Dynamic Point-to-Helical and Point-to-Axial Chirality Transmission and Induction of Optical Activity in Multichromophoric Systems: Basic Principles and Relevant Applications in Chirality Sensing
Abstract
1. Introduction
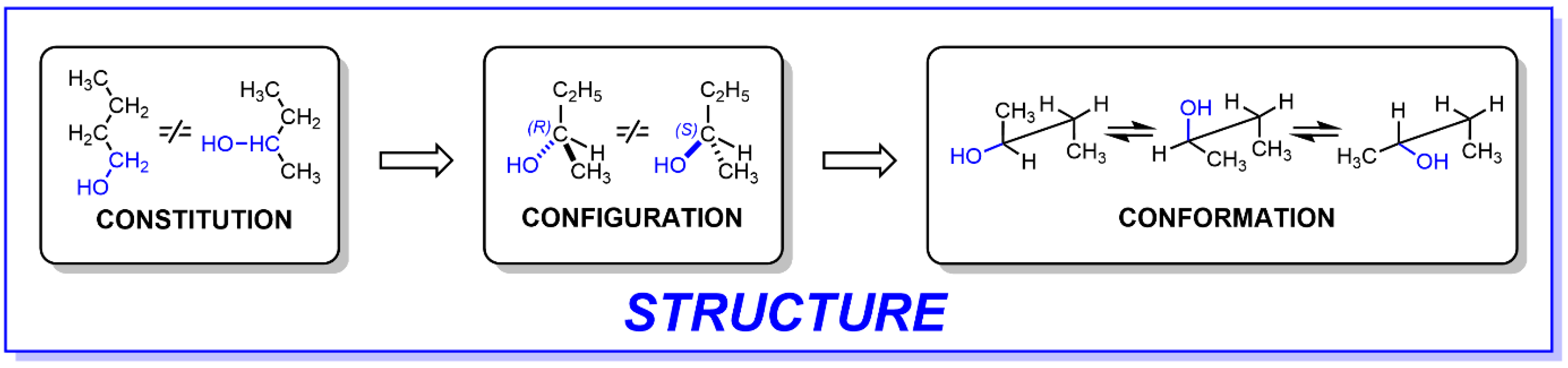
2. Chirality and Isomerism
3. “Permanent” and “Dynamic” Optical Activity
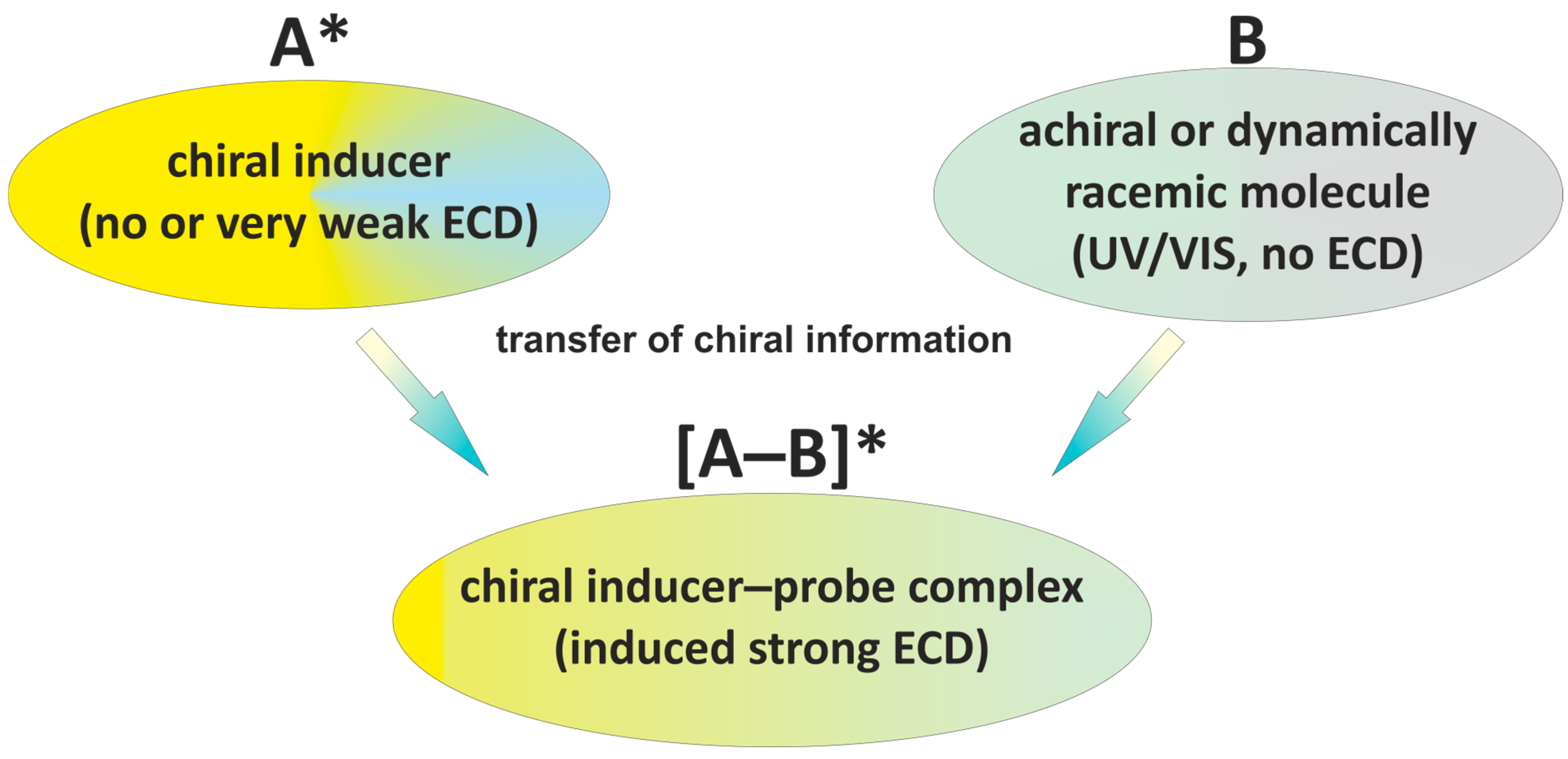
4. Induction, Transfer, Interconversion of Chirality, and Induction of the Optical Activity
5. A Suitable Stereodynamic Chirality Probe
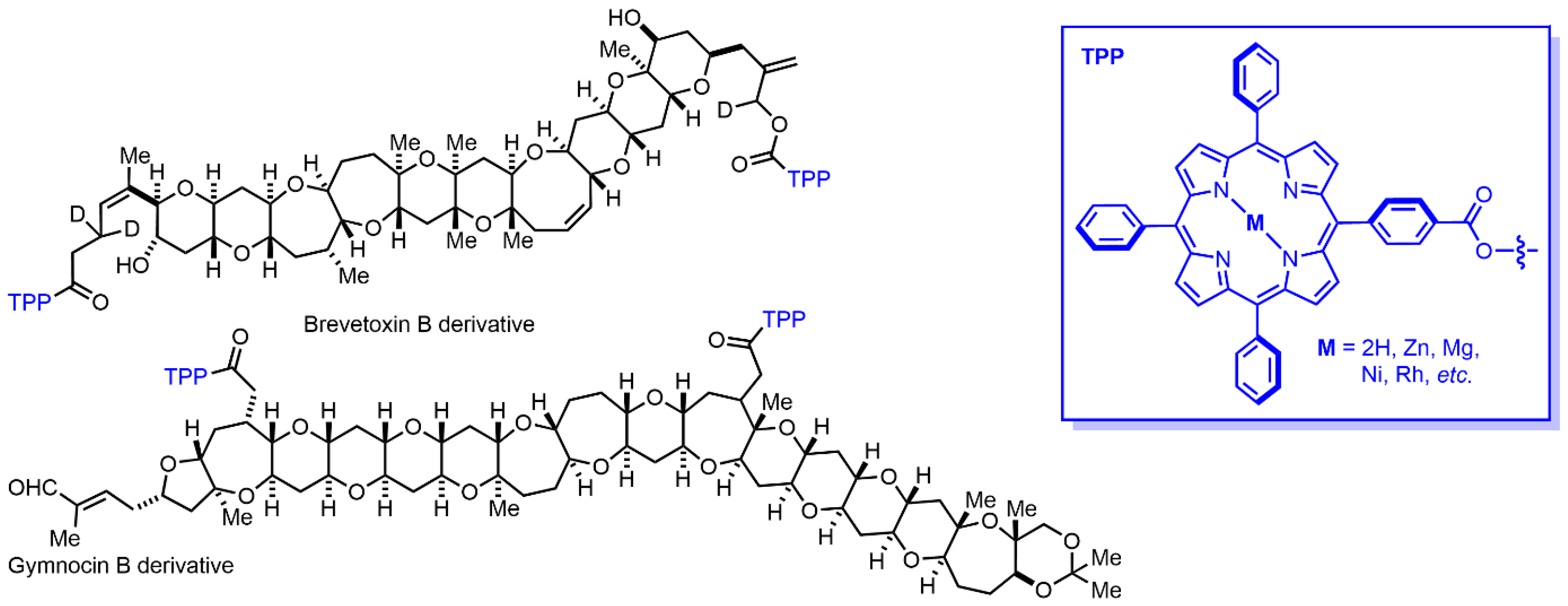
6. Classics in Action—The Most Recognized Porphyrin Probes
7. Point-to-Helix vs. Point-to-Helical Chirality Transmission
7.1. Chirality Transfer in Molecular Propellers of Induced Helicity
7.2. Three Blades Are Good—Two Blades Are Better!
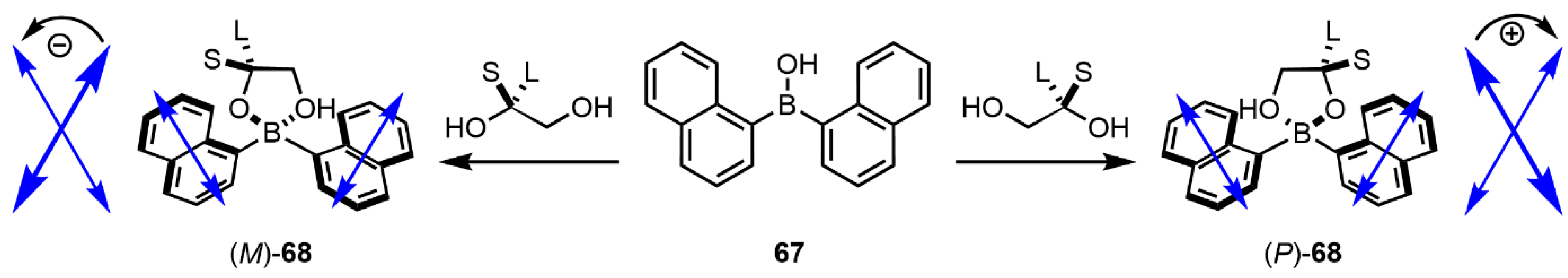
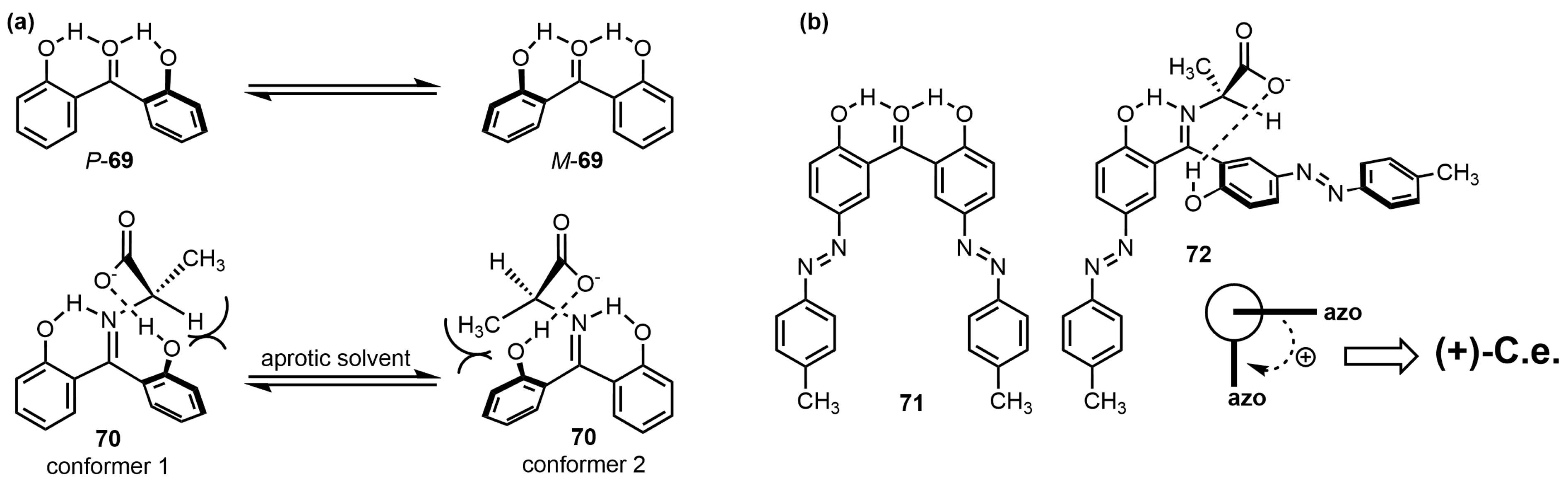
8. “Single Twisted” Aryl Probes—The Case of Biphenyl and Its Congeners
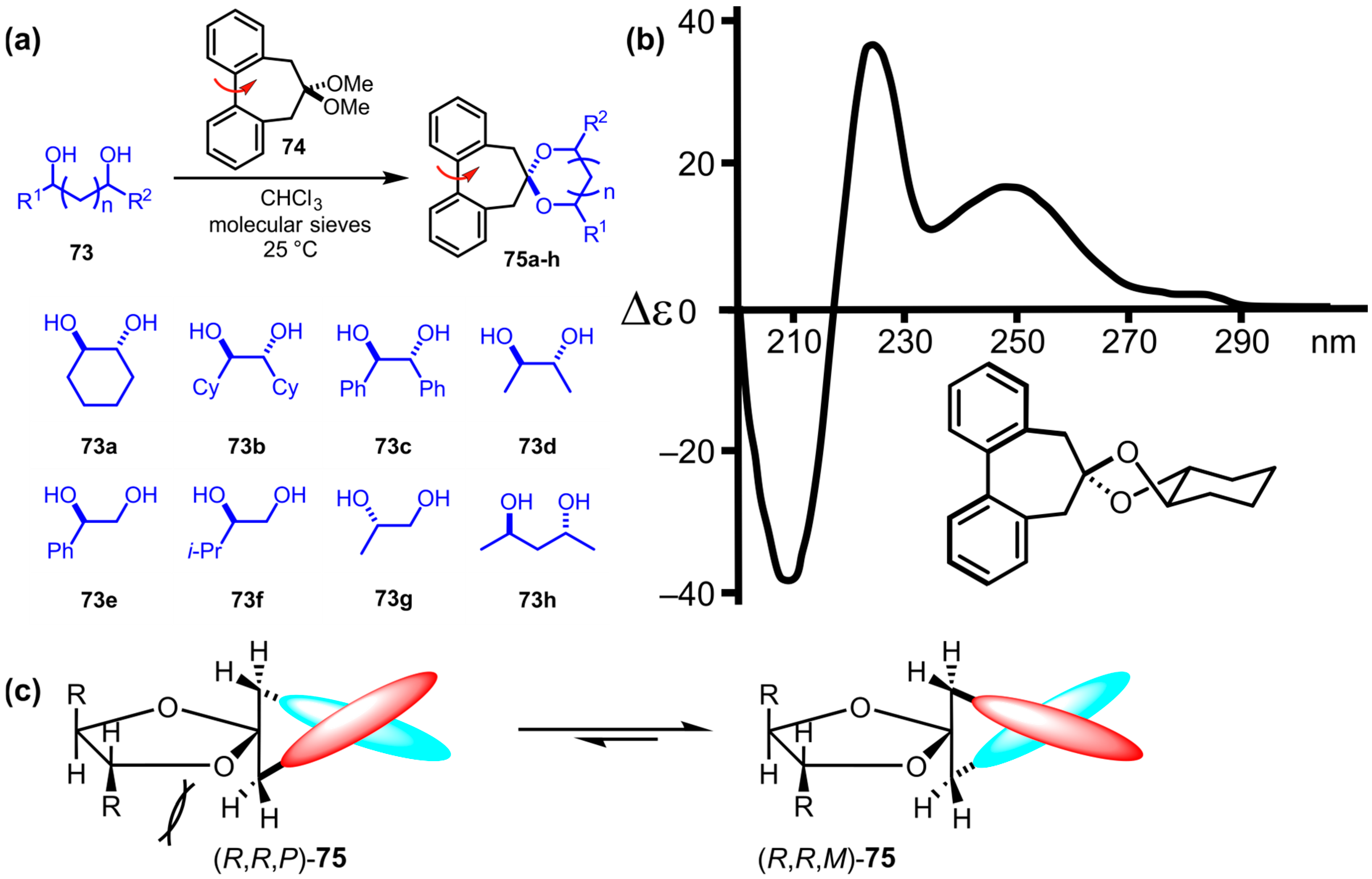
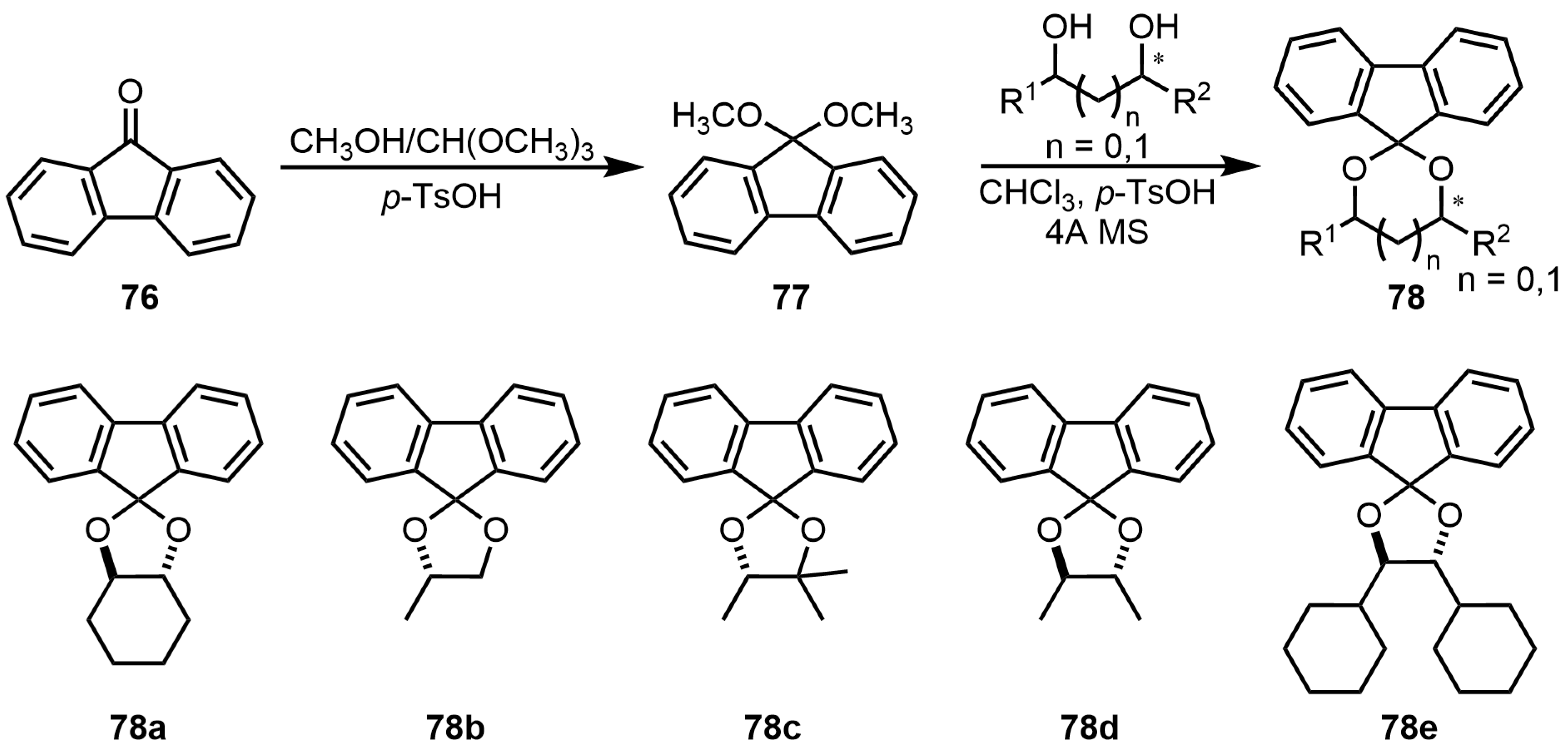
9. Chromophore Probes with “Multiple Twist”
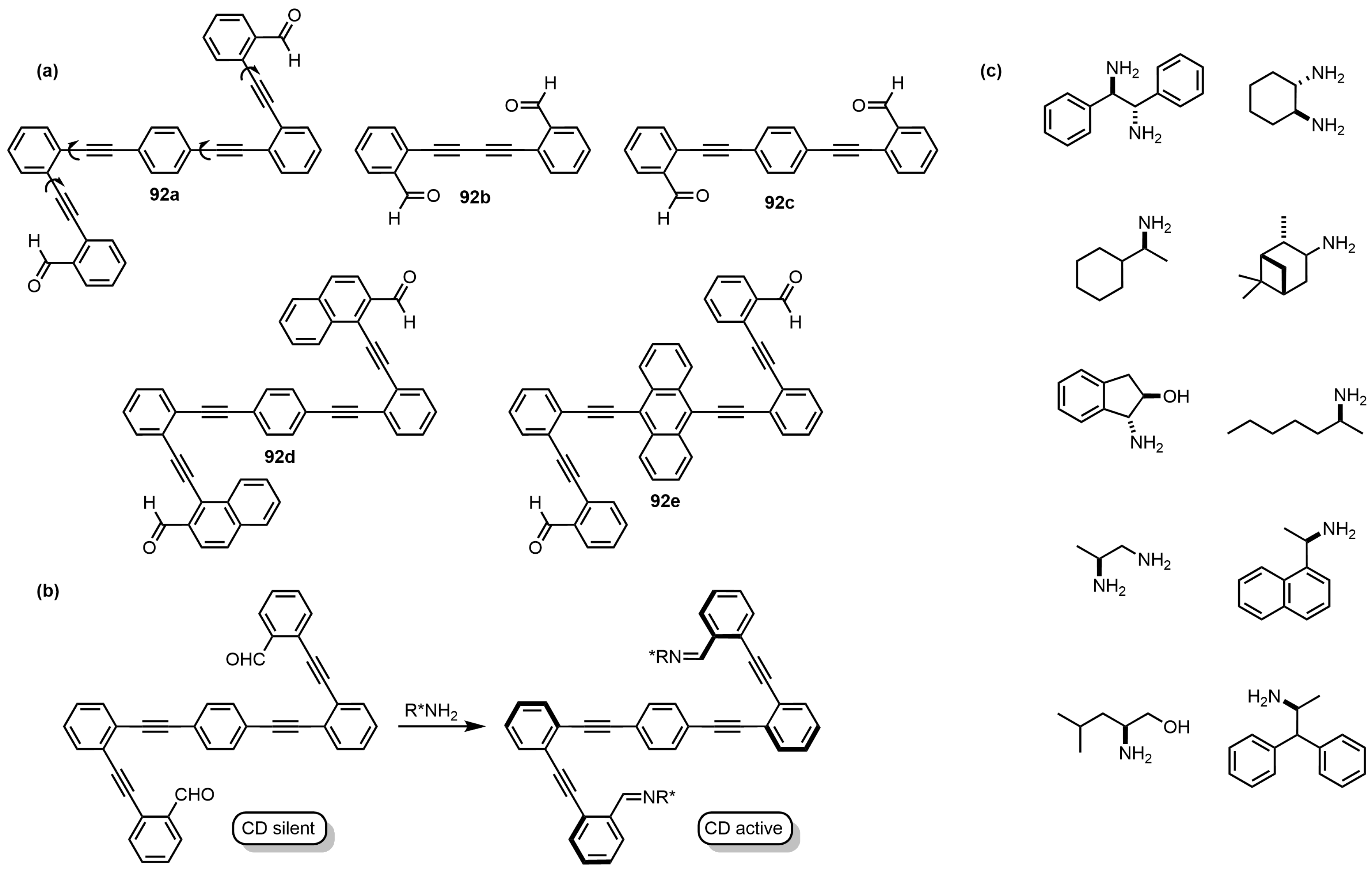
9.1. Imine Folders
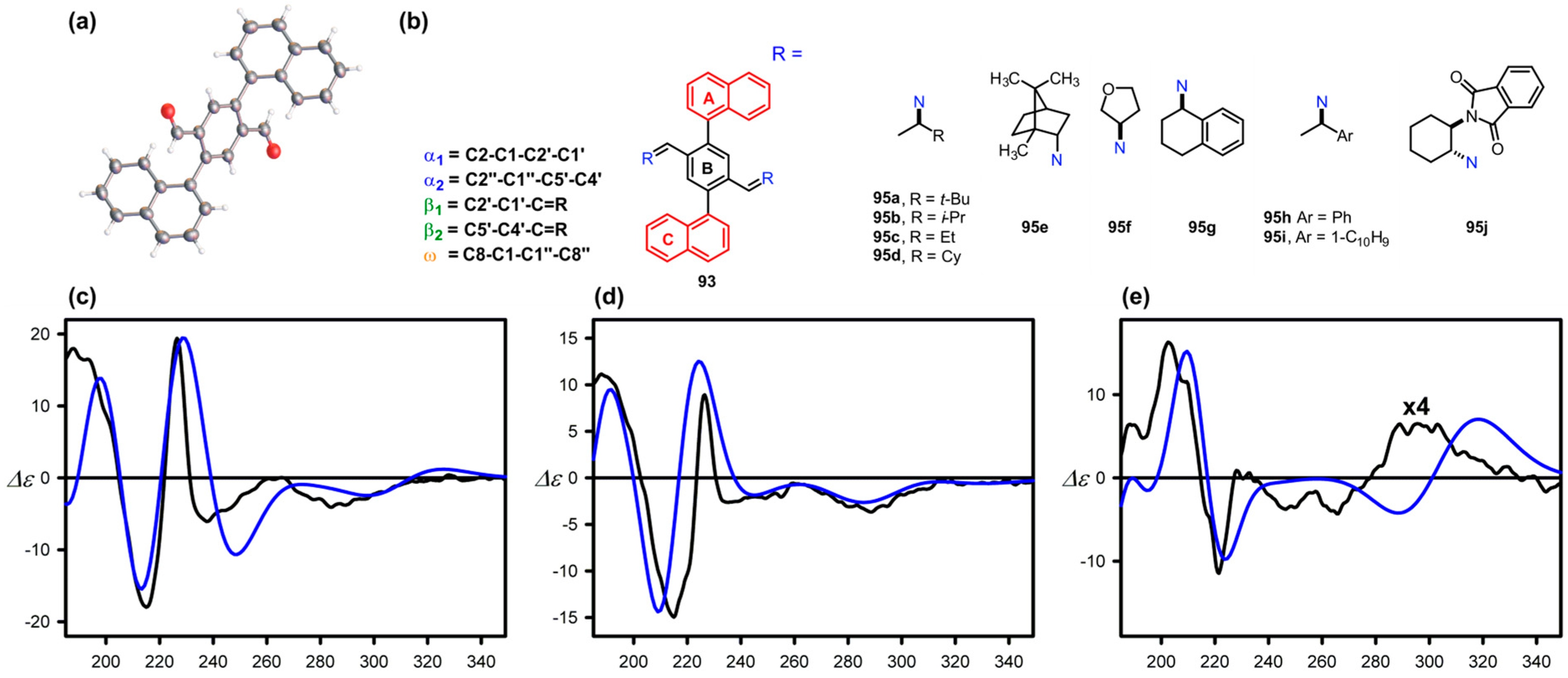
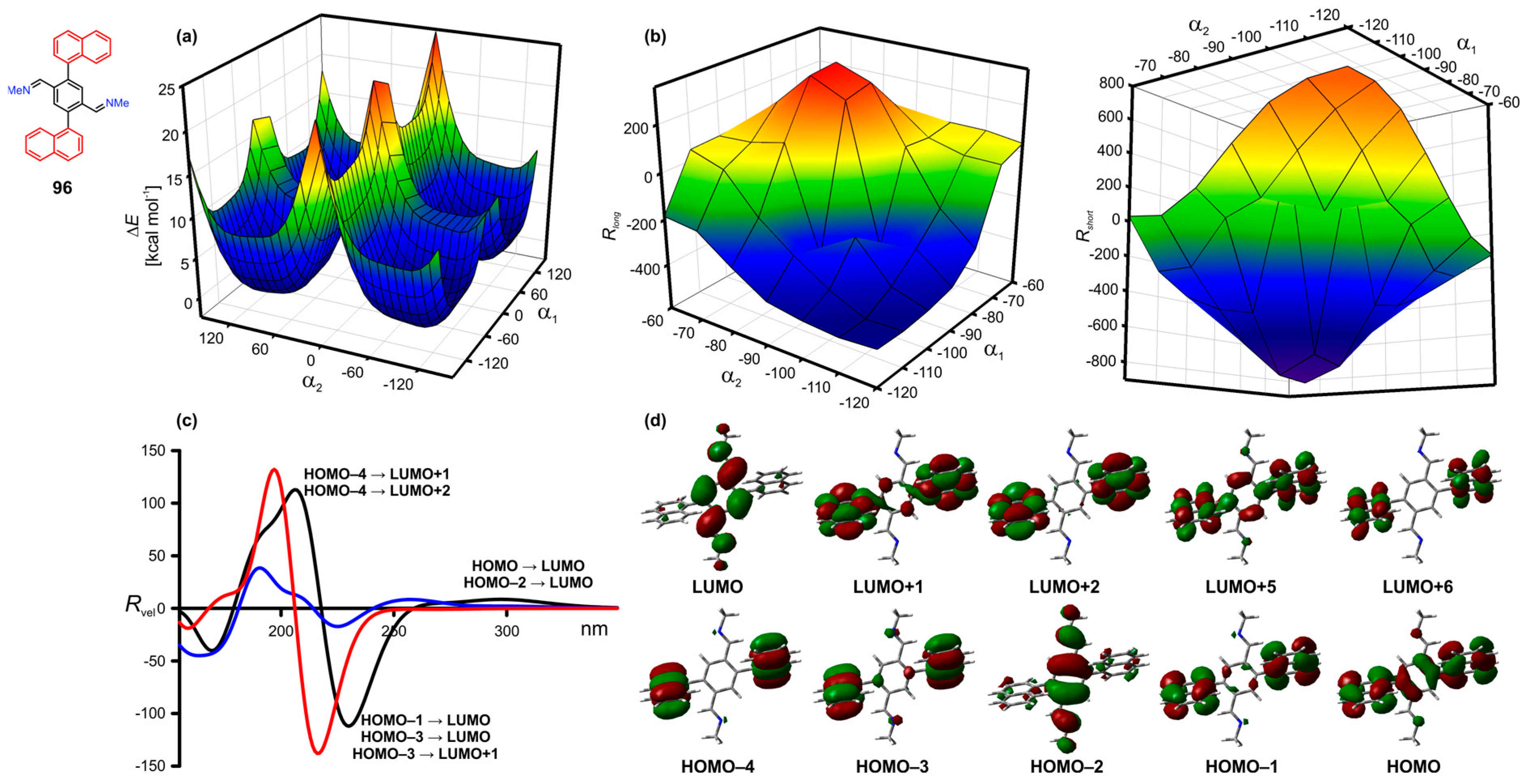
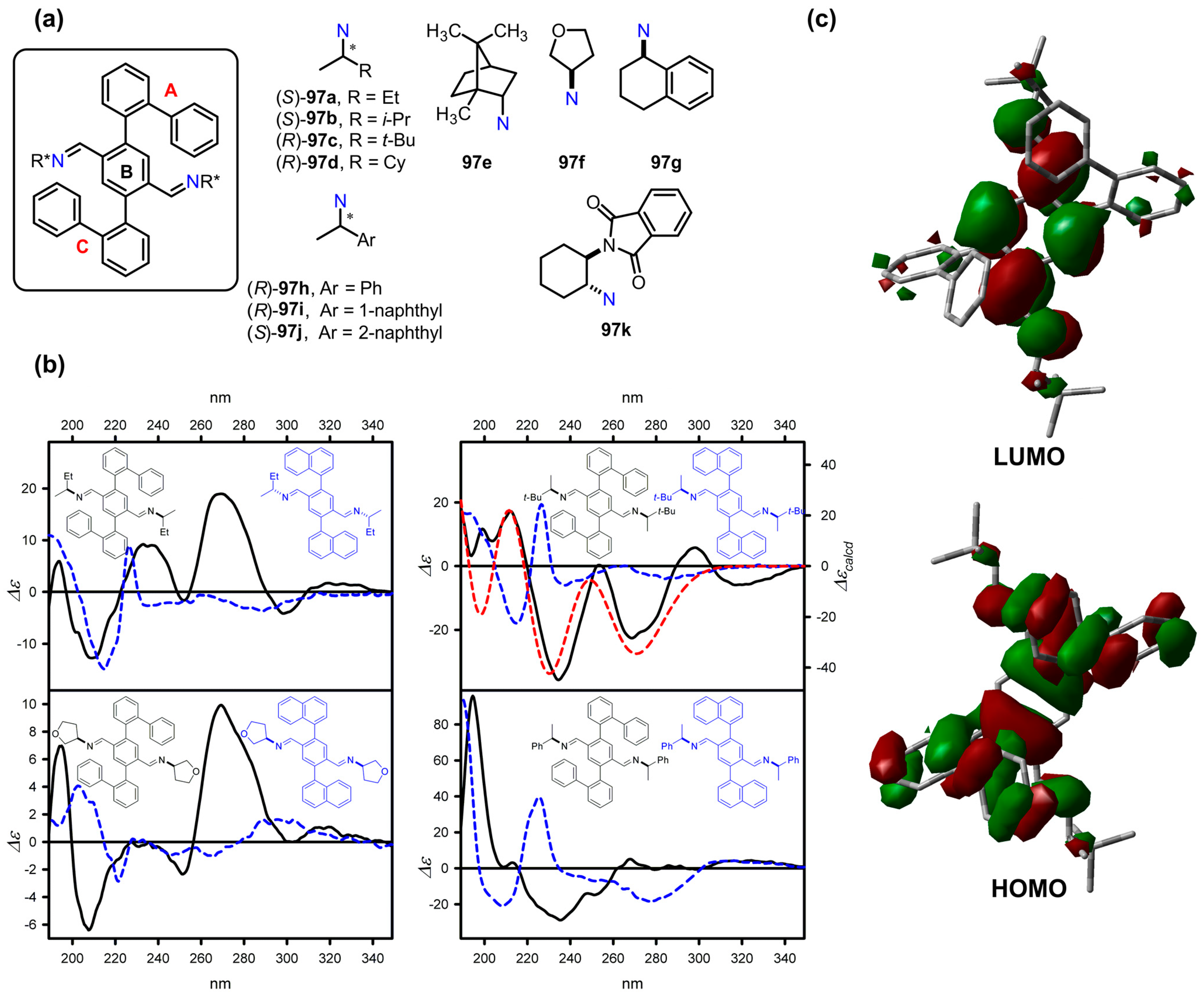
9.2. Tri- and Penta-Aryl Stereodynamic Chirality Probes
10. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman & Co: New York, NY, USA, 2010. [Google Scholar]
- Lough, W.J.; Wainer, I.W. (Eds.) Chirality in Natural and Applied Science; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Francotte, E.; Lindner, W. (Eds.) Chirality in Drug Research, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; Volume 33, ISBN 9783527310760. [Google Scholar]
- Lin, G.-Q.; You, Q.-D.; Cheng, J.-F. (Eds.) Chiral Drugs: Chemistry and Biological Action; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118075647. [Google Scholar]
- Crossley, R. The relevance of chirality to the study of biological activity. Tetrahedron 1992, 48, 8155–8178. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of Chirality in Drug Design and Development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal Activities of Enantiomers via Biological Receptors: Examples of Chiral Drug, Pesticide, and Fragrance Molecules. J. Chem. Educ. 2007, 84, 2012–2018. [Google Scholar] [CrossRef]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective Perception of Chiral Odorants. Tetrahedron Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Engel, K.-H. Chirality: An Important Phenomenon Regarding Biosynthesis, Perception, and Authenticity of Flavor Compounds. J. Agric. Food Chem. 2020, 68, 10265–10274. [Google Scholar] [CrossRef]
- Testa, B.; Caldwell, J.; Kisakürek, M.V. (Eds.) Organic Stereochemistry. Guiding Principles and Biomedical Relevance; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Stephens, T.D.; Brynner, R. Dark Remedy: The Impact of Thalidomide and Its Revival as a Vital Medicine; Perseus Publishing: Cambridge, UK, 2001. [Google Scholar]
- Todd, M. (Ed.) Separation of Enantiomers; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Pellissier, H. Chirality from Dynamic Kinetic Resolution; RSC: Stratford-upon-Avon, UK, 2011. [Google Scholar]
- Helmchen, G.; Hoffmann, R.W.; Mulzer, J.; Schaumann, E. (Eds.) Stereoselective Synthesis; G. Thieme: New York, NY, USA, 1996. [Google Scholar]
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. (Eds.) Comprehensive Asymmetric Catalysis; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. (Eds.) Comprehensive Chiroptical Spectroscopy (Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules); Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lowry, M.T. Optical Rotatory Power; Dover Publications, Inc.: New York, NY, USA, 1964. [Google Scholar]
- Wolf, C. Dynamic Stereochemistry of Chiral Compounds: Principles and Applications; RSC: Cambridge, UK, 2008. [Google Scholar]
- Wolf, C.; Bentley, K.W. Chirality sensing using stereodynamic probes with distinct electronic circular dichroism output. Chem. Soc. Rev. 2013, 42, 5408–5424. [Google Scholar] [CrossRef]
- Ozcelik, A.; Pereira-Cameselle, R.; Poklar Ulrih, N.; Petrovic, A.G.; Alonso-Gómez, J.L. Chiroptical Sensing: A Conceptual Introduction. Sensors 2020, 20, 974. [Google Scholar] [CrossRef]
- Formen, J.S.S.K.; Howard, J.R.; Anslyn, E.V.; Wolf, C. Circular Dichroism Sensing: Strategies and Applications. Angew. Chem. Int. Ed. 2024, 63, e202400767. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Zhu, H.-J. Organic Stereochemistry. Experimental and Computational Methods; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Glaser, R. Symmetry, Spectroscopy, and Crystallography. The Structural Nexus; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Poppe, L.; Nógrádi, M. (Eds.) Stereochemistry and Stereoselective Synthesis. An Introduction; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Ruch, E. Algebraic Aspects of the Chirality Phenomenon in Chemistry. Acc. Chem. Res. 1972, 5, 49–56. [Google Scholar] [CrossRef]
- Mislow, K.; Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984, 106, 3319–3328. [Google Scholar] [CrossRef]
- Buda, A.B.; Mislow, K. A Hausdorff chirality measure. J. Am. Chem. Soc. 1992, 114, 6006–6012. [Google Scholar] [CrossRef]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous symmetry measures. J. Am. Chem. Soc. 1992, 114, 7843–7851. [Google Scholar] [CrossRef]
- Zayit, A.; Pinsky, M.; Elgavi, H.; Dryzun, C.; Avnir, D. A web site for calculating the degree of chirality. Chirality 2011, 23, 17–23. [Google Scholar] [CrossRef]
- Buda, A.B.; Auf der Heyde, T.; Mislow, K. On Quantifying Chirality. Angew. Chem. Int. Ed. 1992, 31, 989–1007. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Moss, G.P. Basic terminology of stereochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Bijvoet, J.M.; Peerdeman, A.F.; van Bommel, A.J. Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays. Nature 1951, 168, 271–272. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Flack, H.D. Chiral and Achiral Crystal Structures. Helv. Chim. Acta 2003, 86, 905–921. [Google Scholar] [CrossRef]
- Harada, N. Chiral molecular science: How were the absolute configurations of chiral molecules determined? Experimental results and theories. Chirality 2017, 29, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Jenkinson, S. 8.3 Perspective and Concepts: Overview of Techniques for Assigning Stereochemistry. In Comprehensive Chirality; Elsevier: Amsterdam, The Netherlands, 2012; pp. 39–53. ISBN 9780080951683. [Google Scholar]
- Polavarapu, P.L. Chiroptical Spectroscopy: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 9781315374888. [Google Scholar]
- Rodger, A.; Nordén, B. Circular Dichroism & Linear Dichroism; Oxford University Press Inc.: New York, NY, USA, 1997. [Google Scholar]
- Nakanishi, K.; Berova, N.; Woody, R.W. Circular Dichroism–Principles and Applications; VCD Publishers, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Lightner, D.A.; Gurst, J.E. Organic Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the Structure Elucidation of Natural Products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.T. Features of electronic circular dichroism and tips for its use in determining absolute configuration. Tetrahedron Asymmetry 2017, 28, 1199–1211. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Why is it important to simultaneously use more than one chiroptical spectroscopic method for determining the structures of chiral molecules? Chirality 2008, 20, 664–672. [Google Scholar] [CrossRef]
- Takaishi, K.; Maeda, C.; Ema, T. Circularly polarized luminescence in molecular recognition systems: Recent achievements. Chirality 2023, 35, 92–103. [Google Scholar] [CrossRef]
- Gennaro Pescitelli, G.; Di Bari, L.; Berova, N. Conformational aspects in the studies of organic compounds by electronic circular dichroism. Chem. Soc. Rev. 2011, 40, 4603–4625. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Renaissance in chiroptical spectroscopic methods for molecular structure determination. Chem. Rec. 2007, 7, 125–136. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Polavarapu, P.L.; Covington, C.L. Comparison of Experimental and Calculated Chiroptical Spectra for Chiral Molecular Structure Determination. Chirality 2014, 26, 539–552. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Sepali, C.; Lafiosca, P.; Gómez, S.; Giovannini, T.; Cappelli, C. Effective fully polarizable QM/MM approaches to compute Raman and Raman Optical Activity spectra in aqueous solution. Spectrochim. Acta A 2024, 305, 123485. [Google Scholar] [CrossRef] [PubMed]
- Groß, J.; Kühlborn, J.; Pusch, S.; Weber, C.; Andernach, L.; Renzer, G.; Eckhardt, P.; Brauer, J.; Opatz, T. Comparison of different density functional theory methods for the calculation of vibrational circular dichroism spectra. Chirality 2023, 35, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Mattiat, J.; Luber, S. Comparison of Length, Velocity, and Symmetric Gauges for the Calculation of Absorption and Electric Circular Dichroism Spectra with Real-Time Time-Dependent Density Functional Theory. J. Chem. Theory Comput. 2022, 18, 5513–5526. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.T. Ab initio calculation of molecular chiroptical properties. Theor. Chem. Acc. 2006, 115, 227–245. [Google Scholar] [CrossRef]
- Neese, F. Prediction of molecular properties and molecular spectroscopy with density functional theory: From fundamental theory to exchange-coupling. Coord. Chem. Rev. 2009, 253, 526–563. [Google Scholar] [CrossRef]
- Superchi, S.; Scafato, P.; Gorecki, M.; Pescitelli, G. Absolute Configuration Determination by Quantum Mechanical Calculation of Chiroptical Spectra: Basics and Applications to Fungal Metabolites. CMC 2018, 25, 287–320. [Google Scholar] [CrossRef]
- Autschbach, J. Computing chiroptical properties with first-principles theoretical methods: Background and illustrative examples. Chirality 2009, 21, E116–E152. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Wipf, P.; Beratan, D.N. Optical Signatures of Molecular Dissymmetry: Combining Theory with Experiments To Address Stereochemical Puzzles. Acc. Chem. Res. 2009, 42, 809–819. [Google Scholar] [CrossRef]
- Hassan, D.S.; Wolf, C. Optical deciphering of multinary chiral compound mixtures through organic reaction based chemometric chirality sensing. Nat. Commun. 2021, 12, 6451. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; MacAvaney, S.; Russell, K.; Wolf, C. Tandem Use of Optical Sensing and Machine Learning for the Determination of Absolute Configuration, Enantiomeric and Diastereomeric Ratios, and Concentration of Chiral Samples. Angew. Chem. Int. Ed. 2020, 59, 2440–2448. [Google Scholar] [CrossRef]
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures on Physics; Addison-Wesley Publishing Company, Inc.: Reading, MA, USA, 1964. [Google Scholar]
- Caldwell, D.J.; Eyring, H. The Theory of Optical Activity; Wiley-Interscience: New York, NY, USA, 1972. [Google Scholar]
- Djerassi, C. Optical Rotatory Dispersion, Applications to Organic Chemistry; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Kirkwood, J.G. On the theory of optical rotatory power. J Chem. Phys. 1937, 5, 479–491. [Google Scholar] [CrossRef]
- Kuhn, W. The physical significance of optical rotatory power. Trans Faraday Soc. 1930, 26, 293–308. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J. Calculation of Optical Rotation Using Density Functional Theory. J. Phys. Chem. A 2001, 105, 5356–5371. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J.; Bortolini, O.; Besse, P. Determination of absolute configuration using ab initio calculation of optical rotation. Chirality 2003, 15, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.M.; Stephens, P.J.; Cheeseman, J.R. Determination of Absolute Configuration Using Density Functional Theory Calculation of Optical Rotation: Chiral Alkanes. J. Org. Chem. 2004, 69, 8709–8717. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Wang, Y.; Vaccaro, P.H.; Cheeseman, J.R.; Luderer, M.R. Conformational Effects on Optical Rotation. 2-Substituted Butanes. J. Phys. Chem. A 2005, 109, 3405–3410. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Vaccaro, P.H.; Cheeseman, J.R. Conformational Effects on Optical Rotation. 3-Substituted 1-Butenes. J. Am. Chem. Soc. 2003, 125, 1888–1896. [Google Scholar] [CrossRef]
- Bunnenberg, E.; Djerassi, C.; Mislow, K.; Moscowitz, A. Inherently Dissymmetric Chromophores and Circular Dichroism. J. Am. Chem. Soc. 1962, 84, 2823–2826. [Google Scholar] [CrossRef]
- Mislow, K.; Bunnenberg, E.; Records, R.; Wellman, K.; Djerassi, C. Inherently Dissymmetric Chromophores and Circular Dichroism. II. J. Am. Chem. Soc. 1963, 85, 1342–1349. [Google Scholar] [CrossRef]
- Moscowitz, A. Theoretical aspects of optical activity part one: Small molecules. Adv. Chem. Phys. 1962, 4, 67–112. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.F.; El-Bayoumi, S.A. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Harada, N.; Nakanishi, K. Circular Dichroism Spectroscopy: Exciton Coupling in Organic Stereochemistry; University Science Books: Mill Valley, CA, USA, 1983. [Google Scholar]
- Harada, N.; Nakanishi, K. Exciton Chirality Method and Its Application to Configurational and Conformational Studies of Natural Products. Acc. Chem. Res. 1972, 5, 257–263. [Google Scholar] [CrossRef]
- Pescitelli, G. ECD exciton chirality method today: A modern tool for determining absolute configurations. Chirality 2022, 34, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Di Bari, L.; Berova, N. Application of electronic circular dichroism in the study of supramolecular systems. Chem. Soc. Rev. 2014, 43, 5211–5233. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; von Zelewsky, A. Charge-transfer excited state properties of chiral transition metal coordination compounds studied by chiroptical spectroscopy. Coord. Chem. Rev. 1998, 177, 257–300. [Google Scholar] [CrossRef]
- Telfer, S.G.; McLean, T.M.; Waterland, M.R. Exciton coupling in coordination compounds. Dalton Trans. 2011, 40, 3097–3108. [Google Scholar] [CrossRef]
- Nehira, T.; Parish, C.A.; Jockusch, S.; Turro, N.J.; Nakanishi, K.; Berova, N. Fluorescence-detected exciton-coupled circular dichroism: Scope and limitation in structural studies of organic molecules. J. Am. Chem. Soc. 1999, 121, 8681–8691. [Google Scholar] [CrossRef]
- Tanaka, K.; Pescitelli, G.; Nakanishi, K.; Berova, N. Fluorescence detected exciton coupled circular dichroism: Development of new fluorescent reporter groups for structural studies. Monatsh Chem. 2005, 136, 367–395. [Google Scholar] [CrossRef]
- Moore, B., II; Autschbach, J. Density functional study of tetraphenylporphyrin long-range exciton coupling. ChemistryOpen 2012, 1, 184–194. [Google Scholar] [CrossRef]
- Tanaka, K.; Itagaki, Y.; Satake, M.; Naoki, H.; Yasumoto, T.; Nakanishi, K.; Berova, N. Three challenges toward the assignment of absolute configuration of gymnocin-B. J. Am. Chem Soc. 2005, 127, 9561–9570. [Google Scholar] [CrossRef]
- Gawronski, J.; Grajewski, J. The Significance of Induced Circular Dichroism. Org. Lett. 2003, 5, 3301–3303. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.P. Open questions on the transfer of chirality. Commun. Chem. 2021, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Lauceri, R.; D’Urso, A.; Mammana, A.; Purrello, R. Transfer of chirality for memory and separation. Top. Curr. Chem. 2011, 298, 143–188. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A Structure and Mechanisms, 2nd ed.; Plenum Press: New York, NY, USA, 1984; ISBN 0-306-41198-9. [Google Scholar]
- Seeman, J.I. Effect of Conformational Change on Reactivity in Organic Chemistry. Evaluations, Application, and Extensions of Cutin–Hammett/Winstein–Holness Kinetics. Chem. Rev. 1983, 83, 83–134. [Google Scholar] [CrossRef]
- Seeman, J.I. The Curtin–Hammett Principle and the Winstein–Holness Equation. J. Chem. Ed. 1986, 63, 42–48. [Google Scholar] [CrossRef]
- Matile, S.; Berova, N.; Nakanishi, K.; Novkova, S.; Philipova, I.; Blagoev, B. Porphyrins: Powerful Chromophores for Structural Studies by Exciton-Coupled Circular Dichroism. J. Am. Chem. Soc. 1995, 117, 7021–7022. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
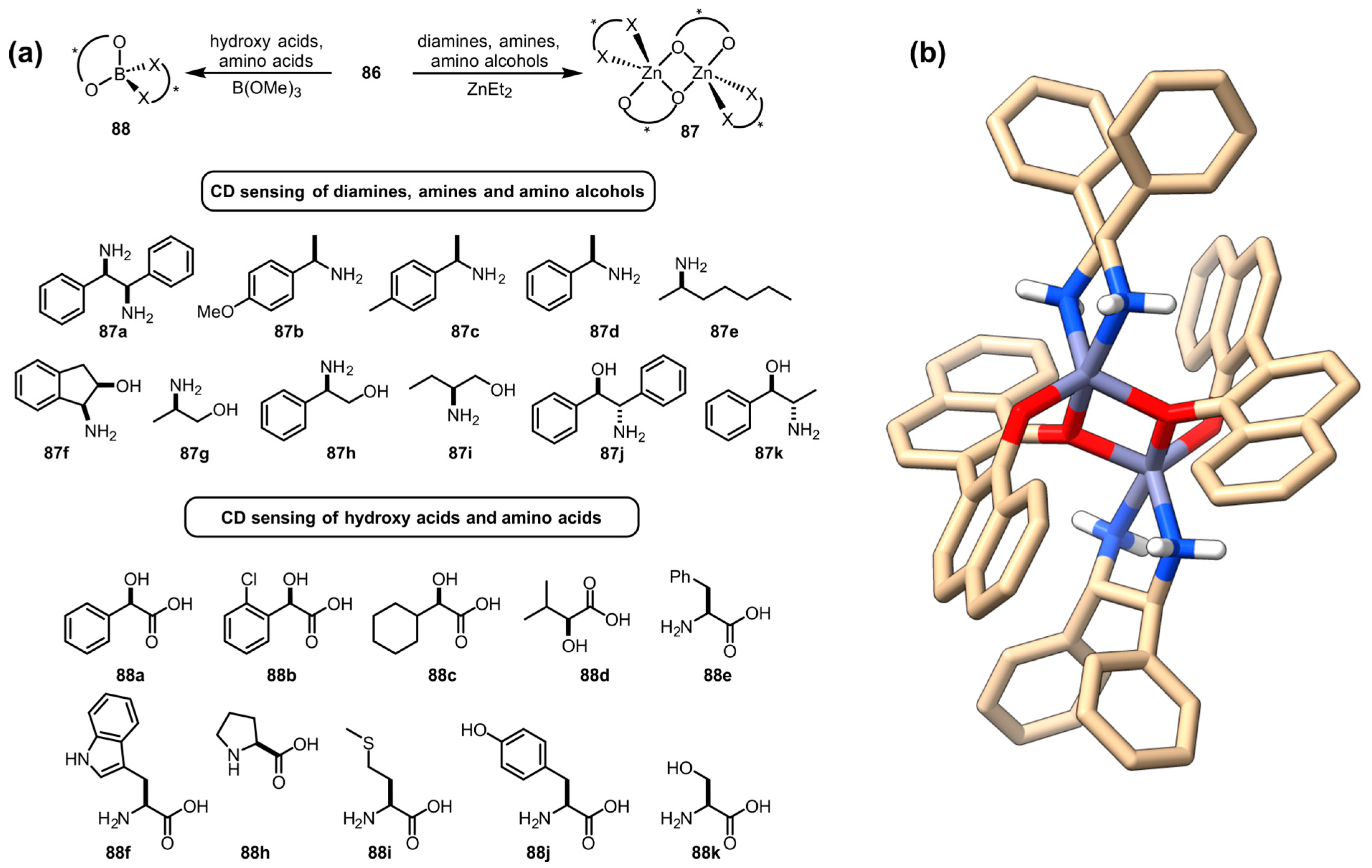
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc porphyrin tweezer in host-guest complexation: Determination of absolute configurations of diamines, amino acids, and amino alcohols by circular dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Berova, N.; Pescitelli, G.; Petrovic, A.G.; Proni, G. Probing molecular chirality by CD-sensitive dimeric metalloporphyrin hosts. Chem. Commun. 2009, 5958–59980. [Google Scholar] [CrossRef]
- Kurtán, T.; Nesnas, N.; Li, Y.Q.; Huang, X.; Nakanishi, K.; Berova, N. Chiral Recognition by CD-Sensitive Dimeric Zinc Porphyrin Host. 1. Chiroptical Protocol for Absolute Configurational Assignments of Monoalcohols and Primary Monoamines. J. Am. Chem. Soc. 2001, 123, 5962–5973. [Google Scholar] [CrossRef]
- Superchi, S.; Bisaccia, R.; Casarini, D.; Laurita, A.; Rosini, C. Flexible Biphenyl Chromophore as a Circular Dichroism Probe for Assignment of the Absolute Configuration of Carboxylic Acids. J. Am. Chem. Soc. 2006, 128, 6893–6902. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, S.; Padula, D.; Scafato, P.; Chiummiento, L.; Rosini, C. A Chemical/Computational Approach to the Determination of Absolute Configuration of Flexible and Transparent Molecules: Aliphatic Diols As a Case Study. J. Org. Chem. 2008, 73, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.T.; Lin, C.Y.; Wright, A.M.; Moor, S.R.; Anslyn, E.V. Mathematical Relationships of Individual Stereocenter er Values to dr Values. J. Org. Chem. 2019, 84, 5922–5926. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.T.; Moor, S.R.; McVeigh, M.; Roesner, E.K.; Marini, F.; Anslyn, E.V. Rapid Optical Determination of Enantiomeric Excess, Diastereomeric Excess, and Total Concentration Using Dynamic-Covalent Assemblies: A Demonstration Using 2-Aminocyclohexanol and Chemometrics. J. Am. Chem. Soc. 2019, 141, 11151–11160. [Google Scholar] [CrossRef]
- Leung, D.; Anslyn, E.V. Rapid Determination of Enantiomeric Excess of α-Chiral Cyclohexanones Using Circular Dichroism Spectroscopy. Org. Lett. 2011, 13, 2298–2301. [Google Scholar] [CrossRef]
- Nieto, S.; Dragna, J.M.; Anslyn, E.V. A Facile Circular Dichroism Protocol for Rapid Determination of Enantiomeric Excess and Concentration of Chiral Primary Amines. Chem.-A Eur. J. 2010, 16, 227–232. [Google Scholar] [CrossRef]
- Gawroński, J.; Kwit, M.; Gawrońska, K. Helicity Induction in a Bichromophore: A Sensitive and Practical Chiroptical Method for the Absolute Configuration Determination of Aliphatic Alcohols. Org. Lett. 2002, 4, 4185–4188. [Google Scholar] [CrossRef]
- Gawroński, J.; Grajewski, J. A superior molecular bichromophore for the determination of absolute configuration of primary amines. Tetrahedron Asymmetry 2004, 15, 1527–1530. [Google Scholar] [CrossRef]
- Dutot, L.; Wright, K.; Gaucher, A.; Wakselman, M.; Mazaleyrat, J.P.; De Zotti, M.; Peggion, C.; Formaggio, F.; Toniolo, C. The Bip Method, Based on the Induced Circular Dichroism of a Flexible Biphenyl Probe in Terminally Protected-Bip-Xaa*Dipeptides, for Assignment of the Absolute Configuration of β-Amino Acids. J. Am. Chem. Soc. 2008, 130, 5986–5992. [Google Scholar] [CrossRef]
- Mazaleyrat, J.-P.; Wright, K.; Gaucher, A.; Toulemonde, N.; Wakselman, M.; Oancea, S.; Peggion, C.; Formaggio, F.; Setnička, V.; Keiderling, T.A.; et al. Induced Axial Chirality in the Biphenyl Core of the Cα-Tetrasubstituted α-Amino Acid Residue Bip and Subsequent Propagation of Chirality in (Bip)n/Val Oligopeptides. J. Am. Chem. Soc. 2004, 126, 12874–12879. [Google Scholar] [CrossRef]
- Holmes, A.E.; Das, D.; Canary, J.W. Chelation-Enhanced Circular Dichroism of Tripodal Bisporphyrin Ligands. J. Am. Chem. Soc. 2007, 129, 1506–1507. [Google Scholar] [CrossRef]
- Canary, J.W.; Mortezaei, S.; Liang, J. Redox-reconfigurable tripodal coordination complexes: Stereodynamic molecular switches. Chem. Commun. 2010, 46, 5850–5860. [Google Scholar] [CrossRef]
- Holmes, A.E.; Zahn, S.; Canary, J.W. Synthesis and circular dichroism studies of N,N-bis(2-quinolylmethyl)amino acid Cu(II) complexes: Determination of absolute configuration and enantiomeric excess by the exciton coupling method. Chirality 2002, 14, 471–477. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Wolf, C. Chiral Amplification with a Stereodynamic Triaryl Probe: Assignment of the Absolute Configuration and Enantiomeric Excess of Amino Alcohols. J. Am. Chem. Soc. 2009, 131, 16360–16361. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Wolf, C. Substrate-Specific Amino Acid Sensing Using a Molecular d/ l-Cysteine Probe for Comprehensive Stereochemical Analysis in Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 7276–7281. [Google Scholar] [CrossRef]
- Hassan, D.S.; Thanzeel, F.Y.; Wolf, C. Stereochemical analysis of chiral amines, diamines, and amino alcohols: Practical chiroptical sensing based on dynamic covalent chemistry. Chirality 2020, 32, 457–463. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Yusin, G.; Wolf, C. Enantioselective sensing of carboxylic acids with a bis(urea)oligo(phenylene)ethynylene foldamer. Tetrahedron 2019, 75, 1504–1509. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Wolf, C. Synthesis, Conformational Stability, and Asymmetric Transformation of Atropisomeric 1,8-Bisphenolnaphthalenes. J. Org. Chem. 2011, 76, 3888–3897. [Google Scholar] [CrossRef]
- Pilicer, S.L.; Bakhshi, P.R.; Bentley, K.W.; Wolf, C. Biomimetic Chirality Sensing with Pyridoxal-5′-phosphate. J. Am. Chem. Soc. 2017, 139, 1758–1761. [Google Scholar] [CrossRef]
- Iwaniuk, D.P.; Wolf, C. Chiroptical sensing of citronellal: Systematic development of a stereodynamic probe using the concept of isostericity. Chem. Commun. 2012, 48, 11226–11228. [Google Scholar] [CrossRef]
- Kim, H.; So, S.M.; Yen, C.P.-H.; Vinhato, E.; Lough, A.J.; Hong, J.-I.; Kim, H.-J.; Chin, J. Highly Stereospecific Generation of Helical Chirality by Imprinting with Amino Acids: A Universal Sensor for Amino Acid Enantiopurity. Angew. Chem. Int. Ed. 2008, 47, 8657–8660. [Google Scholar] [CrossRef]
- Scaramuzzo, F.A.; Badetti, E.; Licini, G.; Zonta, C. Second-Generation Tris(2-pyridylmethyl)amine–Zinc Complexes as Probes for Enantiomeric Excess Determination of Amino Acids. Eur. J. Org. Chem. 2017, 11, 1438–1442. [Google Scholar] [CrossRef]
- Machalska, E.; Hachlica, N.; Zajac, G.; Carraro, D.; Baranska, M.; Licini, G.; Bouř, P.; Zonta, C.; Kaczor, A. Chiral recognition via a stereodynamic vanadium probe using the electronic circular dichroism effect in differential Raman scattering. Phys. Chem. Chem. Phys. 2021, 23, 23336–23340. [Google Scholar] [CrossRef]
- Scaramuzzo, F.A.; Badetti, E.; Licini, G.; Zonta, C. Extending substrate sensing capabilities of zinc tris(2-pyridylmethyl)amine-based stereodynamic probe. Chirality 2019, 31, 375–383. [Google Scholar] [CrossRef]
- Zardi, P.; Wurst, K.; Licini, G.; Zonta, C. Concentration-Independent Stereodynamic g-Probe for Chiroptical Enantiomeric Excess Determination. J. Am. Chem. Soc. 2017, 139, 15616–15619. [Google Scholar] [CrossRef]
- Penasa, R.; Pandey, A.K.; Wurst, K.; Rancan, M.; Armelao, L.; Licini, G.; Zonta, C. Ion-Pairing Stereodynamic Probes for Circular Dichroism Chiral Sensing of Amines. Inorg. Chem. 2024, 63, 17701–17705. [Google Scholar] [CrossRef]
- Pilicer, S.L.; Mancinelli, M.; Mazzanti, A.; Wolf, C. Predictive chirality sensing via Schiff base formation. Org. Biomol. Chem. 2019, 17, 6699–6705. [Google Scholar] [CrossRef]
- Ni, C.; Zha, D.; Ye, H.; Hai, Y.; Zhou, Y.; Anslyn, E.V.; You, L. Dynamic Covalent Chemistry within Biphenyl Scaffolds: Reversible Covalent Bonding, Control of Selectivity, and Chirality Sensing with a Single System. Angew. Chem. Int. Ed. 2018, 57, 1300–1305. [Google Scholar] [CrossRef]
- Hassan, D.S.; Kariapper, F.S.; Lynch, C.C.; Wolf, C. Accelerated Asymmetric Reaction Screening with Optical Assays. Synthesis 2022, 54, 2527–2538. [Google Scholar] [CrossRef]
- Nieto, S.; Lynch, V.M.; Anslyn, E.V.; Kim, H.; Chin, J. High-Throughput Screening of Identity, Enantiomeric Excess, and Concentration Using MLCT Transitions in CD Spectroscopy. J. Am. Chem. Soc. 2008, 130, 9232–9233. [Google Scholar] [CrossRef]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef] [PubMed]
- Borovkov, V.V.; Hembury, G.A.; Inoue, Y. Origin, Control, and Application of Supramolecular Chirogenesis in Bisporphyrin-Based Systems. Acc. Chem. Res. 2004, 37, 449–459. [Google Scholar] [CrossRef]
- Dhamija, A.; Mondal, P.; Saha, B.; Rath, S.P. Induction, control, and rationalization of supramolecular chirogenesis using metalloporphyrin tweezers: A structure-function correlation. Dalton Trans. 2020, 49, 10679–10700. [Google Scholar] [CrossRef]
- Gholami, H.; Chakraborty, D.; Zhang, J.; Borhan, B. Absolute Stereochemical Determination of Organic Molecules through Induction of Helicity in Host−Guest Complexes. Acc. Chem. Res. 2021, 54, 654–667. [Google Scholar] [CrossRef]
- Li, X.; Tanasova, M.; Vasileiou, C.; Borhan, B. Fluorinated porphyrin tweezer: A powerful reporter of absolute configuration for erythro and threo diols, amino alcohols, and diamines. J. Am. Chem. Soc. 2008, 130, 1885–1893. [Google Scholar] [CrossRef]
- Li, X.; Burrell, C.E.; Staples, R.J.; Borhan, B. Absolute configuration for 1,n-glycols: A nonempirical approach to long-range stereochemical determination. J. Am. Chem. Soc. 2012, 134, 9026–9029. [Google Scholar] [CrossRef]
- Tanasova, M.; Anyika, M.; Borhan, B. Sensing Remote Chirality: Stereochemical Determination of β-, γ-, and δ-Chiral Carboxylic Acids. Angew. Chem. Int. Ed. 2015, 54, 4274–4278. [Google Scholar] [CrossRef]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and metalloporphyrins: Versatile circular dichroic reporter groups for structural studies. Chirality 2000, 12, 237–255. [Google Scholar] [CrossRef]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-based nanostructures for sensing applications. J. Sens. 2009, 856053. [Google Scholar] [CrossRef]
- Proni, G.; Pescitelli, G.; Huang, X.; Quraishi, N.Q.; Nakanishi, K.; Berova, N. Configurational assignment of a-chiral carboxylic acids by complexation to dimeric Zn–porphyrin: Host–guest structure, chiral recognition and circular dichroism. Chem. Commun. 2002, 2, 1590–1591. [Google Scholar] [CrossRef]
- Proni, G.; Pescitelli, G.; Huang, X.; Nakanishi, K.; Berova, N. Magnesium Tetraarylporphyrin Tweezer: a CD-Sensitive Host for Absolute Configurational Assignments of α-Chiral Carboxylic Acids. J. Am. Chem. Soc. 2003, 125, 12914–12927. [Google Scholar] [CrossRef] [PubMed]
- Tanasova, M.; Borhan, B. Conformational Preference in Bis(porphyrin) Tweezer Complexes: A Versatile Chirality Sensor for a-Chiral Carboxylic Acids. Eur. J. Org. Chem. 2012, 2012, 3261–3269. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, C.-H.; Jang, W.-D. Absolute Stereochemical Determination of Chiral Carboxylates Using an Achiral Molecular Tweezer. Chem. Eur. J. 2012, 18, 12479–12486. [Google Scholar] [CrossRef]
- Yang, Q.; Olmsted, C.; Borhan, B. Absolute stereochemical determination of chiral carboxylic acids. Org. Lett. 2002, 4, 3423–3426. [Google Scholar] [CrossRef]
- Huang, X.; Fujioka, N.; Pescitelli, G.; Koehn, F.E.; Williamson, R.T.; Nakanishi, K.; Berova, N. Absolute configurational assignments of secondary amines by CD-sensitive dimeric zinc porphyrin host. J. Am. Chem. Soc. 2002, 124, 10320–10335. [Google Scholar] [CrossRef]
- Tanasova, M.; Yang, Q.F.; Olmsted, C.C.; Vasileiou, C.; Li, X.Y.; Anyika, M.; Borhan, B. An Unusual Conformation of alpha-Haloamides Due to Cooperative Binding with Zincated Porphyrins. Eur. J. Org. Chem. 2009, 2009, 4242–4253. [Google Scholar] [CrossRef]
- Xu, T.; Li, J.H.; Momen, R.; Huang, W.J.; Kirk, S.R.; Yasuteru Shigeta, Y.; Jenkins, S. Chirality–Helicity Equivalence in the S and R Stereoisomers: A Theoretical Insight. J. Am. Chem. Soc. 2019, 141, 5497–5503. [Google Scholar] [CrossRef]
- Maeda, K.; Tamaki, S.; Tamura, K.; Yashima, E. Helicity Induction and Memory of the Macromolecular Helicity in a Polyacetylene Bearinga Biphenyl Pendant. Chem. Asian J. 2008, 3, 614–624. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, Y.R.; Jeon, H.G.; Kang, P.; Choi, M.-G.; Jeong, K.-S. A helically twisted imine macrocycle that allows for determining the absolute configuration of α-amino carboxylates. Chem. Commun. 2013, 49, 11412–11414. [Google Scholar] [CrossRef]
- Jeon, H.-G.; Kim, M.J.; Jeong, K.-S. An indolocarbazole dimer as a new stereodynamic probe for chiral 1,2-diamines. Org. Biomol. Chem. 2014, 12, 5464–5468. [Google Scholar] [CrossRef]
- Suk, J.; Naidu, V.R.; Liu, X.; Lah, M.S.; Jeong, K.-S. A Foldamer-Based Chiroptical Molecular Switch That Displays Complete Inversion of the Helical Sense upon Anion Binding. J. Am. Chem. Soc. 2011, 133, 13938–13941. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Cheran, A.; Kumar, J.; Srivastava, A. A Helically-Twisted Stereodynamic Probe for Chiroptical Sensing of Chiral Amines through Point-to-Helical Chirality Transmission. Chem. Asian J. 2025, e202401376. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, R.; Balzani, V.; Credi, A.; Gandolfi, M.T.; Venturi, M. Artificial Molecular-Level Machines: Which Energy To Make Them Work? Acc. Chem. Res. 2001, 34, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.E. Nanotechnology: Molecular Machinery, Manufacturing and Computation; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Koumura, N.; Zijlstra, R.W.J.; van Delden, R.A.; Harada, N.; Feringa, B.L. Light-driven monodirectional molecular rotor. Nature 1999, 401, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feringa, B.L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 2011, 331, 1429–1432. [Google Scholar] [CrossRef]
- Joyce, L.A.; Maynor, M.S.; Dragna, J.M.; da Cruz, G.M.; Lynch, V.M.; Canary, J.W.; Anslyn, E.V. A Simple Method for the Determination of Enantiomeric Excess and Identity of Chiral Carboxylic Acids. J. Am. Chem. Soc. 2011, 133, 13746–13752. [Google Scholar] [CrossRef]
- Setaka, W.; Nirengi, T.; Kabuto, C.; Kira, M. Introduction of Clutch Function into a Molecular Gear System by Silane−Silicate Interconversion. J. Am. Chem. Soc. 2008, 130, 15762–15763. [Google Scholar] [CrossRef]
- Pink, J.H.; Clayden, J. Concerted Rotation in a Tertiary Aromatic Amide: Towards a Simple Molecular Gear. Angew. Chem. Int. Ed. 1998, 37, 1937–1939. [Google Scholar] [CrossRef]
- Katoono, R.; Kawai, H.; Fujiwara, K.; Suzuki, T. Designed molecular propellers based on tetraarylterephthalamide and their chiroptical properties induced by biased helicity through transmission of point chirality. Chem. Comm. 2008, 4906–4908. [Google Scholar] [CrossRef]
- Katoono, R.; Kawai, H.; Fujiwara, K.; Suzuki, T. Dynamic Molecular Propeller: Supramolecular Chirality Sensing by Enhanced Chiroptical Response through the Transmission of Point Chirality to Mobile Helicity. J. Am. Chem. Soc. 2009, 131, 16896–16904. [Google Scholar] [CrossRef]
- Mislow, K. Stereochemical consequences of correlated rotation in molecular propellers. Acc. Chem. Res. 1976, 9, 26–33. [Google Scholar] [CrossRef]
- Iwamura, H.; Mislow, K. Stereochemical consequences of dynamic gearing. Acc. Chem. Res. 1988, 21, 175–182. [Google Scholar] [CrossRef]
- Jones, P.G.; Sheldrick, G.M.; Edwards, M.R.; Kirby, A.J. Bond length and reactivity: 1-arylethyl ethers and esters. 3. Structures of three trityl ethers of 1-arylethanols: 1-phenylethyl, 1-(4-chlorophenyl)ethyl and 1-(4-nitrophenyl)ethyl triphenylmethyl ethers. Acta. Cryst. 1986, C42, 1361–1364. [Google Scholar] [CrossRef]
- Kurland, R.J.; Schuster, I.I.; Colter, A.K. Fluorine Magnetic Resonance Studies of Conformational Equilibria in Triphenylcarbonium Ions. J. Am. Chem. Soc. 1965, 87, 2278–2279. [Google Scholar] [CrossRef]
- Murr, B.L.; Santiago, C.; Wang, S. Stereochemistry of trityl compounds. V. Resolution of phenylbiphenylyl-.alpha.-naphthylcarbinol. J. Am. Chem. Soc. 1969, 91, 3827–3831. [Google Scholar] [CrossRef]
- Ściebura, J.; Skowronek, P.; Gawroński, J. Trityl Ethers: Molecular Bevel Gears Reporting Chirality through Circular Dichroism Spectra. Angew. Chem. Int. Ed. 2009, 48, 7069–7072. [Google Scholar] [CrossRef]
- Mazzeo, G.; Scafato, P.; Superchi, S.; Rosini, C. The absolute configuration of simple aliphatic alcohols through a chemical/computational approach: Triarylether derivatives of (+)-endo-2-norborneol as a case study. Tetrahedron Asymmetry 2009, 20, 2435–2437. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Redden, B.K.; Sheppard, C.I.; Clark, R.W.; Smith, M.D.; Wiskur, S.L. Understanding Internal Chirality Induction of Triarylsilyl Ethers Formed from Enantiopure Alcohols. J. Org. Chem. 2016, 81, 8187–8193. [Google Scholar] [CrossRef]
- Skowronek, P.; Ścianowski, J.; Pacuła, A.J.; Gawroński, J. Chirality transfer through sulfur or selenium to chiral propellers. RSC Adv. 2015, 5, 69441–69444. [Google Scholar] [CrossRef]
- Ściebura, J.; Gawroński, J. Double chirality transmission in trityl amines: Sensing molecular dynamic stereochemistry by circular dichroism and DFT calculations. Chem. Eur. J. 2011, 17, 13138–13141. [Google Scholar] [CrossRef]
- Ściebura, J.; Janiak, A.; Stasiowska, A.; Grajewski, J.; Gawrońska, K.; Rychlewska, U.; Gawroński, J. Intramolecular Interactions of Trityl Groups. ChemPhysChem 2014, 15, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Prusinowska, N.; Bendzińska-Berus, W.; Jelecki, M.; Rychlewska, U.; Kwit, M. Triphenylacetic acid amides: Molecular propellers with induced chirality. Eur. J. Org. Chem. 2015, 4, 738–749. [Google Scholar] [CrossRef]
- Bendzińska-Berus, W.; Jelecki, M.; Kwit, M.; Rychlewska, U. Transfer of chirality in N-triphenylacetylamino acids and chiral derivatives of N-triphenylacetyl Gly–Gly dipeptide and control of their assembly with steric constraints. CrystEngComm 2019, 21, 3420. [Google Scholar] [CrossRef]
- Prusinowska, N.; Bendzińska-Berus, W.; Szymkowiak, J.; Warżajtis, B.; Gajewy, J.; Jelecki, M.; Rychlewska, U.; Kwit, M. Double helicity induction in chiral bis(triphenylacetamides). RSC Adv. 2015, 5, 83448–83458. [Google Scholar] [CrossRef]
- Prusinowska, N.; Czapik, A.; Kwit, M. Chiral triphenylacetic acid esters–residual stereoisomerism and solid-state variability of molecular architectures. J. Org. Chem. 2021, 86, 6433–6448. [Google Scholar] [CrossRef]
- Skowronek, P.; Czapik, A.; Rajska, Z.; Kwit, M. Molecular and supramolecular helicity induction in trityl group-containing compounds: The case of chiral 3,3,3-triphenylpropionic acid derivatives. Tetrahedron 2019, 75, 4497–4505. [Google Scholar] [CrossRef]
- Prusinowska, N.; Czapik, A.; Wojciechowska, M.; Kwit, M. Dynamic optical activity induction in the N-alkyl-N′-trityl ureas and thioureas. Org. Biomol. Chem. 2019, 17, 7782–7793. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Lynch, C.C.; Wolf, C. Dynamic Covalent Optical Chirality Sensing with a Sterically Encumbered Aminoborane. Chem. Eur. J. 2022, 28, e202202028. [Google Scholar] [CrossRef]
- Barnes, J.C.; Paton, J.D.; Damewood, J.R., Jr.; Mislow, K. Crystal and molecular structure of diphenylmethane. J. Org. Chem. 1981, 46, 4975–4979. [Google Scholar] [CrossRef]
- Ściebura, J.; Gawroński, J. Optical activity and helicity enhancement of diphenylmethyl molecular propellers. Tetrahedron Asymmetry 2013, 24, 683–688. [Google Scholar] [CrossRef]
- Grajewski, J.; Mądry, T.; Kwit, M.; Warżajtis, B.; Rychlewska, U.; Gawroński, J. Benzhydryl ethers of tartaric acid derivatives: Stereochemical response of dynamically chiral propeller. ChemPhysChem 2017, 18, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, S.; Pace, F.; Scafato, P.; Rosini, C. A new case of induced helical chirality in a bichromophoric system: Absolute configuration of transparent and flexible diols from the analysis of the electronic circular dichroism spectra of the corresponding di(1-naphthyl)ketals. Org. Lett. 2008, 10, 3421–3424. [Google Scholar] [CrossRef]
- Mądry, T.; Czapik, A.; Kwit, M. Optical activity and helicity enhancement of highly sensitive dinaphthylmethane-based stereodynamic probes for secondary alcohols. ACS Omega 2019, 4, 3244–3256. [Google Scholar] [CrossRef]
- Zhang, J.; Gholami, H.; Ding, X.; Chun, M.; Vasileiou, C.; Nehira, T.; Borhan, B. Computationally Aided Absolute Stereochemical Determination of Enantioenriched Amines. Org. Lett. 2017, 19, 1362–1365. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, W.; Gholami, H.; Nehira, T.; Borhan, B. Di(1-naphthyl) methanol ester of carboxylic acids for absolute stereochemical determination. Chirality 2018, 30, 141–146. [Google Scholar] [CrossRef]
- Kohlbouni, T.S.; Sarkar, A.; Zhang, J.; Li, X.; Borhan, B. Absolute stereochemical determination of 1,2-diols via complexation with dinaphthyl borinic acid. Chirality 2020, 32, 817–823. [Google Scholar] [CrossRef]
- Rubio, M.; Merchán, M.; Ortí, E.; Roos, B.O. A theoretical study of the electronic spectrum of biphenyl. Chem. Phys. Lett. 1995, 234, 373–381. [Google Scholar] [CrossRef]
- Fukuda, R.; Ehara, M. Electronic excited states and electronic spectra of biphenyl: A study using many-body wavefunction methods and density functional theories. Phys. Chem. Chem. Phys. 2013, 15, 17426–17434. [Google Scholar] [CrossRef]
- Sagiv, J.; Yogev, A.; Mazur, Y. Application of Linear Dichroism to the Analysis of Electronic Absorption Spectra of Biphenyl, Fluorene, 9,9′-Spirobifluorene, and [6.6]Vespirene. Interpretation of the Circular Dichroism Spectrum of [6.6]Vespirene. J. Am. Chem. Soc. 1977, 99, 6861–6869. [Google Scholar] [CrossRef]
- Edwards, L.O.; Simpson, W.T. Polarizations of Electronic Transitions from Azimuthal Variation in Fluorescence Intensity—With Application to Biphenyl. J. Chem. Phys. 1970, 53, 4237–4244. [Google Scholar] [CrossRef]
- Rashidi-Ranjbar, P.; Sandström, J. The UV and CD Spectra of a 60° Twisted Bridged Biphenyl: An Experimental and CNDO/S Study. J. Mol. Struct. 1991, 246, 25–32. [Google Scholar] [CrossRef]
- Prusinowska, N.; Szymkowiak, J.; Kwit, M. Unravelling Structural Dynamics, Supramolecular Behavior and Chiroptical Properties of Enantiomerically Pure Macrocyclic Tertiary Ureas and Thioureas. J. Org. Chem. 2023, 88, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Superchi, S.; Casarini, D.; Laurita, A.; Bavoso, A.; Rosini, C. Induction of a preferred twist in a biphenyl core by stereogenic centers: A novel approach to the absolute configuration of 1,2- and 1,3-diols. Angew. Chem. Int. Ed. 2001, 40, 451–454. [Google Scholar] [CrossRef]
- Santoro, E.; Vergura, S.; Scafato, P.; Belviso, S.; Masi, M.; Evidente, A.; Superchi, S. Absolute Configuration Assignment to Chiral Natural Products by Biphenyl Chiroptical Probes: The Case of the Phytotoxins Colletochlorin A and Agropyrenol. J. Nat. Prod. 2020, 83, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kwit, M.; Rychlewska, U.; Gawroński, J. Induced Homohelicity of Diphenimide Bis-propellers. New J. Chem. 2002, 26, 1714–1717. [Google Scholar] [CrossRef]
- Vergura, S.; Pisani, L.; Scafato, P.; Casarini, D.; Superchi, S. Central-to-axial chirality induction in biphenyl chiroptical probes for the stereochemical characterization of chiral primary amines. Org. Biomol. Chem. 2018, 16, 555–565. [Google Scholar] [CrossRef]
- Kuwahara, S.; Nakamura, M.; Yamaguchi, A.; Ikeda, M.; Habata, Y. Combination of a New Chiroptical Probe and Theoretical Calculations for Chirality Detection of Primary Amines. Org. Lett. 2013, 15, 5738–5741. [Google Scholar] [CrossRef]
- Kuwahara, S.; Tasaki, N.; Suzuki, Y.; Nakagawa, M.; Ikeda, M.; Habata, Y. 3-Menthoxybiphenyl-4-carboxylic acid: A versatile resolving agent and reagent for determination of the absolute configuration of benzylic alcohols. Tetrahedron Asymmetry 2017, 28, 945–953. [Google Scholar] [CrossRef]
- Sahnoun, R.; Koseki, S.; Fujimura, Y.J. Density Functional Theoretical Study on Enantiomerization of 2,2′-Biphenol. Phys. Chem. A 2006, 110, 2440–2447. [Google Scholar] [CrossRef]
- Gajewy, J.; Gawroński, J.; Kwit, M. Mechanism and Enantioselectivity of [Zinc(Diamine)(Diol)]-Catalyzed Asymmetric Hydrosilylation of Ketones: DFT, NMR and ECD Study. Eur. J. Org. Chem. 2013, 2013, 307–318. [Google Scholar] [CrossRef]
- Bentley, K.W.; Wolf, C. Chirality Sensing of Amines, Diamines, Amino Acids, Amino Alcohols, and α-Hydroxy Acids with a Single Probe. J. Am. Chem. Soc. 2013, 135, 18052–18055. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. Regioselective Substitution of BINOL. Chem. Rev. 2024, 124, 6643–6689. [Google Scholar] [CrossRef]
- Iwaniuk, D.P.; Wolf, C. A Stereodynamic Probe Providing a Chiroptical Response to Substrate-Controlled Induction of an Axially Chiral Arylacetylene Framework. J. Am. Chem. Soc. 2011, 133, 2414–2417. [Google Scholar] [CrossRef]
- De Los Santos, Z.A.; Joyce, L.A.; Sherer, E.C.; Welch, C.J.; Wolf, C. Optical Chirality Sensing with a Stereodynamic Aluminum Biphenolate Probe. J. Org. Chem. 2019, 84, 4636–4645. [Google Scholar] [CrossRef]
- Bentley, K.W.; Joyce, L.A.; Sherer, E.C.; Sheng, H.; Wolf, C.; Welch, C.J. Antenna Biphenols: Development of Extended Wavelength Chiroptical Reporters. J. Org. Chem. 2016, 81, 1185–1191. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Wolf, C. Enantioselective CD analysis of amino acids based on chiral amplification with a stereodynamic probe. Tetrahedron 2010, 66, 3989–3994. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Wolf, C. Enantioselective recognition of amines with an atropisomeric 1,8-bisphenolnaphthalene. Tetrahedron 2011, 67, 6799–6803. [Google Scholar] [CrossRef]
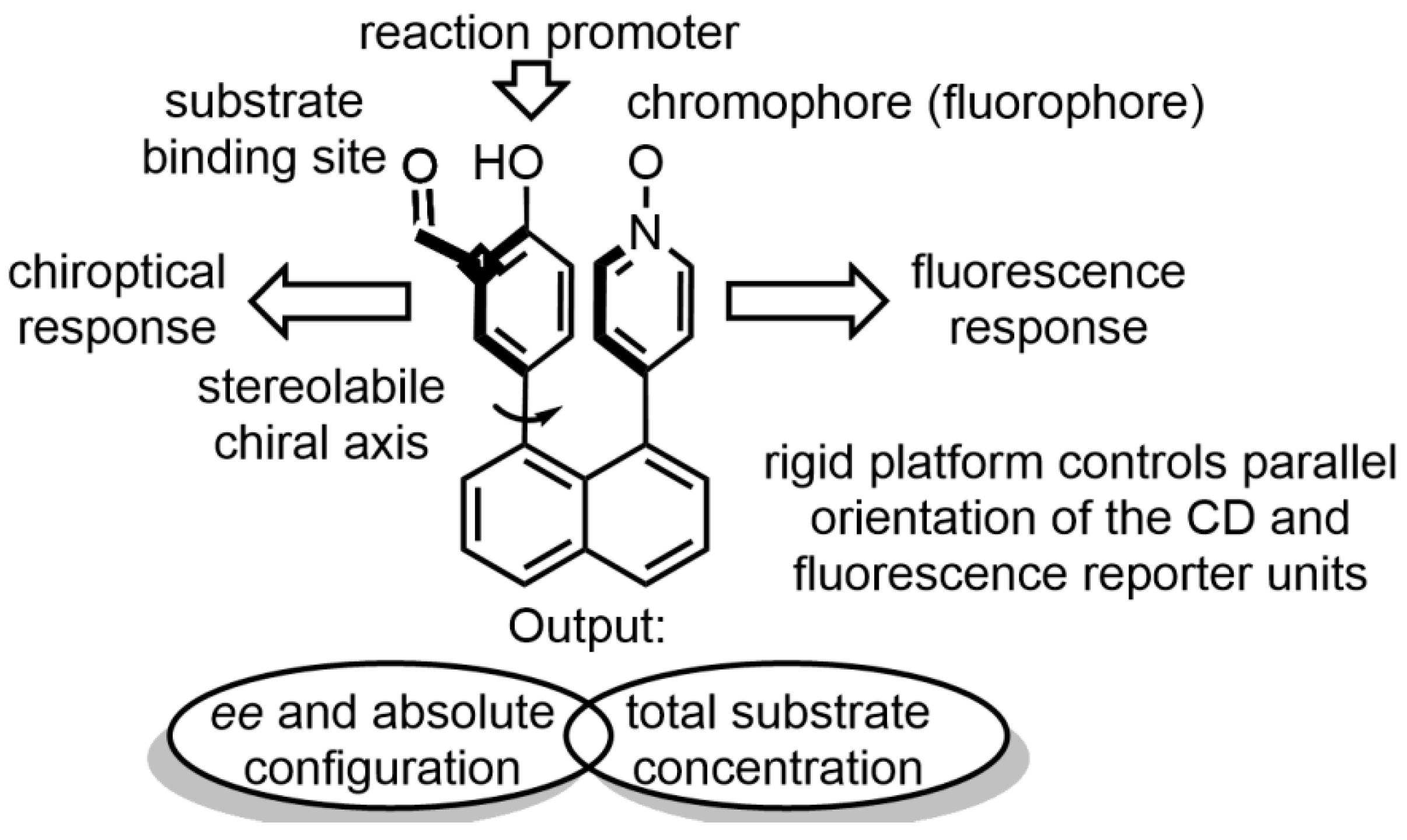
- Bentley, K.W.; Wolf, C. Stereodynamic Chemosensor with Selective Circular Dichroism and Fluorescence Readout for in Situ Determination of Absolute Configuration, Enantiomeric Excess, and Concentration of Chiral Compounds. J. Am. Chem. Soc. 2013, 135, 12200–12203. [Google Scholar] [CrossRef]
- Bentley, K.W.; Wolf, C. Comprehensive Chirality Sensing: Development of Stereodynamic Probes with a Dual (Chir)optical Response. J. Org. Chem. 2014, 79, 6517–6531. [Google Scholar] [CrossRef]
- Iwaniuk, D.P.; Wolf, C. Enantioselective Sensing of Amines Based on [1 + 1]-, [2 + 2]-, and [1 + 2]-Condensation with Fluxional Arylacetylene-Derived Dialdehydes. Org. Lett. 2011, 13, 2602–2605. [Google Scholar] [CrossRef]
- Iwaniuk, D.P.; Bentley, K.W.; Wolf, C. Enantioselective sensing of chiral amino alcohols with a stereodynamic arylacetylene-based probe. Chirality 2012, 24, 584–589. [Google Scholar] [CrossRef] [PubMed]
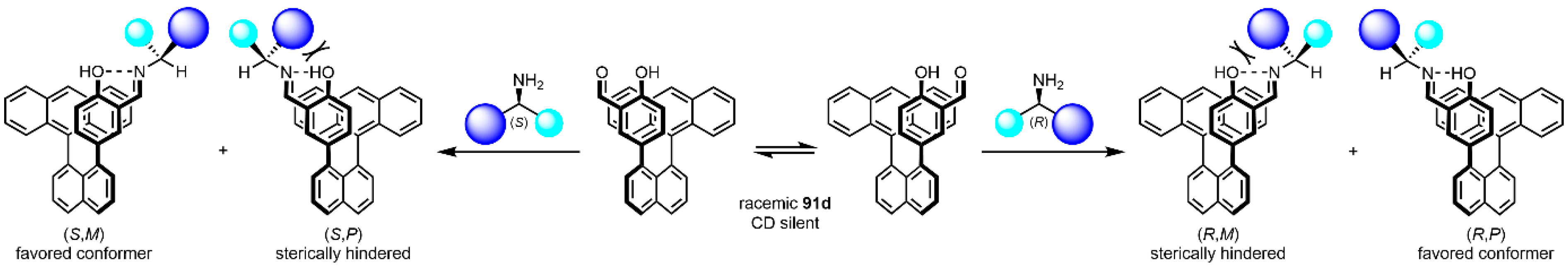
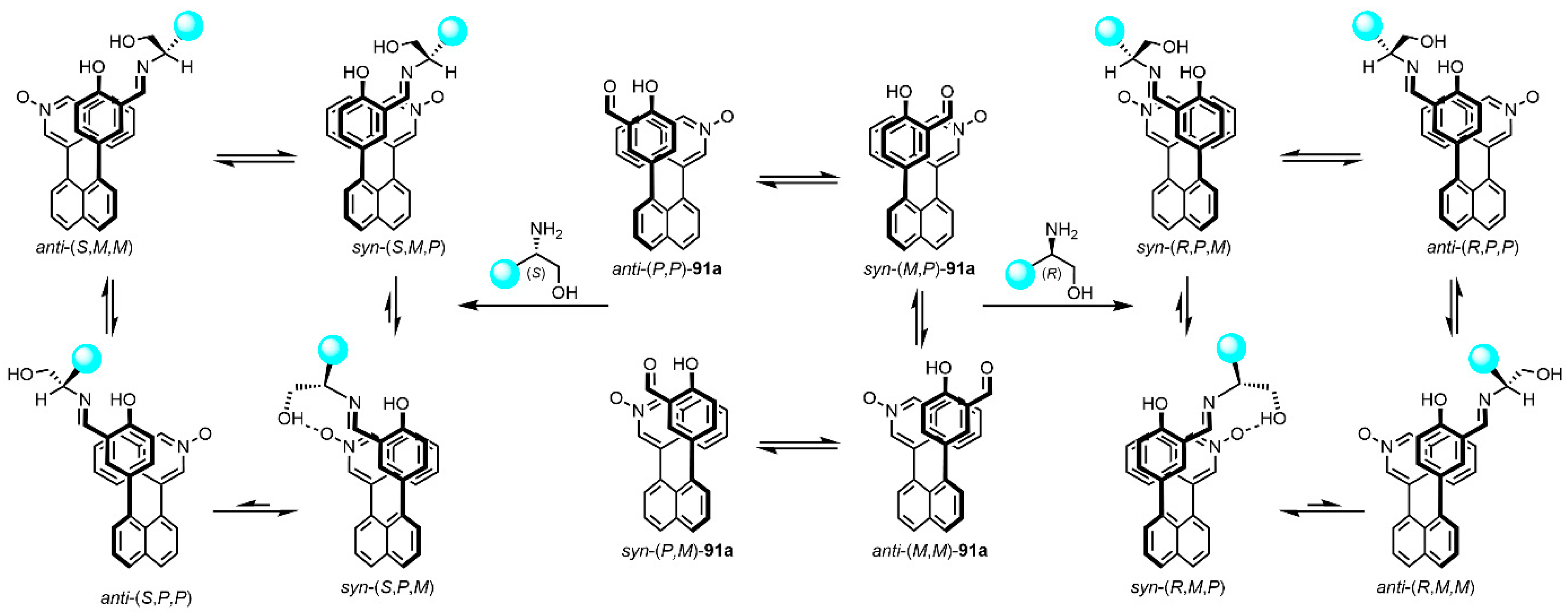
- Mądry, T.; Czapik, A.; Kwit, M. Point-to-axial chirality transmission: A highly sensitive triaryl chirality probe for stereochemical assignments of amines. J. Org. Chem. 2020, 85, 10413–10431. [Google Scholar] [CrossRef] [PubMed]
- Mądry, T.; Czapik, A.; Kwit, M. ‘Double-twist’-based dynamic induction of optical activity in multichromophoric system. Symmetry 2021, 13, 325. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Cheng, Y.; Yao, Y.; Li, R.; Liu, Q.; Yang, H.; Chen, X. Stimuli-Responsive Photoluminescent Molecular Tweezers for Highly Enantioselective Discrimination of Chiral Primary Amines. Anal. Chem. 2024, 96, 19632–19640. [Google Scholar] [CrossRef]
- Chen, H.; Xia, L.; Li, G. Recent progress of chiral metal–organic frameworks in enantioselective separation and detection. Microchim. Acta 2024, 191, 640. [Google Scholar] [CrossRef]
- Huang, L.; Hu, C.; Wang, Y. Chirality Sensing of Chiral Carboxylic Acids by a Ureido-Linked Zinc Bisporphyrinate. Chem. Asian J. 2024, 19, e202400359. [Google Scholar] [CrossRef]
- Della Sala, P.; Calice, U.; Iuliano, V.; Geremia, S.; Hickey, N.; Belviso, S.; Summa, F.F.; Monaco, G.; Gaeta, C.; Superchi, S. Chirality Sensing of Cryptochiral Guests with Prism[n]arenes. Chem. Eur. J. 2024, 30, e202401625. [Google Scholar] [CrossRef]
- Nelson, E.; Bertke, J.A.; Thanzeel, F.Y.; Wolf, C. Organometallic Chirality Sensing via “Click”-like η6-Arene Coordination with an Achiral Cp*Ru(II) Piano Stool Complex. Angew. Chem. Int. Ed. 2024, 63, e202404594. [Google Scholar] [CrossRef]
- Formen, J.S.S.K.; Wolf, C. Optical Relay Sensing of Cryptochiral Alcohols Displaying α-, β-, γ- and δ-Stereocenters or Chirality by Virtue of Isotopic Substitution. Angew. Chem. Int. Ed. 2024, 63, e202409790. [Google Scholar] [CrossRef]
- Formen, J.S.S.K.; Nelson, E.; Wolf, C. Molecular Sensing of Chiral Carboxylic Acid Enantiomers Using CD Inductions in the Visible Light Region. J. Org. Chem. 2025, 90, 994–1000. [Google Scholar] [CrossRef]





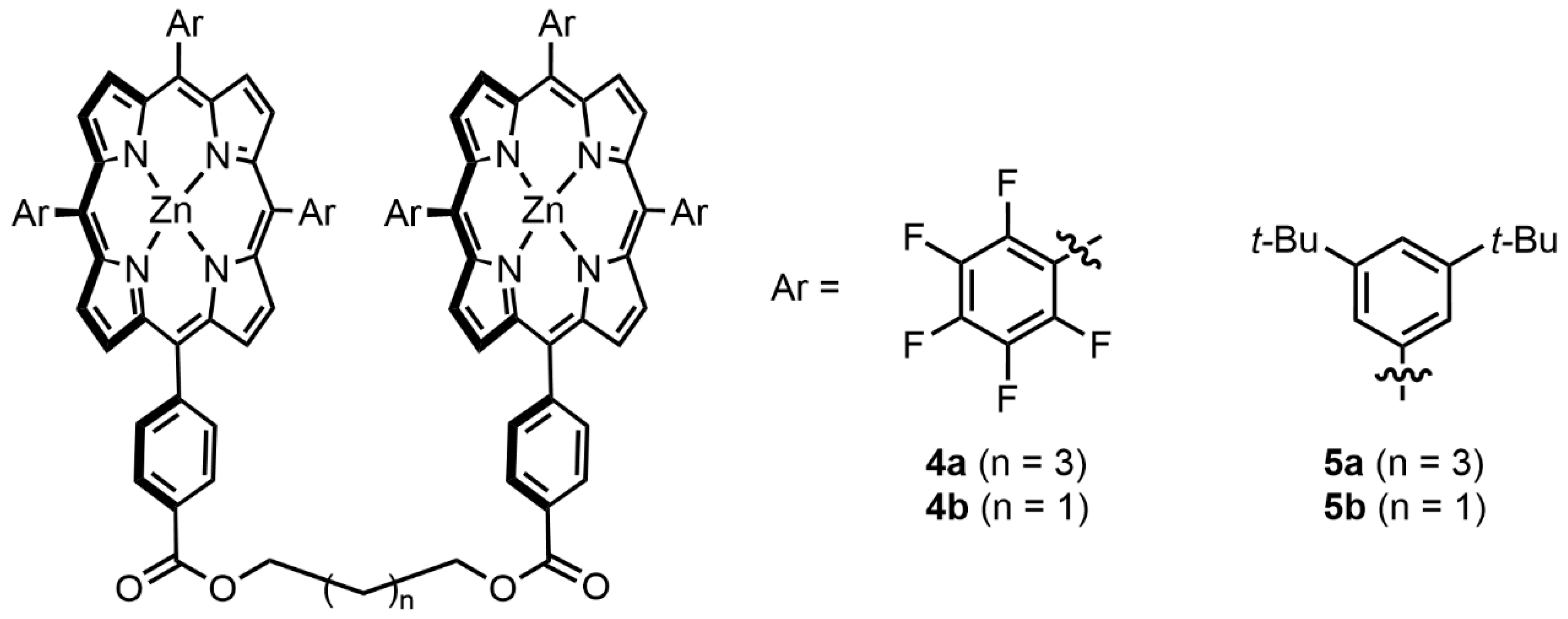
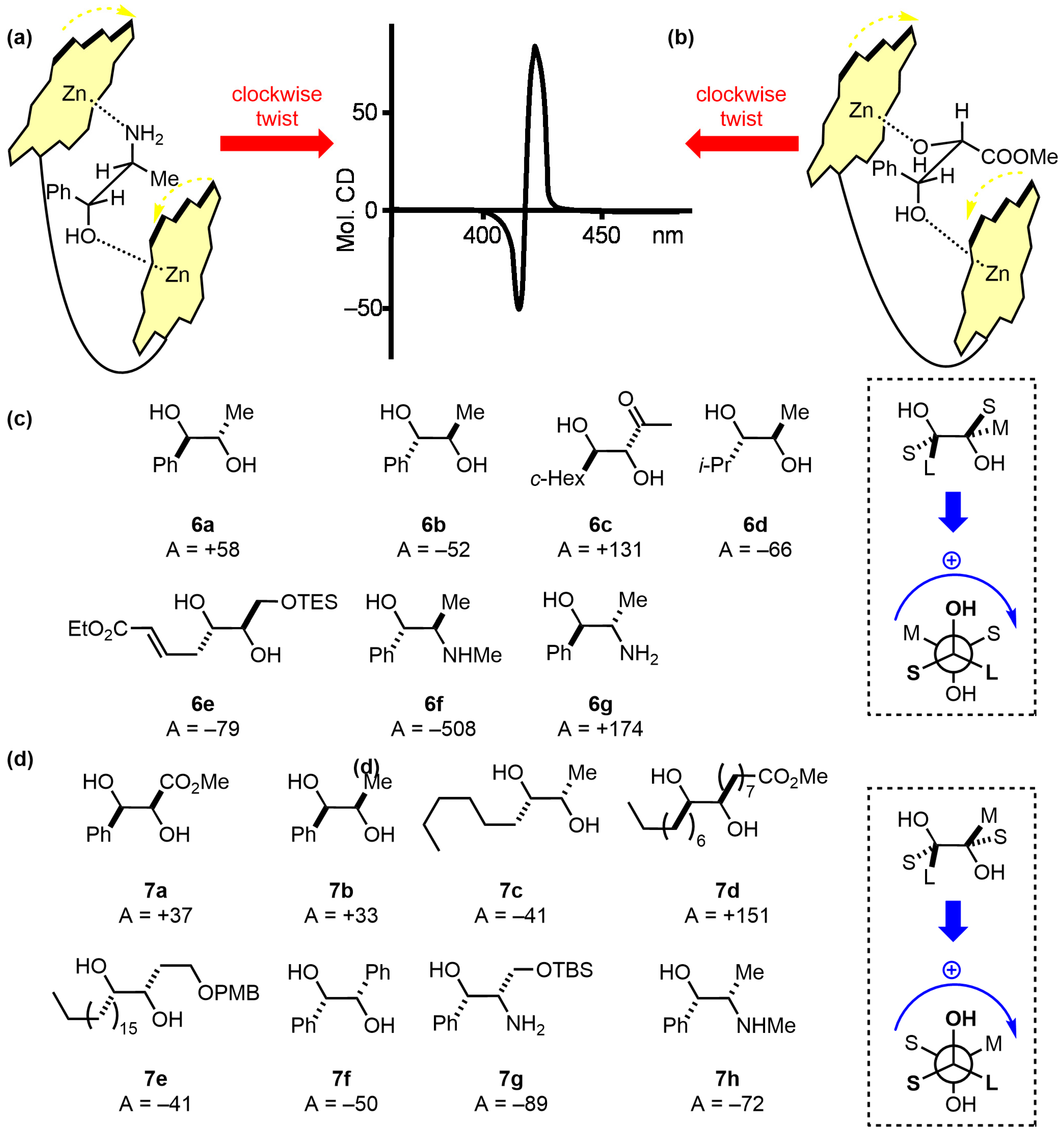
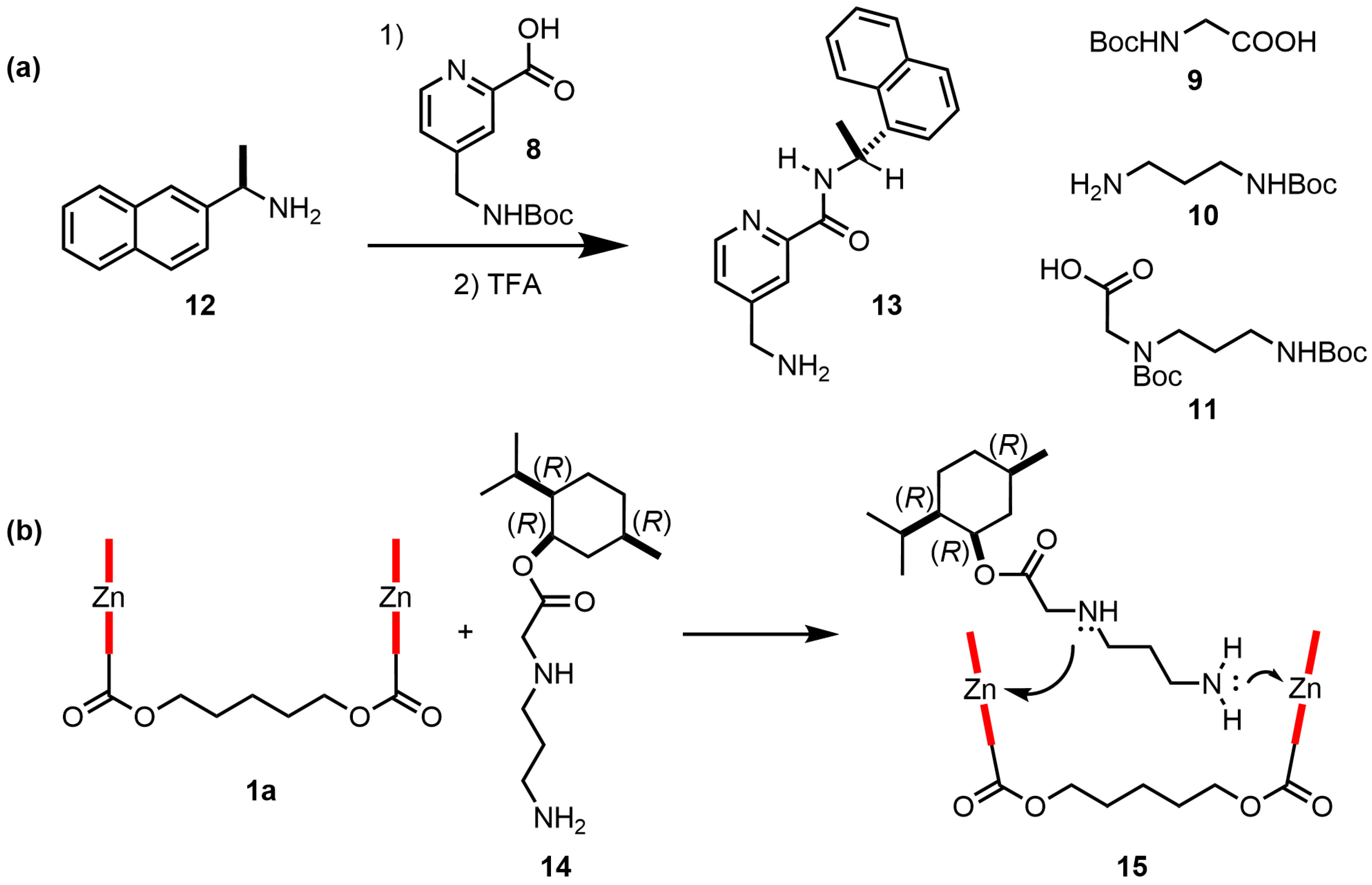
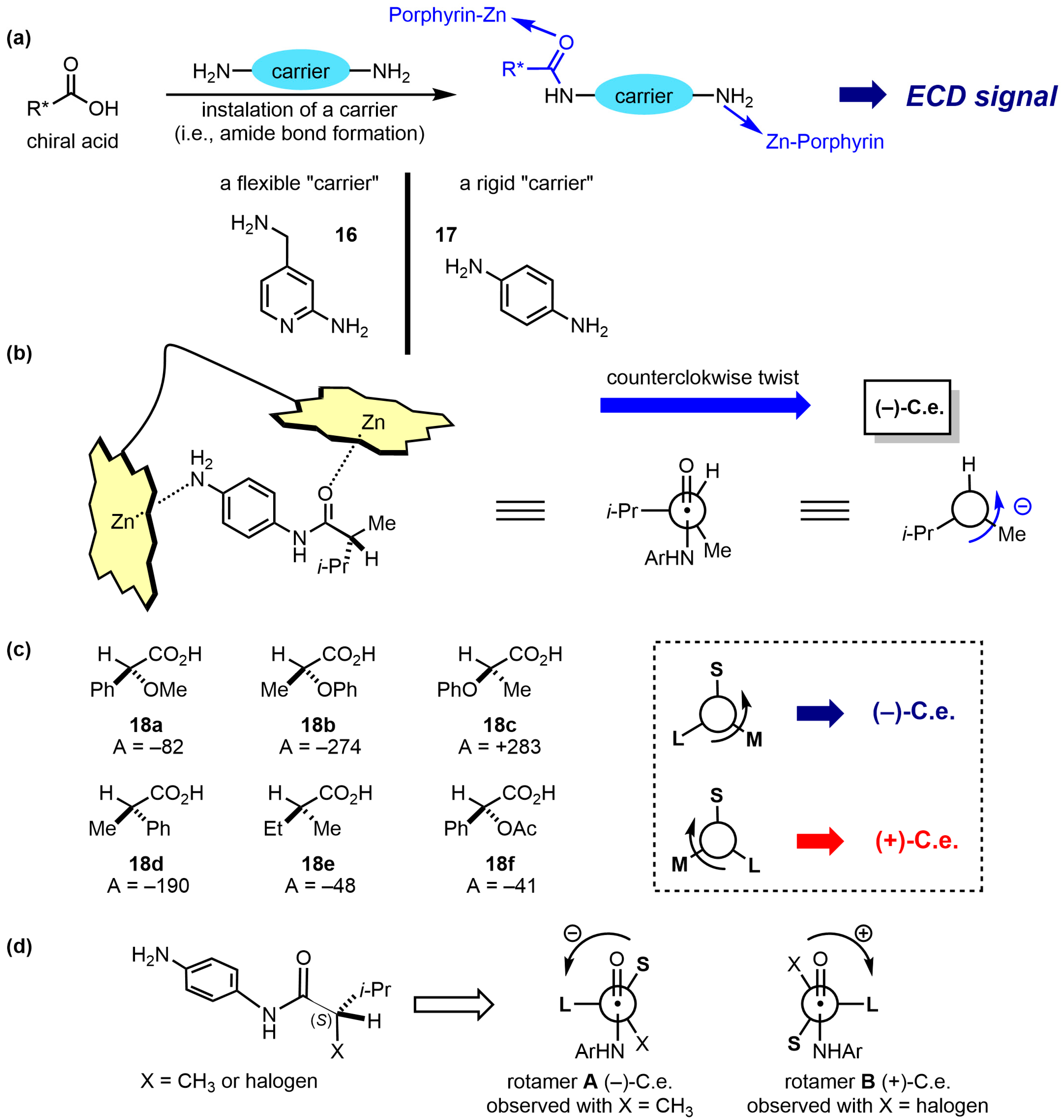

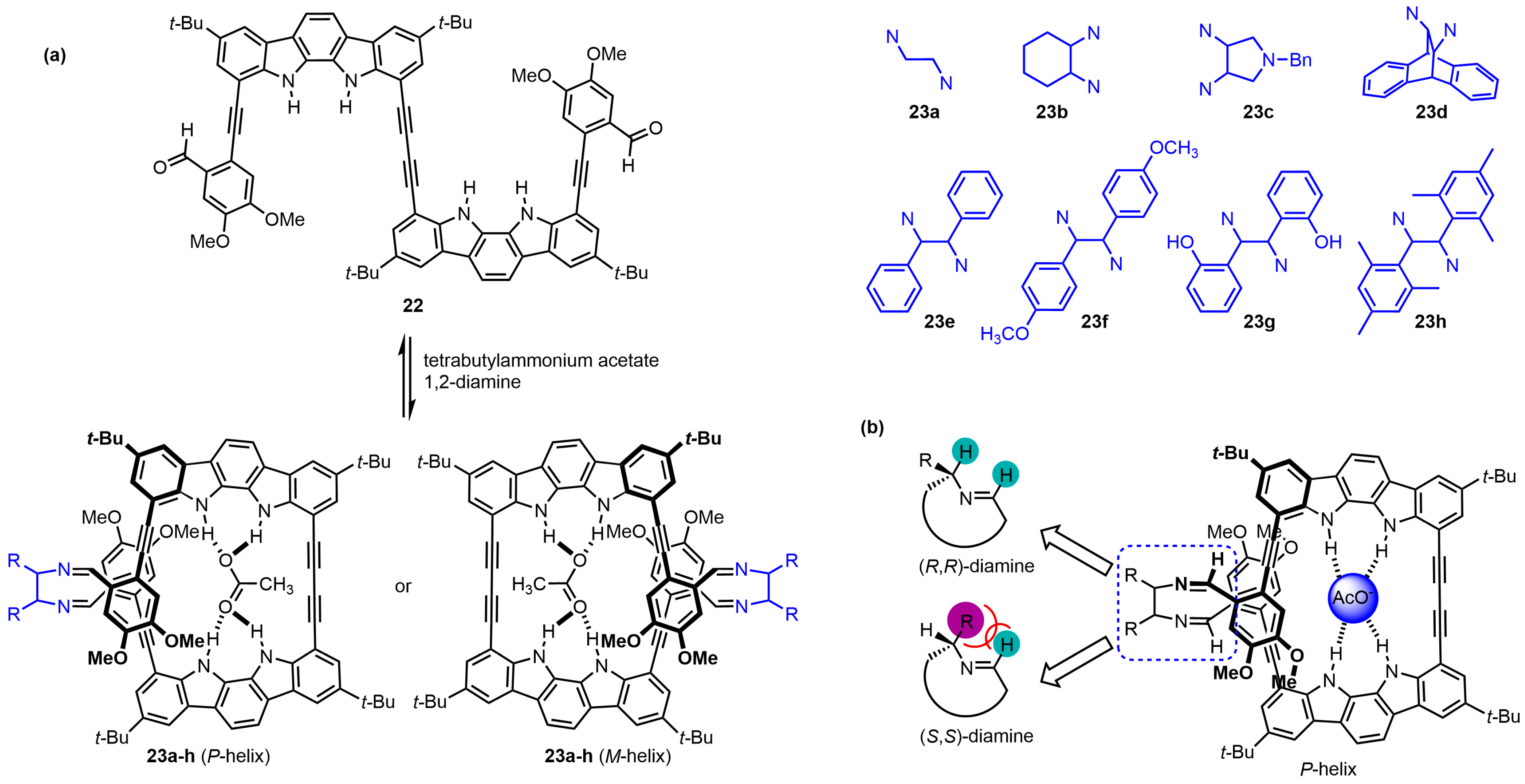
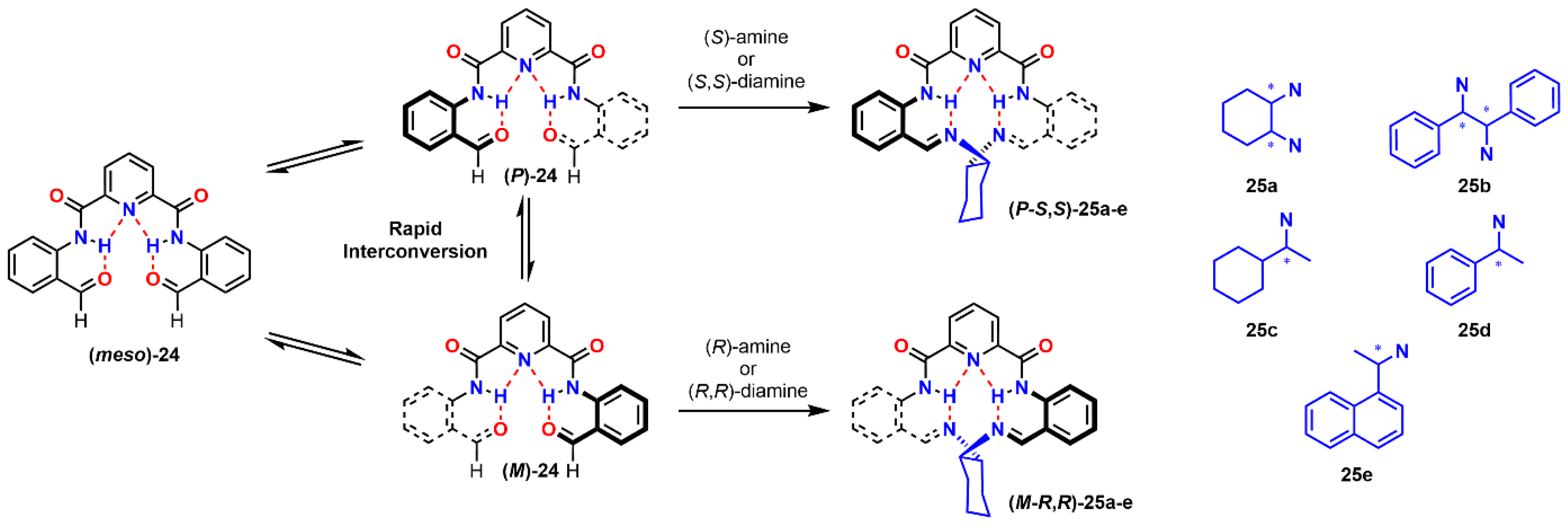

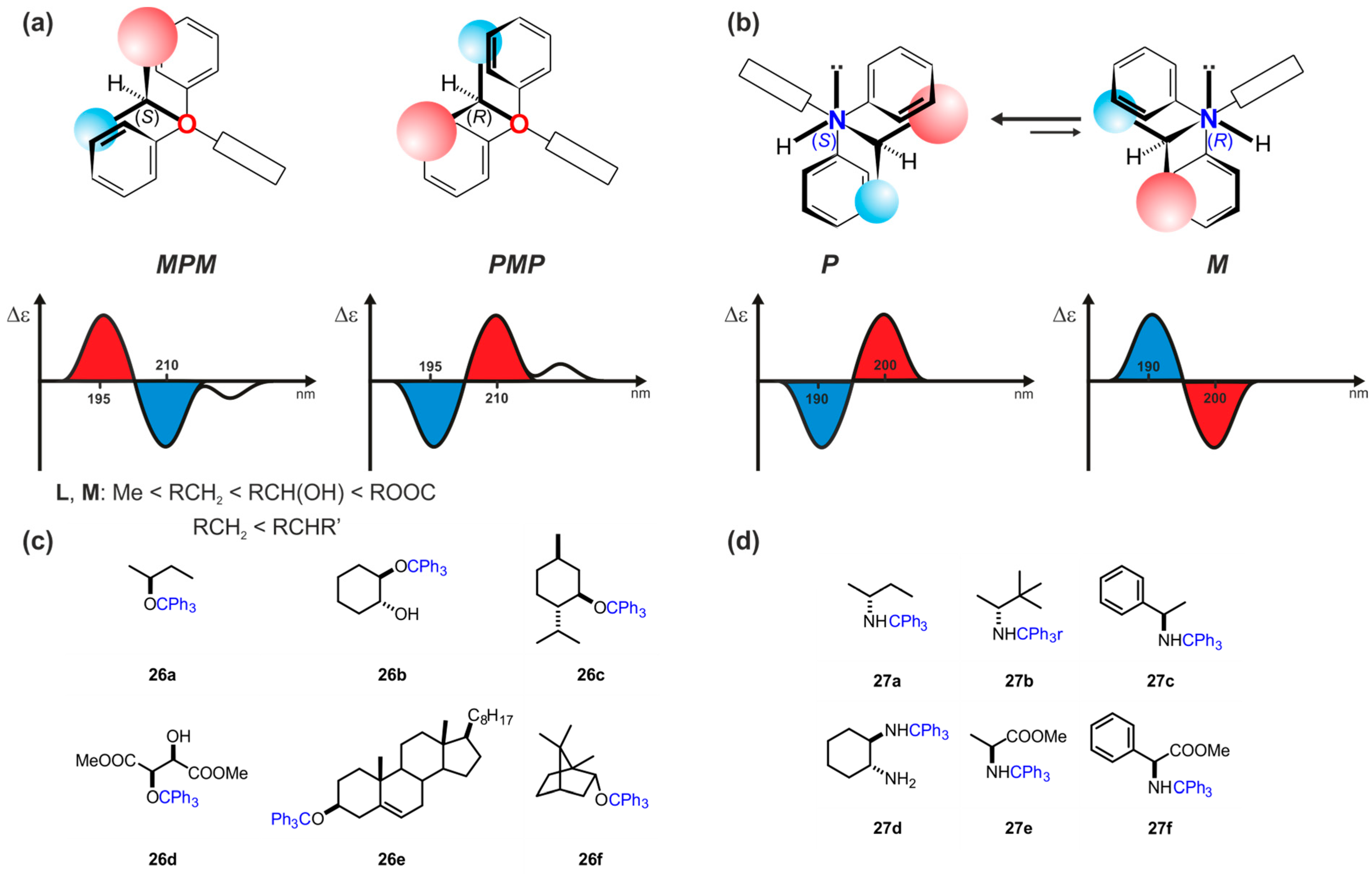



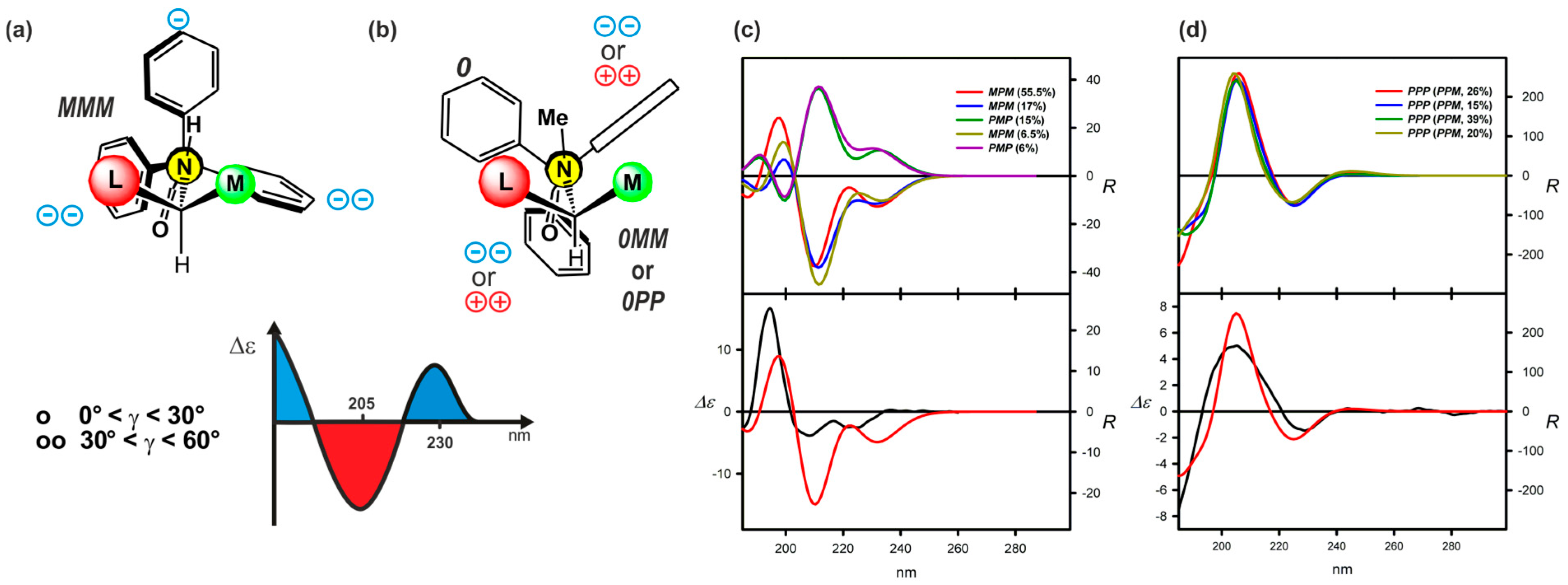


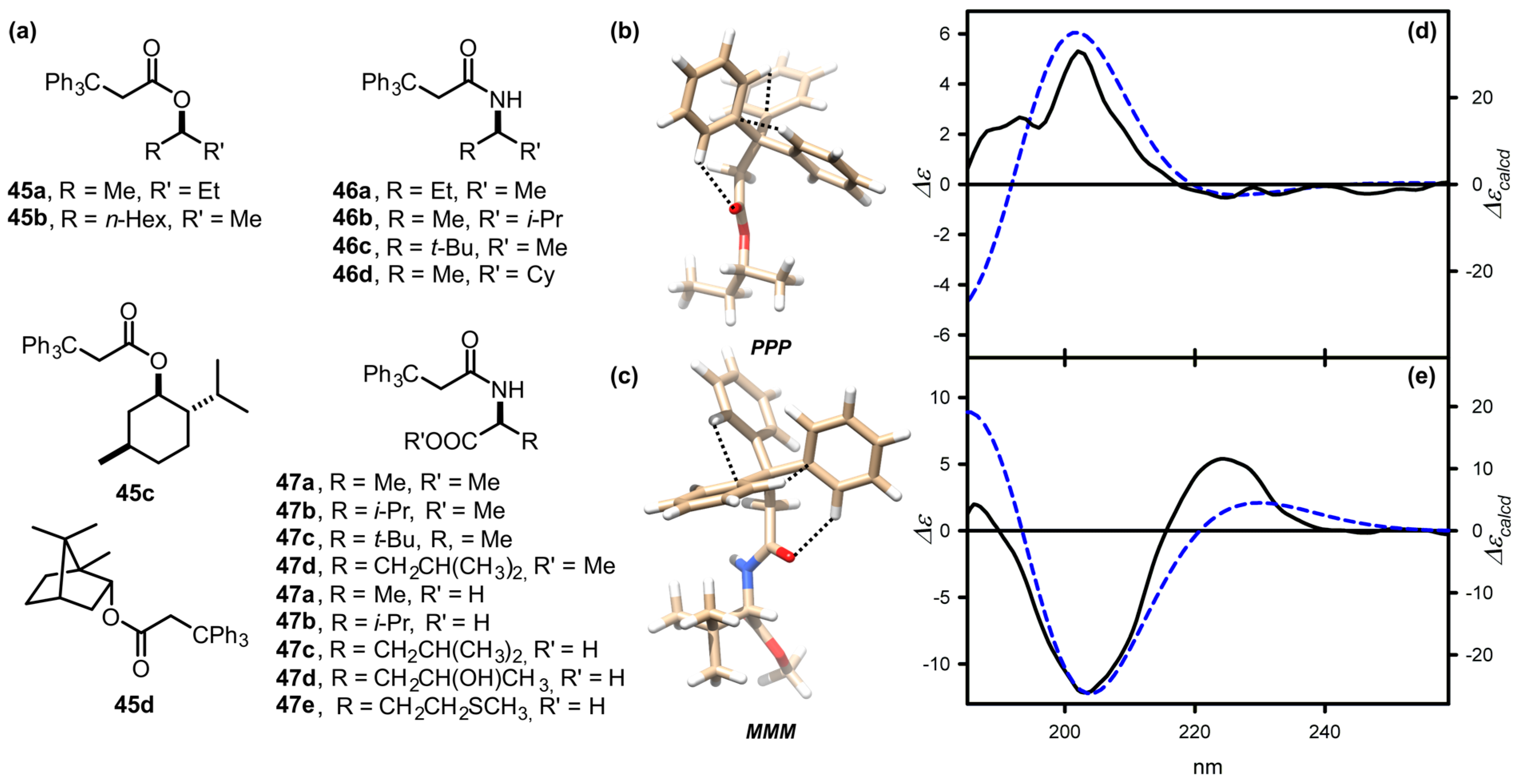



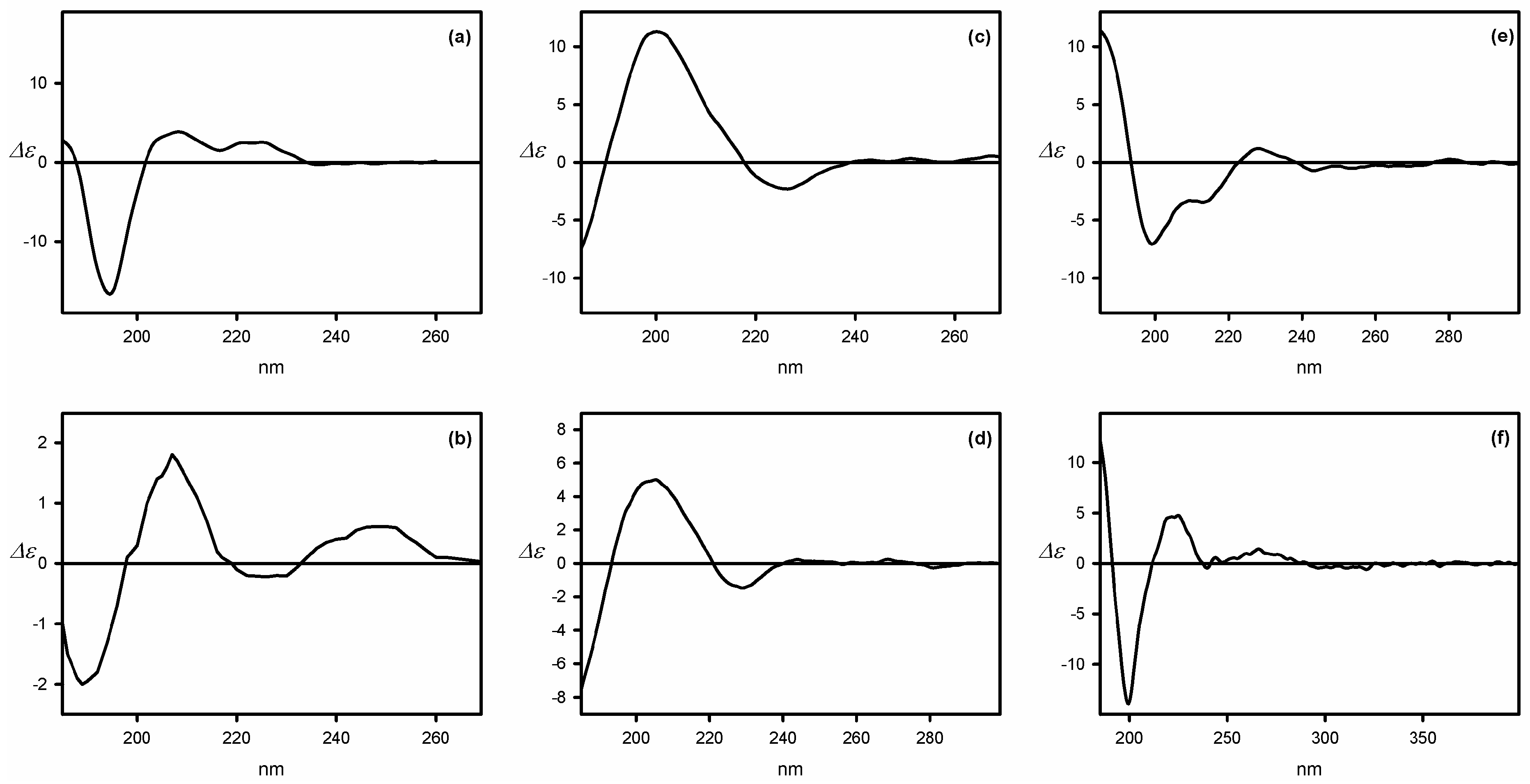

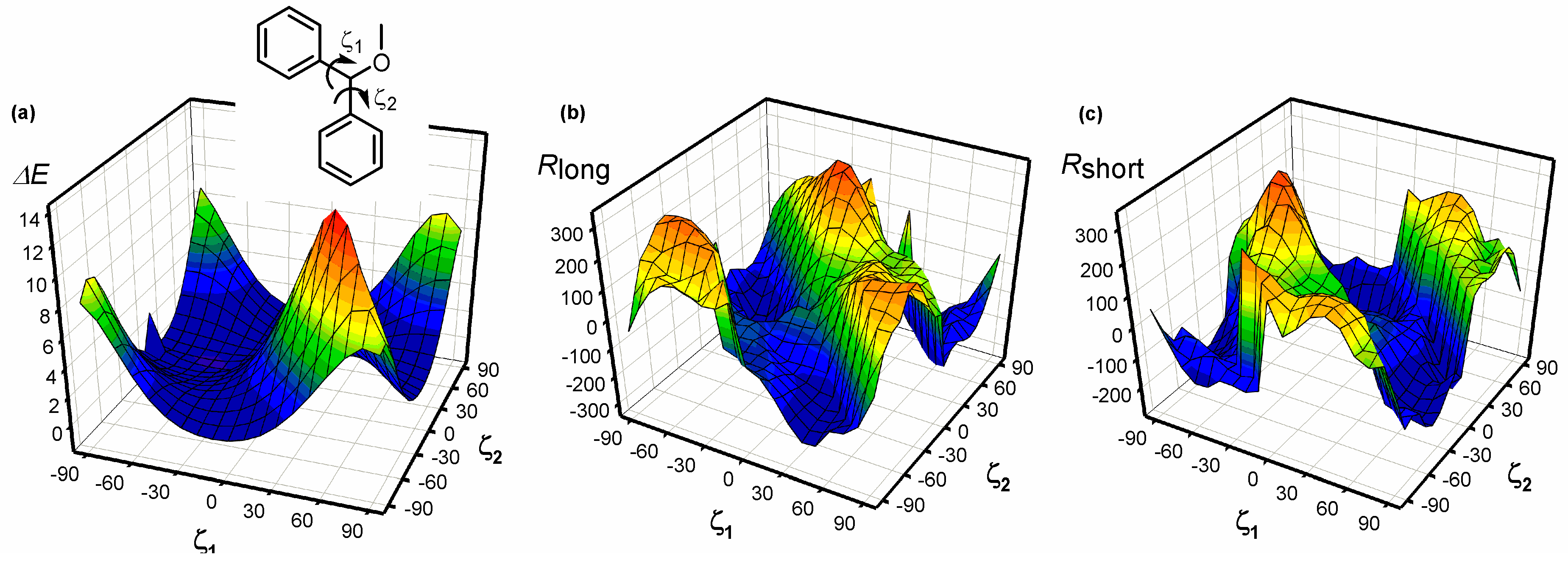
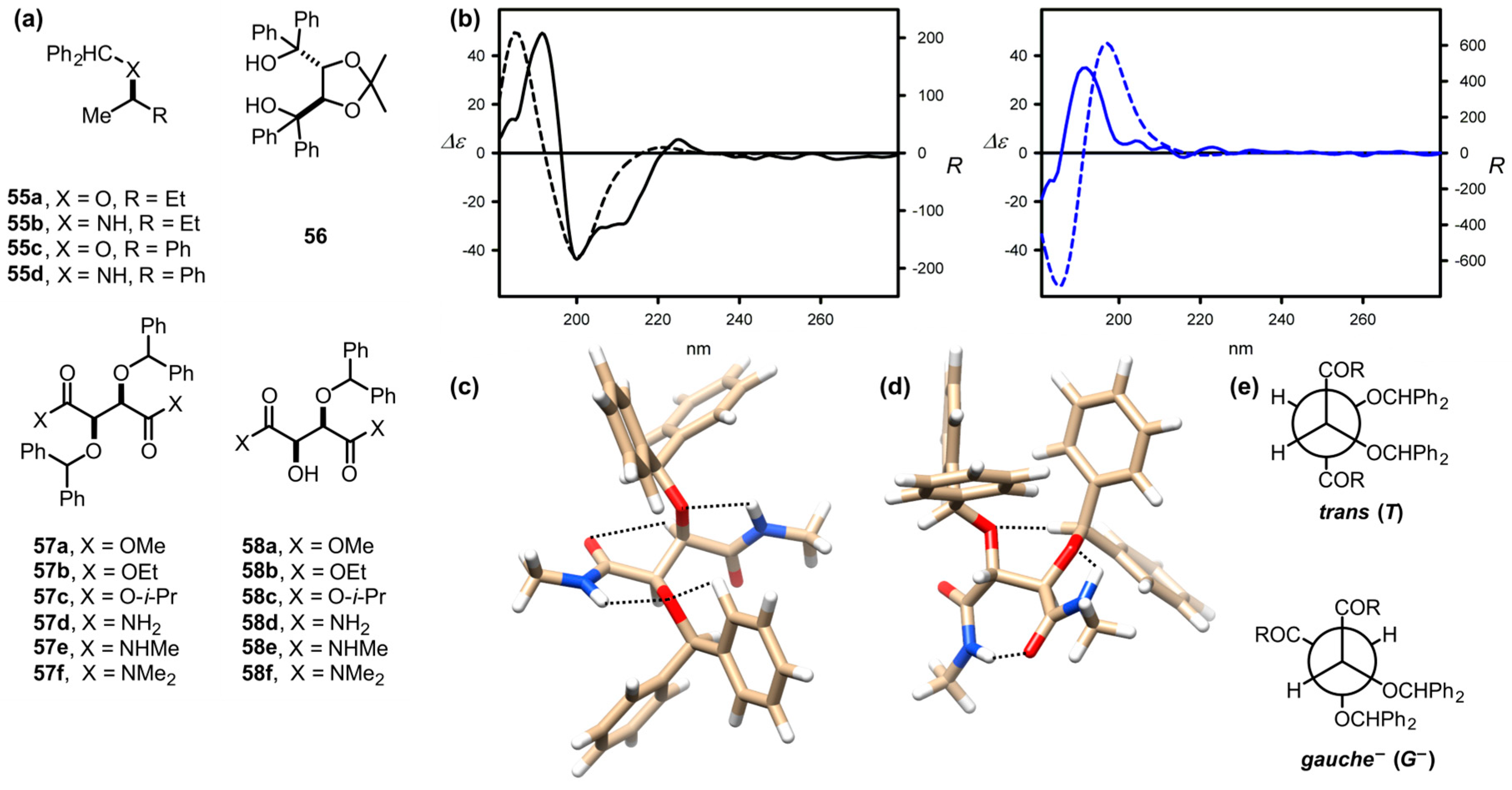





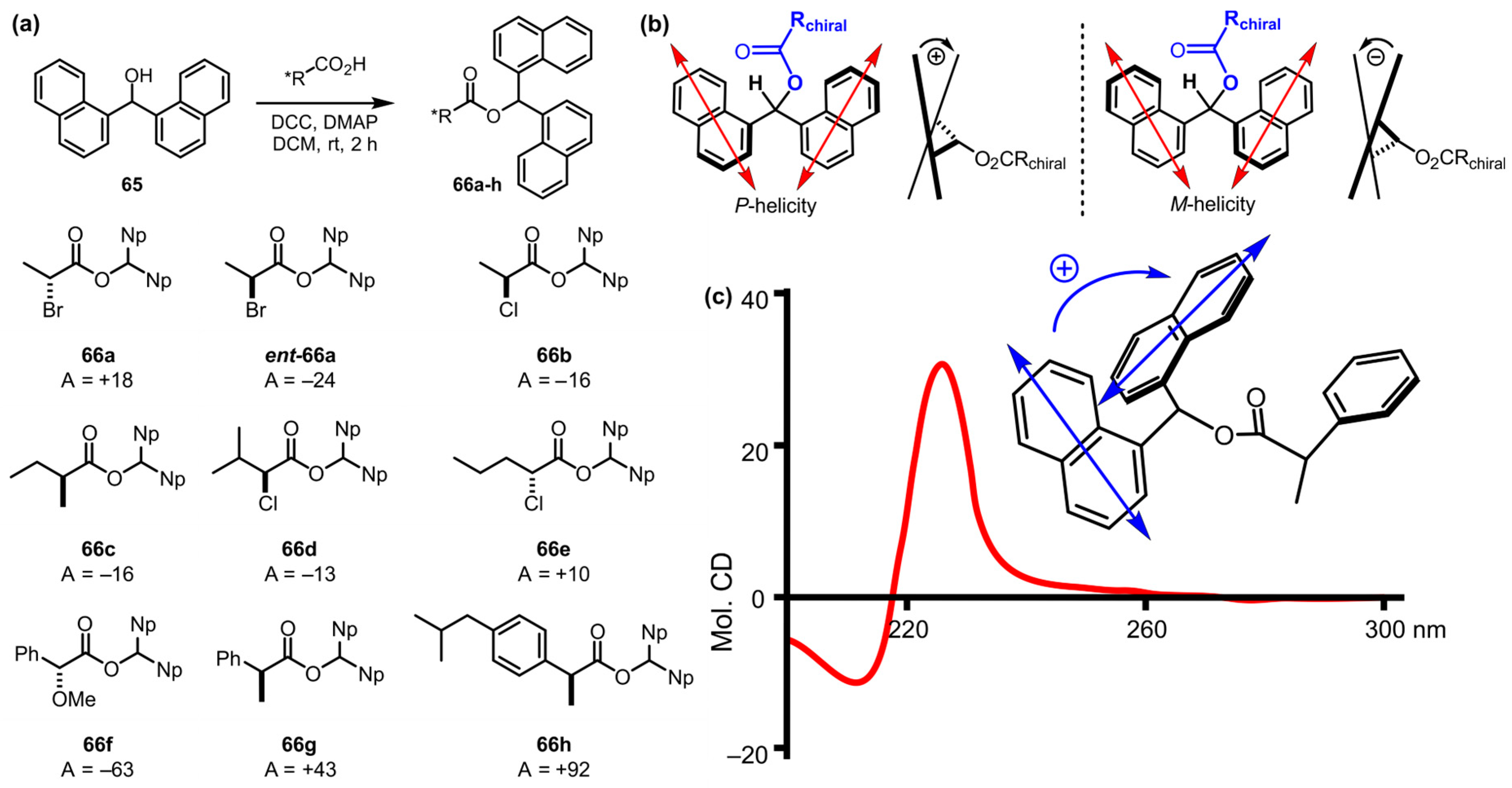

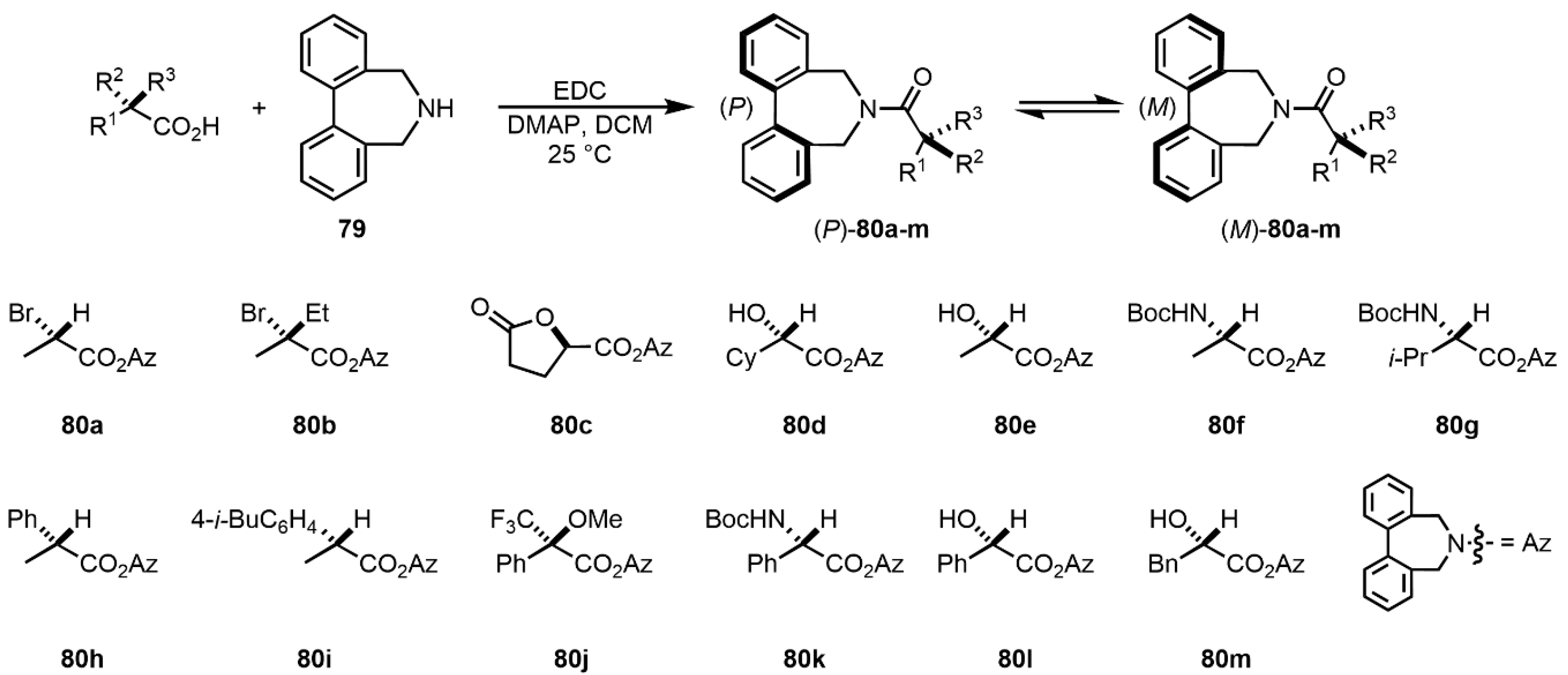
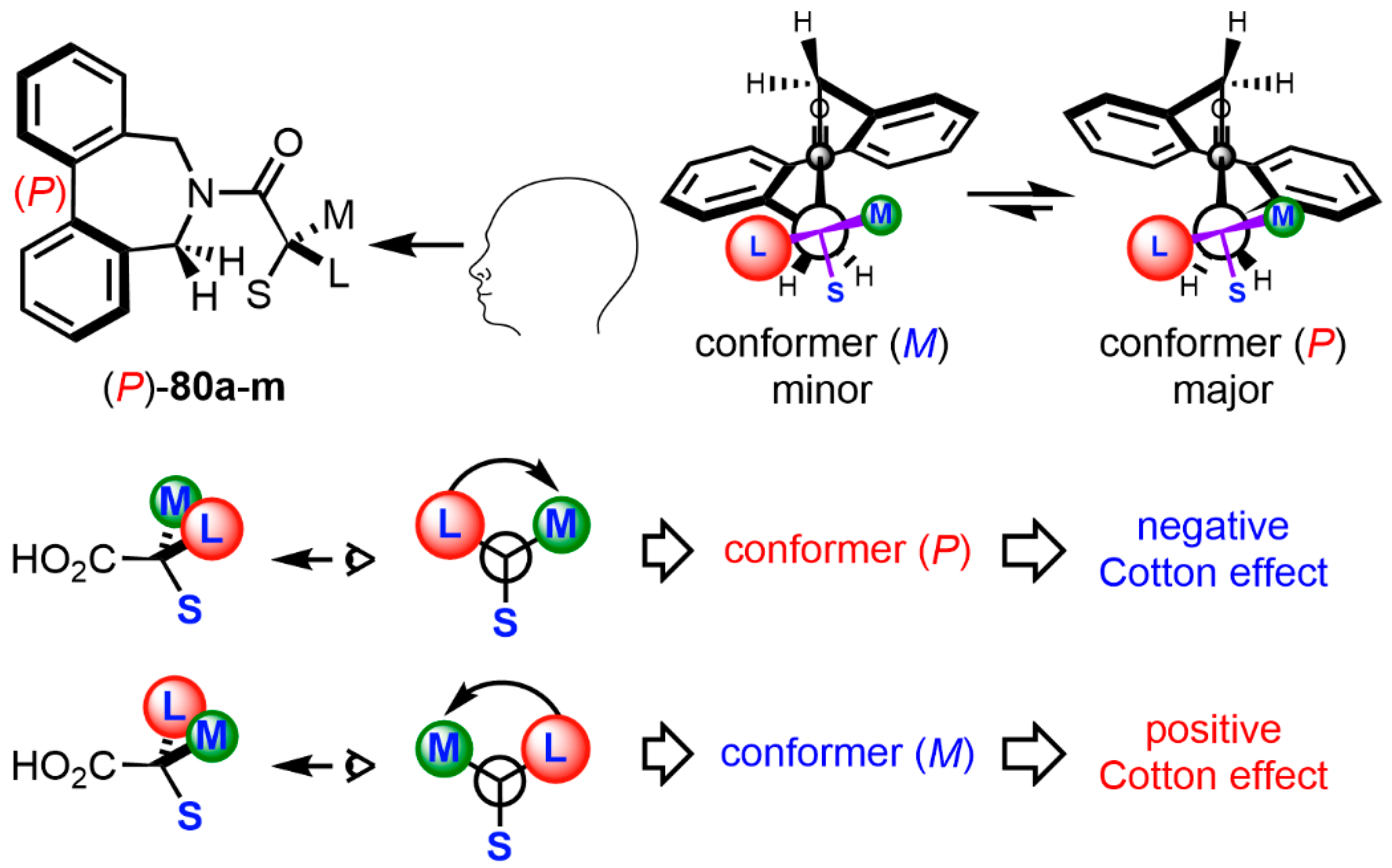


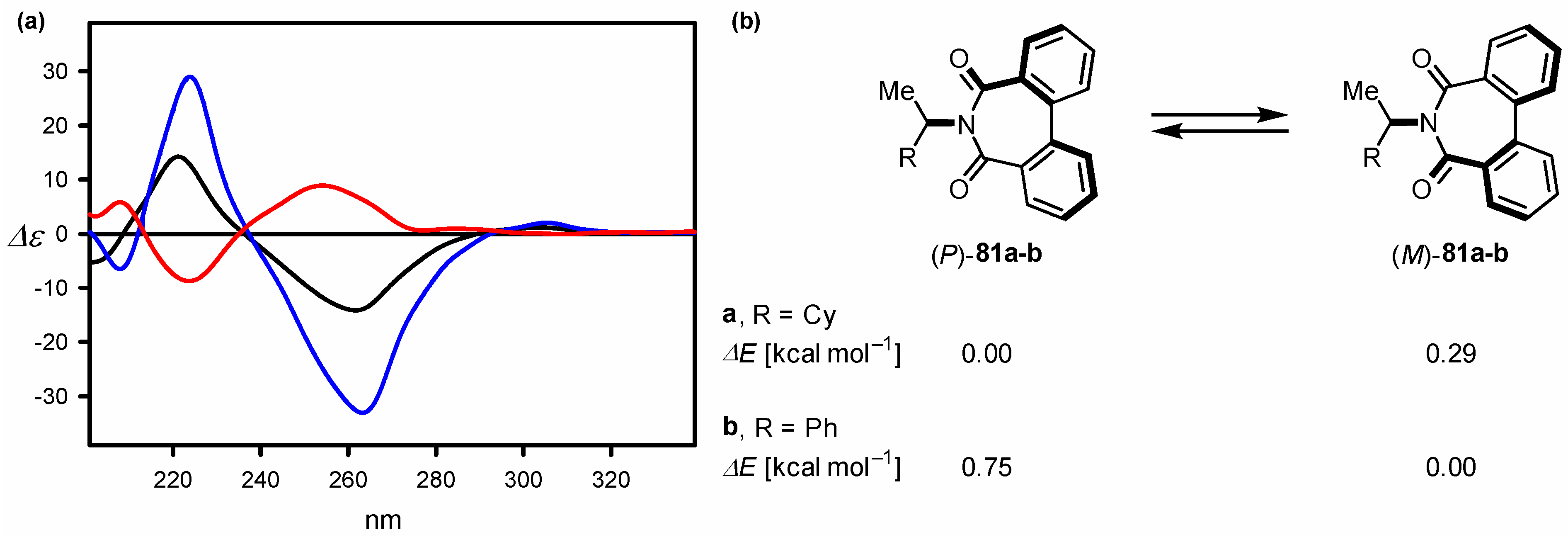


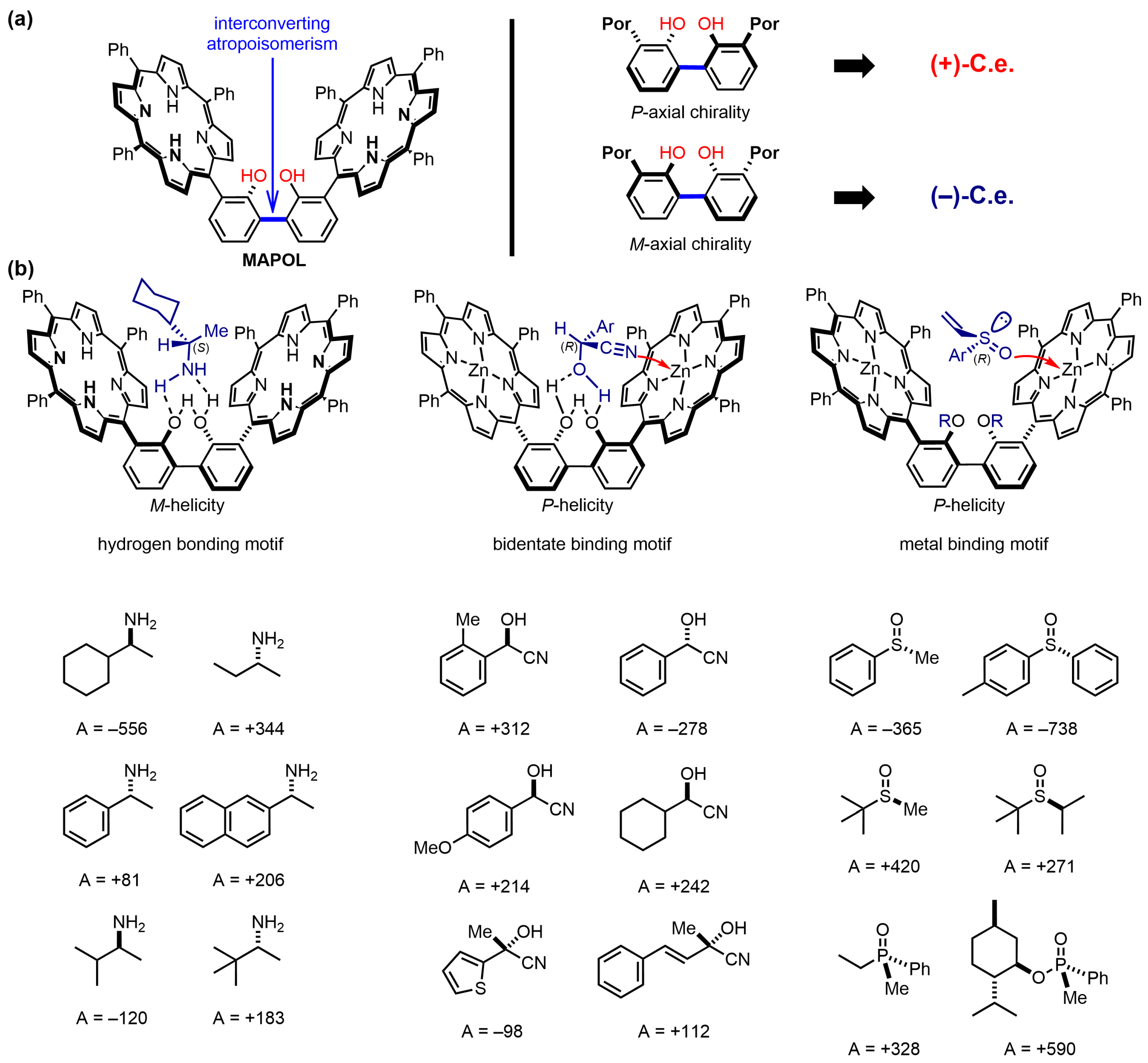





| Compound | ECD, Δε (nm) | |
|---|---|---|
| 55a | −7 (198) | 3 (188) |
| 55b | 3 (202) | −4 (189) |
| 55c | 12 (198) | −7 (190) |
| 55d | 3 (207) | −4 (189) |
| 56 | 41 (198) | −88 (189) |
| 58a | −10.9 (227) | 60.3 (198) |
| 58b | −11.3 (226) | 51.7 (198) |
| 58c | −10.1 (227) | 45.3 (197) |
| 58d | −2.1 (217) | 40.5 (194) |
| 58e | −3.0 (212) | 35 (192) |
| 58f | −20.6 (219) | 26.1 (196) |
| 59a | −9.7 (226) | 38.4 (197) |
| 59b | −10.8 (225) | 34.7 (198) |
| 59c | −8.8 (227) | 26.4 (197) |
| 59d | 3.7 (225) | 29.1 (192) |
| 59e | 3.9 (222) | 18.5 (192) |
| 59f | −7.7 (225) | 39.1 (197) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mądry, T.; Gajewy, J.; Kwit, M. Dynamic Point-to-Helical and Point-to-Axial Chirality Transmission and Induction of Optical Activity in Multichromophoric Systems: Basic Principles and Relevant Applications in Chirality Sensing. Symmetry 2025, 17, 293. https://doi.org/10.3390/sym17020293
Mądry T, Gajewy J, Kwit M. Dynamic Point-to-Helical and Point-to-Axial Chirality Transmission and Induction of Optical Activity in Multichromophoric Systems: Basic Principles and Relevant Applications in Chirality Sensing. Symmetry. 2025; 17(2):293. https://doi.org/10.3390/sym17020293
Chicago/Turabian StyleMądry, Tomasz, Jadwiga Gajewy, and Marcin Kwit. 2025. "Dynamic Point-to-Helical and Point-to-Axial Chirality Transmission and Induction of Optical Activity in Multichromophoric Systems: Basic Principles and Relevant Applications in Chirality Sensing" Symmetry 17, no. 2: 293. https://doi.org/10.3390/sym17020293
APA StyleMądry, T., Gajewy, J., & Kwit, M. (2025). Dynamic Point-to-Helical and Point-to-Axial Chirality Transmission and Induction of Optical Activity in Multichromophoric Systems: Basic Principles and Relevant Applications in Chirality Sensing. Symmetry, 17(2), 293. https://doi.org/10.3390/sym17020293






