Block Copolymer-Based Symmetric Membranes for Direct Methanol Fuel Cells
Abstract
1. Introduction: Setting the Scene
2. Theoretical Background
2.1. How DMFCs Work
2.2. Degradation Mechanisms in DMFCs
2.3. DMFC Challenges
2.4. Beyond Nafion Membranes
3. Block Copolymer Electrolytes
3.1. Synthesis
3.1.1. Postsulfonation of Commercial Block Copolymers
3.1.2. Synthesis of Electrolytes from Poly(arylene Ether)-Based Building Blocks
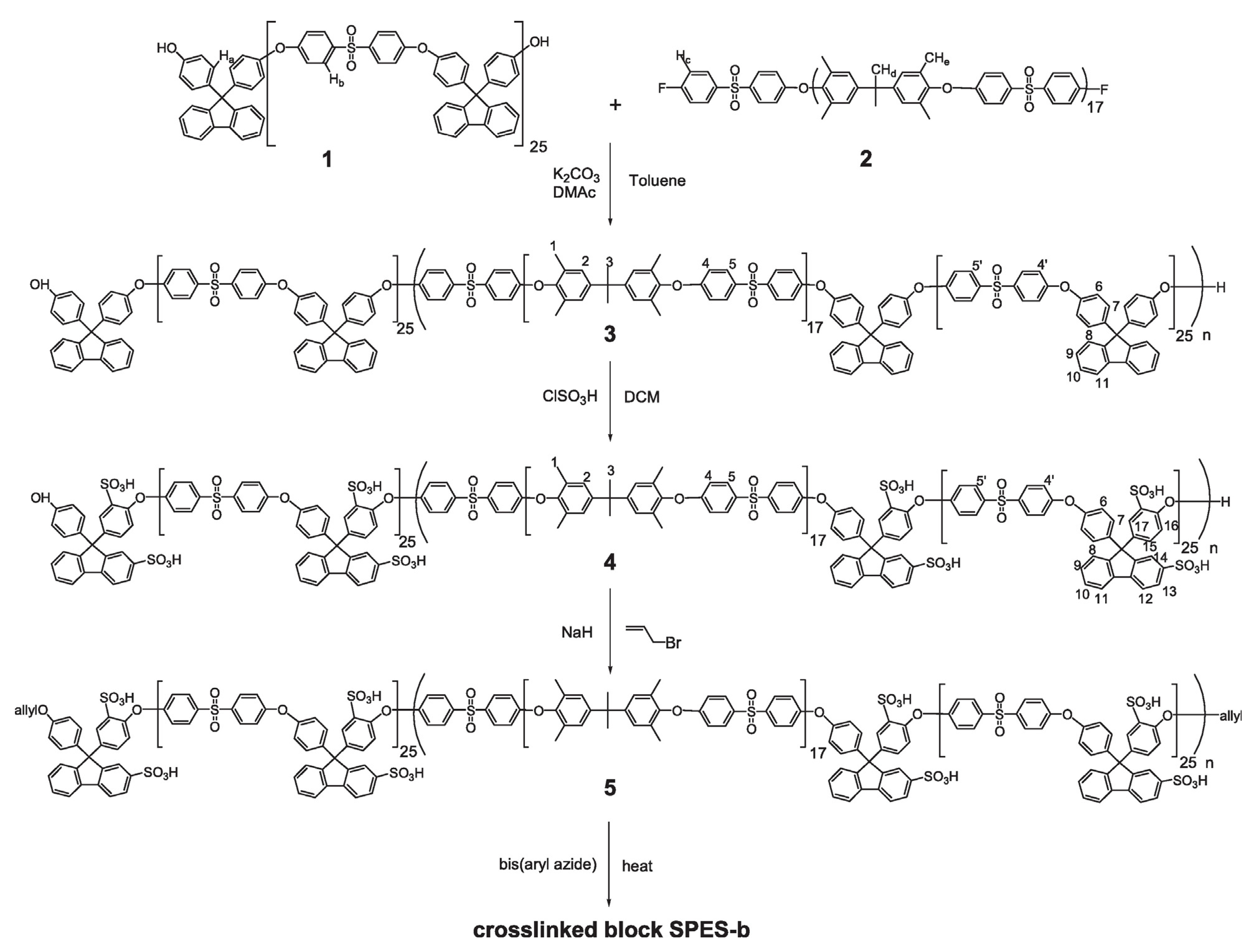
3.1.3. Poly(arylene Ether Sulfone)-Based Copolymers
3.1.4. Poly(ether Sulfone)-Based Copolymers
3.1.5. Other Systems
3.1.6. Synthesis of Electrolytes from Poly(arylene Ether) and Other Similar Building Blocks
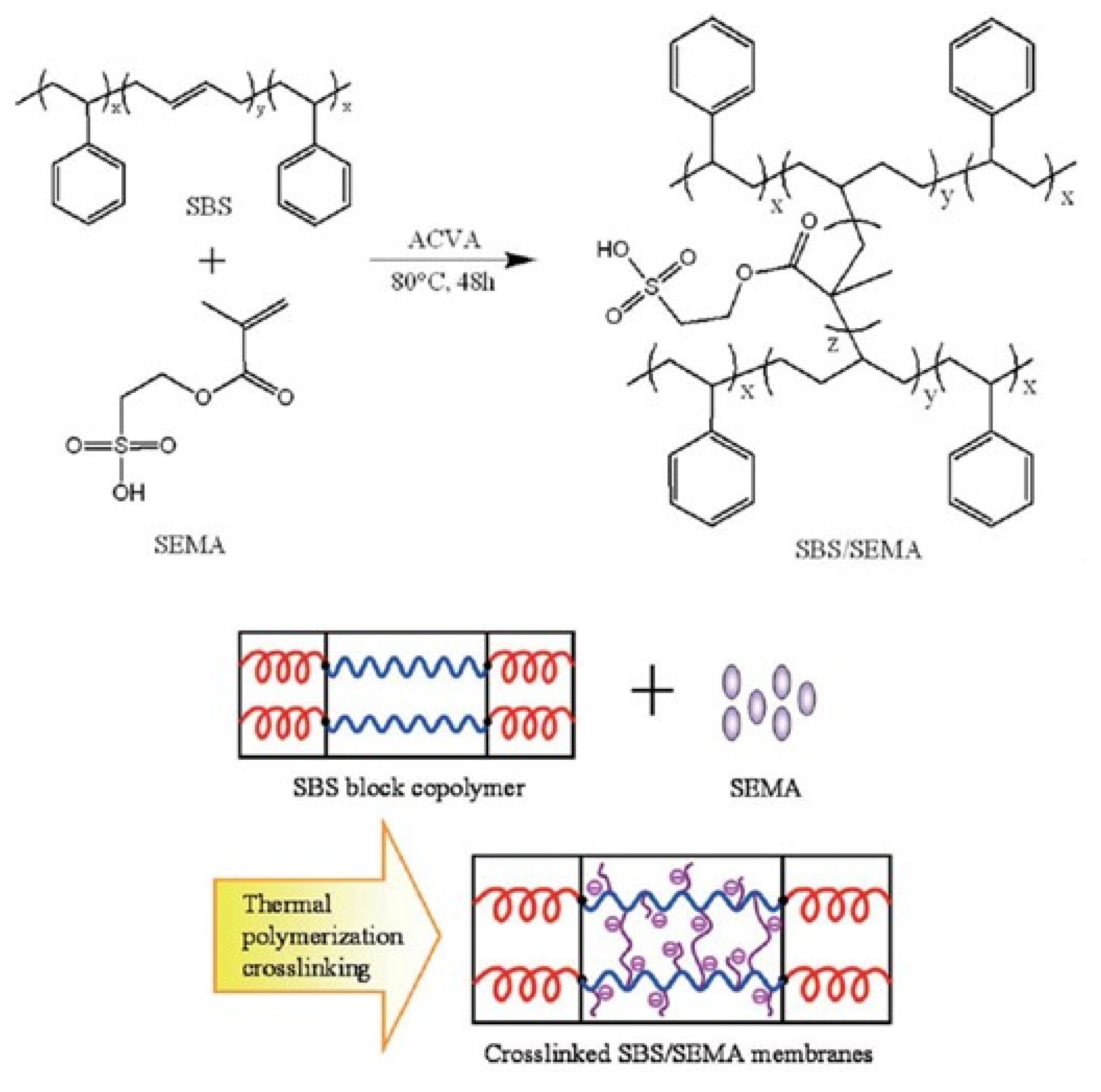
3.1.7. Synthesis of Electrolytes from Poly(styrene) and Other Building Blocks
3.1.8. Synthesis of Electrolytes from Building Blocks Other Than Polystyrene and Poly(arylene Ether)s
3.1.9. Copolymers with Special Shapes
4. Block Copolymer Membranes for DMFCs
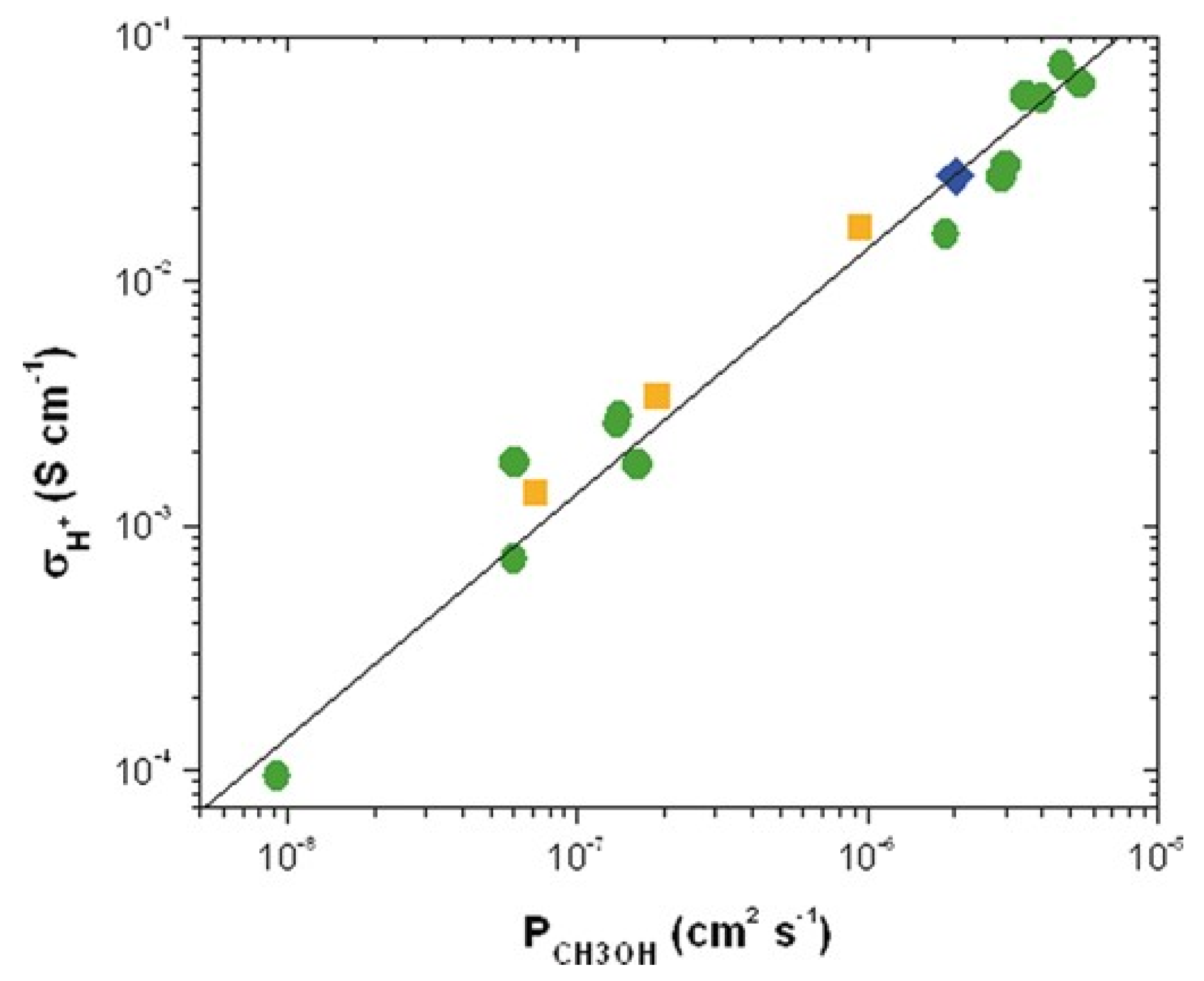
4.1. Some Considerations for Transport Mechanisms
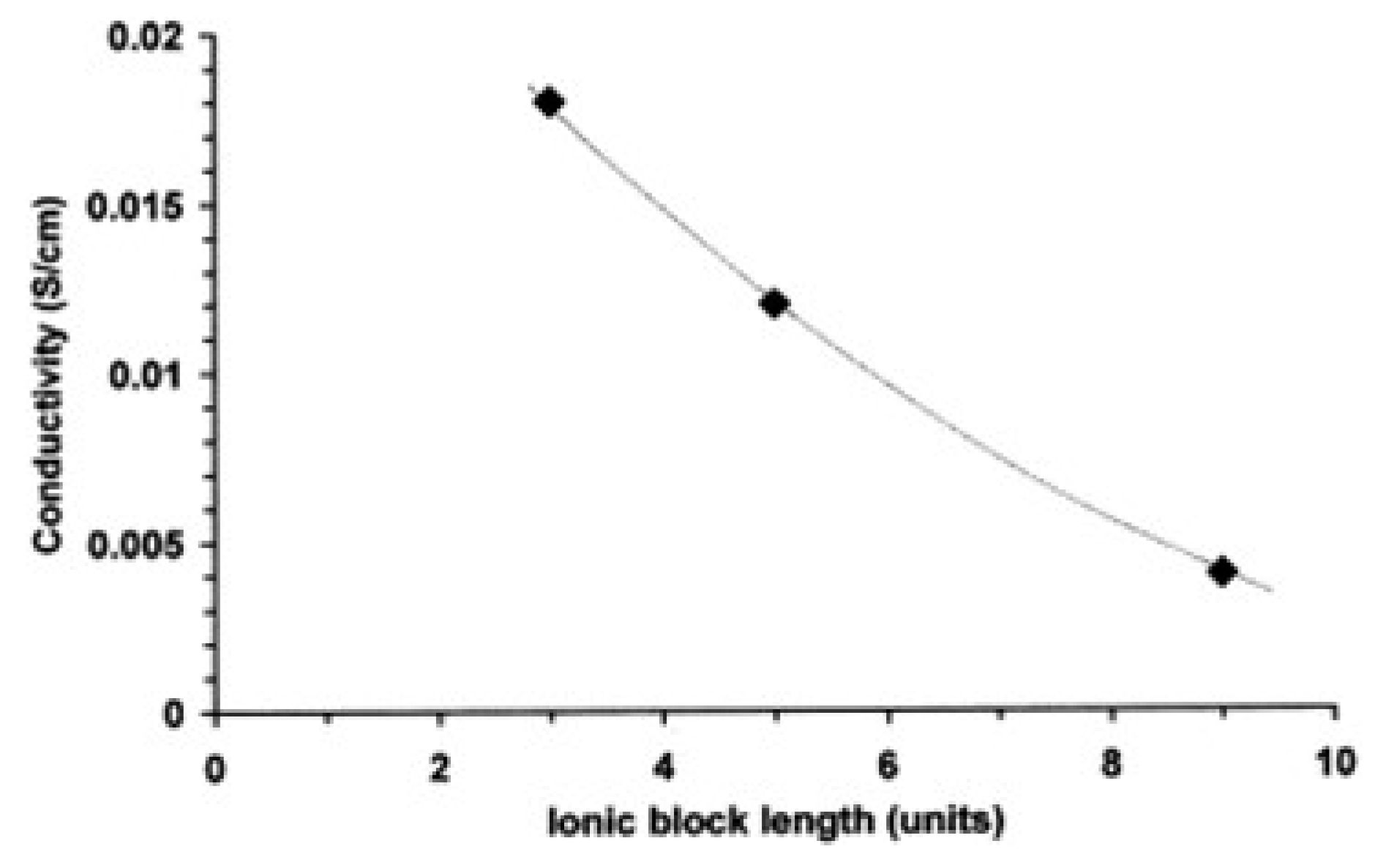
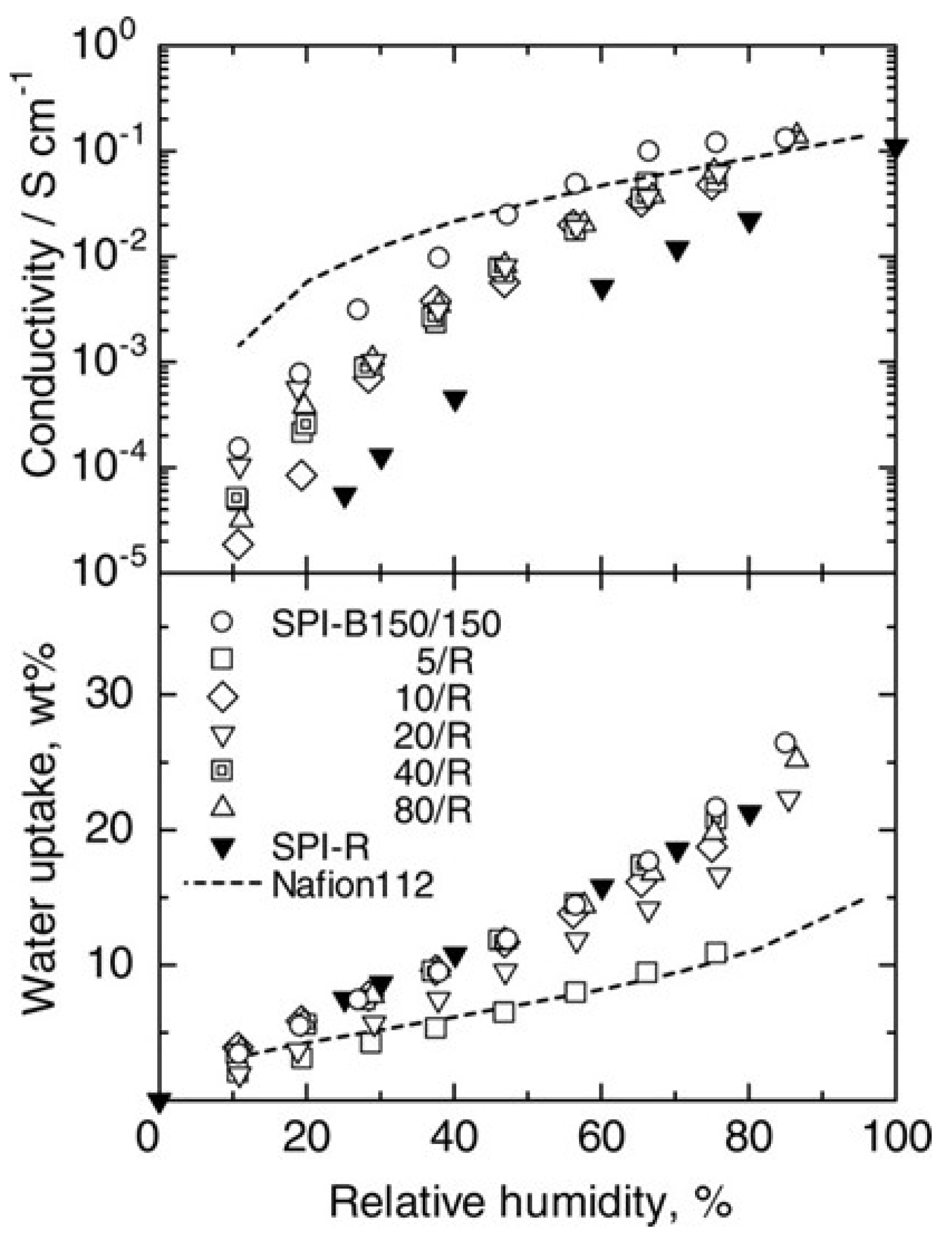
4.2. Role of Block Length
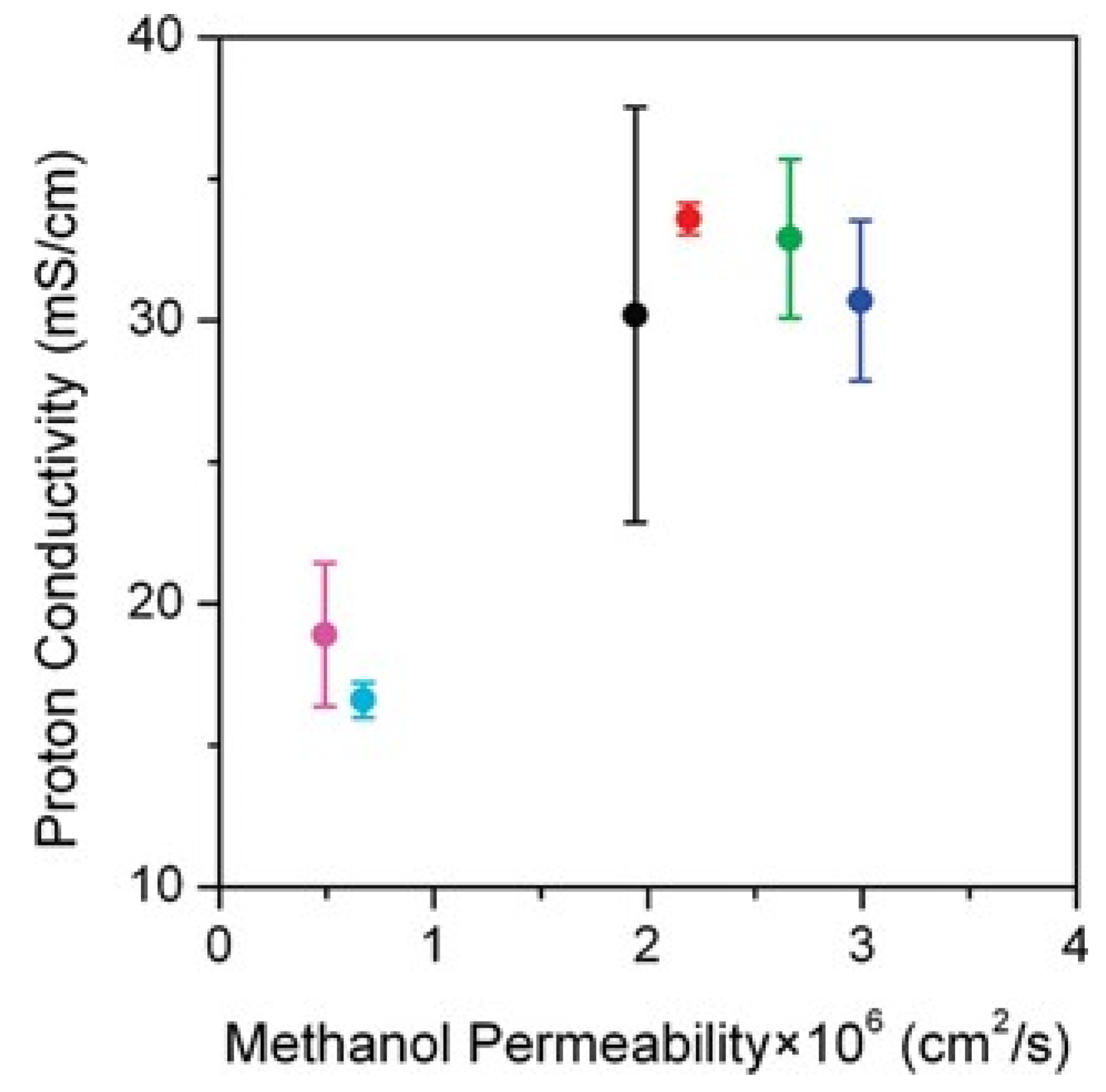
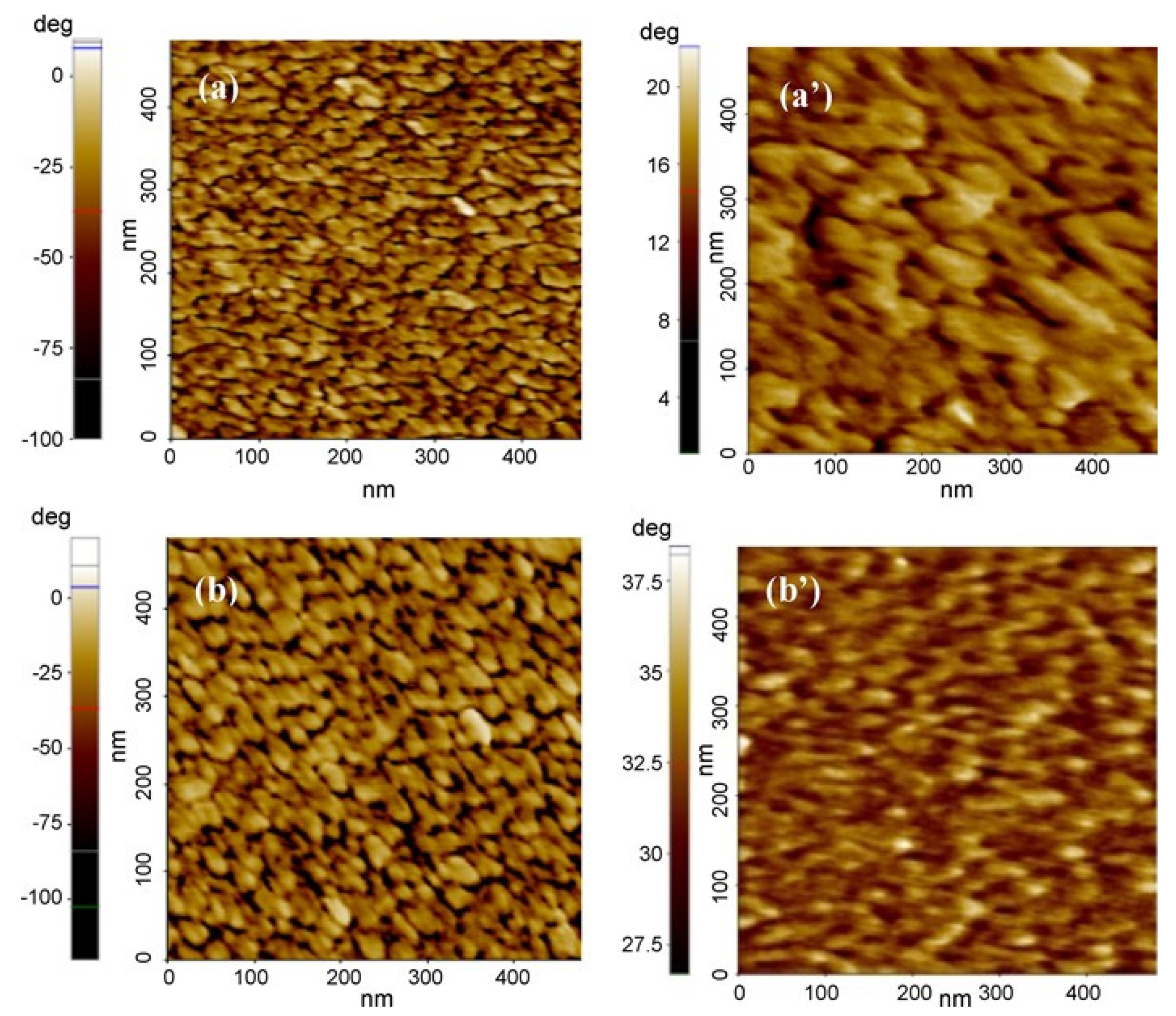
4.3. Role of Crosslinking
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mastoi, M.S.; Zhuang, S.; Munir, H.; Haris, M.; Hassan, M.; Usamn, M.; Bukhari, S.S.H.; Ro, J.-S. An in-depth analysis of electric vehicle charging station infrastructure. Energy Rep. 2022, 8, 11504–11529. [Google Scholar] [CrossRef]
- Tullo, A.H. Tire makers strive to fix their electric car problem. Chem. Eng. News 2024, 102, 18–20. [Google Scholar]
- Graya, N.; O’Shea, R.; Wall, D.; Smythc, B.; Lens, P.N.L.; Murphy, J.D. Batteries, fuel cells, or engines? A probabilistic economic and environmental assessment of electricity and electrofuels for heavy goods vehicles. Adv. Appl. Energy 2022, 8, 100110. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, K. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Available online: https://www.sintef.no/en/latest-news/2021/europes-largest-pem-electrolyser-for-hydrogen-production-in-operation-now-sintef-will-lead-project-where-capacity-is-tenfold/ (accessed on 28 April 2024).
- Hobson, C.; Márquez, C. Renewable Methanol Report; Technical Report; ATA Markets Intelligence S.L. on behalf of the Methanol Institute: Madrid, Spain, 2018. [Google Scholar]
- Goeppert, A.; Czaun, M.; Surya Prakash, G.K.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci. 2012, 5, 7833–7853. [Google Scholar] [CrossRef]
- Brown, A.; Le Feuvre, P. Technology Roadmap: Delivering Sustainable Bioenergy; International Energy Agency (IEA): Paris, France, 2017; p. 94. [Google Scholar]
- Cui, X.; Kær, S.K. Thermodynamic analyses of a moderate-temperature carbon dioxide hydrogenation to methanol via reverse water gas shift process with in situ water removal. Ind. Eng. Chem. Res. 2019, 58, 10559–10569. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic Chemical Carbon Cycle for a Sustainable Future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef] [PubMed]
- Methanol Institute. Methanol|Methanol Institute. Available online: https://www.methanol.org (accessed on 28 April 2024).
- Zhao, K. A Brief Review of China’s Methanol Vehicle Pilot and Policy; Technical Report; Methanol Institute: Alexandria, VA, USA, 2019. [Google Scholar]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Dias, V.; Pochet, M.; Contino, F.; Jeanmart, H. Energy and Economic Costs of Chemical Storage. Front. Mech. Eng. 2020, 6, 00021. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Chapter 1—Methanol Production and Applications: An Overview. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar]
- U.S. Department of Energy. Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 28 April 2024).
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Patil, A.S.; Dubois, T.G.; Sifer, N.; Bostic, E.; Gardner, K.; Quah, M.; Bolton, C. Portable fuel cell systems for America’s army: Technology transition to the field. J. Power Sources 2004, 136, 220–225. [Google Scholar] [CrossRef]
- Hamada, A.T.; Orhan, M.F. An overview of regenerative braking systems. J. Energy Storage 2022, 52, 105033. [Google Scholar] [CrossRef]
- Cowey, K.; Green, K.J.; Mepsted, G.O.; Reeve, R. Portable and military fuel cells. Curr. Opin. Solid State Mater. Sci. 2004, 8, 367–371. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Labadidi, M.A.; Hamada, A.T.; Orhan, M.T. Design and Utilization of a Direct Methanol Fuel Cell. Membranes 2022, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.researchnester.com/reports/direct-methanol-fuel-cell-market/5681 (accessed on 10 August 2024).
- Junoh, H.; Jaafar, J.; Nordin, N.A.H.M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure. Membranes 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Jayakumar, A.; Madheswaran, D.K.; Kumar, N.M.A. Critical Assessment on Functional Attributes and Degradation Mechanism of Membrane Electrode Assembly Components in Direct Methanol Fuel Cells. Sustainability 2021, 13, 13938. [Google Scholar] [CrossRef]
- Wan, N. High Performance Direct Methanol Fuel Cell with Thin Electrolyte Membrane. J. Power Sources 2017, 354, 167–171. [Google Scholar] [CrossRef]
- Iannaci, A.; Mecheri, B.; D’Epifanio, A.; Licoccia, S. Sulfated Zirconium Oxide as Electrode and Electrolyte Additive for Direct Methanol Fuel Cell Applications. Int. J. Hydrogen Energy 2014, 39, 11241–11249. [Google Scholar] [CrossRef]
- Bogolowski, N.; Drillet, J.-F. Appropriate Balance between Methanol Yield and Power Density in Portable Direct Methanol Fuel Cell. Chem. Eng. J. 2015, 270, 91–100. [Google Scholar] [CrossRef]
- Ji, F.; Yang, L.; Sun, H.; Wang, S.; Li, H.; Jiang, L.; Sun, G. A Novel Method for Analysis and Prediction of Methanol Mass Transfer in Direct Methanol Fuel Cell. Energy Convers. Manag. 2017, 154, 482–490. [Google Scholar] [CrossRef]
- Ramesh, V.; Krishnamurthy, B. Modeling the Transient Temperature Distribution in a Direct Methanol Fuel Cell. J. Electroanal. Chem. 2018, 809, 1–7. [Google Scholar] [CrossRef]
- Tsen, W.-C. Composite Proton Exchange Membranes Based on Chitosan and Phosphotungstic Acid Immobilized One-Dimensional Attapulgite for Direct Methanol Fuel Cells. Nanomaterials 2020, 10, 1641. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Deng, Y.; Xu, M.; Artiglia, L.; Wen, W.; Gao, R.; Chen, B.; Yao, S.; Zhang, X.; et al. A Stable Low-Temperature H2-Production Catalyst by Crowding Pt on MoC. Nature 2021, 589, 396–401. [Google Scholar] [CrossRef]
- Sahin, O.; Kivrak, H. A Comparative Study of Electrochemical Methods on Pt–Ru DMFC Anode Catalysts: The Effect of Ru Addition. Int. J. Hydrogen Energy 2013, 38, 901–909. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion Based Composite Membranes on the Performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Synthesis, Preparation, and Performance of Blends and Composites of -Conjugated Polymers and Their Copolymers in DMFCs. Polym. Rev. 2015, 55, 630–677. [Google Scholar] [CrossRef]
- Kludský, M.; Vopička, O.; Matějka, P.; Hovorka, Š.; Friess, K. Nafion® Modified with Primary Amines: Chemical Structure, Sorption Properties and Pervaporative Separation of Methanol-Dimethyl Carbonate Mixtures. Eur. Polym. J. 2018, 99, 268–276. [Google Scholar] [CrossRef]
- Klose, C.; Breitwieser, M.; Vierrath, S.; Klingele, M.; Cho, H.; Büchler, A.; Kerres, J.; Thiele, S. Electrospun Sulfonated Poly(EtherKetone) Nanofibers as Proton Conductive Reinforcement for Durable Nafion Composite Membranes. J. Power Sources 2017, 361, 237–242. [Google Scholar] [CrossRef]
- Pérez, L.C.; Brandão, L.; Sousa, J.M.; Mendes, A. Segmented Polymer Electrolyte Membrane Fuel Cells—A Review. Renew. Sustain. Energy Rev. 2011, 15, 169–185. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of Direct Methanol Fuel Cell Membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Mixed matrix membranes for gas separation. In Nano-Enhanced and Nanostructured Polymer-Based Membranes for Energy Applications; Woodhead Publishing Series; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–254. [Google Scholar]
- Pourzare, K.; Zargar, M.; Farhadi, S.; Hassani Sadrabadi, M.M.; Mansourpanah, Y. Aminosilica-Functionalized Co3O4 Nanostructures in Proton Exchange Mixed Matrix Membranes for Enhanced Separation Efficiency of Direct Methanol Fuel Cells. ACS Appl. Nano Mater. 2023, 6, 296–304. [Google Scholar] [CrossRef]
- Pourzare, K.; Mansourpanah, Y.; Farhadi, S. Advanced nanocomposite membranes for fuel cell applications: A comprehensive review. Biofuel Res. J. 2016, 3, 496–513. [Google Scholar] [CrossRef]
- Simari, C.; Baglio, V.; Lo Vecchio, C.; Aricò, A.S.; Agostino, R.G.; Coppola, L.; Oliviero Rossi, C.; Nicotera, I. Reduced methanol crossover and enhanced proton transport in nanocomposite membranes based on clay—CNTs hybrid materials for direct methanol fuel cells. Ionics 2017, 23, 2113–2123. [Google Scholar] [CrossRef]
- Hasanabadi, N.; Ghaffarian, S.R.; Hasani-Sadrabadi, M.M. Nafion-based magnetically aligned nanocomposite proton exchange membranes for direct methanol fuel cells. Solid State Ion. 2013, 232, 58–67. [Google Scholar] [CrossRef]
- Cozzi, D.; de Bonis, C.; D’Epifanio, A.; Mecheri, B.; Tavares, A.C.; Licoccia, S. Organically functionalized titanium oxide/Nafion composite proton exchange membranes for fuel cells applications. J. Power Sources 2014, 248, 1127–1132. [Google Scholar] [CrossRef]
- Einsla, M.L.; Kim, Y.S.; Hawley, M.; Lee, H.-S.; McGrath, J.E.; Liu, B.; Guiver, M.D.; Pivovar, B.S. Toward improved conductivity of sulfonated aromatic proton exchange membranes at low relative humidity. Chem. Mater. 2008, 20, 5636–5642. [Google Scholar] [CrossRef]
- Won, J.; Park, H.H.; Kim, Y.J.; Choi, S.W.; Ha, H.Y.; Oh, I.H.; Kim, H.S.; Kang, Y.S.; Ihn, K.J. Fixation of Nanosized Proton Transport Channels in Membranes. Macromolecules 2003, 36, 3228–3234. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Li, X.; Na, H. Morphological investigations of block sulfonated poly(arylene ether ketone) copolymers as potential proton exchange membranes. Polym. Adv. Technol. 2011, 22, 2173–2181. [Google Scholar] [CrossRef]
- Nunes, S.P.; Car, A. From Charge-Mosaic to Micelle Self-Assembly: Block Copolymer Membranes in the Last 40 Years. Ind. Eng. Chem. Res. 2012, 52, 993–1003. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
- Yang, Y.; Holdcroft, S. Synthetic Strategies for Controlling the Morphology of Proton Conducting Polymer Membranes. Fuel Cells 2005, 5, 171–186. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.V.; Nunes, S.P.; Scharnagl, N.; et al. Developments in Membrane Research: From Material via Process Design to Industrial Application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Deluca, N.W.; Elabd, Y.A. Polymer electrolyte membranes for the direct methanol fuel cell: A review. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2201–2225. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z. Polymer electrolyte membranes for fuel cells. Prog. Chem. 2008, 20, 602–619. [Google Scholar]
- Elabd, Y.A.; Hickner, M.A. Block copolymers for fuel cells. Macromolecules 2011, 44, 1–11. [Google Scholar] [CrossRef]
- Sutisna, B.; Polymeropoulos, G.; Musteat, V.; Peinemann, K.V.; Avgeropoulos, A.; Smilgies, D.F.; Hadjichristidis, N.; Nunes, S.P. Design of block copolymer membranes using segregation strength trend lines. Mol. Syst. Des. Eng. 2016, 1, 278–289. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Takamuku, S.; Akizuki, K.; Abe, M.; Kanesaka, H. Synthesis and characterization of postsulfonated poly(arylene ether sulfone) diblock copolymers for proton exchange membranes. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 700–712. [Google Scholar] [CrossRef]
- Lee, H.S.; Roy, A.; Lane, O.; Dunn, S.; McGrath, J.E. Hydrophilic–hydrophobic multiblock copolymers based on poly(arylene ether sulfone) via low-temperature coupling reactions for proton exchange membrane fuel cells. Polymer 2008, 49, 715–723. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Synthesis and properties of sulfonated block copolymers having fluorenyl groups for fuel-cell applications. ACS Appl. Mater. Interface 2009, 1, 1279–1286. [Google Scholar] [CrossRef]
- Hou, J.; Li, J.; Madson, L.A. Anisotropy and Transport in Poly(arylene ether sulfone) Hydrophilic–Hydrophobic Block Copolymers. Macromolecules 2010, 43, 347–353. [Google Scholar] [CrossRef]
- Lee, H.S.; Lane, O.; McGrath, J.E. Development of multiblock copolymers with novel hydroquinone-based hydrophilic blocks for proton exchange membrane (PEM) applications. J. Power Sources 2010, 195, 1772–1778. [Google Scholar] [CrossRef]
- Li, H.; Kirk, N.J.; Mauritz, K.A.; Storey, R.F. Poly(arylene Ether Sulfone) Multi-Block Copolymers Bearing Perfluoroalkylsulfonic Acid Groups. Polymer 2011, 52, 3550–3559. [Google Scholar] [CrossRef]
- Takamuku, S.; Jannasch, P. Multiblock Copolymers with Highly Sulfonated Blocks Containing Di- and Tetrasulfonated Arylene Sulfone Segments for Proton Exchange Membrane Fuel Cell Applications. Adv. Energy Mater. 2012, 2, 129–140. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Matsumoto, K.; Ueda, M. Synthesis and properties of sulfonated multiblock copoly(ether sulfone)s by a chain extender. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3947–3957. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Al-Souz, M.A.K.; Gaudet, J.; Guay, D.; Hay, A.S. Ionomers for proton exchange membrane fuel cells by sulfonation of novel dendritic multiblock copoly(ether-sulfone)s. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5461–5473. [Google Scholar] [CrossRef]
- Bai, Z.; Yoonessi, M.; Drummy, L.F.; Durstock, M.F.; Dang, T.D. Synthesis and Characterization of Multiblock Sulfonated Poly(arylenethioethersulfone) Copolymers for Proton Exchange Membranes. Macromolecules 2008, 41, 9483–9486. [Google Scholar] [CrossRef]
- Bae, B.; Yoda, T.; Miyatake, K.; Uchida, H.; Watanabe, M. Proton-Conductive Aromatic Ionomers Containing Highly Sulfonated Blocks for High-Temperature-Operable Fuel Cells. Angew. Chem. Int. Ed. 2010, 49, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Miyatake, K.; Watanabe, M. Sulfonated Poly(arylene ether sulfone ketone) Multiblock Copolymers with Highly Sulfonated Block. Synthesis and Properties. Macromolecules 2010, 43, 2684–2691. [Google Scholar] [CrossRef]
- Umezawa, K.; Oshima, T.; Yoshizawa-Fujita, M.; Takeoka, Y.; Rikukawa, M. Synthesis of hydrophilic-hydrophobic block copolymer ionomers based on polyphenylenes. ACS Macro Lett. 2012, 1, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.A.; Benicewicz, B.C. Synthesis and Properties of Segmented Block Copolymers of Functionalised Polybenzimidazoles for High-Temperature PEM Fuel Cells. Fuel Cells 2011, 11, 222–237. [Google Scholar] [CrossRef]
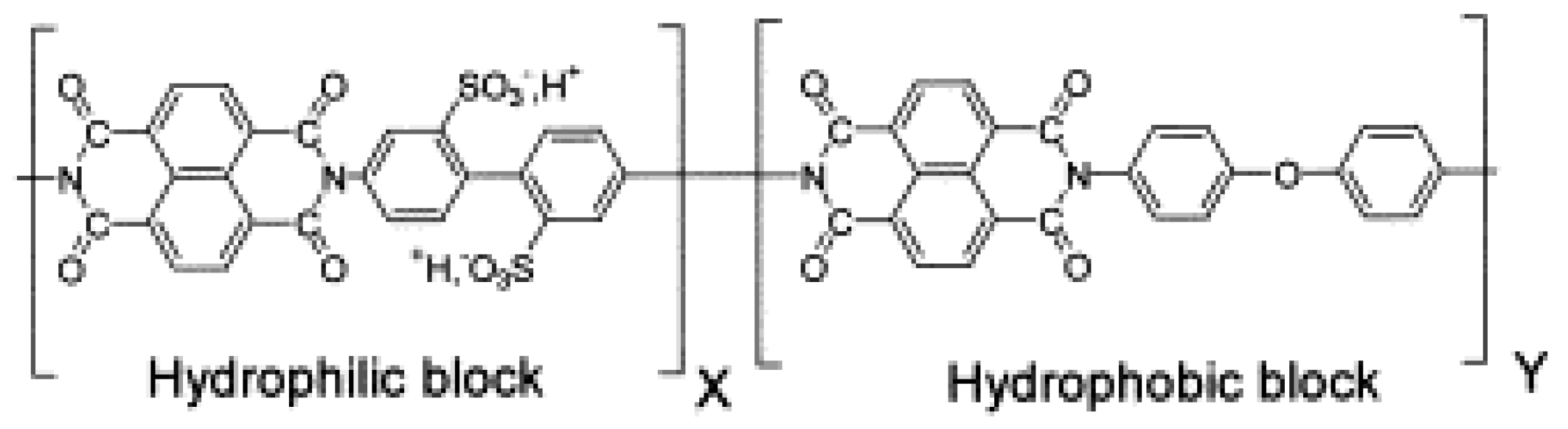
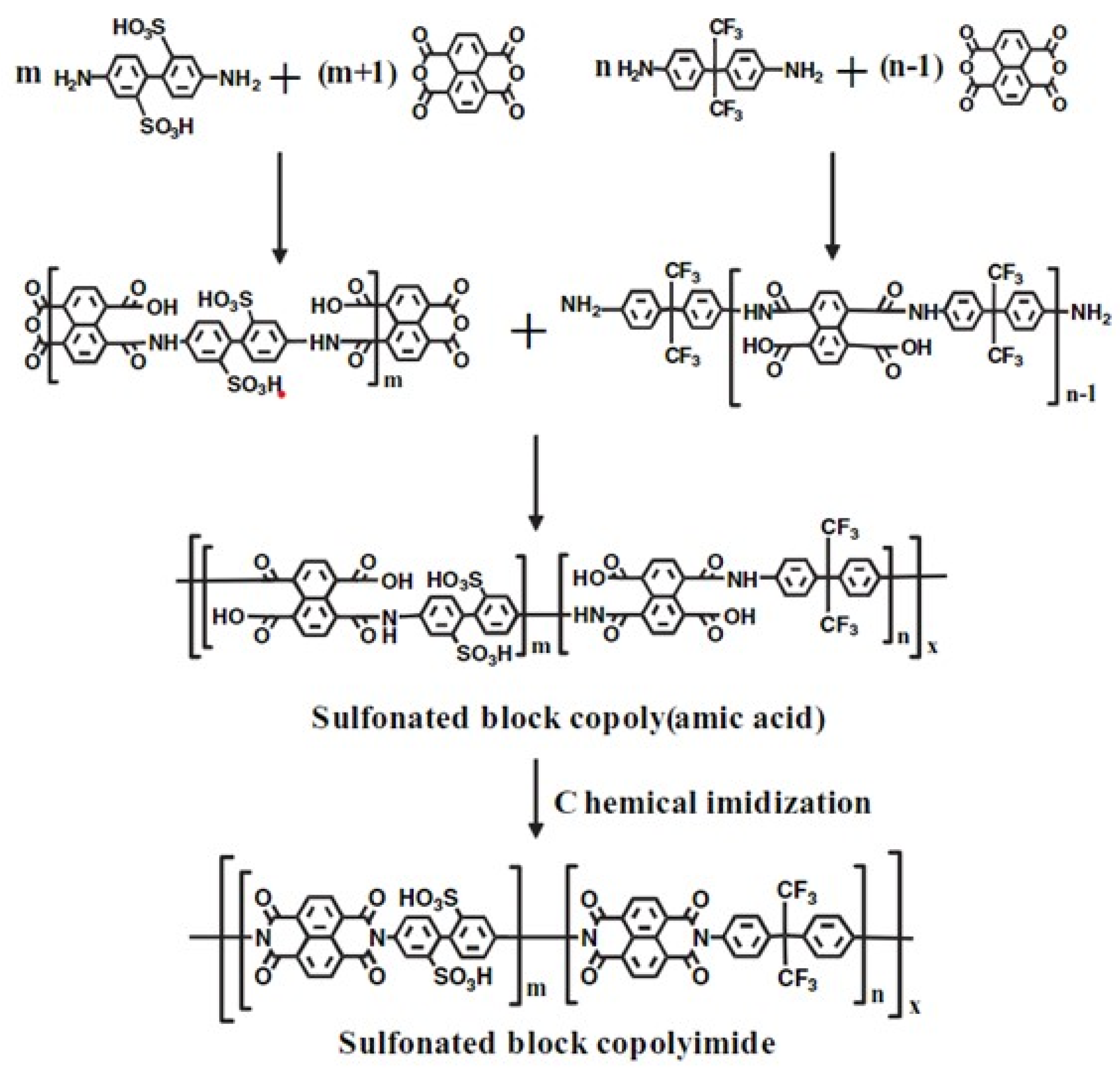
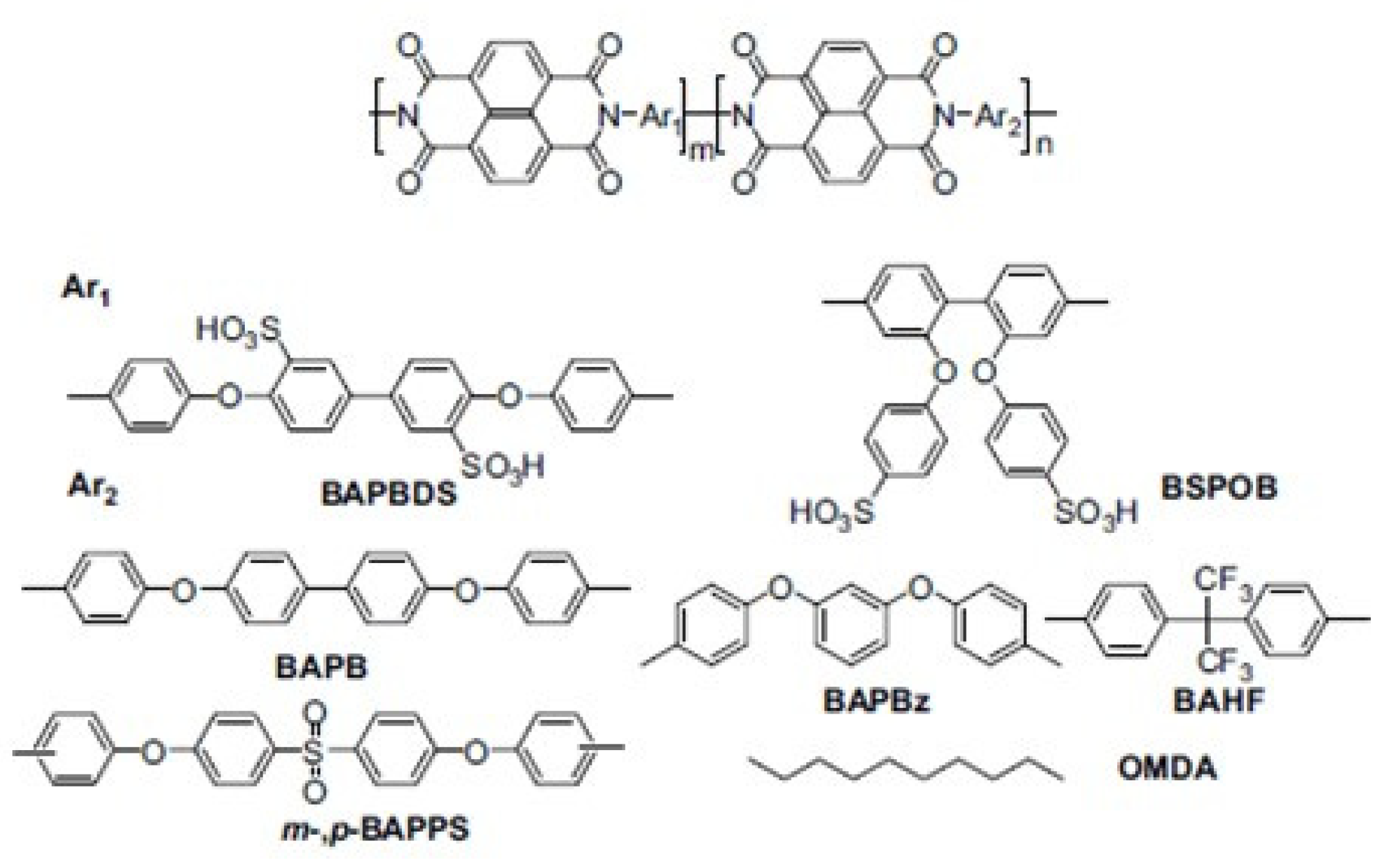
- Asano, N.; Miyatake, K.; Watanabe, M. Sulfonated block polyimide copolymeres as proton-conductive membrane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2744–2748. [Google Scholar] [CrossRef]
- Bi, H.; Chen, S.; Chen, X.; Chen, K.; Endo, N.; Higa, M.; Okamoto, K.; Wang, L. Poly(sulfonated phenylene)-block-Polyimide Copolymers for Fuel Cell Applications. Macromol. Rapid Commun. 2009, 30, 1852–1856. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, C.H.; Rowlett, J.R.; McGrath, J.E. Synthesis and characterization of multiblock semi-crystalline hydrophobic poly(ether ether ketone)–hydrophilic disulfonated poly(arylene ether sulfone) copolymers for proton exchange membranes. Polymer 2012, 53, 3143–3153. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, R.; Lee, C.H.; Lee, M.; McGrath, J.E. Partly fluorinated poly(arylene ether ketone sulfone) hydrophilic–hydrophobic multiblock copolymers for fuel cell membranes. Int. J. Hydrogen Energy 2012, 37, 6132–6139. [Google Scholar] [CrossRef]
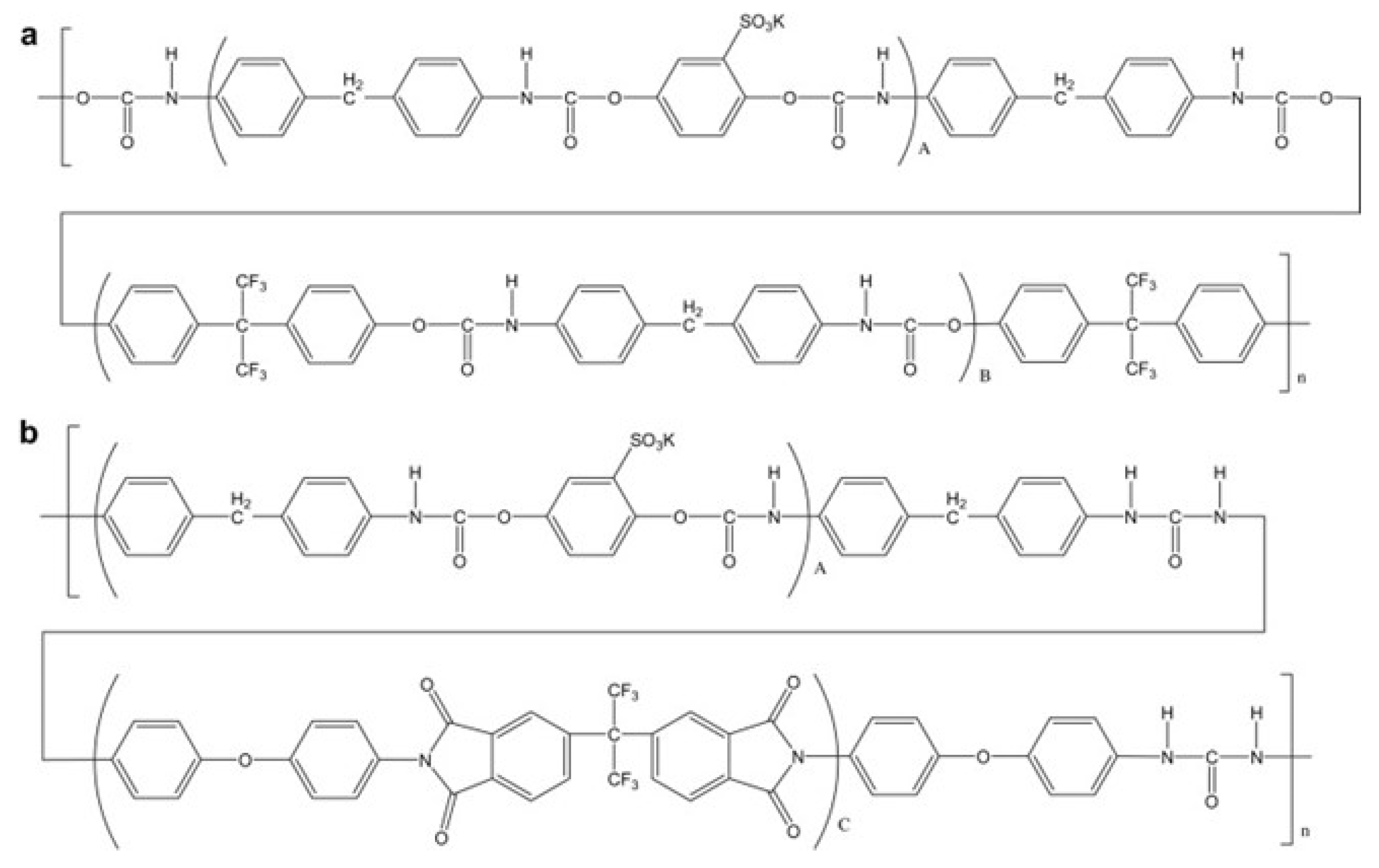
- Lee, H.S.; Roy, A.; Lane, O.; Lee, M.; McGrath, J.E. Synthesis and characterization of multiblock copolymers based on hydrophilic disulfonated poly(arylene ether sulfone) and hydrophobic partially fluorinated poly(arylene ether ketone) for fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 214–222. [Google Scholar] [CrossRef]
- Wang, S.; McGrath, J.E. Synthesis of Poly(Arylene Ether)s: Synthetic Methods in Step-Growth Polymers; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Mikami, T.; Miyatake, K.; Watanabe, M. Synthesis and properties of multiblock copoly(arylene ether)s containing superacid groups for fuel cell membranes. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 452–464. [Google Scholar] [CrossRef]
- Badami, A.S.; Roy, A.; Lee, H.S.; Li, Y.; McGrath, J.E. Morphological investigations of disulfonated poly(arylene ether sulfone)-b-naphthalene dianhydride-based polyimide multiblock copolymers as potential high temperature proton exchange membranes. J. Membr. Sci. 2009, 328, 156–164. [Google Scholar] [CrossRef]
- Lee, H.S.; Badami, A.S.; Roy, A.; McGrath, J.E. Segmented Sulfonated Poly(arylene ether sulfone)-b-polyimide Copolymers for Proton Exchange Membrane Fuel Cells. I. Copolymer Synthesis and Fundamental Properties. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4879–4890. [Google Scholar] [CrossRef]
- Lee, H.S.; Roy, A.; Lane, O.; McGrath, J.E. Synthesis and Characterization of Poly(arylene ether sulfone)-b-polybenzimidazole Copolymers for High Temperature Low Humidity Proton Exchange Membrane Fuel Cells. Polymer 2008, 49, 5387–5396. [Google Scholar] [CrossRef]
- Ng, F.; Bae, B.; Miyatake, K.; Watanabe, M. Polybenzimidazole block sulfonated poly(arylene ether sulfone) ionomers. Chem. Commun. 2011, 47, 8895–8897. [Google Scholar] [CrossRef]
- Wang, H.; Badami, A.S.; Roy, A.; McGrath, J.E. Multiblock copolymers of poly(2,5-benzophenone) and disulfonated poly(arylene ether sulfone) for proton-exchange membranes. I. Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 284–294. [Google Scholar] [CrossRef]
- Matsuno, S.; Watanabe, M.; Miyatake, K. Sulfonated polybenzophenone/poly(arylene ether) block copolymer membranes for fuel cell applications. ACS Appl. Mater. Interfaces 2012, 4, 2881–2884. [Google Scholar]
- Takamuku, S.; Jannasch, P. Multiblock Copolymers Containing Highly Sulfonated Poly(arylene sulfone) Blocks for Proton Conducting Electrolyte Membranes. Macromolecules 2012, 45, 6538–6546. [Google Scholar] [CrossRef]
- Titvinidze, G.; Kreuer, K.D.; Schuster, M.; de Araujo, C.C.; Melchior, J.P.; Meyer, W.H. Proton Conducting Phase-Separated Multiblock Copolymers with Sulfonated Poly(phenylene sulfone) Blocks for Electrochemical Applications: Preparation, Morphology, Hydration Behavior, and Transport. Adv. Funct. Mater. 2012, 22, 4456–4470. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Li, L.; Yu, Y.; Song, Y.; Liu, B.; Jiang, Z. Novel postsulfonated poly(ether ether ketone)-block-poly(ether sulfone)s as proton exchange membranes for fuel cells: Design, preparation and properties. J. Membr. Sci. 2011, 380, 171–180. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, J. Synthesis and properties of poly(ether ether ketone)-block-sulfonated polybutadiene copolymers for PEM applications. Eur. Polym. J. 2010, 46, 592–601. [Google Scholar] [CrossRef]
- Nacher, A.; Escribano, P.; Del Rio, C.; Rodriguez, A.; Acosta, J.L. Polymer proton-conduction systems based on commercial polymers. I. Synthesis and characterization of hydrogenated styrene–butadiene block copolymer and isobutylene isoprene rubber systems. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2809–2815. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Jung, B. Proton conductivities and methanol permeabilities of membranes made from partially sulfonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene copolymers. J. Membr. Sci. 2002, 207, 129–137. [Google Scholar] [CrossRef]
- Elabd, Y.A.; Napadensky, E.; Sloan, J.M.; Crawford, D.M.; Walker, C.W. Triblock copolymer ionomer membranes: Part I. Methanol and proton transport. J. Membr. Sci. 2003, 217, 227–242. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, J.U.; Jo, W.H. Synthesis of fluorinated amphiphilic triblock copolymer and its application in high temperature PEMfuel cells. J. Mater. Chem. 2012, 22, 7187–7193. [Google Scholar] [CrossRef]
- Kim, W.; Jo, H. Synthesis of Nonfluorinated Amphiphilic Rod–Coil Block Copolymer and Its Application to Proton Exchange Membrane. Chem. Mater. 2010, 22, 3646–3652. [Google Scholar] [CrossRef]
- Vargantwar, P.H.; Brannock, M.C.; Smith, S.D.; Spontak, R.J. Midblock sulfonation of a model long-chain poly(p-tert-butylstyrene-b-styrene-b-p-tert-butylstyrene) triblock copolymer. J. Mater. Chem. 2012, 22, 25262–25271. [Google Scholar] [CrossRef]
- Lee, H.C.; Lim, H.; Su, W.F.; Chao, C.Y. Novel Sulfonated Block Copolymer Containing Pendant Alkylsulfonic Acids: Syntheses, Unique Morphologies, and Applications in Proton Exchange Membrane. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2325–2338. [Google Scholar] [CrossRef]
- Goswami, M.; Sumpter, B.G.; Huang, T.; Messman, J.M.; Gido, S.P.; Isaacs-Sodeye, A.I.; Mays, J.W. Tunable morphologies from charged block copolymers. Soft Matter 2010, 6, 6146–6154. [Google Scholar] [CrossRef]
- Perrin, R.; Elomaa, M.; Jannasch, P. Nanostructured Proton Conducting Polystyrene–Poly(vinylphosphonic acid) Block Copolymers Prepared via Sequential Anionic Polymerizations. Macromolecules 2009, 42, 5146–5154. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Holdcroft, S. Synthesis and Proton Conductivity of Partially Sulfonated Poly([vinylidene difluoride-co-hexafluoropropylene]-b-styrene) Block Copolymers. Macromolecules 2005, 38, 4193–4201. [Google Scholar] [CrossRef]
- Tsang, E.M.W.; Shi, Z.; Holdcroft, S. Ionic Purity and Connectivity of Proton-Conducting Channels in Fluorous-Ionic Diblock Copolymers. Macromolecules 2011, 44, 8845–8857. [Google Scholar] [CrossRef]
- Xu, K.; Li, K.; Khanchaitit, P.; Wang, Q. Synthesis and Characterization of Self-Assembled Sulfonated Poly(styrene-b-vinylidene fluoride-b-styrene) Triblock Copolymers for Proton Conductive Membranes. Chem. Mater. 2007, 19, 5937–5945. [Google Scholar] [CrossRef]
- Jankova, K.; Hvilsted, S. Novel fluorinated block copolymer architectures fuelled by atom transfer radical polymerization. J. Fluor. Chem. 2005, 126, 241–250. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ozaki, F.; Matsuoka, H. Synthesis of proton-conducting block copolymer membranes composed of a fluorinated segment and a sulfonic acid segment. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4479–4485. [Google Scholar] [CrossRef]
- Park, M.J.; Balsara, N.P. Phase Behavior of Symmetric Sulfonated Block Copolymers. Macromolecules 2008, 41, 3678–3687. [Google Scholar] [CrossRef]
- Chen, L.; Hallinan, D.T.; Elabd, Y.A.; Hillmyer, M.A. Highly selective polymer electrolyte membranes from reactive block polymers. Macromolecules 2009, 42, 6075–6085. [Google Scholar] [CrossRef]
- Markova, D.; Kumar, A.; Klapper, M.; Müllen, K. Phosphonic acid-containing homo-, AB and BAB block copolymers via ATRP designed for fuel cell applications. Polymer 2009, 50, 3411–3421. [Google Scholar] [CrossRef]
- Moore, H.D.; Saito, T.; Hickner, M.A. Morphology and transport properties of midblock-sulfonated triblock copolymers. J. Mater. Chem. 2010, 20, 6316–6321. [Google Scholar] [CrossRef]
- Rubatat, L.; Li, C.; Dietsch, H.; Nykaenen, A.; Ruokolainen, J.; Mezzenga, R. Structure-Properties Relationship in Proton Conductive Sulfonated Polystyrene-Polymethyl Methacrylate Block Copolymers (sPS-PMMA). Macromolecules 2008, 41, 8130–8137. [Google Scholar] [CrossRef]
- Yang, J.E.; Hong, Y.T.; Lee, J. Synthesis and Characterization of Polystyrene-Poly(arylene ether sulfone)-Polystyrene Triblock Copolymer for Proton Exchange Membrane Applications. Nanosci. Nanotechnol. 2006, 6, 3594–3598. [Google Scholar]
- Yang, Y.S.; Shi, Z.Q.; Holdcroft, S. Synthesis of Sulfonated Polysulfone-block-PVDF Copolymers: Enhancement of Proton Conductivity in Low Ion Exchange Capacity Membranes. Macromolecules 2004, 37, 1678–1681. [Google Scholar] [CrossRef]
- Persson, J.C.; Jannasch, P. Block Copolymers Containing Intrinsically Proton-Conducting Blocks Tethered with Benzimidazole Units. Chem. Mater. 2006, 18, 3096–3102. [Google Scholar] [CrossRef]
- Kumar, A.; Pisula, W.; Markova, D.; Klapper, M.; Müllen, K. Proton-Conducting Poly(phenylene oxide)–Poly(vinyl benzyl phosphonic acid) Block Copolymers via Atom Transfer Radical Polymerization. Macromol. Chem. Phys. 2012, 213, 489–499. [Google Scholar] [CrossRef]
- Zhang, Z.; Chalkova, E.; Fedkin, M.; Wang, C.; Lvov, S.N.; Komarneni, S.; Chung, M.T.C. Synthesis and Characterization of Poly(vinylidene fluoride)-g-sulfonated Polystyrene Graft Copolymers for Proton Exchange Membrane. Macromolecules 2008, 41, 9130–9139. [Google Scholar] [CrossRef]
- Balog, S.; Gasser, U.; Mortensen, K.; Ben Youcef, H.; Gubler, L.; Scherer, G.G. Structure of the ion-rich phase in DVB cross-linked graft-copolymer proton-exchange membranes. Polymer 2012, 53, 175–182. [Google Scholar] [CrossRef]
- Souzy, R.; Boutevin, B.; Ameduri, B. Synthesis and Characterizations of Novel Proton-Conducting Fluoropolymer Electrolyte Membranes Based on Poly(vinylidene fluoride-ter-hexafluoropropylene-ter-α-trifluoromethacrylic acid) Terpolymers Grafted by Aryl Sulfonic Acids. Macromolecules 2012, 45, 3145–3160. [Google Scholar] [CrossRef]
- Osano, K.; Force, L.; Turner, S.R. Synthesis and Properties of Linear Poly(ether sulfone)s with Hyperbranched Terminal Groups. Ind. Eng. Chem. Res. 2010, 49, 12098–12103. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hagashihara, T.; Ueda, M. Star-Shaped Sulfonated Block Copoly(ether ketone)s as Proton Exchange Membranes. Macromolecules 2008, 41, 7560–7565. [Google Scholar] [CrossRef]
- Choi, J.K.; Lee, D.K.; Kim, Y.W.; Min, B.R.; Kim, J.H. Composite polymer electrolyte membranes comprising triblock copolymer and heteropolyacid for fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 691–701. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of proton conductance in polymer electrolyte membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K.S. Fuel Cell Membrane Characterizations. Polym. Rev. 2015, 55, 330–370. [Google Scholar] [CrossRef]
- Pivovar, B.S.; Wang, Y.; Cussler, E.L. Pervaporation membranes in direct methanol fuel cells. J. Membr. Sci. 1999, 154, 155–162. [Google Scholar] [CrossRef]
- Weng, D.; Wainright, J.S.; Landau, U.; Savinell, R.F. Electroosmotic drag coefficient of water and methanol in polymer electrolytes at elevated temperatures. J. Electrochem. Soc. 1996, 143, 1260–1263. [Google Scholar] [CrossRef]
- Hickner, M.A. Transport and Structure in Fuel Cell Proton Exchange Membranes. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2003. Available online: https://vtechworks.lib.vt.edu/server/api/core/bitstreams/0887c64b-e29c-458c-9d92-789de1a1b06c/content (accessed on 28 April 2024).
- Hallinan, D.T.; Elabd, Y.A. Diffusion and sorption of methanol and water in Nafion using time-resolved Fourier transform infrared-attenuated total reflectance spectroscopy. J. Phys. Chem. B 2007, 111, 13221–13230. [Google Scholar] [CrossRef] [PubMed]
- Gassemi, H.; McGrath, J.E.; Zawodzinski, T.A., Jr. Multiblock sulfonated–fluorinated poly(arylene ether)s for a proton exchange membrane fuel cell. Polymer 2006, 47, 4132–4139. [Google Scholar] [CrossRef]
- Fan, Y.; Cornelius, C.J.; Lee, H.S.; McGrath, J.E.; Zhang, M.; Moore, R.; Staiger, C.L. The effect of block length upon structure, physical properties, and transport within a series of sulfonated poly(arylene ether sulfone)s. J. Membr. Sci. 2013, 430, 106–112. [Google Scholar] [CrossRef]
- Chen, L. Synthesis and Characterization of Hydrophobic-Hydrophilic Multiblock Copolymers for Proton Exchange Membrane Applications. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2011. Available online: https://vtechworks.lib.vt.edu/server/api/core/bitstreams/c7786bfc-c130-45a5-a3f8-89b33957d5d9/content (accessed on 28 April 2024).
- Wang, S.J.; Luo, J.J.; Xiao, M.; Han, D.M.; Shen, P.K.; Meng, Y.Z. Design, synthesis and properties of polyaromatics with hydrophobic and hydrophilic long blocks as proton exchange membrane for PEM fuel cell application. Int. J. Hydrogen Energy 2012, 37, 4545–4552. [Google Scholar] [CrossRef]
- Sung, K.A.; Kim, W.K.; Oh, K.H.; Choo, M.J.; Nam, K.W.; Park, J.K. Proton exchange membranes based on hydrophilic–hydrophobic multiblock copolymers using different hydrophobic oligomer. Int. J. Hydrogen Energy 2011, 36, 3956–3964. [Google Scholar] [CrossRef]
- St. Clair, T.L. Structure–Property Relationships in Linear Aromatic Polyimides; Wilson, D., Stenzenberger, H.D., Hergenrother, P.M., Eds.; Polyimides, Chapman and Hall Inc.: New York, NY, USA, 1990; pp. 58–78. [Google Scholar]
- Nakabayashi, K.; Matsumoto, K.; Higashihara, T.; Ueda, M. Influence of adjusted hydrophilic–hydrophobic lengths in sulfonated multiblock copoly(ether sulfone) membranes for fuel cell application. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7332–7341. [Google Scholar] [CrossRef]
- Cornet, N.; Beaudoing, G.; Gebel, G. Influence of the structure of sulfonated polyimide membranes on transport properties. Sep. Purif. Technol. 2001, 22–23, 681–687. [Google Scholar] [CrossRef]
- Cornet, N.; Diat, O.; Gebel, G.; Jousse, F.; Marsacq, D.; Mercier, R.; Piner, M. Sulfonated polyimide membranes: A new type of ion-conducting membrane for electrochemical applications. J. New Mat. Electrochem. Syst. 2000, 3, 33–42. [Google Scholar]
- Nakano, T.; Nagaoka, S.; Kawakami, H. Preparation of novel sulfonated block copolyimides for proton conductivity membranes. Polym. Adv. Technol. 2005, 16, 753–757. [Google Scholar] [CrossRef]
- Wang, H.; Holmberg, B.A.; Huang, L.; Wang, Z.; Mitra, A.; Norbeck, J.M.; Yan, Y. Nafion-bifunctional silica compositeproton conductive membranes. J. Mater. Chem. 2002, 12, 834–837. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Kaliaguine, S. Sulfonated poly(aryl ether ketone)s containing thehexafluoroisopropylidene diphenyl moiety prepared bydirect copolymerization, as proton exchange membranes for fuel fell. Macromolecules 2004, 37, 7960–7967. [Google Scholar] [CrossRef]
- Savett, S.C.; Atkins, J.R.; Sides, C.R.; Harris, J.L.; Thomas, B.H.; Creager, S.E.; Pennington, W.T.; DesMarteau, D.D. A comparison of bis perfluoroalkyl sulfonyl imide ionomers and perfluorosulfonic acid ionomers for applications in PEM fuel-cell technology. J. Electrochem. Soc. 2002, 149, 1527–1532. [Google Scholar] [CrossRef]
- Belkhiri, Z.; Zeroual, M.; Ben Moussa, H.; Zitouni, B. Effect of temperature and water content on the performance of PEM fuel cell. Rev. Energies Renouvelables 2011, 14, 121–130. [Google Scholar]
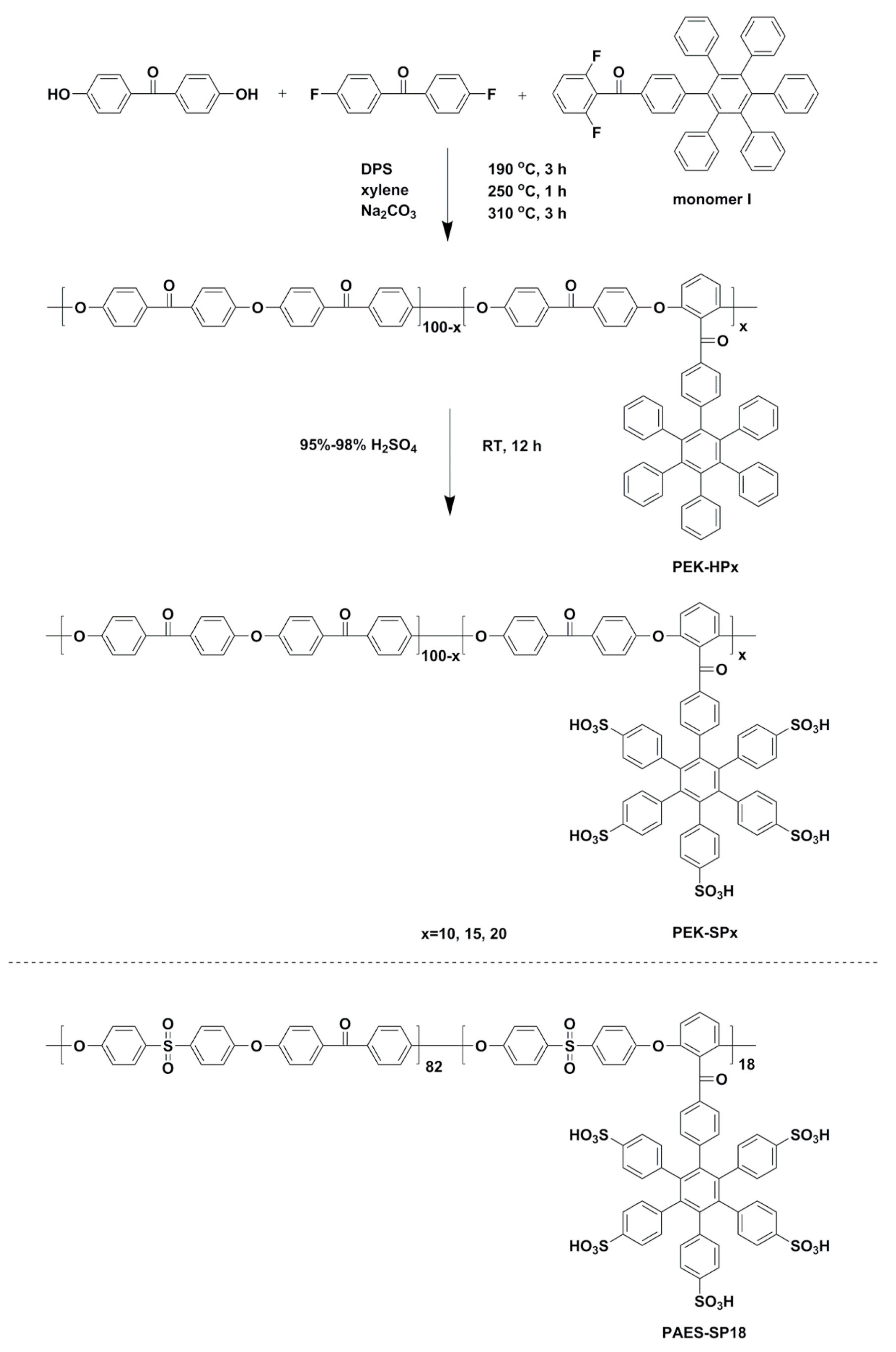
- Liu, D.; Dong, B.; Zhang, H.; Xie, J.; Pang, J.; Jiang, Z. High methanol resistant polyelectrolyte membrane based on semi-crystalline Poly(ether ketone) with densely sulfonated side chain for direct methanol fuel cell. J. Power Sources 2021, 482, 228982. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Yaguchi, K.; Endo, N.; Higa, M.; Okamoto, K. Synthesis and properties of sulfonated multiblock copolynaphthalimides. Polymer 2009, 50, 2933–2943. [Google Scholar] [CrossRef]
- Hou, H.; DiVona, M.; Knauth, P. Building bridges: Crosslinking of sulfonated aromatic polymers—A review. J. Membr. Sci. 2012, 423–424, 113–127. [Google Scholar] [CrossRef]
- Kerres, J.; Zhang, W.; Hearing, T. Covalently cross-linked ionomer (blend) membranes for fuel cells. J. New Mater. Electrochem. Syst. 2004, 7, 299–309. [Google Scholar]
- Fang, J.; Zhai, F.; Guo, X.; Xu, H.; Okamoto, K. A facile approach for the preparation of cross-linked sulfonated polyimide membranes for fuel cell application. J. Mater. Chem. 2007, 17, 1102–1108. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Properties of PEMs based on cross-linked sulfonated poly(ether ether ketone). J. Membr. Sci. 2006, 285, 306–316. [Google Scholar] [CrossRef]
- Gu, S.; He, G.; Wu, X.; Guo, Y.; Liu, H.; Peng, L.; Xiao, G. Preparation and characteristics of crosslinked sulfonated poly(phthalazinone ether sulfone ketone) with poly(vinyl alcohol) for proton exchange membrane. J. Membr. Sci. 2008, 312, 48–58. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Zhao, C.; Na, H. Crosslinked sulfonated poly(ether ether ketone) proton exchange membranes for direct methanol fuel cell applications. J. Power Sources 2007, 164, 65–72. [Google Scholar] [CrossRef]
- Ding, F.C.; Wang, S.J.; Xiao, M.; Li, X.H.; Meng, Y.Z. Fabrication and properties of cross-linked sulfonated fluorene-containing poly(arylene ether ketone) for proton exchange membrane. J. Power Sources 2007, 170, 20–27. [Google Scholar] [CrossRef]
- Feng, S.; Shang, Y.; Xie, X.; Wang, Y.; Xu, J. Synthesis and characterization of crosslinked sulfonated poly(arylene ether sulfone) membranes for DMFC applications. J. Membr. Sci. 2009, 335, 13–20. [Google Scholar] [CrossRef]
- Kerres, J.; Ullrich, A.; Haring, T.; Baldauf, M.; Gebhardt, U.; Preidel, W. Preparation, characterization and fuel cell application of new acid-base blend membranes. J. New Mater. Electrochem. Syst. 2000, 3, 229–239. [Google Scholar]
- Sen, U.; Bozkurt, A.; Ata, A. Nafion/poly(1-vinyl-1,2,4-triazole) blends as proton conducting membranes for polymer electrolyte membrane fuel cells. J. Power Sources 2010, 195, 7720–7726. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Ma, W.; Zhao, C.; Zhang, Y.; Han, M.; Zhu, J.; Liu, Z.; Wu, J.; Na, H. Composite membranes based on a novel benzimidazole grafted PEEK and SPEEK for fuel cells. Int. J. Hydrogen Energy 2010, 35, 11172–11179. [Google Scholar]
- Lin, H.D.; Zhao, C.J.; Ma, W.J.; Li, H.T.; Na, H. Layer-by-layer self-assembly of in situ polymerized polypyrrole on sulfonated poly(arylene ether ketone) membrane with extremely low methanol crossover. Int. J. Hydrogen Energy 2009, 34, 9795–9801. [Google Scholar] [CrossRef]
- Yang, M.; Lu, S.; Lu, J.; Jiang, S.P.; Xiang, Y. Layer-by-layer self-assembly of PDDA/PWA-Nafion composite membranes for direct methanol fuel cells. Chem. Commun. 2010, 46, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Kharlampieva, E.; Kozlovskaya, V.; Sukhishvili, S.A. Layer-bylayer hydrogen-bonded polymer films: From fundamentals to applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- Taylor, M.M.A.; Sekol, R.; Podsiadlo, P.; Ho, P.; Kotov, N.; Thompson, L. High-performance nanostructured membrane electrode assemblies for fuel cells made by layer-by-layer assembly of carbon nanocolloids. Adv. Mater. 2007, 19, 3859–3864. [Google Scholar]
- Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-662-44324-8_1833 (accessed on 16 August 2024).
- Chu, Z.; Zhu, M.; Zhang, W.; Zhao, Y.; Gong, X.; Jiang, Y.; Wu, L.; Zhai, R.; Dai, X.; Fang, X. Layer-by-layer coating and chemical cross-linking of multilayer polysaccharides on silica for mixed-mode HPLC application. Chem. Commun. 2021, 57, 12956–12959. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, R.L.; Won, M.; Kim, T.H. Terminally-crosslinked poly(ether sulfone) block copolymer, as a highly selective and stable polymer electrolyte membrane for DMFC applications. Int. J. Hydrogen Energy 2013, 38, 5084–5091. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Hwang, J.M.; Kim, T.H. Azide-assisted terminal crosslinking of ionomeric blocks: Effects on morphology and proton conductivity. J. Membr. Sci. 2012, 392–393, 58–65. [Google Scholar] [CrossRef]
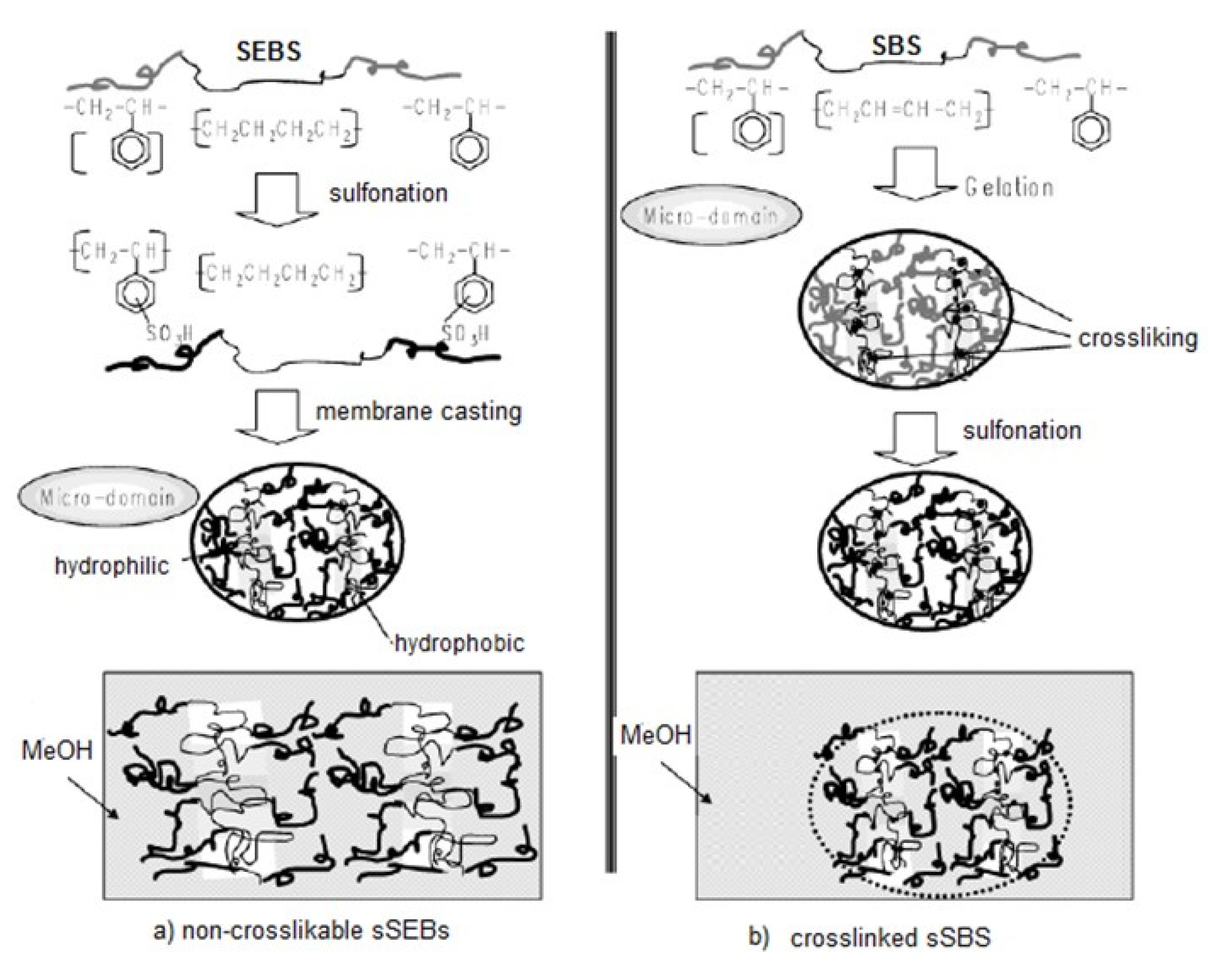
- Roh, D.K.; Koh, J.K.; Chi, W.S.; Shul, Y.G.; Kim, J.H. Proton conducting crosslinked polymer electrolyte membranes based on SBS block copolymer. JAPS 2011, 121, 3283–3291. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Zhang, S.; Chen, S.; Chen, S.; Liu, J.; Wang, L. Preparation and properties of crosslinked multiblock sulfonated poly(arylene ether sulfone) membranes for fuel cell applications. JAPS 2011, 121, 1707–1716. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Ahn, D.; Park, H.W.; Chang, T.; Lee, W. Direct sulfonation and photocrosslinking of unsaturated poly(styrene-b-butadiene-b-styrene) for proton exchange membrane of direct methanol fuel cell. J. Membr. Sci. 2013, 427, 85–91. [Google Scholar] [CrossRef]
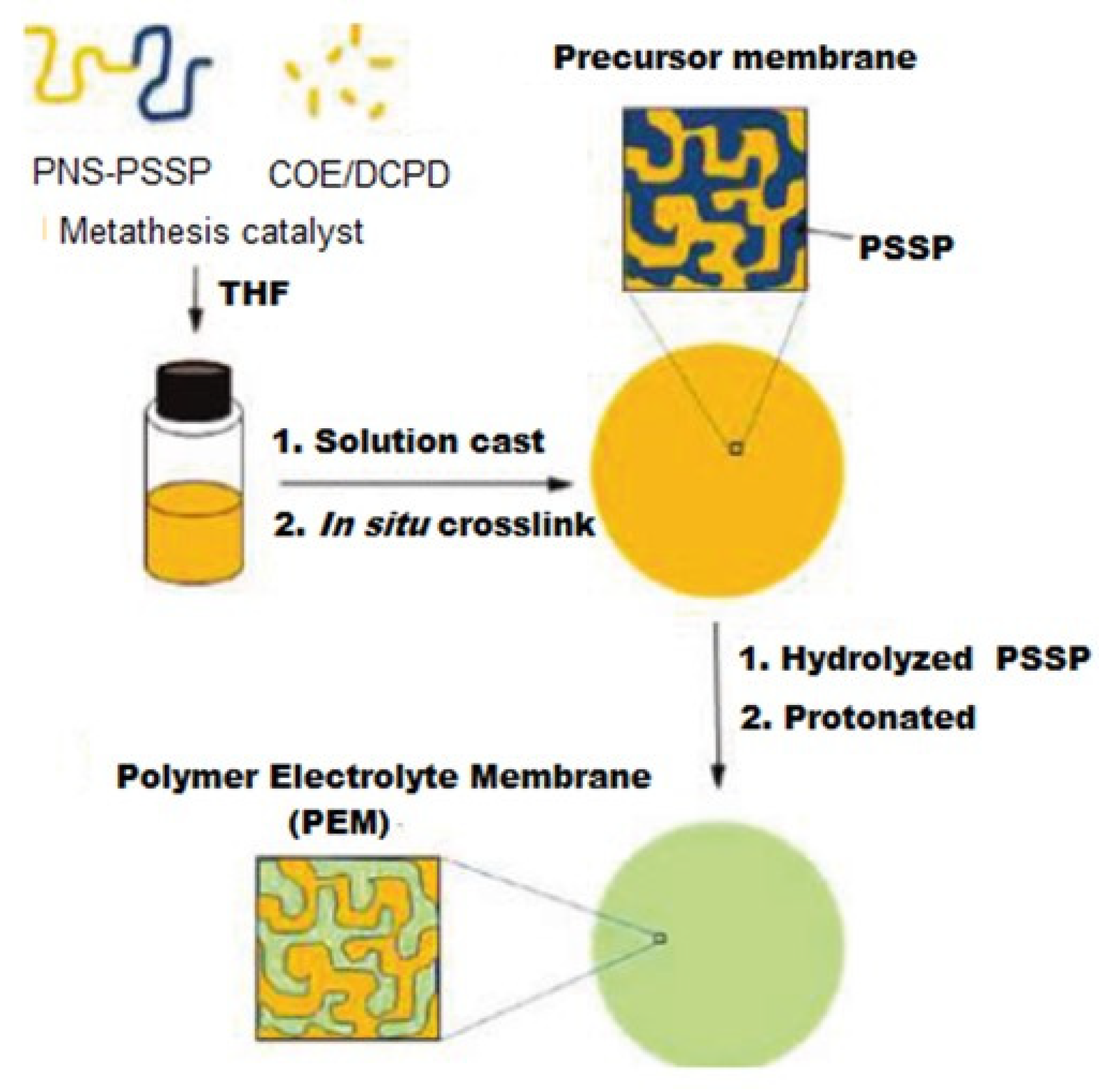
- Julius, D.; Lee, Y.Y.; Hong, L. Hydrophilic Cross-Linked Aliphatic Hydrocarbon Diblock Copolymer as Proton Exchange Membrane for Fuel Cells. Materials 2021, 14, 1617. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, G.; Shao, K.; Li, H.; Zhang, Y.; Li, M.; Wang, S.; Na, H. Carboxyl-terminated benzimidazole-assisted cross-linked sulfonated poly(ether ether ketone)s for highly conductive PEM with low water uptake and methanol permeability. J. Mater. Chem. 2010, 20, 3246–3252. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Li, M.; Wang, S.; Zhang, Y.; Li, H.; Lew, C.M.; Na, H. Considerations of the morphology in the design of proton exchange membranes: Cross-linked sulfonated poly(ether ether ketone)s using a new carboxyl-terminated benzimidazole as the cross-linker for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 2197–2206. [Google Scholar] [CrossRef]
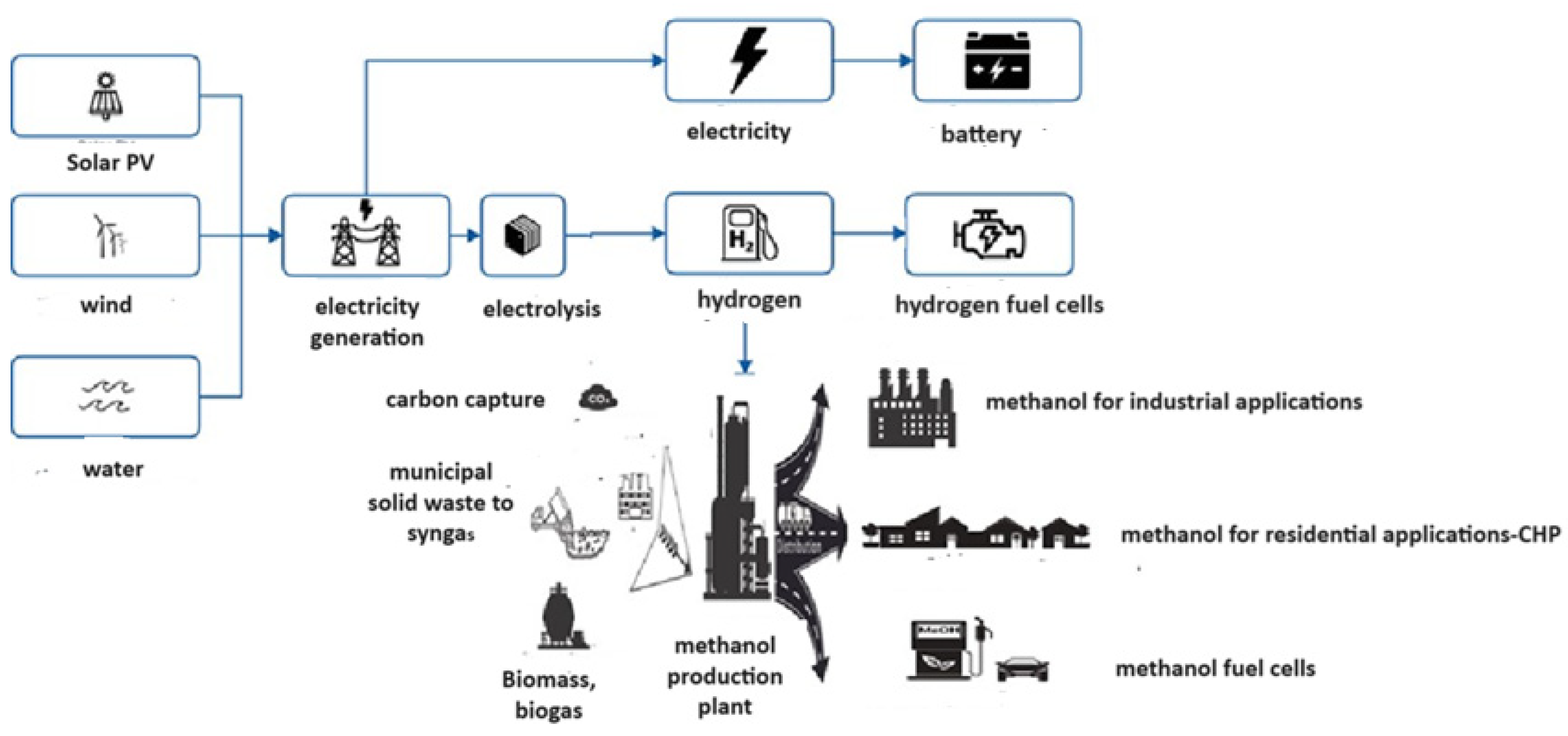



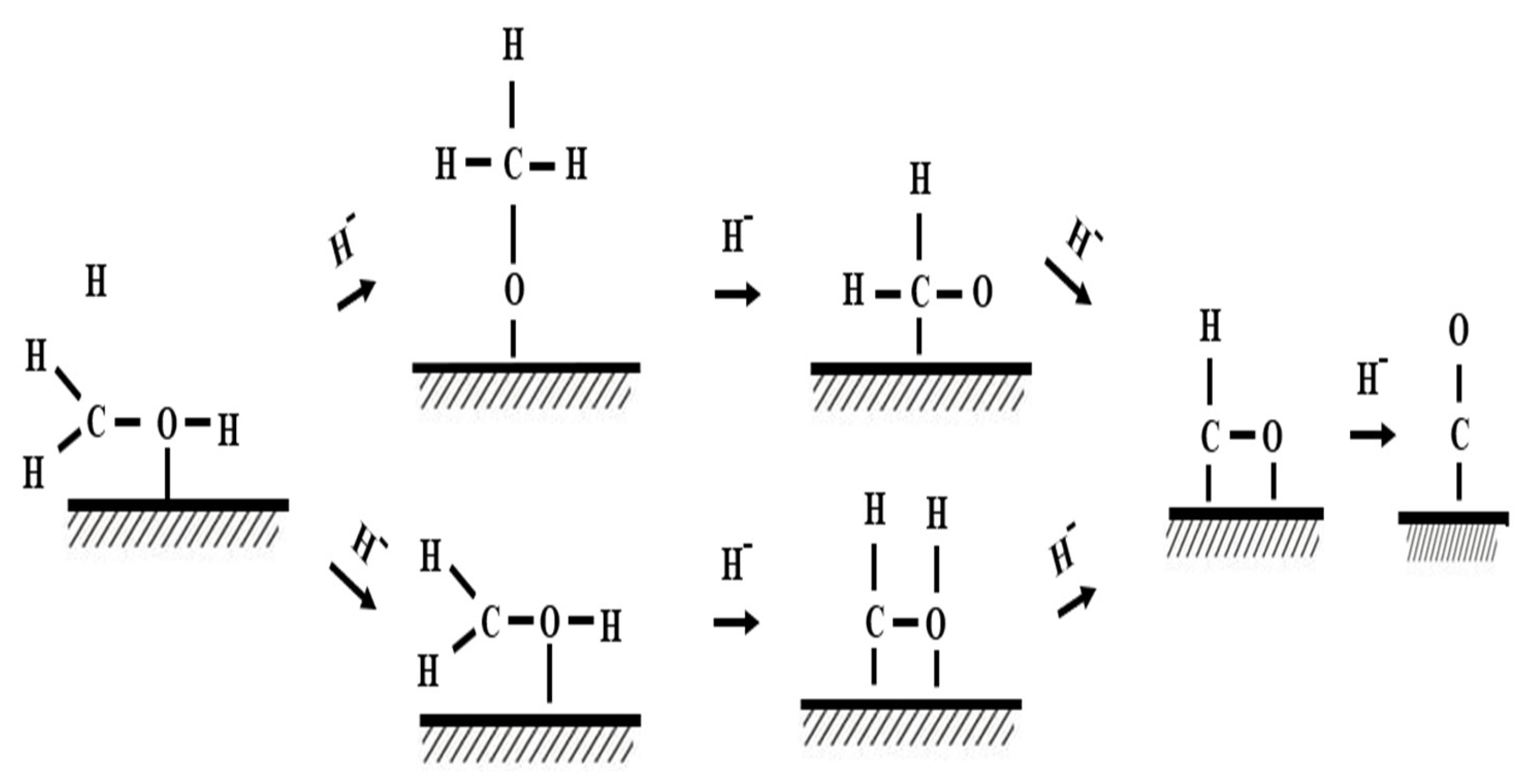
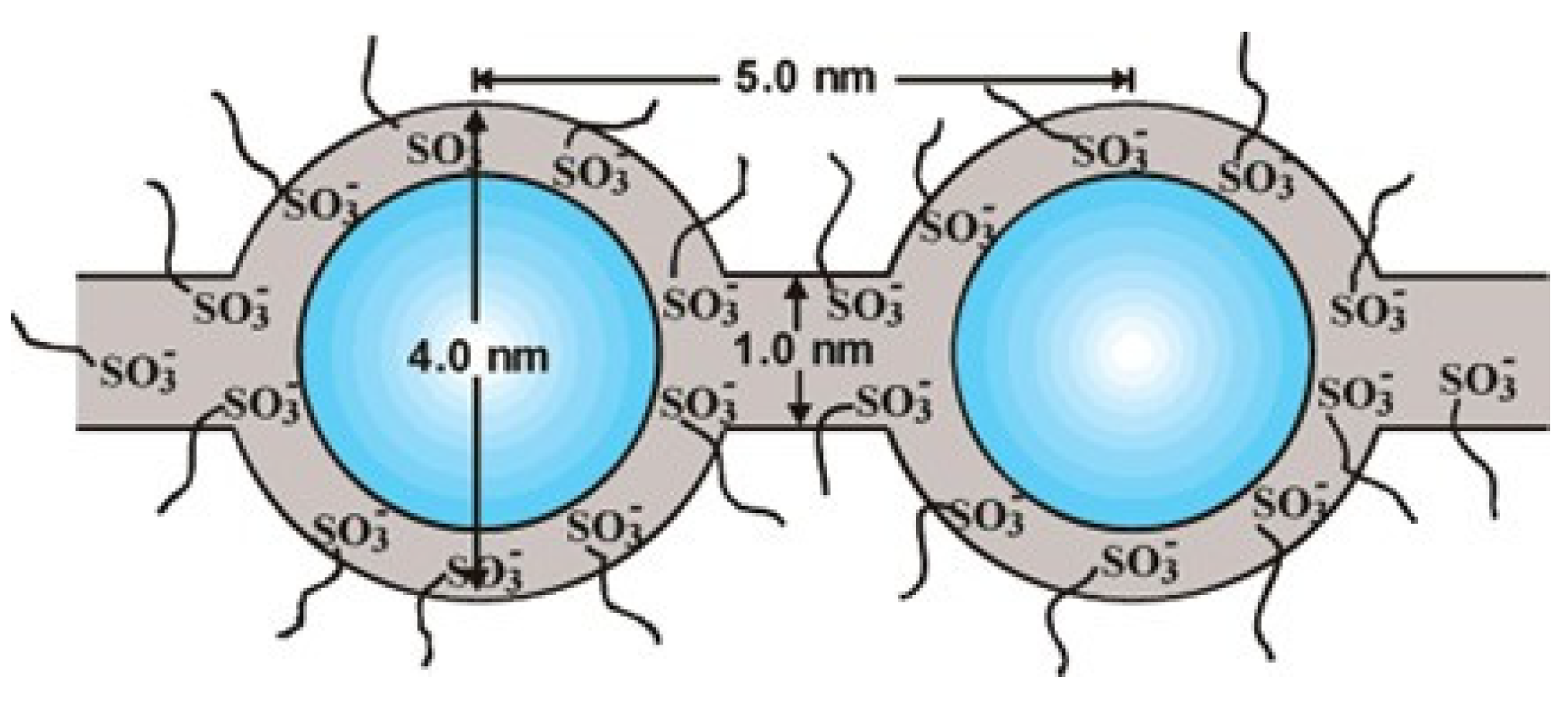
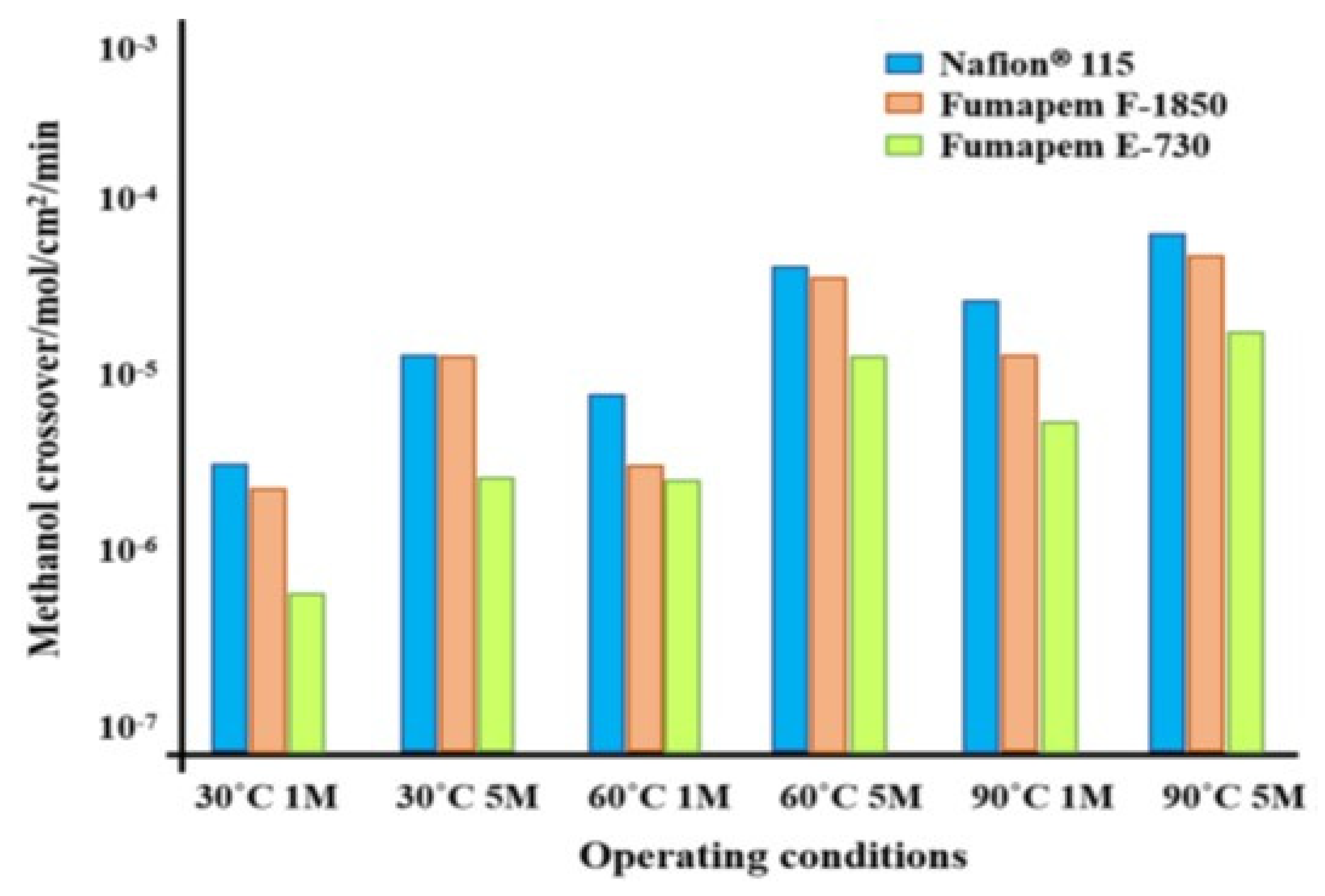
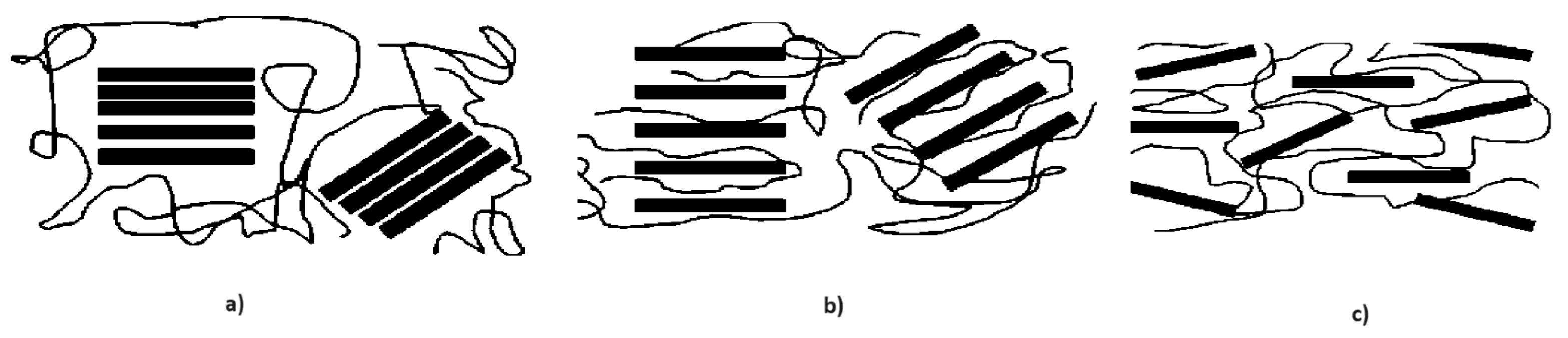
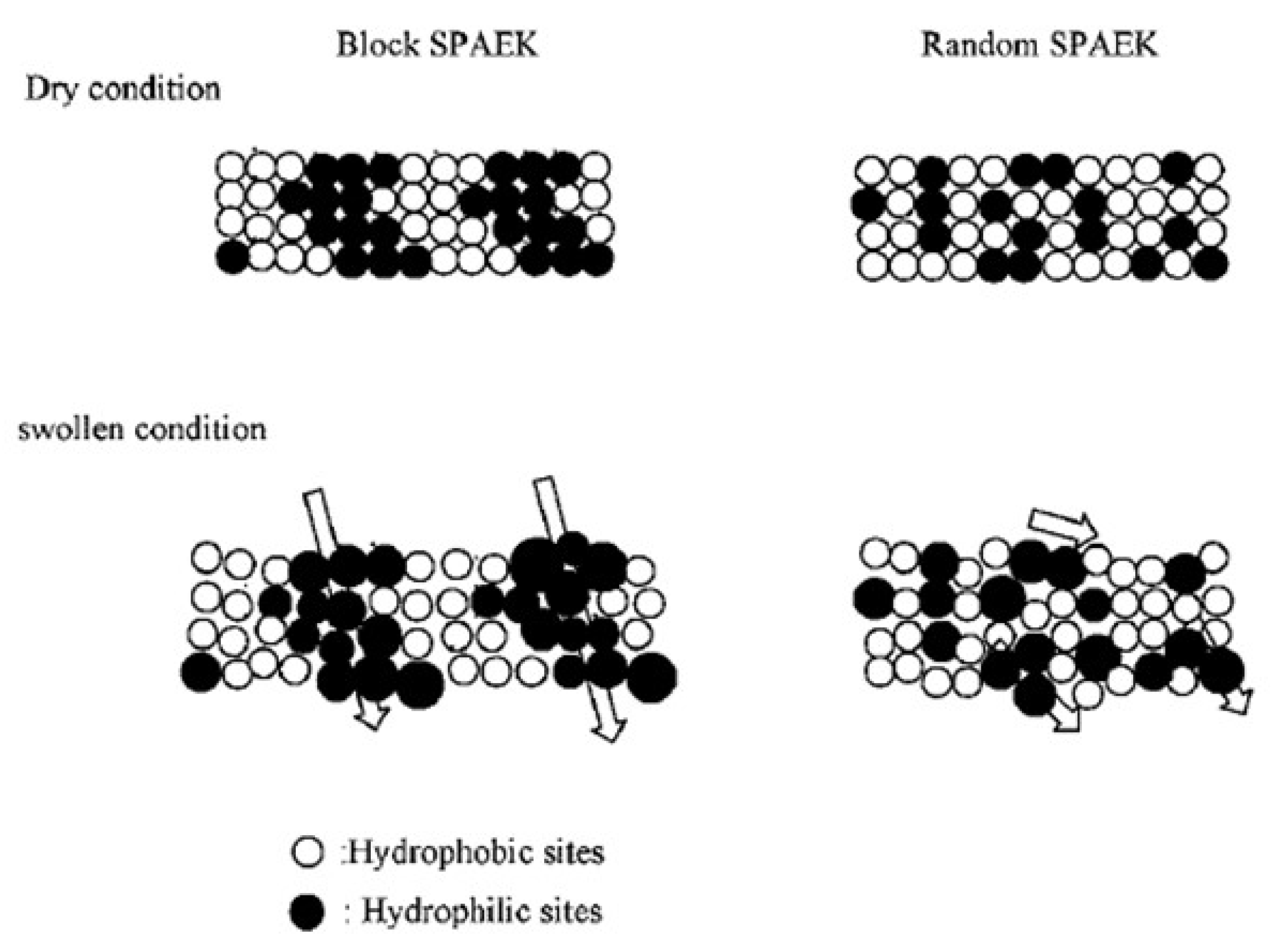
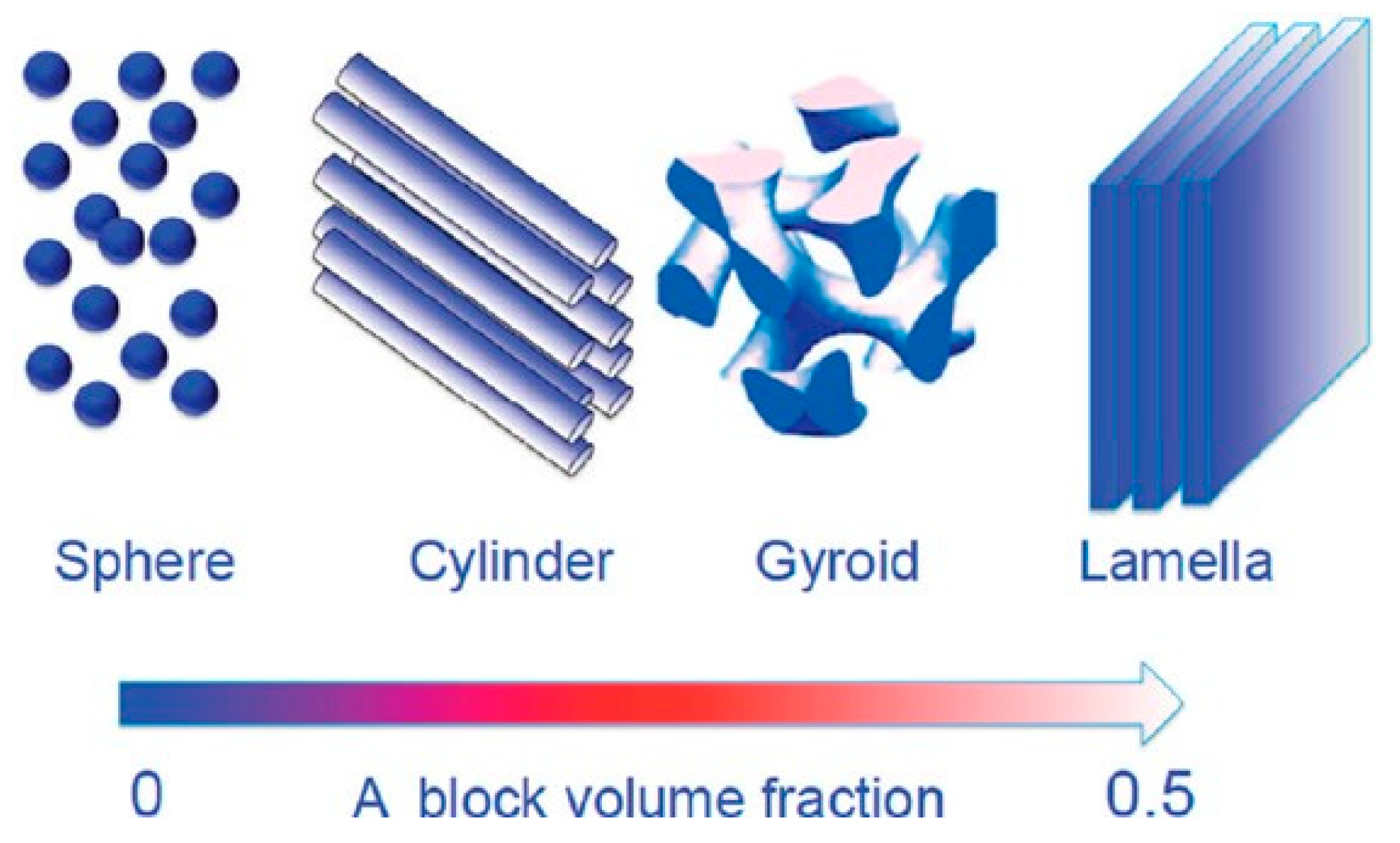
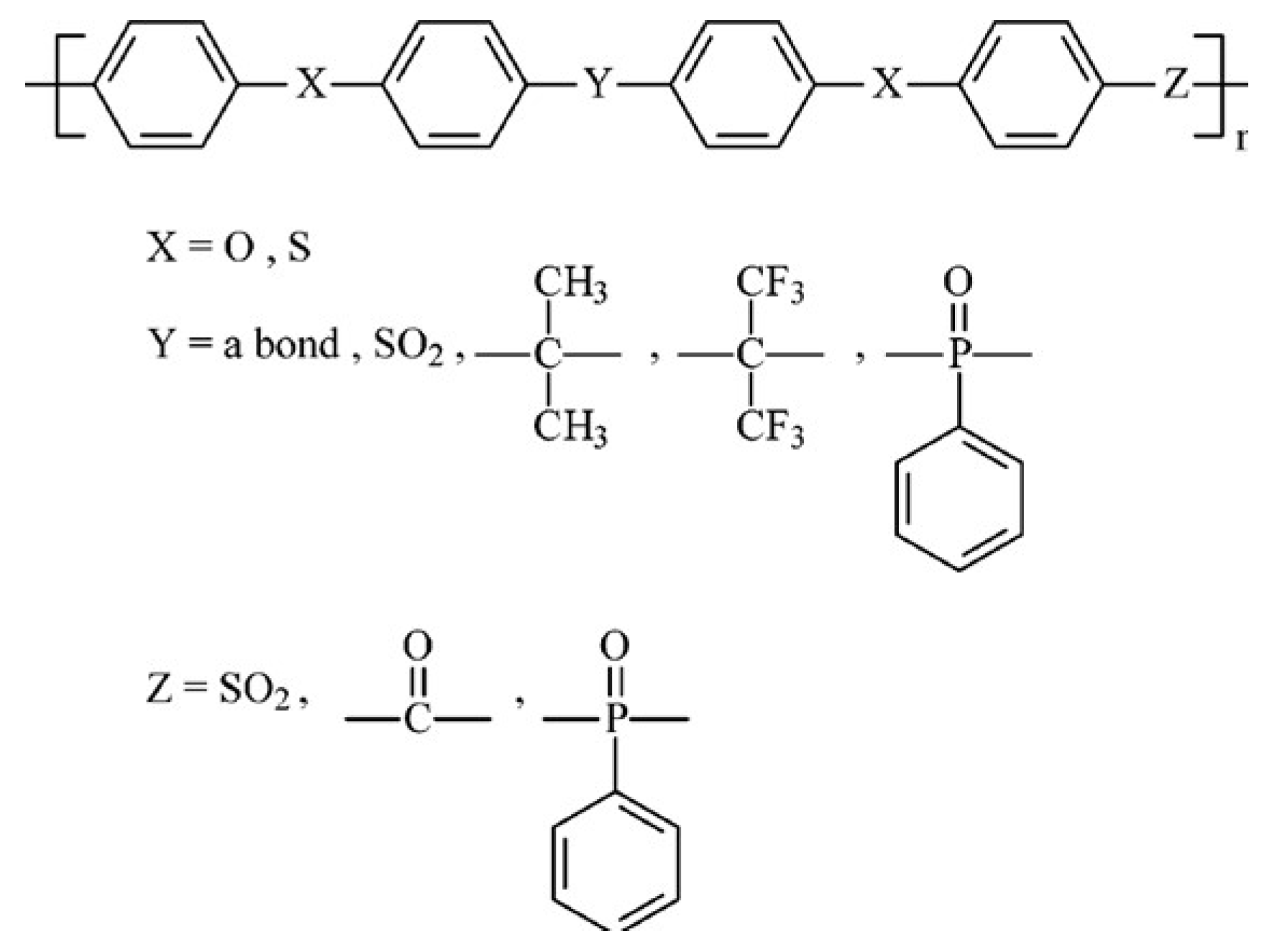



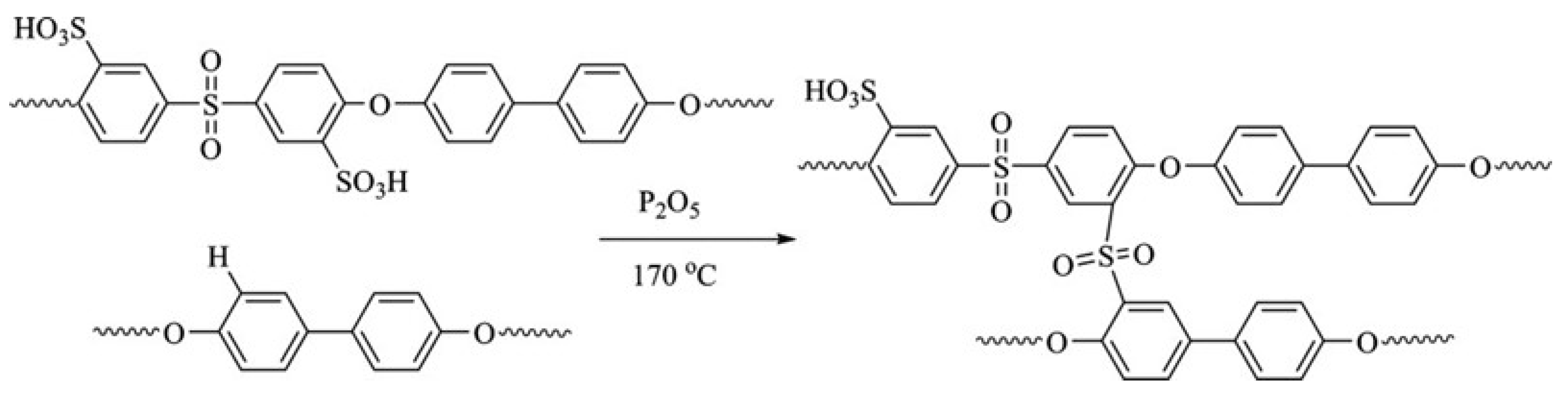

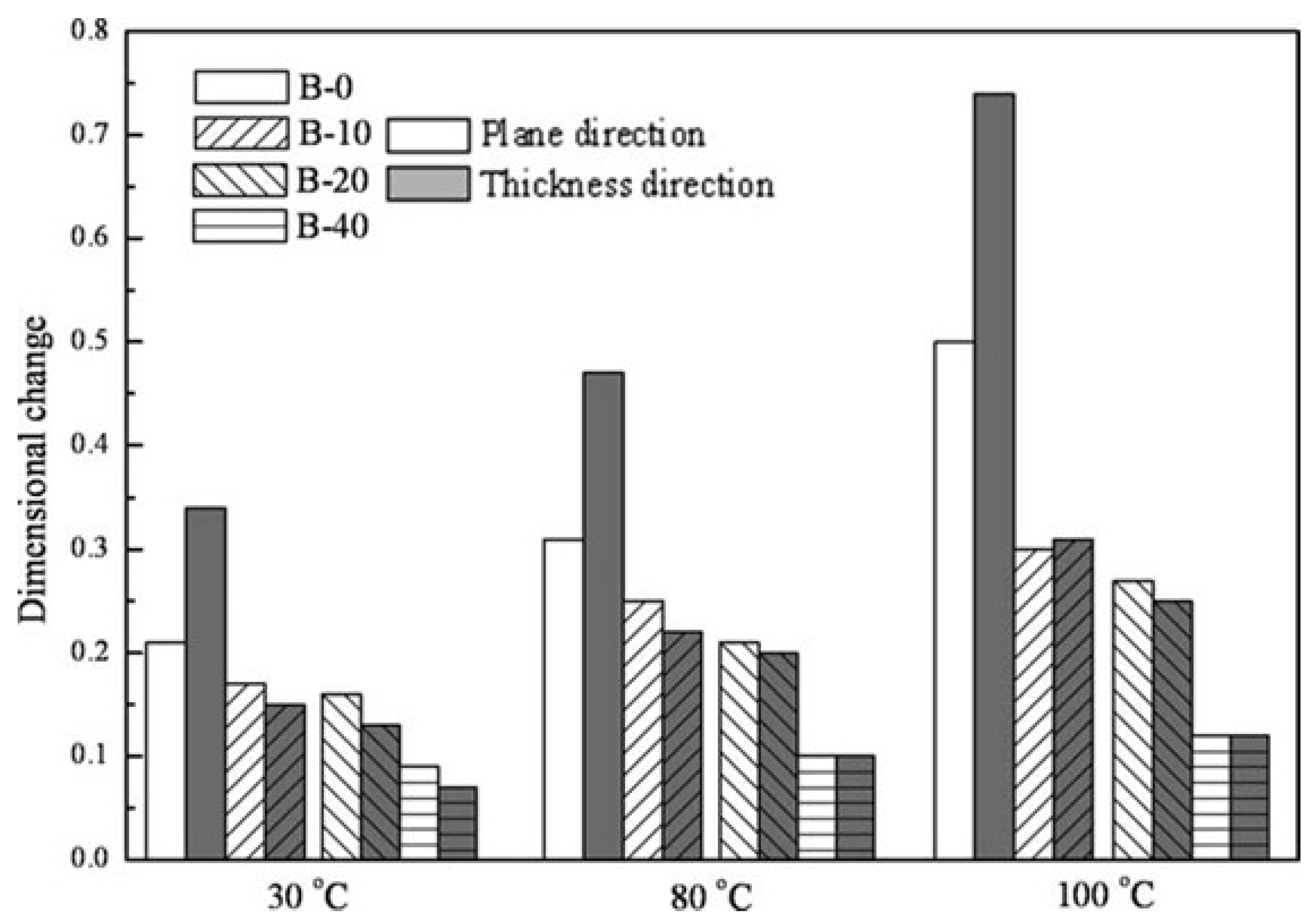
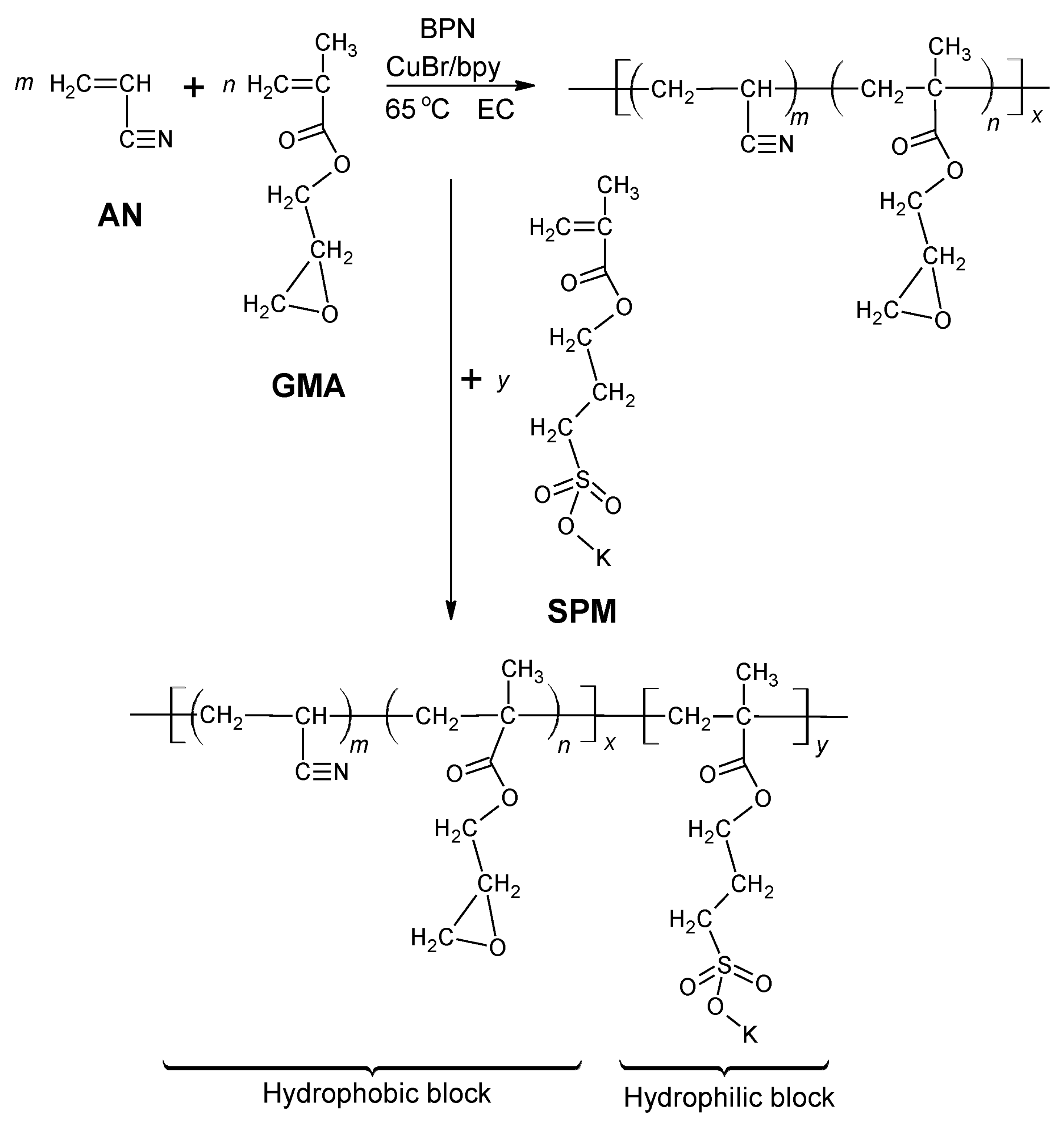
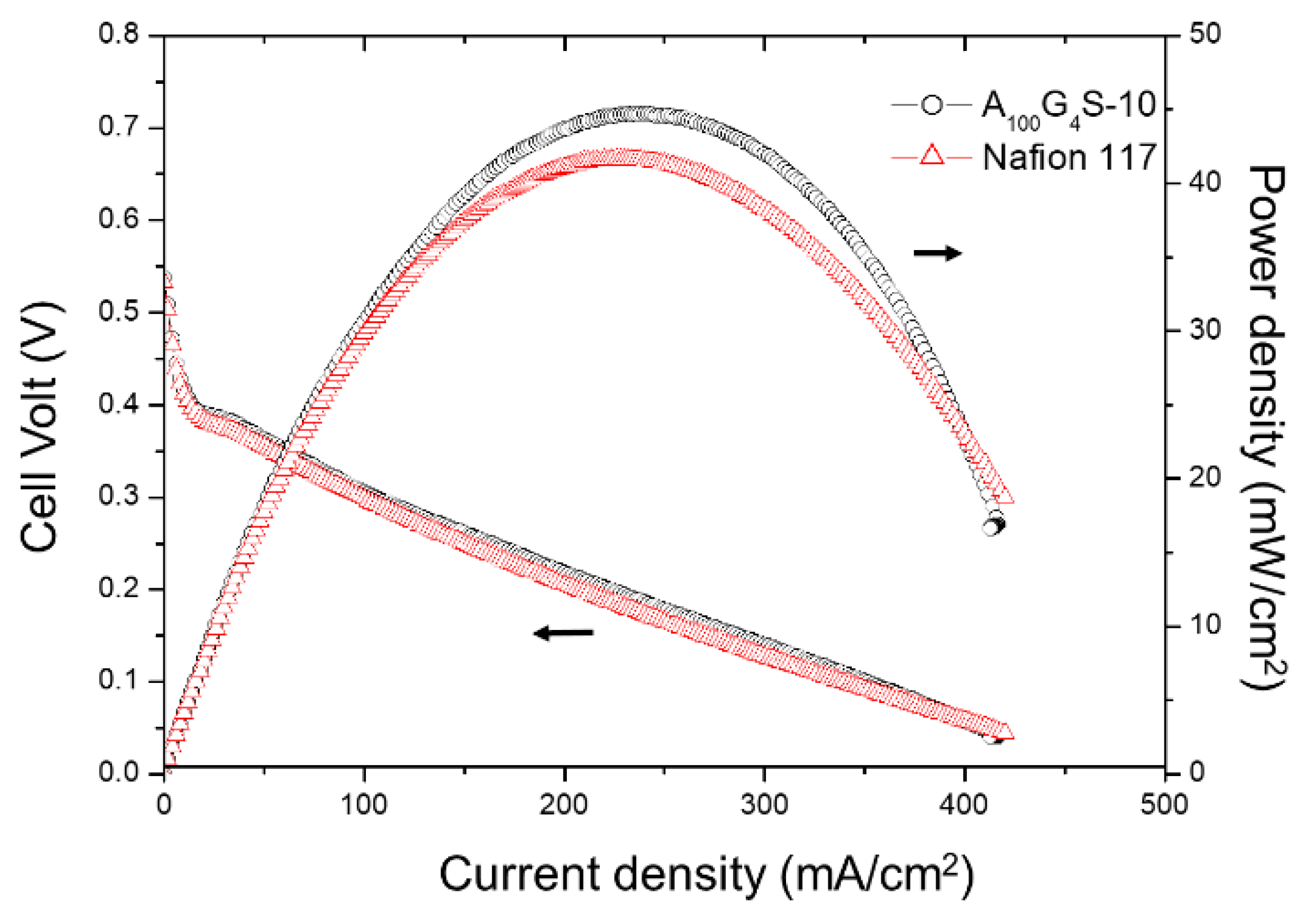
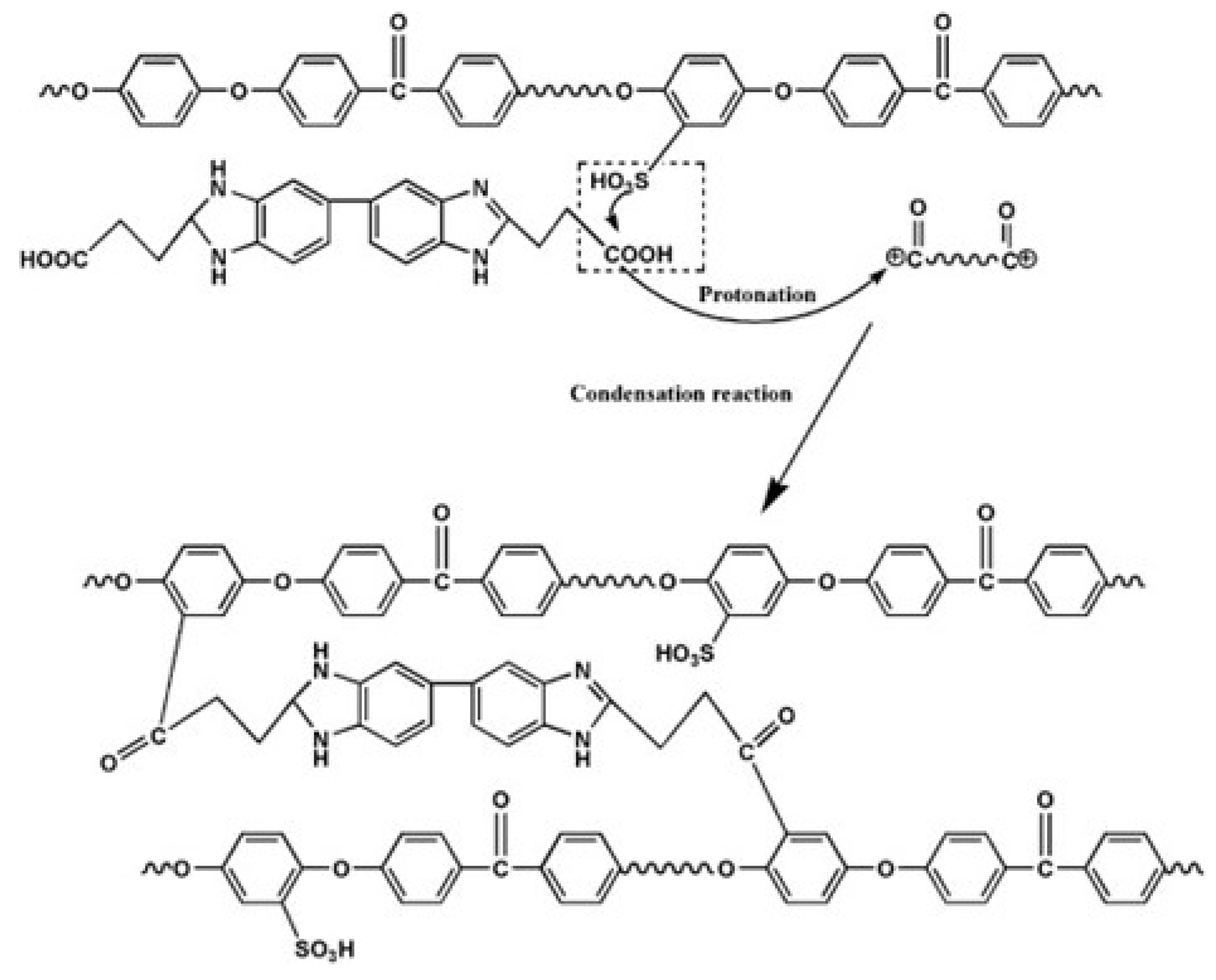
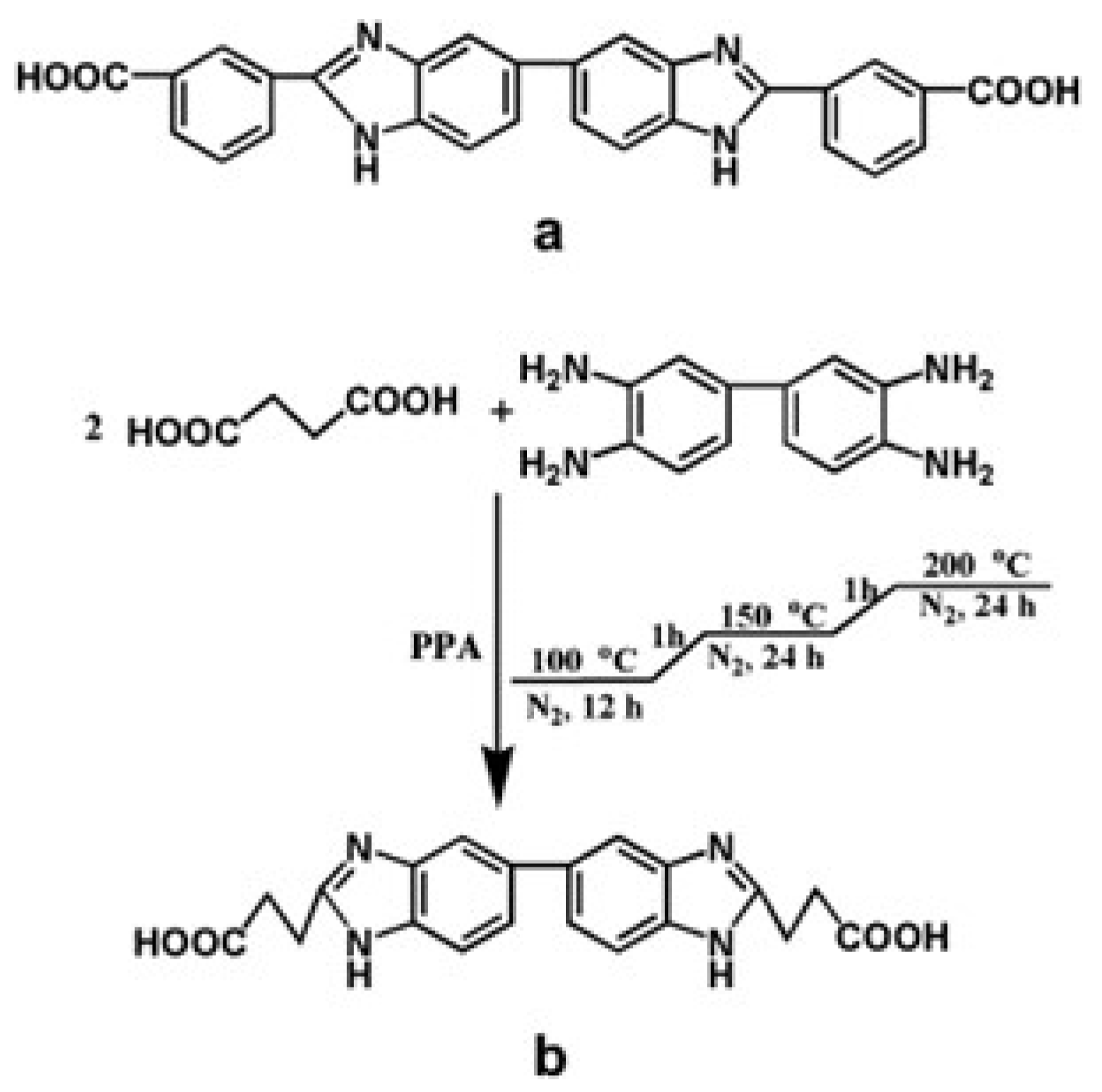
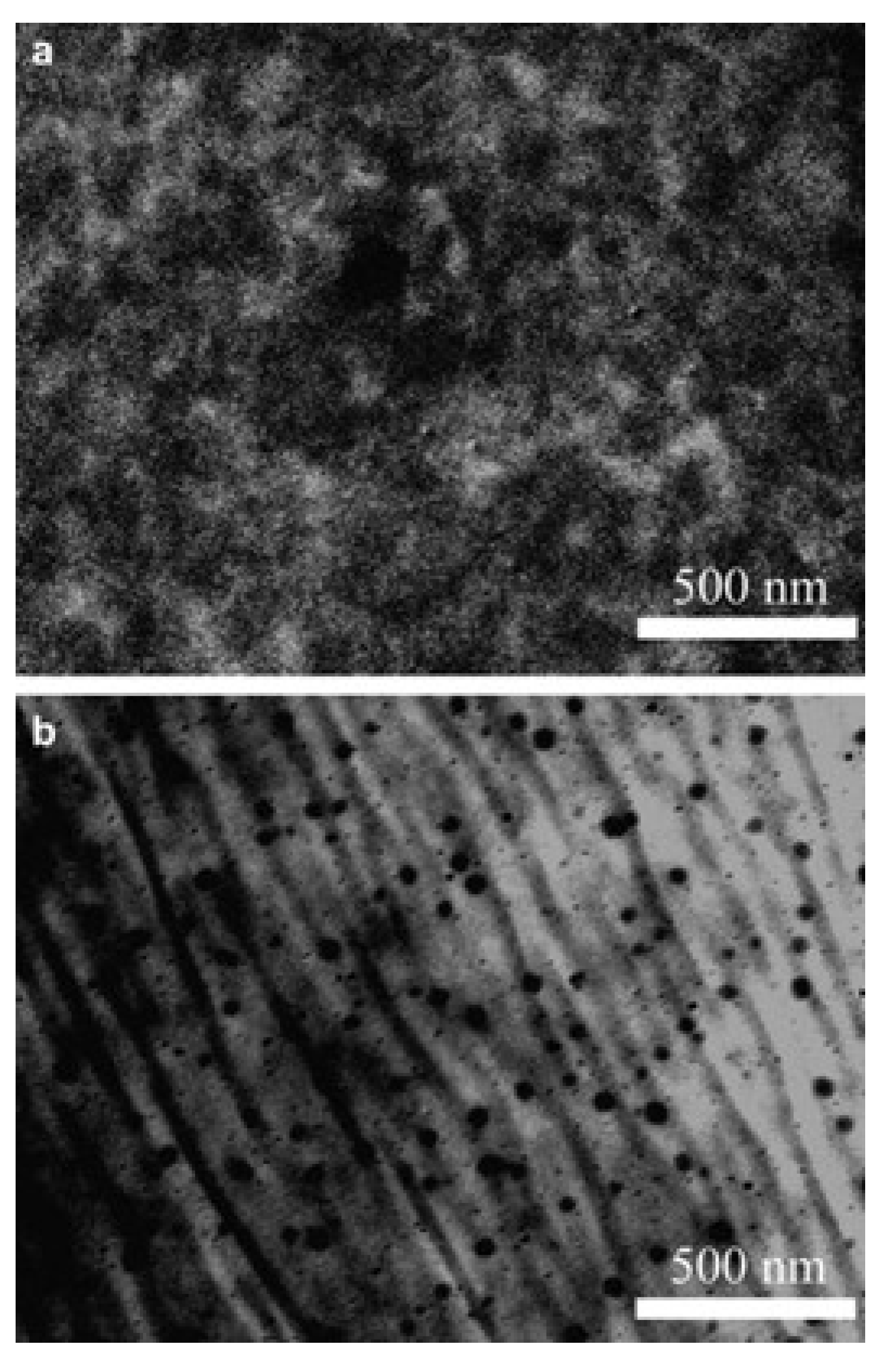
| Storage Properties | Methanol | Hydrogen |
|---|---|---|
| Temperature | 25 °C | −252.8 °C (for liquefied storage) |
| Pressure | Atmospheric pressure | 350–700 bar (for gaseous storage) |
| Density (at 1 bar) | 792 kg/m3 (at room temperature) | 70 kg/m3 (at liquefaction temperature) |
| Specific storage volume | Low | High |
| Cost of storage infrastructure | Low | High |
| Operating cost of storage | Low | High |
| Polymer | Synthesis Reaction | Group | Refs. |
|---|---|---|---|
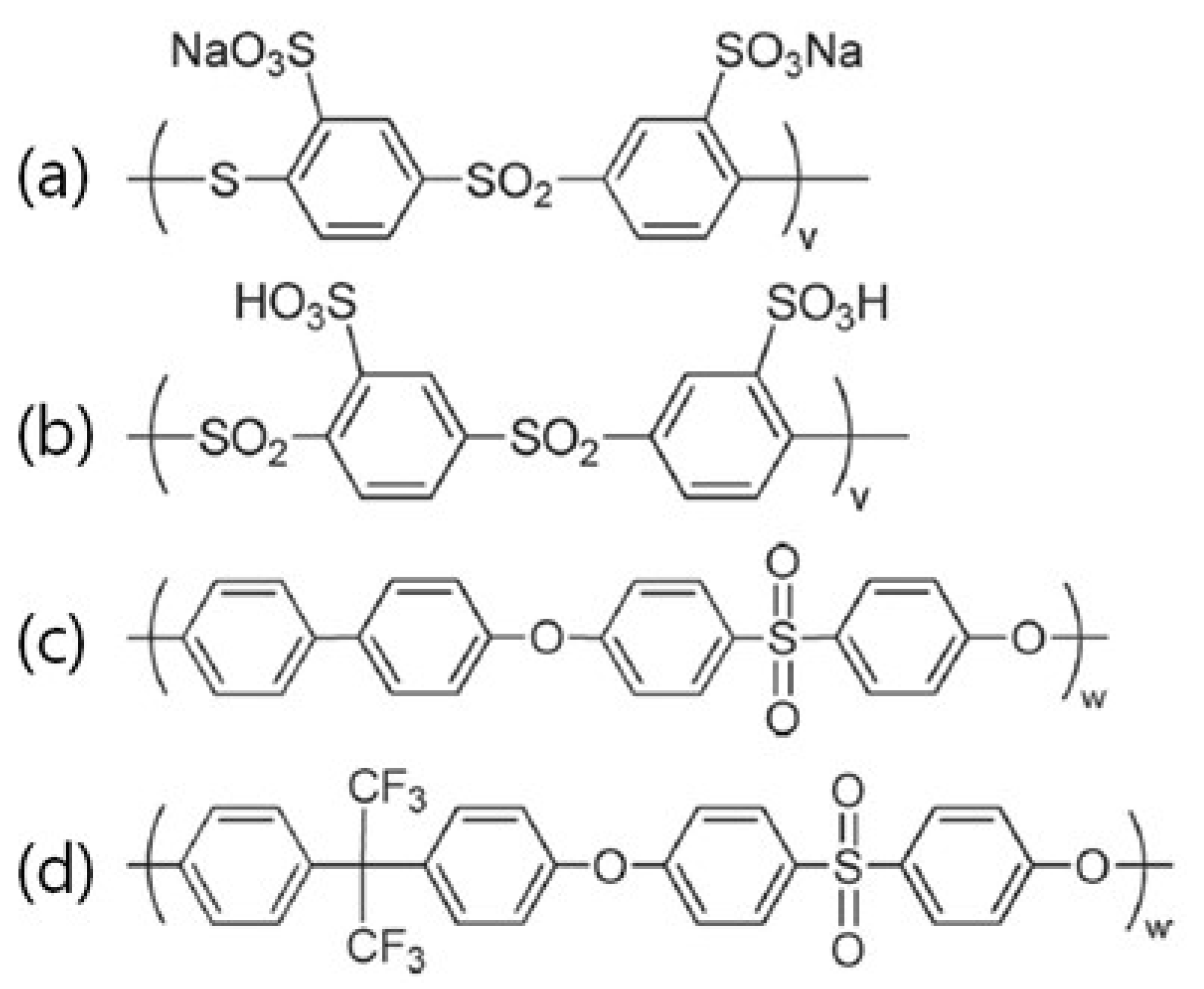
| Poly(arylene ether sulfone) | Coupling | [65,66,67,68,69,70,71] | |
| Poly(ether sulfone) | Coupling | [72,73] | |
| Poly(arylenethioethersulfone) | Coupling | Bai and Dang | [74] |
| Poly(arylene ether sulfone ketone) | Coupling | Watanabe | [75,76] |
| Poly(phenylene) | Polycondensation | Rikukawa | [77] |
| Poly(benzimidazole) | Coupling | Benicewicz | [78] |
| Polyimide | Imidization | Watanabe | [79] |
| Poly(phenylene) and polyimide | Ni catalysis Copolymerization | Okamoto | [80] |
| Poly(arylene ether sulfone) and poly(ether ether ketone) | Nucleophilic coupling | McGrath | [81] |
| Poly(arylene ether sulfone) and poly(arylene ether ketone) | Oligomer coupling | McGrath Watanabe | [81,82,83,84] [85] |
| Poly(arylene ether sulfone) and polyimide | Oligomer coupling | McGrath | [86] |
| Poly(arylene ether sulfone) and polyimide | Imidization coupling | McGrath | [87] |
| Poly(arylene ether sulfone) and polybenzimidazole | Oligomer coupling | McGrath Watanabe | [88] [89] |
| Poly(arylene ether sulfone) and poly(2,5 benzophenone) | Oligomer coupling and nucleophilic aromatic substitution | McGrath | [90] |
| Poly(arylene ether) and poly(benzophenone) | Ni-mediated coupling polymerization | Watanabe | [91] |
| Poly(arylene sulfone, thioether) and poly(arylene ether sulfone) | Coupling and oxidation | Jannasch | [92] |
| Poly(phenylene sulfone) and poly(phenylene ether sulfone) | Coupling | Meyer | [93] |
| Poly(ether ether ketone) and poly(ether sulfone) | Aromatic nucleophilic polycondensation | Liu | [94] |
| Poly(ether ether ketone) and poly(butadiene) | Condensation | Yin | [95] |
| Polystyrene and hydrogenated polybutadiene | Commercial | Nacher | [96] |
| Polystyrene and poly(ethylene-ran-butylene) | Commercial | Kim | [97] |
| Polystyrene and poly(isobutylene) | Commercial | Elabd | [98] |
| Poly(arylene ether) and poly(styrene-co-acrylonitrile) | Atom transfer radical Polymerization | Jo | [99] |
| Poly(arylene sulfone ether ketone) and poly(styrene-co-acrylonitrile) | Condensation and radical polymerization | Jo | [100] |
| Polystyrene and poly(p-tert-butylstyrene) | Living anionic | Spontak | [101] |
| Polystyrene and poly(hydroxystyrene) | Anionic polymerization | Chao | [102] |
| Polystyrene and polyisoprene | Anionic polymerization and sulfonation | Goswami | [103] |
| Polystyrene and poly(vinylphosphonic acid) | Anionic polymerization | Jannasch | [104] |
| Polystyrene and poly(vinylidene difluoride-co- hexafluoropropylene) | Atom transfer radical polymerization and postsulfonation | Holdcroft | [105,106] |
| Polystyrene and poly(vinylidene difluoride) | Atom transfer radical polymerization | Wang | [107] |
| Polystyrene and poly(pentafluorostyrene) | (Atom transfer) radical polymerization | Hvilsted Matsuoka | [108] [109] |
| Polystyrene and polymethylbutylene | Anionic polymerization | Balsara | [110] |
| Poly(norbornenylene ethylstyrene) Poly(styrenesulfonic acid) | Anionic polymerization | Hillmyer | [111] |
| Polystyrene and poly(vinyl benzyl phosphonate) | Atom transfer radical polymerization | Müllen | [112] |
| Polystyrene and poly(methyl methacrylate) | Atom transfer radical polymerization | Hickner Mezzenga | [113] [114] |
| Polystyrene and poly(arylene ether sulfone) | Amide condensation | Lee | [115] |
| Polysulfone and poly(vinylidene difluoride) | Polycondensation and postsulfonation | Holdcroft | [116] |
| Poly(ethylene oxide) and poly(benzimidazole) | Thiol-ene coupling and ring opening | Jannasch | [117] |
| Poly(phenylene oxide) and poly(vinyl benzyl phosphonic acid) | Atom transfer radical Polymerization and Postsulfonation | Müllen | [118] |
| Poly(vinylidene fluoride)-g-polystyrene | Atom transfer radical polymerization Postsulfonation | Chung | [119] |
| Poly(trifluoroethylene)-g- poly(styrene-co-divinylbenzene) | Atom transfer radical polymerization | Mortensen | [120] |
| Poly(vinylidene fluoride)-ter- Poly(hexafluoropropylene)-ter- Trifluoromethacrylic acid Aryl sulfonic acid | Radical terpolymerization and etherification | Ameduri | [121] |
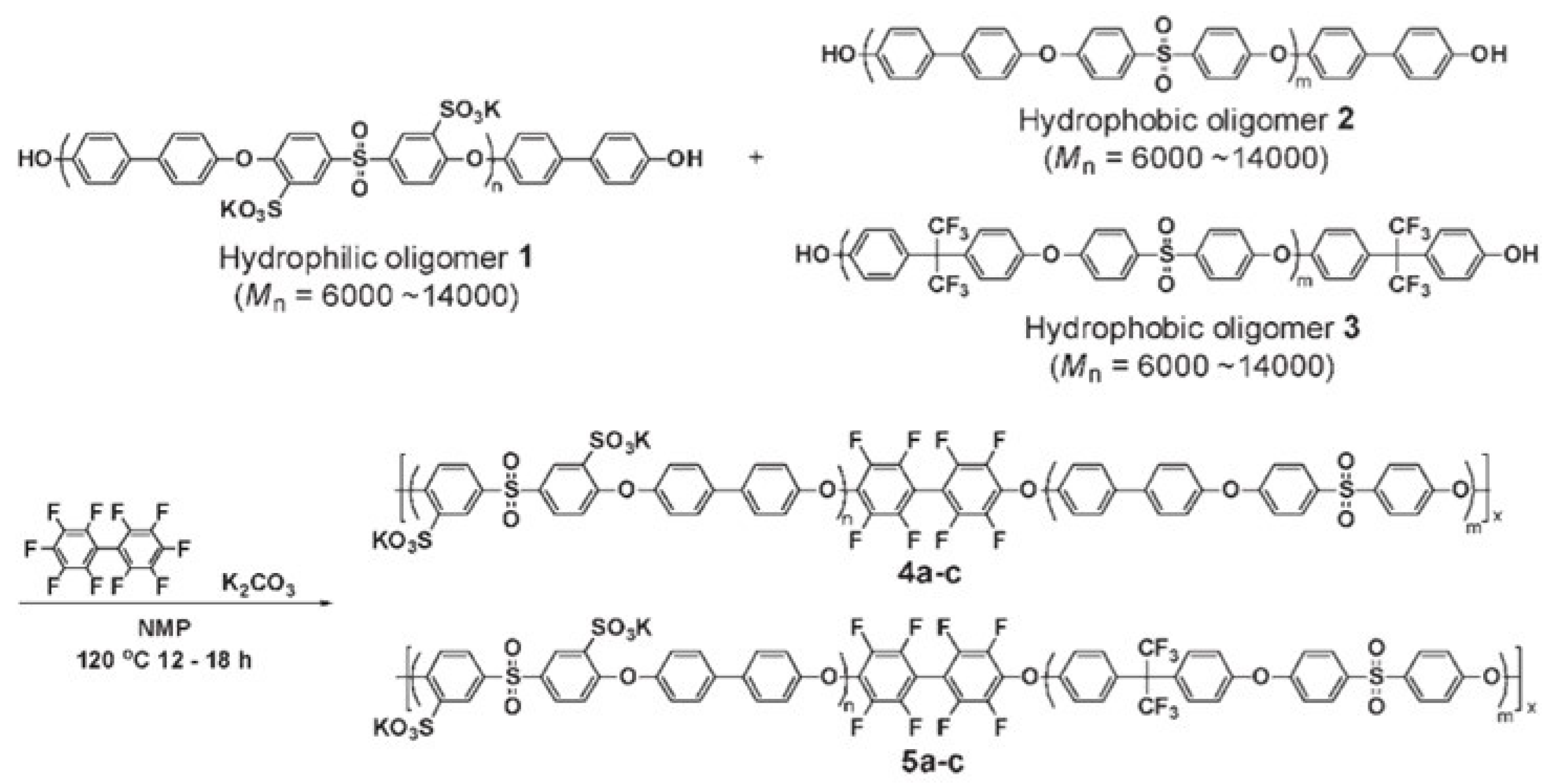
| Block Copolymer | Oligomer Mn Ratio a | Theoretical IEC (meq/g) b | IEC by 1H NMR (meq/g) c | Mn d | Mw/Mn d |
|---|---|---|---|---|---|
| 4a | 14,000/14,000 | 2.10 | 2.00 | 68,000 | 2.5 |
| 4b | 10,000/10,000 | 2.10 | 2.02 | 74,000 | 3.3 |
| 4c | 6000/6000 | 2.10 | 1.99 | 55,000 | 4.4 |
| 5a | 14,000/14,000 | 2.10 | 1.95 | 70,000 | 4.1 |
| 5b | 10,000/10,000 | 2.10 | 1.97 | 60,000 | 3.5 |
| 5c | 6000/6000 | 2.10 | 1.99 | 84,000 | 4.9 |
| Membrane | Proton Conductivity (S cm−1) | Methanol Permeability (10−8 cm2 s−1) |
|---|---|---|
| PEK-SP10 | 0.045 | 8 |
| PEK-SP15 | 0.078 | 22 |
| PEK-SP20 | 0.100 | 36 |
| PAES-SP18 | 0.122 | 34 |
| Nafion 117 | 0.108 | 294 |
| Membrane | PSSA (wt%) | D (nm) | PSSA Width (nm) | IEC (mmol/g) | Water Uptake (%) | λ (mol H2O/mol SO3H) |
|---|---|---|---|---|---|---|
| PEM1a | 36.7 | 41.5 | 18.7 | 1.98 | 118 ± 4 | 33 ± 1 |
| PEM2a | 39.1 | 22.6 | 10.8 | 2.06 | 73 ± 12 | 21 ± 4 |
| PEM3a | 36.6 | 14.4 | 6.6 | 1.93 | 78 ± 5 | 22 ± 1 |
| PEM4a | 36.8 | 28.3 | 13.0 | 1.86 | 73 ± 2 | 20 ± 0.4 |
| PEM5a | 37.3 | 19.5 | 9.0 | 1.96 | 42 ± 0.4 | 12 ± 0.1 |
| Nafion 117 | - | - | - | - | 34 ± 0.4 | 21 ± 0.2 |
| Membrane | IEC | Conductivity (S cm−1) | λ | |||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 40 °C | 60 °C | 80 °C | 100 °C | 20 °C | 100 °C | ||
| SPES-b | 1.54 | 0.09 | 0.19 | 0.27 | 0.43 | 0.77 | 7.81 | 9.02 |
| SPES-bn | 1.92 | 0.11 | 0.16 | 0.20 | 0.25 | 0.30 | 9.44 | 8.94 |
| Nafion 117 | 0.91 | 0.08 | 0.10 | 0.14 | 0.17 | 0.17 | 9.79 | - |
| Crosslinker (DVB) Content (%) | Proton Conductivity (10−2 Scm−1) | MeOH Permeability (10−7 cm2/s) | Water Uptake (%) | Tensile Stress (N/mm2) | Selectivity |
|---|---|---|---|---|---|
| 0 | 4.5 | 8.6 | 75.1 | 4.6 | 1.6 |
| 2 | 3.1 | 3.4 | 35.8 | 11.0 | 2.7 |
| 5 | 3.4 | 6.6 | 23.9 | 13.9 | 1.5 |
| 10 | 2.5 | 4.3 | 25.2 | 17.3 | 1.8 |
| Nafion 115 | 9.0 | 27.0 | 26.7 | 13.9 | 1 |
| Membrane | Proton Conductivity (mS cm−1) | Methanol Permeability (10−7 cm2/s) |
|---|---|---|
| A50G4-S10 | 66 ± 2.7 | 12.7 |
| A100G4-S10 | 62 ± 3.0 | 8.2 |
| A150G4-S10 | 53 ± 2.5 | 5.3 |
| Nafion 117 | 62 ± 3.0 | 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonomenna, M.G.; Bae, J. Block Copolymer-Based Symmetric Membranes for Direct Methanol Fuel Cells. Symmetry 2024, 16, 1079. https://doi.org/10.3390/sym16081079
Buonomenna MG, Bae J. Block Copolymer-Based Symmetric Membranes for Direct Methanol Fuel Cells. Symmetry. 2024; 16(8):1079. https://doi.org/10.3390/sym16081079
Chicago/Turabian StyleBuonomenna, Maria Giovanna, and Joonwon Bae. 2024. "Block Copolymer-Based Symmetric Membranes for Direct Methanol Fuel Cells" Symmetry 16, no. 8: 1079. https://doi.org/10.3390/sym16081079
APA StyleBuonomenna, M. G., & Bae, J. (2024). Block Copolymer-Based Symmetric Membranes for Direct Methanol Fuel Cells. Symmetry, 16(8), 1079. https://doi.org/10.3390/sym16081079








