Optical Dynamics of Picosecond Pulse Trains in Aluminum and Zinc Tetracarboxy-Phthalocyanines
Abstract
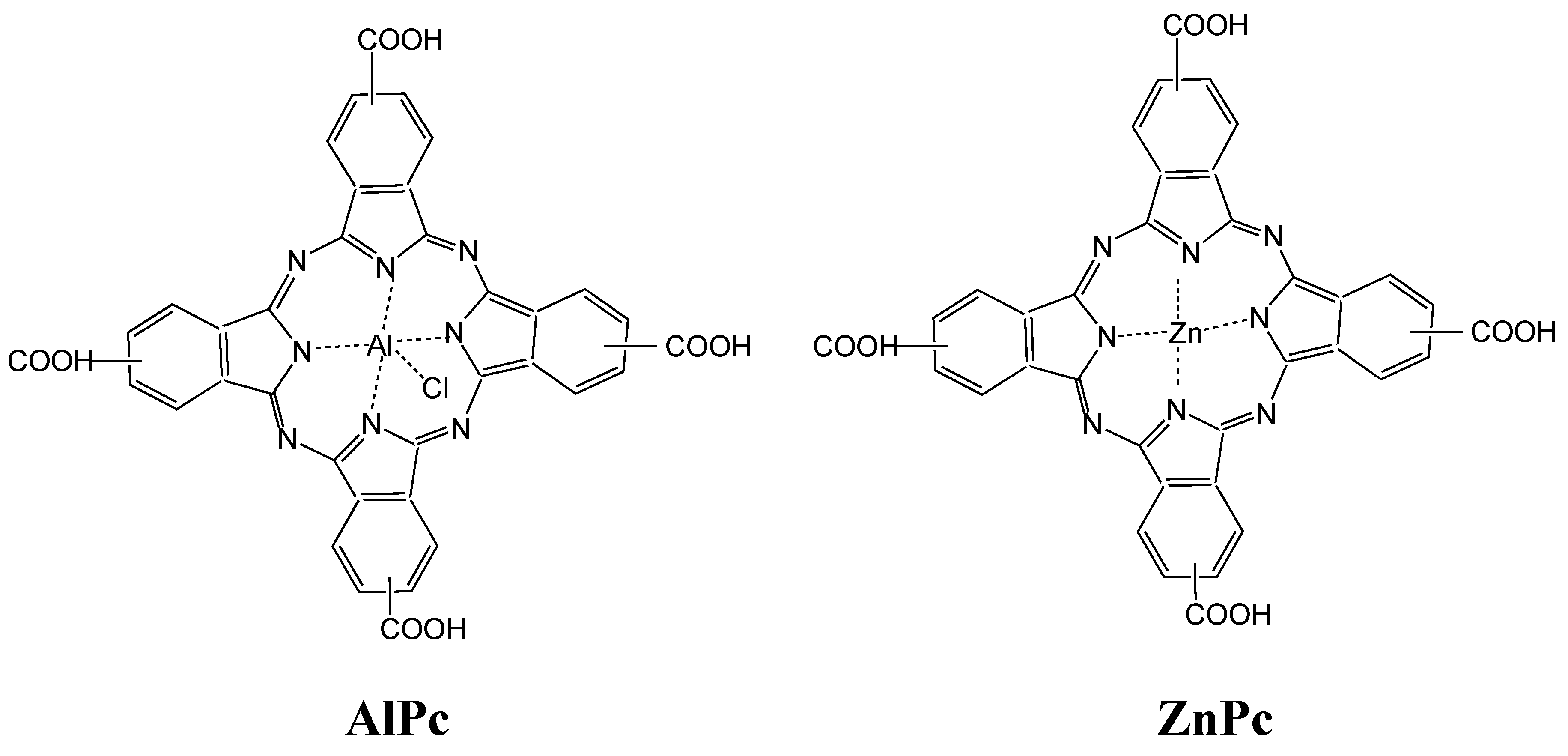
:1. Introduction
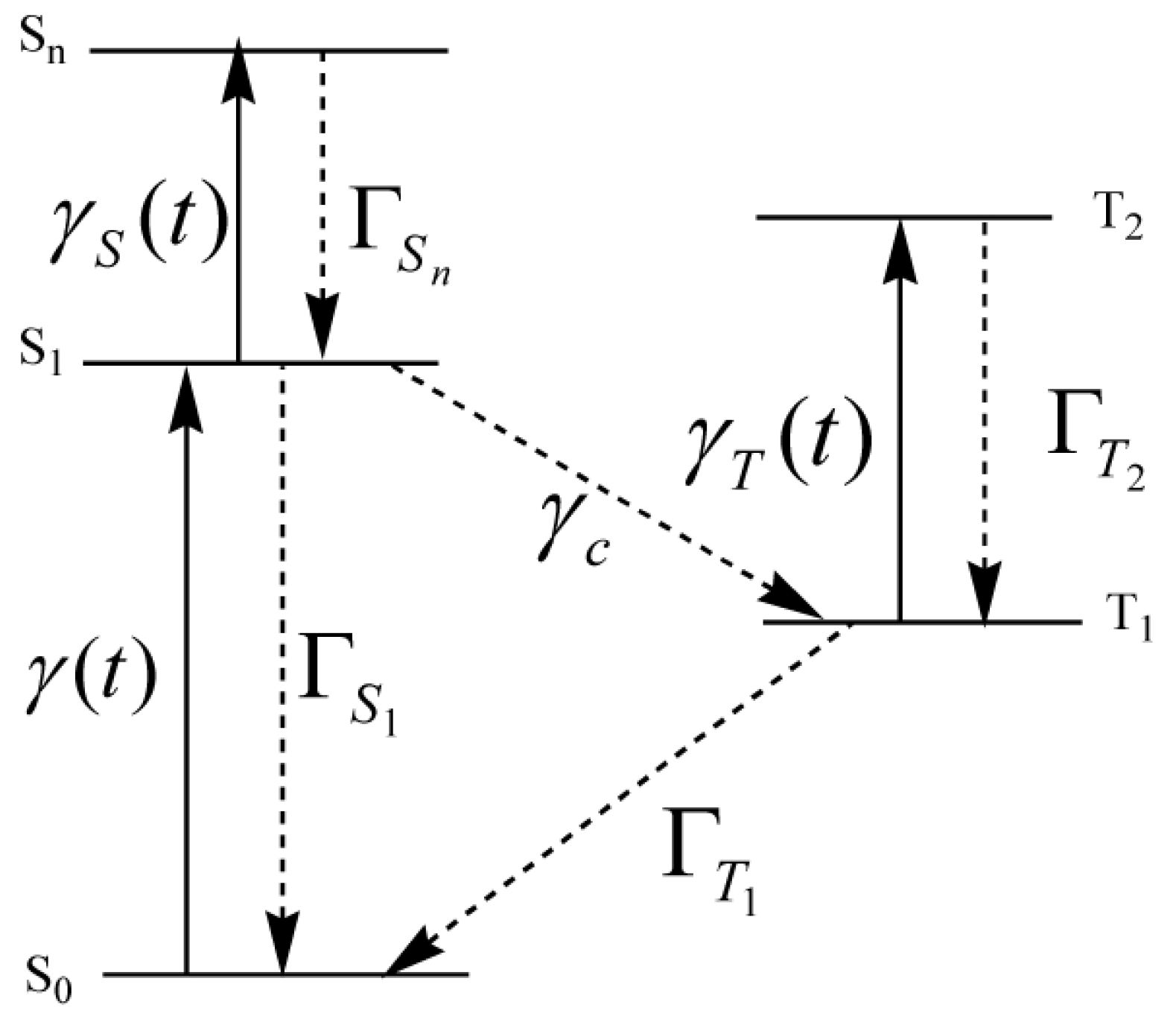
2. Method
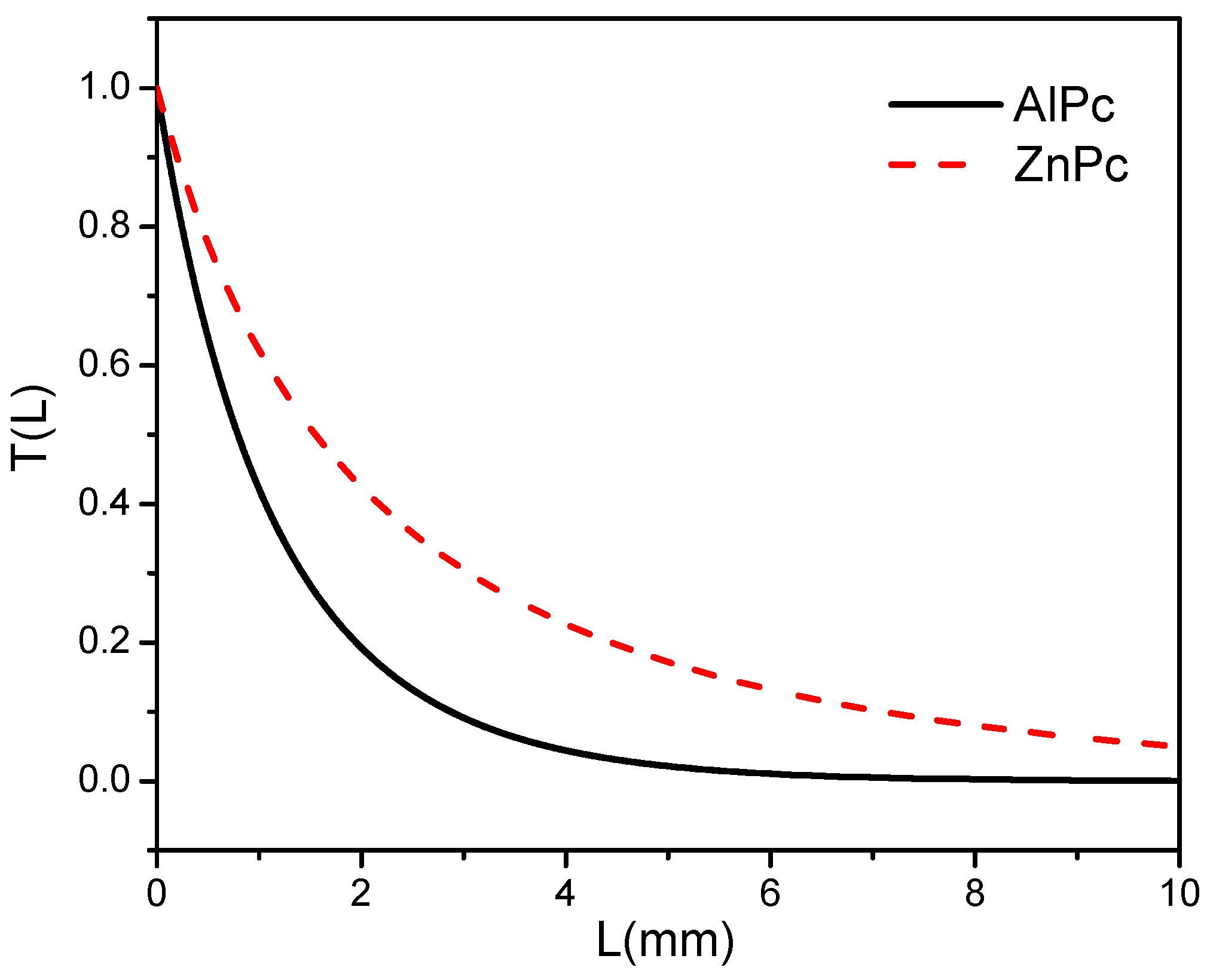
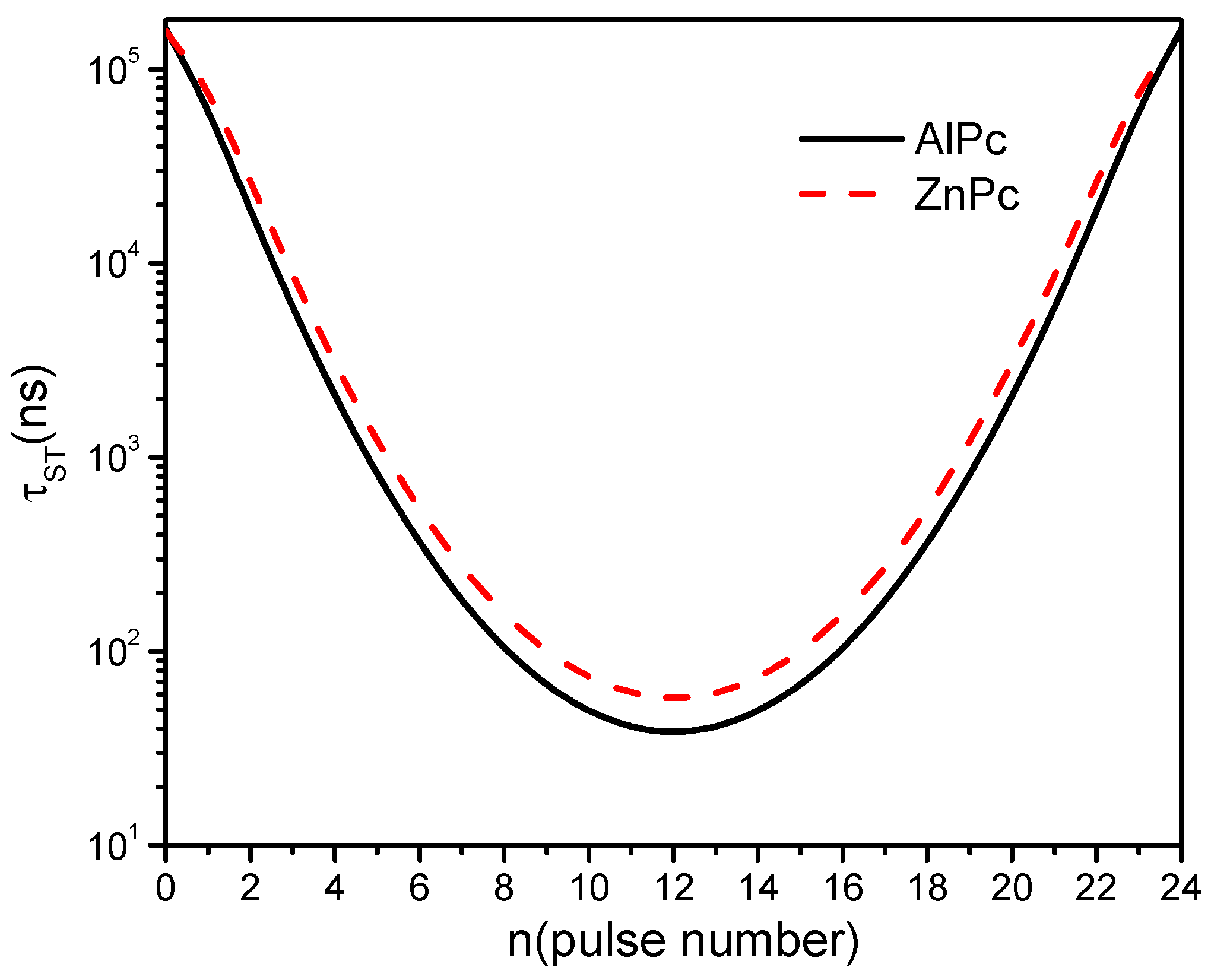
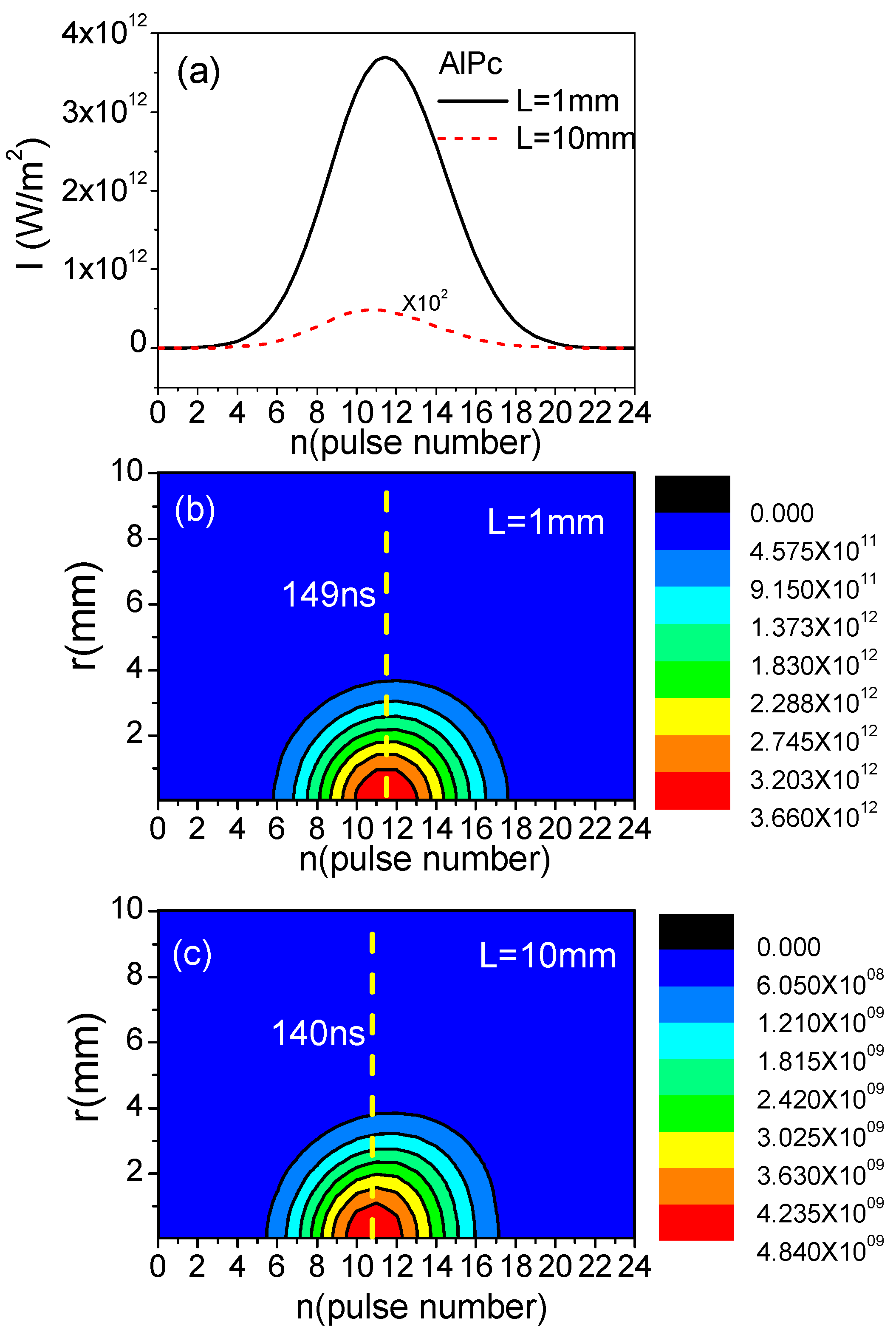
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.F.; Liu, Z.B.; Zhang, X.L.; Wang, Y.; Tian, J.G.; Huang, Y.; Ma, Y.F.; Zhang, X.Y.; Chen, Y.S. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Ren, J.S.; Yang, P.Y.; Wang, A.J.; Zhu, W.H.; Shang, D.H.; Song, Y.L. Synergistic promoted nonlinear optical effects in polyaniline nanohybrids covalently functionalized with tin porphyrin. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129588. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Q.H.; Gu, Z.G.; Zhang, J. Oriented Assembly of 2D Metal-Pyridylporphyrinic Framework Films for Giant Nonlinear Optical Limiting. Nano Lett. 2021, 21, 10012–10018. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Sun, E.; Song, R.; Liang, M.; Xu, Y. Nonlinear optical properties of both sulfonated- and halogen-substituted phenyl porphyrins with the effect of mono or bis halogen substitution and of protonation at different pH values on picosecond pulse trains generation. J. Mol. Struct. 2023, 1293, 136217. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 9, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Naleway, M.A.; Pang, A.J.; Chon, K.J.; Taratula, O. A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol. Pharm. 2013, 10, 3946–3958. [Google Scholar] [CrossRef]
- Sweed, A.M.K.; Ragab, S.S.; Shaker, Y.M. Synthesis and characterization of asymmetric oxyacetamide bridged porphyrin conjugates embedded with N-containing heterocycles. J. Porphyr. Phthalocyanines 2022, 26, 648–655. [Google Scholar] [CrossRef]
- Pucelik, B.; Paczynski, R.; Dubin, G.; Pereira, M.M.; Arnaut, L.G.; Dabrowski, J.M. Properties of halogenated and sulfonated porphyrins relevant for the selection of photosensitizers in anticancer and antimicrobial therapies. PLoS ONE 2017, 12, e0185984. [Google Scholar] [CrossRef]
- Vitkova, A.; Walker, S.J.I.; Sykulska-Lawrence, H. Cryogenically induced signal enhancement of Raman spectra of porphyrin molecules. Anal. Methods 2022, 14, 3307–3314. [Google Scholar] [CrossRef]
- Mondal, S.; Subramaniam, C. Point-of-Care, Cable-Type Electrochemical Zn2+ Sensor with Ultrahigh Sensitivity and Wide Detection Range for Soil and Sweat Analysis. ACS Sustain. Chem. Eng. 2019, 7, 14569–14579. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.F.; Hanack, M. Nonlinear optical materials for the smart filtering of optical radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Di Zazzo, L.; Magna, G.; Lucentini, M.; Stefanelli, M.; Paolesse, R.; Di Natale, C. Sensor-Embedded Face Masks for Detection of Volatiles in Breath: A Proof of Concept Study. Chemosensors 2021, 9, 356. [Google Scholar] [CrossRef]
- Thanopulos, I.; Paspalakis, E.; Yannopapas, V. Optical switching of electric charge transfer pathways in porphyrin: A light-controlled nanoscale current router. Nanotechnology 2008, 19, 445202. [Google Scholar] [CrossRef] [PubMed]
- Szyszko, B. Phenanthrene-Embedded Carbaporphyrinoids and Related Systems: From Ligands to Cages and Molecular Switches. Eur. J. Org. Chem. 2022, 30, e202200714. [Google Scholar] [CrossRef]
- Liu, W.; Yang, S.; Li, J.T.; Su, G.R.; Ren, J.C. One molecule, two states: Single molecular switch on metallic electrodes. Eur. J. Org. Chem. 2020, 11, e1511. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Q.; Roncevic, I.; Christensen, K.E.; Anderson, H.L. Anthracene-Porphyrin Nanoribbons. Angew. Chem. Int. Ed. 2023, 135, e202307035. [Google Scholar] [CrossRef]
- Durantini, J.E.; Rubio, R.; Solis, C.; Macor, L.; Morales, G.M.; Mangione, M.I.; Heredia, D.A.; Durantini, E.N.; Otero, L.; Gervaldo, M. Electrosynthesis of a hyperbranched dendrimeric porphyrin polymer: Optical and electronic characterization as a material for bifunctional electrochromic supercapacitors. Sustain. Energy Fuels 2020, 4, 6125–6140. [Google Scholar] [CrossRef]
- Pandit, S.; Dash, M.R. In pursuit of novel pyriporphyrin-a porphyrin ring expansion congener containing a built-in pyridine moiety by CH radical: A DFT investigation. Struct. Chem. 2023, 34, 1457–1468. [Google Scholar] [CrossRef]
- Gao, H.H.; Sun, Y.N.; Li, S.T.; Ke, X.; Cai, Y.; Wan, X.J.; Zhang, H.T.; Li, C.X.; Chen, Y.S. An all small molecule organic solar cell based on a porphyrin donor and a non-fullerene acceptor with complementary and broad absorption. Dye. Pigment. 2020, 176, 108250. [Google Scholar] [CrossRef]
- Bi, X.H.; Lv, X.; Mu, X.J.; Hai, J.; Cao, J.; Yang, Y.X. Molecular Dipole Modulation of Porphyrins to Enhance Photocatalytic Oxidation Activity for Inactivation of Intracellular Bacteria. ACS Biomater. Sci. Eng. 2023, 9, 617–624. [Google Scholar] [CrossRef]
- He, M.; Yu, X.Q.; Wang, Y.; Bao, M. Tunable Redox Potential Photocatalyst: Aggregates of 2,3-Dicyanopyrazino Phenanthrene Derivatives for the Visible-Light-Induced alpha-Allylation of Amines. J. Org. Chem. 2021, 86, 14720–14731. [Google Scholar] [CrossRef]
- Gavrilyuk, S.; Polyutov, S.; Jha, P.C.; Rinkeviciu, Z.; Ågren, H.; Gel’mukhanov, F. Many-Photon Dynamics of Photobleaching. J. Phys. Chem. A 2007, 111, 11961–11975. [Google Scholar] [CrossRef]
- Eriksson, A.; Bertlisson, K.; Lindgren, M. Simulation of beam propagation with time-dependent nonlinear processes in optical limiting applications. Synth. Met. 2002, 127, 147–150. [Google Scholar] [CrossRef]
- Polyzos, I.; Fakis, G.T.M.; Giannetas, V.; Persephonis, P.; Mikroyannidis, J. Two-photon absorption properties of novel organic materials for three-dimensional optical memories. Chem. Phys. Lett. 2003, 369, 264–268. [Google Scholar] [CrossRef]
- Blau, W.; Byrne, H.; Dennis, W.M.; Dennis, J.M.; Kelly, J.M. Reverse saturable absorption in tetraphenylporphyrins. Opt. Commun. 1985, 56, 25–29. [Google Scholar] [CrossRef]
- Dini, D.; Hanack, M.; Meneghetti, M. Nonlinear Optical Properties of Tetrapyrazinoporphyrazinato Indium Chloride Complexes Due to Excited-State Absorption Processes. J. Phys. Chem. B 2005, 109, 12691–12696. [Google Scholar] [CrossRef]
- Boni, L.D.; Rezende, D.C.J.; Mendonca, C.R. Reverse saturable absorption dynamics in indocyanine green. J. Photochem. Photobiol. A Chem. 2007, 190, 41–44. [Google Scholar] [CrossRef]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef]
- Goncalves, P.J.; Boni, L.D.; Borissevitch, I.E.; Zilio, S.C. Excited State Dynamics of meso-Tetra(sulphonatophenyl) Metalloporphyrins. J. Phys. Chem. A 2008, 112, 6522–6526. [Google Scholar] [CrossRef]
- Goncalves, P.J.; Boni, L.D.; Neto, N.M.B.; Rodrigues, J.J., Jr.; Zilio, S.C.; Borissevitch, I.E. Effect of protonation on the photophysical properties of meso-tetra(sulfonatophenyl) porphyrin. Chem. Phys. Lett. 2005, 407, 236–241. [Google Scholar] [CrossRef]
- Kubat, P.; Mosinger, J. Photophysical properties of metal complexes of meso-tetrakis(4-sulphonatophenyl)porphyrin. J. Photochem. Photobiol. A Chem. 1996, 96, 93–97. [Google Scholar] [CrossRef]
- Goncalves, P.J.; Borissevitch, I.E.; Zilio, S.C. Effect of protonation on the singlet–singlet excited-state absorption of meso-tetrakis(p-sulphonatophenyl) porphyrin. Chem. Phys. Lett. 2009, 469, 270–273. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Gomes, W.R.; Araujo, D.M.S.; Machado, A.E.H.; Silva, R.A.; Marletta, A.; Borissevitch, I.E.; Ito, A.S.; Dinelli, L.R.; Batista, A.A.; et al. Investigation of Ground- and Excited-State Photophysical Properties of 5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphyrin with Ruthenium Outlying Complexes. J. Phys. Chem. A 2012, 116, 18–26. [Google Scholar] [CrossRef]
- Miao, Q.; Sun, E.; Liu, Q.; Song, R.; Liang, M.; Xu, Y. Influence of bovine serum albumin (BSA) on photodynamic properties of meso-tetrakis(sulfonatophenyl) porphyrin (TPPS4) for picosecond pulse trains. Phys. Scr. 2023, 98, 035401. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Bezerra, F.C.; Almeida, L.M.; Alonso, L.; Souza, G.R.L.; Alonso, A.; Zilio, S.C.; Borissevitch, I.E. Effects of bovine serum albumin (BSA) on the excited-state properties of meso tetrakis(sulfonatophenyl) porphyrin (TPPS4). Eur. Biophys. J. 2019, 48, 721–729. [Google Scholar] [CrossRef]
- Borissevitch, I.E.; Tominaga, T.T.; Schmitt, C.C. Photophysical studies on the interaction of two water soluble porphyrins with bowine serum albumin. Effects upon the porphyrin triplet state characteristics. J. Photochem. Phototobiol. A 1998, 114, 201–207. [Google Scholar] [CrossRef]
- Andrade, S.M.; Costa, S.M.B. Spectroscopic studies on the interaction of a water soluble porphyrin and two drug carrier proteins. Biophys. J. 2002, 82, 1607–1619. [Google Scholar] [CrossRef]
- Dubbelman, T.M.A.R. Porphyrin-protein interaction. In Photosensitisation; Moreno, G., Pottier, R.H., Truscott, T.G., Eds.; NATO ASI Series (Series H: Cell Biology); Springer: Berlin, Germany, 1988; Volume 15. [Google Scholar]
- Morgan, W.T.; Smith, A.; Koskelo, P. The interaction of human serum albumin and hemopexin with porphyrins. BBA Protein Struct. 1980, 624, 271–285. [Google Scholar] [CrossRef]
- Kadish, K.M.; Maiya, G.B.; Araullo, C.; Guillard, R. Micellar effects on the aggregation of tetraanionic porphyrins. Spectroscopic characterization of free-base meso-tetrakis(4-sulfonatophenyl)porphyrin, (TPPS)H2, and (TPPS)M (M = zinc(II), copper(II), and vanadyl) in aqueous micellar media. Inorg. Chem. 1989, 28, 2725–2731. [Google Scholar] [CrossRef]
- Kadish, K.M.; Maiya, B.G.; Araullo-McAdams, C. Spectroscopic characterization of meso-tetrakis(1-methylpyridinium-4-yl)porphyrins, [(TMpyP)H2]4+ and [(TMpyP)M]4+, in aqueous micellar media, where M = VO2+, Cu(II), and Zn(II). J. Phys. Chem. 1991, 95, 427–431. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Yushmanov, V.E.; Tabak, M. Interaction of Fe(III)- and Zn(II)-tetra(4-sulfonatophenyl) porphyrins with ionic and nonionic surfactants: Aggregation and binding. J. Inorg. Biochem. 2001, 85, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Ma, R.; An, Y.; Shi, L. Stability enhancement of ZnTPPS in acidic aqueous solutions by polymeric micelle. Chem. Commun. 2010, 46, 6560–6562. [Google Scholar] [CrossRef] [PubMed]
- Szmytkowski, J.; Brunet, S.M.K.; Tripathy, U.; O’Brien, J.A.; Paige, M.F.; Steer, R.P. Photophysics and halide quenching of Soret-excited ZnTPPS4− in aqueous media. Chem. Phys. Lett. 2011, 501, 278–282. [Google Scholar] [CrossRef]
- Lebold, T.P.; Yeow, K.L.; Steer, R.P. Fluorescence quenching of the S1 and S2 states of zinc meso-tetrakis(4-sulfonatophenyl)porphyrin by halide ions. Photochem. Photobiol. Sci. 2004, 3, 160–166. [Google Scholar] [CrossRef]
- Goncalvesa, P.J.; Franzen, P.L.; Correa, D.S.; Almeida, L.M.; Takara, M.; Ito, A.S.; Zilio, S.C.; Borissevitch, I.E. Effects of environment on the photophysical characteristics of mesotetrakis methylpyridiniumyl porphyrin (TMPyP). Spectrochim. Acta Part A 2011, 79, 1532–1539. [Google Scholar] [CrossRef]
- Reddi, E.; Ceccon, M.; Valduga, G.; Jori, G.; Bommer, J.C.; Elisei, F.; Latterini, L.; Mazzucato, U. Photophysical Properties and Antibacterial Activity of Meso-substituted Cationic Porphyrins. Photochem. Photobiol. 2002, 75, 462–470. [Google Scholar] [CrossRef]
- Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. 5,10,15,20-Tetrakis(N-Methyl-4-Pyridyl)-21 H,23H-Porphine (TMPyP) as a Sensitizer for Singlet Oxygen Imaging in Cells: Characterizing the Irradiation-dependent Behavior of TMPyP in a Single Cell. Photochem. Photobiol. 2006, 82, 177–184. [Google Scholar] [CrossRef]
- Jimenez-Banzo, A.; Sagrista, M.L.; Mora, M.; Nonell, S. Kinetics of singlet oxygen photosensitization in human skin fibroblasts. Free Radic. Biol. Med. 2008, 44, 1926–1934. [Google Scholar] [CrossRef]
- Cocca, L.H.Z.; Oliveira, T.M.A.; Gotardo, F.; Teles, A.V.; Menegatti, R.; Siqueira, J.P.; Mendonca, C.R.; Bataus, L.A.M.; Ribeiro, A.O.; Souza, T.F.M.; et al. Tetracarboxy-phthalocyanines: From excited state dynamics to photodynamic inactivation against Bovine herpesvirus type. J. Photochem. Photobiol. B Biol. 2017, 175, 1–8. [Google Scholar] [CrossRef]
- Idowu, M.; Ogunsipe, A.; Nyokong, T. Excited state dynamics of zinc and aluminum phthalocyanine carboxylates. Spectrochim. Acta Part A 2007, 68, 995–999. [Google Scholar] [CrossRef]
- Hendow, S.T.; Shakir, S.A. Recursive numerical solution for nonlinear wave propagation in fibers and cylindrically symmetric systems. Appl. Opt. 1986, 25, 1759. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Sun, Y.-P.; Gavrilyuk, S.; Liu, J.-C.; Wang, C.-K.; Ågren, H.; Gel’mukhanov, F. Optical limiting and pulse reshaping of picosecond pulse trains by fullerene C60. J. Electron. Spectrosc. Relat. Phenom. 2009, 174, 125–130. [Google Scholar] [CrossRef]
- Gel’mukhanov, F.; Ågren, H. Resonant X-ray Raman scattering. Phys. Rep. 1999, 312, 87–330. [Google Scholar] [CrossRef]
- Gavrilyuk, S.; Liu, J.C.; Kamada, K.; Ågren, H.; Gel’mukhanov, F. Optical limiting for microsecond pulses. J. Chem. Phys. 2009, 130, 054114. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Q.; Sun, E.; Xu, Y. Optical Dynamics of Picosecond Pulse Trains in Aluminum and Zinc Tetracarboxy-Phthalocyanines. Symmetry 2024, 16, 1337. https://doi.org/10.3390/sym16101337
Miao Q, Sun E, Xu Y. Optical Dynamics of Picosecond Pulse Trains in Aluminum and Zinc Tetracarboxy-Phthalocyanines. Symmetry. 2024; 16(10):1337. https://doi.org/10.3390/sym16101337
Chicago/Turabian StyleMiao, Quan, Erping Sun, and Yan Xu. 2024. "Optical Dynamics of Picosecond Pulse Trains in Aluminum and Zinc Tetracarboxy-Phthalocyanines" Symmetry 16, no. 10: 1337. https://doi.org/10.3390/sym16101337








