1. Introduction
Organoboron compounds have many applications, including pharmaceuticals, advanced materials, and even reagents for synthesis [
1,
2,
3,
4,
5,
6,
7]. The same can be said of the azaborines, boron, and nitrogen-containing heterocyclic analogs of benzene and other aromatic hydrocarbons [
8,
9,
10,
11]. The versatility of boron lies in its chemical properties, namely its electron deficiency and its coordination behavior, that allow a change from a three-coordinate trigonal planar geometry to a four-coordinate tetrahedral geometry upon coordination to a suitable heteroatom.
The hydrolytic and oxidative sensitivity of many boron reagents were initially an obstacle [
4], but this has now been surpassed by the development of stable reagents and robust reactions, such as the palladium-catalyzed borylation or the Suzuki–Miyaura cross-coupling reaction [
6,
7]. Boron compounds displaying central chirality are well-known, although still few in number [
12], with the first example of asymmetric synthesis, that of a highly fluorescent chiral boron dipyrrole (a BODIPY), dating back to 2021 [
13]. The reason for this slow progress is the fact that the synthesis of compounds containing a boron stereocenter is quite challenging since ligands attached to a tetracoordinate boron atom are labile, which makes it configurationally unstable [
12,
14,
15].
Axial chirality is even rarer [
16], and the first example of an atropselective synthesis, described in
Section 3 of this review, was only reported in 2020 [
17]. Axial chirality has emerged as an important field of research in recent years, since the successes attained in asymmetric synthesis by C
2-symmetric chiral ligands such as BINOL (1,1′-bi-2-naphthol) [
18], BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphyl) [
19,
20,
21] or BINAM (1,1′-binaphyl-2,2′-diamine) [
22] and C
2-symmetric organocatalysts like the BINOL-derived chiral phosphoric acids [
23,
24] or the even more acidic chiral binaphthyl-based disulfonimides [
25,
26]. Other aspects that brought to light atropisomerism was the increased awareness of the importance of rotational isomers in drug development [
27,
28,
29,
30,
31,
32], as well as in materials science [
33,
34] since atropisomers can vary drastically in their biological activities and functions.
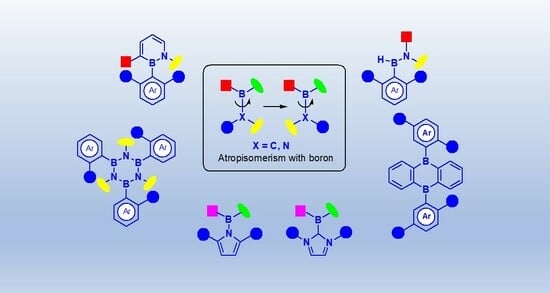
Atropisomerism is chirality in the absence of chiral centers, as shown in
Figure 1a. Due to a restriction of rotation around a single bond, the axis of rotation, different conformations can be identified. A stable atropisomer has a very long lifetime due to the presence of a very high energy barrier to rotation and, hence, racemization. An energy barrier of 24 kcal mol
−1 is sufficient to allow for the isolation of atropisomers at +25 °C [
33,
35], but if the temperature is only raised by 20 °C, racemization can occur. Atropisomers can be divided into groups according to their stabilities based on their half-life of racemization at 37 °C: class 1 (
t½ > 60 s), class 2 (
t½ = 60 s to 4.5 years), and class 3 (
t½ > 4.5 years) [
36]. Class 3 atropisomers are those generally considered to be stable enough for drug development [
33].
The boron-carbon bond is, on average, longer than a carbon–carbon bond [Csp
2–B bond: 1.58 Å, Csp
2–Csp
2 bond: 1.46 Å (biaryl compounds)], which can partially explain the low rotational barrier that exists in boron-containing compounds and this is probably one of the reasons for the slow development of this field of research (
Figure 1b)) [
37]. Other factors that have been suggested to account for the scarcity of examples of axially chiral boron compounds are the limited structural variability of known
B-aryl-1,2-azaborine scaffolds and the lack of established chirality induction modes to obtain this class of compounds [
27].
Figure 2 shows examples of very well-known atropisomers and some boron-containing analogs.
In this review, we survey the literature on the synthesis of atropisomers, specifically those in which boron is one of the atoms in the axis of rotation. Included are both racemic methods that produce atropisomers that could be clearly identified even if only partial separation was achieved, as well as the more recent examples involving atropselective synthesis catalyzed by a metal complexed to a chiral ligand or by an organocatalyst. Atropisomers are classified in this review using the Cahn–Ingold–Prelog rules, i.e., as R (or Ra) and S (or Sa), or by the rules of nomenclature applied for helicity, viz., as P (positive helix) and as M (negative helix).
2. Synthesis of Atropisomeric Racemates with Atropisomer Resolution
The fact that the empty p-orbital of a tricoordinate boron atom can overlap with an adjacent organic
π-system (e.g., aryl, vinyl, and alkynyl) or heteroatom (e.g., O, N) has led to the development of many compounds with interesting properties [
3,
8,
9,
10,
11]. This overlap leads to a strong
π-acceptor effect and extension of
π-conjugation, which have been demonstrated using
11B NMR, photophysical and electrochemical studies, and theoretical calculations. As a consequence, aromatic compounds doped with boron can have very desirable electronic and photophysical characteristics such as strong photoluminescence, electroluminescence, nonlinear optical properties,
n-type semi-conductivity, etc. Other applications have been the development of sensory materials for anions, neutral nucleophiles such as toxic amines, or biologically relevant species such as saccharides or dopamine. Even further, boron-doped polymers containing chromophores have found applications as luminescent materials for biomedical imaging and organic light-emitting devices (OLEDs).
A C–C bond and a B–N bond also have an isoelectronic relationship [
8], a fact that has been known since the studies of Dewar and coworkers in the 1950s [
38]. Hence, when a B–N unit is incorporated into an aromatic system, retention of the aromatic character is expected to occur, although this is still a subject of debate [
8,
39]. The B–N fragment is dipolar, but the B and N atoms are not significantly polarized due to the opposing σ- and π-electron polarizations, even though there is a net negative charge at nitrogen. Since the polarization is not significant, there is electron delocalization. The quintessential BN hydrocarbon analog is borazine (B
3N
3H
6), commonly known as “inorganic benzene”, in which all C–C bonds have been substituted by B–N units. Some
N-aryl and
B-aryl substituted borazines, in which there is enough steric hindrance to prevent free rotation, have been known to exist as mixtures of atropisomers as far back as 1974 when
B-tri-
o-tolyl-
N-triethylborazine
trans-
1 was described by Johnson and Mellon [
40]. The rotational isomerism was shown either with variable temperature NMR studies or multinuclear NMR.
Figure 3 shows borazine
1 as well as other examples of related compounds reported to exist as atropisomeric mixtures [
41,
42,
43].
In 2012, Wagner and coworkers described the synthesis and characterization of boron-doped tri(9,10-anthrylene)s which, when substituted at boron by
tert-butyl groups, showed atropisomerism due to restricted rotation about the exocyclic B–C bonds [
44] (
Figure 4). X-ray crystallography suggested that the overall interactions between facing
tert-butyl groups in compound
8 are attractive and not repulsive. The atropisomers
8 and
8′ were stable toward air and moisture for several hours, even in solution (
Figure 4). The two rotamers of the 9,10-dianthryl-9,10-dihydro-9,10-diboraanthracenes
8 could only be partially separated, although not on a preparative scale, and hence their configurations could not be determined. One of them (
8′) could be obtained as single crystals. In the solid state,
8′ has
Cs symmetry, with the two symmetry-related 9-anthryl moieties nearly coplanar to each other (dihedral angle = 2.3(1)°).
The UV/Vis absorption spectra have broad charge-transfer bands in the range λmax = 510–556 nm; the corresponding fluorescence bands show strong positive solvatochromism. This information suggests that the electronic spectra are dominated by twisted intramolecular charge transfer (TICT) emissive properties due to interactions between the anthryl donors and the DBA acceptor. The bright fluorescence obtained with solid samples suggests they may be interesting for solid-state applications. Other related compounds were prepared in this study, although the others did not show atropisomerism.
Diboron-doped analogs of alkenes, alkynes, and (hetero)aromatic molecules are particularly well suited to the activation of small molecules (e.g., H
2, CO, CO
2, O
2, etc.) owing to the cooperation of the two empty p orbitals at the boron atoms [
45,
46,
47]. The reduction of elemental chalcogens by low-valent diboron compounds allows for the atom-efficient synthesis of boron-chalcogen heterocycles. Braunschweig and coworkers recently reported the synthesis of bicyclic chalcogen-bridged compounds using this approach [
48]. Thus, the reaction of oxygen, elemental sulfur, and selenium with the doubly NHC-stabilised 9,10-diboraanthracene
11 yielded the endoperoxo-bridged
12 or S/Se-bridged bicyclic compounds
13 or
14 of which atropisomers could be distinguished (
Figure 5).
They are produced as a result of hindered rotation of the cyclic alkyl(amino)carbene (CAAC) ligands about the B–C bonds caused by the edge-to-face CH/π interaction of two
ortho protons of the diboraanthracene core with the aromatic CAAC ligands (
Figure 6). Proof for this came from the strong magnetic shielding observed for these
ortho protons, which appear in the 4.45 to 4.70 ppm region of the
1H NMR spectrum, as well as by the solid-state structures of the
rac-
13 or
rac-
14 derivatives. Variable temperature NMR provided further information. An experiment on rac/meso-
14-Se suggested that there is a very high energetic barrier to the interconversion of the two atropisomers with a coalescence temperature above 110 °C. In the case of the dioxygen derivatives,
1H NMR spectroscopy revealed the presence of a 71:21:6:2 mixture of four different atropisomers, which were identified through comparison with the NMR data of rac/meso-
13/14-S/Se, as well as through other 1D and 2D NMR experiments and also from the solid-state crystal structure of syn-
12. However, unlike
13-S and
14-Se,
12-O
2 was not stable in solution, but decomposed slowly at room temperature into a mixture of unidentifiable compounds.
Azaborines, substances partially substituted with boron and nitrogen, are aromatic due to the isoelectronic relationship between the B-N and the C=C bonds. They have attracted a great deal of attention in recent years due to their usefulness for potential use in biomedical and materials science applications [
8,
9,
10,
11,
33]. The usefulness of these compounds stems from the fact that because the B–N bond is partially ionic, they possess at least local dipole moments. These properties can alter both molecular and solid-state electronic and optical properties since they modify the character of the frontier molecular orbitals and intermolecular interactions in the solid phases. In 2016, Mancinelli and coworkers reported the synthesis of azaborines containing a thermally stable boron-carbon chiral axis [
37]. Although single crystals of azaborines have seldom been reported up to this date, they could be obtained for compound
17. Its X-ray structure revealed that the 2,1-borazaronaphthalene ring is planar and the B–N bond length is 1.427 Å, shorter than the C4–C5 bond length, which supports the existence of a double bond between nitrogen and boron due to the presence of the nitrogen lone pair. A B–C3 bond length of 1.515 Å is more consistent with a B–C single bond, and the C3–C4 length is typical of an isolated C=C double bond. The structure adopts a twisted conformation, with the
m-tolyl group displaced from the azaborine plane, and the
p-nitrophenyl group is almost perpendicular to the same plane. Since the
m-tolyl ring cannot develop conjugation with boron, a B–Cq bond length of 1.581 Å is observed. The rotational barrier was calculated to be lower than that of a classical aryl–aryl compound, i.e., 19.0 kcal mol
−1 vs. 25.2 kcal mol
−1 in the C–C isostere. However, when the more highly hindered compound
23, containing a 2-methylnaphthyl substituent, was prepared, the two atropisomers could be effectively resolved by CSP-HPLC (
Figure 7). Using semi-preparative HPLC, good amounts of the two atropisomers could be obtained. The absolute configuration was determined using the Time-Dependent DFT (TD-DFT) simulation of the Electronic Circular Dichroism (ECD) spectra. The B–aryl rotational barrier was determined to be 33.0 kcal mol
−1 by monitoring the racemization of an enantiopure sample at +130 °C and +140 °C in C
2D
2Cl
4. The results obtained in this study showed that the presence of the longer C-B bond has a large influence on the thermal stability of C–B atropisomers since this energy barrier is about 12–13 kcal mol
−1 lower than in the corresponding C–C analogs of type
24 [
37].
In pursuit of new materials with unique functions, the Mancinelli group studied more extended conjugated π-systems doped with boron. The replacement of one or more of the carbon atoms in polycyclic aromatic hydrocarbons with boron decreases the HOMO–LUMO gap, and molecules with chemiluminescent properties may be obtained or potential semiconductors due to the unbalanced electron distribution. Azaborines have been shown to be effective organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs) [
49,
50]. Moreover, 6-aryl-5,6-dihydrodibenzo[c,e][1,2]azaborinines, substances which are analogs of 9-arylphenanthrene and 1-arylnaphthalenes but contain the B-N moiety with a reverse geometry in relation to the azaborines described above, e.g., those in
Figure 7, were studied next [
51]. When these compounds contain an unsymmetrically
ortho-substituted aryl ring attached to boron, this ring is also skewed out of the plane because of the steric hindrance between the
ortho substituent and the NH and CH in positions 5 and 7, and two enantiomeric conformations are possible. Four of these compounds were prepared from 2,4,6-triphenylaniline
25 and
26 through a Friedel–Crafts reaction using BCl
3 and AlCl
3, followed by a Grignard reaction to introduce the different aryl groups (
Figure 8). In the case of
27d, with the largest steric hindrance to rotation, thermally stable atropisomers could be isolated by semi-preparative chiral HPLC. The racemization barrier was determined to be 26.0 ± 0.2 kcal·mol−1 from the rate constants determined at the two different temperatures and the Eyring equation, which corresponds to a half-life time of 30 days at +25 °C. DFT calculations supported this value; 25.3 kcal·mol−1 was obtained. A ΔΔG
⧧ > 12 kcal·mol
−1 with respect to the isoster 9-(2-methylnaphthalen-1-yl)phenanthrene was found (ΔG
⧧rac > 38 kcal·mol
−1). This energy difference was attributed by the authors to the presence of the longer C-B bond in comparison with C-C. The absolute configuration of the two atropisomers of
27d was assigned using the simulation of the ECD spectrum based on time-dependent density functional theory (TD-DFT).
N-Heterocyclic carbene boranes (NHC-boranes) are another class of compounds that have gained interest in recent years due to their chemical stability and interesting reactivity profile. They are analogous to other Lewis acid/Lewis base complexes with boranes (ethers, sulfides, amines, etc.), although they are much more stable [
52]. They are also different from their closest relatives, such as the amine and phosphineboranes. They are tetravalent, neutral complexes with a rich chemistry. They were rare before 2008 [
52].
In 2015, Curran and coworkers prepared some
B,
B-disubstituted 1,3-dimethylimidazol-2-ylidene boranes and studied their properties using dynamic NMR spectroscopy, e.g.,
29–
31 (
Figure 9) [
53]. Compounds with one primary and either one secondary or one tertiary substituent on the boron atom were found to have substantial rotational barriers due to slow rotation around the exocyclic N–B bond. They range from 56 kJ mol
−1 with a secondary boron substituent up to 75–86 kJ mol
−1 when a tertiary boron substituent is present. The highest was present when there were bonds to boron atoms bearing a thexyl (1,1,2-trimethylpropyl) group. Stable atropisomers were formed.
Most of the compounds prepared by Curran and coworkers had stereocenters at boron, but because of the presence of a local plane of symmetry in the NHC ring, the two rotamers were identical. If the symmetry of the NHC ring is broken, i.e., if the two substituents, the imidazolyl nitrogen or the carbon substituents, are different, then the slow rotation should produce diastereomers. Based on the results obtained for 29 and 30, with the highest rotational barriers at 82 and 86 kJ mol−1 just below the level at which chromatographic separation of atropisomers is possible at room temperature, a new, more hindered borane was prepared, 31, in the hope of producing the desired atropisomers. Moreover, 31 was obtained using 1-isopropyl-1-methylimadozol-2-ylidene borane as a starting material through a two-step sequence of borenium-catalyzed hydroboration of tetramethylethylene and Rh-catalyzed B–H insertion with ethyl diazoacetate. However, although 1H and 13C NMR analysis showed that this borane consisted of a rotamer 70/30 mixture, resolution at rt was not possible. Partial separation could be observed by HPLC, with the atropisomers equilibrating partially during the time scale of the experiment. These were the first measurements of rotation barriers for bonds between N-heterocyclic carbenes and boron.
Non-biaryl atropisomers containing a C–B bond about which there is restricted rotation were described by Chabaud and Pucheault in 2020 [
54]. Produced by a reaction between Grignards prepared from 1-bromo-2-alkylnaphthalenes
32 and an appropriate borane
33 (
Figure 10), aminoarylboranes
34 may be seen as isosteres of vinyl styrene derivatives since electron delocalization about the C-B bond is possible as in
34a and
34a′. The synthesis and separation of this type of atropisomers had never been previously reported. The presence of bulky substituents at both the dialkylaminoborane moiety and the aromatic ring was necessary to restrict the rotation around the C–B bond. A variable temperature NMR spectroscopic analysis of
34a in [D
6]DMSO showed no coalescence even at 105 °C, which indicated that the enantiomers could be separated, and this was indeed accomplished by performing the chromatography at 0 °C. The kinetics for racemization were subsequently determined using circular dichroism spectroscopy, following the decay at λ
max of its chiroptical signal in hexane at 10 °C. A half-life for racemization of 5 min was calculated, with a barrier of ΔG
rac = 83.4 kJ mol
−1. Other aminoboranes could be prepared in good to high yields and those bearing bulky substituents, i.e., Bn,
i-Pr, and Et, next to the aminoborane moiety, displayed atropisomerism. In contrast, when a methoxy group was present, rapid interconversion took place, with a 5 ms half-life at 10 °C. This observation is probably due to the smaller size of methoxy versus methyl and/or lone pair delocalization into the empty orbital of the boron atom, which may stabilize the transition state for atropisomer interconversion. More crowded aminoboranes, e.g.,
34c, obtained by changing DIPAB by dicyclohexylaminoborane, showed an increased ΔG
rac‡ value up to 92.6 kJ mol
−1 with a half-life for racemization of 244 min at 10 °C. DFT calculations supported the experimental results obtained.
Biarylaminoboranes, or more precisely bis-mesityl carbazole boranes, that display atropisomerism have also been described [
55]. Many of these substances have been reported in more recent years because they possess TICT emissive properties [
15]. For example, in a series of highly twisted benzocarbazole boranes, it was found that the presence of a carbazole ring system as the heteroaromatic component provided the highest
π-contribution to the boron–nitrogen bond (24 kcal mol
−1) [
51]. The twist angle observed in the ground state (GS) between the heteroaromatic ring and the borane branch was directly related to the emissive properties of the compounds. In general, strong TICT phenomena and noticeable solvatochromic effects were correlated to the large geometric differences between the GS and the relaxed excited state, where the B–N twist angle between the donor and acceptor is the result of a less efficient
π-contribution. New compounds were prepared with a more extended aromatic system, aiming to modify the TICT rearrangement properties and the nature of the HOMO and LUMO to change the photophysical properties. It was also hoped that atropisomeric aminoboranes with a C–B chiral axis could be circularly polarized luminescence (CPL)-active. Chiral
bis-aryl carbazole boranes bearing more sterically demanding aromatic systems than those previously described, e.g., naphthyl, 2-methyl-naphthyl (1b), or anthracene (1c), in addition to the mesityl group, needed to preserve the chemical stability, were prepared (e.g.,
39,
Figure 11). The synthesis was achieved with cheap reagents such as aryl bromines and a stable and easy-to-handle boron source, MesBF
3K, an advantage over the methods previously used that relied on the corrosive BF
3OEt
2 (previously recognized as the most efficient reagent [
56]) and a larger number of steps.
Carbazole borane 39 was found to be stable enough to be resolved into atropisomers by CSP-HPLC. An isomerization barrier of 26.1 kcal mol−1 was measured using an enantiopure sample in tetrachloroethane at 50 °C, with CSP-HPLC monitoring, and an M absolute configuration could be assigned. A search for emissive properties of different solvents showed solvatochromism in the emission spectra owing to the TICT process, with very large Stokes shifts (>10,000 cm−1) for 39 and also for an analogous compound bearing a naphthyl substituent instead of 2-methylnaphthyl. CPL spectra were acquired for the stable atropisomers of 39, showing the maximum intensity in apolar solvents. The results show that 39 is a new example of a CPL-active bis-aryl aminoborane with an exocyclic B–N bond.
3. Enantioselective Synthesis
The enantioselective synthesis of boron atropisomers was only developed in this decade, and so far, very few reports have appeared [
12,
57,
58]. After the description by Chabaud, Pucheault, and coworkers of the first non-biaryl compounds displaying slow rotation about the B–C bond and the partial separation of these B–C atropisomers in 2020 [
54], Song and coworkers developed an atroposelective Miyaura borylation, which gave, for the first time, direct access to axially chiral arylborons with high yields and good ees [
59]. Instead of the typical B
2pin
2 (bis(pinacolato)diboron) reagent normally used to create arylboron compounds by reaction with aryl halides [
9,
60,
61], a set of unsymmetrical diboron reagents
41 bearing different substituents at boron were used, which were key to the success obtained, along with the nature of the chiral ligand used in this palladium-catalyzed reactions (
Figure 12). Various functional groups were compatible with the reaction conditions, and R
2 could be varied, but the highest yield of
42 was obtained when R = Me, although the selectivity was not much affected.
There was also no change in ee and efficiency when the reaction was performed on a 10-fold scale. Racemization experiments were also performed to demonstrate the stereochemical stability of arylboron atropisomers 42. A larger stability was obtained for 42b over 42a, which probably reflects the larger steric hindrance of the methyl group over the methide in naphthalene. Additional derivatizations of the initially obtained products were also performed. One of these, tetra-ortho-substituted axial chiral arylboron 3d, obtained with 95% ee after treatment of 42a with n-BuLi/MeI, also showed good configurational stability. It did not racemize even after incubation at 150 °C for 24 h.
In 2021, the first enantioselective procedure to synthesize 1,2-azaborines with a C–B stereogenic axis was also described. Xiang, Zhang, Tan, and coworkers selected as starting materials prochiral
B-aryl-1,2-azaborines
43, which, if functionalized with groups capable of blocking free rotation, could lead to the desired products (
Figure 13) [
17]. Such a strategy is more difficult to implement with
B-aryl-1,2-azaborines than with their congeners with C–C or C–N stereogenic axes since the longer bond length of the Csp
2–B bond leads to lower configurational stability. A strategy relying on electrophilic aromatic substitution of phenols was planned, which was implemented using a chiral phosphoric acid-catalyzed desymmetrization reaction with suitable electrophiles (
Figure 13a). Although
45 and
47–
48 (
Figure 13b) did not afford the desired product, and
49 and
50 reacted to give products with moderate ees, diazodicarboxamide
51 afforded excellent results, particularly with
CPA-1. The long-range enantiocontrol obtained was excellent for a large range of compounds, presumably through hydrogen bond formation between the catalyst and both substrates. Control experiments showed that in the absence of a phenolic OH group on the azaborine framework, i.e., when the hydroxyl group was replaced with a methoxy group, no reaction occurred. This observation supports the idea that the phenolic hydroxyl group is directly involved in the desymmetrization reaction, presumably via hydrogen bond formation with the catalyst, according to the model shown in TS1 (
Figure 13b). A gram-scale synthesis of
46a (
46: R
1 = Me, R
2 = Ph) followed by N-N bond cleavage under hydrogenation conditions with Raney nickel catalysis, afforded carbamate
46b, which was shown by an X-ray crystal structure to have a B–Cq bond length of 1.592 Å, larger than was originally predicted by Mancinelli and coworkers for a B-C bond [
33,
37]. The involvement of the NH group was also confirmed since when the nitrogen was methylated, the reaction yield dropped considerably. The gram-scale synthesis of the 1,2-azaborine showed no deterioration of the results.
The atropselective synthesis of B-aryl-2,1-azaborines was recently accomplished for the first time. You, Song, and coworkers developed a catalytic dynamic kinetic (DyKAT) asymmetric palladium-catalyzed cross-coupling reaction involving a tetracoordinate boron intermediate (
Figure 14a,b) [
62]. Hence, racemic, configurationally stable 3-bromo-2,1-azaborines
52 were reacted with boronic acid derivatives
53 or
54 to produce the desired chiral compounds
55 in very high yields and drs and ees when P-chiral monophosphorus ligand
L2 was used as catalyst. The configuration of product
55a was determined using X-ray crystallography to be (
R). The success of this method was due to the fact that the tetracoordinate boron intermediate
I1 that is formed in the reaction can reduce the rotation barrier and facilitate rotation of the aryl group on the B atom around the C-B stereogenic axis, allowing a fast equilibration between intermediate
(R)-I and
(S)-I. Since one of the intermediates undergoes transmetallation faster than the other, DyKAT is achieved (
Figure 14b). This procedure could also be used to prepare atropisomers bearing adjacent C-B and C-C diaxes with excellent drs and ees.
Atropisomers bearing two or more different kinds of stereogenic axes are, in general, still rare [
63]. Diaxially chiral B,N-heterocycles bearing C-B and C-N axes were first described in 2022 [
64]. He and coworkers reported an asymmetric vinylation of 2,1-borazaronaphthalenes
56, which displayed both the C-B and C-N axis of rotation (
Figure 15). The enantioenriched allylic compounds
58 were generated; they were subjected to a stereospecific isomerization to afford axially chiral molecules
59 with the two stereogenic axes. For the allylic substitution reaction, DBU or
tBuOLi did not afford the desired product, presumably for failing to deprotonate NH, while KHMDS or LiHMDS did. If a methyl was used instead of an aryl B-substituent, a methyl was used, there would be no reaction. The products were obtained with good yields and very high ees. The configuration of
59a was determined to be
S using single-crystal X-ray diffraction. The isomerization proved to be trickier, and inorganic bases did not work. However, the transformation could be successfully achieved with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD). This base promoted reaction via a stereospecific 1,3-proton transfer process, and for the first time, no H-bond assistance or directing groups were required, in contrast to previously reported examples of stepwise asymmetric allylic substitution-isomerization (
AASI) of axially chiral alkenes [
65]. By using ortho-substituted arenes, axially chiral B,N-heterocycles with higher configurational stability were produced. DFT studies were also undertaken and showed that the NH–π interactions played an important role in promoting stereospecific isomerization. With bulkier B,N-heterocycles, a stereodivergent synthesis of diaxially chiral B,N-heterocycles was also achieved.