, K.; Fei, H. Ultrafast Joule heating synthesis of hierarchically porous graphene-based Co-N-C single-atom monoliths. Nano Res. 2022, 15, 3913–3919. [Google Scholar] [CrossRef]
- Xi, D.; Li, J.; Low, J.; Mao, K.; Long, R.; Li, J.; Dai, Z.; Shao, T.; Zhong, Y.; Li, Y.; et al. Limiting the Uncoordinated N Species in M–Nx Single-Atom Catalysts toward Electrocatalytic CO2 Reduction in Broad Voltage Range. Adv. Mater. 2022, 34, 2104090. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Wu, L.; Ma, L.; Li, T.; Pang, Z.; Jiao, M.; Liang, Z.; Gao, J.; et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 2019, 14, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Farooqi, A.S.; Ying, Y.X.; Keong, L.K.; Alam, M.A.; Hellgardt, K.; Abdullah, B. Syngas production employing nickel on alumina-magnesia supported catalyst via dry methane reforming. Materialwiss. Werkstofftech. 2021, 52, 1090–1100. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, Y.; Pang, Z.; Yuan, Y.; Li, T.; He, K.; Hu, X.; Cheng, J.; Yao, W.; Liu, Y.; et al. Direct observation of the formation and stabilization of metallic nanoparticles on carbon supports. Nat. Commun. 2020, 11, 6373. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, Z.; Li, H.; Ren, Q.; Chen, Y.; Hu, S. Hybrid electrocatalyst Ag/Co/C via flash Joule heating for oxygen reduction reaction in alkaline media. Chem. Eng. J. 2022, 430, 132769. [Google Scholar] [CrossRef]
- Wu, H.; Lu, Q.; Li, Y.; Wang, J.; Li, Y.; Jiang, R.; Zhang, J.; Zheng, X.; Han, X.; Zhao, N.; et al. Rapid Joule-Heating Synthesis for Manufacturing High-Entropy Oxides as Efficient Electrocatalysts. Nano Lett. 2022, 22, 6492–6500. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Peng, Z.; Zhang, Z.; Zhou, H.; Zhang, X.; Yakobson, B.I.; Goddard, W.A., III; Guo, X.; Hauge, R.H.; et al. Atomic H-Induced Mo2C Hybrid as an Active and Stable Bifunctional Electrocatalyst. ACS Nano 2017, 11, 384–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Wang, Z.; Chen, W.; Li, J.T.; Luong, D.X.; Carter, R.A.; Gao, G.; Yakobson, B.I.; Zhao, Y.; Tour, J.M. Phase controlled synthesis of transition metal carbide nanocrystals by ultrafast flash Joule heating. Nat. Commun. 2022, 13, 262. [Google Scholar] [CrossRef]
- Óvári, L.; Kiss, J.; Farkas, A.P.; Solymosi, F. Surface and Subsurface Oxidation of Mo2C/Mo(100): Low-Energy Ion-Scattering, Auger Electron, Angle-Resolved X-ray Photoelectron, and Mass Spectroscopy Studies. J. Phys. Chem. B 2005, 109, 4638–4645. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Yang, D.; Hu, Y.; He, J.; Chen, Y.; He, W. Mo2C Nanodots Anchored on N-Doped Porous CNT Microspheres as Electrode for Efficient Li-Ion Storage. Small Methods 2019, 3, 1800287. [Google Scholar] [CrossRef]
- Saha, A.; Paul, A.; Srivastava, D.N.; Panda, A.B. Porous carbon incorporated β-Mo2C hollow sphere: An efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 21655–21664. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Liu, M.; Wang, E.; Wang, B.; Xu, L.; Jiang, K.; Fan, S.; Sun, Y.; Li, J.; et al. Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction. Nat. Commun. 2022, 13, 3338. [Google Scholar] [CrossRef] [PubMed]
- Sundara Venkatesh, P.; Kannan, N.; Ganesh Babu, M.; Paulraj, G.; Jeganathan, K. Transition metal doped MoS2 nanosheets for electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 37256–37263. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, B.; Lin, Z.; Xu, Y.; Lin, Y.; Meng, F.; Zhang, Q.; Gu, L.; Fang, B.; Guo, S.; et al. PtSe2/Pt Heterointerface with Reduced Coordination for Boosted Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 23388–23393. [Google Scholar] [CrossRef]
- Ruan, Q.; Luo, W.; Xie, J.; Wang, Y.; Liu, X.; Bai, Z.; Carmalt, C.J.; Tang, J. A Nanojunction Polymer Photoelectrode for Efficient Charge Transport and Separation. Angew. Chem. Int. Ed. 2017, 56, 8221–8225. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yan, M.; Meng, J.; Jiang, G.; Qu, L.; Pan, X.; Liu, J.Z.; Mai, L. Oxygen evolution reaction dynamics monitored by an individual nanosheet-based electronic circuit. Nat. Commun. 2017, 8, 645. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tan, X.; Hocking, R.K.; Bo, X.; Ren, H.; Johannessen, B.; Smith, S.C.; Zhao, C. Implanting Ni-O-VOx sites into Cu-doped Ni for low-overpotential alkaline hydrogen evolution. Nat. Commun. 2020, 11, 2720. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, A.; Kim, J.M.; Lim, D.; Chae, K.H.; Cho, E.N.; Han, H.J.; Jeon, K.U.; Kim, M.; Lee, G.H.; et al. Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts. Nat. Commun. 2020, 11, 4921. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Xu, Z.; Wang, H.; Fan, W.; Zong, X.; Li, C. Metal phosphide catalysts anchored on metal-caged graphitic carbon towards efficient and durable hydrogen evolution electrocatalysis. Nano Energy 2018, 48, 500–509. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Ding, C.; Xu, Z.; Wang, H.; Zong, X.; Li, C. Amorphous Multi-elements Electrocatalysts with Tunable Bifunctionality toward Overall Water Splitting. ACS Catal. 2018, 8, 9926–9935. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Qi, S.; Zhu, K.; Wang, H.; Zhang, G.; Ma, W.; Zong, X. Ultrafast Synthesis of Mo2C-Based Catalyst by Joule Heating towards Electrocatalytic Hydrogen Evolution Reaction. Symmetry 2023, 15, 801. https://doi.org/10.3390/sym15040801
Zhang H, Qi S, Zhu K, Wang H, Zhang G, Ma W, Zong X. Ultrafast Synthesis of Mo2C-Based Catalyst by Joule Heating towards Electrocatalytic Hydrogen Evolution Reaction. Symmetry. 2023; 15(4):801. https://doi.org/10.3390/sym15040801
Chicago/Turabian StyleZhang, Hefeng, Shengliang Qi, Kaixin Zhu, Haidong Wang, Guanghui Zhang, Weiguang Ma, and Xu Zong. 2023. "Ultrafast Synthesis of Mo2C-Based Catalyst by Joule Heating towards Electrocatalytic Hydrogen Evolution Reaction" Symmetry 15, no. 4: 801. https://doi.org/10.3390/sym15040801






