Abstract
Immersion tests with 5 wt.% Na2SO4 solution were carried out to investigate the deterioration of calcium silicate hydrate (C-S-H) gel in a sulfate environment. C-S-H gels with different Ca/(Si + Al) molar ratios were used for comparison. Particular attention was paid to the changes in element composition and Si-O-Si chain structure of the C-S-H gel. The results show that the C-S-H gels with a low Ca/(Si + Al) ratio (0.8–1.0) and appropriate Si-O-Si chain length (14.8) presented better stability in a severe sulfate environment. The C-S-H gels with a higher Ca/(Si + Al) ratio (>2.0) were more sensitive to the attack of sulfate ions. Calcium ions dissolved rapidly during the immersion process, causing the loss of cohesive strength of the C-S-H gel, and then decomposed into fine particles. The C-S-H gel with a lower Ca/(Si + Al) ratio (about 1.1) rarely leached out calcium ions and maintained good micromorphology. The 29Si MAS-NMR results indicate that the Si-O-Si chains with too short or too long chain lengths will break and recombine under the attack of sulfate ions. The Si-O-Si chains with an appropriate chain length (14.8) maintained the stability of the structure of the C-S-H gel in a sulfate environment. These changes are closely related to the asymmetric layered structure of amorphous C-S-H gel. Partial calcium ions between the layers of the main chain structure of Si-O-Si are easily taken away by sulfate ions, leading to the structural instability of the C-S-H gel.
1. Introduction
Sulfate attack has always been an important issue in concrete durability research. The resistance of concrete to sulfates is an important factor affecting the long-term durability of concrete [1,2]. Sulfate attack typically occurs when concrete is exposed to a sulfate-bearing environment, such as seawater, groundwater and saline soil [3]. For decades, research has focused on the mechanisms of expansive sulfate attack [4,5,6,7] and the improvement of the sulfate resistance of concrete [8,9]. Most of the studies on sulfate attack mainly focused on the following aspects: (1) the formation of ettringite or gypsum in small pores generates high crystallization pressure, which causes expansion, peeling and cracking of the concrete [10,11,12]. (2) Thaumasite can be formed in the presence of carbonate ions at low temperature, which causes the de-cohesion of the aggregate and cement paste [13,14]. (3) Improving the pore structure of concrete can reduce the penetration rate of sulfate ions with the use of supplementary cementitious materials (SCMs) [8,15,16].
Few studies have been reported on the behavior of C-S-H gel in a sulfate environment. C-S-H gel is the main product of cement hydration, which accounts for about 60% of the total volume [17,18,19]. It is also the main source of the structural strength of concrete. C-S-H gel is an amorphous substance with long-range disorder and short-range order [20,21,22]. Its chemical composition and microstructure will change with the change in the surrounding solution environment [23,24]. During the sulfate attack process, the C-S-H gel can be decomposed by sulfate ions, causing the loss of cohesive strength of cement paste. The stability of C-S-H gel is a decisive factor in the durability and long-term service life of the concrete. Therefore, it is important to study the deterioration of C-S-H gel in a sulfate environment. Such studies may have a reference value for improving the resistance of concrete to sulfate attack.
Furthermore, most studies on sulfate attack are carried out with hardened cement pastes or mortar specimens. The resistance of concrete to sulfate attack is determined by measuring the changes in the physical parameters of the immersed specimens over time, such as length, strength, mass, etc. The results are influenced by many factors. For example, the infiltration rate of sulfate ions and the precipitation rate of erosion products may be affected by the specific surface area, porosity and pore size distribution of the sample. The dissolution and precipitation kinetics may be affected by the presence or absence of accelerators, inhibitors, and superplasticizers [25,26,27]. Therefore, powdered samples were used in some studies to avoid such problems [28,29]. Using powdered samples allows researchers to analyze the influence of individual chemical and physical parameters.
The solid-state nuclear magnetic resonance (NMR) technique is mainly used in studies of the environment of the small region around the nucleus. This technique can quantitatively analyze and calculate the structure of solid materials and amorphous materials with low crystallinity. Other long-range diffraction methods, such as X-ray diffraction (XRD) and electron microscopy, are mainly used for the analysis of the crystalline phase, and it is difficult to quantitatively analyze amorphous substances such as C-S-H gel in cement hydration products. Thus, solid-state NMR techniques can be complementary to these diffraction techniques. In addition, 29Si magic-angle spinning nuclear magnetic resonance (MAS-NMR) is usually used to characterize the environment of small regions around a silicon nucleus. This technique can determine the structure using the chemical shift of the silica tetrahedron, and further determine the relative content of each coordination structure, so as to characterize the parameters of the amorphous C-S-H gel.
The present study aims to analyze the deterioration of C-S-H gel immersed in 5 wt.% Na2SO4 solution. The changes in element composition and Si-O-Si chain structure of C-S-H gel were emphasized. To achieve this, C-S-H gels with different Ca/(Si + Al) molar ratios were prepared by various hydration methods. In the immersion process, powdered C-S-H gel samples were used to avoid the influence of some unstable factors of bulk specimens, such as pore size distribution, porosity, and so on.
2. Experimental Work
2.1. Material and Sample Preparation
The Portland cement (PC, PII 52.5) and fly ash (FA) used in this study were obtained from Jiangnan-Onoda cement Co., Ltd., Nanjing, China, and Nanjing Huaneng Thermal Power Plant, Nanjing, China, respectively. Their chemical compositions, measured by X-ray fluorescence (XRF), are provided in Table 1.

Table 1.
Chemical compositions (wt.%) of Portland cement and fly ash.
Fly ash cement has been widely used in practical engineering. The current Chinese standard (GB 175-2007 [30]) stipulates in detail that the content of fly ash in fly ash cement should be between 20 wt.% and 40 wt.%. To ensure the research was representative, the middle value of the standard specified range (30 wt.%) was chosen to prepare PF in this paper.
In order to obtain C-S-H gels with different compositions and constructions, various hydration methods were used in the experiment. All the C-S-H gels were prepared with a water to solid ratio of 5:1 by mass. The C-S-H gel labeled as PII was obtained by continuous magnetic stirring at room temperature with PC for 3 days. PII120 and PII180 were prepared by hydrothermal treatment at 120 °C or 180 °C with PC for 24 h in a PTFE-lined stainless steel autoclave, respectively. And PF180 was also prepared by hydrothermal treatment at 180 °C with PF for 24 h in a stainless steel autoclave with a PTFE lining. After the hydration process, all slurries were centrifuged and each solid product was dried in a vacuum drying oven at 45 °C.
The immersion procedure was carried out in a centrifuge tube with a constant temperature water bath of 80 °C. Each C-S-H gel was immersed in 5 wt.% Na2SO4 solution with a liquid to solid ratio of 35:1 by mass. The Na2SO4 solution was subjected to high-speed centrifugation for the first 24 h and then every 48 h. Centrifuge sampling after immersed for a certain durations.
2.2. Characterization
X-ray fluorescence (XRF, ADVANT’XP, ARL, Geneva, Switzerland) with a 4GN rhodium target was utilized to measure the chemical compositions of raw materials.
X-ray diffraction (XRD, Rigaku D/Max-2500, Rigaku Ltd., Tokyo, Japan) with Cu-Kα radiation was carried out to determine the phase composition. Data were collected over the angular range 2θ from 5° to 70°, with a step size of 0.02° and scan speed of 10°/min.
A scanning electron microscope (SEM, JSM-6510, JEOL, Tokyo, Japan) coupled with an energy dispersive spectrometer (EDS, EDAX, Warrendale, PA, USA) with an acceleration voltage of 15 kV was utilized to analyze the micro-morphology and elementary composition.
The 29Si and 27Al magic-angle spinning nuclear magnetic resonance (MAS-NMR, Bruker AV-400D, Bruker, Billerica, MA, USA) spectra were obtained at 300 K with a frequency of 79.49 MHz using acetone as the solvent to characterize the structure of Si-O-Si chains.
3. Results and Discussion
3.1. XRD Analysis
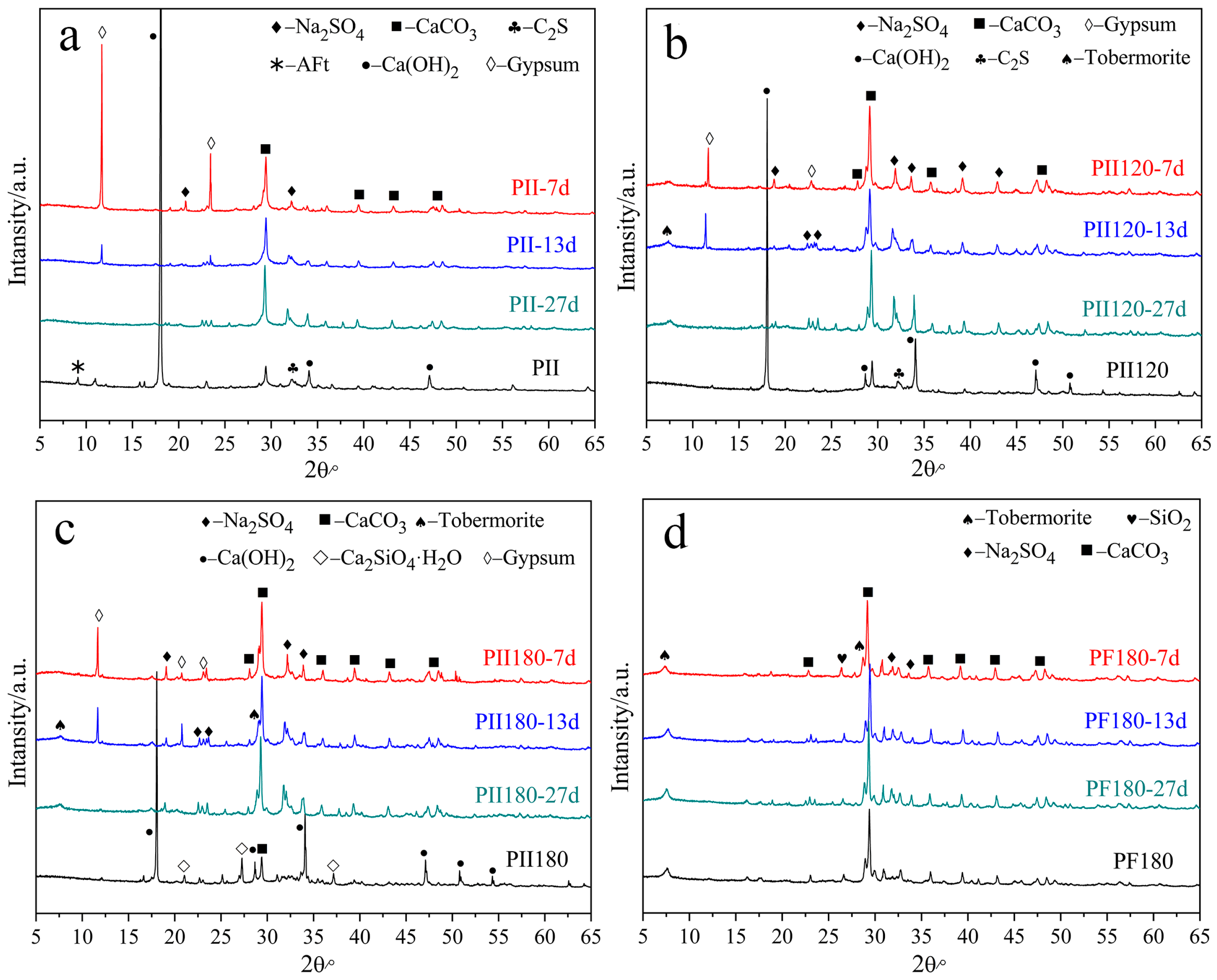
Figure 1 shows the XRD patterns of different C-S-H gels before and after being immersed in Na2SO4 solution for several days.
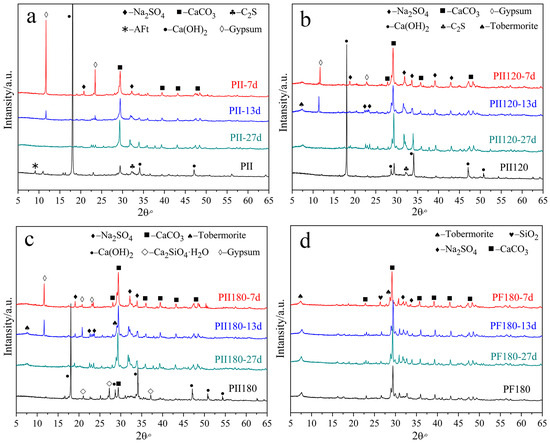
Figure 1.
XRD patterns of different C-S-H gels immersed in Na2SO4 solution for different durations. (a) PII, (b) PII120, (c) PII180; (d) PF180.
For PII, the main crystalline hydration products were calcium hydroxide (CH), calcium carbonate and ettringite (AFt) before immersion. For PII120 and PII180, AFt was no longer detected due to high-temperature hydrothermal treatment. And the hydrothermal treatment promoted cement hydration, which led to a lower CH content in PII120 and PII180. Few dicalcium silicate hydrates were detected in PII180. Comparing PF180 to PII180, CH was not detected and minimal tobermorite was generated. It suggests that the pozzolanic reaction of FA occurs under high-temperature hydrothermal treatment. CH was completely consumed by SiO2 microspheres that participated in cement hydration.
After immersion in Na2SO4 solution for 7 days, CH was totally consumed by sulfate ions and plenty of gypsum was generated in PII, PII120 and PII180. But gypsum was still detected after being immersed for 13 days. It indicates that calcium ions leached out from the C-S-H gel following the erosion of sulfate ions. No gypsum was generated after being immersed for 27 days. For PF180, no significant changes can be seen from the XRD patterns up to 27 days of immersion, which means the C-S-H gel in PF180 was relatively stable in Na2SO4 solution.
3.2. SEM-EDS Analysis
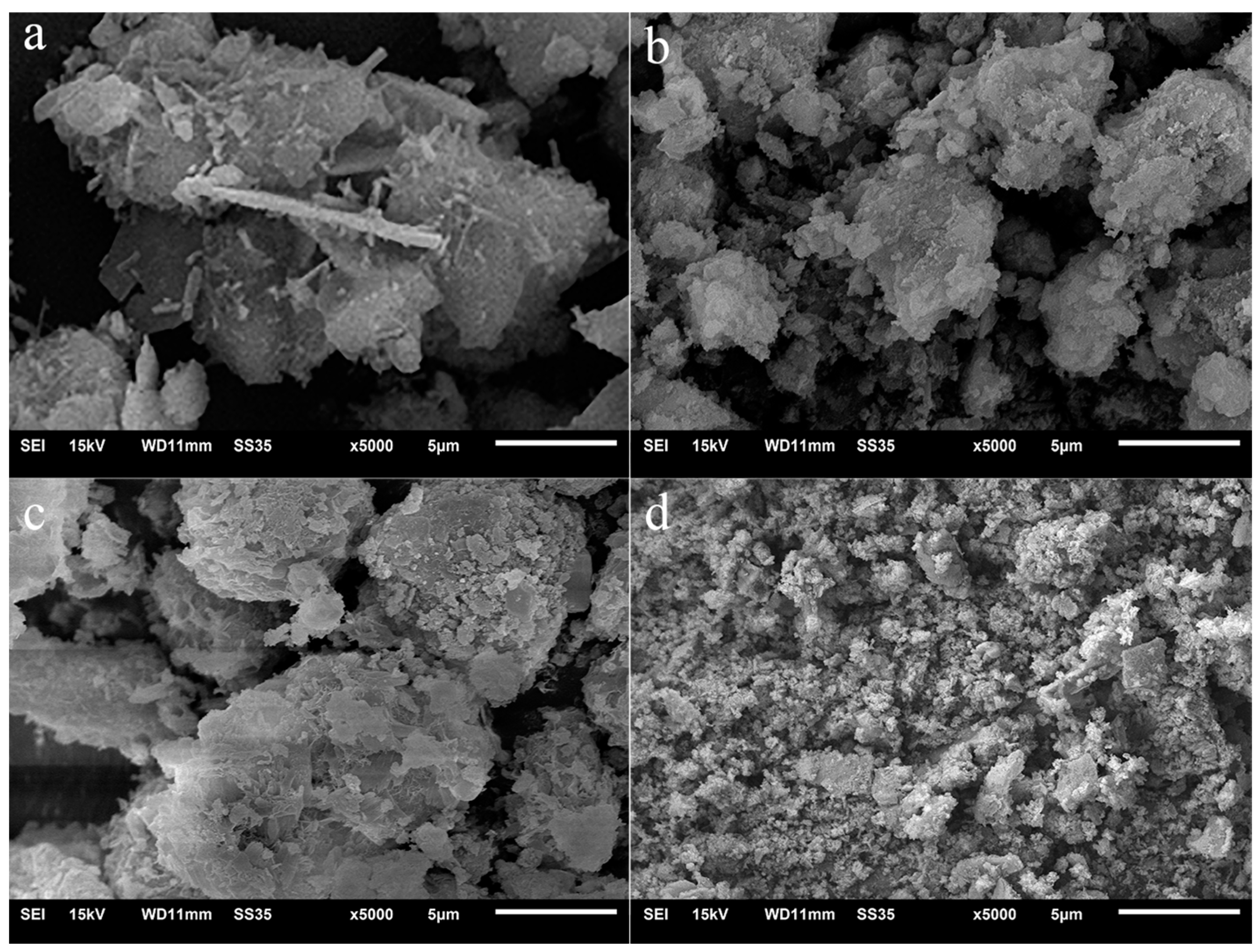
Figure 2 shows the SEM images of PII before and after being immersed in Na2SO4 solution for several days. For PII, large particles formed by amorphous C-S-H gel agglomeration can be clearly found, as well as some needle-like AFt and plate-like CH. After 7 days of immersion in Na2SO4 solution, AFt and CH were no longer observed and only the outermost layer of C-S-H gel was attacked by sulfate ions. When the immersion duration was extended to 13 days, the outer layer of amorphous C-S-H gel particles changed into a lamellar structure. It indicated that the amorphous C-S-H was gradually eroded by sulfate ions from the outside to the inside. Then, the agglomerated C-S-H gel particle was decomposed into fine particles after 27 days of immersion, which means that the C-S-H gel lost its cohesive strength following the attack of sulfate ions.
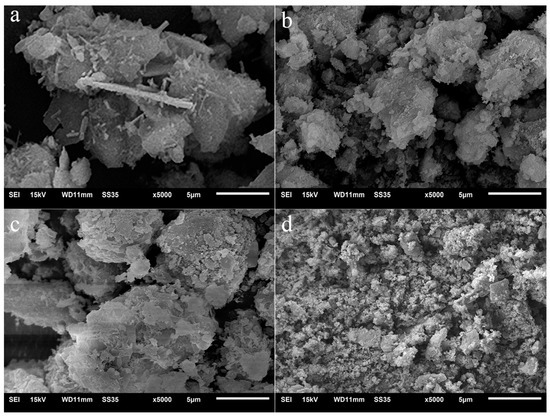
Figure 2.
SEM images of PII immersed in Na2SO4 solution for different durations. (a) 0 d, (b) 7 d, (c) 13 d; (d) 27d.
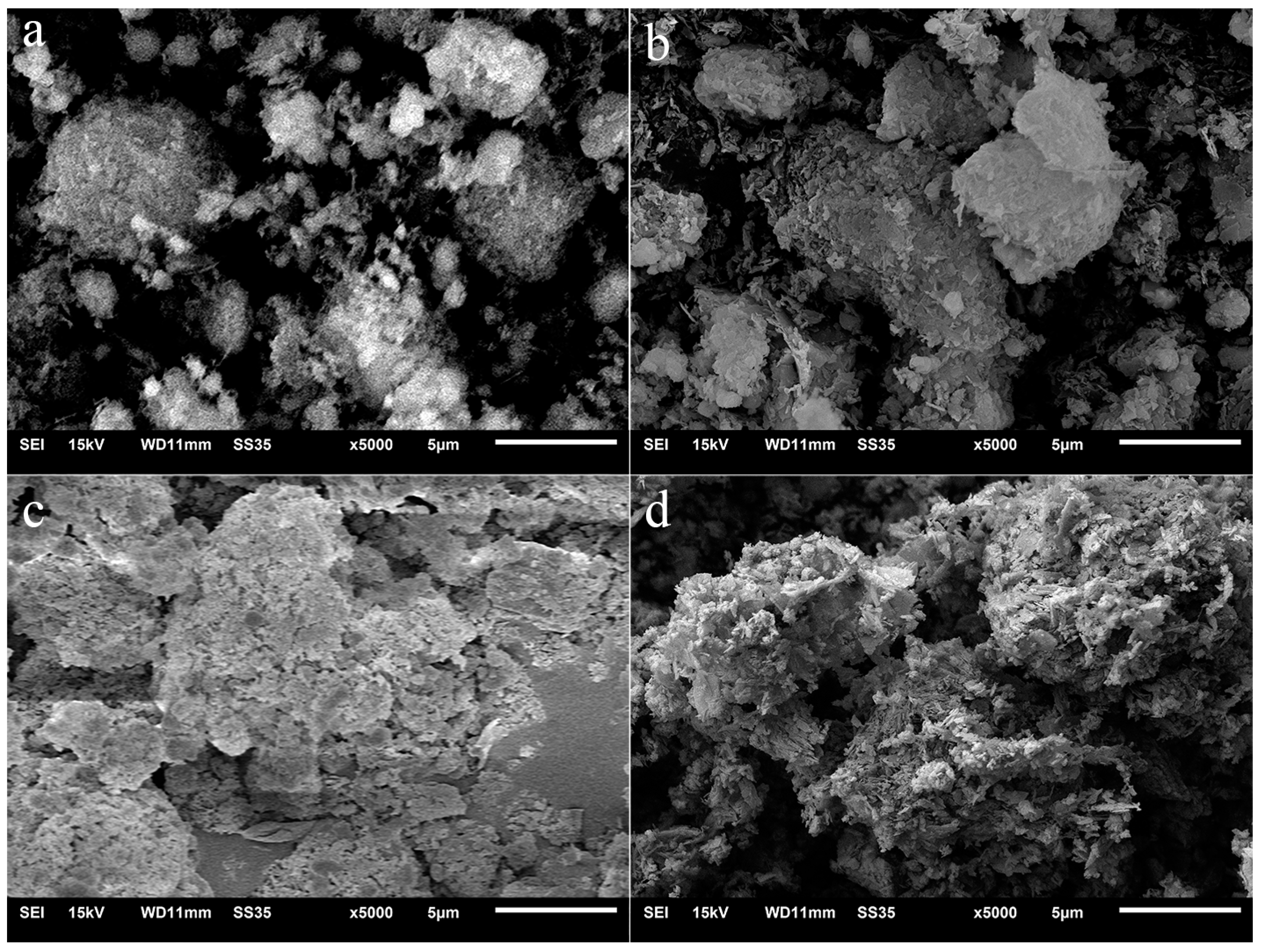
Figure 3 shows the SEM images of PF180 before and after immersed in Na2SO4 solution for several days. For PF180, a large amount of amorphous C-S-H gel was generated on the spherical SiO2 surface due to the pozzolanic reaction of FA. Comparing PF180 to PII, the size of C-S-H agglomeration particles did not change significantly with the increase in immersion time. Only the surface C-S-H gel was eroded by sulfate ions even after 27 days of immersion. It suggests that the reaction of FA somehow improves the stability of C-S-H gel in Na2SO4 solution. This improvement prevents C-S-H gel agglomeration particles from being fully decomposed by sulfate ions.
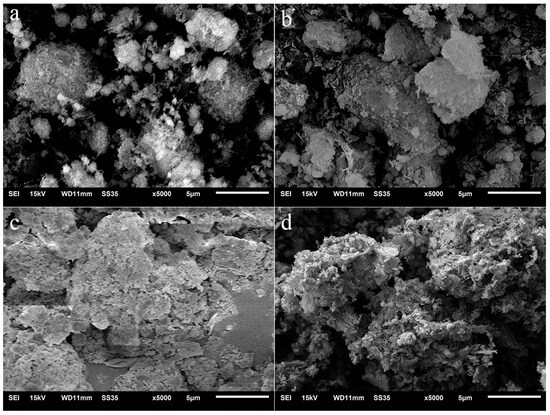
Figure 3.
SEM images of PF180 immersed in Na2SO4 solution for different durations. (a) 0 d, (b) 7 d, (c) 13 d; (d) 27d.
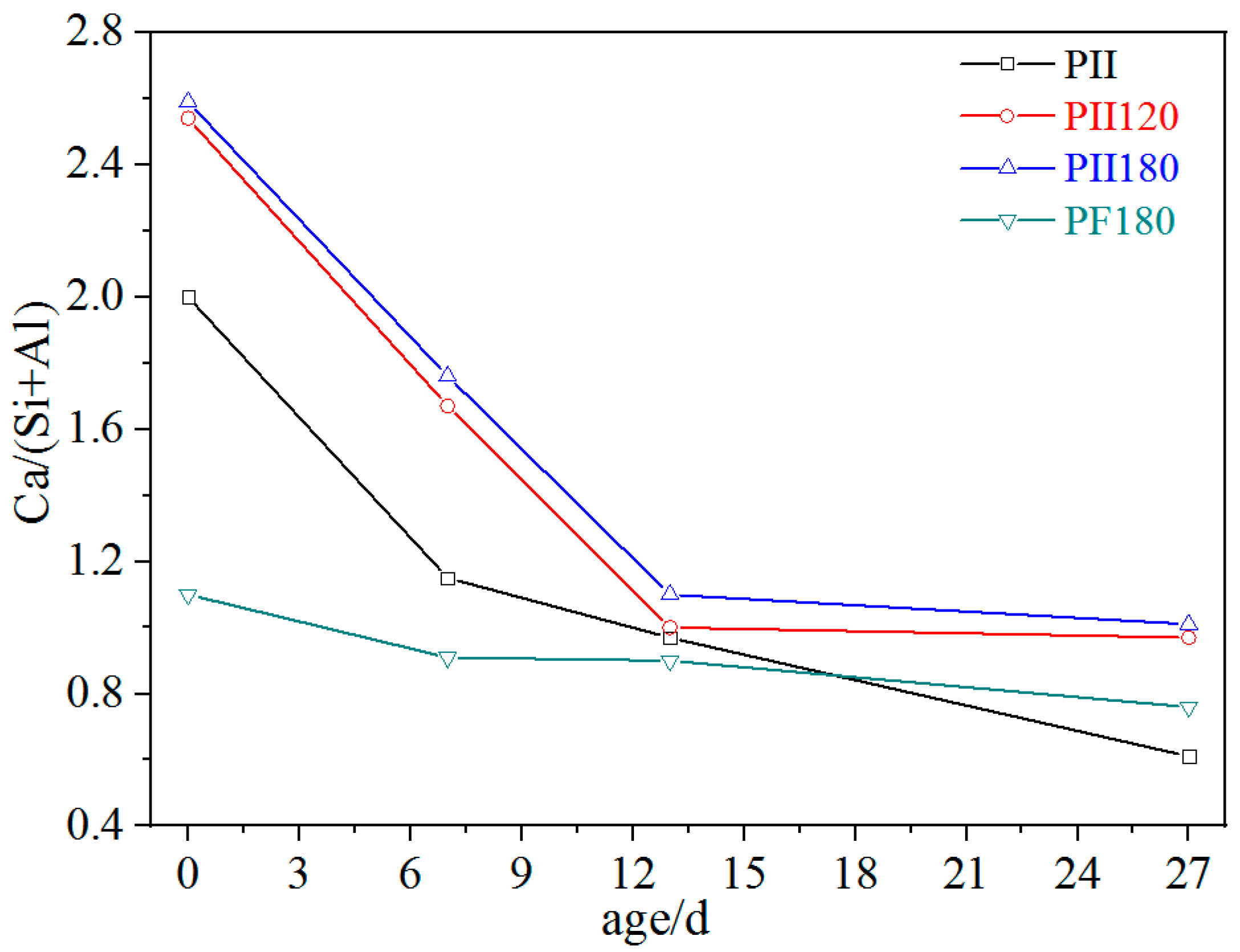
EDS was carried out to characterize changes in the Ca/(Si + Al) molar ratio of C-S-H gel during immersion. Each data is an average value of 15 to 20 detecting points. As shown in Figure 4, the Ca/(Si + Al) molar ratio of PII, PII120 and PII180 rapidly decreased from above 2.0 to around 1.0 during the first 13 days of immersion. Then, it remained stable around 1.0 after being immersed for 27 days. By combining the XRD results (Figure 1), it can be inferred that plenty of calcium ions leached out from C-S-H gel due to the erosion of sulfate ions during the first 13 days of immersion. After that, only a few calcium ions were released and the structure of C-S-H gel was further destroyed, which led to the decomposition of agglomerated particles (Figure 2). For PF180, plenty of SiO2 microspheres in FA participated in the hydration of cement under hydrothermal treatment to generate C-S-H gel with a lower Ca/(Si + Al) molar ratio. Thus, the initial Ca/(Si + Al) molar ratio of PF180 was around 1.1 before immersion. And over the course of up to 27 days of immersion, the Ca/(Si + Al) molar ratio of PF180 decreased only slightly. This indicates that the calcium ions in C-S-H gels with lower Ca/(Si + Al) molar ratios are relatively more stable and less susceptible to the erosion of sulfate ions. The leaching out of calcium ions is mostly related to the asymmetric structure of C-S-H gel, which is similar to the layered structure of 1.4 nm tobermorite. Calcium ions are located between the layers of the Si-O-Si main chain structure in the form of Ca-OH. Some calcium ions are used to balance the asymmetrical charges caused by the defects in the Si-O-Si chain. And Ca-OH has a weak interaction force with the Si-O-Si chain, meaning that calcium ions can easily be taken away by sulfate ions. Therefore, the C-S-H gel has to change its structure to prevent more calcium ions from leaching out (further discussed in Section 3.3).
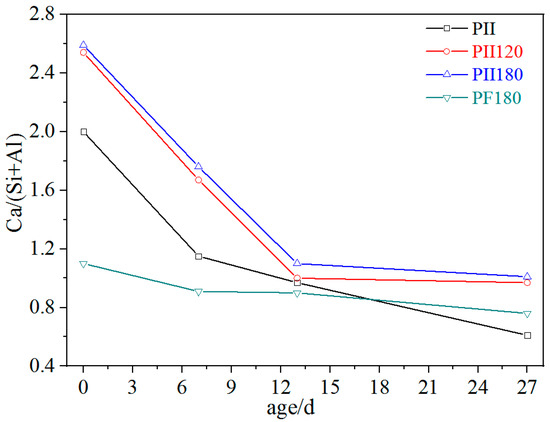
Figure 4.
Ca/(Si + Al) ratio of different C-S-H gels immersed in Na2SO4 solution for different durations.
3.3. 29Si and 27Al MAS-NMR Analysis
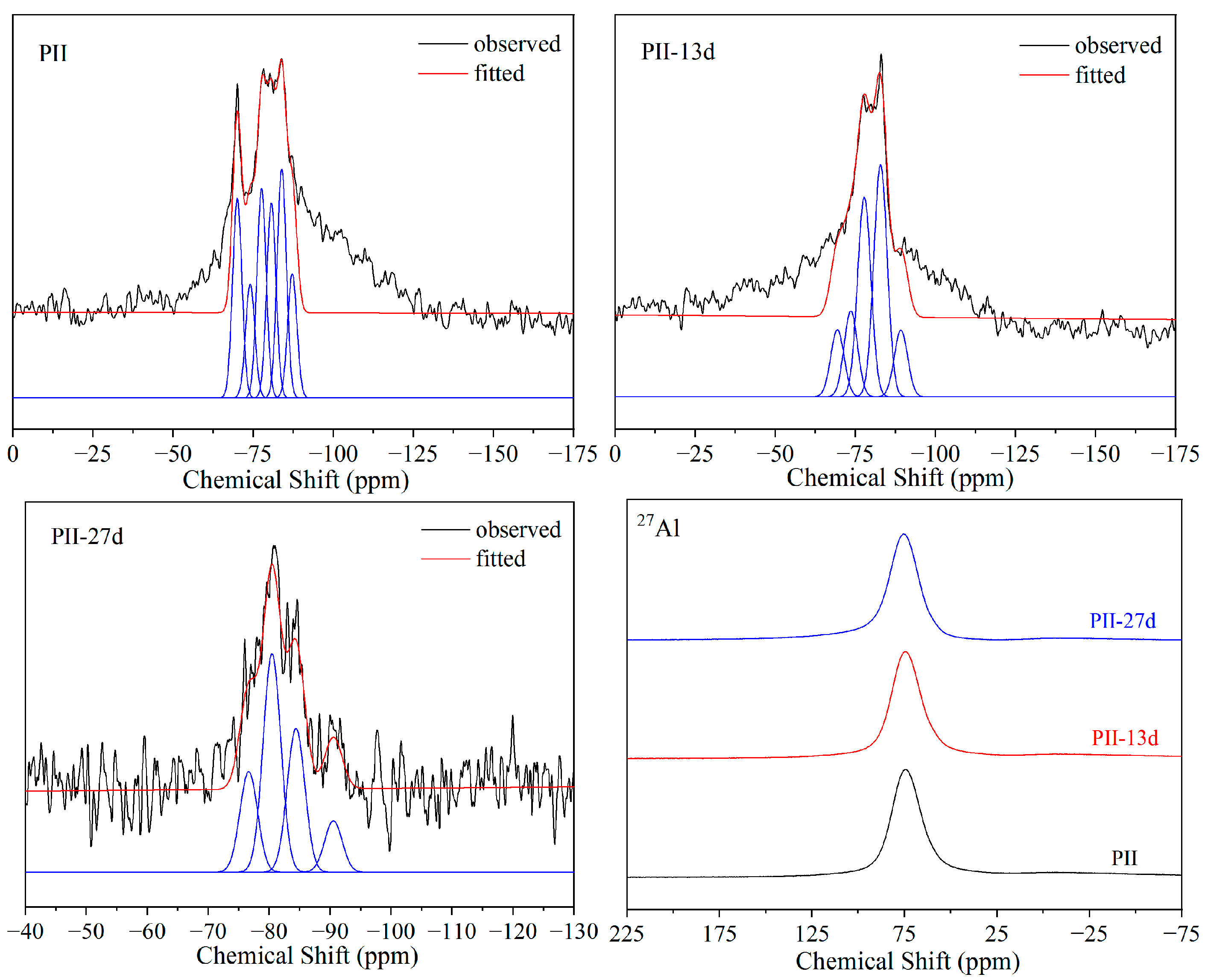
The 29Si and 27Al MAS-NMR spectra of PII immersed in Na2SO4 solution for different durations are presented in Figure 5. In 27Al MAS-NMR spectra, only one resonance peak at 75 ppm was detected, which was assigned to IVAl. In addition, IVAl should be present in the bridging position that links two silicate chains together to form Q2(1Al). And this IVAl resonance peak barely changed even after being immersed for 27 days, which suggests that the chemical structure of Al did not change during immersion.
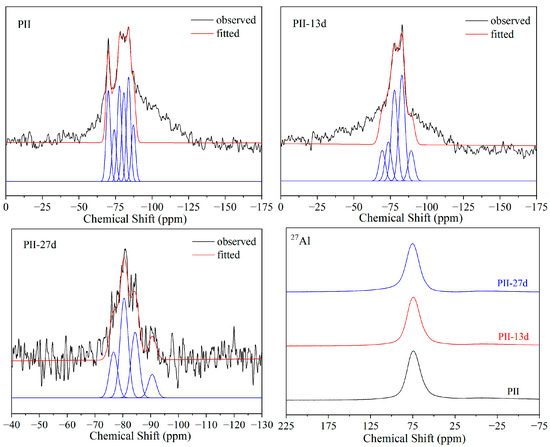
Figure 5.
29Si and 27Al MAS-NMR spectra of PII immersed for different durations.
Before immersion, the 29Si MAS-NMR spectra of PII displayed well-resolved signals between −70 and −90 ppm. The peak at −70 ppm can be assigned to Q0 from the free silica tetrahedron formed during the hydration of cement. The peaks at −77, −80, −85 and −90 ppm are assigned to Q1, Q2(1Al), Q2 and Q3 in the structure of C-S-H gel, respectively. The relatively low intensity of the Q3 peak indicates that the C-S-H gel is dominated by the Si-O-Si chain structure. During the immersion process, the relative content of Q0 and Q1 decreased while the relative content of Q2(1Al) and Q2 increased significantly. It suggests that the short Si-O-Si chain was polymerized to form longer Si-O-Si chains during the immersion process, and the free silica tetrahedron (Q0) participated in this polymerization.
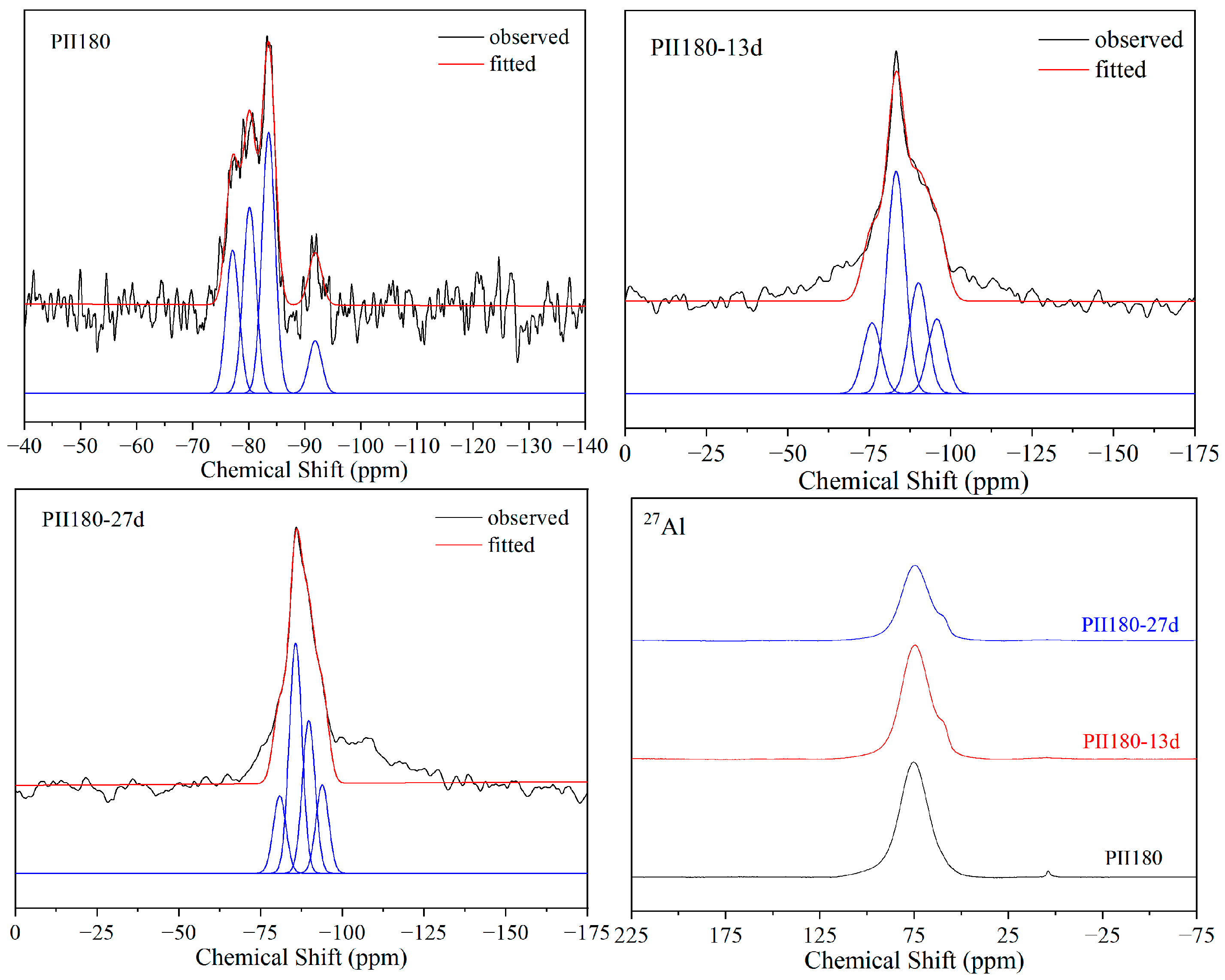
As shown in Figure 6, the 29Si and 27Al MAS-NMR spectra of PII180 present almost the same changes as PII during immersion. The relative content of Q2(1Al) and Q2 increased significantly during immersion. The only difference is that no Q0 resonate peak was detected before immersion. Hydrothermal treatment promoted the hydration of cement, ensuring the participation of silica tetrahedrons in the formation of C-S-H gel.
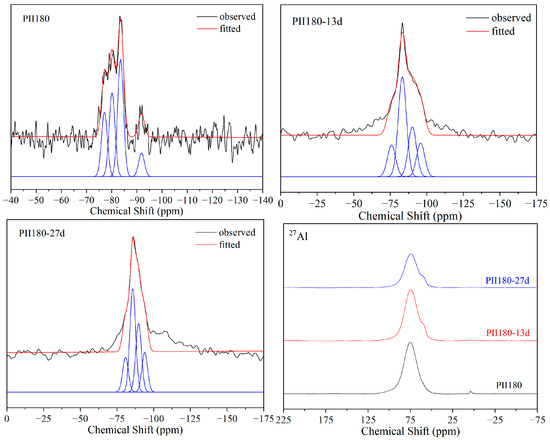
Figure 6.
29Si and 27Al MAS-NMR spectra of PII180 immersed for different durations.
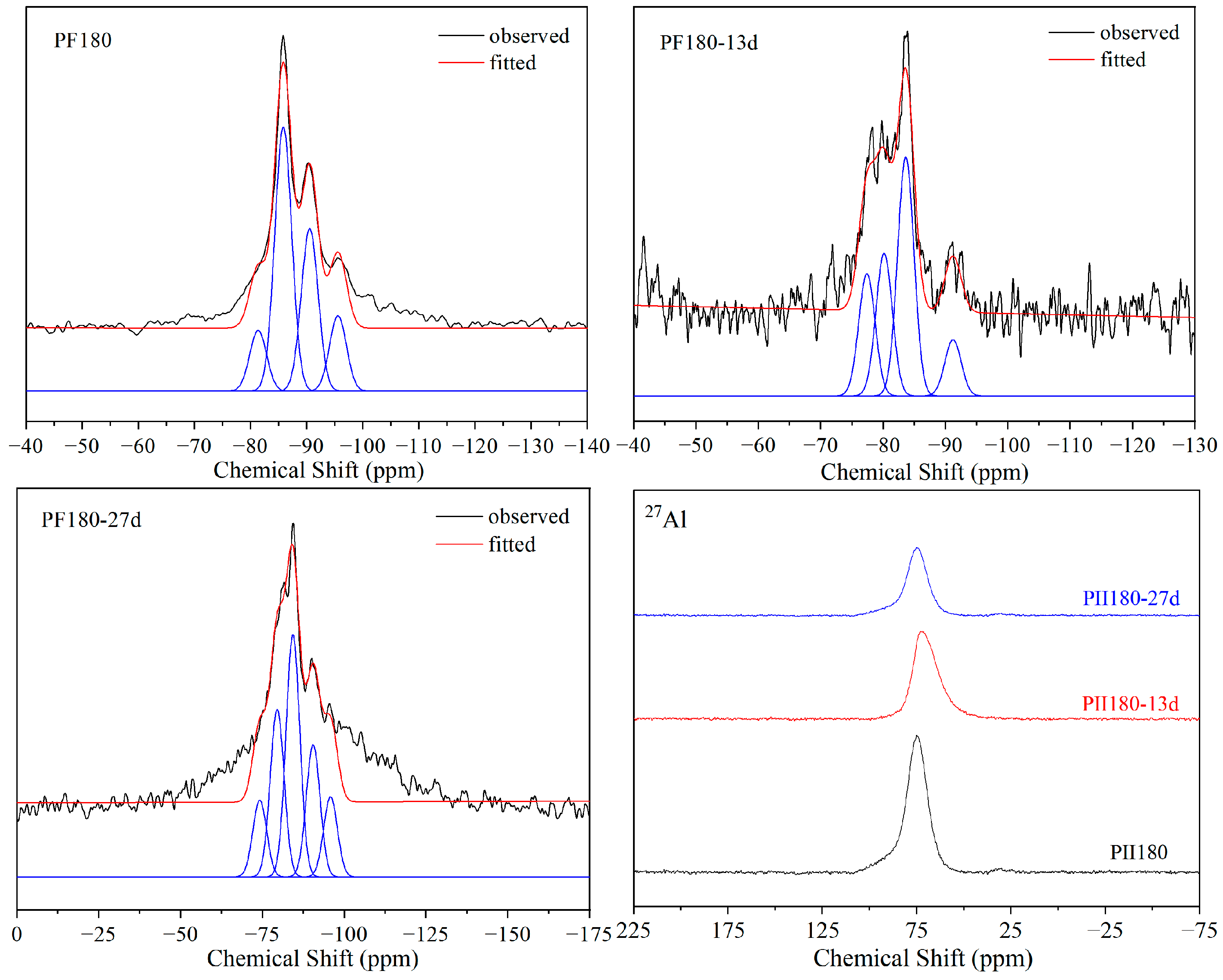
Figure 7 shows the 29Si and 27Al MAS-NMR spectra of PF180 immersed in Na2SO4 solution for different durations. Before immersion, the relative content of Q1 was much lower than that in PII and PII180 due to a large amount of SiO2 microspheres in FA that participated in cement hydration under hydrothermal treatment. It can be clearly seen that the relative content of Q1 increased in the first 13 days of immersion and then decreased after being immersed for 27 days. This indicates that the Si-O-Si chain breaks into short chains first, and then recombines to form longer Si-O-Si chains during the immersion process.
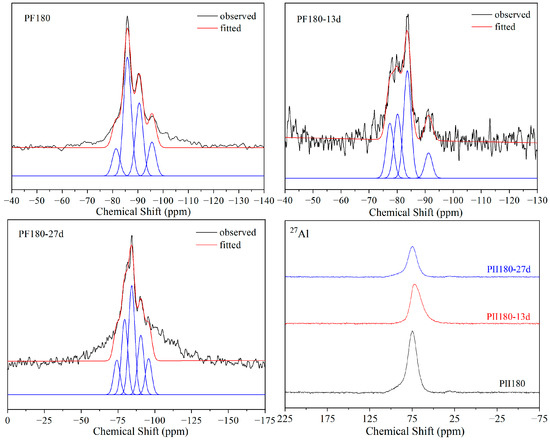
Figure 7.
29Si and 27Al MAS-NMR spectra of PF180 immersed for different durations.
The relative contents of each resonance peak and Si-O-Si chain lengths calculated from the fitted results are shown in Table 2. The chain length was calculated via Equation (1) [31].
CL = 2 × [Q1 + Q2(0Al) + 1.5 × Q2(1Al)]/Q1

Table 2.
Relative content of each resonance peak and chain length in the 29Si MAS-NMR of PII, PII180 and PF180.
As shown in Table 1, the CLs of PII and PII180 continuously increased from 6.98 to 11.37 and from 9.55 to 14.85 during immersion, respectively. This suggests that with the continuous leaching of Ca2+ in C-S-H gel during the immersion process, the short Si-O-Si chain recombines to form a long Si-O-Si chain. Thus, more silicate tetrahedrons enter the chain structure to form longer Si-O-Si chains, increasing the degree of polymerization to improve the stability of the C-S-H structure in a sulfate environment. Comparing PF180 to PII and PII180, the CL is much longer before immersion due to the pozzolanic reaction of FA. However, this Si-O-Si chain with a longer CL did not exhibit better stability in Na2SO4 solutions. After 13 days of immersion, the CL decreased from 20.42 to 9.41, which means that the longer Si-O-Si chain was broken into short chains under the erosion of sulfate ions. As the immersion time extended to 27 days, the short Si-O-Si chain was polymerized again. The CL increased from 9.41 to 14.84 to maintain better stability of the structure of C-S-H gel.
From the above discussion, it can be seen that C-S-H gels with too long or too short chain lengths are sensitive to the sulfate environment because the asymmetrical structure makes the calcium ions between the layered Si-O-Si chain structure unstable. During the attack by sulfate ions, the Si-O-Si chain in C-S-H gel breaks and recombines along with the leaching of calcium ions to prevent further leaching of calcium ions. Eventually, C-S-H gels with an appropriate chain length and Ca/(Si + Al) molar ratio are formed to keep the remaining calcium ions stable in the structure and ensure the structural stability of C-S-H gel in a sulfate environment. This may provide some reference value for improving the resistance of concrete to sulfate attack.
4. Conclusions
The deterioration of C-S-H gel with different Ca/(Si + Al) molar ratios in 5 wt.% Na2SO4 solution was studied. According to the experimental results, the following conclusions can be drawn. For C-S-H gel with a higher Ca/(Si + Al) ratio (PII, PII120, PII180), calcium ions rapidly leached out under the erosion of sulfate ions, which led to a decrease in the Ca/(Si + Al) ratio from above 2.0 to 1.0 after 13 days of immersion. At the same time, short Si-O-Si chains recombined to form C-S-H gel with a longer chain length. For C-S-H gel with a lower Ca/(Si + Al) ratio (PF180), the calcium ions seemed to be relatively stable. After 27 days of immersion, only a few calcium ions leached out from C-S-H gel and the Ca/(Si + Al) ratio only decreased by 0.3. However, the initial long Si-O-Si chain broke into short chains during the first 13 days of immersion, and then recombined into Si-O-Si chains with an appropriate chain length (14.85) after 27 days of immersion.
Research has shown that C-S-H gels with a low Ca/(Si + Al) ratio (0.8–1.0) and appropriate Si-O-Si chain length (14.8) present better stability in a severe sulfate environment. Therefore, cementitious materials with C-S-H gel as the main binder should demonstrate good resistance to sulfate attack.
Author Contributions
Conceptualization: C.L. and Z.X.; funding acquisition: C.L. and Z.X.; methodology: W.L.; writing—original draft: W.L.; writing—review and editing: W.L. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD), the Qing Lan Project, Six Talent Peaks Project in Jiangsu Province (no. XCL-029), the Natural Science Foundation of Jiangsu Province (grant no. BK20140606) and the Natural Science Foundation of Jiangsu Province (BK20180714).
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hossain, M.M.; Karim, M.R.; Hasan, M.; Hossain, M.K.; Zain, M.F.M. Durability of mortar and concrete made up of pozzolans as a partial replacement of cement: A review. Constr. Build. Mater. 2016, 116, 128–140. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Niu, D.T.; De Wang, Y.; Ma, R.; Wang, J.B.; Xu, S.H. Experiment study on the failure mechanism of dry-mix shotcrete under the combined actions of sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2015, 81, 74–80. [Google Scholar] [CrossRef]
- Whittaker, M.; Black, L. Current knowledge of external sulfate attack. Adv. Cem. Res. 2015, 27, 532–545. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attack research—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Neuenschwander, J.; Scrivener, K. Physical and microstructural aspects of sulfate attack on ordinary and limestone blended Portland cements. Cem. Concr. Res. 2009, 39, 1111–1121. [Google Scholar] [CrossRef]
- Hansen, W.C. Attack of Portland cement concrete by alkali soils and waters—A critical review. Highw. Res. Rec. 1966, 113, 1–32. [Google Scholar]
- Ding, Q.; Yang, J.; Hou, D.; Zhang, G. Insight on the mechanism of sulfate attacking on the cement paste with granulated blast furnace slag: An experimental and molecular dynamics study. Constr. Build. Mater. 2018, 169, 601–611. [Google Scholar] [CrossRef]
- Hou, D.; Li, T.; Wang, P. Molecular Dynamics Study on the Structure and Dynamics of NaCl Solution Transport in the Nanometer Channel of CASH Gel. ACS Sustain. Chem. Eng. 2018, 6, 9498–9509. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in Pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Zhu, H.; Chen, Y.; Xu, L.; Wang, P.; Lai, Y. Thaumasite form of sulfate attack in ettringite rich-ternary systems: Effects of limestone filler, etching solutions and exposure temperature. Dev. Built Environ. 2023, 15, 100208. [Google Scholar] [CrossRef]
- Crammond, N. The occurrence of thaumasite in modern construction—A review. Cem. Concr. Compos. 2002, 24, 393–402. [Google Scholar] [CrossRef]
- Shafaghat, J.; Allahverdi, A. Enhancing Concrete Properties by Using Silica Fume as Reactive Powder and Portland Cement-Clinker as Reactive Aggregate. J. Mater. Civ. Eng. 2019, 31, 04019278. [Google Scholar] [CrossRef]
- Dolado, J.S.; Campillo, I.; Erkizia, E.; Ibáñez, J.A.; Porro, A.; Guerrero, A.; Goñi, S. Effect of nanosilica additions on belite cement pastes held in sulfate solutions. J. Am. Ceram. Soc. 2007, 90, 3973–3976. [Google Scholar] [CrossRef]
- Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Pellenq, R.J.M.; Lequeux, N.; van Damme, H. Engineering the bonding scheme in C-S-H: The iono-covalent framework. Cem. Concr. Res. 2008, 38, 159–174. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nonat, A. Calcium silicate hydrates: Solid and liquid phase composition. Cem. Concr. Res. 2015, 78, 57–70. [Google Scholar] [CrossRef]
- Tennis, P.D.; Jennings, H.M. Model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem. Concr. Res. 2000, 30, 855–863. [Google Scholar] [CrossRef]
- Jennings, H.M. Refinements to colloid model of C-S-H in cement: CM-II. Cem. Concr. Res. 2008, 38, 275–289. [Google Scholar] [CrossRef]
- Mondai, P.; Shah, S.R.; Marks, L.D. Nanoscale characterization of cementitious materials. ACI Mater. J. 2008, 105, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Pomberger, R.; Sarc, R. Use of Solid Recovered Fuels in the Cement Industry. Waste Manag. 2014, 4, 471–488. [Google Scholar]
- Zschiesche, W.; Menzel, K. System for the use of alternative fuels in cement production, part one: Alternative fuels and raw materials. World Cem. 2014, 17, 225–276. [Google Scholar]
- Ferrari, L.; Bernard, L.; Deschner, F.; Kaufmann, J.; Winnefeld, F.; Plank, J. Characterization of polycarboxylate-ether based superplasticizer on cement clinker surfaces. J. Am. Ceram. Soc. 2012, 95, 2189–2195. [Google Scholar] [CrossRef]
- Plank, J.; Zhimin, D.; Keller, H.; Hössle, F.V.; Seidl, W. Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement. Cem. Concr. Res. 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Impact of particle size on interaction forces between ettringite and dispersing comb-polymers in various electrolyte solutions. J. Colloid Interface Sci. 2014, 419, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in C-A-S-H during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- Steindl, F.R.; Baldermann, A.; Galan, I.; Sakoparnig, M.; Briendl, L.; Dietzel, M.; Mittermayr, F. Chemical resistance of eco-concrete–Experimental approach on Ca-leaching and sulphate attack. Constr. Build. Mater. 2019, 223, 55–68. [Google Scholar] [CrossRef]
- GB2007; Common Portland Cement. China Standards Press: Beijing, China, 2008.
- Richardson, I.G. Nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).