Brain Lateralization for Language, Vocabulary Development and Handedness at 18 Months
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Ethics and Informed Consent
2.3. Procedure
2.3.1. Evaluation of Brain Lateralization for Language
2.3.2. Handedness Evaluation
2.3.3. Language Evaluation
2.4. Data Analyses
2.4.1. EEG Processing
2.4.2. Handedness
2.4.3. Language Development
2.5. Statistical Analyses
3. Results
3.1. Handedness
3.2. Language Development
3.3. ERP Lateralization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryden, M.; Hécaen, H.; DeAgostini, M. Patterns of cerebral organization. Brain Lang. 1983, 20, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.P.; Johnstone, L.T. Quantifying cerebral asymmetries for language in dextrals and adextrals with random-effects meta analysis. Front. Psychol. 2014, 5, 1128. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.A.; Binder, J.R.; Hammeke, T.A.; Swanson, S.J.; Frost, J.A.; Bellgowan, P.S.; Brewer, C.C.; Perry, H.M.; Morris, G.L.; Mueller, W.M. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain A J. Neurol. 1999, 122, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.-B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Deus, J.; Losilla, J.M.; Capdevila, A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology 1999, 52, 1038. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Binder, J.R.; Possing, E.T.; McKiernan, K.A.; Ward, B.D.; Hammeke, T.A. Language lateralization in left-handed and ambidextrous people: FMRI data. Neurology 2002, 59, 238–244. [Google Scholar] [CrossRef]
- Fagard, J.; Chapelain, A.; Bonnet, P. How should “ambidexterity” be estimated? Laterality 2015, 20, 543–570. [Google Scholar] [CrossRef]
- Higuchi, S.; Chaminade, T.; Imamizu, H.; Kawato, M. Shared neural correlates for language and tool use in Broca’s area. Neuroreport 2009, 20, 1376–1381. [Google Scholar] [CrossRef]
- Greenfield, P.M.; Nelson, K.; Saltzman, E. The development of rulebound strategies for manipulating seriated cups: A parallel between action and grammar. Cogn. Psychol. 1972, 3, 291–310. [Google Scholar] [CrossRef]
- Packheiser, J.; Schmitz, J.; Arning, L.; Beste, C.; Gunturkun, O.; Ocklenburg, S. A large-scale estimate on the relationship between language and motor lateralization. Sci. Rep. 2020, 10, 13027. [Google Scholar] [CrossRef]
- Francks, C.; Maegawa, S.; Laurén, J.; Abrahams, B.S.; Velayos-Baeza, A.; Medland, S.; Colella, S.; Groszer, M.; McAuley, E.Z.; Caffrey, T.M.; et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol. Psychiatry 2007, 12, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Beste, C.; Güntürkün, O. Handedness: A neurogenetic shift of perspective. Neurosci. Biobehav. Rev. 2013, 37, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.L.; Davison, A.; McManus, I. Genome-wide association study of handedness excludes simple genetic models. Heredity 2014, 112, 221–225. [Google Scholar] [CrossRef] [PubMed]
- McManus, I.C.; Davison, A.; Armour, J.A.L. Multilocus genetic models of handedness closely resemble single-locus models in explaining family data and are compatible with genome-wide association studies. Ann. N. Y. Acad. Sci. 2013, 1288, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hepper, P.G.; Shahidullah, S.; White, R. Handedness in the human fetus. Neuropsychologia 1991, 29, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- McCartney, G.; Hepper, P. Development of lateralized behaviour in the human fetus from 12 to 27 weeks’ gestation. Dev. Med. Child Neurol. 1999, 41, 83–86. [Google Scholar] [CrossRef]
- Kurjak, A.; Vecek, N.; Hafner, T.; Bozek, T.; Funduk-Kurjak, B.; Ujevic, B. Prenatal diagnosis: What does four-dimensional ultrasound add? J. Perinat. Med. 2002, 30, 57–62. [Google Scholar] [CrossRef]
- de Vries, J.; Wimmers, R.; Ververs, I.; Hopkins, B.; Savelsbergh, G.; van Geijn, H. Fetal handedness and head position preference: A developmental study. Dev. Psychobiol. 2001, 39, 171–178. [Google Scholar] [CrossRef]
- Myowa-Yamakoshi, M.; Takeshita, H. Do Human Fetuses Anticipate Self-Oriented Actions? A Study by Four-Dimensional (4D) Ultrasonography. Infancy 2006, 10, 289–301. [Google Scholar] [CrossRef]
- Ververs, I.A.; de Vries, J.I.; van Geijn, H.P.; Hopkins, B. Prenatal head position from 12-38 weeks. weeks. I. Developmental aspects. Early Hum. Dev. 1994, 39, 83–91. [Google Scholar] [CrossRef]
- Van der Meer, A.L. Keeping the arm in the limelight: Advanced visual control of arm movements in neonates. Eur. J. Paediatr. Neurol. 1997, 4, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.F.; Harkins, D.A. Postural and lateral asymmetries in the ontogeny of handedness during infancy. Dev. Psychobiol. 1986, 19, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, D.; Bojczyk, K.E. Infants Return to Two-Handed Reaching When They Are Learning to Walk. J. Mot. Behav. 2002, 34, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.F.; Tyler, A.N.; Ferre, C.; Sheu, C.-F. The manifestation of infant hand-use preferences when reaching for objects during the seven- to thirteen-month age period. Dev. Psychobiol. 2006, 48, 436–443. [Google Scholar] [CrossRef]
- Nelson, E.L.; Campbell, J.M.; Michel, G.F. Unimanual to bimanual: Tracking the development of handedness from 6 to 24 months. Infant Behav. Dev. 2013, 36, 181–188. [Google Scholar] [CrossRef]
- Fagard, J. The nature and nurture of human infant hand preference. Ann. N. Y. Acad. Sci. 2013, 1288, 114–123. [Google Scholar] [CrossRef]
- Ferre, C.L.; Babik, I.; Michel, G.F. Development of infant prehension handedness: A longitudinal analysis during the 6- to 14-month age period. Infant Behav. Dev. 2010, 33, 492–502. [Google Scholar] [CrossRef]
- Fagard, J.; Margules, S.; Lopez, C.; Granjon, L.; Huet, V. How should we test infant handedness? Laterality 2016, 22, 294–312. [Google Scholar] [CrossRef]
- Michel, G.F.; Babik, I.; Sheu, C.-F.; Campbell, J.M. Latent classes in the developmental trajectories of infant handedness. Dev. Psychol. 2014, 50, 349–359. [Google Scholar] [CrossRef]
- Jacquet, A.-Y.; Esseily, R.; Rider, D.; Fagard, J. Handedness for grasping objects and declarative pointing: A longitudinal study. Dev. Psychobiol. 2012, 54, 36–46. [Google Scholar] [CrossRef]
- Nelson, E.L.; Gonzalez, S.L. Measuring infant handedness reliably from reaching: A systematic review. Laterality 2020, 25, 430–454. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, M.; Dehaene-Lambertz, G.; Fournier, M.; Kongolo, G.; Goudjil, S.; Dubois, J.; Grebe, R.; Wallois, F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc. Natl. Acad. Sci. USA. 2013, 110, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Dehaene-Lambertz, G.; Spelke, E. The Infancy of the Human Brain. Neuron 2015, 88, 93–109. [Google Scholar] [CrossRef]
- Gervain, J.; Macagno, F.; Cogoi, S.; Pena, M.; Mehler, J. The neonate brain detects speech structure. Proc. Natl. Acad. Sci. USA. 2008, 105, 14222–14227. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.B.; Schild, U.; Friedrich, C.K. ERP correlates of word onset priming in infants and young children. Dev. Cogn. Neurosci. 2014, 9, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Arimitsu, T.; Uchida-Ota, M.; Yagihashi, T.; Kojima, S.; Watanabe, S.; Hokuto, I.; Ikeda, K.; Takahashi, T.; Minagawa-Kawai, Y. Functional Hemispheric Specialization in Processing Phonemic and Prosodic Auditory Changes in Neonates. Front. Psychol. 2011, 2, 202. [Google Scholar] [CrossRef]
- Bertoncini, J.; Morais, J.; Bijeljac-Babic, R.; McAdams, S.; Peretz, I.; Mehler, J. Dichotic perception and laterality in neonates. Brain Lang. 1989, 37, 591–605. [Google Scholar] [CrossRef]
- DeCasper, A.J.; Prescott, P. Lateralized processes constrain auditory reinforcement in human newborns. Hear. Res. 2009, 255, 135–141. [Google Scholar] [CrossRef]
- Shultz, S.; Vouloumanos, A.; Bennett, R.H.; Pelphrey, K. Neural specialization for speech in the first months of life. Dev. Sci. 2014, 17, 766–774. [Google Scholar] [CrossRef]
- Perani, D.; Saccuman, M.C.; Scifo, P.; Anwander, A.; Spada, D.; Baldoli, C.; Poloniato, A.; Lohmann, G.; Friederici, A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. USA 2011, 108, 16056–16061. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G.; Montavont, A.; Jobert, A.; Allirol, L.; Dubois, J.; Hertz-Pannier, L.; Dehaene, S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang. 2009, 114, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, C.; Nazzi, T.; Gervain, J. Hemispheric Asymmetries in Repetition Enhancement and Suppression Effects in the Newborn Brain. PLoS ONE 2015, 10, e0140160. [Google Scholar] [CrossRef] [PubMed]
- Cristia, A.; Minagawa-Kawai, Y.; Egorova, N.; Gervain, J.; Filippin, L.; Cabrol, D.; Dupoux, E. Neural correlates of infant accent discrimination: An fNIRS study. Dev. Sci. 2014, 17, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, Y.; Naoi, N.; Nishijima, N.; Kojima, S.; Dupoux, E. Developmental changes in cerebral responses to native and non-native vowels: A NIRS Study. In Proceedings of the ICPhS XVI Symposium, Saarbrucken, Germany, 6–10 August 2007. ID 1487. [Google Scholar]
- Bisiacchi, P.; Cainelli, E. Structural and functional brain asymmetries in the early phases of life: A scoping review. Anat. Embryol. 2022, 227, 479–496. [Google Scholar] [CrossRef]
- Esseily, R.; Jacquet, A.-Y.; Fagard, J. Handedness for grasping objects and pointing and the development of language in 14-month-old infants. Laterality 2011, 16, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Kühn-Popp, N.; Kristen, S.; Paulus, M.; Meinhardt, J.; Sodian, B. Left hemisphere EEG coherence in infancy predicts infant declarative pointing and preschool epistemic language. Soc. Neurosci. 2016, 11, 49–59. [Google Scholar] [CrossRef]
- Kohler, M.; Keage, H.; Spooner, R.; Flitton, A.; Hofmann, J.; Churches, O.; Elliott, S.; Badcock, N. Variability in lateralised blood flow response to language is associated with language development in children aged 1–5 years. Brain Lang. 2015, 145-146, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.L.; Campbell, J.M.; Michel, G.F. Early handedness in infancy predicts language ability in toddlers. Dev. Psychol. 2014, 50, 809–814. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Hirst, R.; Hudson, J.M. Hemispheric speech lateralisation in the developing brain is related to motor praxis ability. Dev. Cogn. Neurosci. 2016, 22, 9–17. [Google Scholar] [CrossRef]
- Goldfield, B.A.; Reznick, J.S. Early lexical acquisition: Rate, content, and the vocabulary spurt. J. Child Lang. 1990, 17, 171–183. [Google Scholar] [CrossRef]
- Cochet, H.; Jover, M.; Vauclair, J. Hand preference for pointing gestures and bimanual manipulation around the vocabulary spurt period. J. Exp. Child Psychol. 2011, 110, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Ganger, J.; Brent, M.R. Reexamining the Vocabulary Spurt. Dev. Psychol. 2004, 40, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, E.; Salomon, L.; Fagard, J. Hand movements in communicative and non-communicative situations in very young infants: A preliminary study. J. Mot. Learn. Dev. 2021, 9, 132–152. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G.; Dehaene, S. Speed and cerebral correlates of syllable discrimination in infants. Nature 1994, 370, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Fagard, J.; Corbetta, D.; Somogyi, E.; Safar, A.; Bernard, C. Right-handed one day, right-handed the next day? Laterality 2020, 1–14. [Google Scholar] [CrossRef]
- Fenson, L.; Dale, P.S.; Reznick, J.S.; Thal, D.; Bates, E.; Hartung, J.P.; Pethick, S.; Reilly, J.S. The MacArthur Development Inventories: User’s Guide and Technical Manual; Singular Publishing Group: San Diego, CA, USA, 1993. [Google Scholar]
- Bovet, F.; Danjou, G.; Langue, J.; Moretto, M.; Tockert, E.; Kern, S. Un nouvel outil d’évaluation du développement communicatif du nourrisson. Médecine Enfance 2005, 25, 67–74. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Adibpour, P.; Lebenberg, J.; Kabdebon, C.; Dehaene-Lambertz, G.; Dubois, J. Anatomo-functional correlates of auditory development in infancy. Dev. Cogn. Neurosci. 2020, 42, 100752. [Google Scholar] [CrossRef]
- Bristow, D.; Dehaene-Lambertz, G.; Mattout, J.; Soares, C.; Gliga, T.; Baillet, S.; Mangin, J.-F. Hearing Faces: How the Infant Brain Matches the Face It Sees with the Speech It Hears. J. Cogn. Neurosci. 2009, 21, 905–921. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G. Cerebral Specialization for Speech and Non-Speech Stimuli in Infants. J. Cogn. Neurosci. 2000, 12, 449–460. [Google Scholar] [CrossRef]
- Wunderlich, J.L.; Cone-Wesson, B.K.; Shepherd, R. Maturation of the cortical auditory evoked potential in infants and young children. Hear. Res. 2006, 212, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kotilahti, K.; Nissilä, I.; Näsi, T.; Lipiäinen, L.; Noponen, T.; Meriläinen, P.; Huotilainen, M.; Fellman, V. Hemodynamic responses to speech and music in newborn infants. Hum. Brain Mapp. 2009, 31, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Vannasing, P.; Florea, O.; González-Frankenberger, B.; Tremblay, J.; Paquette, N.; Safi, D.; Wallois, F.; Lepore, F.; Béland, R.; Lassonde, M.; et al. Distinct hemispheric specializations for native and non-native languages in one-day-old newborns identified by fNIRS. Neuropsychologia 2016, 84, 63–69. [Google Scholar] [CrossRef]
- Pena, M.; Maki, A.; Kovacic, D.; Dehaene-Lambertz, G.; Koizumi, H.; Bouquet, F.; Mehler, J. Sounds and silence: An optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. USA 2003, 100, 11702–11705. [Google Scholar] [CrossRef]
- Bisiacchi, P.; Marzi, C.; Nicoletti, R.; Carena, G.; Mucignat, C.; Tomaiuolo, F. Left-right asymmetry of callosal transfer in normal human subjects. Behav. Brain Res. 1994, 64, 173–178. [Google Scholar] [CrossRef]
- Zhang, F.; Gervain, J.; Roeyers, H. Developmental changes in the brain response to speech during the first year of life: A near-infrared spectroscopy study of dutch-learning infants. Infant Behav. Dev. 2022, 67, 101724. [Google Scholar] [CrossRef]
- Molfese, D.L.; Freeman, R.B.; Palermo, D.S. The ontogeny of brain lateralization for speech and nonspeech stimuli. Brain Lang. 1975, 2, 356–368. [Google Scholar] [CrossRef]
- Telkemeyer, S.; Rossi, S.; Koch, S.P.; Nierhaus, T.; Steinbrink, J.; Poeppel, D.; Obrig, H.; Wartenburger, I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 2009, 29, 14726–14733. [Google Scholar] [CrossRef]
- Arimitsu, T.; Minagawa, Y.; Yagihashi, T.; Uchida, M.O.; Matsuzaki, A.; Ikeda, K.; Takahashi, T. The cerebral hemodynamic response to phonetic changes of speech in preterm and term infants: The impact of postmenstrual age. NeuroImage Clin. 2018, 19, 599–606. [Google Scholar] [CrossRef]
- Minagawa-Kawai, Y.; Cristià, A.; Vendelin, I.; Cabrol, D.; Dupoux, E. Assessing Signal-Driven Mechanisms in Neonates: Brain Responses to Temporally and Spectrally Different Sounds. Front. Psychol. 2011, 2, 135. [Google Scholar] [CrossRef] [PubMed]
- De Vareilles, H.; Rivière, D.; Pascucci, M.; Sun, Z.-Y.; Fischer, C.; Leroy, F.; Tataranno, M.-L.; Benders, M.J.; Dubois, J.; Mangin, J.-F. Exploring the emergence of morphological asymmetries around the brain’s Sylvian fissure: A longitudinal study of shape variability in preterm infants. Cereb. Cortex 2023, bhac533. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Benders, M.; Cachia, A.; Lazeyras, F.; Leuchter, R.H.-V.; Sizonenko, S.V.; Borradori-Tolsa, C.; Mangin, J.F.; Huppi, P.S. Mapping the Early Cortical Folding Process in the Preterm Newborn Brain. Cereb. Cortex 2008, 18, 1444–1454. [Google Scholar] [CrossRef]
- Habas, P.A.; Scott, J.A.; Roosta, A.; Rajagopalan, V.; Kim, K.; Rousseau, F.; Barkovich, A.J.; Glenn, O.A.; Studholme, C. Early Folding Patterns and Asymmetries of the Normal Human Brain Detected from in Utero MRI. Cereb. Cortex 2012, 22, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Dierker, D.; Neil, J.; Inder, T.; Knutsen, A.; Harwell, J.; Coalson, T.; Van Essen, D. A Surface-Based Analysis of Hemispheric Asymmetries and Folding of Cerebral Cortex in Term-Born Human Infants. J. Neurosci. 2010, 30, 2268–2276. [Google Scholar] [CrossRef]
- Glasel, H.; Leroy, F.; Dubois, J.; Hertz-Pannier, L.; Mangin, J.; Dehaene-Lambertz, G. A robust cerebral asymmetry in the infant brain: The rightward superior temporal sulcus. Neuroimage 2011, 58, 716–723. [Google Scholar] [CrossRef]
- Leroy, F.; Cai, Q.; Bogart, S.L.; Dubois, J.; Coulon, O.; Monzalvo, K.; Fischer, C.; Glasel, H.; Van der Haegen, L.; Benezit, A.; et al. New human-specific brain landmark: The depth asymmetry of superior temporal sulcus. Proc. Natl. Acad. Sci. USA. 2015, 112, 1208–1213. [Google Scholar] [CrossRef]
- Leroy, F.; Glasel, H.; Dubois, J.; Hertz-Pannier, L.; Thirion, B.; Mangin, J.F.; Dehaene-Lambertz, G. Early maturation of the linguistic dorsal pathway in human infants. J. Neurosci. 2011, 31, 1500–1506. [Google Scholar] [CrossRef]
- Rolland, C.; Lebenberg, J.; Leroy, F.; Moulton, E.; Adibpour, P.; Riviere, D.; Poupon, C.; Hertz-Pannier, L.; Mangin, J.-F.; Dehaene-Lambertz, G.; et al. Exploring Microstructure Asymmetries in the Infant Brain Cortex: A Methodological Framework Combining Structural and Diffusion Mri. In Proceedings of the 019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 426–429. [Google Scholar]
- Dubois, J.; Hertz-Pannier, L.; Cachia, A.; Mangin, J.F.; Le Bihan, D.; Dehaene-Lambertz, G. Structural Asymmetries in the Infant Language and Sensori-Motor Networks. Cereb. Cortex 2009, 19, 414–423. [Google Scholar] [CrossRef]
- Dubois, J.; Poupon, C.; Thirion, B.; Simonnet, H.; Kulikova, S.; Leroy, F.; Hertz-Pannier, L.; Dehaene-Lambertz, G. Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb. Cortex 2016, 26, 2283–2298. [Google Scholar] [CrossRef]
- Adibpour, P.; Dubois, J.; Moutard, M.-L.; Dehaene-Lambertz, G. Early asymmetric inter-hemispheric transfer in the auditory network: Insights from infants with corpus callosum agenesis. Anat. Embryol. 2018, 223, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Klöppel, S.; Rivière, D.; Perrot, M.; Frackowiak, R.; Siebner, H.; Mangin, J.-F. The effect of handedness on the shape of the central sulcus. Neuroimage 2012, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cantiani, C.; Riva, V.; Piazza, C.; Bettoni, R.; Molteni, M.; Choudhury, N.; Marino, C.; Benasich, A.A. Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Dev. Cogn. Neurosci. 2016, 20, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cantiani, C.; Ortiz-Mantilla, S.; Riva, V.; Piazza, C.; Bettoni, R.; Musacchia, G.; Molteni, M.; Marino, C.; Benasich, A.A. Reduced left-lateralized pattern of event-related EEG oscillations in infants at familial risk for language and learning impairment. NeuroImage Clin. 2019, 22, 101778. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.S.; Beach, S.D.; Eddy, M.D.; McWeeny, S.; Ozernov-Palchik, O.; Gaab, N.; Gabrieli, J.D.E. ERP Mismatch Negativity Amplitude and Asymmetry Reflect Phonological and Rapid Automatized Naming Skills in English-Speaking Kindergartners. Front. Hum. Neurosci. 2021, 15, 624617. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ferradal, S.L.; Sliva, D.D.; Dunstan, J.; Carruthers, C.; Sanfilippo, J.; Zuk, J.; Zöllei, L.; Boyd, E.; Gagoski, B.; et al. Functional Connectivity in Infancy and Toddlerhood Predicts Long-Term Language and Preliteracy Outcomes. Cereb. Cortex 2021, bhab230. [Google Scholar] [CrossRef]
- Zuk, J.; Yu, X.; Sanfilippo, J.; Figuccio, M.J.; Dunstan, J.; Carruthers, C.; Sideridis, G.; Turesky, T.K.; Gagoski, B.; Grant, P.E.; et al. White matter in infancy is prospectively associated with language outcomes in kindergarten. Dev. Cogn. Neurosci. 2021, 50, 100973. [Google Scholar] [CrossRef]
- Aeby, A.; De Tiège, X.; Creuzil, M.; David, P.; Balériaux, D.; Van Overmeire, B.; Metens, T.; Van Bogaert, P. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: A diffusion tensor imaging study. Neuroimage 2013, 78, 145–151. [Google Scholar] [CrossRef]
- Groen, M.A.; Whitehouse, A.J.O.; Badcock, N.A.; Bishop, D.V.M. Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain Behav. 2012, 2, 256–269. [Google Scholar] [CrossRef] [PubMed]
- O’Muircheartaigh, J.; Dean, D.C., 3rd; Dirks, H.; Waskiewicz, N.; Lehman, K.; Jerskey, B.A.; Deoni, S.C. Interactions between white matter asymmetry and language during neurodevelopment. J. Neurosci. 2013, 33, 16170–16177. [Google Scholar] [CrossRef]
- Fagard, J.; Sirri, L.; Rã¤Mã¤, P. Effect of handedness on the occurrence of semantic N400 priming effect in 18- and 24-month-old children. Front. Psychol. 2014, 5, 355. [Google Scholar] [CrossRef] [PubMed]
- Provins, K.A. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychol. Rev. 1997, 104, 554–571. [Google Scholar] [CrossRef] [PubMed]
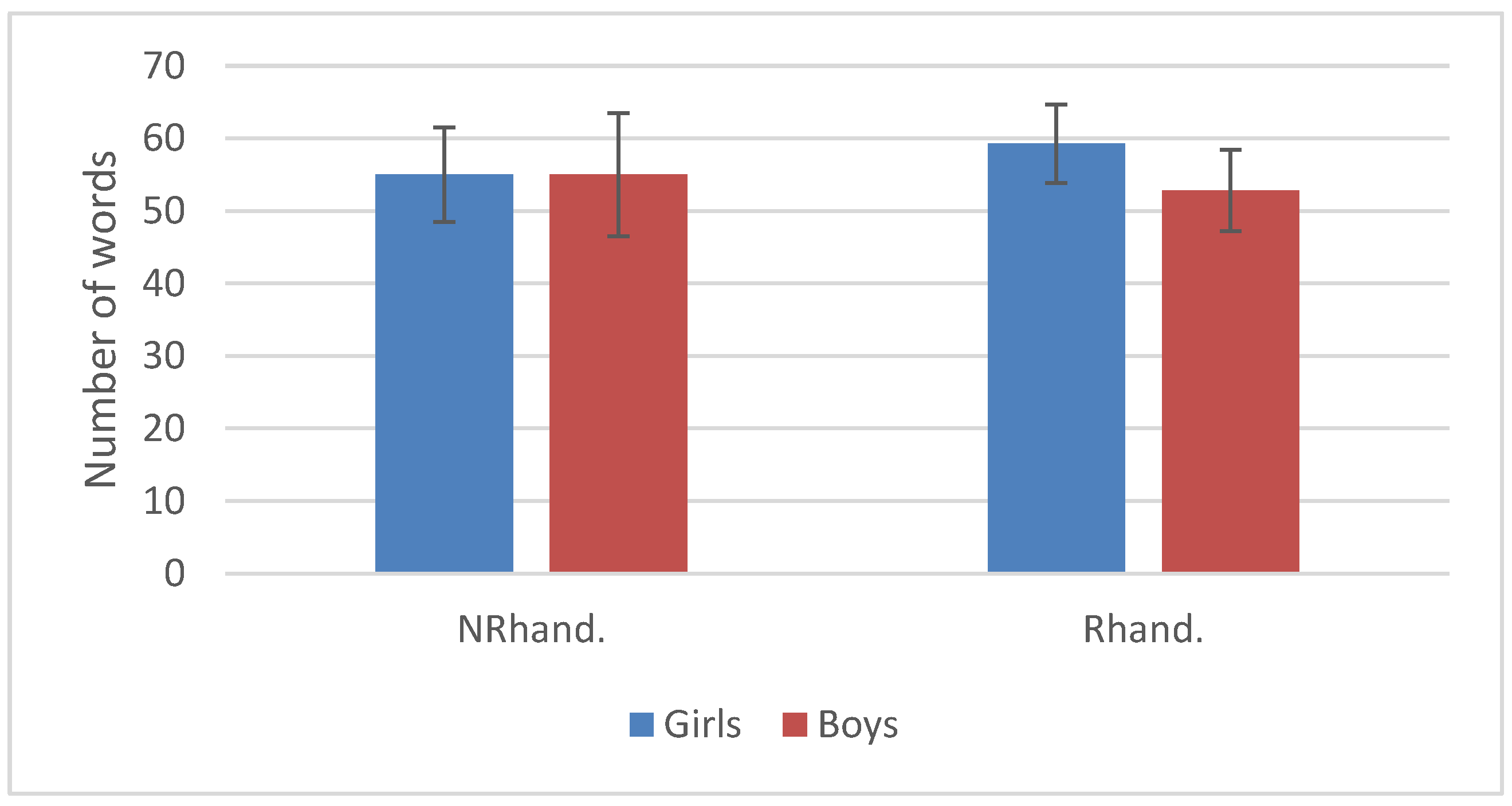
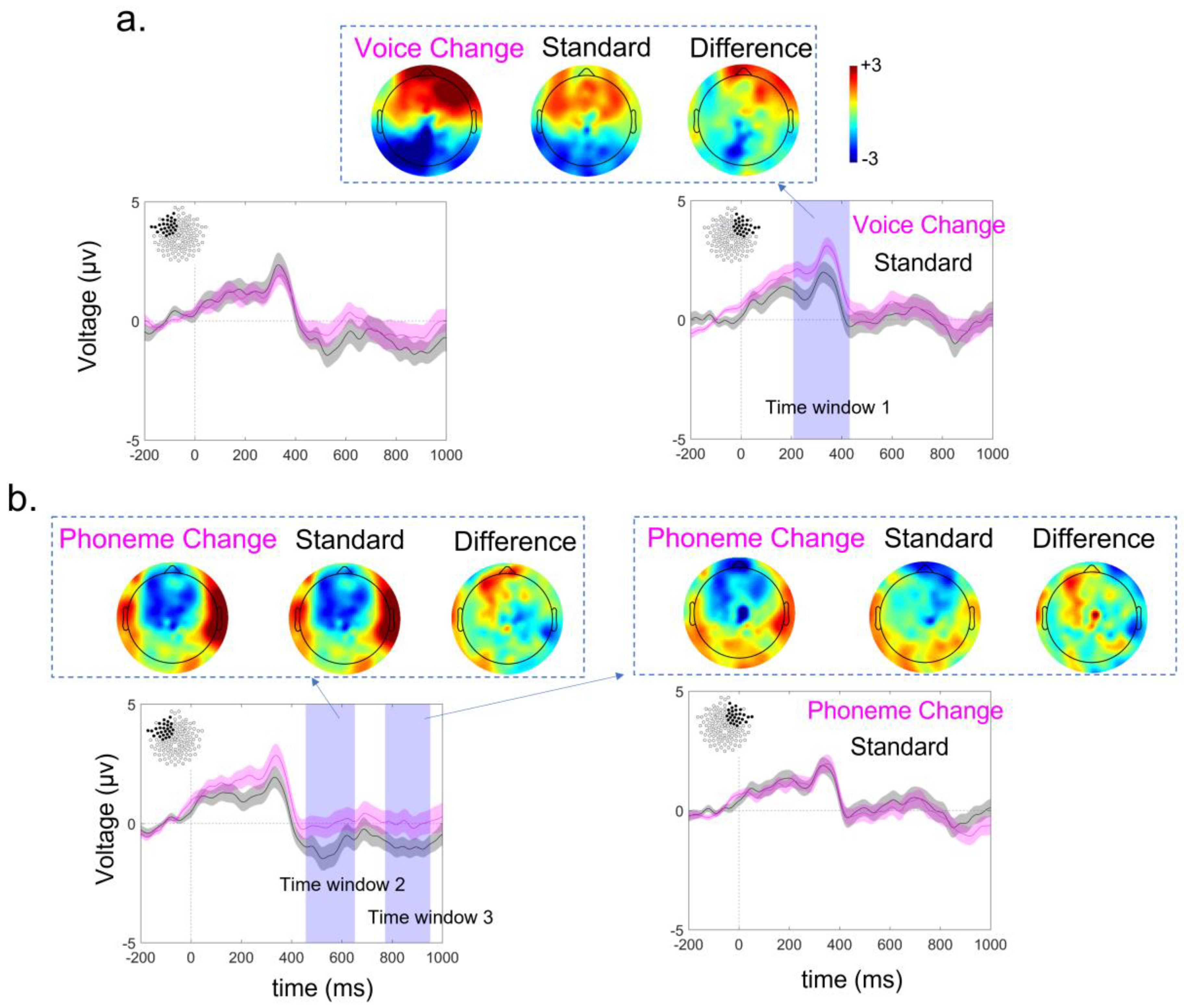
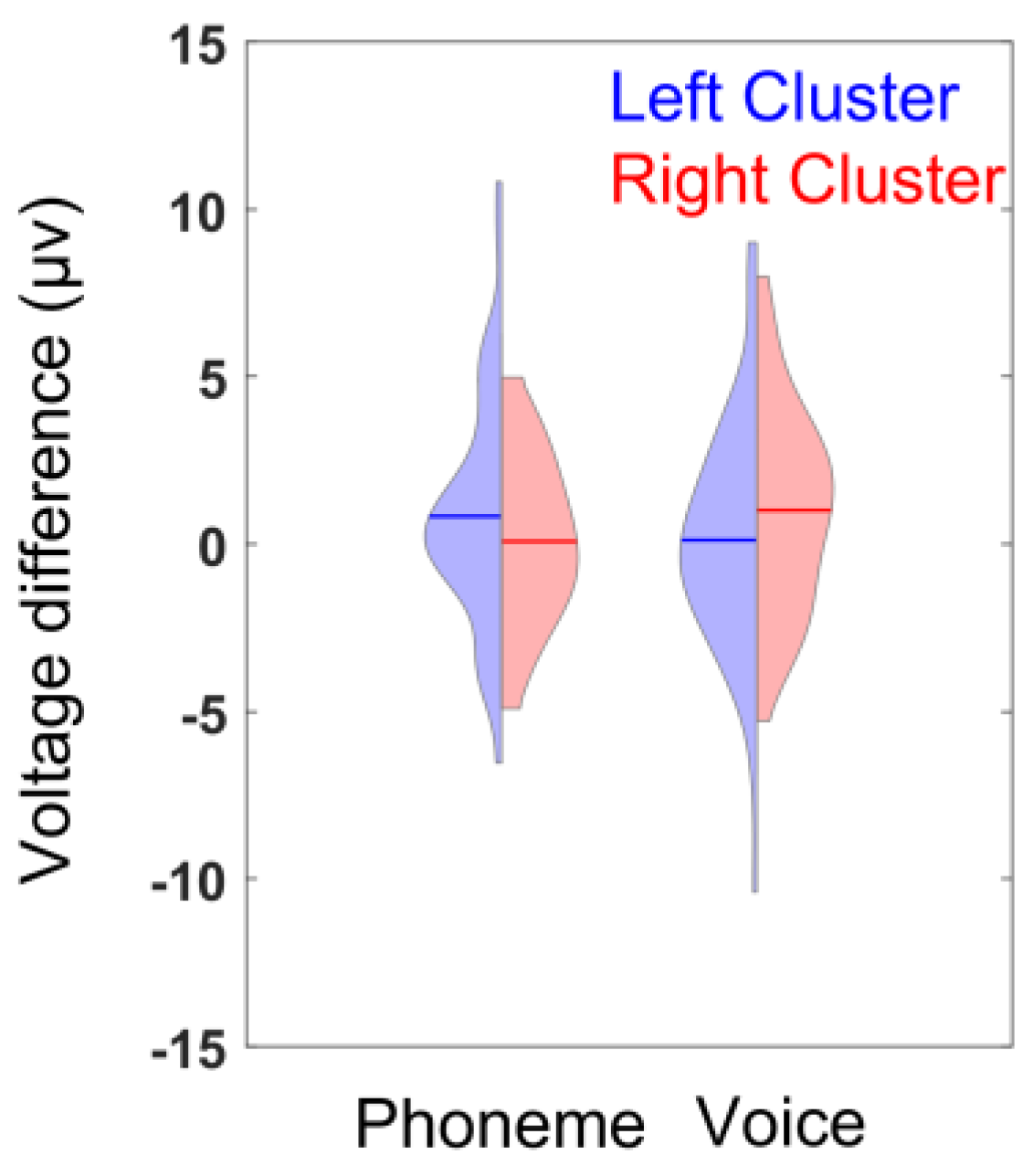



| Mean HI | SD | SE | N | |
|---|---|---|---|---|
| Handedness (HI) | 0.39 | 0.38 | 0.05 | 48 |
| Handedness (+Voc) | 0.39 | 0.38 | 0.06 | 46 |
| Voc (Number of words) | 55.69 | 12.84 | 1.85 | 48 |
| Voc (+Handedness) | 55.83 | 12.75 | 1.88 | 46 |
| ANCOVA for Time Window T1 | Statistics | ƞ2 |
|---|---|---|
| handedness | F(1,42) = 0.8, p = 0.373 | 0.18 |
| vocabulary | F(1,42) = 0.1, p = 0.766 | 0.02 |
| cluster | F(1,42) = 0.3, p = 0.558 | 0.10 |
| condition | F(1,42) = 0.0, p = 0.925 | 0.00 |
| handedness × vocabulary | F(1,42) = 3.7, p = 0.061 | 0.81 |
| handedness × cluster | F(1,42) = 1.9, p = 0.177 | 0.53 |
| handedness × condition | F(1,42) = 0.2, p = 0.695 | 0.07 |
| vocabulary × cluster | F(1,42) = 1.2, p = 0.289 | 0.32 |
| vocabulary × condition | F(1,42) = 1.5, p = 0.231 | 0.66 |
| cluster × condition | F(1,42) = 6.5, p = 0.015 * | 0.96 |
| handedness × vocabulary × cluster | F(1,42) = 0.2, p = 0.671 | 0.05 |
| handedness × vocabulary × condition | F(1,42) = 0.6, p = 0.448 | 0.26 |
| handedness × cluster × condition | F(1,42) = 0.2, p = 0.660 | 0.03 |
| vocabulary × cluster × condition | F(1,42) = 0.0, p = 0.989 | 0.00 |
| handedness × vocabulary × cluster × condition | F(1,42) = 0.1, p = 0.801 | 0.01 |
| ANCOVA for Time Window T2 | Statistics | ƞ2 |
|---|---|---|
| handedness | F(1,42) = 0.4, p = 0.515 | 0.06 |
| vocabulary | F(1,42) = 1.2, p = 0.276 | 0.18 |
| cluster | F(1,42) = 1.2, p = 0.270 | 0.30 |
| condition | F(1,42) = 0.1, p = 0.744 | 0.02 |
| handedness × vocabulary | F(1,42) = 5.3, p = 0.026 * | 0.76 |
| handedness × cluster | F(1,42) = 2.8, p = 0.104 | 0.66 |
| handedness × condition | F(1,42) = 0.1, p = 0.769 | 0.02 |
| vocabulary × cluster | F(1,42) = 0.0, p = 0.954 | 0.00 |
| vocabulary × condition | F(1,42) = 0.6, p = 0.457 | 0.11 |
| cluster × condition | F(1,42) = 0.1, p = 0.784 | 0.05 |
| handedness × vocabulary × cluster | F(1,42) = 0.2, p = 0.685 | 0.04 |
| handedness × vocabulary × condition | F(1,42) = 4.5, p = 0.040 * | 0.86 |
| handedness × cluster × condition | F(1,42) = 0.0, p = 0.862 | 0.02 |
| vocabulary × cluster × condition | F(1,42) = 0.5, p = 0.486 | 0.32 |
| handedness × vocabulary × cluster × condition | F(1,42) = 0.9, p = 0.340 | 0.61 |
| ANCOVA for Time Window T3 | Statistics | ƞ2 |
|---|---|---|
| handedness | F(1,42) = 0.3, p = 0.603 | 0.05 |
| vocabulary | F(1,42) = 4.7, p = 0.035 * | 0.81 |
| cluster | F(1,42) = 1.0, p = 0.315 | 0.13 |
| condition | F(1,42) = 0.1, p = 0.811 | 0.01 |
| handedness × vocabulary | F(1,42) = 0.8, p = 0.367 | 0.14 |
| handedness × cluster | F(1,42) = 4.5, p = 0.041 * | 0.56 |
| handedness × condition | F(1,42) = 0.9, p = 0.336 | 0.24 |
| vocabulary × cluster | F(1,42) = 0.6, p = 0.441 | 0.08 |
| vocabulary × condition | F(1,42) = 0.2, p = 0.656 | 0.05 |
| cluster × condition | F(1,42) = 0.5, p = 0.505 | 0.12 |
| handedness × vocabulary × cluster | F(1,42) = 1.9, p = 0.177 | 0.24 |
| handedness × vocabulary × condition | F(1,42) = 2.7, p = 0.105 | 0.70 |
| handedness × cluster × condition | F(1,42) = 1.0, p = 0.314 | 0.27 |
| vocabulary × cluster × condition | F(1,42) = 1.9, p = 0.176 | 0.49 |
| handedness × vocabulary × cluster × condition | F(1,42) = 0.5, p = 0.492 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potdevin, D.; Adibpour, P.; Garric, C.; Somogyi, E.; Dehaene-Lambertz, G.; Rämä, P.; Dubois, J.; Fagard, J. Brain Lateralization for Language, Vocabulary Development and Handedness at 18 Months. Symmetry 2023, 15, 989. https://doi.org/10.3390/sym15050989
Potdevin D, Adibpour P, Garric C, Somogyi E, Dehaene-Lambertz G, Rämä P, Dubois J, Fagard J. Brain Lateralization for Language, Vocabulary Development and Handedness at 18 Months. Symmetry. 2023; 15(5):989. https://doi.org/10.3390/sym15050989
Chicago/Turabian StylePotdevin, Delphine, Parvaneh Adibpour, Clémentine Garric, Eszter Somogyi, Ghislaine Dehaene-Lambertz, Pia Rämä, Jessica Dubois, and Jacqueline Fagard. 2023. "Brain Lateralization for Language, Vocabulary Development and Handedness at 18 Months" Symmetry 15, no. 5: 989. https://doi.org/10.3390/sym15050989
APA StylePotdevin, D., Adibpour, P., Garric, C., Somogyi, E., Dehaene-Lambertz, G., Rämä, P., Dubois, J., & Fagard, J. (2023). Brain Lateralization for Language, Vocabulary Development and Handedness at 18 Months. Symmetry, 15(5), 989. https://doi.org/10.3390/sym15050989








