Molecular Dynamics Simulations of CeO2 Nano-Fuel: Thermodynamic and Kinetic Properties
Abstract
1. Introduction
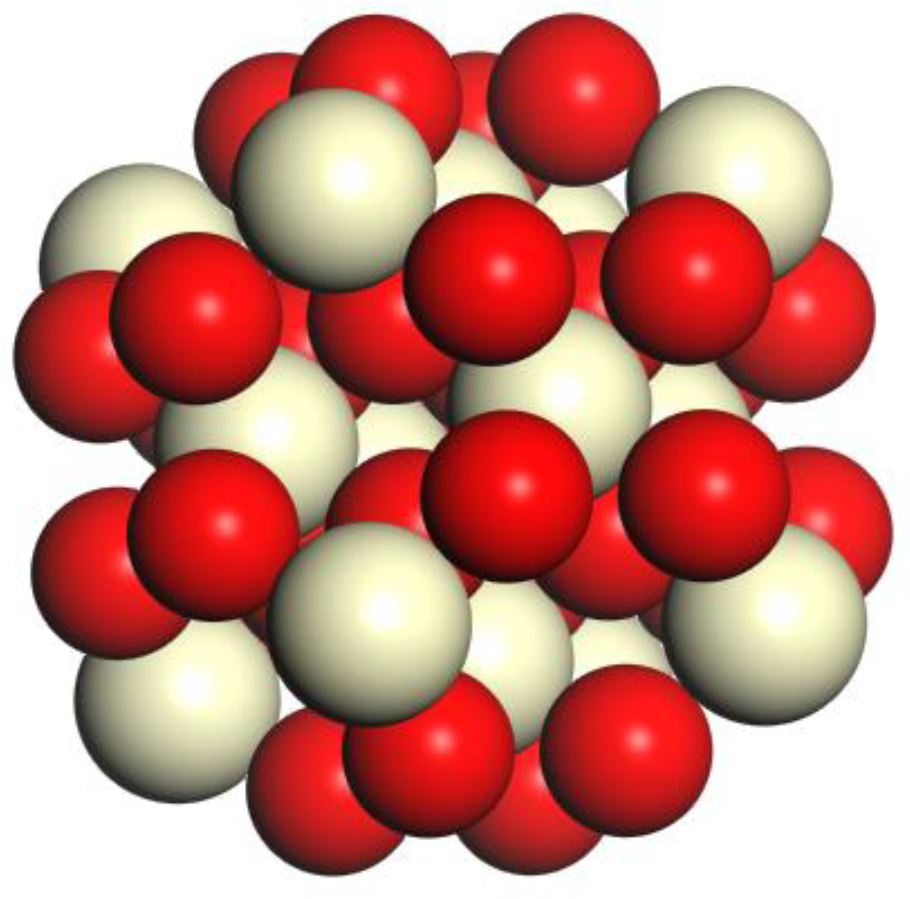
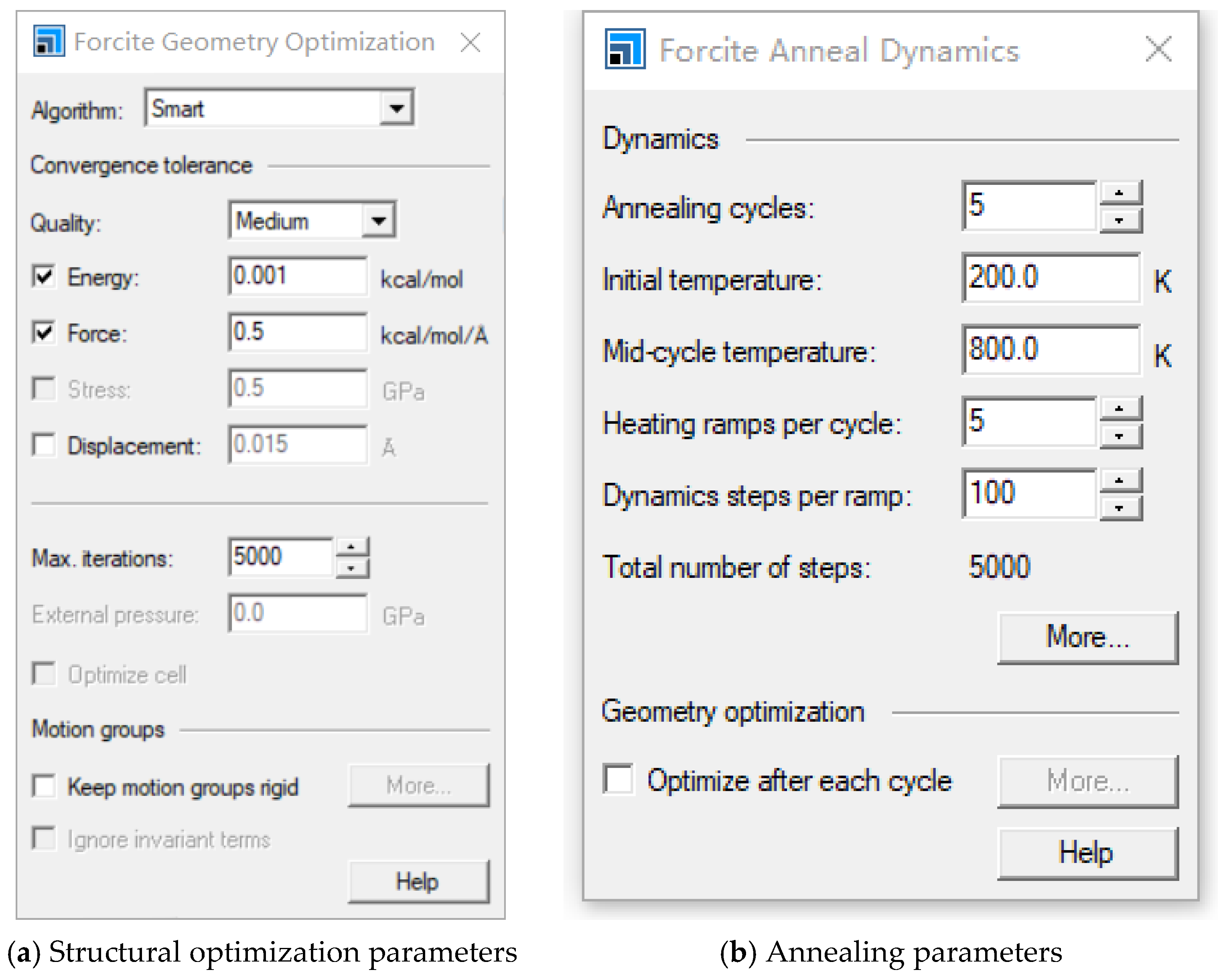
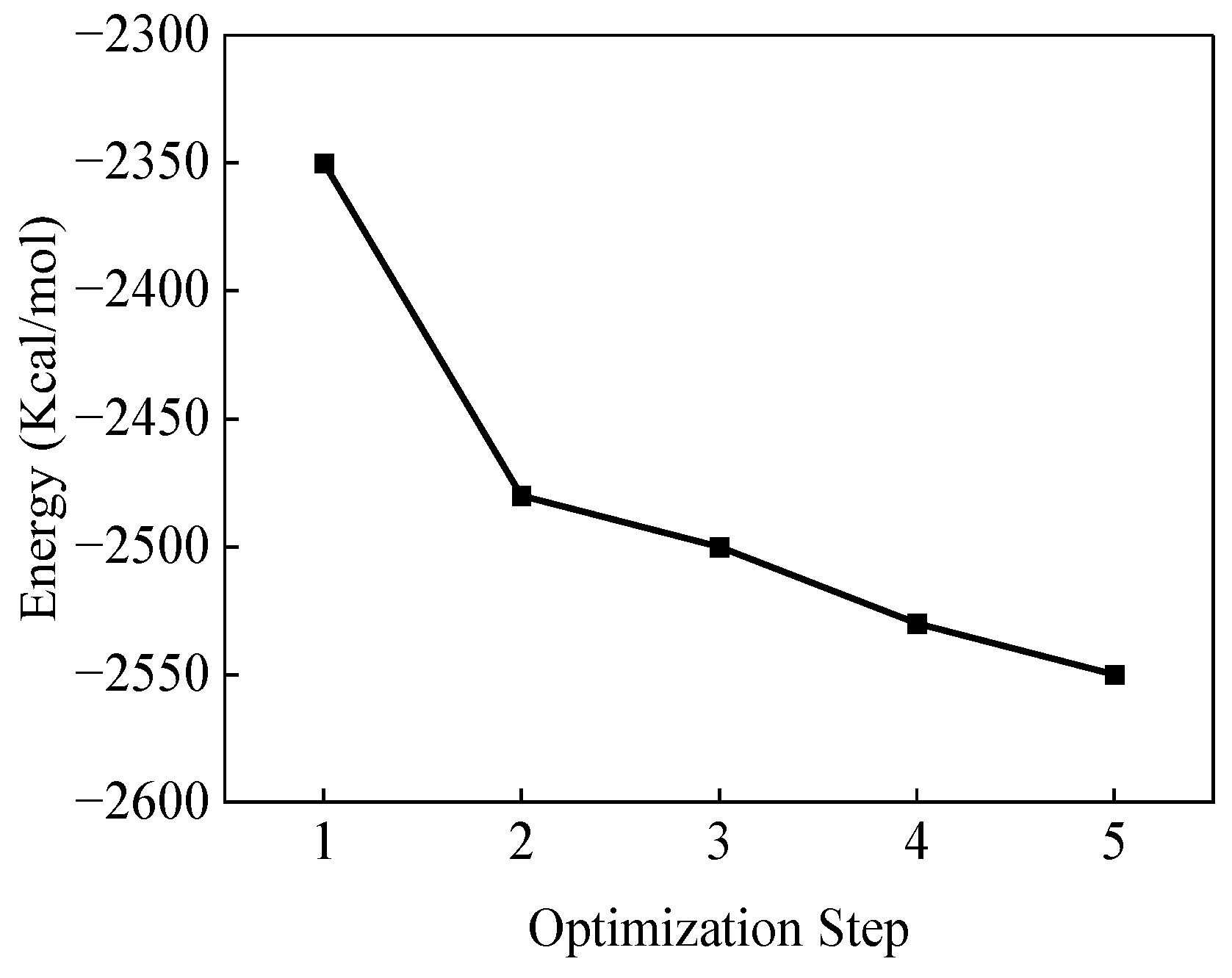
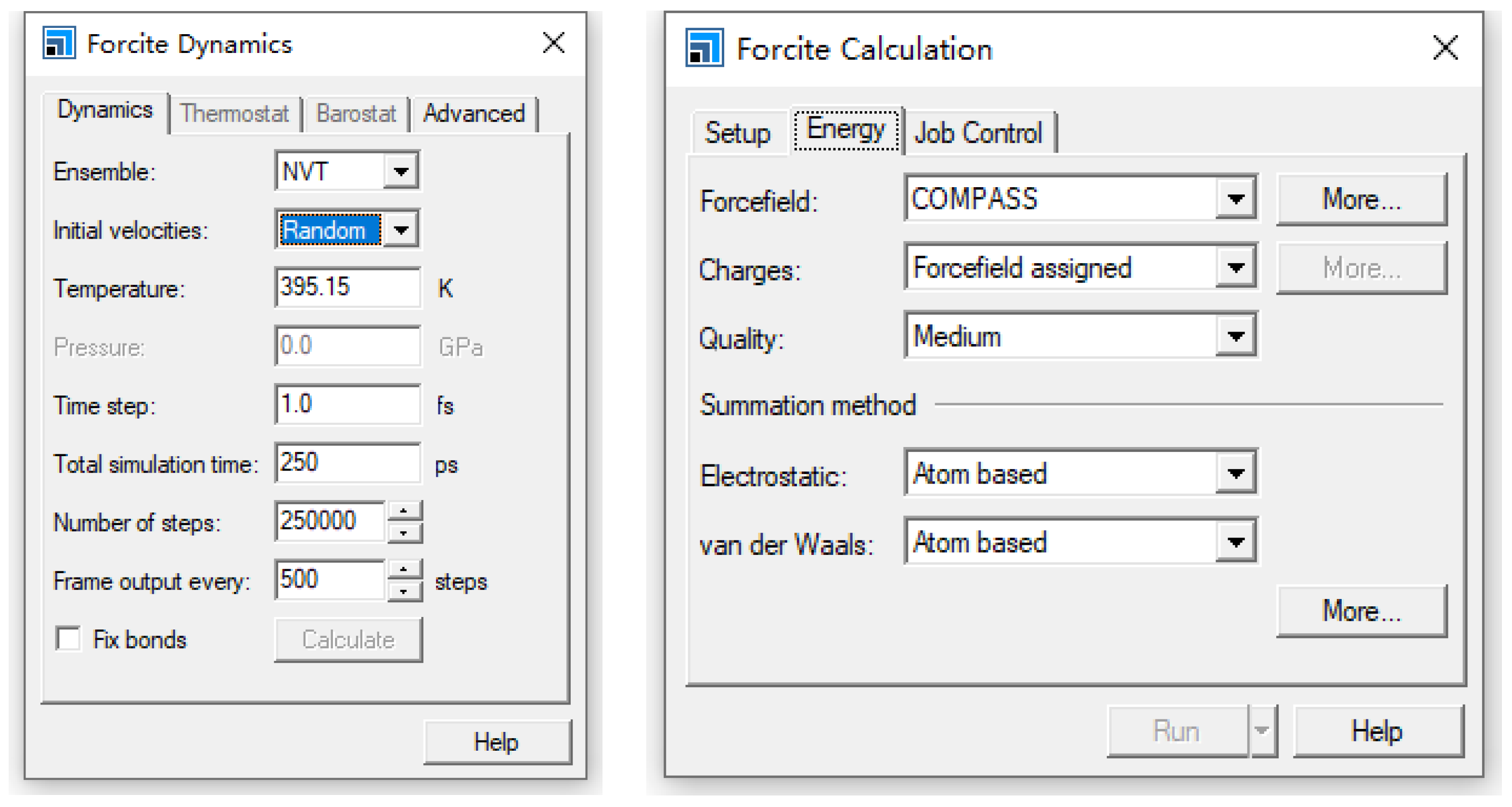
2. Model Construction and Optimization
3. Simulation Results and Analysis
3.1. Thermal Conductivity Calculation
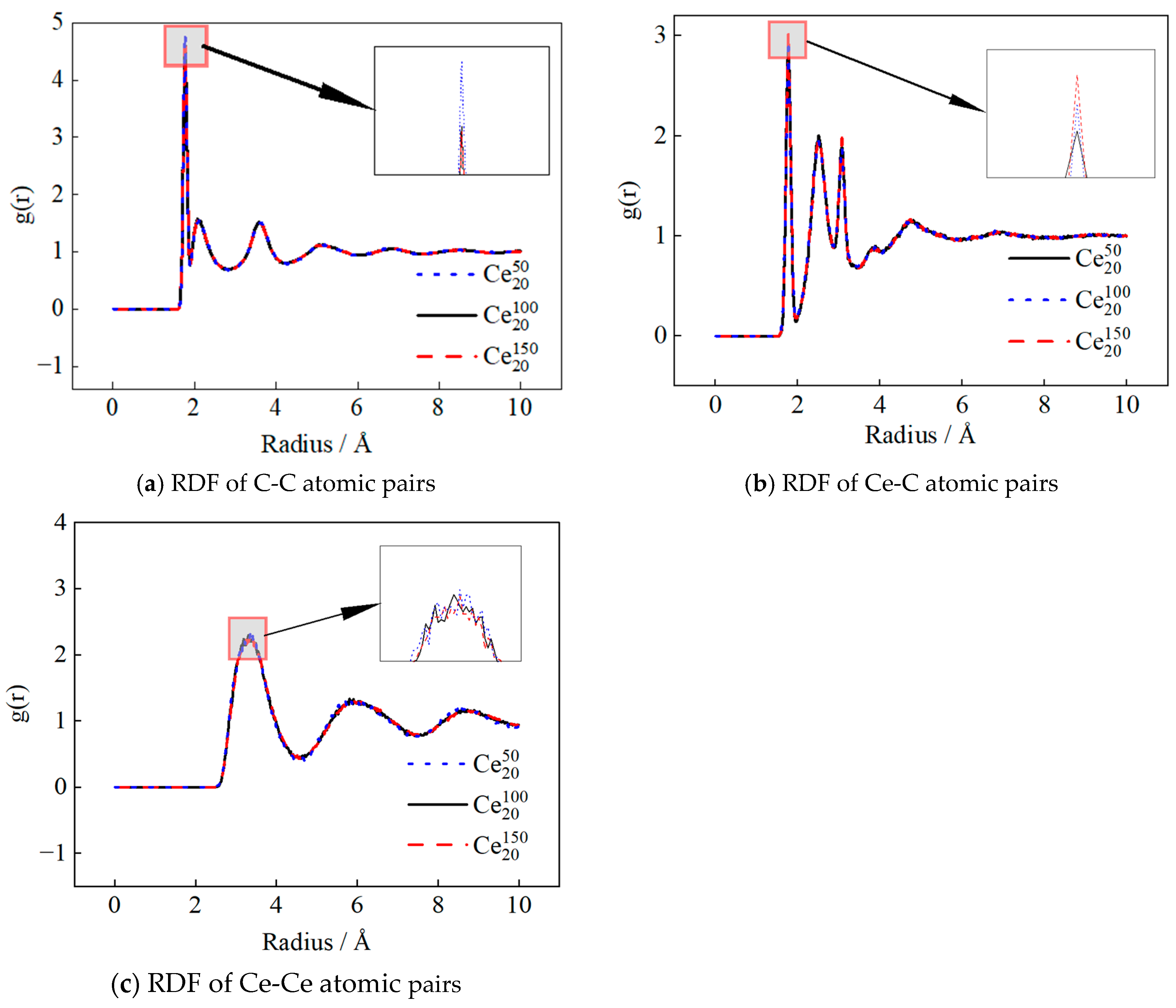
3.2. Radial Distribution Function
3.2.1. Impact of Mass Concentration
3.2.2. Effect of Nanoparticle Size
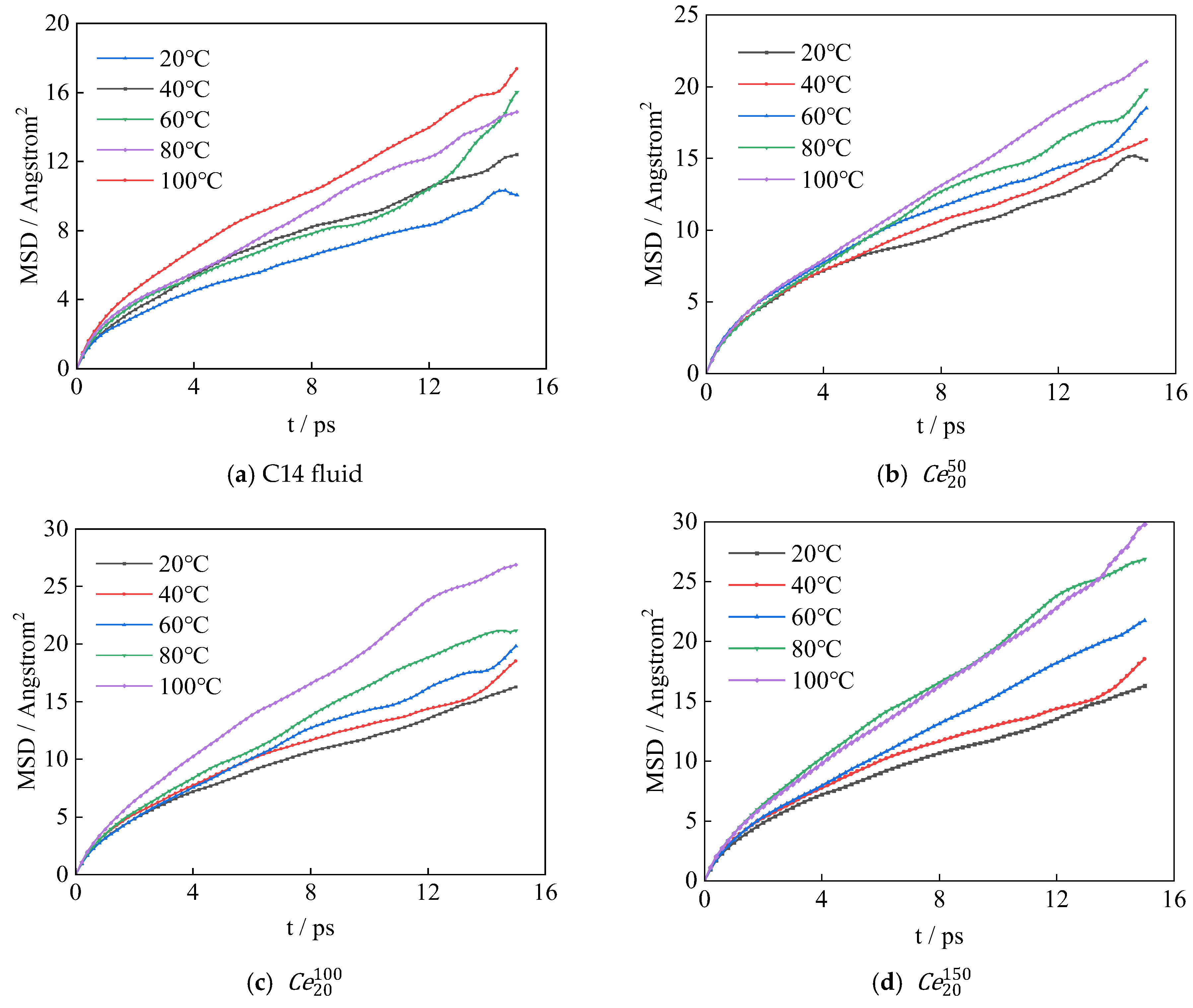
3.3. Diffusion Coefficient
3.3.1. Effect of Mass Concentration
3.3.2. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G.; Kumar, A. Experimental assessment of performance, combustion and emissions of a compression ignition engine fueled with Spirulina platensis biodiesel. Energy 2020, 193, 116861. [Google Scholar] [CrossRef]
- Chen, A.F.; Adzmi, M.A.; Adam, A.; Othman, M.F.; Kamaruzzaman, M.K.; Mrwan, A.G. Combustion characteristics, engine performances and emissions of a diesel engine using nanoparticle-diesel fuel blends with aluminium oxide, carbon nanotubes and silicon oxide. Energy Convers. Manag. 2018, 171, 461–477. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, X.; Wang, L.; Wang, R.Z.; Wang, Y.D.; Roskilly, A.P. Investigation on an innovative sorption system to reduce nitrogen oxides of diesel engine by using carbon nanoparticle. Appl. Therm. Eng. 2018, 134, 29–38. [Google Scholar] [CrossRef]
- Antonov, D.V.; Kuznetsov, G.V.; Sazhin, S.S.; Strizhak, P.A. Puffing/micro-explosion in droplets of rapeseed oil with coal micro-particles and water. Fuel 2022, 3, 123009–123017. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Y.; Liu, J.; Li, K. Light-Fueled Self-Propulsion of Liquid Crystal Elastomer-Engined Automobiles in Zero-Energy Modes. Mathematics 2024, 12, 2109. [Google Scholar] [CrossRef]
- Ashraf, M.Z.; Rehman, S.U.; Farid, S.; Hussein, A.K.; Ali, B.; Shah, N.A.; Weera, W. Insight into Significance of Bioconvection on MHD Tangent Hyperbolic Nanofluid Flow of Irregular Thickness across a Slender Elastic Surface. Mathematics 2022, 10, 2592. [Google Scholar] [CrossRef]
- Alahmer, A.; Rezk, H.; Aladayleh, W.; Mostafa, A.O.; Abu-Zaid, M.; Alahmer, H.; Gomaa, M.R.; Alhussan, A.A.; Ghoniem, R.M. Modeling and Optimization of a Compression Ignition Engine Fueled with Biodiesel Blends for Performance Improvement. Mathematics 2022, 10, 420. [Google Scholar] [CrossRef]
- Rimkus, A.; Žaglinskis, J. Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel. J. Mar. Sci. Eng. 2024, 12, 2192. [Google Scholar] [CrossRef]
- Gao, Z.; Xiao, Y.; Mao, J.; Zhou, L.; Li, X.; Li, Z. Optimization of Second-Generation Biodiesel Blends to Enhance Diesel Engine Performance and Reduce Pollutant Emissions. Energies 2024, 17, 5829. [Google Scholar] [CrossRef]
- Man, D.; Pytel, B. Effect of Ionic and Nonionic Compounds Structure on the Fluidity of Model Lipid Membranes: Computer Simulation and EPR Experiment. Membranes 2024, 14, 257. [Google Scholar] [CrossRef]
- Yu, Q.; Li, C.; Peng, B.; Tang, H.; Yang, T.; Yu, Y.; Zhang, K.; Chen, Z. Research Progress and Outlook of Molecular Dynamics Simulation on Carbon Dioxide Applied for Methane Exploitation from Hydrates. Molecules 2024, 29, 5579. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, K.; Luo, L.; Ma, Z.; Hu, Z.; Li, X.; Qu, P.; Pei, Y.; An, Y.; Gao, Z. A Numerical Simulation of Mixture Formation in a Hydrogen Direct-Injection Internal Combustion Engine. Appl. Sci. 2024, 14, 11317. [Google Scholar] [CrossRef]
- Fang, Z.; Song, X. The Effect of H2O2 Pretreatment on TiO2-Supported Ruthenium Catalysts for the Gas Phase Catalytic Combustion of Dichloromethane (CH2Cl2). Catalysts. 2024, 14, 886. [Google Scholar] [CrossRef]
- Van, K. Stochastic Processes in Physics and Chemistry; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Tian, L. Molecular Dynamics Simulation of Thermal Conductivity of Nanofluids; Yanshan University: Qinhuangdao, China, 2021. [Google Scholar]
- Kumar, R.; Gupta, A. Role of Brownian motion on the thermal conductivity enhancement of nanofluids. Appl. Phys. Lett. 2007, 91, 223102–223123. [Google Scholar]
- Yang, P. Molecular Dynamics Simulation of Enhanced Heat Transfer and Flow Characteristics of Nanofluids; Chongqing University: Chongqing, China, 2017. [Google Scholar]
- Sundar, L.S.; Otero-Irurueta, G.; Singh, M.K.; Sousa, A.C. Heat transfer and friction factor of multi-walled carbon nanotubes–Fe3O4 nanocomposite nanofluids flow in a tube with/without longitudinal strip inserts. Int. J. Heat Mass Transf. 2016, 100, 691–703. [Google Scholar] [CrossRef]
- Nasrin, R.; Rahim, N.A.; Fayaz, H.; Hasanuzzaman, M. Water/MWCNT nanofluid based cooling system of PVT: Experimental and numerical research. Renew. Energy 2018, 121, 286–300. [Google Scholar] [CrossRef]
- Omid, M.; Lioua, K.; Mohammad, A.; Estellé, P.; Ahmadi, G.; Kleinstreuer, C.; Pop, I. Recent advances in modeling and simulation of nanofluid flows—Part II: Applications. Phys. Rep. 2019, 791, 1–59. [Google Scholar]
- Mohammad, K.; Hossein, H. Lattice Boltzmann simulation of nanofluid free convection heat transfer in an L-shaped enclosure. Superlattices Microstruct. 2014, 66, 112–128. [Google Scholar]
- Wei, M.; Song, Y.; Wang, Y. Heat transfer of nanofluidics in hydrophilic pores: Insights from molecule dynamics simulations. Chin. J. Chem. Eng. 2016, 24, 1117–1121. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Q.; Dong, Y.; Jin, H.; Bawaa, B.; Guo, L. Molecular dynamics simulation of sub- and supercritical water extraction shale oil in slit nanopores. J. Supercrit. Fluids 2023, 195, 105862. [Google Scholar]
- Tang, X.; Xiao, S.; Lei, Q.; Yuan, L.; Peng, B.; He, L.; Luo, J.; Pei, Y. Molecular Dynamics Simulation of Surfactant Flooding Driven Oil-Detachment in Nano-Silica Channels. J. Phys. Chem. B 2019, 123, 277–288. [Google Scholar] [CrossRef]
- Fu, T.; Mao, Y.; Tang, Y.; Zhang, Y.; Yuan, W. Effect of nanostructure on rapid boiling of water on a hot copper plate: A molecule dynamics study. Heat Mass Transf. 2016, 52, 1469–1478. [Google Scholar] [CrossRef]
- Azimi, S.S.; Kalbasi, M. A molecule dynamics simulation of brownian motion of a nanoparticle in a nanofluid. Nanoscale Microscale Thermophys. Eng. 2017, 21, 263–277. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.E.; Prasher, R.; Peck, R.; Lee, T.; Pacheco, J.R.; Arentzen, P. Increased hot-plate ignition probability for nanoparticle-laden diesel fuel. Nano Lett. 2008, 8, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Dai, B.; Liu, J.; Sun, Y.; Zhu, B.; Liu, X. Ignition and combustion characteristics of heptane-based nanofluid fuel droplets. Energy Fuels 2019, 33, 10282–10289. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, B.; Sun, Y.; Guo, P.; Liu, J. Nano-sized copper oxide enhancing the combustion of aluminum/kerosene-based nanofluid fuel droplets. Combust. Flame 2022, 240, 112028. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.U.; Saidur, R.; Amalina, M.A.; Mostafizur, R.M.; Islam, A.K.M.S. Effect of nanoparticles concentration and their sizes on surface tension of nanofluids. Procedia Eng. 2015, 105, 431–437. [Google Scholar] [CrossRef]
- Hu, M.Y.; Yin, K.; Wen, Y.F.; Song, S.; Xiong, Y.; Dai, Y.; Sun, L. Combining in-situ TEM observations and theoretical calculation for revealing the thermal stability of CeO2 nanoflowers. Nano Res. 2022, 15, 1319–1326. [Google Scholar]
- Wei, Y.Y.; Wang, X.Y.; Dong, L.H.; Liu, G.; Xia, Q.; Yuan, S. Molecule dynamics study on the effect of surfactant mixture on their packing states in mixed micelles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 43, 11–20. [Google Scholar]
- Muller-Plathe, F. A simple nonequilibrium molecule dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 2878–2891. [Google Scholar] [CrossRef]
- Zhao, Q.; Buens, S.E. Molecule dynamics simulation of secondary sorption behavior of montmorillonite modified by single chain quaternary ammonium cations. Environ. Sci. Technol. 2012, 46, 3999–4007. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Qiu, X.; Xiao, S.; Hu, G.; Wang, F.; Yuan, J. Molecular dynamic investigations on the adhesion behaviors of asphalt mastic-aggregate interface. Materials 2020, 13, 5061–5071. [Google Scholar] [CrossRef]
- Porath, L.; Derkaloustian, M.; Ewoldt, R.H.; Huang, J.; Ramlawi, N.; Evans, C.M. Relaxation of vitrimers with kinetically distinct mixed dynamic bonds. Macromolecules 2022, 55, 4450–4458. [Google Scholar] [CrossRef]







| Parameter | Unit | Value |
|---|---|---|
| Molar Mass | g/mol | 198.39 |
| Standard Boiling Point | °C | 253.58 |
| Density | g/cm3 | 0.76 |
| Critical Pressure | MPa | 1.57 |
| Fuel Type | System Size (Å) | Number of C14 Molecules | Number of CeO2 Molecules |
|---|---|---|---|
| 72 | 1132 | 4 | |
| 57.3 | 570 | 4 | |
| 50 | 380 | 4 | |
| 97.7 | 2830 | 10 | |
| 77.5 | 1414 | 10 | |
| 67.7 | 942 | 10 |
| Temperature (°C) | Thermal Conductivity (W/(m·K)) |
|---|---|
| 20 | 0.1312 |
| 40 | 0.1386 |
| 60 | 0.1440 |
| 80 | 0.1547 |
| 100 | 0.1622 |
| Mass Concentration (mg/L) | Temperature (°C) | Diffusion Coefficient (10−4 cm2/s) |
|---|---|---|
| 0 | 20 | 0.1139 |
| 60 | 0.1411 | |
| 100 | 0.1778 | |
| 50 | 20 | 0.1211 |
| 60 | 0.1954 | |
| 100 | 0.2912 | |
| 100 | 20 | 0.1451 |
| 60 | 0.2663 | |
| 100 | 0.3602 | |
| 150 | 20 | 0.1677 |
| 60 | 0.3317 | |
| 100 | 0.4602 |
| Temperature (°C) | Mass Concentration (mg/L) | Diffusion Coefficient (10−4 cm2/s) |
|---|---|---|
| 20 | 0 | 0.1139 |
| 50 | 0.1211 | |
| 150 | 0.1677 | |
| 40 | 0 | 0.1231 |
| 50 | 0.1624 | |
| 150 | 0.2468 | |
| 60 | 0 | 0.1411 |
| 50 | 0.1954 | |
| 150 | 0.3317 | |
| 80 | 0 | 0.1591 |
| 50 | 0.2418 | |
| 150 | 0.3865 | |
| 100 | 0 | 0.1778 |
| 50 | 0.2912 | |
| 150 | 0.4602 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhou, J.; Zhao, Y.; He, Z.; Xi, W.; Zhao, W. Molecular Dynamics Simulations of CeO2 Nano-Fuel: Thermodynamic and Kinetic Properties. Symmetry 2025, 17, 296. https://doi.org/10.3390/sym17020296
Zhang R, Zhou J, Zhao Y, He Z, Xi W, Zhao W. Molecular Dynamics Simulations of CeO2 Nano-Fuel: Thermodynamic and Kinetic Properties. Symmetry. 2025; 17(2):296. https://doi.org/10.3390/sym17020296
Chicago/Turabian StyleZhang, Rui, Jianbo Zhou, Yingjie Zhao, Zhen He, Wenxiong Xi, and Weidong Zhao. 2025. "Molecular Dynamics Simulations of CeO2 Nano-Fuel: Thermodynamic and Kinetic Properties" Symmetry 17, no. 2: 296. https://doi.org/10.3390/sym17020296
APA StyleZhang, R., Zhou, J., Zhao, Y., He, Z., Xi, W., & Zhao, W. (2025). Molecular Dynamics Simulations of CeO2 Nano-Fuel: Thermodynamic and Kinetic Properties. Symmetry, 17(2), 296. https://doi.org/10.3390/sym17020296






