Conformational and Chiroptical Properties of Salicylamide-Based Peptidomimetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Conformational Analysis and Geometry Optimizations
2.3. Calculations of Optical Rotation
3. Results and Discussion
3.1. Conformational Analysis
3.2. Calculations of Optical Rotation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devinsky, F. Chirality and the origin of life. Symmetry 2021, 13, 2277. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A look at the importance of chirality in drug activity: Some significative examples. Appl. Sci. 2022, 12, 10909. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Zhou, H.; Huang, H.; Zhao, J.; Deng, Y.; Wang, H.; Yang, Y.; Yang, J.; Luo, L. Chiral pharmaceuticals: Environment sources, potential human health impacts, remediation technologies and future perspective. Environ. Int. 2018, 121, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, I.P.; Colbon, P.J.J. The growing importance of chirality in 3D chemical space exploration and modern drug discovery approaches for Hit-ID: Topical innovations. ACS Med. Chem. Lett. 2021, 12, 1220–1229. [Google Scholar] [CrossRef]
- Abram, M.; Jakubiec, M.; Kaminski, K. Chirality as an important factor for the development of new antiepileptic drugs. ChemMedChem 2019, 14, 1744–1761. [Google Scholar] [CrossRef] [PubMed]
- McGown, A.; Nafie, J.; Otayfah, M.; Hassell-Hart, S.; Tizzard, G.J.; Coles, S.J.; Banks, R.; Marsh, G.P.; Maple, H.J.; Kostakis, G.E.; et al. Chirality: A key parameter in chemical probes. RSC Chem. Biol. 2023, 4, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Kwon, J.; Cho, W.; Yeom, J. Chiral biomaterials for nanomedicines: From molecules to supraparticles. Pharmaceutics 2022, 14, 1951. [Google Scholar] [CrossRef]
- Cho, N.H.; Guerrero-Martinez, A.; Ma, J.; Bals, S.; Kotov, N.A.; Liz-Marzan, L.M.; Nam, K.T. Bioinspired chiral inorganic nanomaterials. Nat. Rev. Bioeng. 2023, 1, 88–106. [Google Scholar] [CrossRef]
- Rosenfeld, L. Quantenmechanische Theorie der Natürlichen OptischenAktivität von Flüssigkeiten und Gasen. Z. Phys. 1928, 52, 161–174. [Google Scholar] [CrossRef]
- Grauso, L.; Teta, R.; Esposito, G.; Menna, M.; Mangoni, A. Computational prediction of chiroptical properties in structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 1005–1030. [Google Scholar] [CrossRef]
- Crawford, T.D.; Tam, M.C.; Abrams, M.L. The current state of ab initio calculations of optical rotation and electronic circular dichroism spectra. J. Phys. Chem. A 2007, 111, 12057–12068. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef] [PubMed]
- Salicylic Acid, DrugBank. Available online: https://go.drugbank.com/drugs/DB00936 (accessed on 5 December 2023).
- Dotto, C.; Lombarte Serrat, A.; Ledesma, M.; Vay, C.; Ehling-Schulz, M.; Sordelli, D.O.; Grunert, T.; Buzzola, F. Salicylic acid stabilizes Staphylococcus aureus biofilm by impairing the agr quorum-sensing system. Sci. Rep. 2021, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Almolhim, H.; Elhassanny, A.E.M.; Abutaleb, N.S.; Abdelsattar, A.S.; Seleem, M.N.; Carlier, P.R. Substituted salicylic acid analogs offer improved potency against multidrug-resistant Neisseria gonorrhoeae and good selectivity against commensal vaginal bacteria. Sci Rep. 2023, 13, 14468. [Google Scholar] [CrossRef]
- Sykes, E.M.E.; White, D.; McLaughlin, S.; Kumar, A. Salicylic acids and pathogenic bacteria: New perspectives on an old compound. Can. J. Microbiol. 2024, 70, 1–14. [Google Scholar] [CrossRef]
- Aspirin, DrugBank. Available online: https://go.drugbank.com/drugs/DB00945 (accessed on 5 December 2023).
- Mesalazine, DrugBank. Available online: https://go.drugbank.com/drugs/DB00244 (accessed on 5 December 2023).
- Niclosamide, DrugBank. Available online: https://go.drugbank.com/drugs/DB06803 (accessed on 5 December 2023).
- Rafoxanide, NIH—Inxight Drugs. Available online: https://drugs.ncats.io/drug/22F4FLA7DH (accessed on 5 December 2023).
- Closantel, NIH—Inxight Drugs. Available online: https://drugs.ncats.io/drug/EUL532EI54 (accessed on 5 December 2023).
- Jiang, H.; Li, A.M.; Ye, J. The magic bullet: Niclosamide. Front. Oncol. 2022, 12, 1004978. [Google Scholar] [CrossRef]
- Singh, S.; Weiss, A.; Goodman, J.; Fisk, M.; Kulkarni, S.; Lu, I.; Gray, J.; Smith, R.; Sommer, M.; Cheriyan, J. Niclosamide—A promising treatment for COVID-19. Br. J. Pharmacol. 2022, 179, 3250–3267. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Wsol, V.; Trejtnar, F.; Ulmann, V.; Stolarikova, J.; Fernandes, S.; Bhat, S.; et al. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis 2012, 92, 434–439. [Google Scholar] [CrossRef]
- Wang, W.; Qin, Z.; Zhu, D.; Wei, Y.; Li, S.; Duan, L. Synthesis, bioactivity evaluation, and toxicity assessment of novel salicylanilide ester derivatives as cercaricides against Schistosoma japonicum and molluscicides against Oncomelania hupensis. Antimicrob. Agents Chemother. 2015, 60, 323–331. [Google Scholar] [CrossRef]
- He, P.; Wang, W.; Sanogo, B.; Zeng, X.; Sun, X.; Lv, Z.; Yuan, D.; Duan, L.; Wu, Z. Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasit. Vectors 2017, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Burgain, A.; Chaillot, J.; Pic, E.; Khemiri, I.; Sellam, A. A phenotypic small-molecule screen identifies halogenated salicylanilides as inhibitors of fungal morphogenesis, biofilm formation and host cell invasion. Sci. Rep. 2018, 8, 11559. [Google Scholar] [CrossRef] [PubMed]
- Angom, R.S.; Zhu, J.; Wu, A.T.H.; Sumitra, M.R.; Pham, V.; Dutta, S.; Wang, E.; Madamsetty, V.S.; Perez-Cordero, G.D.; Huang, H.S.; et al. LCC-09, a novel salicylanilide derivative, exerts anti-inflammatory effect in vascular endothelial cells. J. Inflamm. Res. 2021, 14, 4551–4565. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Ferriz, J.M.; Pauk, K.; Kratky, M.; Vinsova, J. Synthetic route for the preparation of 2-hydroxy-N-[1-(2-hydroxyphenylamino)-1-oxoalkan-2-yl]benzamides. J. Comb. Chem. 2010, 12, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Alazemi, A.M.; Dawood, K.M.; Al-Matar, H.M.; Tohamy, W.M. Microwave-assisted chemoselective synthesis and photophysical properties of 2-arylazo-biphenyl-4-carboxamides from hydrazonals. RSC Adv. 2023, 13, 25054–25068. [Google Scholar] [CrossRef] [PubMed]
- Lal, J.; Ramalingam, K.; Meena, R.; Ansari, S.B.; Saxena, D.; Chopra, S.; Goyal, N.; Reddy, D.N. Design and synthesis of novel halogen rich salicylanilides as potential antileishmanial agents. Eur. J. Med. Chem. 2023, 246, 114996. [Google Scholar] [CrossRef] [PubMed]
- Kauerova, T.; Perez-Perez, M.J.; Kollar, P. Salicylanilides and their anticancer properties. Int. J. Mol. Sci. 2023, 24, 1728. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Imramovsky, A.; Stepankova, S.; Vanco, J.; Pauk, K.; Monreal-Ferriz, J.; Vinsova, J.; Jampilek, J. Acetylcholinesterase-inhibiting activity of salicylanilide N-alkylcarbamates and their molecular docking. Molecules 2012, 17, 10142–10158. [Google Scholar] [CrossRef]
- Song, Q.; Li, Y.; Cao, Z.; Qiang, X.; Tan, Z.; Deng, Y. Novel salicylamide derivatives as potent multifunctional agents for the treatment of Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 84, 137–149. [Google Scholar] [CrossRef]
- Kratky, M.; Jaklova, K.; Stepankova, S.; Svrckova, K.; Pflegr, V.; Vinsova, J. N-[3,5-Bis(trifluoromethyl)phenyl]-5-bromo-2-hydroxybenzamide analogues: Novel acetyl- and butyrylcholinesterase inhibitors. Curr. Top. Med. Chem. 2020, 20, 2094–2105. [Google Scholar] [CrossRef]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russell, R.K.; Sui, Z.; Weidner-Wells, M.A.; Werblood, H.; et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.Y.; Gruber, T.D.; Samuels, A.; Yun, M.; Nam, B.; Kang, M.; Crowley, K.; Winterroth, B.; Boshoff, H.I.; Barry, C.E. Structure-activity relationships of antitubercular salicylanilides consistent with disruption of the proton gradient via proton shuttling. Bioorg. Med. Chem. 2013, 21, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Vinsova, J.; Kozic, J.; Kratky, M.; Stolarikova, J.; Mandikova, J.; Trejtnar, F.; Buchta, V. Salicylanilide diethyl phosphates: Synthesis, antimicrobial activity and cytotoxicity. Bioorg. Med. Chem. 2014, 22, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Kollar, P.; Ferreira, A.L.; Palma, D.; Duarte, A.; Lopes, M.M.; Bartos, M.; Pauk, K.; Imramovsky, A.; Jampilek, J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016, 14, 125–130. [Google Scholar] [CrossRef]
- Paraskevopoulos, G.; Monteiro, S.; Vosatka, R.; Kratky, M.; Navratilova, L.; Trejtnar, F.; Stolarikova, J.; Vinsova, J. Novel salicylanilides from 4,5-dihalogenated salicylic acids: Synthesis, antimicrobial activity and cytotoxicity. Bioorg. Med. Chem. 2017, 25, 1524–1532. [Google Scholar] [CrossRef]
- Alhashimi, M.; Mayhoub, A.; Seleem, M.N. Repurposing salicylamide for combating multidrug-resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2019, 63, e01225-19. [Google Scholar] [CrossRef]
- Copp, J.N.; Pletzer, D.; Brown, A.S.; Van der Heijden, J.; Miton, C.M.; Edgar, R.J.; Rich, M.H.; Little, R.F.; Williams, E.M.; Hancock, R.E.W.; et al. Mechanistic understanding enables the rational design of salicylanilide combination therapies for Gram-negative infections. mBio 2020, 11, e02068-20. [Google Scholar] [CrossRef]
- Pop, B.; Ionuț, I.; Marc, G.; Vodnar, D.C.; Pîrnau, A.; Vlase, L.; Oniga, O. Development of new 2-methyl-4-salicylamide thiazole derivatives: Synthesis, antimicrobial activity evaluation, lipophilicity and molecular docking study. Farmacia 2021, 69, 724–731. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J. Salicylanilide N-monosubstituted carbamates: Synthesis and in vitro antimicrobial activity. Bioorg. Med. Chem. 2016, 24, 1322–1330. [Google Scholar] [CrossRef]
- Baranyai, Z.; Kratky, M.; Vinsova, J.; Szabo, N.; Senoner, Z.; Horvati, K.; Stolarikova, J.; David, S.; Bosze, S. Combating highly resistant emerging pathogen Mycobacterium abscessus and Mycobacterium tuberculosis with novel salicylanilide esters and carbamates. Eur. J. Med. Chem. 2015, 101, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Ienascu, I.M.C.; Obistioiu, D.; Popescu, I.M.; Stefanut, M.N.; Gyongyi, O.; Jurca, C.; Ciavoi, G.; Bechir, E.S.; Bechir, F.; Cata, A. In vitro testing of salicylanilide derivatives against some fungal and bacterial strains. Rev. Chim. 2019, 70, 1496–1499. [Google Scholar] [CrossRef]
- Ienascu, I.M.C.; Cata, A.; Stefanuț, M.N.; Popescu, I.; Rusu, G.; Sfirloaga, P.; Ursu, D.; Mosoarca, C.; Dabici, A.; Danciu, C.; et al. Novel chloro-substituted salicylanilide derivatives and their β-cyclodextrin complexes: Synthesis, characterization, and antibacterial activity. Biomedicines 2022, 10, 1740. [Google Scholar] [CrossRef] [PubMed]
- Pindjakova, D.; Pilarova, E.; Pauk, K.; Michnova, H.; Hosek, J.; Magar, P.; Cizek, A.; Imramovsky, A.; Jampilek, J. Study of biological activities and ADMET-related properties of salicylanilide-based peptidomimetics. Int. J. Mol. Sci. 2022, 23, 11648. [Google Scholar] [CrossRef]
- Panda, S.S.; Kumari, S.; Dixit, M.; Sharma, N.K. N-Salicyl-AAn-picolamide foldameric peptides exhibit quorum sensing inhibition of Pseudomonas aeruginosa (PA14). ACS Omega 2023, 8, 30349–30358. [Google Scholar] [CrossRef]
- Jorda, R.; Magar, P.; Hendrychova, D.; Pauk, K.; Dibus, M.; Pilarova, E.; Imramovsky, A.; Krystof, V. Novel modified leucine and phenylalanine dipeptides modulate viability and attachment of cancer cells. Eur. J. Med. Chem. 2020, 188, 112036. [Google Scholar] [CrossRef]
- Xu, J.; Kim, H.; Dong, J.; Chen, H.; Xu, J.; Ma, R.; Zhou, M.; Wang, T.; Shen, Q.; Zhou, J. Structure-activity relationship studies on O-alkylamino-tethered salicylamide derivatives with various amino acid linkers as potent anticancer agents. Eur. J. Med. Chem. 2022, 234, 114229. [Google Scholar] [CrossRef]
- Soukaina, E.; Al-Zaqri, N.; Warad, I.; Ichou, H.; Yassine, K.; Guenoun, F.; Bouachrine, M. Novel antiproliferative inhibitors from salicylamide derivatives with dipeptide moieties using 3D-QSAR, molecular docking, molecular dynamic simulation and ADMET studies. J. Mol. Struct. 2023, 1282, 135219. [Google Scholar] [CrossRef]
- Advanced Chemistry Development, Inc. ACD/Name, ver. 12.01; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2009. [Google Scholar]
- Hypercube Inc. HyperChem, Release 8.0.3 for Windows, Molecular Modeling System; Hypercube Inc.: Waterloo, ON, Canada, 2007. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J.; Mennucci, B.; Tomasi, J. Prediction of optical rotation using density functional theory: 6,8-dioxabicyclo [3.2.1]octanes. Tetrahedron Asymmetry 2000, 11, 2443–2448. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Frisch, M.J.; Devlin, F.J.; Stephens, P.J. Hartree-Fock and density functional theory ab initio calculation of optical rotation using GIAOs: Basis set dependence. J. Phys. Chem. A 2000, 104, 1039–1046. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J. Calculation of optical rotation using density functional theory. J. Phys. Chem. A 2001, 105, 5356–5371. [Google Scholar] [CrossRef]
- Stephens, P.J.; McCann, D.M.; Cheeseman, J.R.; Frisch, M.J. Determination of absolute configurations of chiral molecules using ab initio time-dependent density functional theory calculations of optical rotation: How reliable are absolute configurations obtained for molecules with small rotations? Chirality 2005, 17, S52–S64. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J.; Cammi, R.; Cheeseman, J.R.; Frisch, M.J.; Devlin, F.J.; Gabriel, S.; Stephens, P.J. Polarizable continuum model (PCM) calculations of solvent effects on optical rotations of chiral molecules. J. Phys. Chem. A 2002, 106, 6102–6113. [Google Scholar] [CrossRef]
| Comp. | Structure | Name | Molecular Formula /Weight | Refs. |
|---|---|---|---|---|
| 1 | 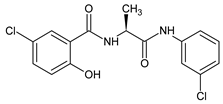 | 5-chloro-N-{(2S)-1-[(3-chlorophenyl)amino]-1-oxopropan-2-yl}-2-hydroxybenzamide | C16H14Cl2N2O3 [353.200] | [30,47] |
| 2 | 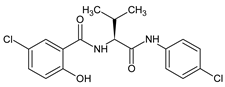 | 5-chloro-N-{(2S)-1-[(4-chloro phenyl)amino]-3-methyl -1-oxobutan-2-yl}-2-hydroxybenzamide | C18H18Cl2N2O3 [381.253] | [47] |
| 3 | 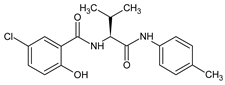 | 5-chloro-2-hydroxy-N-{(2S) -3-methyl-1-[(4-methylphenyl)amino] -1-oxobutan-2-yl}benzamide | C19H21ClN2O3 [360.835] | [30,47] |
| 4 | 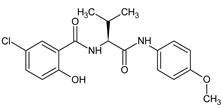 | 5-chloro-2-hydroxy-N-{(2S)- 1-[(4-methoxyphenyl)amino]-3-methyl -1-oxobutan-2-yl}benzamide | C19H21ClN2O4 [376.834] | [30,47] |
| 5 | 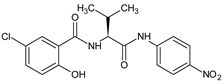 | 5-chloro-2-hydroxy-N-{(2S) -3-methyl-1-[(4-nitrophenyl)amino] -1-oxobutan-2-yl}benzamide | C18H18ClN3O5 [391.806] | [30] |
| 6 |  | 5-chloro-2-hydroxy-N-[(2S) -3-methyl-1-oxo-1-{[4-(trifluoromethyl)phenyl]amino}butan-2-yl]benzamide | C19H18ClF3N2O3 [414.806] | [30,47] |
| Comp. | Conformations after Conformation Search | Conformations after Preoptimization |
|---|---|---|
| 1 | 268 | 22 |
| 2 | 349 | 25 |
| 3 | 1268 | 10 |
| 4 | 1219 | 22 |
| 5 | 1180 | 13 |
| 6 | 518 | 24 |
| Comp. | Conformer Designation | Geometry | Population |
|---|---|---|---|
| 1 | a | 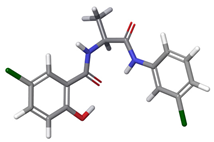 | 99.95% |
| 2 | a | 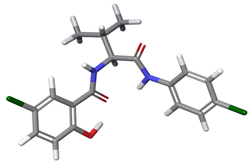 | 60.74% |
| b | 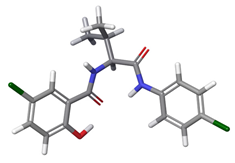 | 23.13% | |
| c | 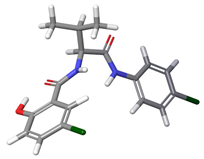 | 16.09% | |
| 3 | a | 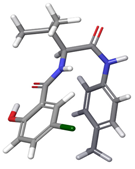 | 79.29% |
| b | 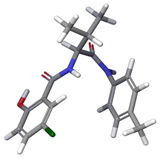 | 16.40% | |
| c | 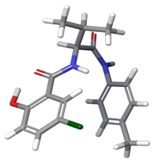 | 4.30% | |
| 4 | a |  | 69.25% a |
| b | 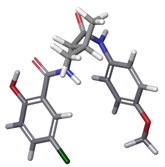 | 17.52% a | |
| c | 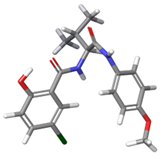 | 7.54% a | |
| 5 | a | 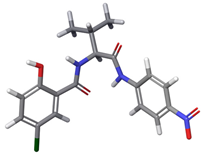 | 67.90% |
| b | 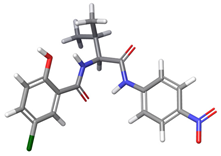 | 32.09% | |
| 6 | a | 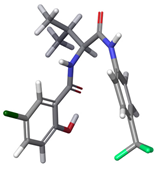 | 34.50% |
| b |  | 33.11% | |
| c | 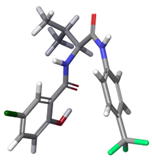 | 27.79% | |
| d |  | 14.10% |
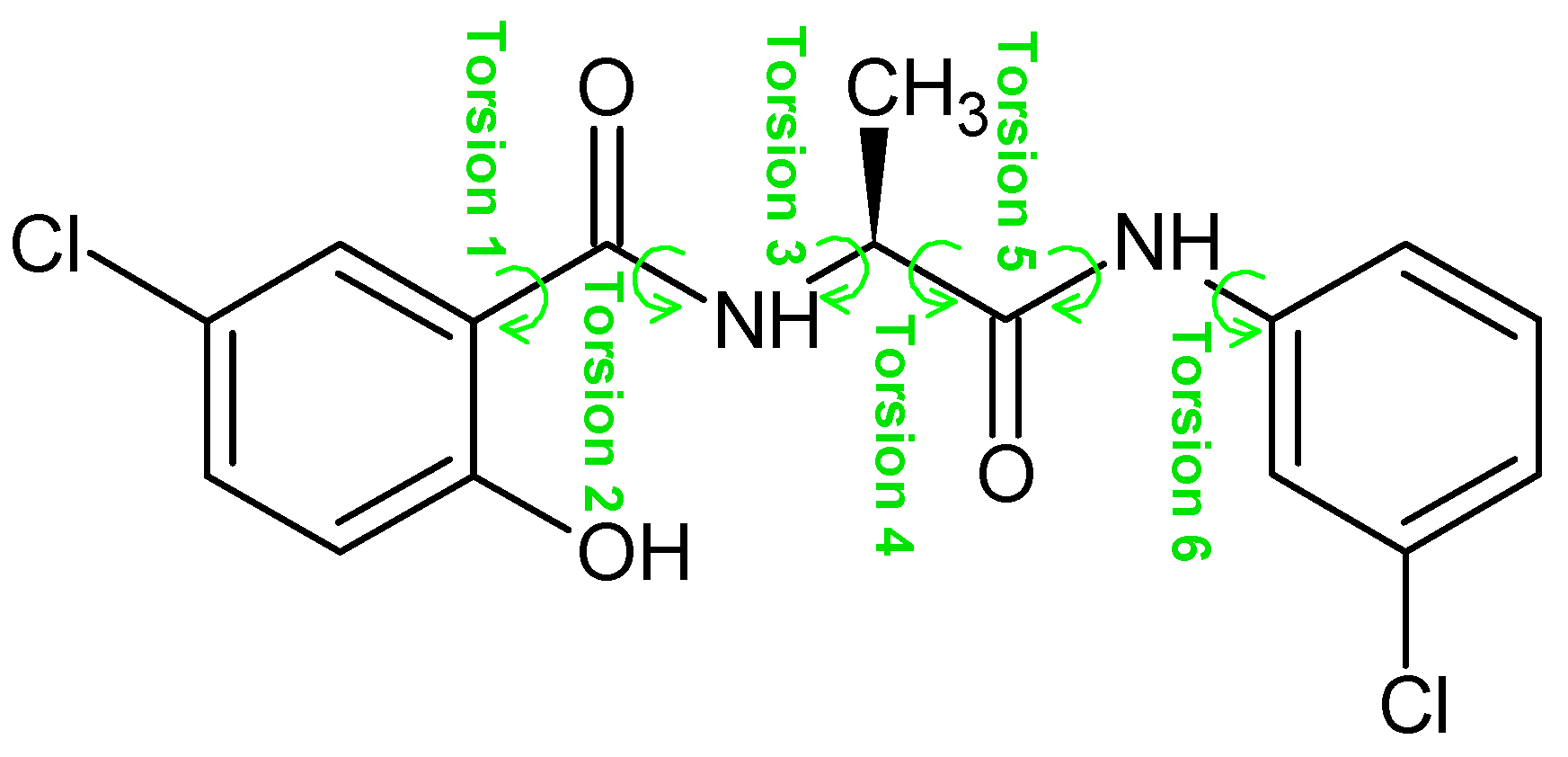
| Conformer | Torsion 1 | Torsion 2 | Torsion 3 | Torsion 4 | Torsion 5 | Torsion 6 |
|---|---|---|---|---|---|---|
| 1a | −169.7 | −174.1 | −87.4 | 67.0 | −178.1 | 177.7 |
| 2a | −170.0 | −177.6 | −87.9 | 76.7 | −176.4 | 174.6 |
| 2b | −168.7 | −173.7 | −88.1 | 61.4 | −179.1 | 179.9 |
| 2c | 172.9 | −173.2 | −117.5 | 12.8 | 176.7 | −178.1 |
| 3a | 173.1 | −178.7 | −132.6 | 41.4 | 16.1 | 51.4 |
| 3b | −173.7 | −178.8 | 149.7 | −37.1 | −11.6 | 117.1 |
| 3c | −171.1 | −174.1 | −106.8 | −3.6 | −14.9 | 114.7 |
| 4a | −168.2 | −178.6 | −130.6 | 40.0 | 14.3 | 55.4 |
| 4b | −172.4 | −178.1 | −84.4 | −43.2 | −8.0 | 106.6 |
| 4c | −171.8 | 179.6 | 57.7 | 56.0 | 8.4 | 64.8 |
| 5a | 1.3 | −174.8 | −86.8 | 72.1 | −176.7 | 174.4 |
| 5b | 0.4 | −172.5 | −85.9 | 60.0 | −178.8 | 179.4 |
| 6a | −174.6 | −176.2 | −133.2 | 39.5 | 18.4 | 42.6 |
| 6b | 174.0 | −176.4 | −133.8 | 39.4 | 16.9 | 43.7 |
| 6c | 173.5 | −176.9 | −133.4 | 39.4 | 17.1 | 44.8 |
| 6d | 171.2 | 176.2 | 55.2 | 51.5 | 6.8 | 52.4 |
| Conformer | Population | Torsion 1 | Torsion 2 | Torsion 3 | Torsion 4 | Torsion 5 | Torsion 6 |
|---|---|---|---|---|---|---|---|
| 1a | 92.17% | −164.8 | −175.9 | −85.5 | 65.3 | −174.6 | 170.2 |
| 2a | 48.37% | −166.2 | −178.0 | −87.0 | 73.9 | −172.8 | 167.4 |
| 2b | 29.67% | −164.4 | −174.1 | −87.0 | 58.7 | −176.2 | 174.6 |
| 2c | 11.36% | −164.2 | 167.4 | −124.0 | 34.3 | 15.2 | 53.9 |
| 2d | 8.45% | 170.0 | −174.1 | −125.7 | 17.0 | 177.8 | −179.1 |
| 3a | 90.63% | −163.1 | 169.0 | −125.9 | 36.2 | 14.3 | −124.3 |
| 3b | 7.32% | −164.8 | 172.2 | −75.6 | −7.3 | −5.6 | 112.2 |
| 4a | 95.70% | −164.8 | 169.0 | −125.0 | 34.7 | 12.3 | −132.2 |
| 4b | 4.20% | −162.3 | 170.3 | −59.6 | −33.2 | 2.1 | 109.2 |
| 5a | 94.07% | −167.5 | 164.4 | −124.9 | 31.9 | 13.9 | −131.6 |
| 5b | 2.44% | 162.5 | −164.9 | 36.4 | 42.7 | −10.8 | −122.6 |
| 6a | 89.84% | −167.0 | 166.3 | −124.3 | 32.6 | 14.8 | −129.2 |
| 6d | 7.47% | −160.3 | 169.4 | −56.3 | −30.8 | 1.1 | 115.5 |
| Comp. | Solvent | 6-311++G(2d,p) | aug-cc-pVDZ | aug-cc-pVTZ |
|---|---|---|---|---|
| 1 | chloroform | −0.4 | 6.9 | 14.0 |
| ethyl acetate | 1.7 | 22.6 | 31.5 | |
| 2 | chloroform | −90.3 | −85.1 | −78.8 |
| ethyl acetate | −81.1 | −73.9 | −70.2 |
| Comp. | [α]d | Refs. |
|---|---|---|
| 1 | 37.0 (c 1.6) | [30,47] |
| 2 | −71.7 (c 1.5) | [47] |
| 3 | −56.2 (c 0.3) | [30,47] |
| 4 | −63.2 (c 0.2) | [30,47] |
| 5 | −64.8 (c 0.2) | [30] |
| 6 | −73.8 (c 0.2) | [30,47] |
| Comp. | Conformer | 6-311++G(2d,p) | aug-cc-pVDZ | aug-cc-pVTZ | aug-cc-pVQZ |
|---|---|---|---|---|---|
| 1 | a | 1.7 | 22.6 | 31.5 | 32.2 |
| 2 | a | −98.6 | −90.3 | −84.5 | −86.1 |
| b | −214.9 | −207.0 | −200.6 | −203.2 | |
| c | 177.2 | 179.1 | 179.9 | 180.8 | |
| w. a. 1 | −81.1 | −73.9 | −68.8 | −70.2 | |
| 3 | a | −161.9 | −158.7 | −151.8 | −132.8 |
| b | 108.4 | 110.9 | 111.8 | 121.4 | |
| c | 238.3 | 236.7 | 239.9 | 239.9 | |
| w. a. | −100.4 | −97.5 | −91.7 | −75.1 | |
| 4 | a | −66.9 | −60.1 | −54.2 | −53.8 |
| b | 51.5 | 55.0 | 55.4 | 57.3 | |
| c | −185.3 | −185.2 | −184.5 | −201.7 | |
| w. a. | −54.4 | −48.7 | −44.2 | −45.0 | |
| 5 | a | −230.6 | −225.0 | −226.9 | −228.0 |
| b | −363.8 | −357.1 | −356.8 | −340.0 | |
| w. a. | −273.3 | −267.3 | −268.6 | −263.9 | |
| 6 | a | −247.0 | −241.0 | −234.3 | −226.1 |
| b | −200.9 | −202.2 | −195.4 | −187.6 | |
| c | −188.9 | −189.7 | −183.6 | −179.3 | |
| d | 160.7 | 161.5 | 159.1 | 167.3 | |
| w. a. | −160.0 | −158.7 | −153.5 | −146.4 |
| Comp. | Conformer | aug-cc-pVDZ |
|---|---|---|
| 1 | a | 24.3 |
| 2 | a | 101.7 |
| b | −222.9 | |
| c | −341.7 | |
| d | 185.5 | |
| w. a. 1 | −139.9 | |
| 3 | a | −26.0 |
| b | 179.2 | |
| w. a. | −10.6 | |
| 4 | a | −60.1 |
| b | 55.0 | |
| c | −185.2 | |
| w. a. | −55.3 | |
| 5 | a | −257.2 |
| b | −191.6 | |
| w. a. | −255.5 | |
| 6 | a | −335.4 |
| b | 217.5 | |
| w. a. | −292.9 |
| Basis Set | Time [d] |
|---|---|
| 6-311++G(2d,p) | 0.5 |
| aug-cc-pVDZ | 0.5 |
| aug-cc-pVTZ | 10 |
| aug-cc-pVQZ | >70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raich, I.; Pauk, K.; Imramovsky, A.; Jampílek, J. Conformational and Chiroptical Properties of Salicylamide-Based Peptidomimetics. Symmetry 2024, 16, 138. https://doi.org/10.3390/sym16020138
Raich I, Pauk K, Imramovsky A, Jampílek J. Conformational and Chiroptical Properties of Salicylamide-Based Peptidomimetics. Symmetry. 2024; 16(2):138. https://doi.org/10.3390/sym16020138
Chicago/Turabian StyleRaich, Ivan, Karel Pauk, Ales Imramovsky, and Josef Jampílek. 2024. "Conformational and Chiroptical Properties of Salicylamide-Based Peptidomimetics" Symmetry 16, no. 2: 138. https://doi.org/10.3390/sym16020138
APA StyleRaich, I., Pauk, K., Imramovsky, A., & Jampílek, J. (2024). Conformational and Chiroptical Properties of Salicylamide-Based Peptidomimetics. Symmetry, 16(2), 138. https://doi.org/10.3390/sym16020138







