Abstract
The mechanical properties and crack propagation behavior of aluminum alloys, both with and without corroded surfaces, were thoroughly investigated through molecular dynamic (MD) simulations. The study delved into the effects of corrosion depth and width on the mechanical properties of corroded aluminum alloys. It was found that as the corrosion depth increases, the yield strength experiences an initial decrease followed by a subsequent increase. This can be attributed to the impact of increased corrosion depth on the healing of surface roughness, which ultimately leads to significant changes in yield strength. Furthermore, the presence of corrosion pits was identified as a key factor in regulating the local microstructure evolution within the material, leading to pronounced differences in stress distribution localization. This, in turn, influenced the path of crack propagation within the material. These findings not only contribute to a deeper understanding of the behavior of aluminum alloys under corrosion, but also provide valuable insights for the development of aluminum alloys with enhanced mechanical properties.
1. Introduction
Corrosion poses a serious threat to the integrity of engineering equipment structures, compromising their safety, reliability, and incurring higher maintenance or repair costs [1,2,3,4,5]. This is particularly true for equipment operating in marine environments, where metal structures are more susceptible to severe corrosion compared to those in land-based settings. For instance, in corrosive marine conditions, aluminum alloy surfaces are vulnerable to extensive and haphazard corrosion damage, resulting in stress concentration and ultimately reducing the structure’s lifespan [6,7,8,9,10]. The aluminum alloys are extensively used in the aerospace, automotive, shipbuilding, and high-speed rail industries due to their high strength-to-weight ratio, which is particularly beneficial for reducing structural weight [11,12,13,14,15,16,17,18]. Moreover, these alloys are commonly employed as medium-strength structural materials, offering advantages such as excellent weldability, corrosion resistance, and immunity to stress-corrosion cracking.
Recently, some research has made significant advancements in understanding how corroded surfaces affect the strength and rate of crack propagation in aluminum alloys [19,20,21]. Most studies have concentrated on macroscopic crack propagation mechanisms and methods for corrosion prevention [22,23,24,25]. The corrosion fatigue crack growth rate in Al-Li alloys under cyclical loading can be accurately predicted using a general corrosion fatigue model. This model is built on an equation that calculates the potential drop from the external surface to the crack tip [19]. The stress corrosion cracking behavior of Mg-Al alloys in distilled water is investigated through constant extension rate tests and linearly increasing stress tests [20]. The influence of Zn and second phase particles on diffusivity is most notable within the matrix, as it is linked to a lower threshold stress. The fatigue crack propagation properties of aluminum alloy in corrosive environments are being assessed [23]. The aluminum alloy exhibited superior fatigue performance in both air and aqueous saline environments due to its non-homogeneous deformation and increased corrosion resistance. The fatigue micro-cracking behavior of pre-corroded aluminum alloy was analyzed through in situ scanning electron microscopy observation. The results of the experiment show that fatigue micro-cracks predominantly stem from inclusions at the specimen notch and corrosion pits on the specimen surface. Additionally, the severity of pre-corrosion and the maximum stress level have a significant impact on the extent and timing of micro-crack initiation [25]. However, there is a lack of research on the influence of microscale corrosion surfaces on the corrosion resistance of metal structures. Basic research on the impact of corroded surfaces on the lifespan, failure modes, and crack propagation behavior of metal structures, particularly from a mechanical perspective, is scarce. This limited knowledge seriously hinders the safety and potential applications of corrosion in various industries.
The molecular dynamic (MD) simulations can offer comprehensive insights into plastic deformation and the corresponding microstructural changes at the atomic level [26,27,28]. For instance, the segregation behavior of Cu and Mg solutes on the mechanical properties in Al-Cu-Mg ternary alloys is investigated through MD stimulation [26]. Additionally, the solid–liquid interfacial free energy and its associated anisotropy are determined for the Al-Mg binary alloy system using MD simulations combined with the capillary fluctuation method [27]. The effects of various factors such as temperature, loading and unloading velocities, holding/dwelling time, and the composition of Ni-Al alloys during the nanoimprinting process are examined in terms of molecular trajectories, imprinting force, potential energy, stress, slip vector, and elastic recovery ratio [28]. Therefore, it would be advantageous to investigate the crack propagation behavior of the aluminum alloy at the nanoscale.
In this work, we systematically investigate the crack propagation and mechanical behavior of aluminum alloy with corrosion surface using MD simulation. Various types of specimens were designed and manufactured with single-sided notch clamping under different corrosion conditions. Tensile tests were conducted under varied surface states, including corrosion depth and corrosion width, to determine the impact of fracture modes and mechanisms of aluminum alloy under these different surface conditions. The corrosion coupling fracture mechanism has been investigated by MD simulation for the first time at the atomic scale. This work not only provides deep insights into the understanding of the interaction mechanism between corroded surfaces and crack propagation, but also would be conducive to the design and development of new corrosion-resistant high-toughness aluminum alloys.
2. Method
The crack evaluation under the mode I loading condition is studied by atomic simulations, as shown in Figure 1. In the compositions of the aluminum alloy, 97 at.% is made up of Al and 3 at.% is comprised of Cu [29,30]. In order to produce the random aluminum alloy, aluminum atoms are randomly chosen and substituted with Cu atoms until the desired composition of Al97Cu3 is attained. The aluminum alloy possesses a face-centered cubic (fcc) structure with a specific lattice constant of 4.049 Å. The size of the sample is 60.2 × 88.6 × 4.0 nm3 along the x direction, the y direction, and the z direction, respectively, and it contains about 1,316,220 atoms (see Table 1). The edge crack is built by removing atoms. The length of a crack is 10.0 nm, and the maximum opening displacement of a crack is 2.0 nm. The crack width is larger than the cut-off radius of 0.5 nm for avoiding the interplay of forces between the atoms located on both sides of the crack surface. To create the corrosive surface, the local atoms at the surface are deleted. The depth and width of the corrosive surface are presented in Figure 1e, where the depth and width of the corrosive surface are defined as the distance along the x direction and the y direction. The corrosion depth is set to 0.5 nm, 1 nm, and 2 nm, and the corrosion width is set to 0.8 nm, 1.2 nm, and 2 nm. The corrosion depth and width selected is less than half of the crack length to minimize the influence of corroded surfaces on mechanical properties. By adhering to this principle, the current atomic simulation can provide a more accurate description of crack propagation behavior in corrosive environments. The periodic boundary condition is applied in the y and z directions, and the free boundary condition is applied in the x direction. The deformation is along the y direction with a constant of 1 × 108 s−1. Thus, Figure 1e also further verifies that the boundary conditions applied are accurate. Firstly, the sample system is minimum energy. The temperature is 300 K. The interaction of the Al-Cu atoms is simulated using the embedded-atom method (EAM) interatomic potentials [31]. The open-source MD code, the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) (version 23 June 2022, Sandia National Laboratories, Albuquerque, NM, USA) [32] is used in the atomic simulation, and the open visualization tool (OVITO) (version 2.9.0, Alexander Stukowski, Wiesbaden, Germany) [33] is used in the post-processing of the simulation data.
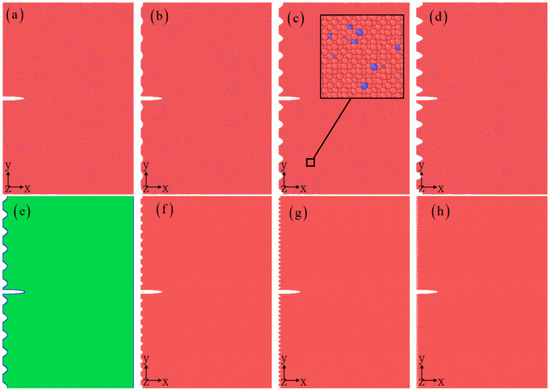
Figure 1.
Atomic model of corrosive aluminum alloys for different corrosion depths: 0 nm, 1 nm, 2 nm, and 3 nm (a–d). The atomic model of corrosive aluminum alloys with a corrosion depth of 2 nm and corrosion width of 4 nm is colored according to the CNA value (e). The sample of the corrosive aluminum alloys for different corrosion widths: 2 nm, 1.2 nm, and 0.8 nm (f–h). The atomic model of corrosive aluminum alloys is colored according to the atomic types, where  is Al, and
is Al, and  is Cu (a–d,f–h).
is Cu (a–d,f–h).
 is Al, and
is Al, and  is Cu (a–d,f–h).
is Cu (a–d,f–h).

Table 1.
Computational parameters used in the MD simulations.
When exploring different types of alloys, it is important to adjust the composition and crystal structure of the material accordingly, as well as to use appropriate interatomic potentials. It is also recommended to utilize the same simulation parameters as those used in the current aluminum alloy system.
3. Result and Discussion
3.1. Corrosion Depth
Figure 2 illustrates the impact of corrosion depth on the mechanical properties of corroded aluminum alloys with cracks. The sample is stretched in the y direction to analyze the propagation of mode I cracks, as shown in Figure 2. The analysis of mode II and mixed mode I–II crack propagation [34,35] will be discussed in future work due to the complexity involved. In this study, the focus is on the impact of corrosion depth and width on mode I crack propagation. Analysis of the stress–strain curves indicates that corrosion depth has a minor effect on the elastic modulus, but a significant effect on strength [12,13,14,15]. As depicted in Figure 2, as the strain increases, the stress initially rises linearly until reaching a yield point, after which it rapidly declines. Interestingly, the yield strength initially decreases and then increases as corrosion depth increases, peaking at 3 nm. These findings agree with the previous experiment in the 5083 Al alloy, where the results in NaCl solution show relatively high strength compared with the sample in air [21,36]. However, at the nanoscale, increased corrosion depth can contribute to the local healing of surface roughness, leading to substantial changes in yield strength. Understanding the propagation of nanocracks at the atomic level is essential for comprehending the damage mechanism of corroded aluminum alloys [37,38]. The molecular dynamic simulations are conducted to study the evolution of nanocracks induced by plastic deformation under loading conditions in single-crystal and bicrystal copper [38]. The compressive stress generated by defects such as dislocations, 9R phases, and deformation twinning plays a key role in driving crack closure. Interestingly, after subjecting both single-crystal and bicrystal copper to one cyclic shear loading, no crack nucleation is observed in the crack-closure zone. Therefore, further exploration of atomic-scale microstructure evolution is necessary to uncover the underlying strengthening mechanisms influencing the yield strength trends.
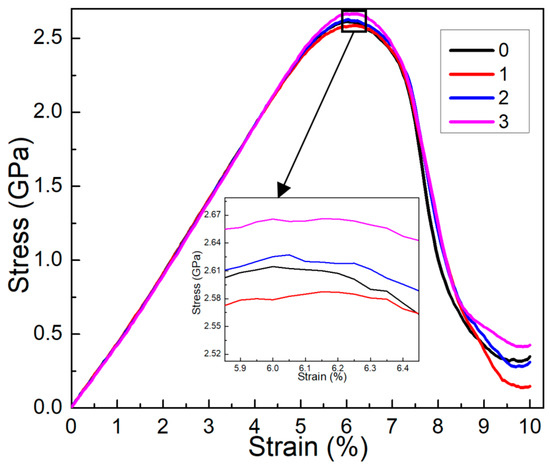
Figure 2.
The stress–strain curves for different corrosion depths along the y direction. The corrosion depth of the corrosive aluminum alloys is 0 nm, 1 nm, 2 nm, and 3 nm, and the corrosion width is 4 nm.
Figure 3 illustrates the microstructure evaluation as the strain increases. The crack tip initially develops a localized plastic region dominated by dislocations when subjected to loading stress. During the initial stages of deformation, amorphization occurs at the crack tip at strains less than 6% [39], as shown in Figure 4a. A nanoscale amorphous bridge can be found experimentally at the tip of a crack in the equiatomic CrMnFeCoNi high-entropy alloy [40]. Subsequently, the corroded aluminum alloys experience localized dislocation nucleation and continuous dislocation emission at the crack tip [37,38]. The accumulation of a significant number of dislocations near the crack tip results in the formation of a distinct plastic region, with two distinct dislocation channel regions present at the crack tip. These channels create a clear shear band, inducing local deformation similar to the traditional crack propagation path and facilitating the transition from brittle to ductile fracture. Moreover, deformation twinning is observed away from the crack region (see Figure 4c), activating a more intricate deformation mechanism characterized by fine dislocation behavior. The previous experimental work demonstrates high-density stacking faults and twinning formed in aluminum alloys [41,42,43], consistent with the atomic simulation results (Figure 4b,c).
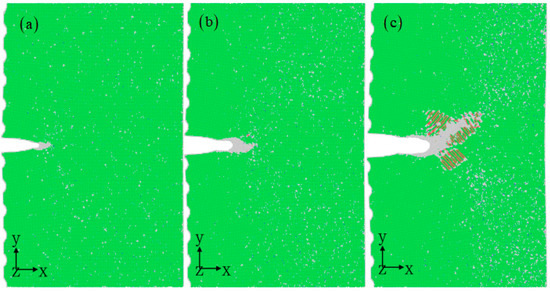
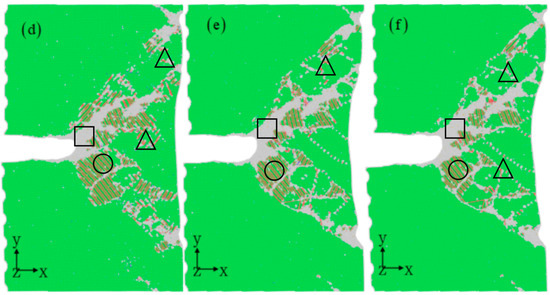
Figure 3.
Nanocrack propagation process with the increasing strain of 5% (a), 6% (b), 7% (c), 8% (d), 9% (e), and 10% (f) at the corrosion depth of 1 nm. The atomic model of corrosive aluminum alloys is colored according to the CNA value. The rectangles represent amorphous structures, circles represent stacking faults, and triangles represent twins.
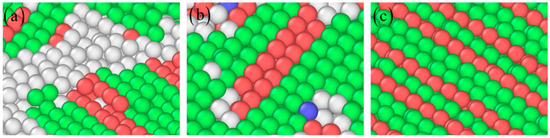
Figure 4.
Partial zoom of amorphous structure (a), stacking fault (b), and deformation twinning (c) at the strain of 8%. The atom is colored according to the CNA value.
Figure 5 displays the microstructure near the crack with varying depths of corrosion. In the absence of corrosion, cracks typically propagate in a single direction, with amorphous regions forming at the crack tip. Deformation twins are abundant along these regions, aiding in stress relief. On the other hand, when corrosion is present, two distinct plastic deformation zones emerge in the vicinity of the crack. Consequently, the depth of corrosion significantly impacts microstructural evolution. Greater corrosion depths result in smaller crack opening displacement and sharper crack tips. This, in turn, affects various geometric features crucial for crack propagation, such as crack length, opening displacement, curvature radius of the crack tip, and crack turning angle [37,38,39]. From the previous work [39], the formation of deformation twinning and the activation of multi-slip systems are observed during the propagation of cracks with the orientation, which is consistent with previous experiments. When the crack propagates with the orientation, an amorphous region forms throughout the crack growth, a phenomenon that is rare in traditional metal materials. In contrast, for this orientation , blunting and slip bands occur at the front of the crack tip by switching the slip mode from planar to wavy slip, as observed in the transmission electron microscopy experiments. Notably, the influence of corrosion depth on these parameters is highly sensitive, showcasing a complex impact on the crack propagation process. For instance, a decrease in the curvature radius of the crack tip may lead to brittle fracture development, whereas an increase enhances crack propagation ability.
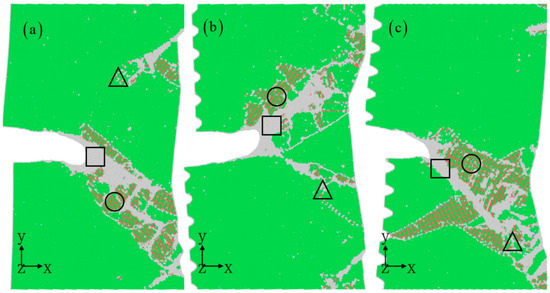
Figure 5.
Nanocrack propagation for different corrosion depths: 0 nm (a), 2 nm (b), and 3 nm (c) at a strain of 10%. The corrosion width is 4 nm. The atomic model of corrosive aluminum alloys is colored according to the CNA value. The rectangles represent amorphous structures, circles represent stacking faults, and triangles represent twins.
The strain distribution shown in Figure 6 clearly highlights the presence of a shear band, which is more prominently observed with larger corrosion depths resulting in slender shear bands that accelerate crack propagation. From previous in situ SEM compression tests on Al alloys, it is observed that high-density shear bands are formed during successive serrations [44]. This suggests that shear bands are more likely to occur in conditions where the corrosion is coupled with the fracture. Furthermore, a significant amount of local plastic deformation occurs in the key influence zone along the crack path, releasing stress concentrations within the crystal by generating dislocations, stacking faults, and twinning. The depth of corrosion plays a crucial role in regulating the evolution of microstructure, consequently determining the paths of crack propagation. Under the influence of large strain, local crack propagation and blunting occur. The distribution of strain in Figure 6 vividly reflects the dynamic changes during the deformation process and their impact on crack propagation. This results in the formation of local high strain stripes, such as single, double, and multiple beams, which play a pivotal role in facilitating further crack propagation. Previous studies have suggested that cracks tend to propagate towards regions with higher strain, allowing for the release of stored strain energy within the matrix and enhancing structural stability [39,45]. It is evident that larger corrosion depths lead to more pronounced high strain areas, indicating a less stable crystal state and a higher likelihood of crack propagation. The concentration of stress is notably higher in the shear band, as depicted in Figure 7, and the stress distribution map in front of the crack reinforces this observation. Higher stress concentrations are found in the early stages of the potential crack propagation path with larger corrosion depths, leading to an increased enrichment of high stress areas along the crack path. This ultimately results in deeper corrosion and easier fracture.
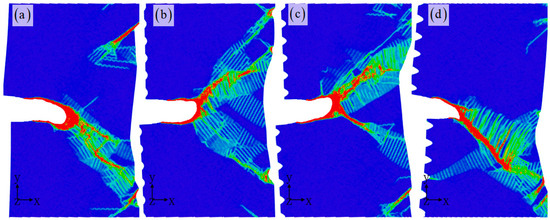
Figure 6.
The strain distribution for different corrosion depths: 0 nm (a), 1 nm (b), 2 nm (c), and 3 nm (d). The corrosion width is 4 nm. The atomic model of corrosive aluminum alloys is colored according to the strain value.
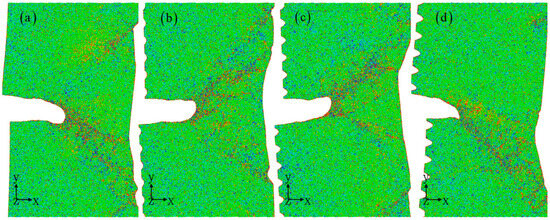
Figure 7.
The stress distribution for different corrosion depths: 0 nm (a), 1 nm (b), 2 nm (c), and 3 nm (d). The corrosion width is 4 nm. The atomic model of corrosive aluminum alloys is colored according to the stress value.
3.2. Corrosion Width
Figure 8 demonstrates the influence of corrosion width on mechanical properties. The graph shows that the impact of corrosion width on the elastic region of the stress–strain curve is minimal; however, once the material enters the plastic region, noticeable changes in yield strength can be observed. This suggests that in corrosive marine environments, corrosion width significantly affects material strength, while having little impact on the elastic modulus [37,38,39]. When larger corrosion pits form in a corrosive setting, it is crucial to focus on material properties beyond the yield stage. The evolution of crack tip propagation area during plastic deformation plays a key role in understanding future service life. Therefore, future research will concentrate on identifying the root cause of stress corrosion mechanisms in corrosive materials through structural evolution [46,47,48]. The previous experiment shows that the strength and plasticity of aluminum alloy are the main factors influencing the fatigue crack propagation [46]. The impact of adding copper on the initiation and propagation of cracks is studied through in situ cyclic testing in the as-cast state, and the findings reveal that the presence of Cu-rich intermetallic compounds does not influence the fatigue characteristics and the strain accumulation is most prominent at the grain boundaries [48]. Additionally, it is evident that material strength does not show a significant correlation with corrosion width. The conventional macroscopic understanding implies that an increase in corrosion width results in a substantial decrease in material properties, implying that wider corrosion pits lead to reduced strength. From Figure 8, it is evident that as the width of corrosion increases, the strength of the material initially decreases before rising again. This indicates that as the corrosion depth increases, there is a dual effect on the material performance: a general weakening overall, but also local strengthening in certain areas. This local strengthening creates zones of plastic deformation within the material, leading to high strain gradient effects and localized strengthening. Therefore, it is crucial in the later stages of significant deformation to establish a clear understanding of the relationship between strain distribution, stress distribution, and the resulting microstructure [45,46,47]. This deep understanding is essential for gaining insight into the material’s performance and longevity.
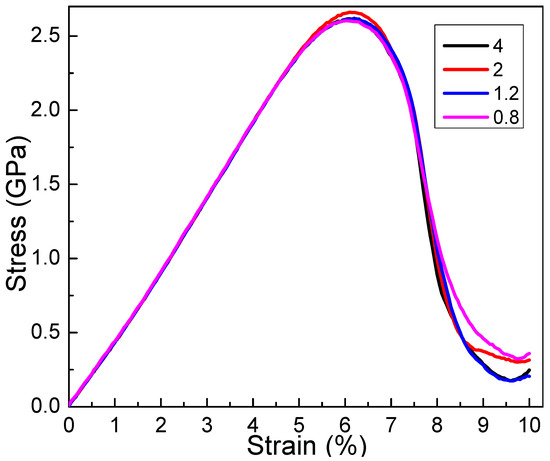
Figure 8.
The stress strain curves for different corrosion widths along the y direction. The corrosion width of the corrosive aluminum alloys is 0.8 nm, 1.2 nm, 2 nm, and 4 nm, and the corrosion depth is 1 nm.
Furthermore, Figure 2 and Figure 7 demonstrate the impact of corrosion width and depth on mechanical response. The minimal variations in parameters have led to intricate relationships between corrosion and performance. The distinction between corroded and non-corroded conditions is barely noticeable. This can be attributed to the fact that corrosion-induced cracks typically develop on the surface, thereby exerting limited influence on mechanical properties. The corrosion morphology on the surface primarily affects crack propagation, as opposed to the substrate. However, under the conditions of fatigue, the role of corrosion becomes significantly critical, as evidenced by extensive validation in prior experiments [19,23,25].
Figure 9 illustrates the impact of corrosion width on the propagation of nanocracks. The results demonstrate that while corrosion width has a minor influence on the propagation path of cracks, it plays a significant role in shaping the evolution of microstructure and consequently, affecting the mechanical properties of aluminum alloys. Further, this strain distribution (Figure 10) clearly illustrates the crack propagation process, showing that the corrosion pit does not typically result in brittle fracture failure, but rather creates a local amorphous zone at the crack tip. This pattern leads to a transition from brittle to ductile behavior, effectively halting further crack propagation and enhancing the material’s resistance to cracking [39]. However, the accumulation of amorphous regions at the crack tip introduces a new issue by promoting stress concentration (Figure 9). This localized stress concentration can trigger crack nucleation within the amorphous zone, extending to form twin deformation zones and eventually spreading to other areas of the corroded sample surface [46,47,48]. This leads to significant local necking on the sample surface, causing a sharp decrease in the material’s mechanical properties.
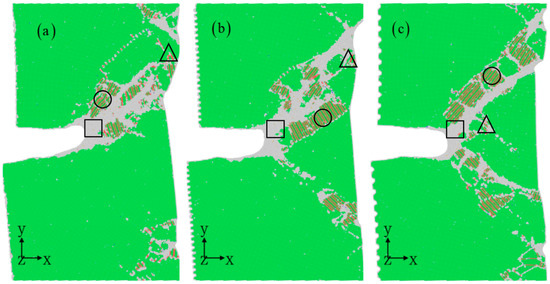
Figure 9.
Nanocrack propagation for different corrosion widths: 0.8 nm (a), 1.2 nm (b), and 2 nm (c). The corrosion depth is 1 nm. The atomic model of corrosive aluminum alloys is colored according to the CNA value. The rectangles represent amorphous structures, circles represent stacking faults, and triangles represent twins.
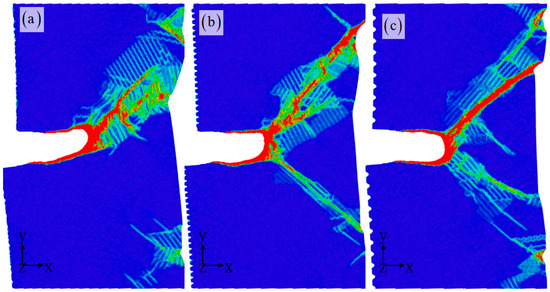
Figure 10.
The strain distribution for different corrosion widths: 0.8 nm (a), 1.2 nm (b), and 2 nm (c). The corrosion depth is 1 nm. The atomic model of corrosive aluminum alloys is colored according to the strain value.
In Figure 10, the strain–shear band is defined according to the strain value. The strain–shear band effectively illustrates the evolution process of the damage area of materials, allowing for a more intuitive assessment of the influence of corrosion width on the performance of corrosion samples. As the corrosion width increases, noticeable changes in the path of the strain–shear band expansion can be observed. It is important to consider that crystal materials have a symmetrical structure and loading conditions involve single uniaxial tension [46,47,48]. The shape of prefabricated cracks remains consistent. According to traditional fracture mechanic principles, one would expect the path of crack propagation to be relatively similar. However, our simulation results reveal significant impacts from changes in corrosion width. Therefore, it is evident that relying solely on the assumption of macroscopic isotropic material properties is inadequate in understanding the atomic-scale propagation mechanism of corrosion cracks [39,45]. Furthermore, the stress distribution displayed in Figure 11 aligns with previous discussions. It is clear that corrosion width plays a crucial role in regulating local microstructure evolution, resulting in notable differences in stress distribution localization [49,50]. The previous work suggests that the elevated stress ratio results in a higher rate of fatigue crack propagation, as well as lower threshold and fracture toughness. Examination of the microstructure along the crack path reveals that the crack tends to propagate towards grains with low-twist angles and high Schmid factors as the stress ratio increases [50]. Ultimately, this leads to the establishment of the dominant path of crack propagation. Hence, the distribution of stress and strain induced by the corrosive surface plays a critical role in determining the mechanical properties. The properties of polycrystalline metals are closely tied to grain boundaries, which are essential structural components where there is a sudden change in crystallographic orientations and often display a certain level of symmetry [50,51,52]. The microstructural transformation of aluminum alloys demonstrates a notable rise in the abundance of coincidence site lattice boundaries. In future, the effect of corrosion environment on mechanical properties and the synergistic microstructure evolution mechanism of polycrystalline aluminum alloys should be further investigated. On the other hand, the material composition remains consistent for both the corroded and non-corroded regions. Therefore, the potential impact of corrosion-induced material composition changes on mechanical properties should be considered. Given the size limitations of atomic simulations, an analytical model should be developed by combining experimental and simulation results. This work is attempting to explore the problem of crack propagation under corrosive conditions from an atomic scale simulation perspective.
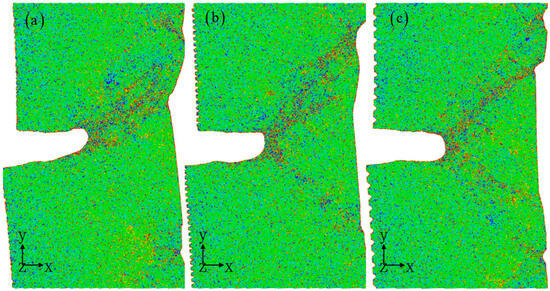
Figure 11.
The stress distribution for different corrosion widths: 0.8 nm (a), 1.2 nm (b), and 2 nm (c). The corrosion depth is 1 nm. The atomic model of corrosive aluminum alloys is colored according to the stress value.
4. Conclusions
In this work, atomic simulations were employed to investigate the mechanical properties and crack propagation of aluminum alloys, both with and without corroded surfaces. The depth of corrosion has a minor impact on the elastic modulus, but it significantly affects the strength of the material. Initially, stress increases linearly until it reaches a yield point, after which it rapidly decreases. Interestingly, the yield strength exhibits a decrease followed by an increase as the corrosion depth increases. The accumulation of a considerable number of dislocations near the crack tip leads to the formation of a distinct plastic region, with two separate dislocation channel regions at the crack tip. Deeper corrosion depths result in smaller crack opening displacements and sharper crack tips, influencing important geometric features crucial for crack propagation, such as crack length, opening displacement, curvature radius of the crack tip, and crack turning angle. With an increase in corrosion width, the material strength initially decreases before rising again, suggesting a dual effect on material performance as corrosion depth increases: an overall weakening and local strengthening in specific areas. This shift leads to a transition from brittle to ductile behavior, effectively stopping further crack propagation and enhancing the material’s resistance to cracking. As the corrosion width expands, noticeable changes in the direction of strain–shear band expansion become apparent. These insights will be invaluable in refining existing models and enhancing the development of advanced aluminum alloys with improved durability and damage tolerance properties.
Author Contributions
Conceptualization, Y.Z. and J.L.; methodology, A.W.; software, W.Y.; validation, W.F., Q.F. and Y.Z.; formal analysis, A.W. and W.Y.; investigation, Q.F.; resources, W.F.; data curation, J.L.; writing—original draft preparation, Y.Z.; writing—review and editing, J.L.; visualization, A.W.; supervision, Q.F.; project administration, W.Y.; funding acquisition, Y.Z. and W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the supports from the Natural Science Foundation of Shandong Province (ZR2020ME130), the National Natural Science Foundation of China (52101392, and 12172123).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, H.; Sohn, S.S.; Lu, W.; Li, L.; Li, X.; Soundararajan, C.K.; Raabe, D. A strong and ductile medium-entropy alloy resists hydrogen embrittlement and corrosion. Nat. Commun. 2020, 11, 3081. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Huang, Y.; Li, L.; Wei, G.; Li, H.; Shang, Q.; Tao, X. A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries. Nat. Commun. 2023, 14, 8269. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92. [Google Scholar] [CrossRef]
- Wu, P.; Gan, K.; Yan, D.; Fu, Z.; Li, Z. A non-equiatomic FeNiCoCr high-entropy alloy with excellent anti-corrosion performance and strength-ductility synergy. Corros. Sci. 2021, 183, 109341. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Zheng, G.; Woller, K.B.; Stahle, P.W.; Minor, A.M.; Short, M.P. Proton irradiation-decelerated intergranular corrosion of Ni-Cr alloys in molten salt. Nat. Commun. 2020, 11, 3430. [Google Scholar] [CrossRef]
- López Freixes, M.; Zhou, X.; Zhao, H.; Godin, H.; Peguet, L.; Warner, T.; Gault, B. Revisiting stress-corrosion cracking and hydrogen embrittlement in 7xxx-Al alloys at the near-atomic-scale. Nat. Commun. 2022, 13, 4290. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Gupta, R.K. Corrosion-resistant metastable Al alloys: An overview of corrosion mechanisms. J. Electrochem. Soc. 2020, 167, 081504. [Google Scholar] [CrossRef]
- Ji, Y.; Li, N.; Cheng, Z.; Fu, X.; Ao, M.; Li, M.; Dong, C. Random forest incorporating ab-initio calculations for corrosion rate prediction with small sample Al alloys data. npj Mater. Degrad. 2022, 6, 83. [Google Scholar] [CrossRef]
- Guo, X.H.; Ren, Y.J.; Shen, J.; Dai, T.; Lv, Y.L.; Niu, Y. The corrosion behavior of Co-Cr-Al alloys exposed to mixed oxygen-sulfur atmospheres at 900 °C. Corros. Sci. 2021, 188, 109530. [Google Scholar] [CrossRef]
- Tan, W.; Li, T.; Li, S.; Fang, D.; Ding, X.; Sun, J. High strength-ductility and rapid degradation rate of as-cast Mg-Cu-Al alloys for application in fracturing balls. J. Mater. Sci. Technol. 2021, 94, 22. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Y.; Marceau, R.; Wang, L.; Zhang, Q.; Gao, X.; Hutchinson, C. Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity. Science 2019, 363, 972. [Google Scholar] [CrossRef] [PubMed]
- Asadikiya, M.; Yang, S.; Zhang, Y.; Lemay, C.; Apelian, D.; Zhong, Y. A review of the design of high-entropy aluminum alloys: A pathway for novel Al alloys. J. Mater. Sci. 2021, 56, 12093. [Google Scholar] [CrossRef]
- Zhao, H.; Chakraborty, P.; Ponge, D.; Hickel, T.; Sun, B.; Wu, C.H.; Raabe, D. Hydrogen trapping and embrittlement in high-strength Al alloys. Nature 2022, 602, 437. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.; Euesden, R.; Yao, Y.; Aboura, Y.; Zhao, H.; Donoghue, J.; Prangnell, P.B. Multiscale analysis of grain boundary microstructure in high strength 7xxx Al alloys. Acta Mater. 2021, 202, 190. [Google Scholar] [CrossRef]
- Wang, Y.; Freiberg, D.; Huo, Y.; Zhu, W.; Williams, R.; Li, M.; Wang, Y. Shapes of nano Al6Mn precipitates in Mn-containing Al-alloys. Acta Mater. 2023, 249, 118819. [Google Scholar] [CrossRef]
- Bousser, E.; Rogov, A.; Shashkov, P.; Gholinia, A.; Laugel, N.; Slater, T.J.; Yerokhin, A. Phase transitions in alumina films during post-sparking anodising of Al alloys. Acta Mater. 2023, 244, 118587. [Google Scholar] [CrossRef]
- Gao, P.F.; Fei, M.Y.; Zhan, M.; Fu, M.W. Microstructure-and damage-nucleation-based crystal plasticity finite element modeling for the nucleation of multi-type voids during plastic deformation of Al alloys. Int. J. Plast. 2023, 165, 103609. [Google Scholar] [CrossRef]
- Zha, M.; Zhang, H.; Jia, H.; Gao, Y.; Jin, S.; Sha, G.; Li, Y. Prominent role of multi-scale microstructural heterogeneities on superplastic deformation of a high solid solution Al–7Mg alloy. Int. J. Plast. 2021, 146, 103108. [Google Scholar] [CrossRef]
- Kovalov, D.; Fekete, B.; Engelhardt, G.R.; Macdonald, D.D. Prediction of corrosion fatigue crack growth rate in alloys. Part I: General corrosion fatigue model for aero-space aluminum alloys. Corros. Sci. 2018, 141, 22. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Raja, V.S.; Song, G.; Kainer, K.U. Characterisation of stress corrosion cracking (SCC) of Mg–Al alloys. Mater. Sci. Eng. A 2008, 488, 339. [Google Scholar] [CrossRef]
- Qin, J.; Li, Z.; Yi, D.; Wang, B. Diversity of intergranular corrosion and stress corrosion cracking for 5083 Al alloy with different grain sizes. Trans. Nonferrous Met. Soc. China 2022, 32, 765. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Dietzel, W.; Song, G.; Kainer, K.U. Fractography of stress corrosion cracking of Mg-Al alloys. Metall. Mater. Trans. A 2008, 39, 1157. [Google Scholar] [CrossRef]
- Cavalcante, T.R.F.; Pereira, G.S.; Koga, G.Y.; Bolfarini, C.; Bose Filho, W.W.; Avila, J.A. Fatigue crack propagation of aeronautic AA7050-T7451 and AA2050-T84 aluminum alloys in air and saline environments. Int. J. Fatigue 2022, 154, 106519. [Google Scholar] [CrossRef]
- Scully, J.C. Stress corrosion crack propagation: A constant charge criterion. Corros. Sci. 1975, 15, 207. [Google Scholar] [CrossRef]
- Song, H.; Liu, C.; Zhang, H.; Du, J.; Yang, X.; Leen, S.B. In-Situ SEM study of fatigue micro-crack initiation and propagation behavior in pre-corroded AA7075-T7651. Int. J. Fatigue 2020, 137, 105655. [Google Scholar] [CrossRef]
- Cui, Y.; Song, K.; Bao, Y.; Zhu, Y.; Liu, Q.; Qian, P. Effect of Cu and Mg co-segregation on the strength of the Al grain boundaries: A molecular dynamics simulation. Comput. Mater. Sci. 2023, 229, 112391. [Google Scholar] [CrossRef]
- Dolce, D.; Swamy, A.; Hoyt, J.; Choudhury, P. Computing the solid-liquid interfacial free energy and anisotropy of the Al-Mg system using a MEAM potential with atomistic simulations. Comput. Mater. Sci. 2023, 217, 111901. [Google Scholar] [CrossRef]
- Wu, C.D.; Fang, T.H.; Sung, P.H.; Hsu, Q.C. Critical size, recovery, and mechanical property of nanoimprinted Ni–Al alloys investigation using molecular dynamics simulation. Comput. Mater. Sci. 2012, 53, 321. [Google Scholar] [CrossRef]
- Yang, R.; Feng, Z.; Huang, T.; Wu, G.; Godfrey, A.; Huang, X. Unprecedented age-hardening and its structural requirement in a severely deformed Al-Cu-Mg alloy. Scr. Mater. 2022, 206, 114240. [Google Scholar] [CrossRef]
- Bansal, U.; Singh, M.P.; Sinha, S.K.; Sahu, D.K.; Mondol, S.; Makineni, S.K.; Chattopadhyay, K. Strength and stability through variable micro segregation behaviour of Ta and Zr solutes at intermetallic interfaces in Al-Cu alloys. Acta Mater. 2023, 259, 119254. [Google Scholar] [CrossRef]
- Lee, B.J.; Baskes, M.I. Second nearest-neighbor modified embedded-atom-method potential. Phys. Rev. B 2000, 62, 8564. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Jia, M.; Wu, Z.; Wang, H.; Rena, C.Y.; Zhang, X. Analytical method for predicting mode I crack propagation process of concrete under low-cycle fatigue loading. Eng. Fract. Mech. 2023, 286, 109320. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Ma, G. Experimental investigation on Mode I crack growth and damage mechanism of sandstone subjected to cyclic loading. Eng. Fract. Mech. 2022, 267, 108429. [Google Scholar] [CrossRef]
- Zhao, G.L.; Qi, H.Y.; Li, S.L.; Liu, Y.; Yang, X.G.; Shi, D.Q.; Sun, Y.T. Low-temperature hot corrosion fatigue damage mechanism, life model, and corrosion resistance design method of hot section components. Adv. Mech. 2022, 52, 809. [Google Scholar]
- Li, J.; Fang, Q.H.; Liu, B.; Liu, Y.; Liu, Y.W.; Wen, P.H. Mechanism of crack healing at room temperature revealed by atomistic simulations. Acta Mater. 2015, 95, 291. [Google Scholar] [CrossRef]
- Fang, Q.; Li, J.; Luo, H.; Du, J.; Liu, B. Atomic scale investigation of nanocrack evolution in single-crystal and bicrystal metals under compression and shear deformation. J. Alloys Compd. 2017, 710, 281. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; He, Q.; Fang, Q.; Liu, B.; Jiang, C.; Liaw, P.K. Unveiling the atomic-scale origins of high damage tolerance of single-crystal high entropy alloys. Phys. Rev. Mater. 2020, 4, 103612. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; An, X.; Zhang, Y.; Sun, S.; Tian, Y.; Liao, X. Deformation-induced crystalline-to-amorphous phase transformation in a CrMnFeCoNi high-entropy alloy. Sci. Adv. 2021, 7, eabe3105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, C.; Wu, R.; Sun, L.; Lu, C.; Xiao, Y.; Wu, G. Synergistic strengthening of Al–SiC composites by nano-spaced SiC-nanowires and the induced high-density stacking faults. Compos. Part B Eng. 2023, 250, 110458. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Zekri, A.; Youssef, K.M. The role of twinning and stacking fault-induced plasticity on the mechanical properties of aluminum-lithium-graphene nanocomposites. Nanocomposites 2024, 10, 91–107. [Google Scholar] [CrossRef]
- Peng, P.; Wang, W.; Jin, Y.; Liu, Q.; Zhang, T.; Qiao, K.; Wang, K. Experimental investigation on fatigue crack initiation and propagation mechanism of friction stir lap welded dissimilar joints of magnesium and aluminum alloys. Mater. Charact. 2021, 177, 111176. [Google Scholar] [CrossRef]
- Li, Q.; Shang, Z.; Sun, X.; Fan, C.; Su, R.; Richter, N.A.; Zhang, X. High-strength and tunable plasticity in sputtered Al–Cr alloys with multistage phase transformations. Int. J. Plast. 2021, 137, 102915. [Google Scholar] [CrossRef]
- Li, J.; Fang, Q.; Liu, Y. Crack interaction with a second phase nanoscale circular inclusion in an elastic matrix. Int. J. Eng. Sci. 2013, 72, 89. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, Z.; Duan, Q.; Wang, H.; Wang, X.; Liu, H.; Zhang, Z. Comparison on the Fatigue Crack Propagation of High-Strength Aluminum Alloys. Adv. Eng. Mater. 2023, 25, 2301083. [Google Scholar] [CrossRef]
- Lu, D.; Lin, B.; Liu, T.; Deng, S.; Guo, Y.; Li, J.; Liu, D. Effect of grain structure on fatigue crack propagation behavior of Al-Cu-Li alloys. J. Mater. Sci. Technol. 2023, 148, 75. [Google Scholar] [CrossRef]
- Bogdanoff, T.; Lattanzi, L.; Merlin, M.; Ghassemali, E.; Seifeddine, S. The influence of copper addition on crack initiation and propagation in an Al–Si–Mg alloy during cyclic testing. Materialia 2020, 12, 100787. [Google Scholar] [CrossRef]
- Wang, P.; Ye, L.; Liu, X.; Dong, Y.; Zhao, L. Effects of grain structures on fatigue crack propagation behavior of an Al-Cu-Li alloy. Int. J. Fatigue 2023, 177, 107927. [Google Scholar] [CrossRef]
- Ma, M.; Wang, B.; Liu, H.; Yi, D.; Shen, F.; Zhai, T. Investigation of fatigue crack propagation behavior of 5083 aluminum alloy under various stress ratios: Role of grain boundary and Schmid factor. Mater. Sci. Eng. A 2020, 773, 138871. [Google Scholar] [CrossRef]
- Snopiński, P. Electron Microscopy Study of Structural Defects Formed in Additively Manufactured AlSi10Mg Alloy Processed by Equal Channel Angular Pressing. Symmetry 2023, 15, 860. [Google Scholar] [CrossRef]
- Snopiński, P. Effects of KoBo-Processing and Subsequent Annealing Treatment on Grain Boundary Network and Texture Development in Laser Powder Bed Fusion (LPBF) AlSi10Mg Alloy. Symmetry 2024, 16, 122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).