A Symmetry Concept for the Self-Assembly Synthesis of Mn-MIL-100 Using a Capping Agent and Its Adsorption Performance with Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mn-MIL-100
2.3. Synthesis of Mn-MIL-100-CA
2.4. General Characterization
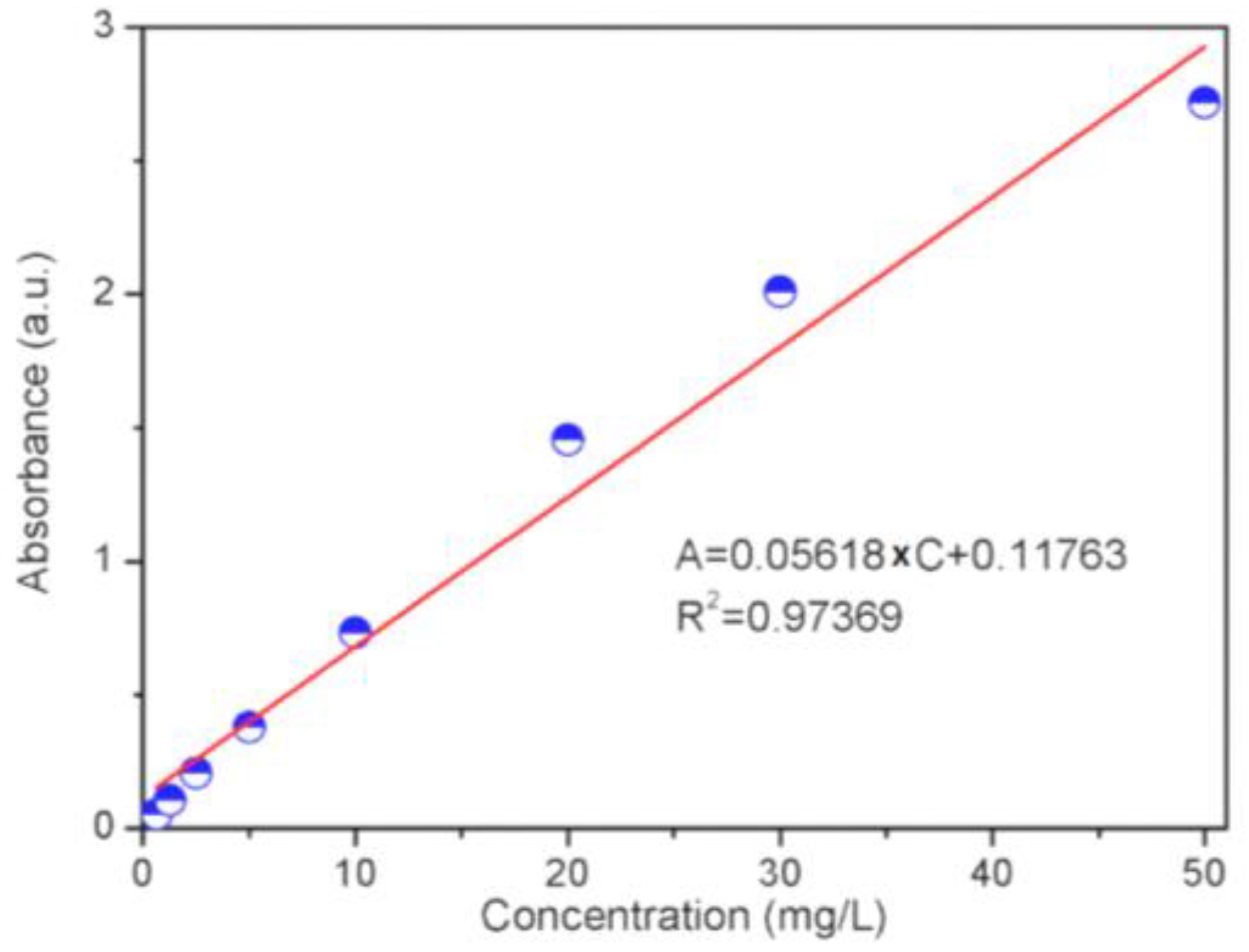
2.5. Adsorption Performance Evaluation
3. Results and Discussion
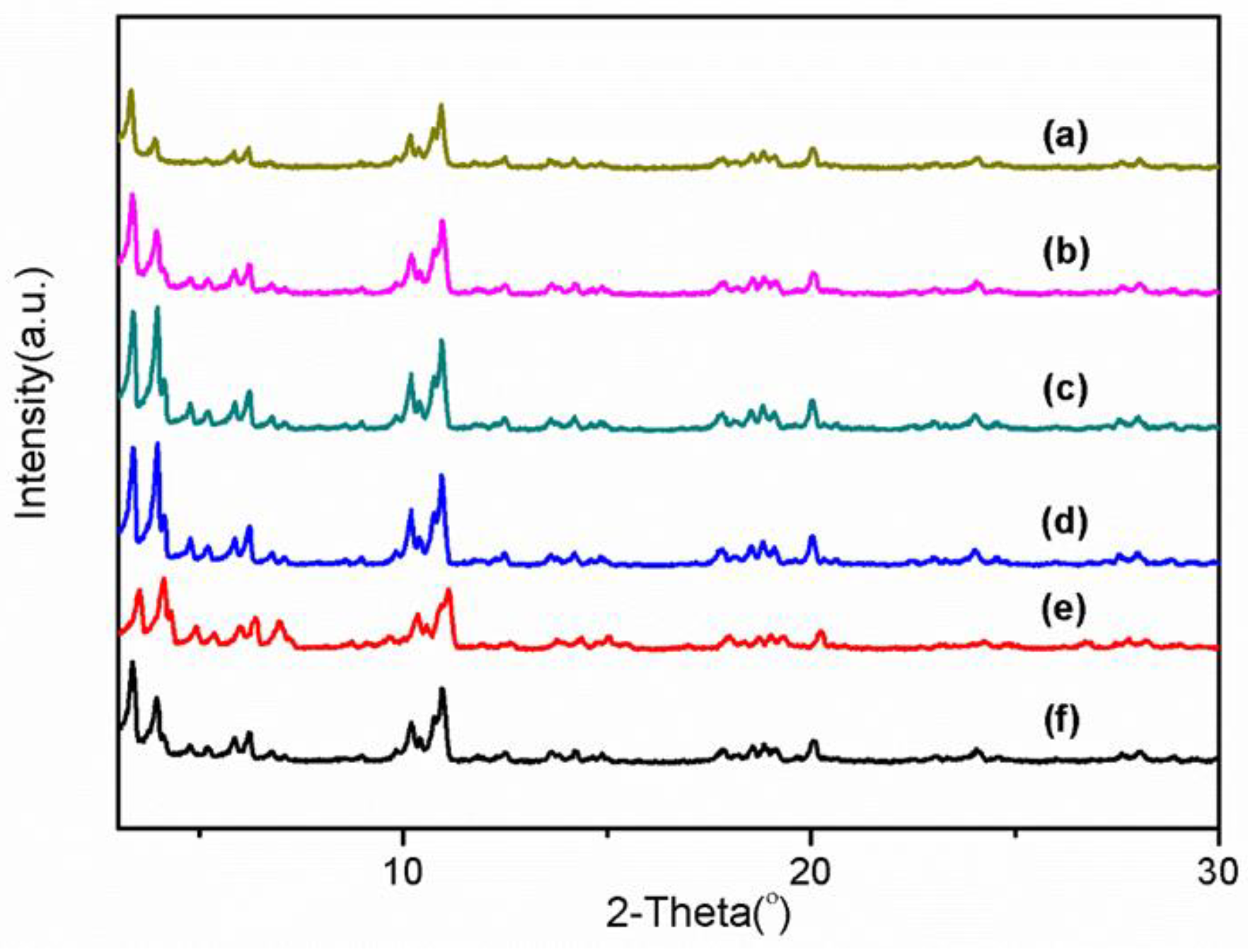
3.1. Structural Properties
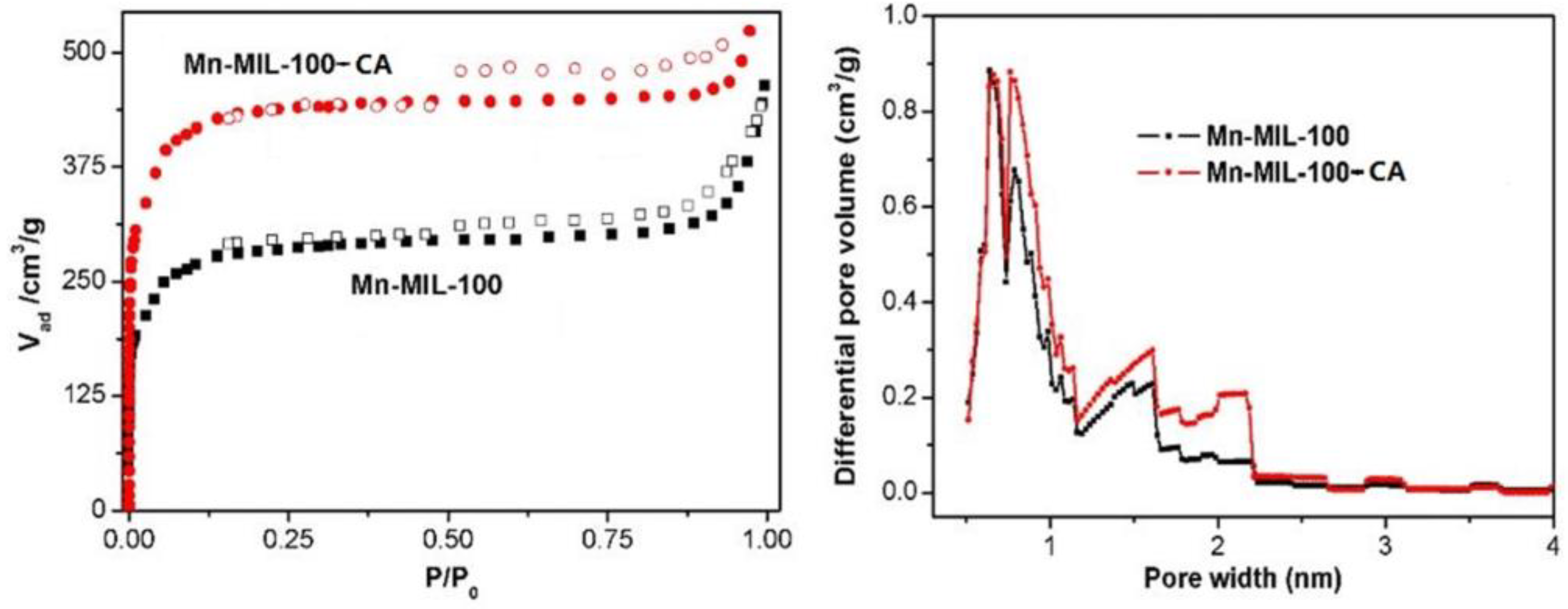
3.2. Isotherms Study
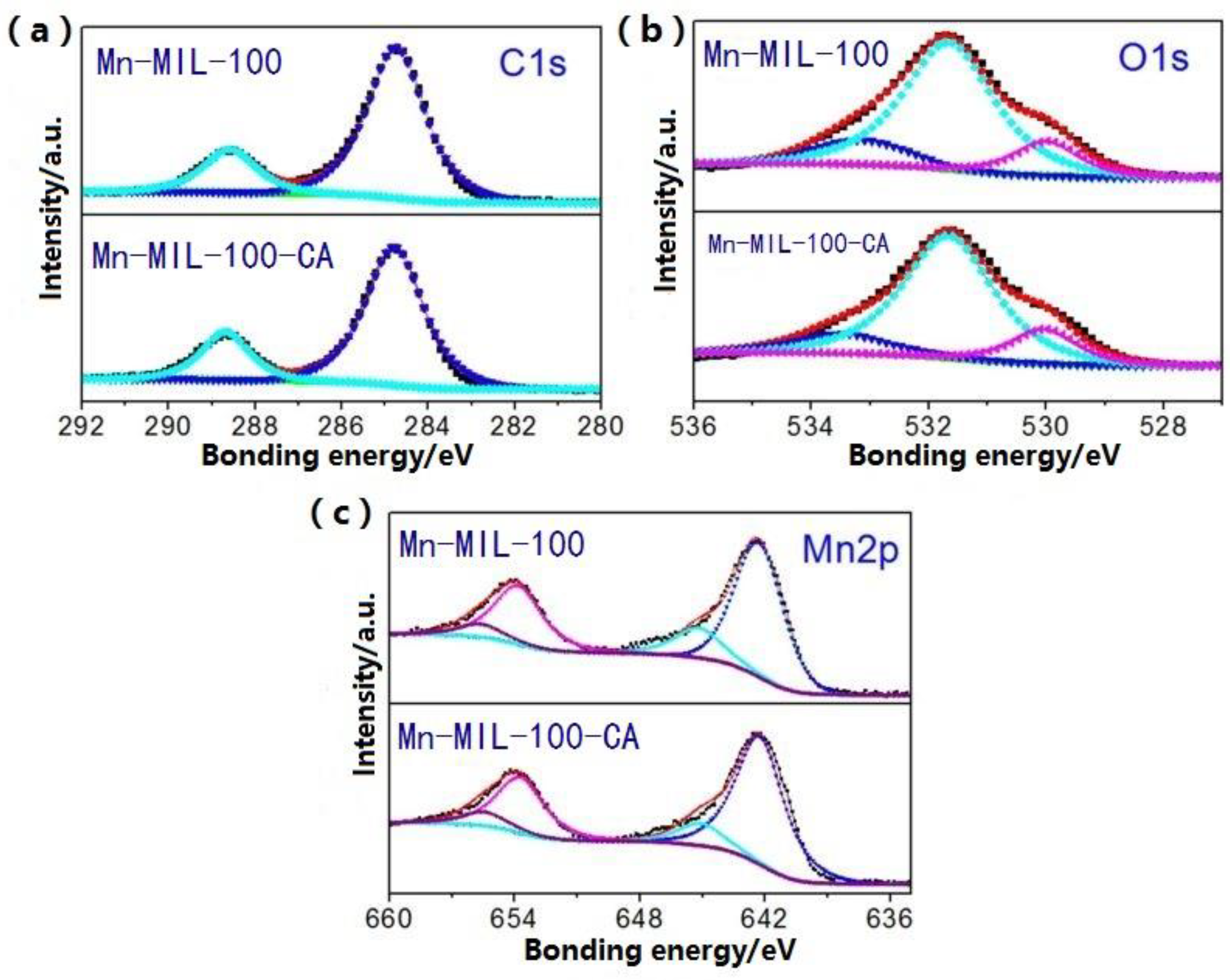
3.3. Surface Components
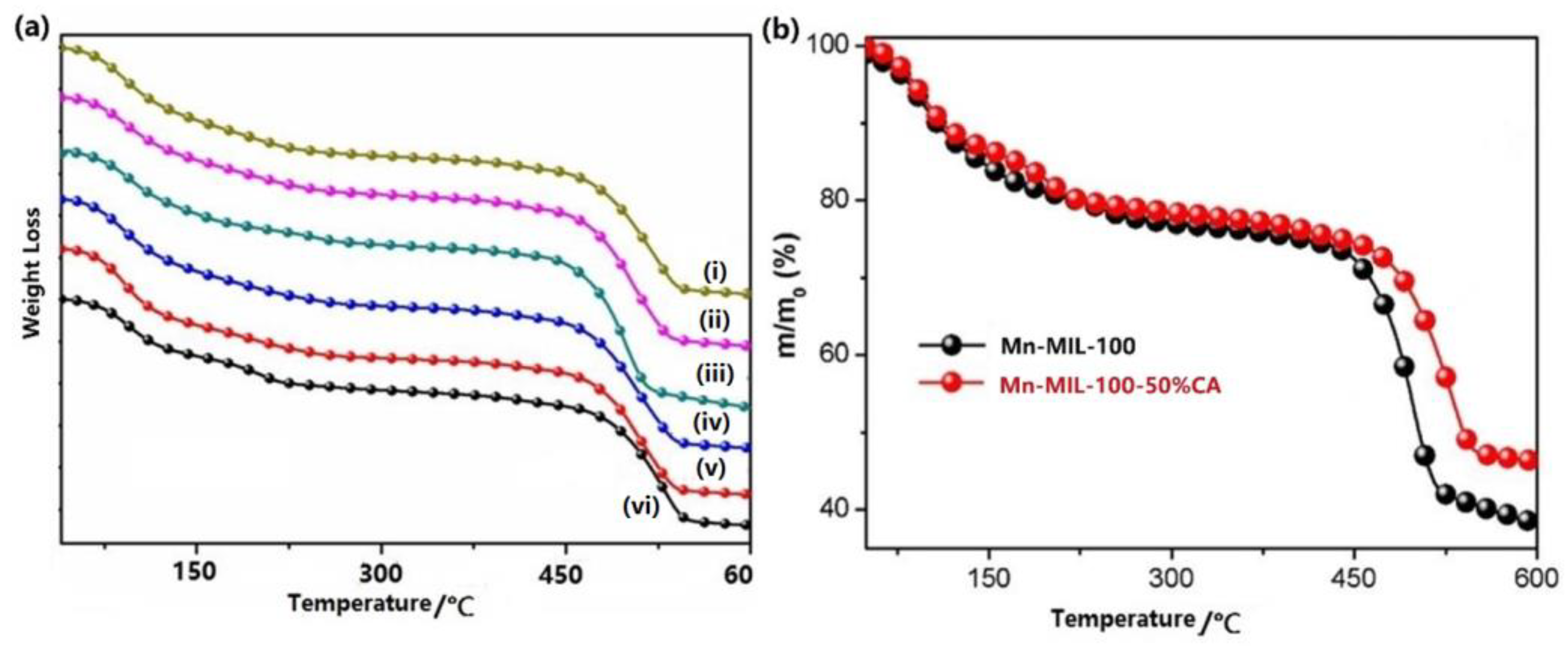
3.4. Thermal and Water Stability
3.5. Adsorption Ability with Regard to MB
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Primack, R.B.; Morrison, R.A. Encyclopedia of Biodiversity, 2nd ed.; Elsevier Inc.: Oxford, UK, 2013; pp. 401–412. [Google Scholar]
- Wang, X.; Deng, B.W.; Yu, L.Z.; Cui, E.B.; Xiang, Z.; Lu, W. Degradation of azo dyes Congo red by MnBi alloy powders: Performance, kinetics and mechanism. Mater. Chem. Phys. 2020, 251, 123096. [Google Scholar] [CrossRef]
- Zhao, G.D.; Li, Z.L.; Cheng, B.W.; Zhuang, X.P.; Lin, T. Hierarchical porous metal organic framework aerogel for highly efficient CO2 adsorption. Sep. Purif. Technol. 2023, 315, 123754. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Z.; Reimus, P.; Ma, F.N.; Soltanian, M.R.; Xing, B.S.; Zang, J.Z.; Wang, Y.; Dai, Z.X. Plutonium reactive transport in fractured granite: Multi-species experiments and simulations. Water Res. 2022, 224, 119068. [Google Scholar] [CrossRef]
- Jiang, Z.S.; Huang, X.F.; Wu, Q.F.; Li, M.; Xie, Q.L.; Liu, Z.W.; Zou, X.M. Adsorption of sulfonamides on polyamide microplastics in an aqueous solution: Behavior, structural effects, and its mechanism. Chem. Eng. J. 2023, 454, 140452. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Dai, L.Y.; Yao, J.C.; Guo, T.J.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 2021, 281, 130718. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Solińska, A.; Bajda, T. Modified zeolite as a sorbent for removal of contaminants from wet flue gas desulphurization wastewater. Chemosphere 2022, 286, 131772. [Google Scholar] [CrossRef]
- Shen, C.Z.; Song, G.L.; Tang, G.Y. A facile modification method of activated carbon by spark discharge of atmospheric pressure plasma jets to improve its adsorption performance of methylene blue. Surf. Coat. Technol. 2018, 354, 126–133. [Google Scholar] [CrossRef]
- Iturbe-Ek, J.; Andrade-Martínez, J.; Gómez, R.; Rodríguez-González, V. A functional assembly of SiO2 nanospheres/graphene oxide composites. Mater. Lett. 2015, 142, 75–79. [Google Scholar] [CrossRef]
- Maria, L.C.D.S.; Aguiar, M.R.M.P.; D’Elia, P.; Ferreira, L.O.; Wang, S.H. Comparative adsorptive removal of biperidene and sibutramine chlorhydrates from methanolic solutions by using active coal, clay and polymeric resins. Mater. Lett. 2007, 61, 3395–3399. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Günter, C.; Weber, J.; Lubahn, S.; Taubert, A. Hybrid clay: A new highly efficient adsorbent for water treatment. ACS Sustain. Chem. Eng. 2013, 1, 966–973. [Google Scholar] [CrossRef]
- Liu, X.G.; Shan, Y.Y.; Zhang, S.T.; Kong, Q.Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energ. Environ. 2022, 8, 698–721. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Agboola, O.S.; Ogunmodede, J.; Araoye, A.O.; Bello, O.S. Metal-organic frameworks as adsorbents for sequestering organic pollutants from wastewater. Mater. Chem. Phys. 2020, 253, 123246. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Li, A.H.L.; Yaghi, O.M. Highly porous and stable metal-organic frameworks: structure design and sorption properties. J. Am. Chem. Soc. 2000, 122, 1391–1397. [Google Scholar] [CrossRef]
- Kim, J.; Chen, B.L.; Reineke, T.M.; Li, H.L.; Eddaoudi, M.; Moler, D.B.; O’Keeffe, M.; Yaghi, O.M. Assembly of metal−organic frameworks from large organic and inorganic secondary building units: new examples and simplifying principles for complex structures. J. Am. Chem. Soc. 2001, 123, 8239–8247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.L.; Chen, K.J.; Xing, H.B.; Yang, Q.W.; Krishna, R.; Bao, Z.B.; Wu, H.; Zhou, W.; Dong, X.L.; Han, Y.; et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef]
- Lieb, A.; Leclerc, H.; Devic, T.; Serre, C.; Margiolaki, I.; Mahjoubi, F.; Lee, J.S.; Vimont, A.; Daturi, M.; Chang, J.S. MIL-100(V)—A mesoporous vanadium metal organic framework with accessible metal sites. Microporous Mesoporous Mater. 2012, 157, 18–23. [Google Scholar] [CrossRef]
- Ferey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surble, S.; Dutour, J.; Margiolaki, I. A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction. Angew. Chem. Int. Ed. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Horcajada, P.; Surble, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Greneche, J.M.; Margiolaki, I.; Ferey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Volkringer, C.; Popov, D.; Loiseau, T.; Fe’rey, G.; Burghammer, M.; Riekel, C.; Haouas, M.; Taulelle, F. Synthesis, single-crystal X-ray microdiffraction, and NMR characterizations of the giant pore metal-organic framework aluminum trimesate MIL-100. Chem. Mater. 2009, 21, 5695–5697. [Google Scholar] [CrossRef]
- Mowat, J.P.S.; Miller, S.R.; Slawin, A.M.Z.; Seymour, V.R.; Ashbrook, S.E.; Wright, P.A. Synthesis, characterization and adsorption properties of microporous scandium carboxylates with rigid and flexible frameworks. Microporous Mesoporous Mater. 2011, 142, 322–333. [Google Scholar] [CrossRef]
- Reinsch, H.; Stock, N. Formation and characterization of Mn-MIL-100. Cryst. Eng. Comm. 2013, 15, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.K.; Yoon, J.W.; Lee, J.S.; Hwang, Y.K.; Jun, C.H.; Chang, J.S.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; et al. Energy-efficient dehumidification over hierachically porous metal–organic frameworks as advanced water adsorbents. Adv. Mater. 2012, 24, 806–810. [Google Scholar] [CrossRef]
- Jeremias, F.; Khutia, A.; Henninger, S.K.; Janiak, C. MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—A promising application. J. Mater. Chem. 2012, 22, 10148–10151. [Google Scholar] [CrossRef]
- Guo, H.L.; Zhu, Y.Z.; Qiu, S.L.; Lercher, J.A.; Zhang, H.J. Coordination modulation induced synthesis of nanoscale Eu1-xTbx-metal-organic frameworks for luminescent thin films. Adv. Mater. 2010, 22, 4190–4192. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal–organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Wang, K.Y.; Powell, J.; Zhou, H.C. Controllable synthesis of metal-organic frameworks and their hierarchical assemblies. Matter 2019, 1, 801–824. [Google Scholar] [CrossRef] [Green Version]
- Bunzen, H.; Grzywa, M.; Hambach, M.; Spirkl, S.; Volkmer, D. From micro to nano: A toolbox for tuning crystal size and morphology of benzotriazolate-based metal–organic frameworks. Cryst. Growth Des. 2016, 16, 3190–3197. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.H.; Guo, Y.; Zhang, L.G.; Zhang, Y.; Wang, J.D. A general and efficient approach for tuning the crystal morphology of classical MOFs. Chem. Commun. 2018, 54, 252–255. [Google Scholar] [CrossRef]
- Ji, H.; Lee, S.; Park, J.; Kim, T.; Choi, S.; Oh, M. Improvement in crystallinity and porosity of poorly crystalline metal-organic frameworks (MOFs) through their induced growth on a well-crystalline MOF Template. Inorg. Chem. 2018, 57, 9048–9054. [Google Scholar] [CrossRef]
- Qi, Z.P.; Yang, J.M.; Kang, Y.S.; Guo, F.; Sun, W.Y. Facile water-stability evaluation of metal–organic frameworks and the property of selective removal of dyes from aqueous solution. Dalton Trans. 2016, 45, 8753–8759. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T. Water stable MOFs as emerging class of porous materials for potential environmental applications. Chemosphere 2023, 313, 137607. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.L.; Cai, X.C.; Jiang, H.L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209. [Google Scholar] [CrossRef] [Green Version]
- Hermes, S.; Witte, T.; Hikov, T.; Zacher, D.; Bahnmuller, S.; Langstein, G.; Huber, K.; Fischer, R.A. Trapping metal-organic framework nanocrystals: an in-situ time-resolved light scattering study on the crystal growth of MOF-5 in solution. J. Am. Chem. Soc. 2007, 129, 5324–5325. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Timmons, D.J.; Yuan, D.Q.; Zhou, H.C. Tuning the topology and functionality of Metal−Organic Frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef]
- Kim, D.W.; Liu, X.F.; Lah, M.S. Topology analysis of metal–organic frameworks based on metal–organic polyhedra as secondary or tertiary building units. Inorg. Chem. Front. 2015, 2, 336–360. [Google Scholar] [CrossRef]
- Ha, Y.; Mu, M.M.; Liu, Q.L.; Ji, N.; Song, C.F.; Ma, D.G. Mn-MIL-100 heterogeneous catalyst for the selective oxidative cleavage of alkenes to aldehydes. Catal. Commun. 2018, 103, 51–55. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Li, C.Y.; Zhao, Q.D.; Li, X.Y. Synthesis of bimetallic MOFs MIL-100 (Fe-Mn) as an efficient catalyst for selective catalytic reduction of NOx with NH3. Catal. Lett. 2016, 146, 1956–1964. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Zhang, A.F.; Hou, K.K.; Liu, M.; Wang, Y.X.; Song, C.S.; Zhang, G.L.; Guo, X.W. Size- and morphology-controlled NH2-MIL-53(Al) prepared in DMF–water mixed solvents. Dalton Trans. 2013, 42, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef] [Green Version]
- Andreas, S.; Frank, H.; Michael, F.; Lorenz, K.; Viola, D.; Matthias, T.; Christian, S.; Gérard, F.; Norbert, S. Giant pores in a chromium 2,6-naphthalenedicarboxylate open-framework structure with MIL-101 topology. Angew. Chem. Int. Ed. Engl. 2009, 48, 3791–3794. [Google Scholar]
- Liu, Y.; Che, R.C.; Chen, G.; Fan, J.W.; Sun, Z.K.; Wu, Z.X.; Wang, M.H.; Li, B.; Wei, J.; Wei, Y.; et al. Radially oriented mesoporous TiO2 microspheres with single-crystal–like anatase walls for high-efficiency optoelectronic devices. Sci. Adv. 2015, 1, e1500166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, J.R.; Verdegaal, W.M.; Liu, T.F.; Zhou, H.C. Isostructural metal-organic frameworks assembled from functionalized diisophthalate ligands through a ligand-truncation strategy. Chem.—A Euro. J. 2013, 19, 5637–5643. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Rychlicki, G. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: The temperature dependence of adsorption at the neutral pH. Colloids Surf. A 2000, 163, 135–150. [Google Scholar] [CrossRef]
- Liu, S.C.; Xu, J.; Dai, E.B.; Qiu, J.J.; Yi, L. Synthesis and properties of ferrocene confined within UiO-67 MOFs. Microporous Mesoporous Mater. 2018, 264, 133–138. [Google Scholar] [CrossRef]
- Li, D.D.; Yang, G.L.; Li, P.L.; Wang, J.L.; Zhang, P.Y. Promotion of formaldehyde oxidation over Ag catalyst by Fe doped MnOx support at room temperature. Catal. Today 2016, 277, 257–265. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, H.G.; Hou, F.L.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.X.; Cui, L.F. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. App. Sur. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.M.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [Green Version]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 2000, 38, 1423–1436. [Google Scholar] [CrossRef]



| Sample | BTC (mmol) | CA (mmol) | Methanol (mL) |
|---|---|---|---|
| Mn-MIL-100 | 2.0 | 0.0 | 35 |
| Mn-MIL-100-10%CA | 2.0 | 0.2 | 35 |
| Mn-MIL-100-30%CA | 2.0 | 0.6 | 35 |
| Mn-MIL-100-50%CA | 2.0 | 1.0 | 35 |
| Mn-MIL-100-70%CA | 2.0 | 1.4 | 35 |
| Mn-MIL-100-100%CA | 2.0 | 2.0 | 35 |
| Sample | Specific Surface Area (m2/g) | Mean Pore Diameter (nm) | Pore Volume (m3/g) |
|---|---|---|---|
| Mn-MIL-100 | 1050.1 | 2.55 | 0.67 |
| Mn-MIL-100-10%CA | 1280.4 | 2.51 | 0.83 |
| Mn-MIL-100-30%CA | 1424.2 | 2.04 | 0.73 |
| Mn-MIL-100-50%CA | 1671.3 | 2.51 | 1.05 |
| Mn-MIL-100-70%CA | 1230.8 | 2.33 | 0.76 |
| Mn-MIL-100-100%CA | 878.8 | 1.67 | 0.37 |
| Sample | Element Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Mn | Mn3+ | Mn4+ | O (C=O) | O (Ligand) | O (Others) | |
| Mn-MIL-100 | 49.2 | 37.4 | 13.1 | 80.4 | 19.6 | 14.4 | 72.5 | 13.1 |
| Mn-MIL-100-50%CA | 53.4 | 34.4 | 11.8 | 85.6 | 14.4 | 15.3 | 72.7 | 12.0 |
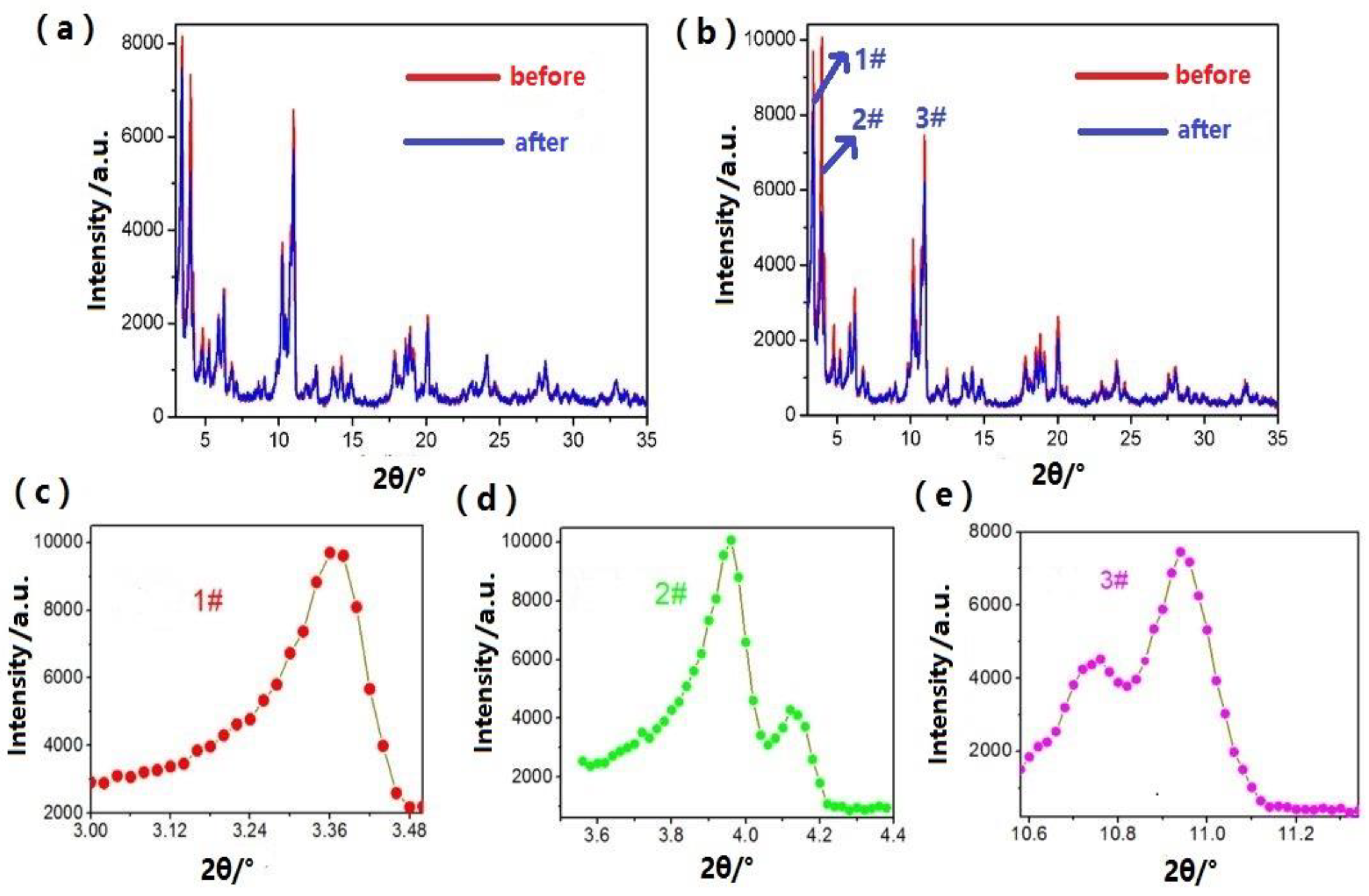
| Sample | 1# | 2# | 3# | BET (m2/g) |
|---|---|---|---|---|
| Mn-MIL-100-before | 1160.1 | 1464.2 | 1540.3 | 1050.1 |
| Mn-MIL-100-after | 1034.9 | 909.1 | 1034.9 | 812.8 |
| Mn-MIL-100-50%CA-before | 1173.3 | 1641.3 | 1552.1 | 1671.3 |
| Mn-MIL-100-50%CA-after | 1101.5 | 906.2 | 1337.8 | 1514.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Shen, C.; Bassir, D.; Li, Q. A Symmetry Concept for the Self-Assembly Synthesis of Mn-MIL-100 Using a Capping Agent and Its Adsorption Performance with Methylene Blue. Symmetry 2023, 15, 1334. https://doi.org/10.3390/sym15071334
Song G, Shen C, Bassir D, Li Q. A Symmetry Concept for the Self-Assembly Synthesis of Mn-MIL-100 Using a Capping Agent and Its Adsorption Performance with Methylene Blue. Symmetry. 2023; 15(7):1334. https://doi.org/10.3390/sym15071334
Chicago/Turabian StyleSong, Guolin, Chengzhu Shen, David Bassir, and Qiulin Li. 2023. "A Symmetry Concept for the Self-Assembly Synthesis of Mn-MIL-100 Using a Capping Agent and Its Adsorption Performance with Methylene Blue" Symmetry 15, no. 7: 1334. https://doi.org/10.3390/sym15071334





