A Century of Azulene Chemistry; A Brief Look at Azulenes Building
Abstract
1. Introduction
2. Beginnings
3. Obtaining of Azulene Seven-Membered Ring from Precursors Containing Cyclopentadienyl or Cyclopentane Structure
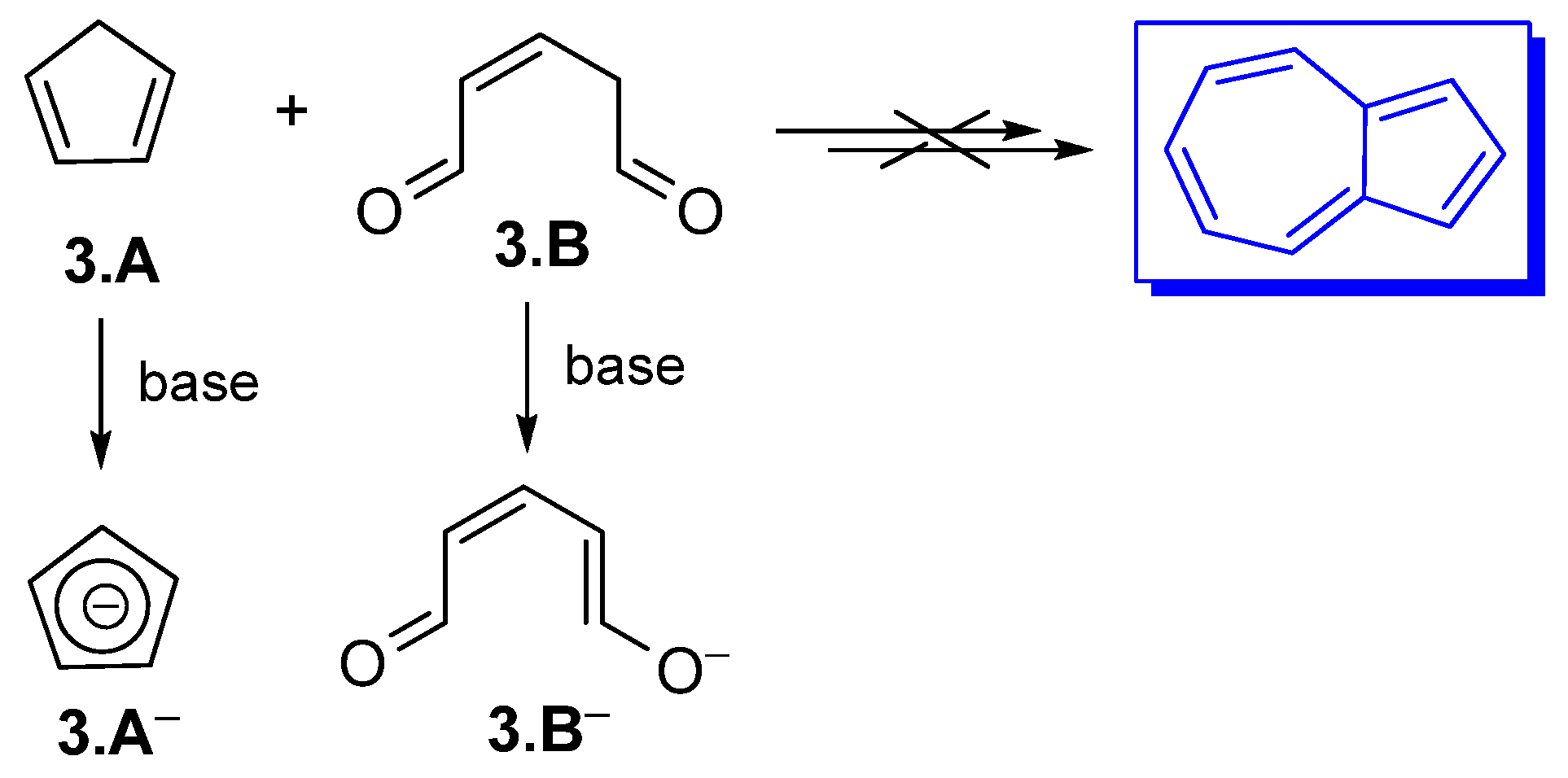
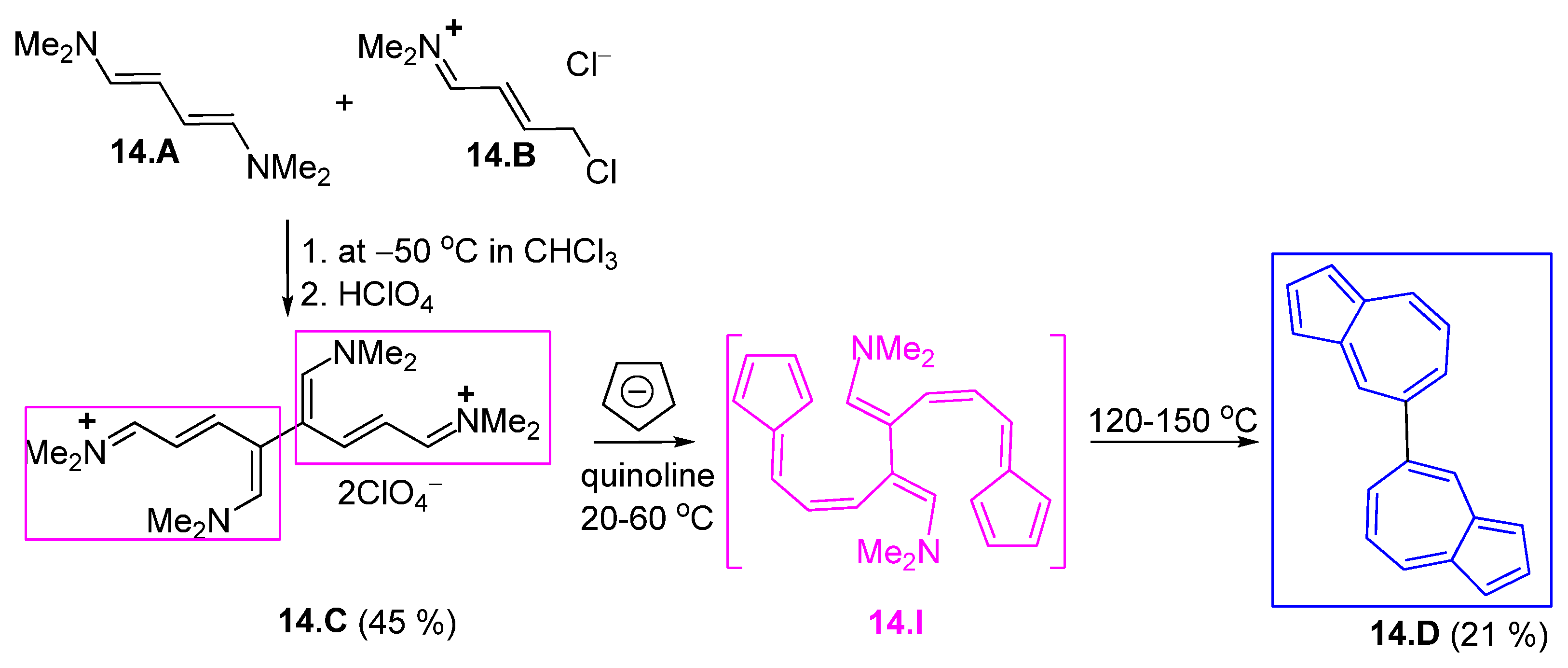
3.1. Condensation Between Cyclopentadienyl Anions and Zincke Iminium Salts Glutaconic Aldehyde Derivativ
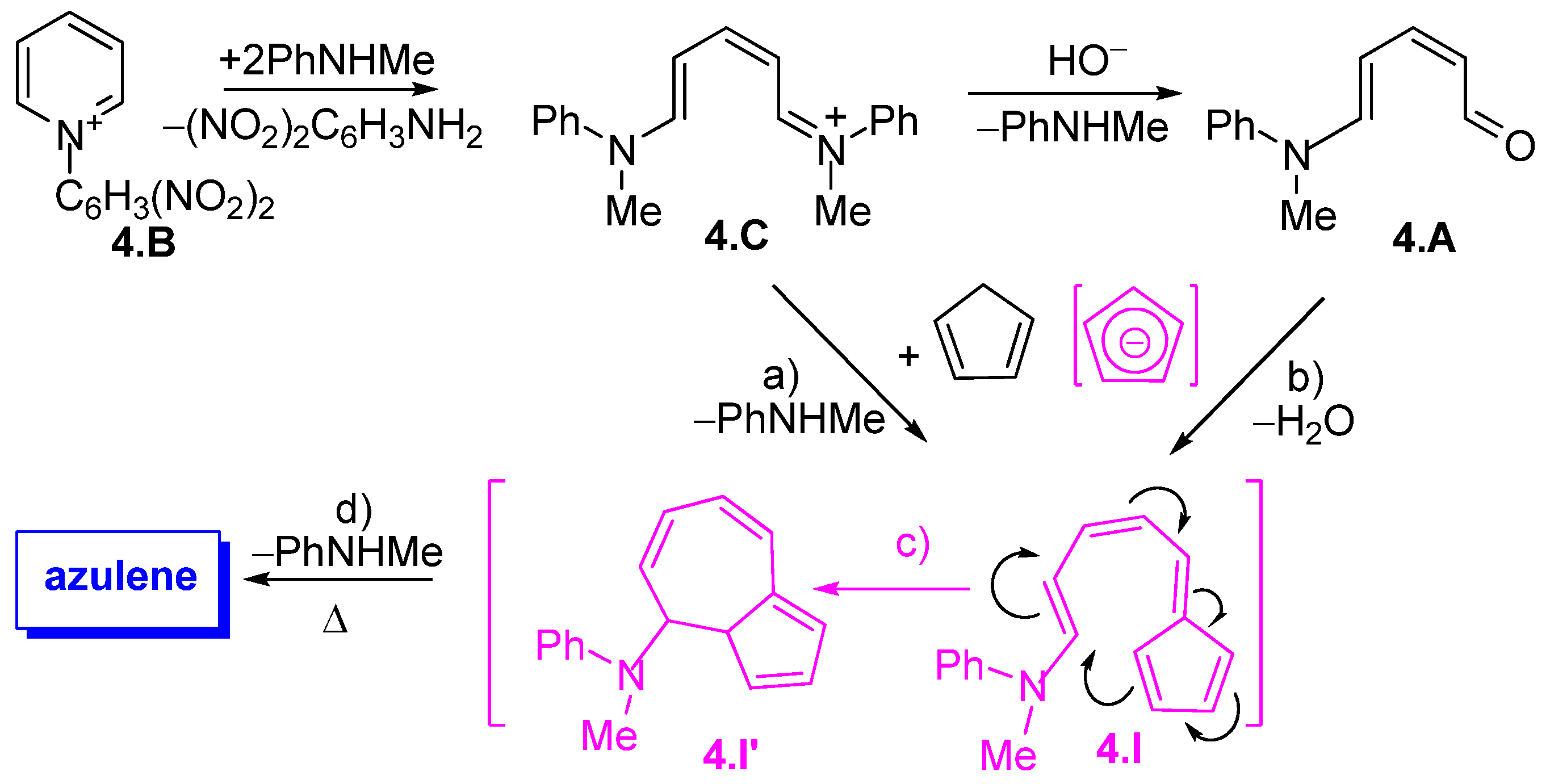
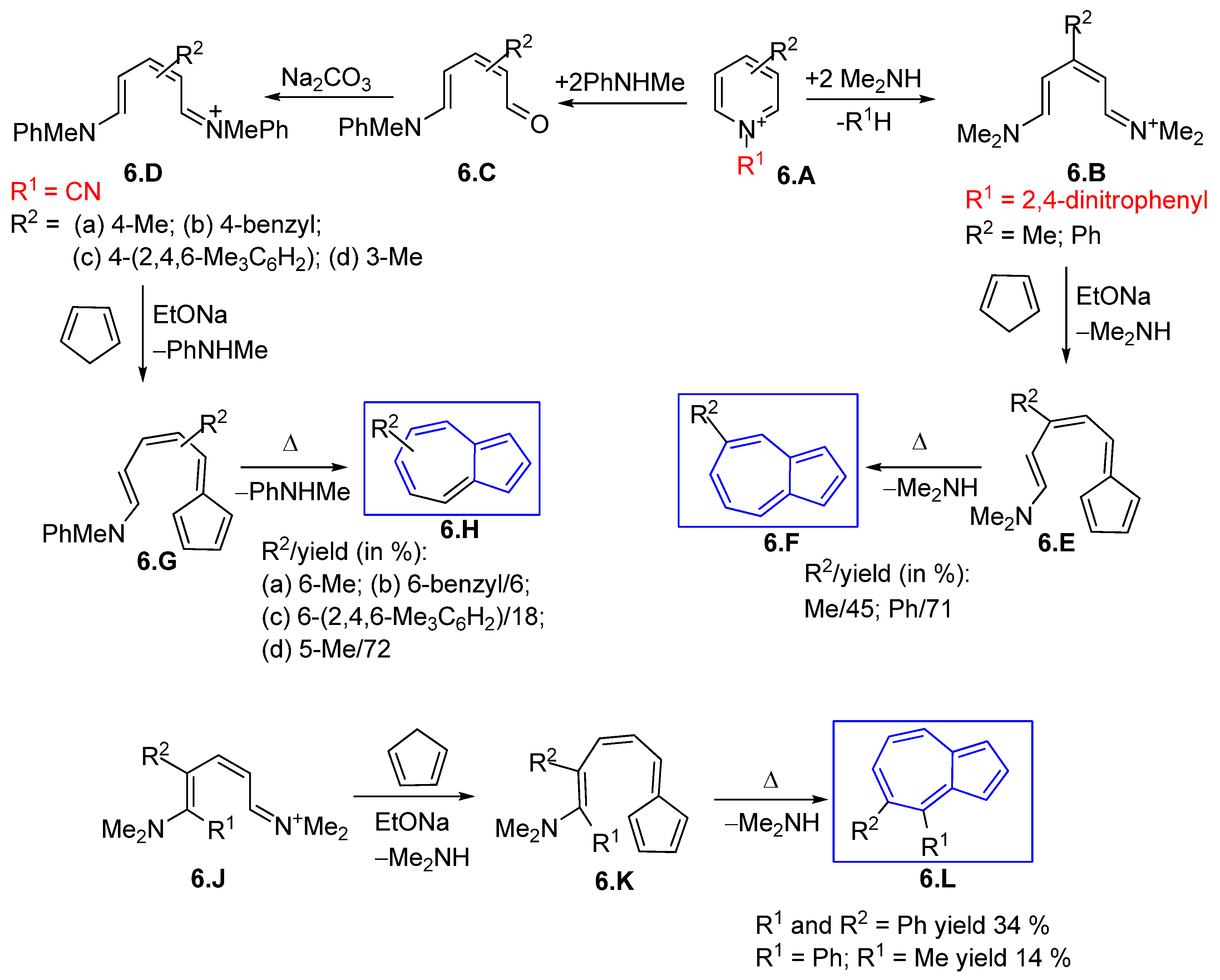
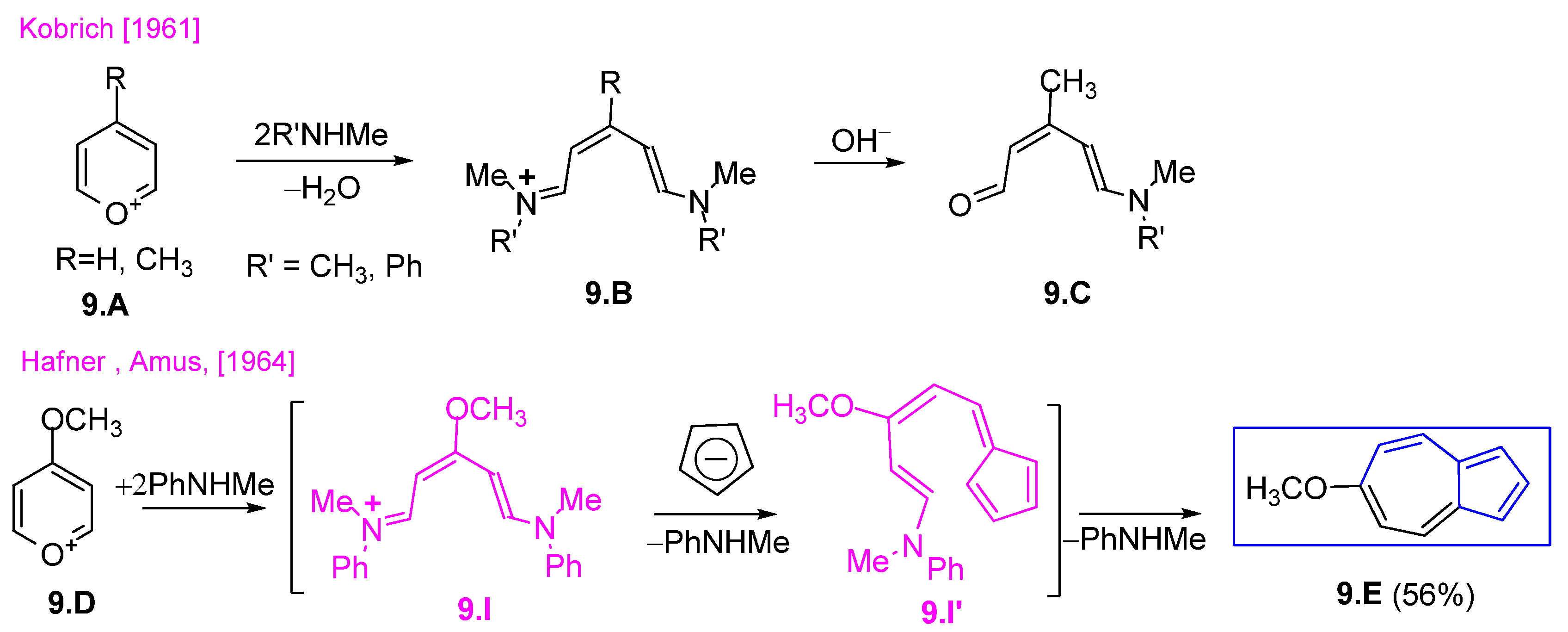
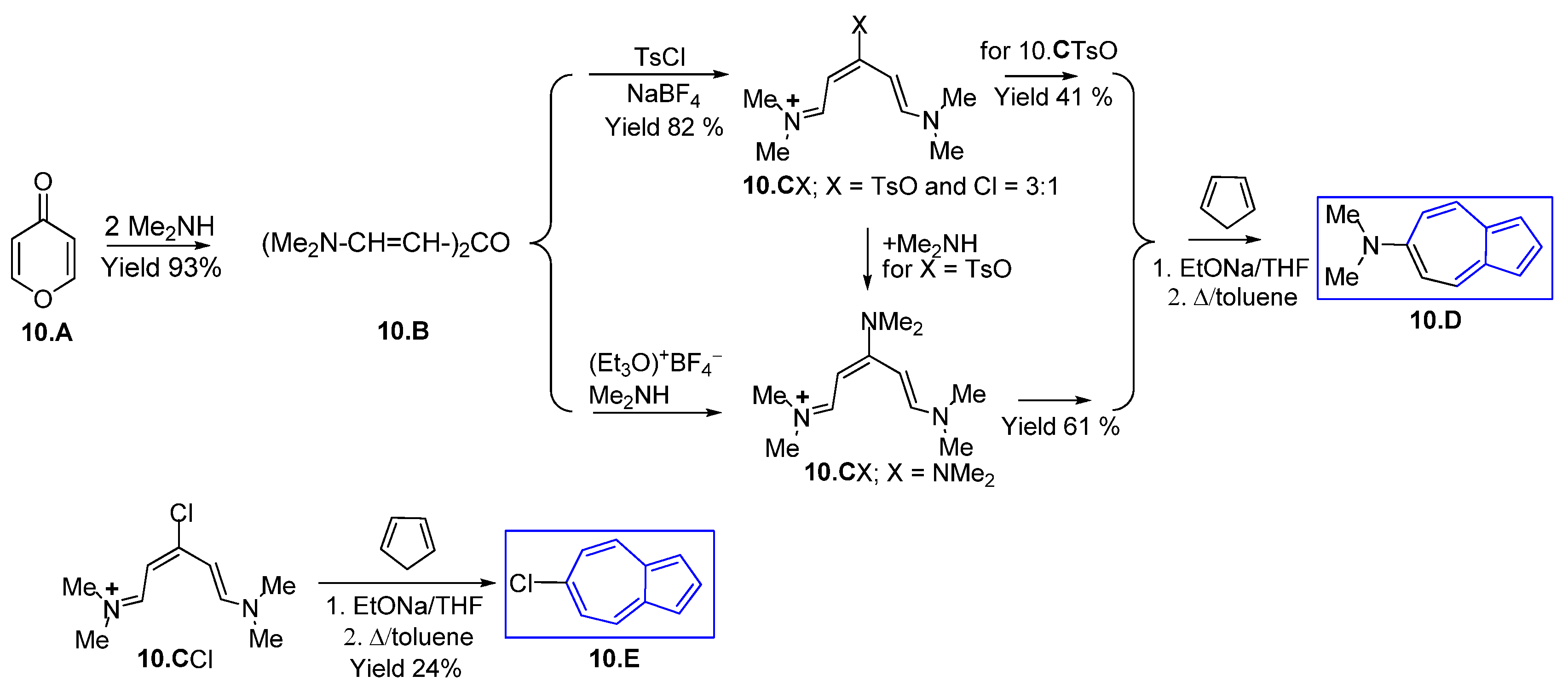
3.1.1. Glutaconic Aldehyde Derivatives from Pyridinium Salts
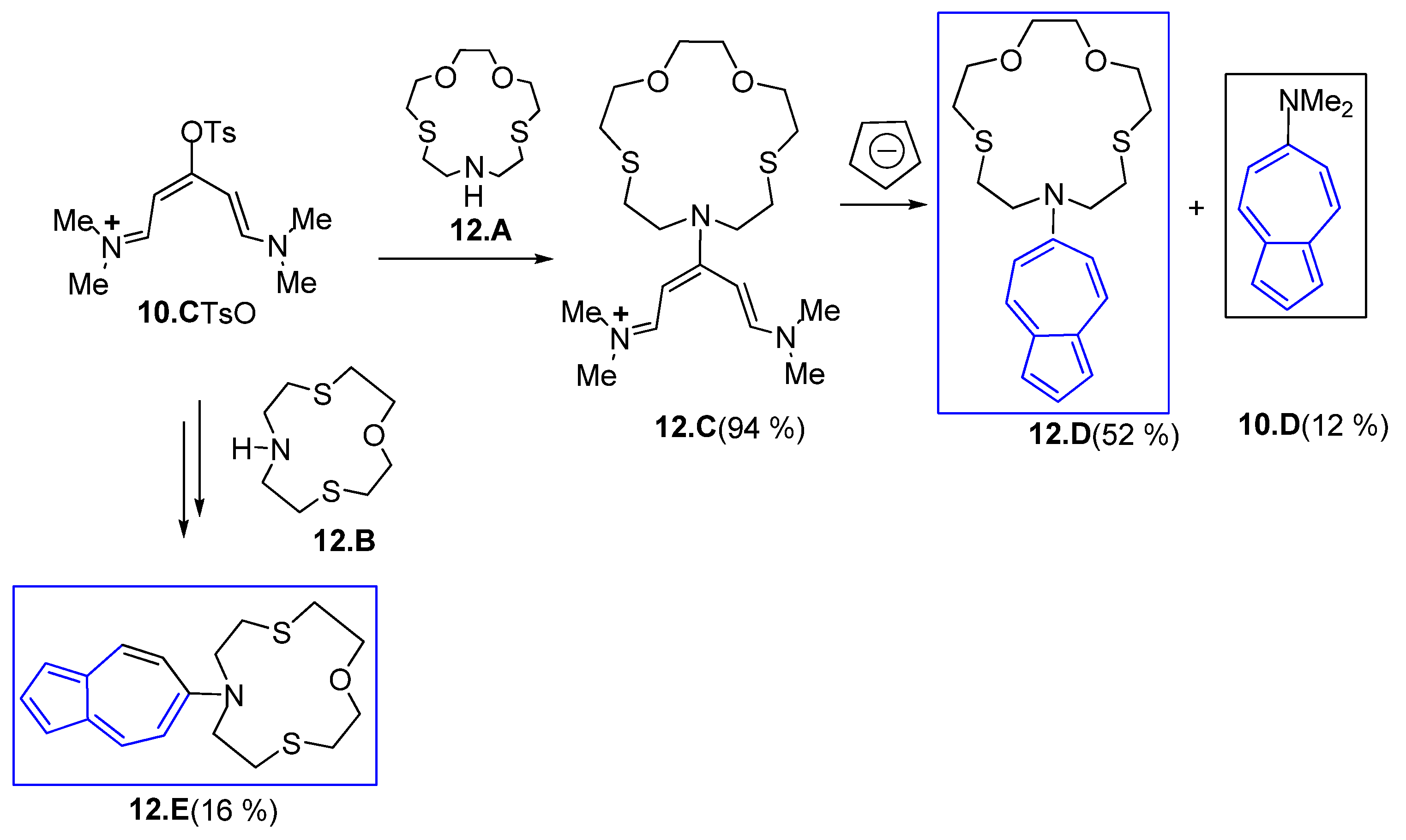
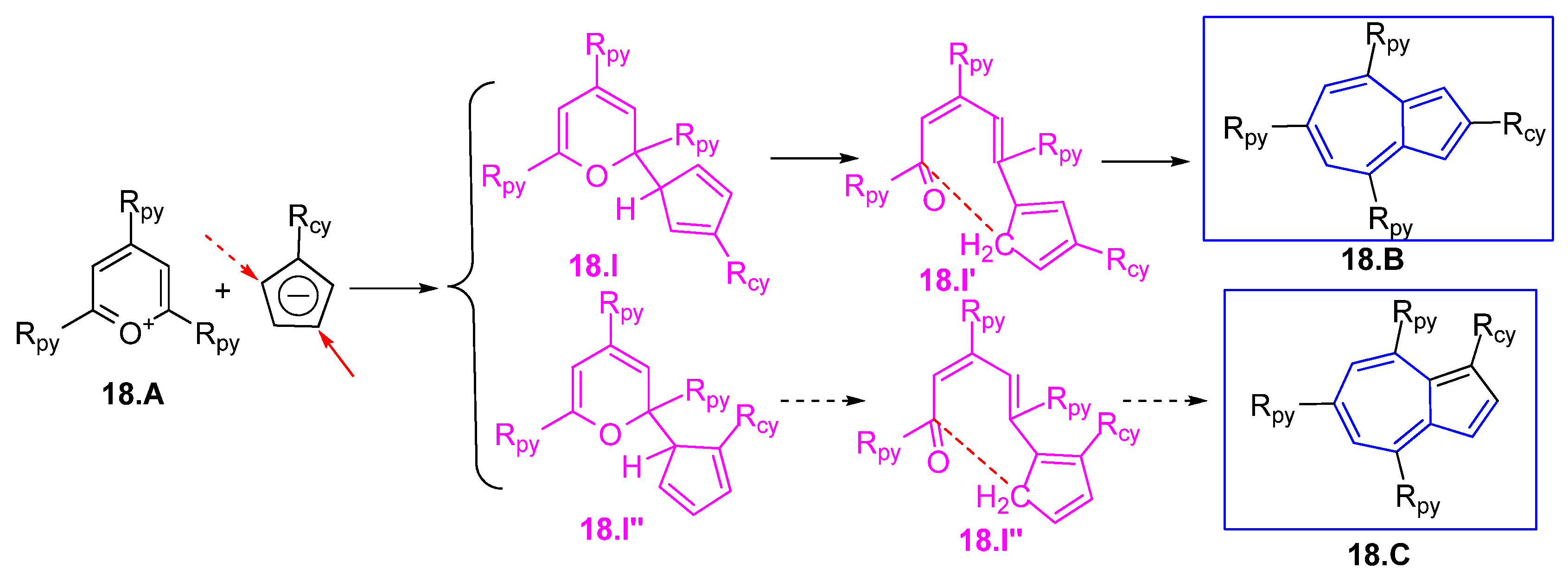
3.1.2. Glutaconic Aldehyde Derivatives from Pyrylium Salts
3.1.3. Glutaconic Aldehyde Derivatives from 4-Pyrone
3.1.4. Iminium Salt Derivatives on Other Routes
3.2. Fulvenes as Starting Compounds in the Building of Seven-Ring Azulene
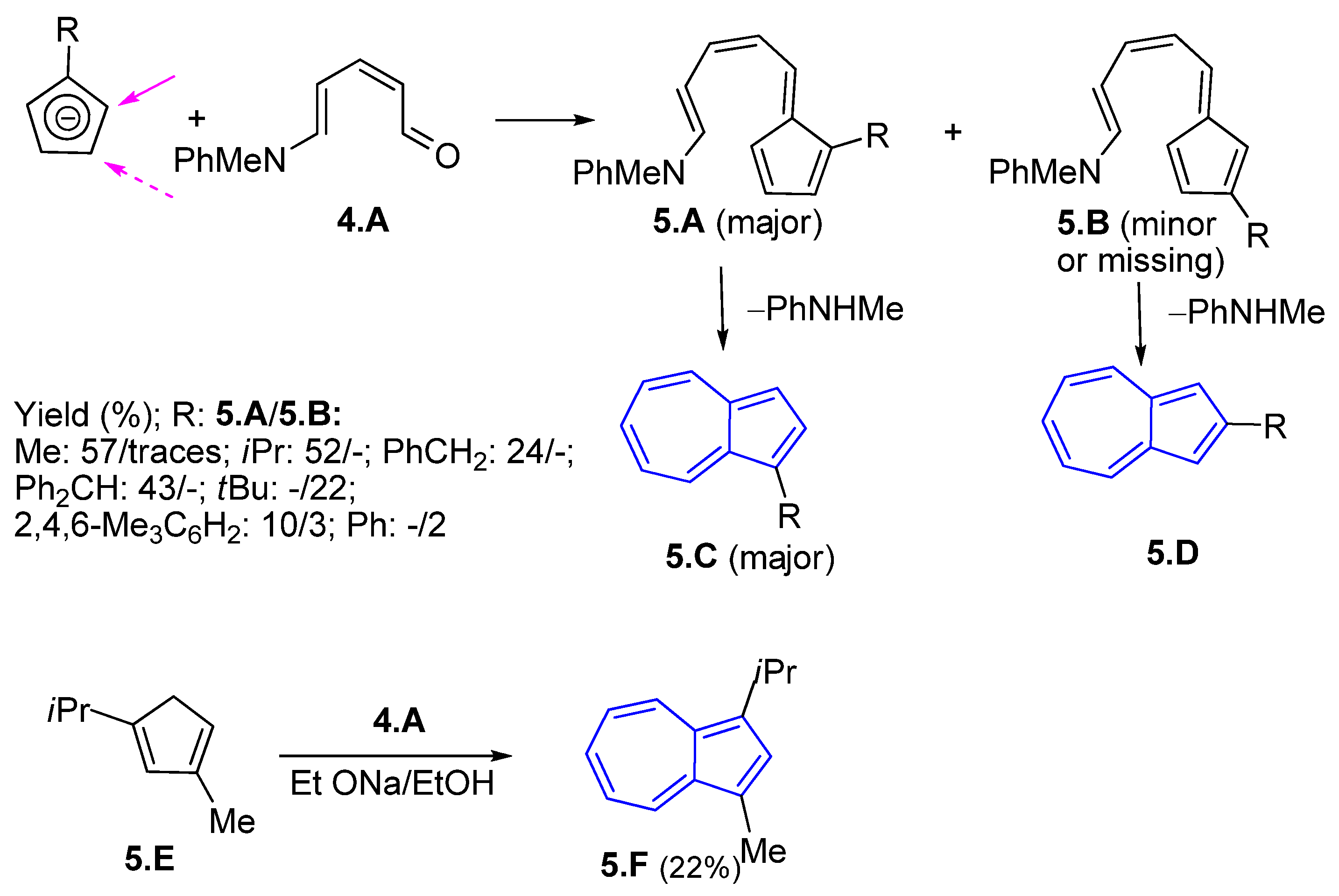
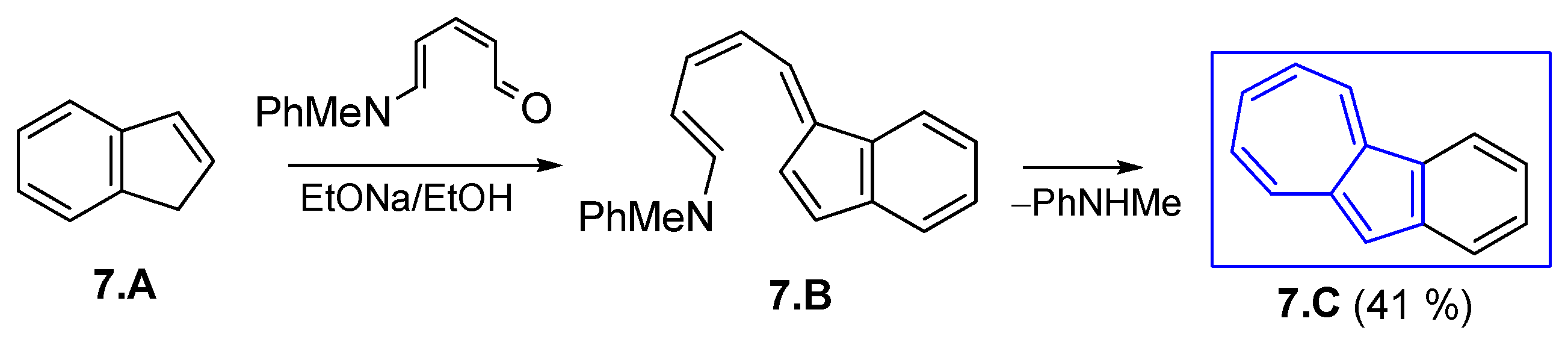
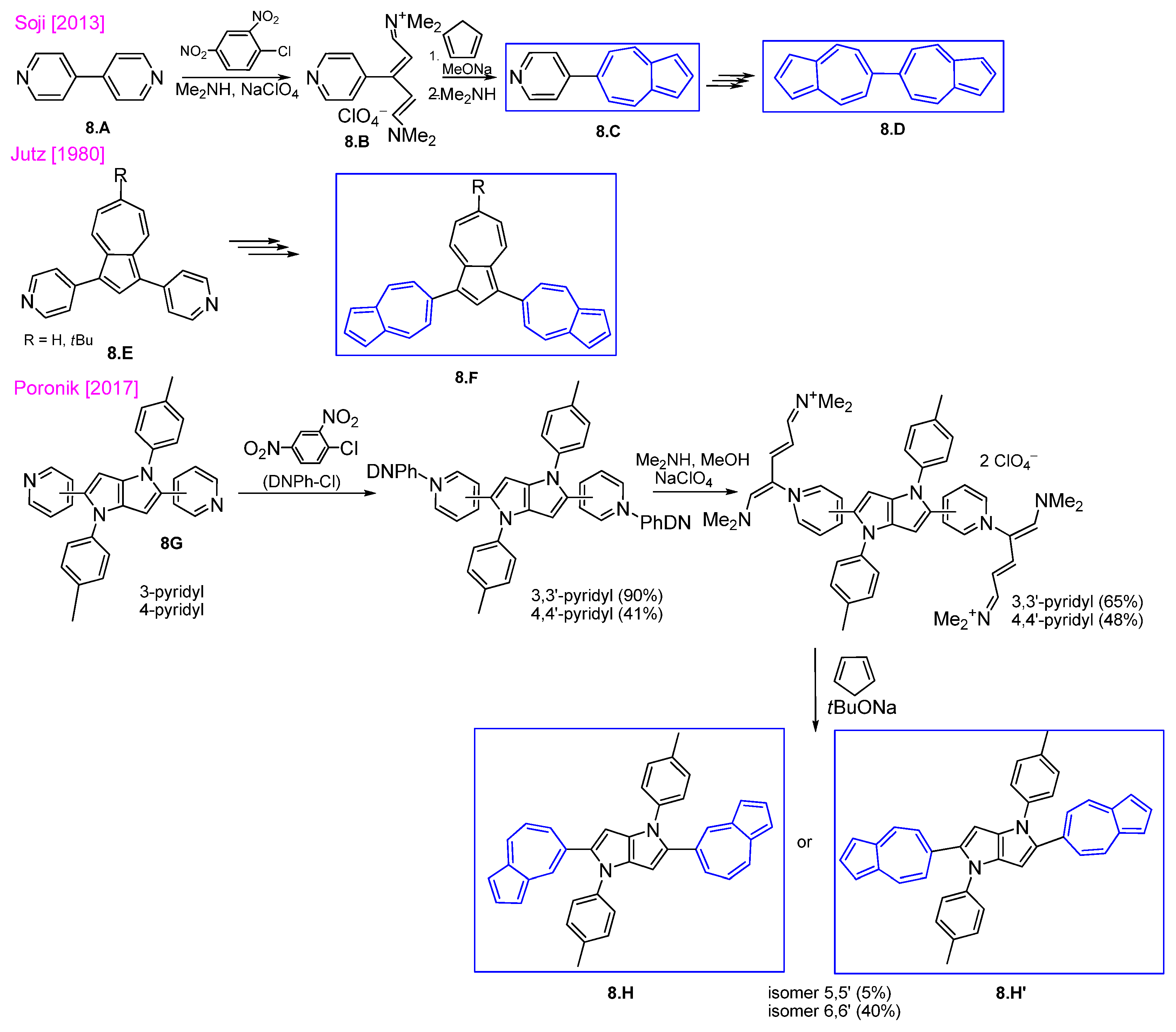
3.2.1. Fulvenes After Nucleophilic Substitution of Pyridinium Salts with Cyclopentadienyl Anion
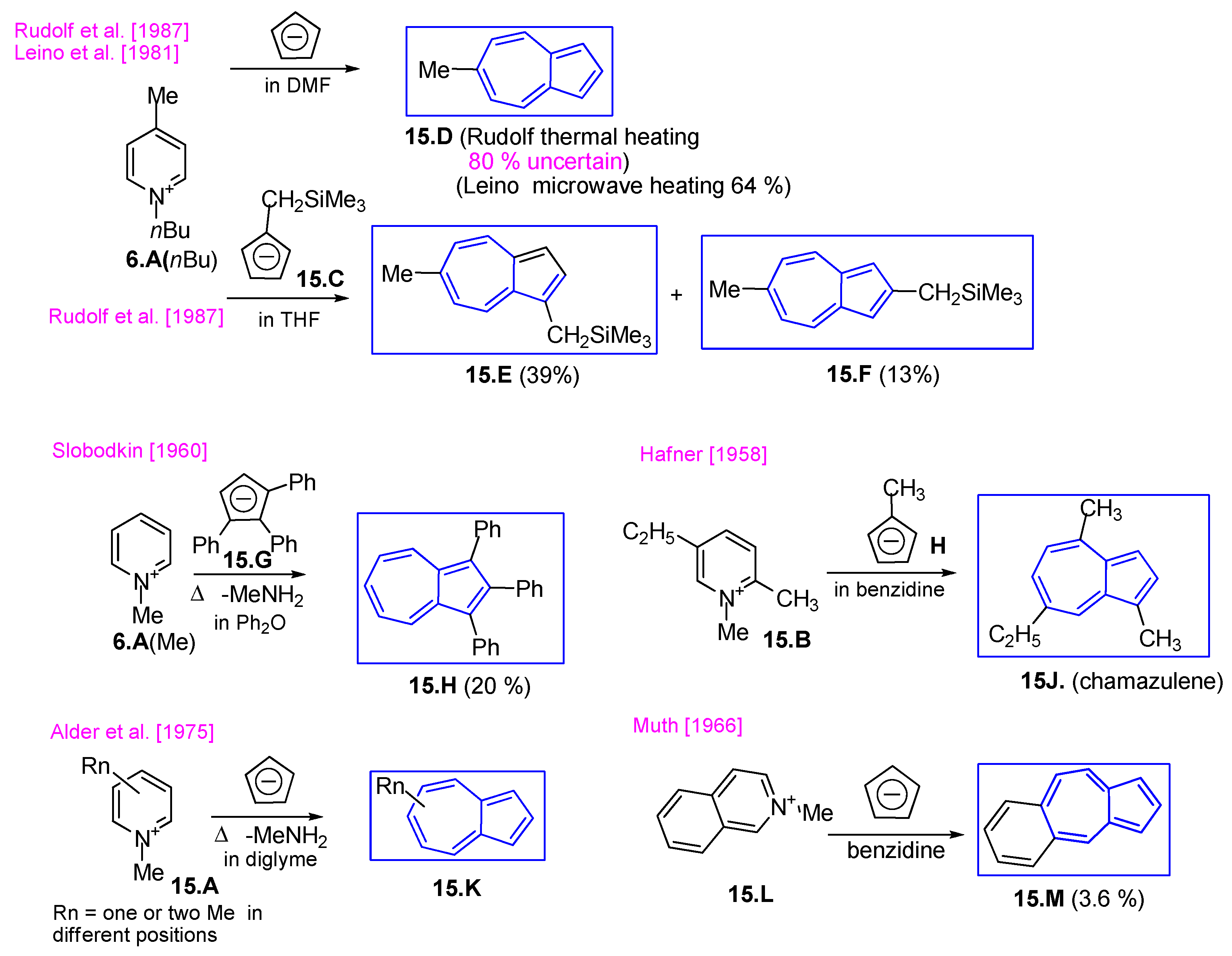
3.2.2. Fulvenes After Nucleophilic Substitution of Pyrylium Salts with Cyclopentadienyl Anion
3.3. Fulvenes as Starting Compounds with a Five-Azulenic Ring
3.3.1. Fulvenes in [6+4]Cycloadditions with Cyclic Dienes
3.3.2. Fulvenes in [6+4]Cycloadditions with Acyclic Dienes
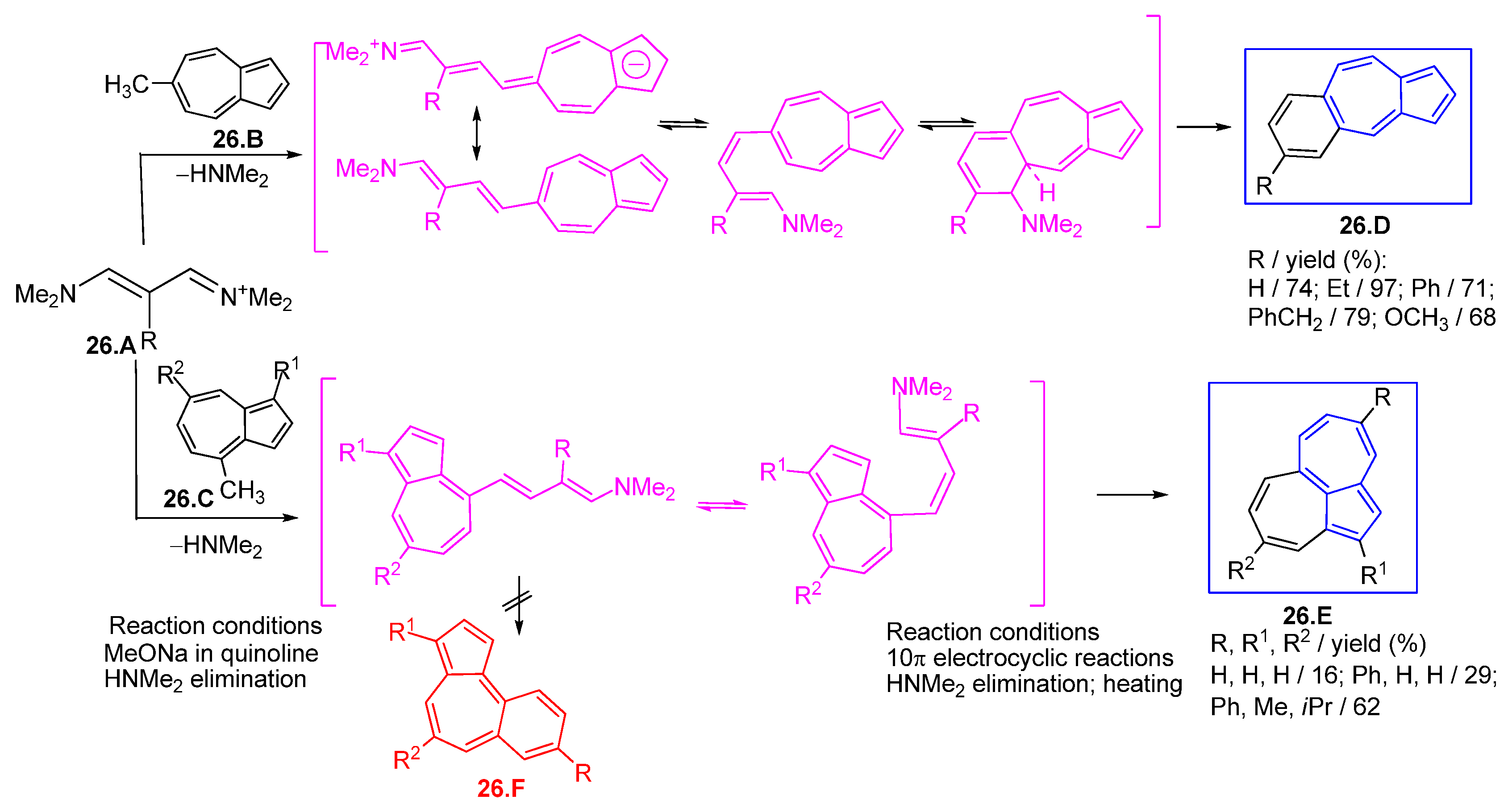
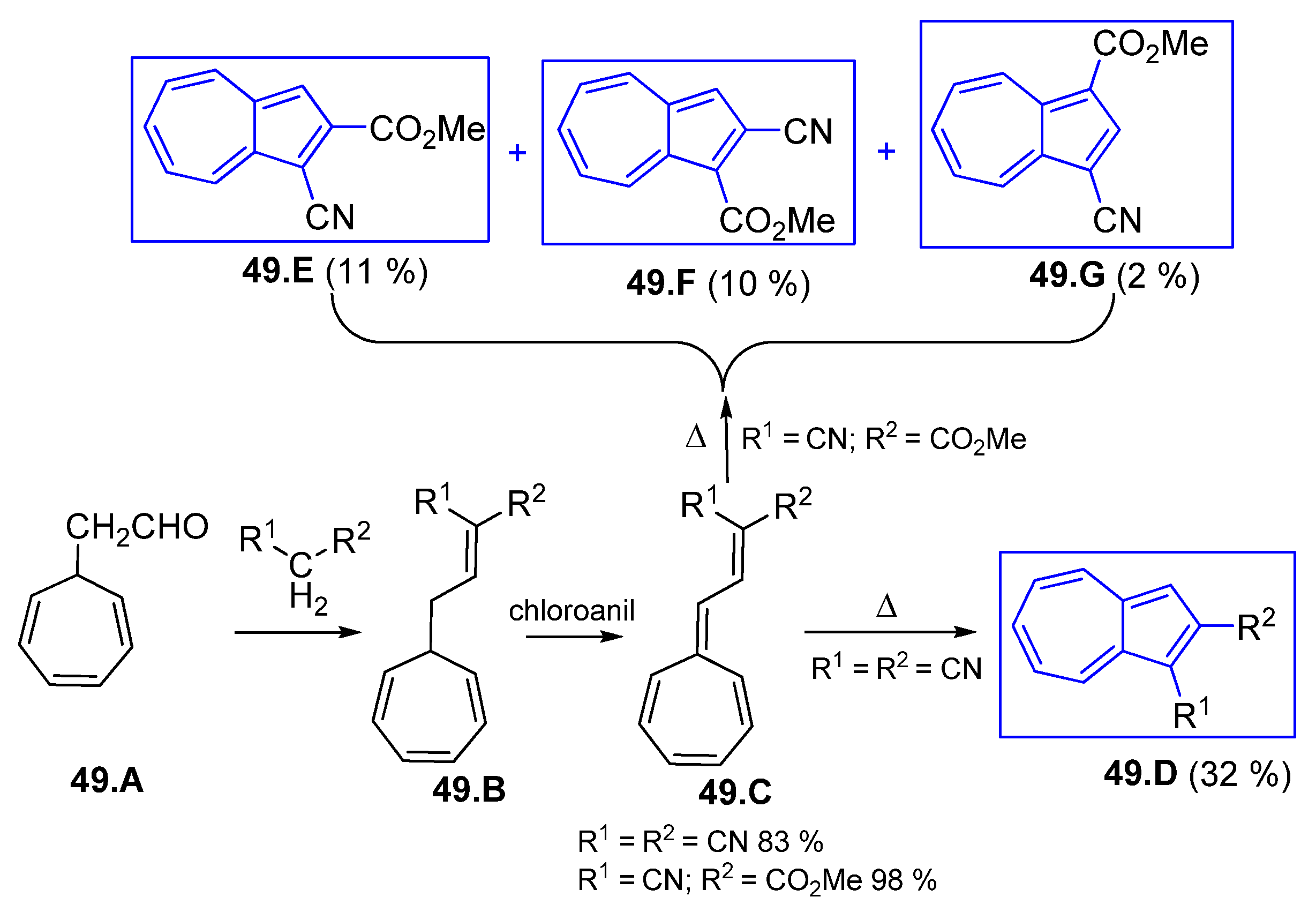
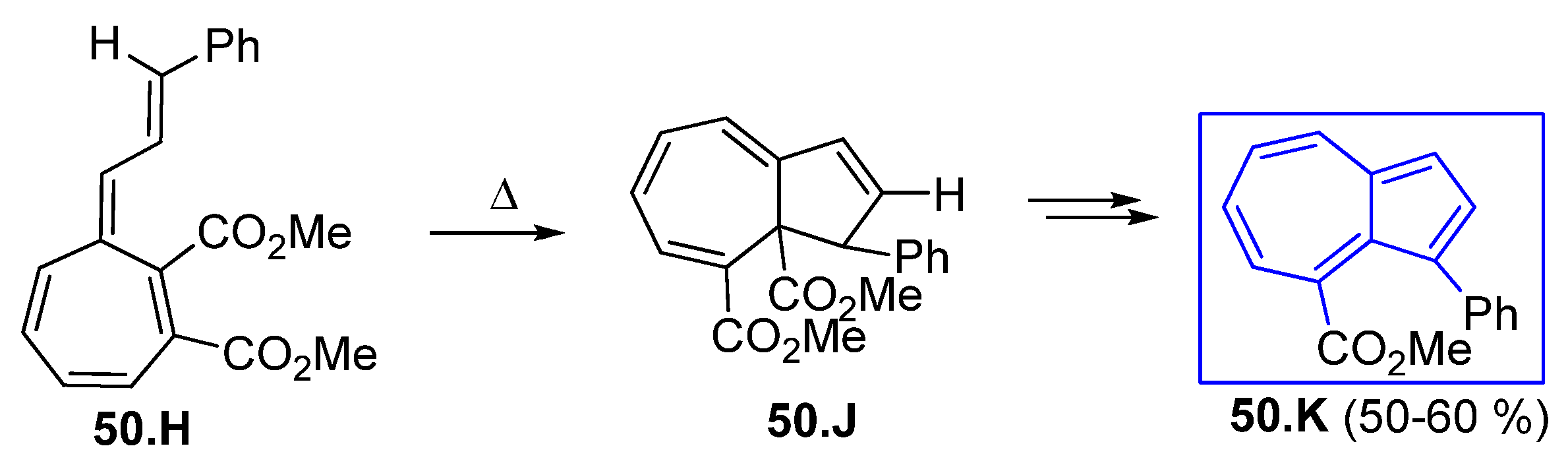
3.3.3. Fulvenes and Fulvenoid Derivatives Involved in 10π Electrocyclic Reactions; Polycyclic Azulenes Building
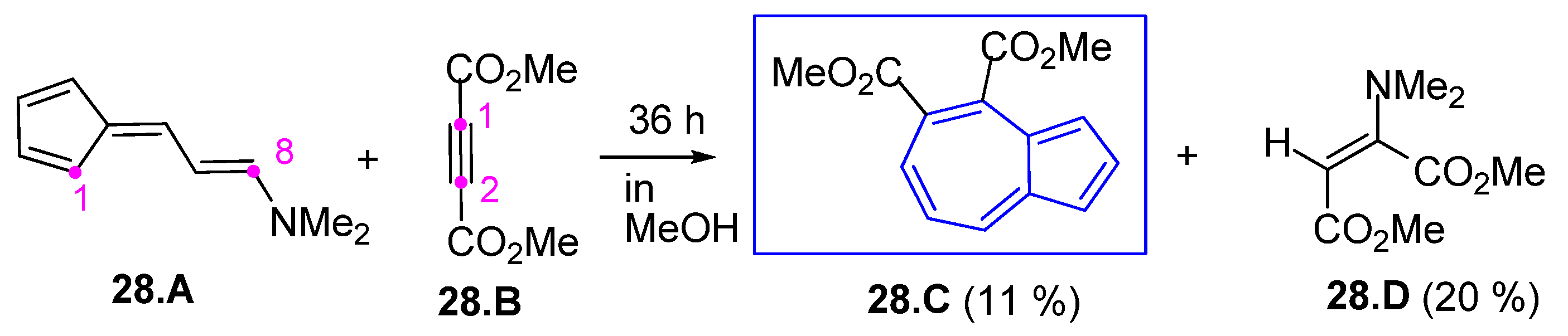
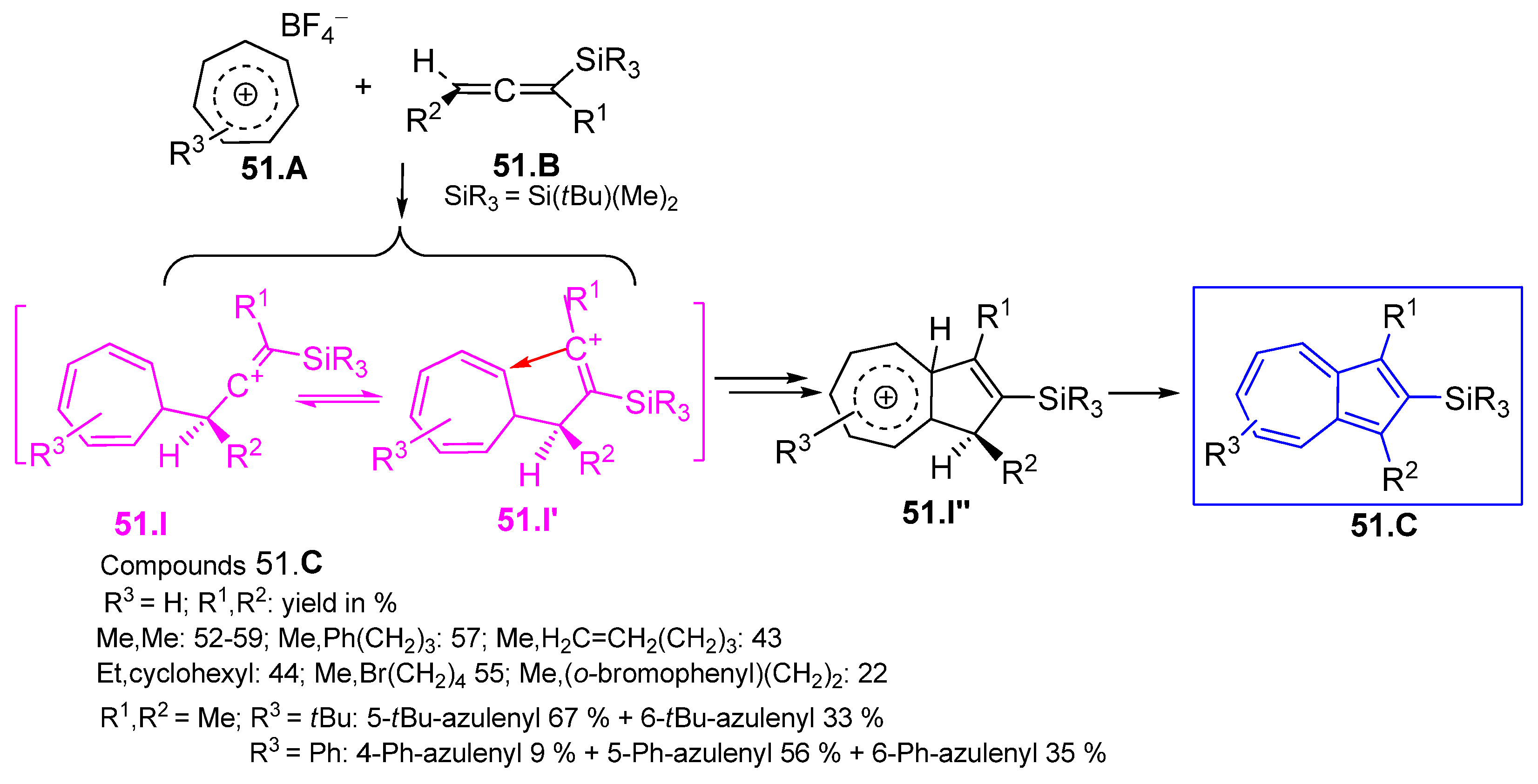
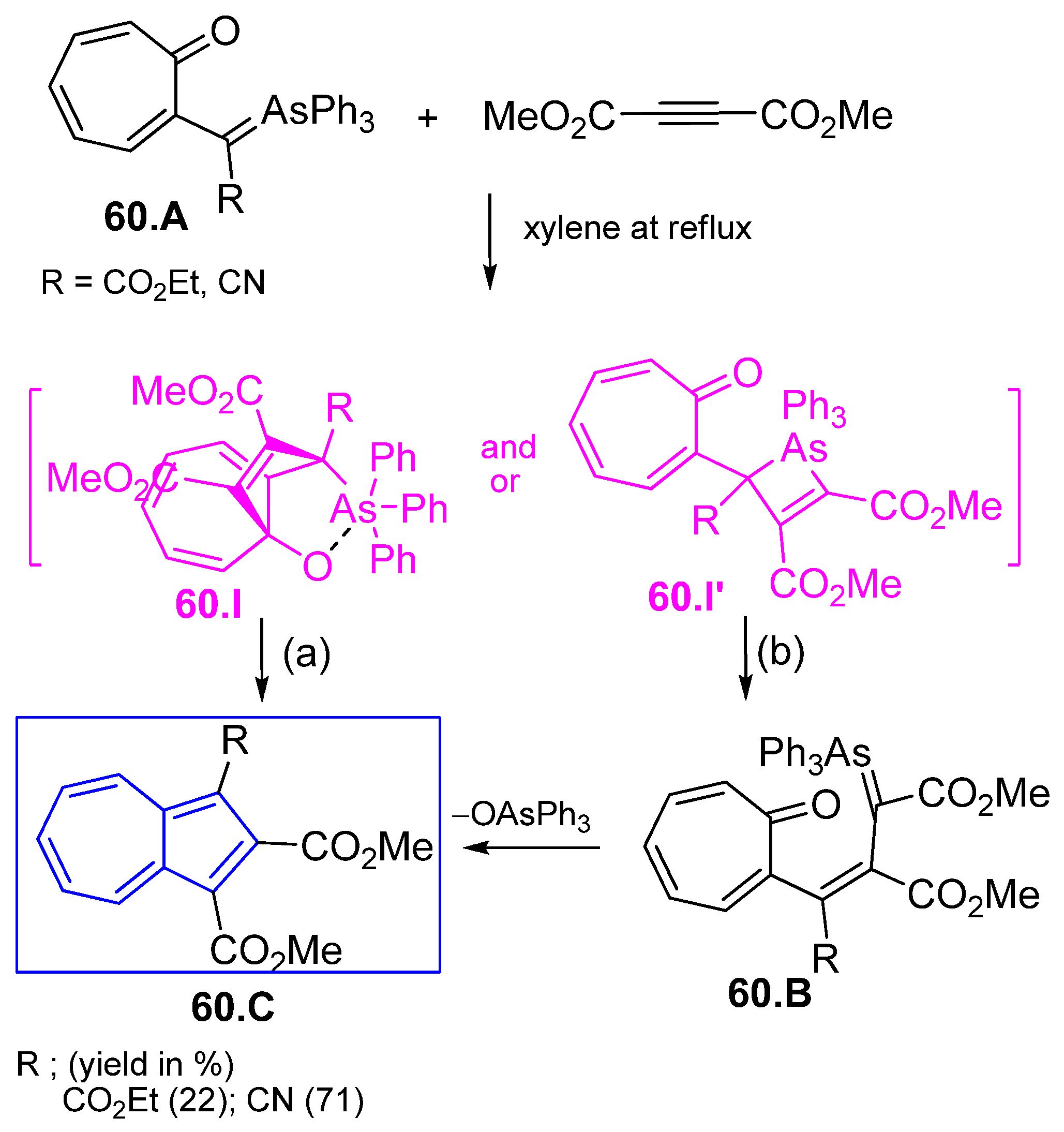
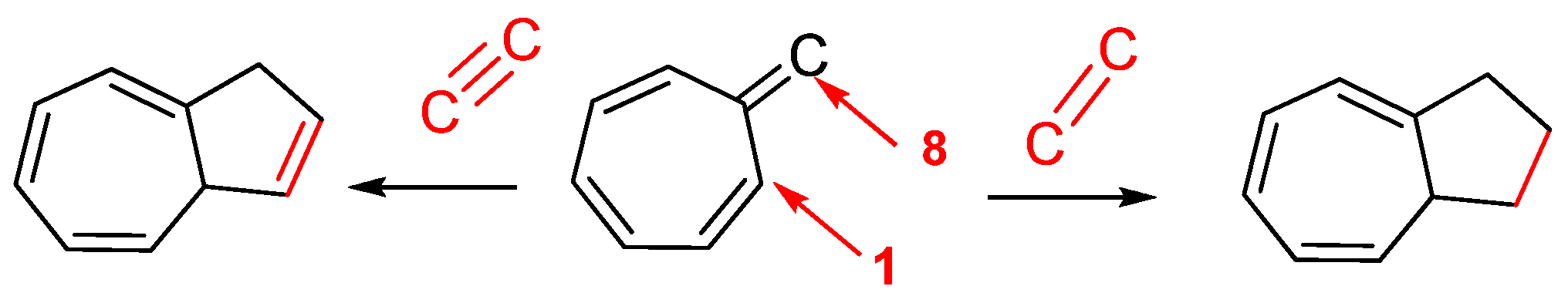
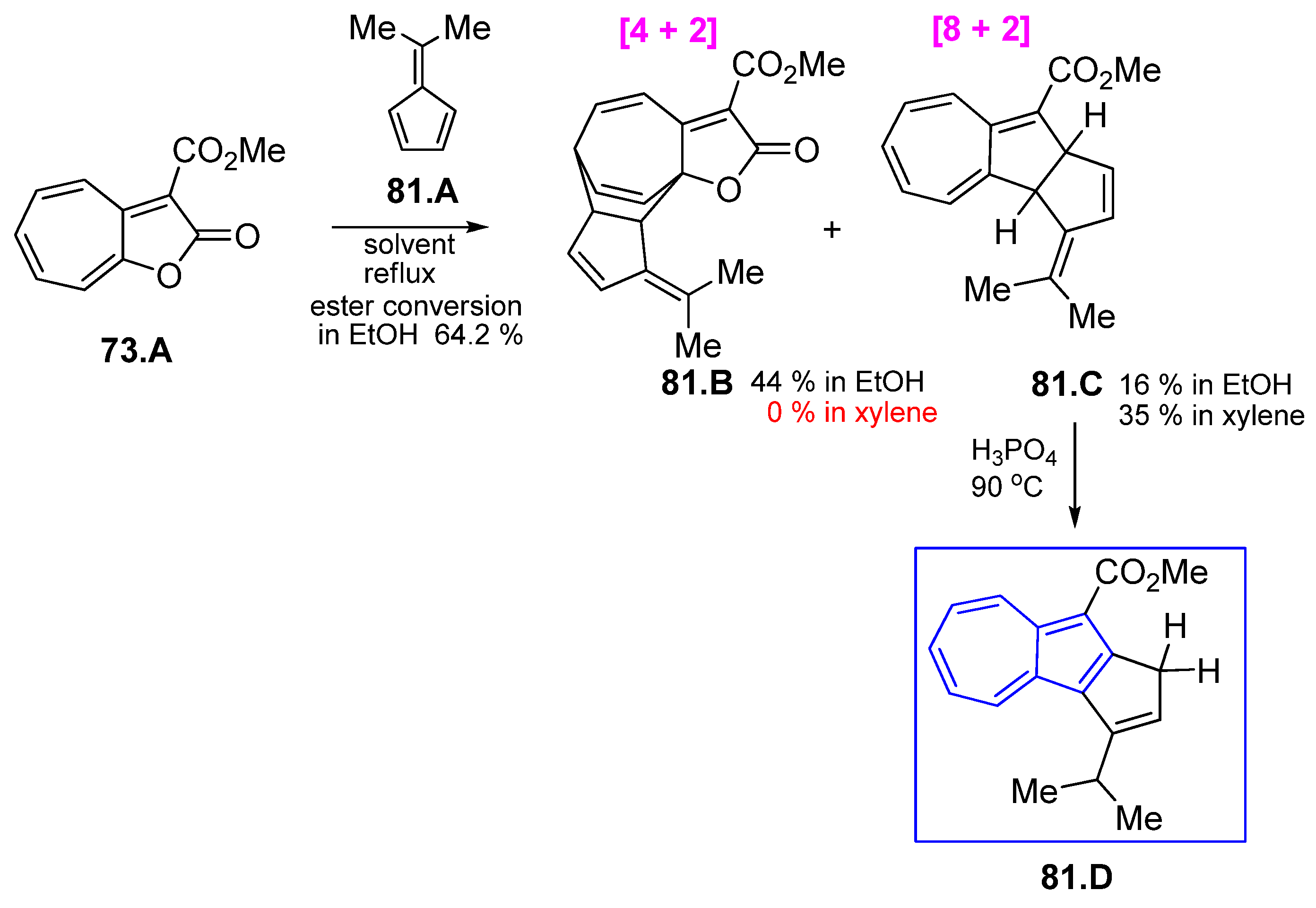
3.3.4. Fulvenes in [8+2]Cycloadditions with Acetylenes
3.4. Other Azulene Precursors Containing Cyclopentadienyl or Cyclopentane System
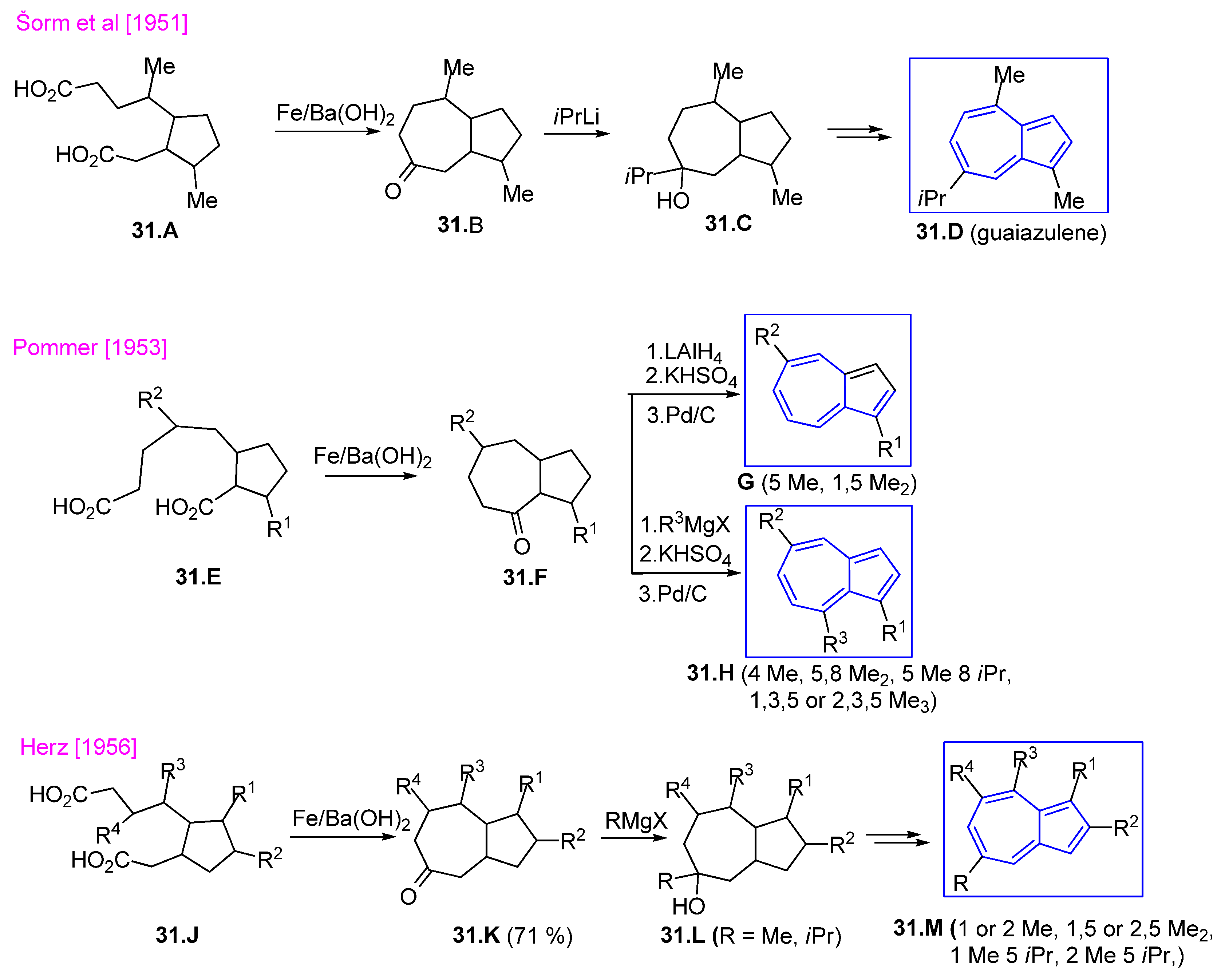
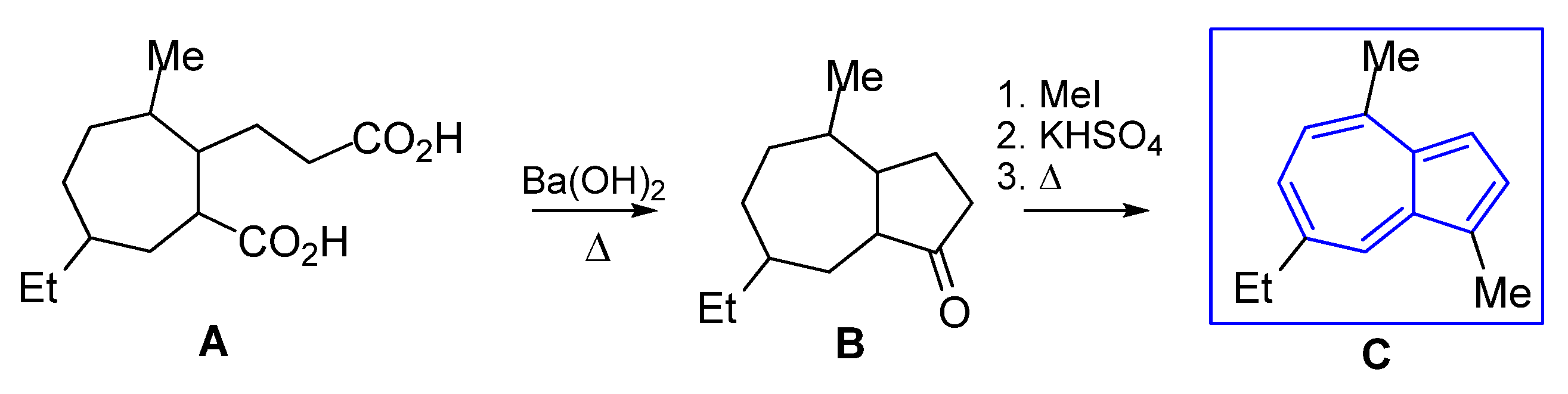
3.4.1. Seven Ring Building by Cyclocondensation of Dicarboxylic Groups
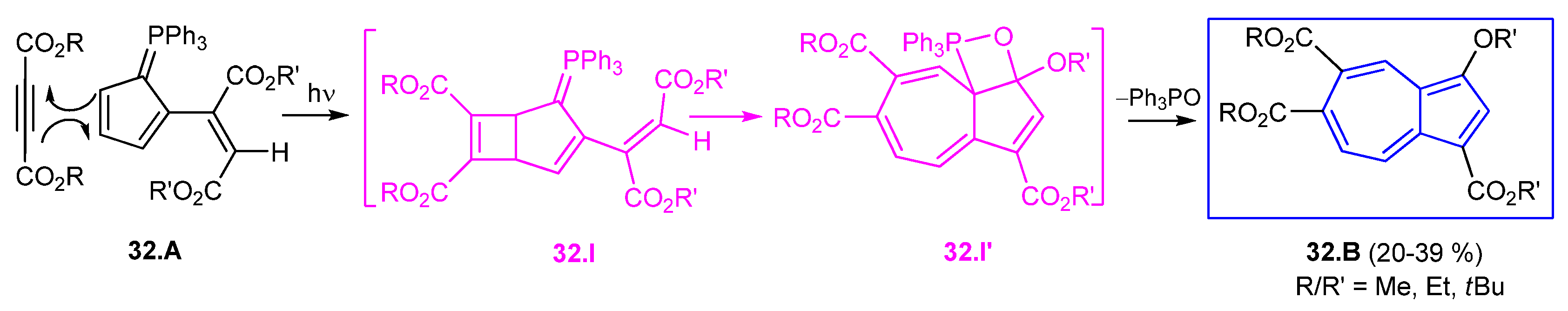
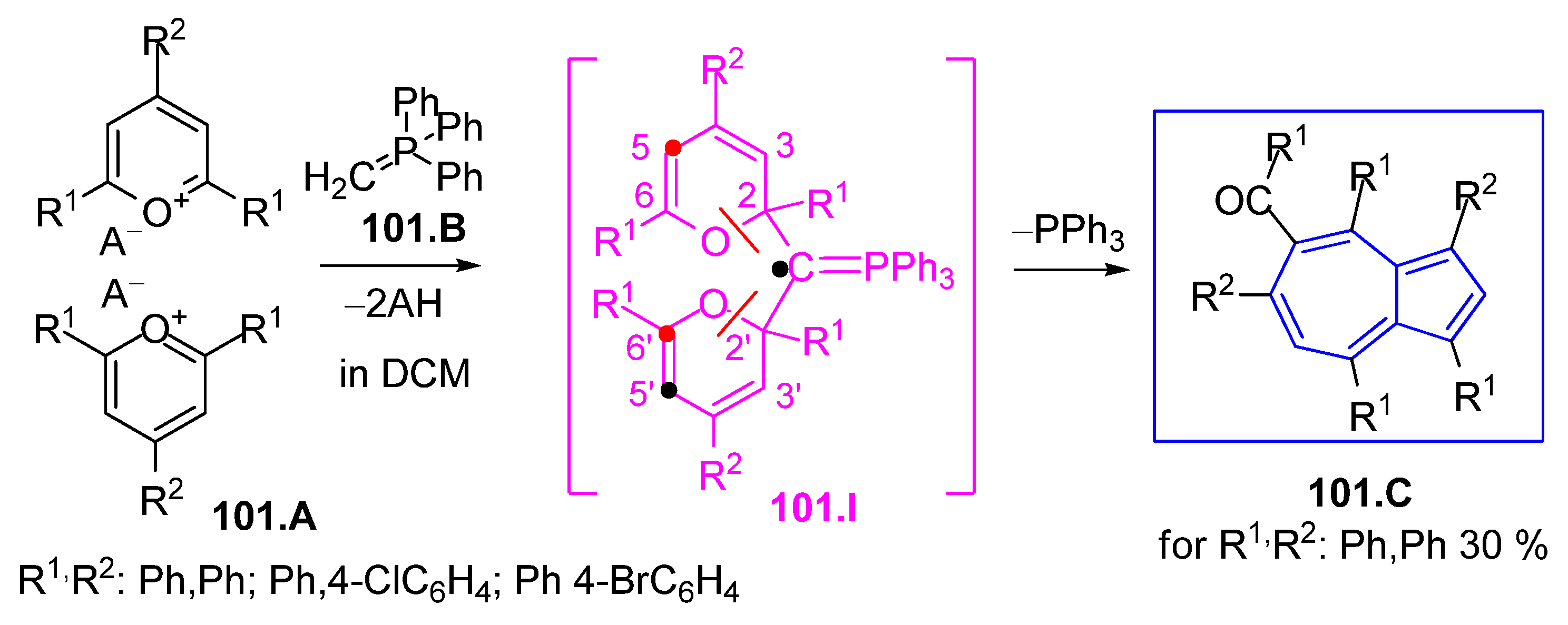
3.4.2. Azulene Synthesis from the Ramirez Ylide
3.4.3. Divinylcyclopropane-Rearrangement for 4-Cyanotetrahydroazulenes and 4-Cyanoazulenes Generation
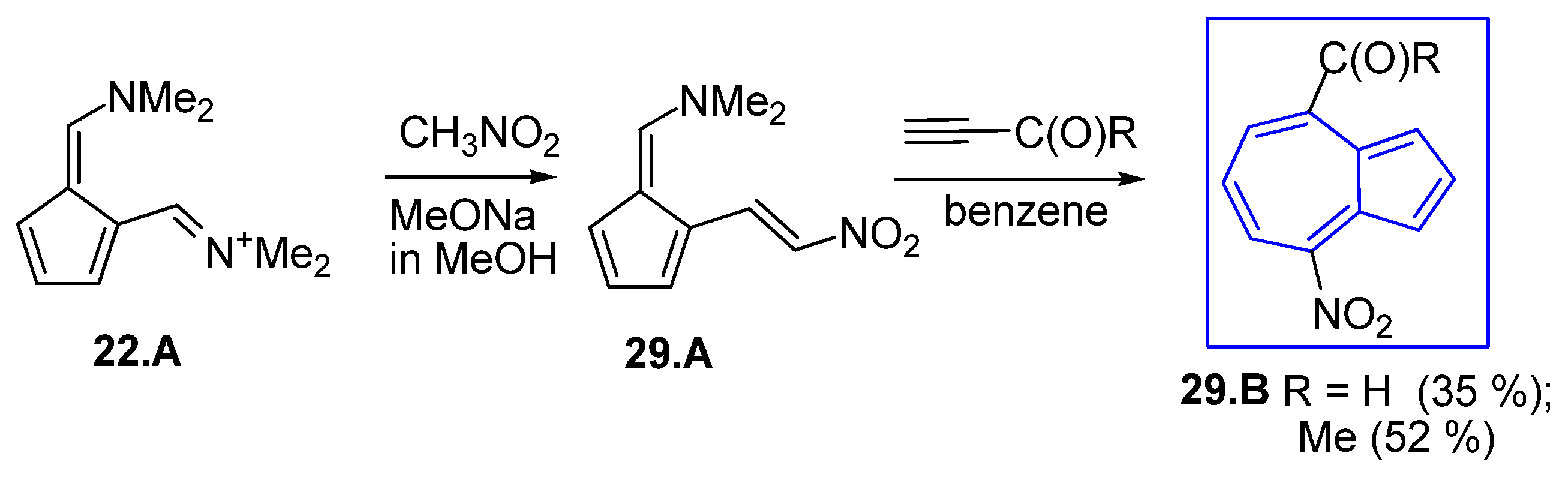
4. Obtaining Azulene Five-Membered Ring from Precursors Containing Seven-Membered Ring
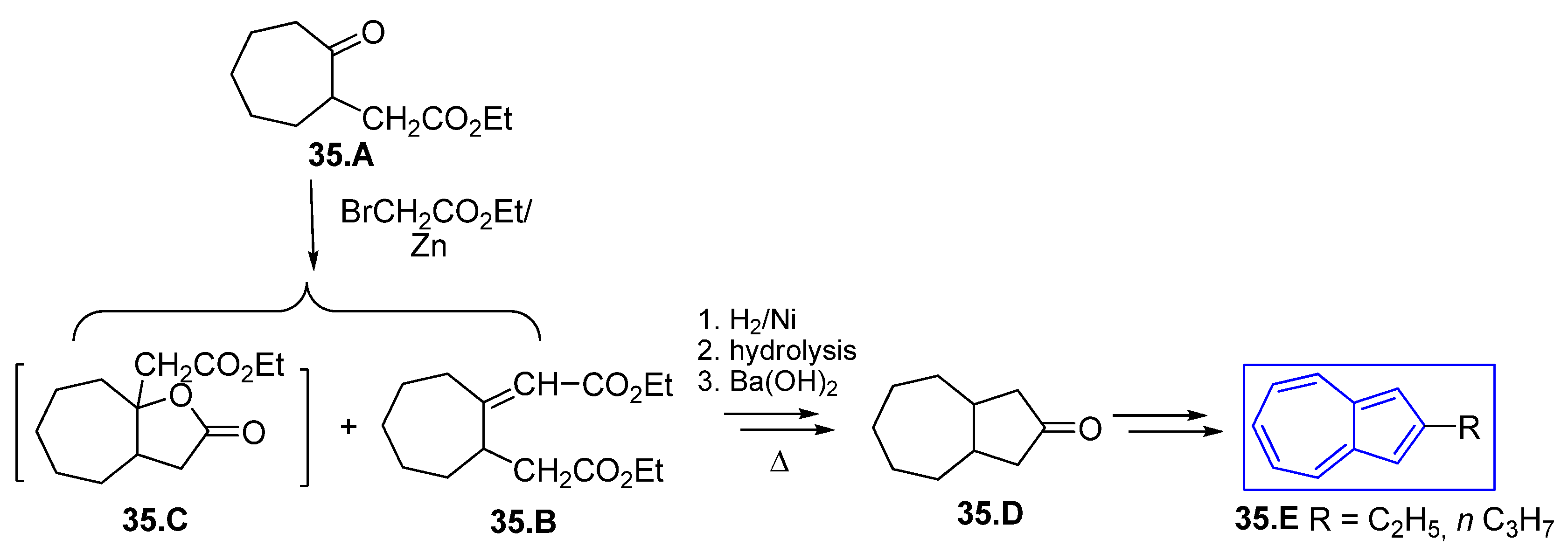
4.1. Five Ring Building by Cyclocondensation of Dicarboxylic Groups
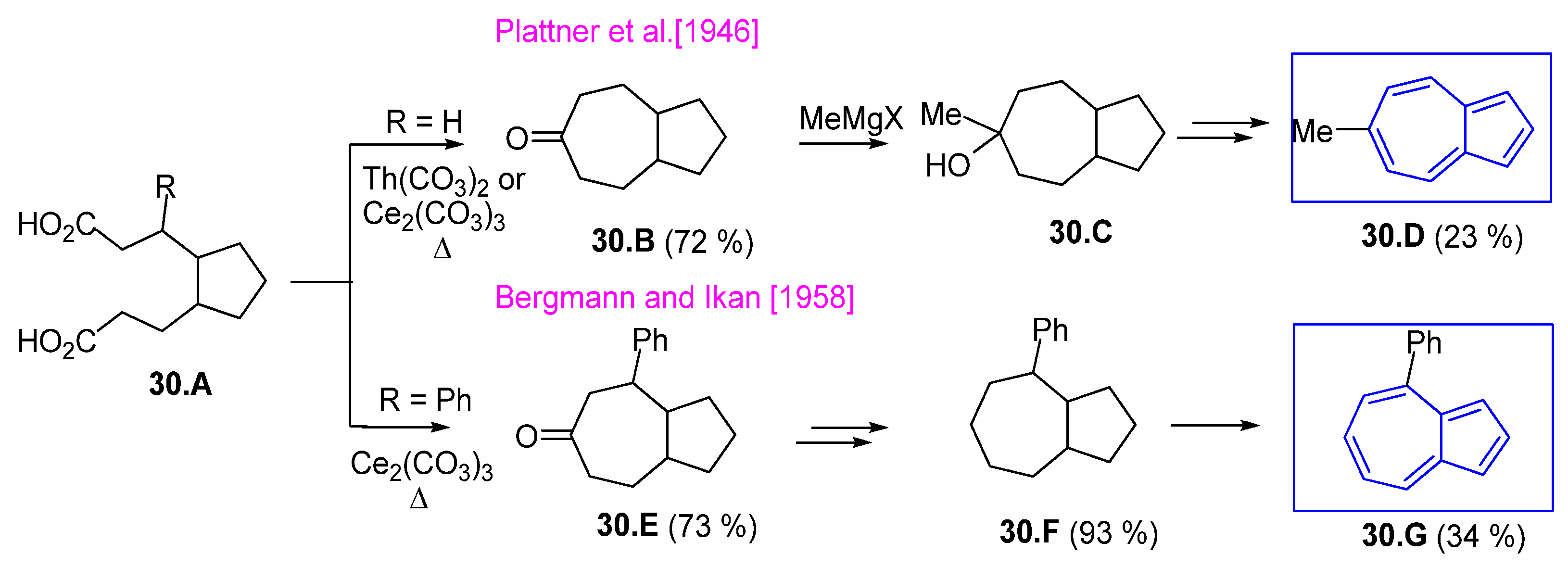
4.2. Cycloheptanone and Derivatives as Starting Materials
4.3. Cycloheptene and Derivatives as Starting Materials
4.4. Cycloheptatrienes, Tropylium Ions and Their Derivatives as Starting Materials
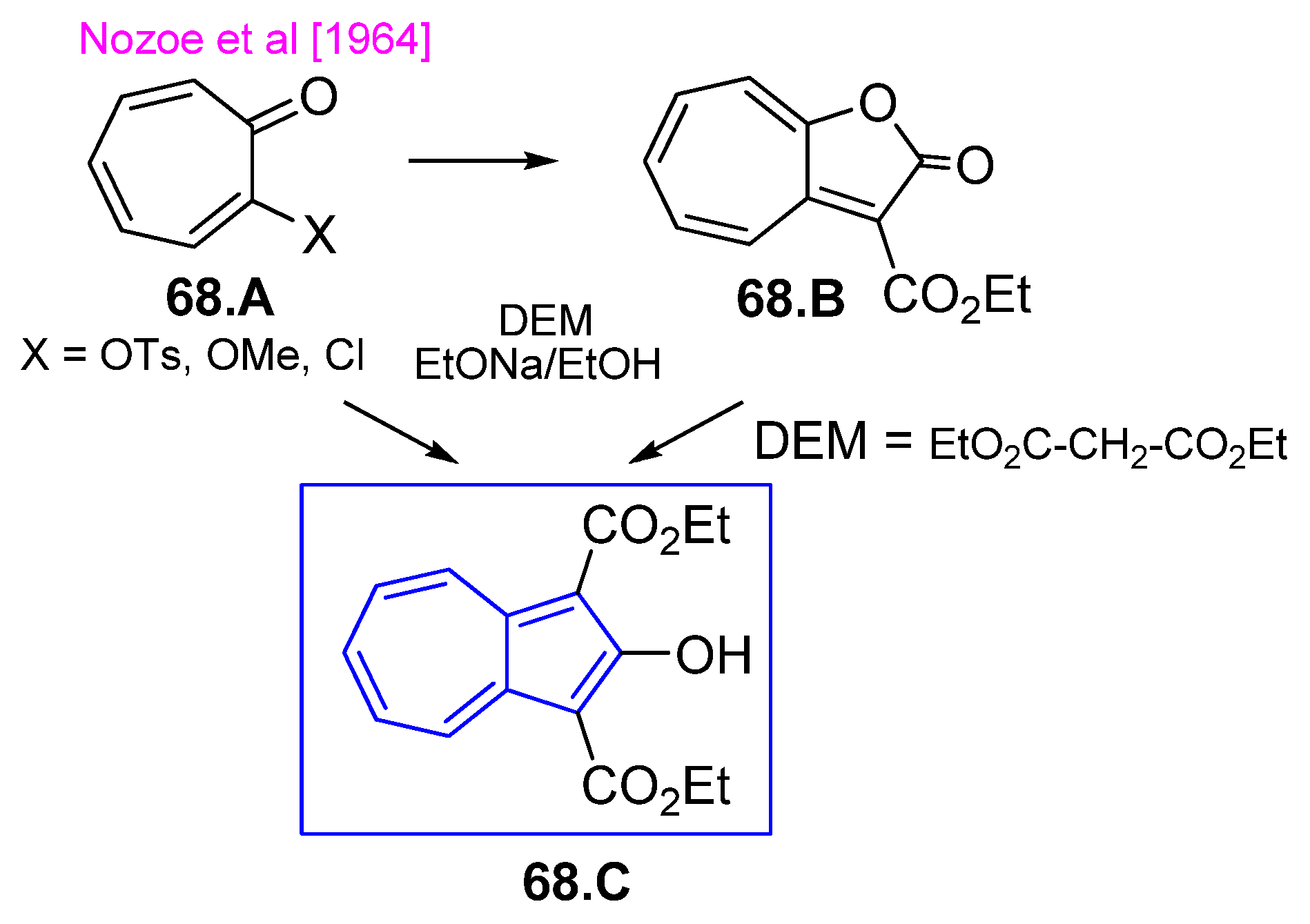
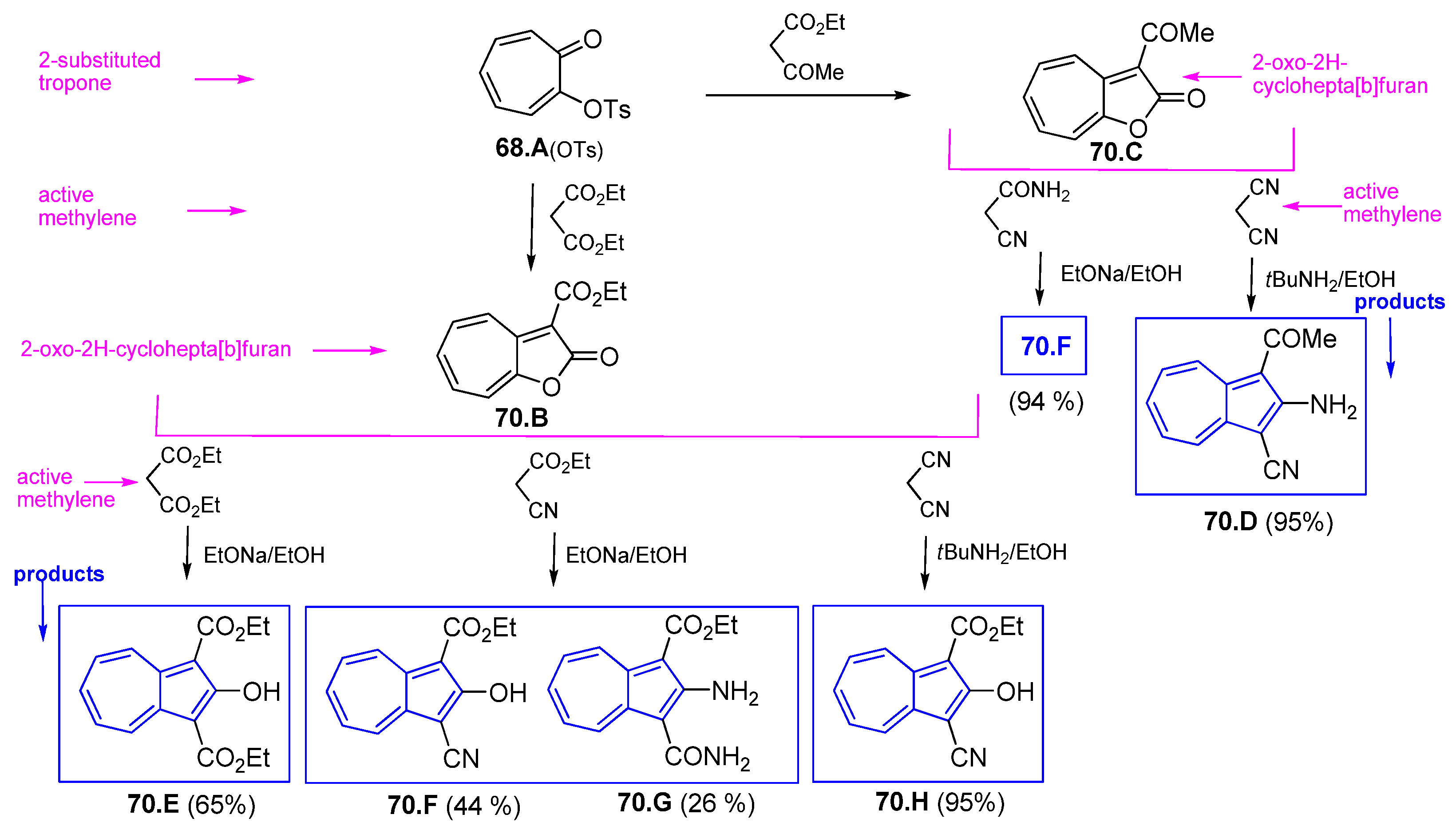
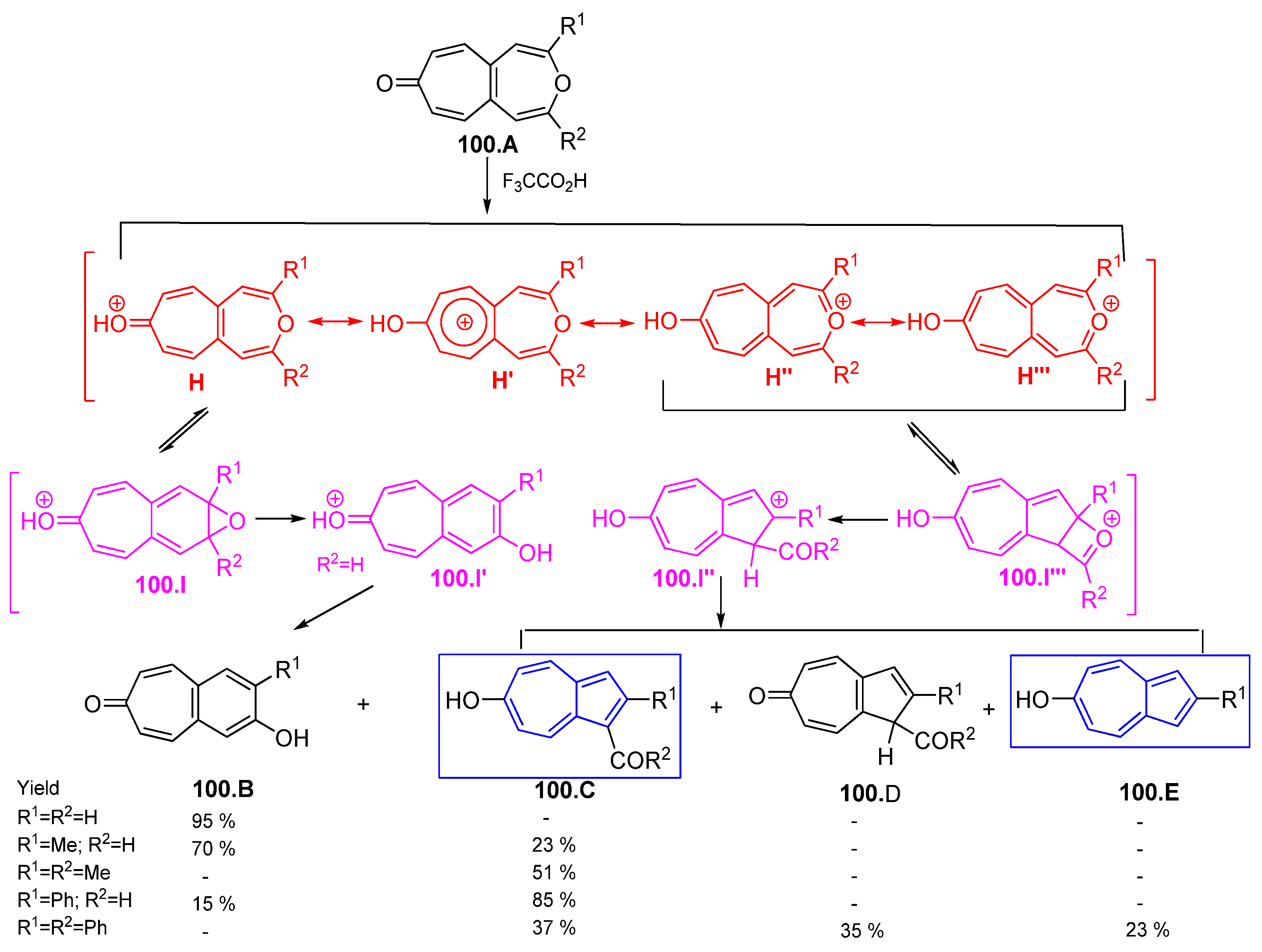
4.5. Azulene Five-Membered Ring from Troponoid Compunds
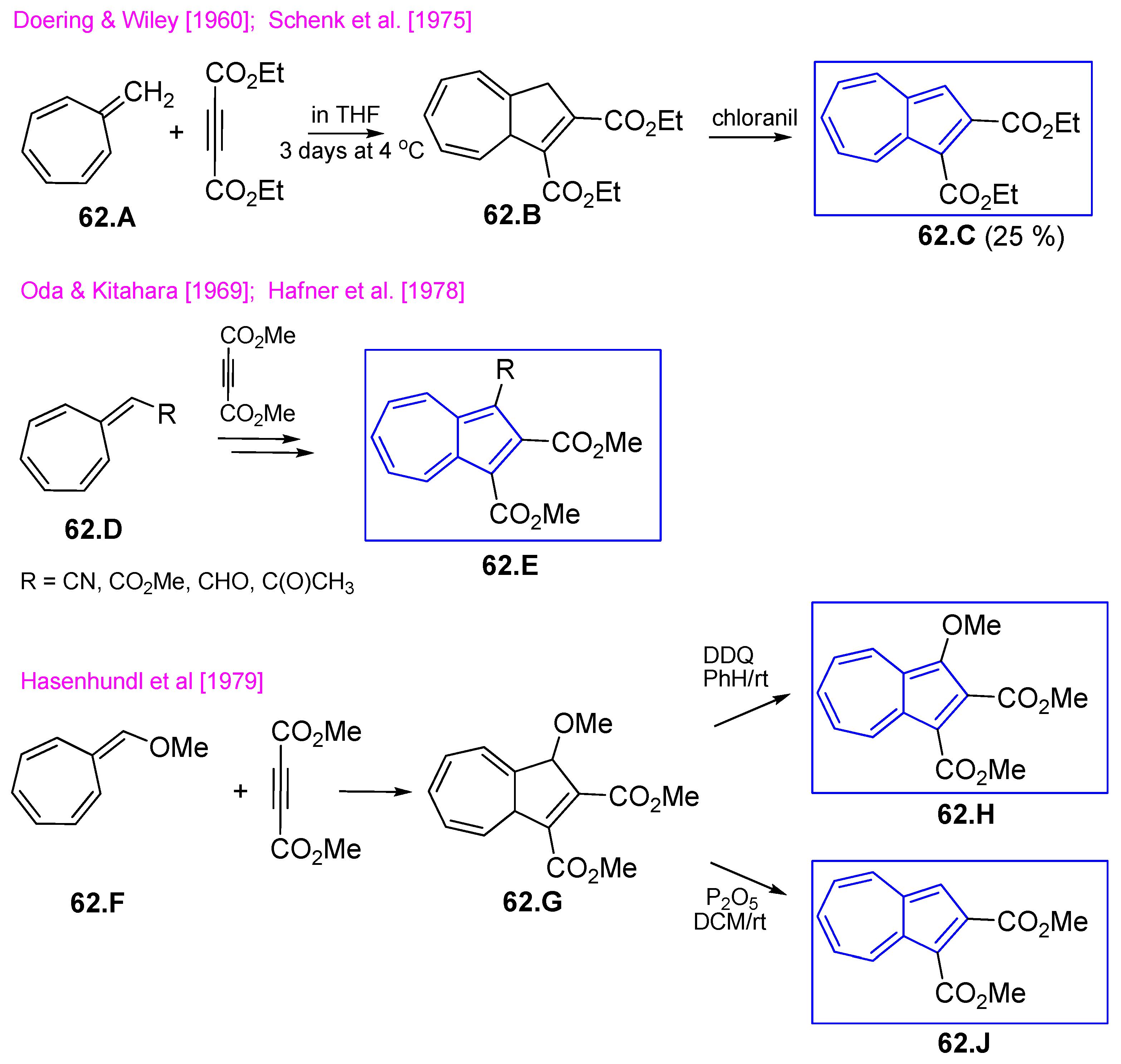
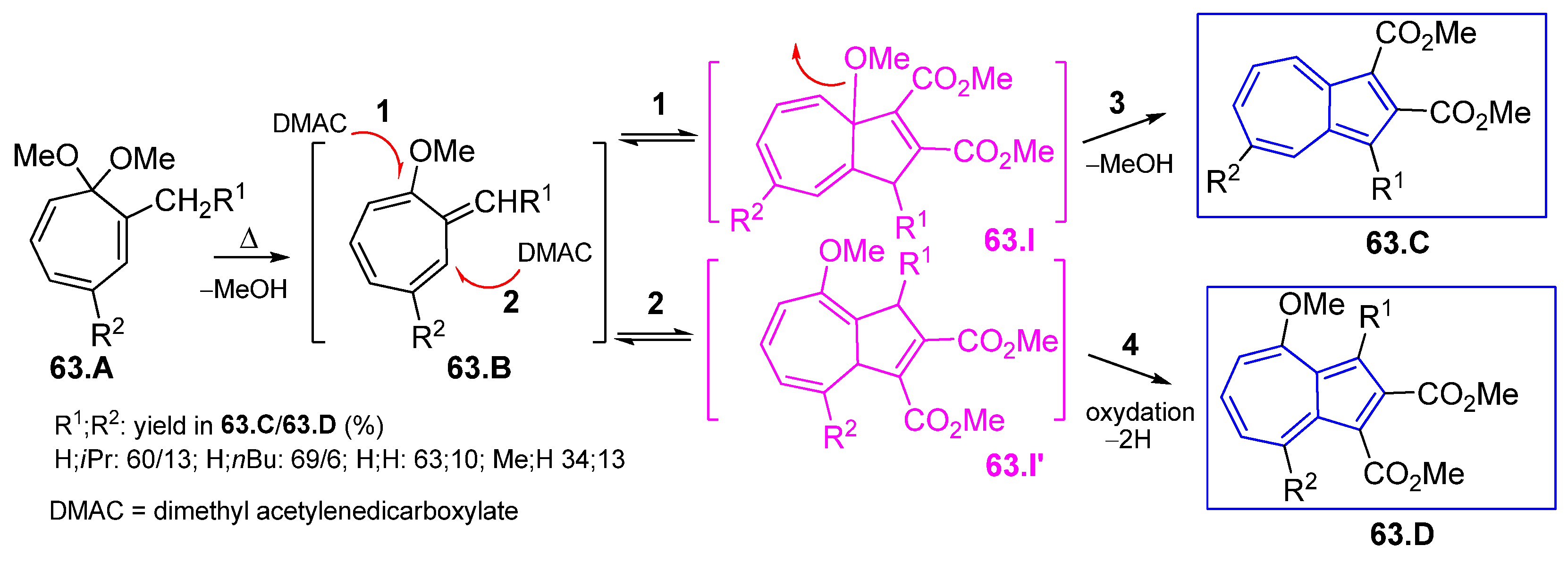
4.6. Azulene Five-Membered Ring from Cycloheptafulvene Derivatives
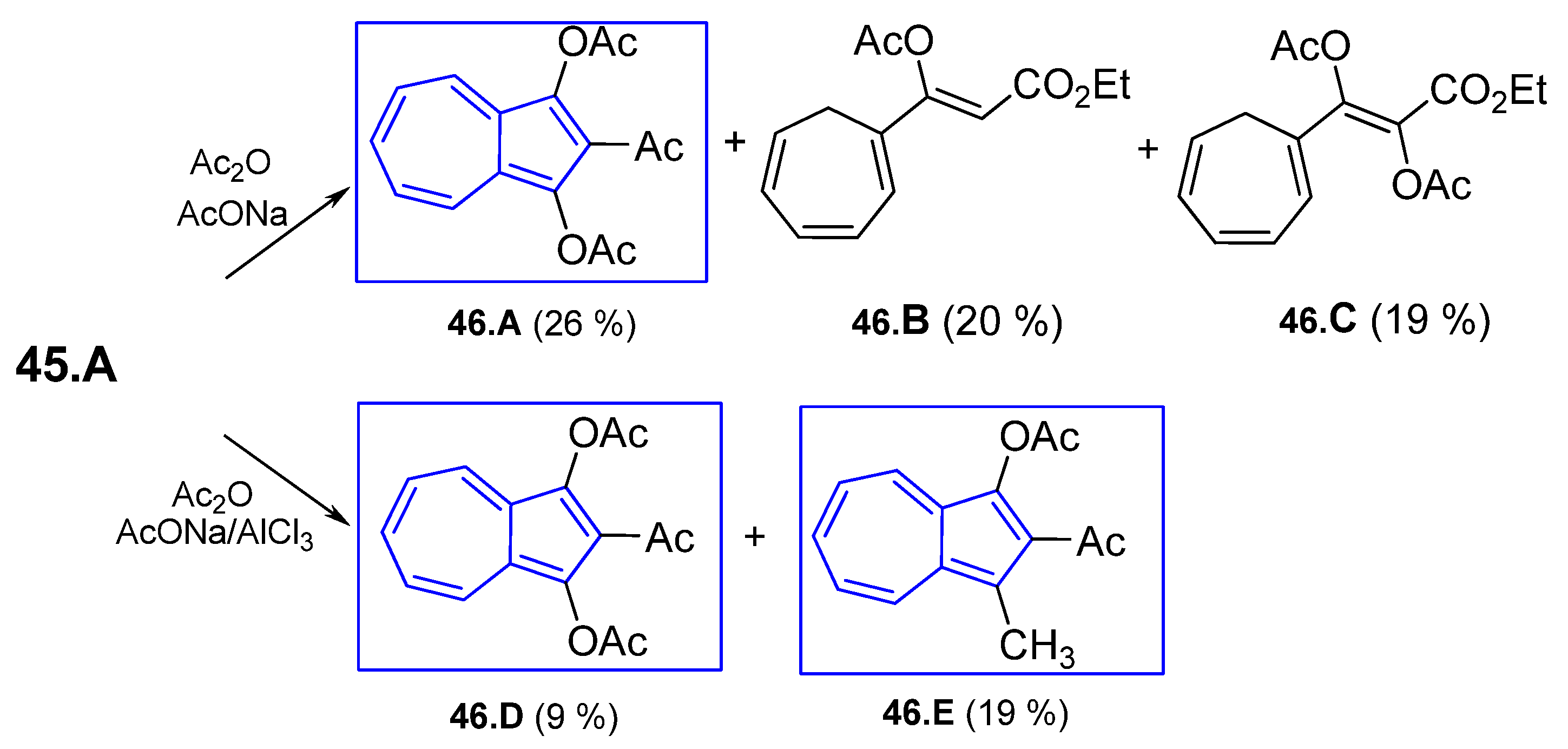
4.6.1. Reaction of Cycloheptafulvene Derivatives with Dialkyl Acetylenedicarboxylate
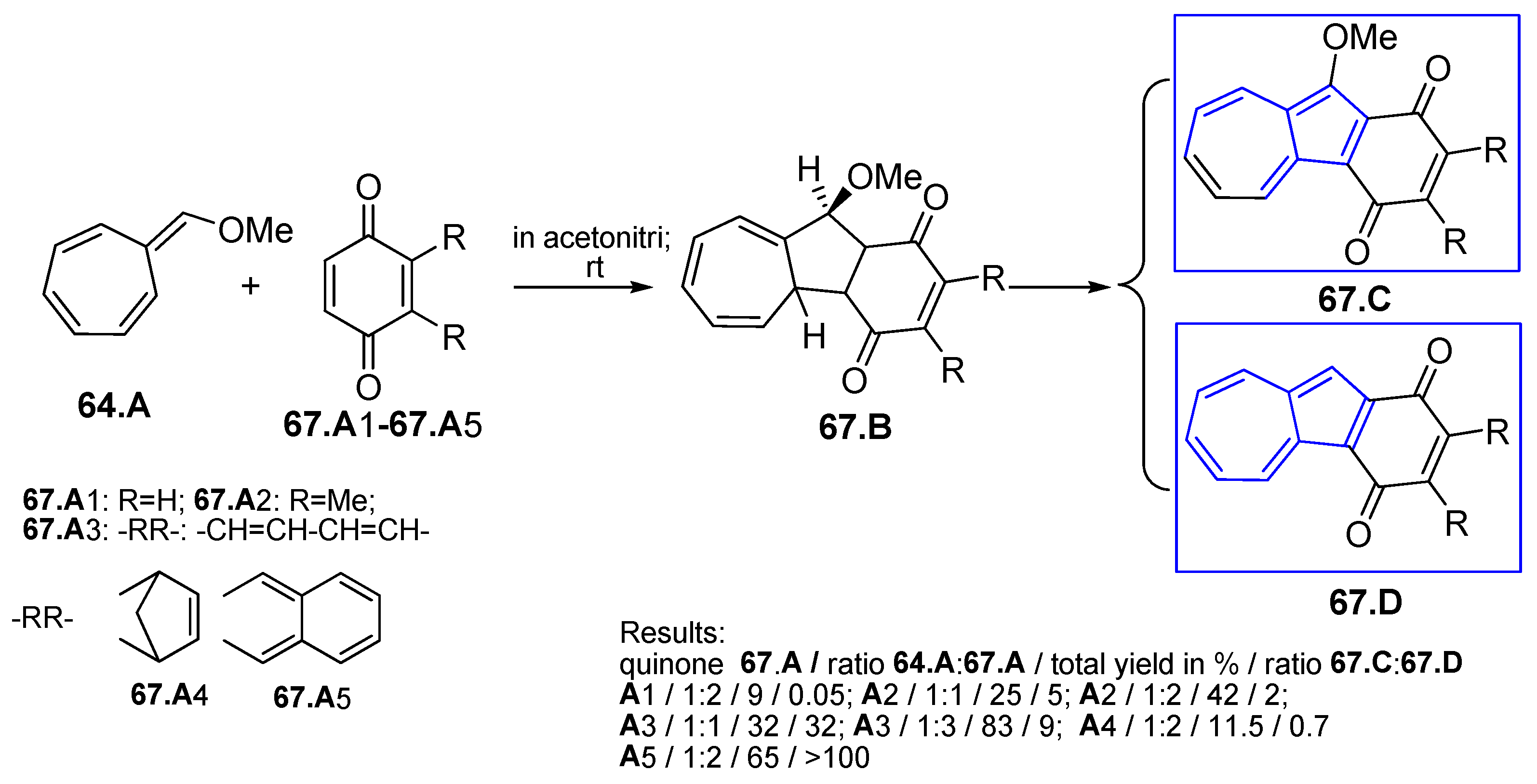
4.6.2. Reaction Between Cycloheptafulvene Derivatives and Compounds with C=C Bond
4.6.3. Embedded Azulene Polycyclic Molecules Starting from Cycloheptafulvene Derivatives
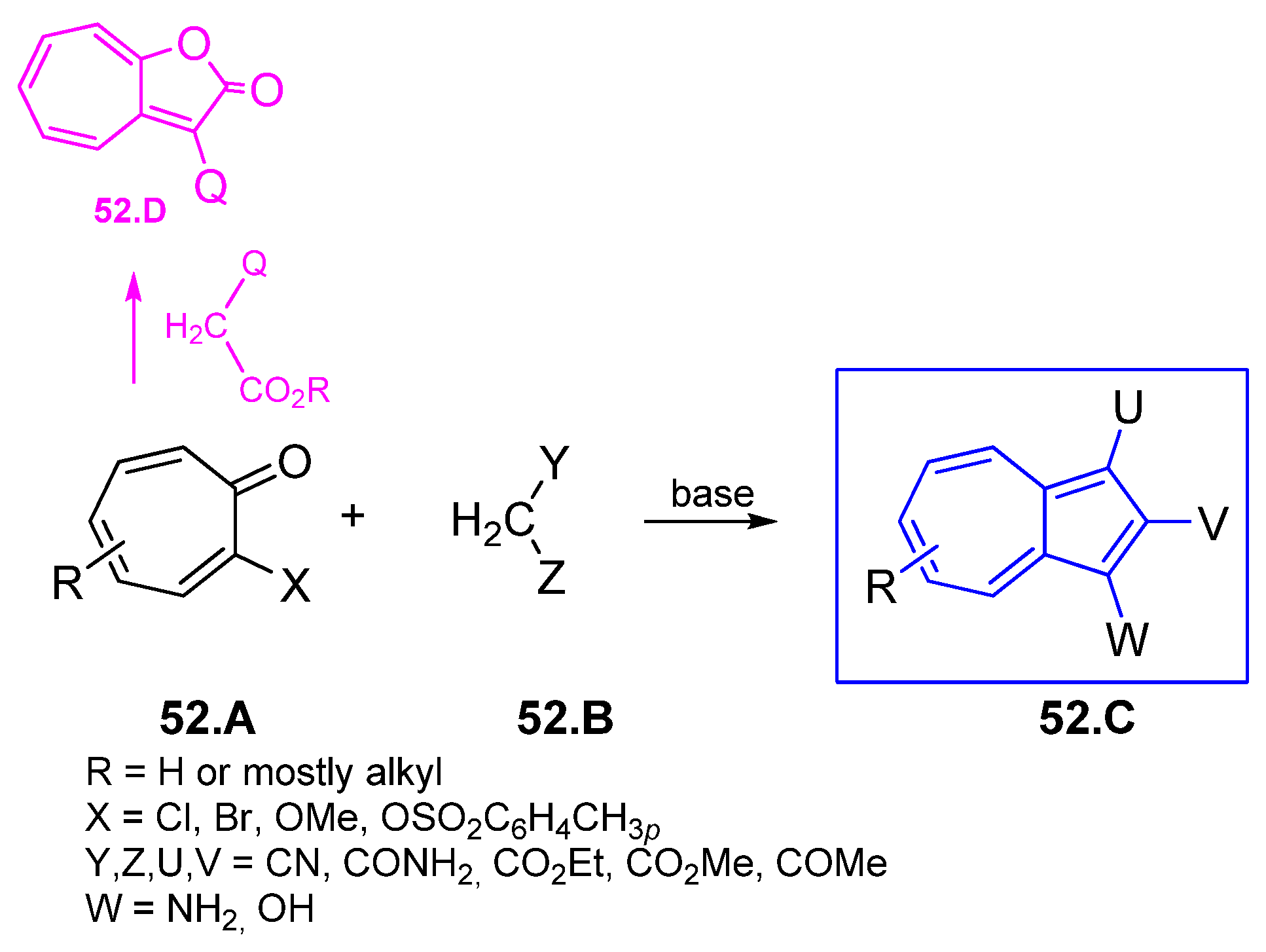
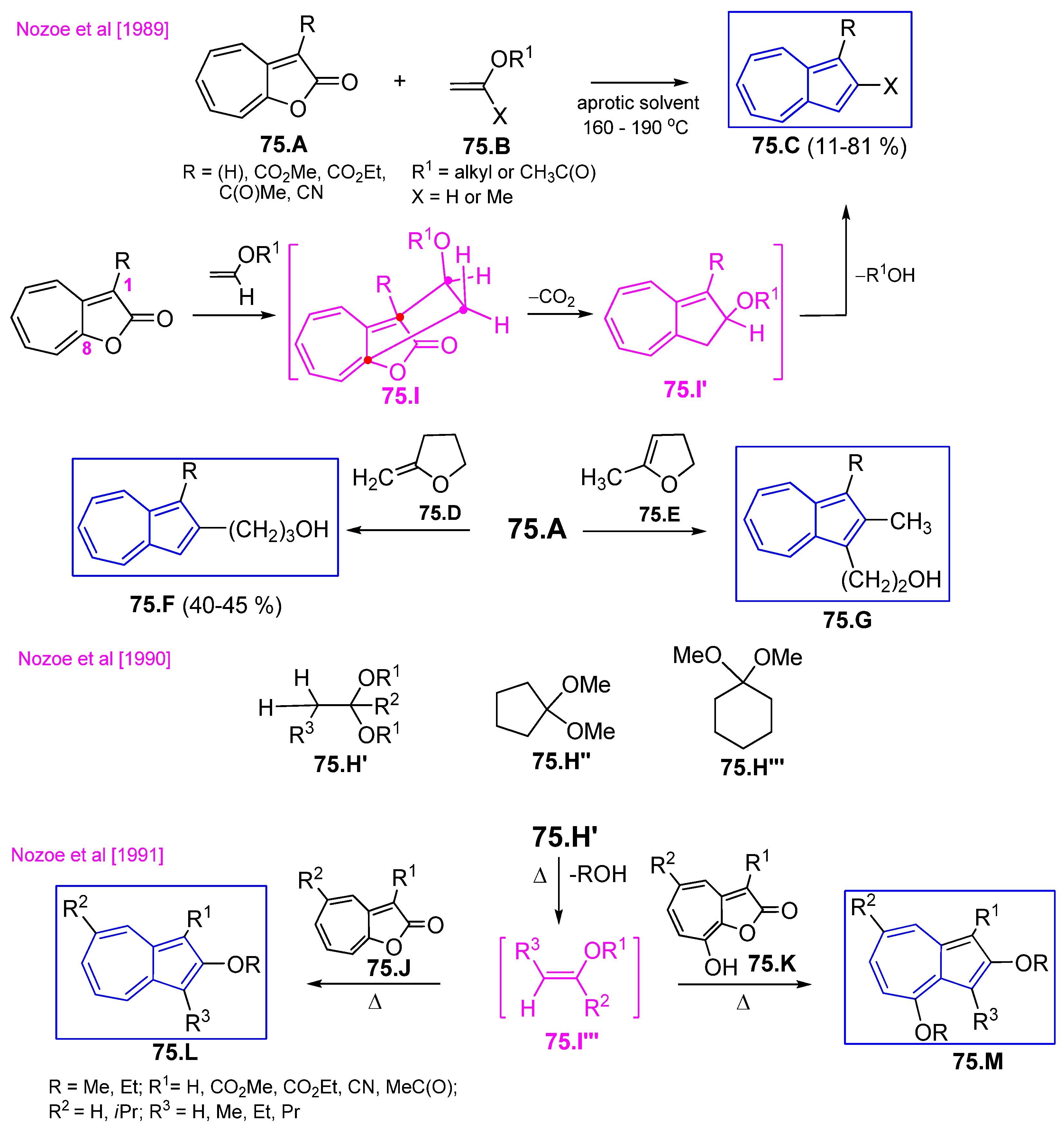
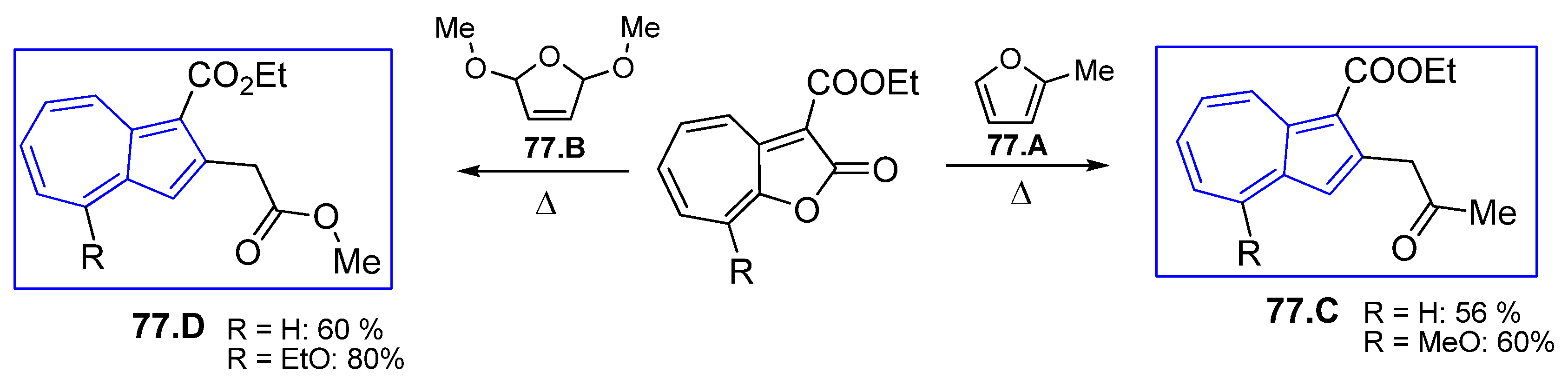
4.7. [8+2] Cycloaddition Starting from 2H-Cyclohepta[b]furan2-ones
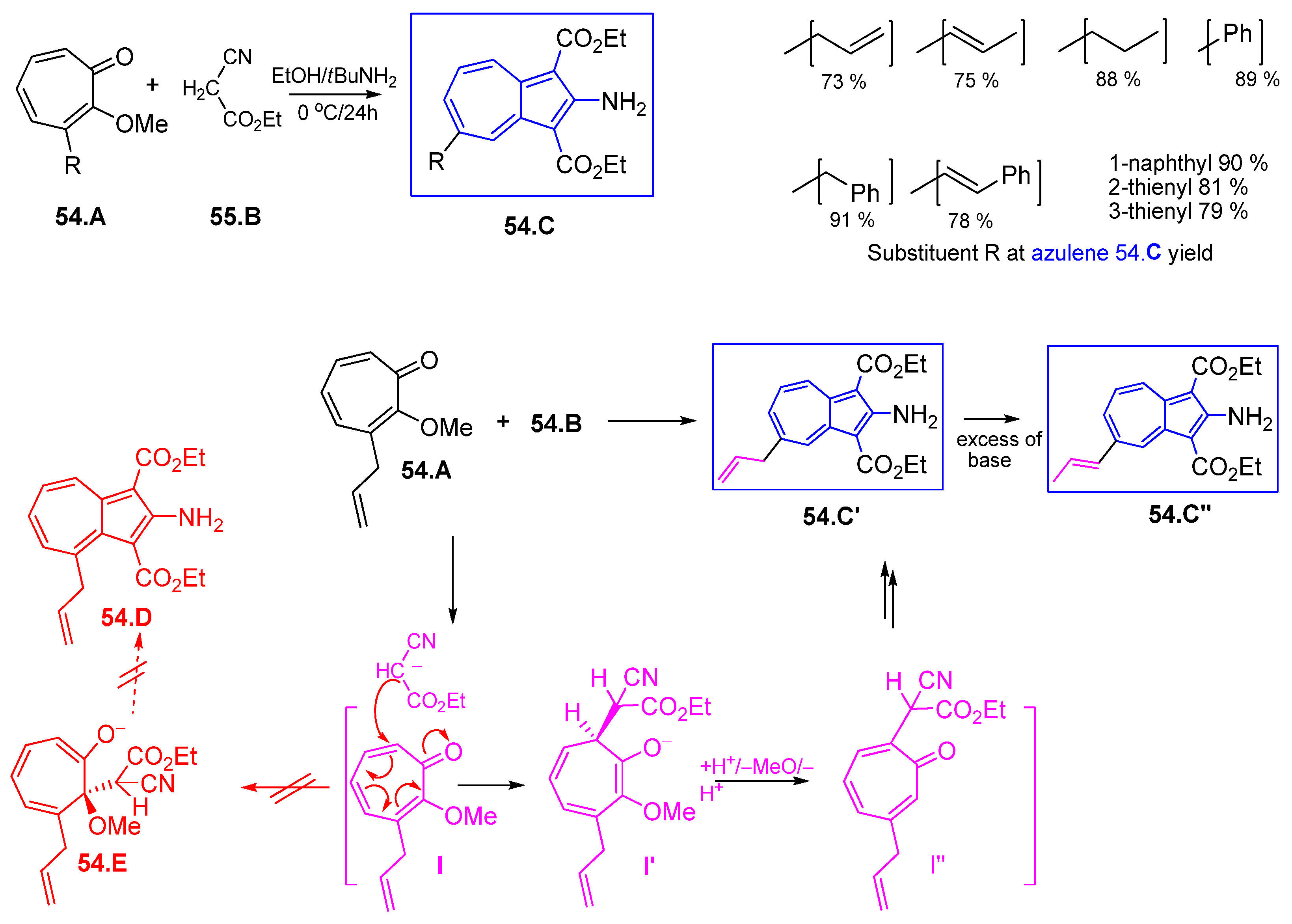
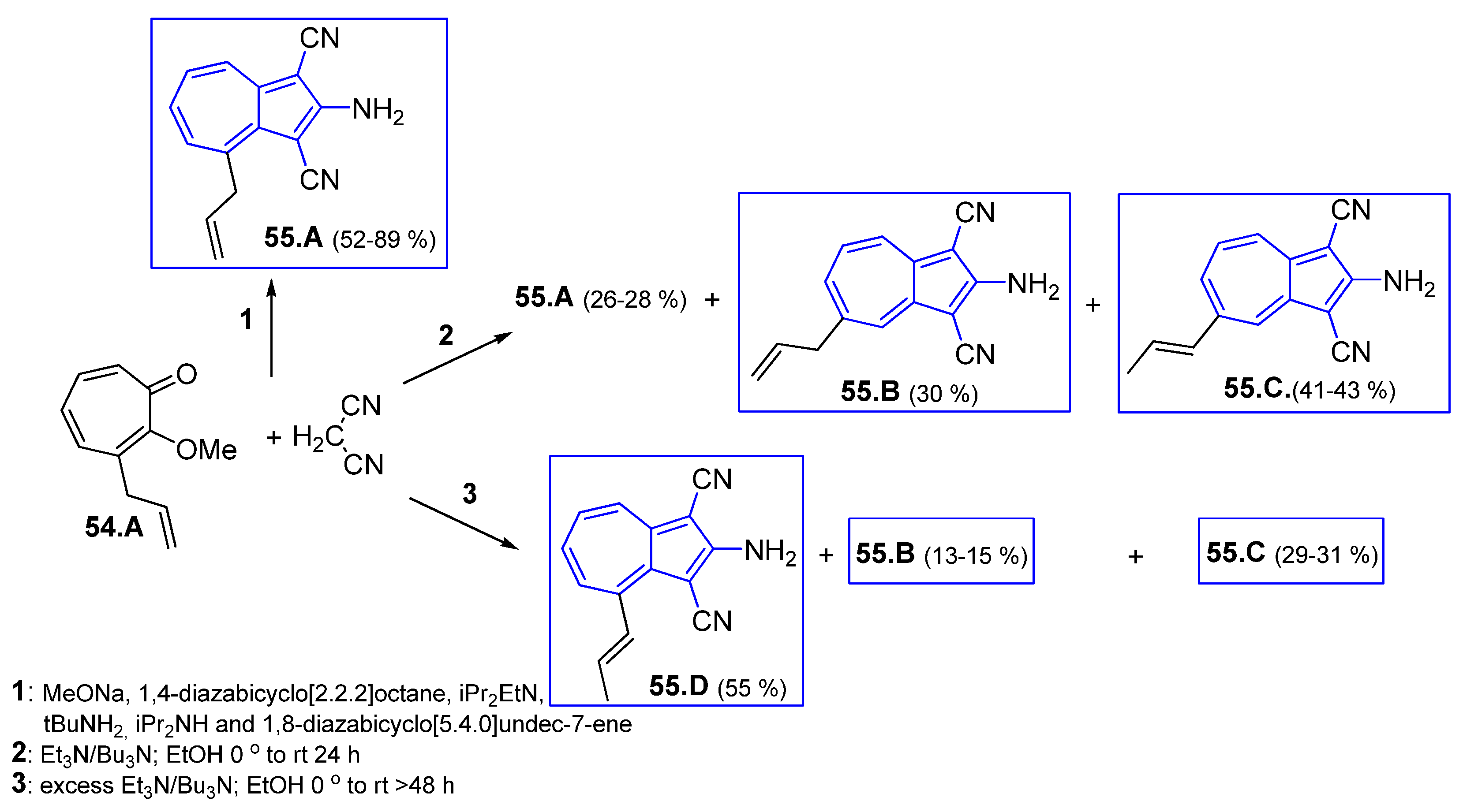
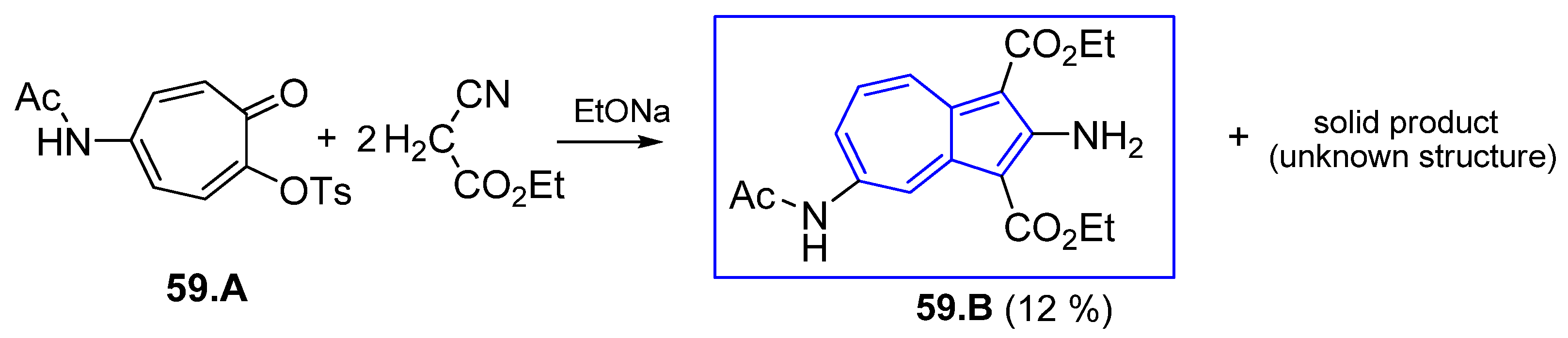
4.7.1. Reaction of 2H-Cyclohepta[b]furan2-ones with Active Methylenes
4.7.2. Reaction with Enol Ethers and Its Analogs
4.7.3. Reaction with Enamines and Its Analogs
4.7.4. Reaction with Other Compounds
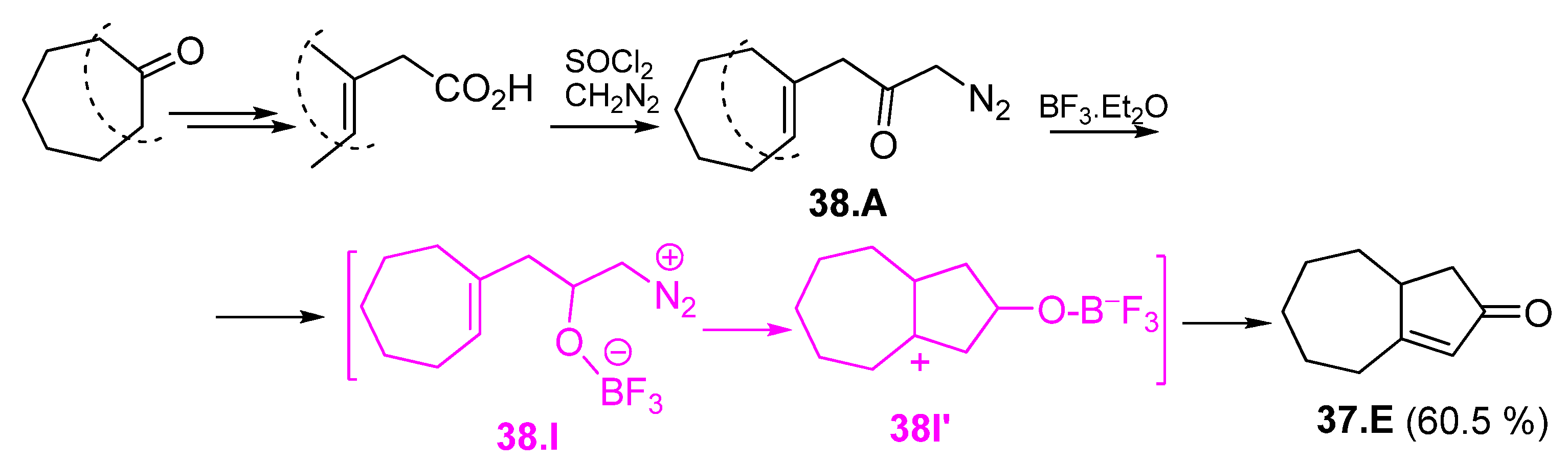
5. Enlarging of Six to Seven-Membered Ring by the Insertion of Carbenes
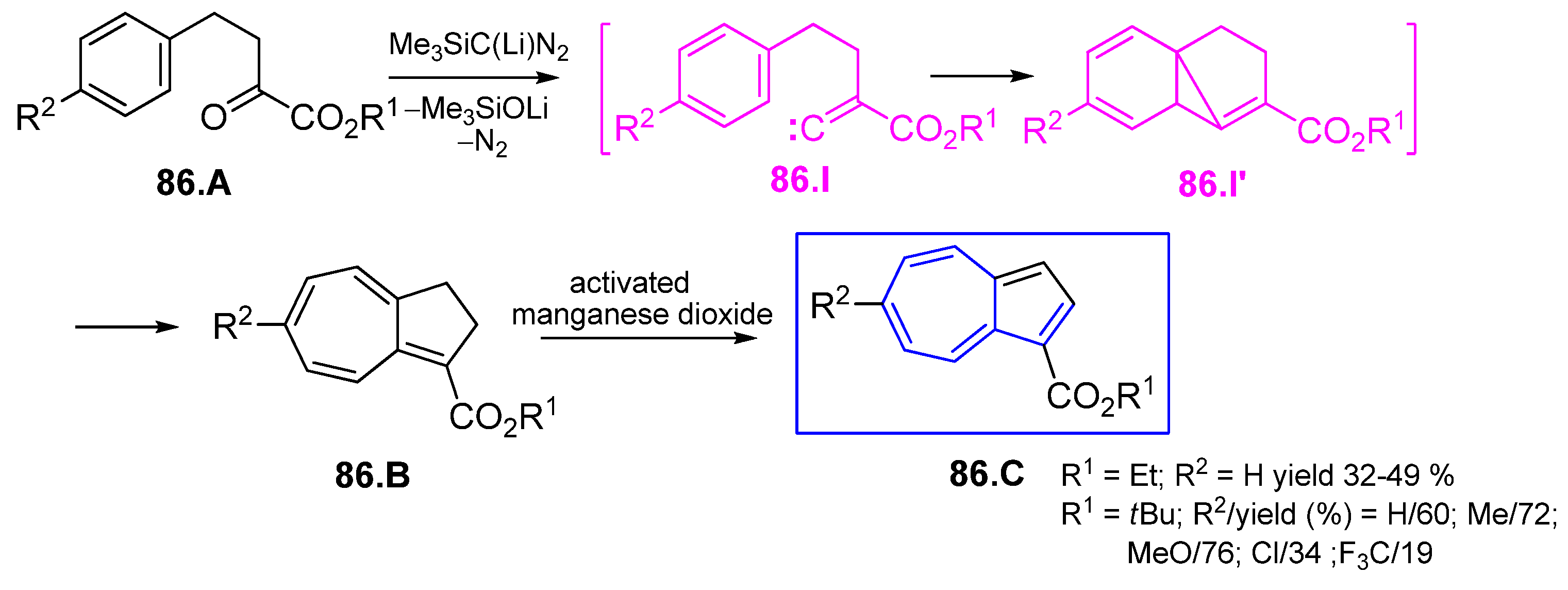
5.1. Carbenes Derived from Diazomethane or Diazoacetic Ester
5.2. Intramolecular Carbene Insertion
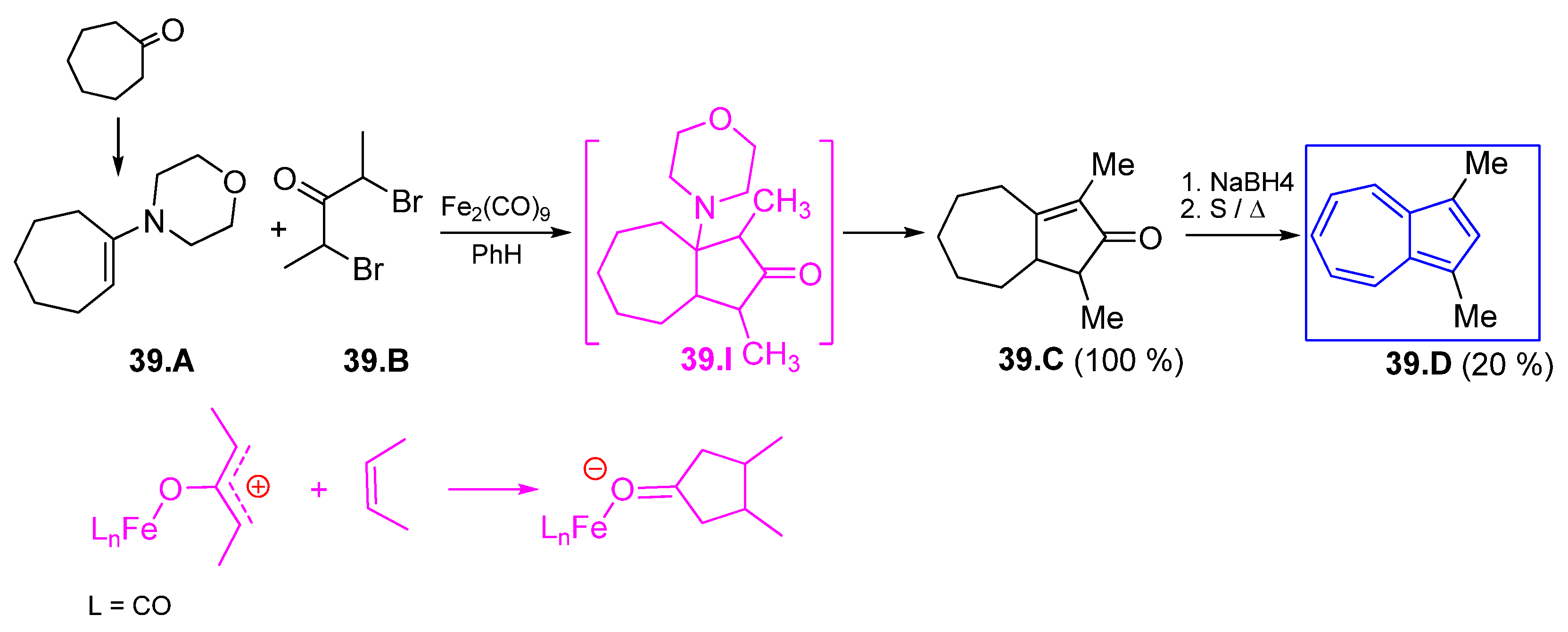
6. Different Examples of Azulenes Building
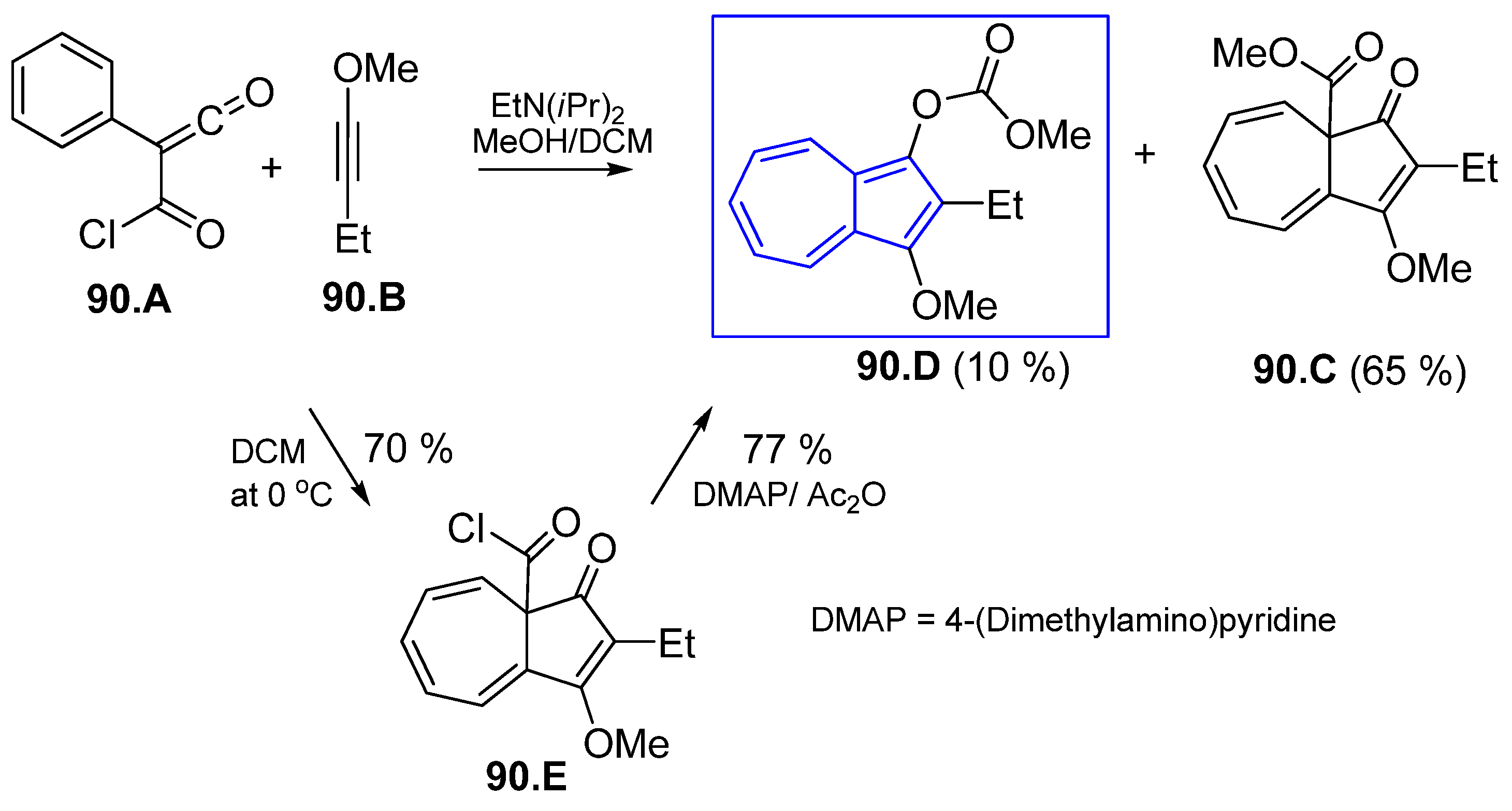
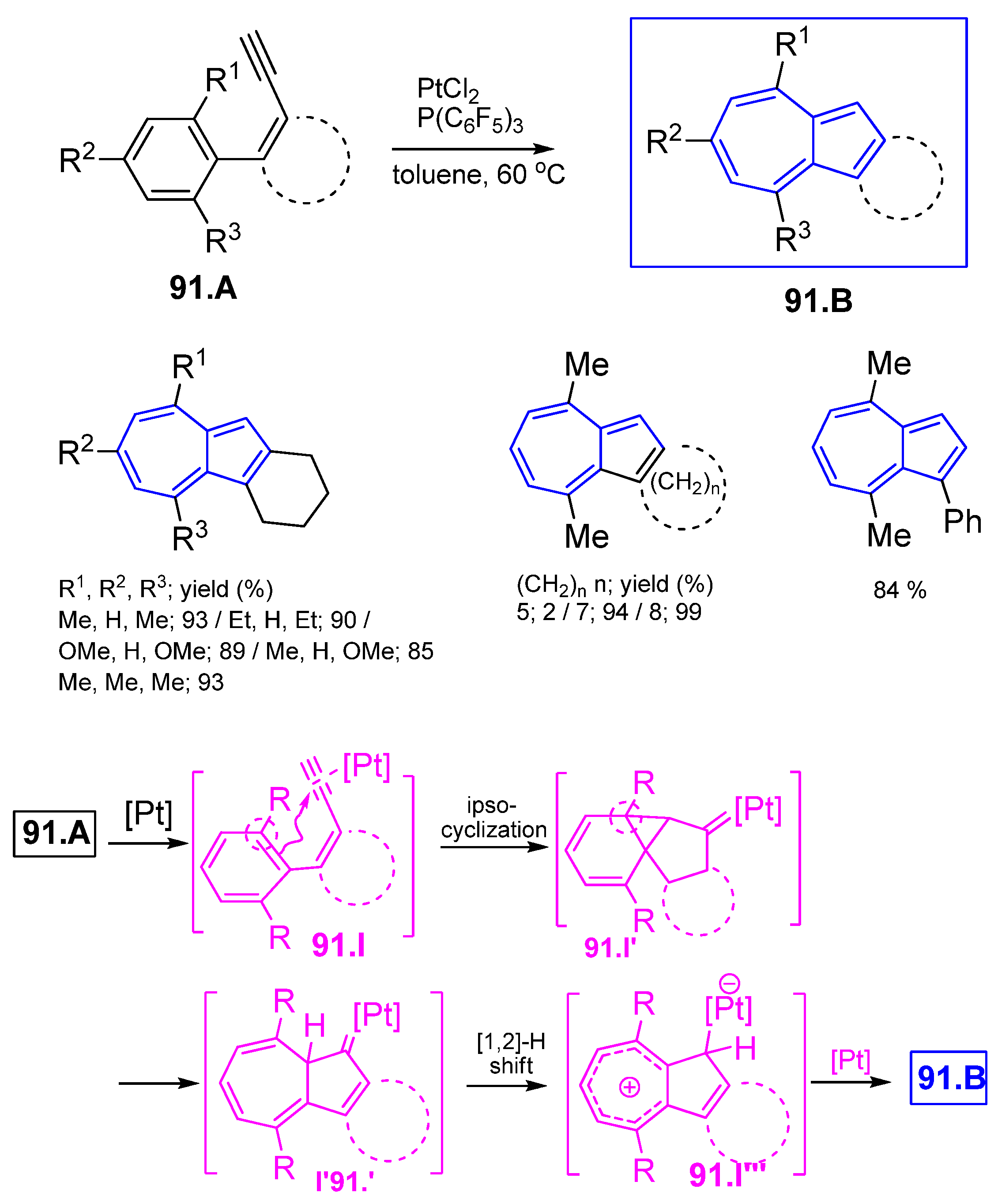
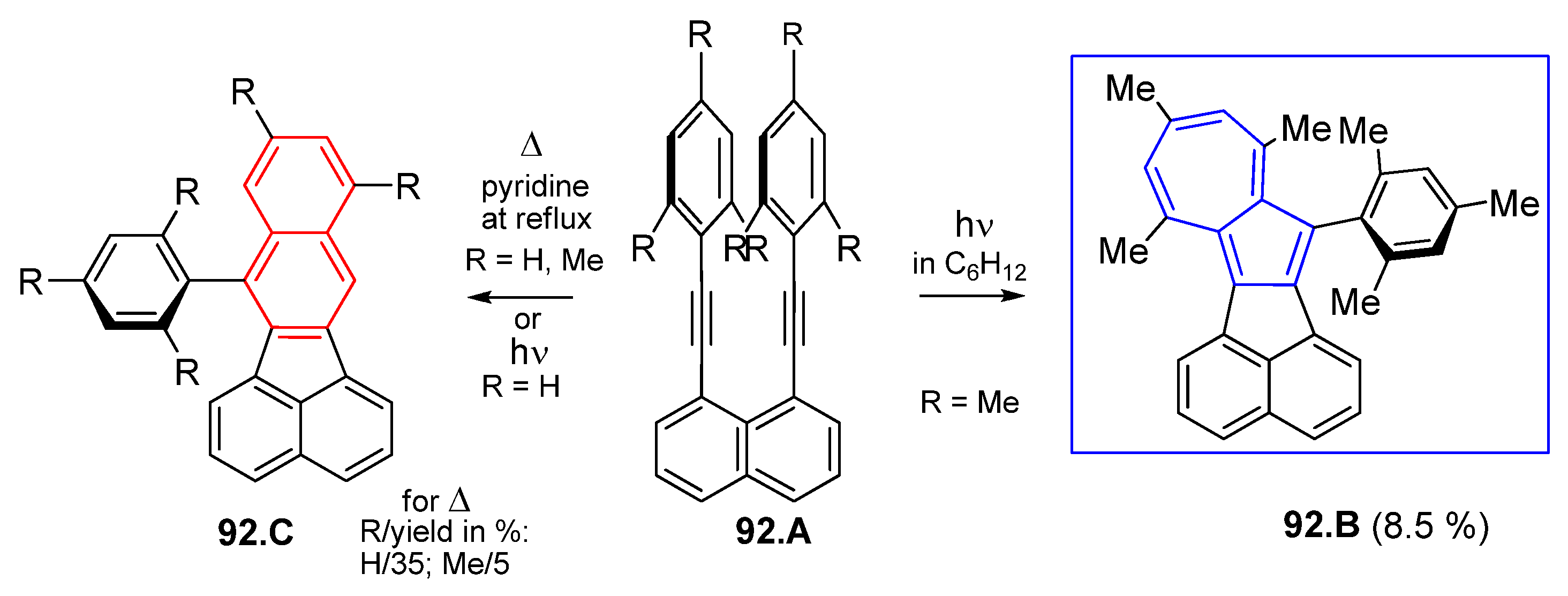
6.1. Obtaining Azulenes Involving Alkyne Compounds
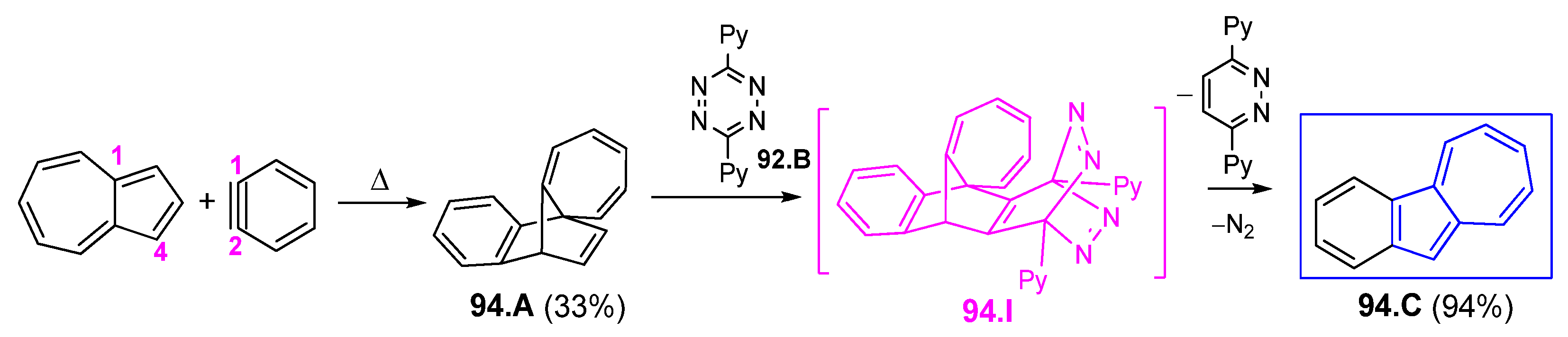
6.2. Diels Alder Condensations and [2+2] Cycloadditions
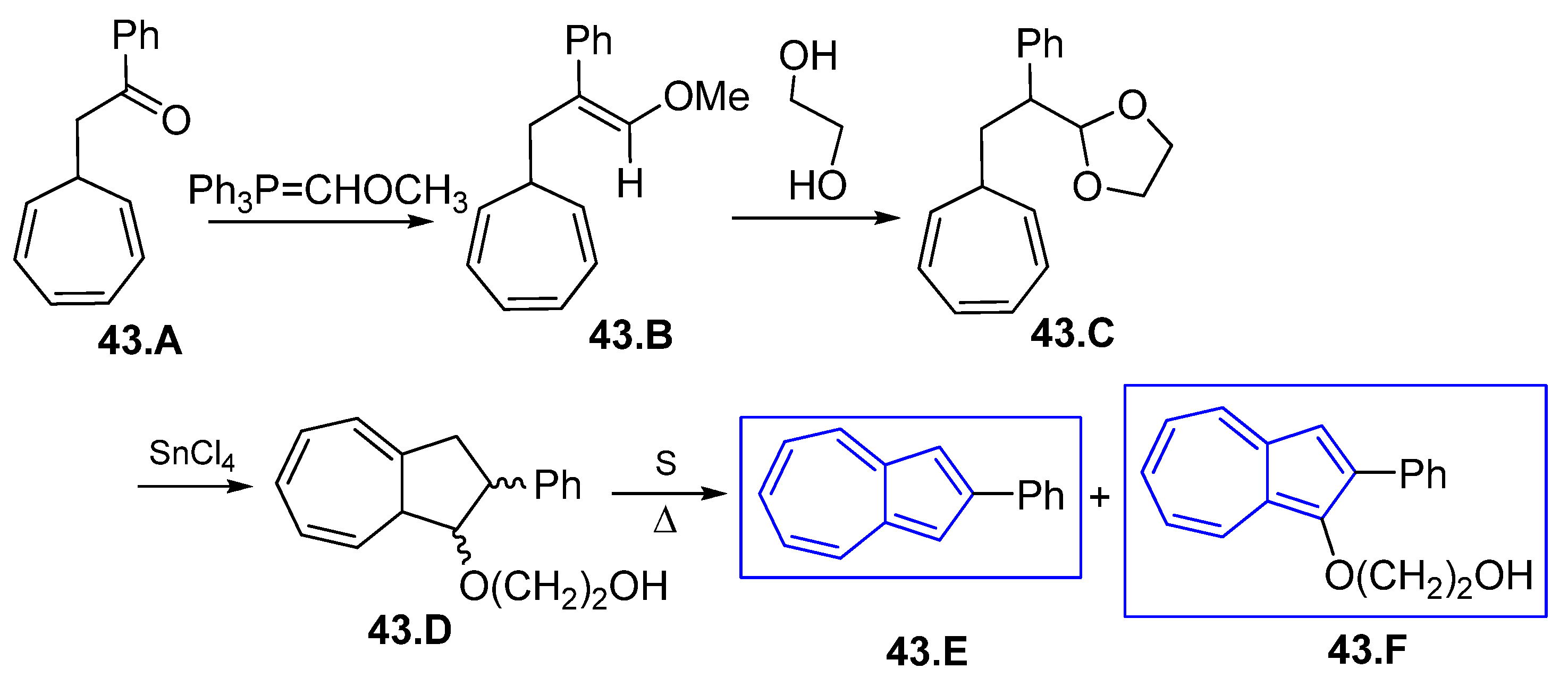
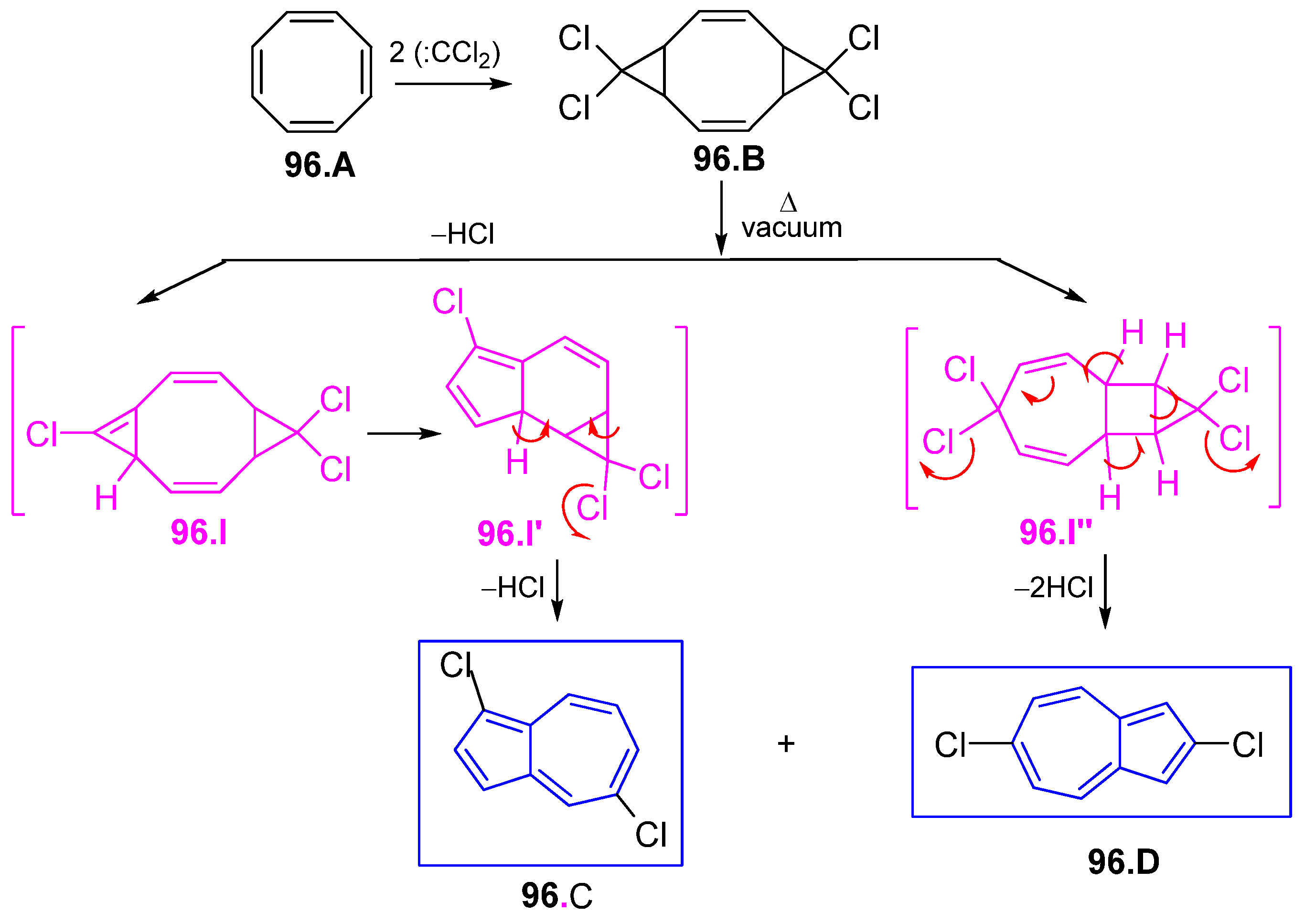
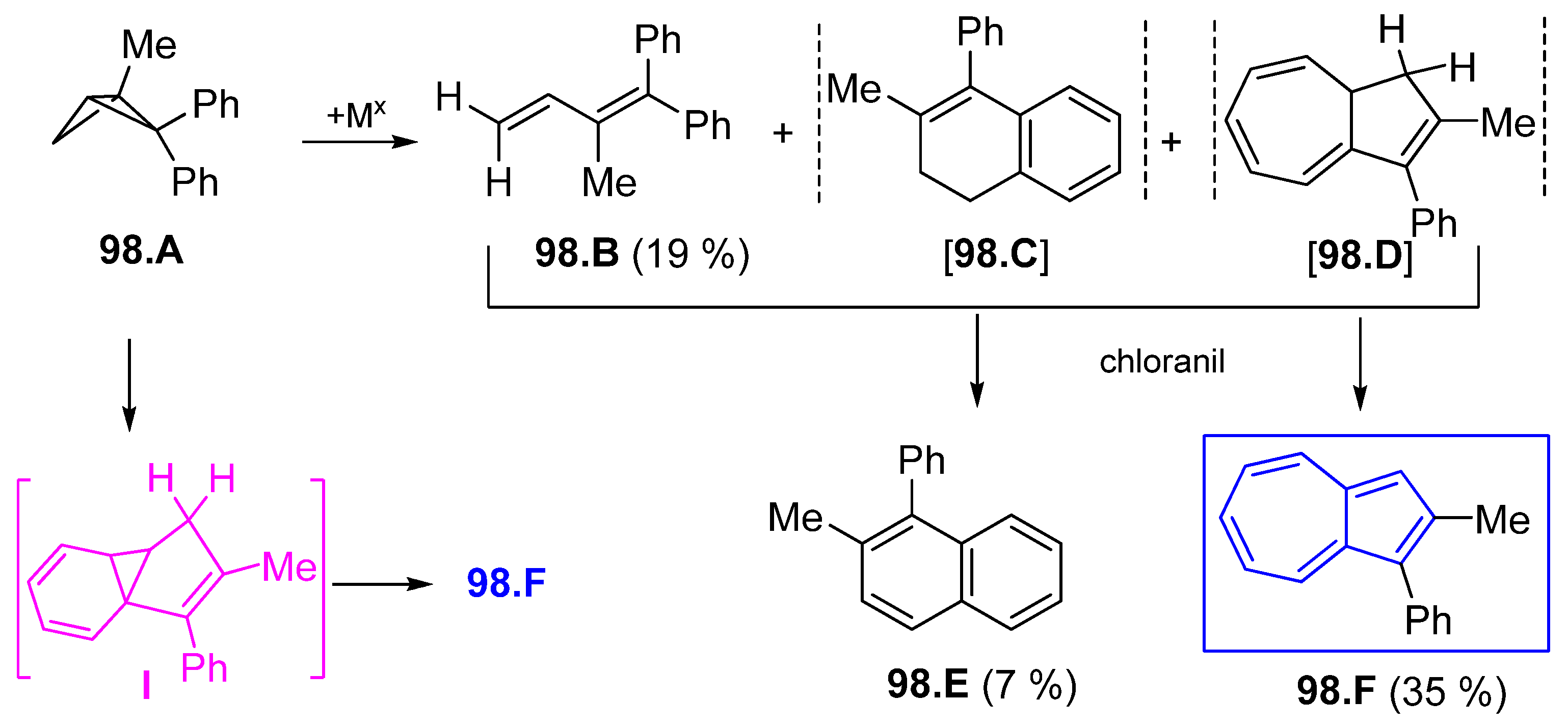
6.3. Azulene Syntheses Involving Strained and Antiaromatic Systems
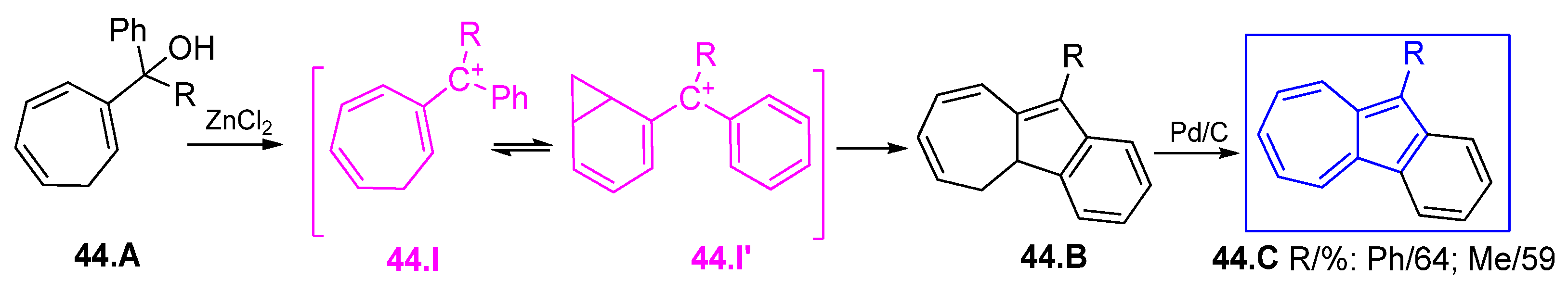
6.4. Various Azulenes Syntheses
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruzicka, L.; Rudolph, E.A. Höhere Terpenverbindungen XXVII. Zur Kenntnis der Azulene. Helv. Chim. Acta 1926, 9, 118–140. [Google Scholar] [CrossRef]
- Pfau, A.S.; Plattner, P. Zur Kenntnis der fluehtigen Pflanzenstoffe. IV. Uber die Konstitution der Azulene. Helv. Chim. Acta 1936, 19, 858–879. [Google Scholar] [CrossRef]
- Plattner, P.A.; Pfau, A.S. Zur Kenntnis der flüchtigen Pflanzenstoffe V. Über die Darstellung des Grundkörpers der Azulen-Reihe. Helv. Chim. Acta 1937, 19, 224–232. [Google Scholar] [CrossRef]
- Gordon, M. The Azulenes. Chem. Rev. 1952, 50, 127–200. [Google Scholar] [CrossRef]
- Mochalin, V.B.; Porshnev, Y.N. Advances in the Chemistry of Azulene. Russ. Chem. Rev. 1977, 46, 530–547. [Google Scholar] [CrossRef]
- Ito, S.; Shoji, T.; Morita, N. Recent Advances in the Development of Methods for the Preparation of Functionalized Azulenes for Electrochromic Applications. Synlett 2011, 2011, 2279–2298. [Google Scholar] [CrossRef]
- Zeller, K.-P. Methoden der Organischen Chemie-Houben-Weil, 4th ed.; Thieme Verlag Stuttgart: New York, NY, USA, 1985; Volume V/2c, pp. 258–263. [Google Scholar]
- Hückel, W.; Schnitzspahn, L. Zur Stereochemie bicyclischer Ringsysteme. IX. Derivate des Cyclopentano-cycloheptans. Ann. Chem. 1933, 505, 274–282. [Google Scholar] [CrossRef]
- Günthard, H.H.; Süess, R.; Marti, L.; Fürst, A.; Plattner, P.A. Zur Kenntnis der Sesquiterpene und Azulene. 95. Mitteilung. Dehydrierung von Decahydro-naphtalinen und Hydro-azulenen. Helv. Chim. Acta 1951, 34, 959–970. [Google Scholar] [CrossRef]
- Kovats, E.; Gunthard, H.H.; Plattner, P.A. Katalytische Dehydrierungen mit Schwefelkohlenstoff. I. Dehydrierung von Oktahydro-azulen. Helv. Chim. Acta 1954, 37, 2123–2133. [Google Scholar] [CrossRef]
- Hafner, K. Einer neue Azulen-synthese. Liebigs Ann. Chem. 1957, 606, 79–89. [Google Scholar] [CrossRef]
- Zincke, T.; Heuser, G.; Moller, W. Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte. Liebigs Ann. Chem. 1904, 333, 296–345. [Google Scholar] [CrossRef]
- Hafner, K. Neuere Ergebnisse der Azulen-Chemie. Angew. Chem. 1958, 70, 419–430. [Google Scholar] [CrossRef]
- Jutz, J.C. Organic Chemistry. Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1978; Volume 73, p. 125. [Google Scholar] [CrossRef]
- Ziegler, K.; Hafner, K. Eine rationelle Synthese des Azulens. Angew. Chem. 1955, 67, 301. [Google Scholar] [CrossRef]
- Hafelinger, G.; Ott, G. Synthesen mesitylsubstituierter Azulene. Liebigs Ann. Chem. 1984, 1984, 1605–1615. [Google Scholar] [CrossRef]
- Köbrich, G. 4-Methyl-pyryliumperchlorat und dessen Abwandlung zu Pentamethinderivaten. Liebigs Ann. Chem. 1961, 648, 114–123. [Google Scholar] [CrossRef]
- Tilney-Bassett, J.F.; Waters, W.A. The substitution of azulene by benzyl radicals. J. Chem. Soc. 1959, 633, 3123–3129. [Google Scholar] [CrossRef]
- Köbrich, G. Ungesättigte Ketone aus Pyrylium-Verbindungen. Angew. Chem. 1960, 72, 348. [Google Scholar] [CrossRef]
- Grewe, R.; von Bonn, W. Pentadienal aus Pyridin. Chem. Ber. 1961, 94, 234–241. [Google Scholar] [CrossRef]
- Hanke, M.; Jutz, C. Synthese von 6,6′-Biazulenyl. Synthesis 1980, 1980, 31–32. [Google Scholar] [CrossRef]
- Shoji, T.; Yamamoto, A.; Shimomura, E.; Maruyama, M.; Ito, S.; Okujima, T.; Toyota, K.; Morita, N. Synthesis of 1,6′-Bi- and 1,6′:3,6″-Terazulenes from 1-Pyridyl- and 1,3-Di(pyridyl)azulenes by the Ziegler–Hafner Method. Chem. Lett. 2013, 42, 638–640. [Google Scholar] [CrossRef]
- Poronik, Y.M.; Mazur, L.M.; Samoć, M.; Jacquemin, D.; Gryko, D.T. Bis(azulenyl)pyrrolo[3,2-b]pyrroles—The key influence of the linkage position on the linear and nonlinear optical properties. J. Mater. Chem. C 2017, 5, 2620–2628. [Google Scholar] [CrossRef]
- Hafner, K.; Asmus, K.-D. Zur Kenntnis der Azulene, XIV. Darstellung substituierter Glutacondialdehyd-Derivate und ihre Überführung in Azulene. Liebigs Ann. Chem. 1964, 671, 31–40. [Google Scholar] [CrossRef]
- Bauer, W.; Muller-Westerhoff, U. Reactions of 3-substituted pentacyanines with cyclopentadienide and cyclononatetraenide anions. Tetrahedron Lett. 1972, 13, 1021–1024. [Google Scholar] [CrossRef]
- Löhr, H.-G.; Vögtle, F.; Chromoinophore, V.I. Neue Chromoionophore mit Azulen-Bausteinen und hoher Ba2+-Farbselektivität. Chem. Ber. 1985, 118, 905–913. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Uchida, M.; Tanaka, R.; Habata, Y.; Shimizu, M. Synthesis of Azulene Derivatives That Have an Azathiacrown Ether Moiety and Their Selective Color Reaction Towards Silver Ions. Asian J. Org. Chem. 2013, 2, 786–791. [Google Scholar] [CrossRef]
- Jutz, C.; Schweiger, E.; Löbering, H.-G.; Kraatz, A.; Kosbahn, W. Synthese polycyclischer Azulene. Chem. Ber. 1974, 107, 2956–2975. [Google Scholar] [CrossRef]
- Hanke, M.; Jutz, C. Synthesis of 5,5′-Biazulenyl. Angew. Chem. lnt. Ed. Engl. 1979, 18, 214–215. [Google Scholar] [CrossRef]
- Rudolf, K.; Robinette, D.; Koenig, T. Synthesis, characterization and flash vacuum pyrolysis studies of anti-[2.2](1,6)azulenophane. J. Org. Chem. 1987, 52, 641–647. [Google Scholar] [CrossRef]
- Leino, T.O.; Baumann, M.; Yli-Kauhaluoma, J.; Baxendale, I.R.; Wallén, E.A.A. Synthesis of 1,3,6-Trisubstituted Azulenes. J. Org. Chem. 2015, 80, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, R. A Fifth Route to 1,2,3-Triphenylazulene. J. Org. Chem. 1960, 25, 273–274. [Google Scholar] [CrossRef]
- Alder, R.W.; Whittaker, G. The thermal rearrangements of azulenes to naphthalenes. J. Chem. Soc. Perkin 1975, 2, 714–723. [Google Scholar] [CrossRef]
- Muth, C.W.; DeMatte, M.L.; Urbanik, A.R.; Isner, W.G. Some Synthetic Studies for Benzazulenes1. J. Org. Chem. 1966, 31, 3013–3015. [Google Scholar] [CrossRef]
- Kost, A.N.; Gromov, S.P.; Sagitullin, R.S. Pyridine ring nucleophilic recyclizations. Tetrahedron 1981, 37, 3423–3454. [Google Scholar] [CrossRef]
- Hafner, K.; Kaiser, H. Zur Kenntnis der Azulene, III. Eine einfache synthese substituierter azulene. Ann. Chem. 1958, 618, 140–152. [Google Scholar] [CrossRef]
- Dorofeenko, G.N.; Koblik, A.V.; Polyakova, T.I.; Murad’yan, L.A. Synthesis of 6-hetaryl-substituted azulenes and their reactions with 2,6-diphenylpyrylium perchlorate. Chem. Heterocycl. Compd. 1980, 16, 807–810. [Google Scholar] [CrossRef]
- Anderson, A.G., Jr.; Hollander, G.T. The Reaction of Some 1-Trihaloacetyl-8-methylazuleneswith Base. J. Org. Chem. 1965, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Briquet, A.A.S.; Hansen, H.-J. Radical Arylation Reactions of 4, 6, 8-Trimethylazulene. Helv. Chim. Acta 1994, 77, 1577–1584. [Google Scholar] [CrossRef]
- Rippert, A.J.; Hansen, H.-J. Synthesis of 4,6,8-Trisubstituted Methyl Azulene-2-carboxylates. Helv. Chim. Acta 1995, 78, 238–241. [Google Scholar] [CrossRef]
- Gee, A.P.; Cosham, S.D.; Johnson, A.L.; Lewis, S.E. Phosphorus-Substituted Azulenes Accessed via Direct Hafner Reaction of a Phosphino Cyclopentadienide. Synlett 2017, 28, 973–975. [Google Scholar] [CrossRef]
- Razus, A.C.; Pavel, C.; Lehadus, O.; Nica, S.; Birzan, L. Synthesis and properties of [1,6′]biazulenyl compounds. Tetrahedron 2008, 64, 1792–1797. [Google Scholar] [CrossRef]
- Sato, M.; Ebine, S.; Tsunetsug, Y. Cycloaddition of 6-aminofulvenes with coumalic esters. A novel azulene formation. Tetrahedron Lett. 1974, 15, 2769–2770. [Google Scholar] [CrossRef]
- Preethalayam, P.; Krishnan, K.S.; Thulasi, S.; Chand, S.S.; Joseph, J.; Nair, V.; Jaroschik, F.; Radhakrishnan, K.V. Recent Advances in the Chemistry of Pentafulvenes. Chem. Rev. 2017, 117, 3930–3989. [Google Scholar] [CrossRef]
- Hong, B.-C. Constructing Molecular Complexity and Diversity by Cycloaddition Reactions of Fulvenes. In Cross Conjugation: Dendralene, Radialene and Fulvene Chemistry, 1st ed.; Hopf, H., Sherburn, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2016; pp. 249–300. [Google Scholar] [CrossRef]
- Hong, B.-C.; Jiang, Y.-F.; Kumar, E.S. Microwave-assisted [6+4]-cycloaddition of fulvenes and α-pyrones to azulene–indoles: Facile syntheses of novel antineoplastic agents. Bioorg. Med. Chem. Lett. 2001, 11, 1981–1984. [Google Scholar] [CrossRef]
- Copland, D.; Leaver, D.; Menzies, W.B. A new and convenient synthesis of azulenes from 6-N,N-Dimethyl-aminofulvene and thiophene 1,1-dioxides. Tetrahedron Lett. 1977, 18, 639–640. [Google Scholar] [CrossRef]
- Reiter, S.E.; Dunn, L.C.; Houk, K.N. Synthesis of azulenes by the [6+4]cycloadditions of 6-aminofulvenes to thiophene S,S-dioxides. J. Am. Chem. Soc. 1977, 101, 4199–4201. [Google Scholar] [CrossRef]
- Mukherjee, D.; Dunn, L.C.; Houk, K.N. Efficient Guaiazulene and Chamazulene Syntheses Involving [6+4] Cycloadditions. J. Am. Chem. Soc. 1979, 101, 251–252. [Google Scholar] [CrossRef]
- Amir, E.; Amir, R.J.; Campos, L.M.; Hawker, C.J. Stimuli-Responsive Azulene-Based Conjugated Oligomers with Polyaniline-like Properties. J. Am. Chem. Soc. 2011, 133, 10046–10049. [Google Scholar] [CrossRef]
- Houk, K.N. Generalized frontier orbitals of alkenes and dienes. J. Am. Chem. Soc. 1973, 95, 4092–4094. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, J.; Jorgensen, J.; Lemal, D.M. Octachloroazulene. J. Amer. Chem. Soc. 2002, 124, 15302–15307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.N.; Mani, S.R.; Houk, K.N. Synthesis of azulene from 6-acyloxyfulvenes. Tetrahedron Lett. 1982, 23, 495–498. [Google Scholar] [CrossRef]
- Dunn, L.C.; Houk, K.N. The regioselectivity of [6+4]cycloadditions of dienamines to fulvenes. Tetrahedron Lett. 1978, 37, 3411–3414. [Google Scholar] [CrossRef]
- Razus, A.C. Syntheses of Azulene Embedded Polycyclic Compounds. Symmetry 2024, 16, 382. [Google Scholar] [CrossRef]
- Jutz, C.; Kirchlechner, R. Azuleno[5,6,7-cd]phenalen, ein neues quasiaromatisches System. Angew. Chem. 1966, 78, 493–494. [Google Scholar] [CrossRef]
- Jutz, C.; Kirchlechner, R.; Seidel, A.J. Über die Umsetzung von Phenalen mit vinylogen Formamidinium-Salzen: Synthese substituierter Pyrene und des Azuleno[5.6.7-cd]phenalens. Chem. Ber. 1969, 102, 2301–2318. [Google Scholar] [CrossRef]
- Jutz, C.; Penker, H.G.; Kosbach, W. Azuleno[1,2-f]azulen, ein tetracyclischer, nichtbenzoider, aromatischer Kohlenwasserstoff. Synthesis 1976, 1976, 673–675. [Google Scholar] [CrossRef]
- Jutz, C.; Schweiger, E. Darstellung von Benzazulenen aus Azulenderivaten. Chem. Ber. 1974, 107, 2383–2396. [Google Scholar] [CrossRef]
- Jutz, C.; Schweiger, E. Über eine verbesserte Synthese des Dicyclopenta [ef,kl] heptalens. Synthesis 1974, 1974, 193–196. [Google Scholar] [CrossRef]
- Alder, R.W.; Whittaker, G.J. A new azulene synthesis. J. Chem. Soc. Chem. Commun. 1971, 776. [Google Scholar] [CrossRef]
- Severin, T.; Ipach, I. Umsetzungen mit Nitroenaminen; XVII1. Synthese von 5-Nitroazulenen. Synthesis 1978, 1978, 592–593. [Google Scholar] [CrossRef]
- Plattner, P.A.; Studer, A. Zur Kenntnis der Sesquiterpene. (70. Mitteilung†) Über 6-Methyl-azulen. Helv. Chim. Acta. 1946, 29, 1432–1438. [Google Scholar] [CrossRef]
- Bergmann, E.D.; Ikan, R.J. 4- and 5-Phenylazulenes. J. Am. Chem. Soc. 1958, 80, 3135–3139. [Google Scholar] [CrossRef]
- Šorm, F.; Gut, J.; Hlavnička, J.; Kučera, J.; Šedivý, L. On terpenes. XXV. The total synthesis of S-guaiazulene. Collect. Czech. Chem. Commun. 1951, 16, 168–183. [Google Scholar] [CrossRef]
- Pommer, H. Ein neuer Weg zur Synthese von alkylierten Azulenen. Ann. Chem. 1953, 579, 47–76. [Google Scholar] [CrossRef]
- Herz, J. A Novel Rearrangement in the Synthesis of Azulenes. J. Amer. Chem. Soc. 1956, 78, 1485–1494. [Google Scholar] [CrossRef]
- Ramirez, F.; Levy, S. Phosphinemethylenes. III. A New Class of Azo Dyes Containing Phosphorus. J. Amer. Chem. Soc. 1957, 79, 6167–6172. [Google Scholar] [CrossRef]
- Higham, L.J.; Kelly, P.G.; Corr, D.M.; Müller-Bunz, H.; Walker, B.J.; Gilheany, D.G. A novel azulene synthesis from the Ramirez ylide involving two different modes of its reaction with activated alkynes. Chem. Commun. 2004, 684–685. [Google Scholar] [CrossRef] [PubMed]
- Tanino, K.; Yamada, T.; Yoshimura, F.; Suzuki, T. Cyanoazulene-based Multistage Redox Systems Prepared from Vinylcyclopropanecarbonitrile and Cyclopentenone via Divinylcyclopropane-rearrangement Approach. Chem. Lett. 2014, 43, 607–609. [Google Scholar] [CrossRef]
- Novák, J.; Šorm, F.; Sicher, J. On terpenes. LXIII. Total synthesis of chamazulene. A simple general synthesis of 1,4,7-trisubstituted azulenes. Collect. Czech. Chem. Commun. 1954, 19, 1264–1273. [Google Scholar] [CrossRef]
- Plattner, P.A.; Furst, A.; Jirasek, K. Zur Kenntnis der Sesquiterpene. (68. Mitteilung) Über das Bicyclo-[0, 3, 5]-decan. Helv. Chim. Acta 1946, 29, 730–739. [Google Scholar] [CrossRef]
- Plattner, P.A.; Furst, A.; Jirasek, K. Zur Kenntnis der Sesquiterpene. (69. Mitteilung). Helv. Chim. Acta 1946, 29, 740–742. [Google Scholar] [CrossRef]
- Plattner, P.A.; Büchi, G. Zur Kenntnis der Sesquiterpene. (72. Mitteilung†)). Über eine einfache, von Cycloheptanon ausgehende Azulen-Synthese. Helv. Chim. Acta 1946, 29, 1608–1611. [Google Scholar] [CrossRef]
- Anderson, A.G., Jr.; Tazumsa, J.J. Azulene. II. Synthesis of Methyl 1-Azuloate1. J. Am. Chem. Soc. 1953, 75, 4979–4980. [Google Scholar] [CrossRef]
- Braude, E.A.; Forbes, W.F. A new route to azulenes. J. Chem. Soc. 1953, 2208–2216. [Google Scholar] [CrossRef]
- Jacobson, R.M.; Lahm, G.P. Three-carbon annelations. New routes to the Nazarov cyclization via protected cyanohydrins. J. Org. Chem. 1979, 44, 462–465. [Google Scholar] [CrossRef]
- Islam, A.M.; Raphael, R.A. A convenient synthesis of bicyclo[5: 3: 0]dec-1(7)-en-8-one and related compounds. J. Chem. Soc. 1953, 75, 2247–2250. [Google Scholar] [CrossRef]
- Lloyd, D.; Rowe, F. Azulenes. Part I. A Synthesis of Azulene. J. Chem. Soc. 1953, 3718–3719. [Google Scholar] [CrossRef]
- Islam, A.M.; Raphael, R.A. Bicyclo[4: 3: 0]Non-6-en-8-on. J. Chem. Soc. 1952, 4086–4087. [Google Scholar] [CrossRef]
- Hamlet, J.C.; Henbest, H.B.; Jones, E.R.H. Researches on acetylenic compounds. Part XXXII. Dehydration of acetylphenyl- and vinylacetylenyl-alcohols derived from cyclohexanone, and the hydration of the resultant hydrocarbons. J. Chem. Soc. 1951, 589, 2652. [Google Scholar] [CrossRef]
- Prelog, V.; Wirth, M.M.; Ruzicka, L. Zur Kenntnis des Kohlenstoff-Ringes. (39. Mitteilung†)). Bicyclische Verbindungen mit einem vielgliedrigen Ring. Helv. Chim. Acta 1946, 29, 1425–1432. [Google Scholar] [CrossRef]
- Lloyd, D.; Rowe, F. Azulenes. Part II. Application of the Pinacone Reduction to the synthesis of 1-Substituted Azulenes. J. Chem. Soc. 1954, 4232–4234. [Google Scholar] [CrossRef]
- Smith, A.B.; Toder, B.H.; Branca, S.J.; Dieter, R.K. Lewis acid promoted decomposition of unsaturated alpha-diazo ketones. 1. An efficient approach to simple and annulated cyclopentenones. J. Am. Chem. Soc. 1981, 103, 1996–2008. [Google Scholar] [CrossRef]
- Noyori, R.; Yokoyama, K.; Makino, S.; Hayakawa, Y. Carbon-carbon bond formations promoted by transition metal carbonyls. II. Reaction of alpha,alpha.1-dibromo ketones and enamines with the aid of iron carbonyls. Novel cyclopentenone synthesis. J. Am. Chem. Soc. 1972, 84, 1772–1773. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Yokoyama, K.; Noyori, R. Carbon-carbon bond formation promoted by transition metal carbonyls. 20. Iron carbonyl promoted reaction of alpha,alpha-dibromo ketones and aromatic olefins leading to 3-arylcyclopentanones. The [3+2] cycloaddition involving an allylic cation. J. Am. Chem. Soc. 1978, 100, 1791–1799. [Google Scholar] [CrossRef]
- Noyori, R. Organic syntheses via the polybromo ketone-iron carbonyl reaction. Acc. Chem. Res. 1979, 12, 61–66. [Google Scholar] [CrossRef]
- Trost, B.M.; Bogdanowicz, M.J. New synthetic reactions. Cyclopentane annelation. J. Am. Chem. Soc. 1973, 95, 289–290. [Google Scholar] [CrossRef]
- Marino, J.P.; Landick, R.C. 1-Phenylthiocyclopropyltriphenylphosphonium fluoborate: A new synthon for cyclopentanone synthesis. Tetrahedron Lett. 1975, 16, 4531–4534. [Google Scholar] [CrossRef]
- Hiyama, T.; Shinoda, M.; Tsukanaka, M.; Nozaki, H. Cyclopentenone Synthesis from 1,1-Dichloroallyllithium–Ketone Adducts as Well as from Dichlorocarbene–Allyl Alcohol Adducts. Bull. Chem. Soc. Jpn. 1980, 53, 1010–1014. [Google Scholar] [CrossRef]
- Danheiser, R.L.; Carini, D.J.; Basak, A. (Trimethylsilyl)cyclopentene annulation: A regiocontrolled approach to the synthesis of five-membered rings. J. Am. Chem. Soc. 1981, 103, 1604–1606. [Google Scholar] [CrossRef]
- Danishefsky, S.; Etheredge, S. Annulative route to enediones. J. Org. Chem. 1982, 47, 4791–4793. [Google Scholar] [CrossRef]
- Furuta, K.; Misumi, A.; Mori, A.; Ikeda, N.; Yamamoto, H. A new cyclopentanone annulation of α-bromomethylacrylates. Tetrahedron Lett. 1984, 25, 669–670. [Google Scholar] [CrossRef]
- Wada, E.; Nakai, T.; Okawara, M. A new method for cyclopentenone annelation via the rearrangement of bicyclic cyclopropyl ketones. Syntheses of bicyclo[4.3.0] non-6-en-8-one and bicyclo[5.3.0]dec-7-en-9-one. Chem. Lett. 1976, 5, 1025–1028. [Google Scholar] [CrossRef]
- Monti, S.A.; Cowherd, F.G.; McAninch, T.W. Thermal Rearrangement of Trimethylsilyl Enol Ethers of Cyclopropyl Methyl Ketones. A Cyclopentanone Annelation Procedure. J. Org. Chem. 1975, 40, 858–862. [Google Scholar] [CrossRef]
- Jung, M.E.; Hudspeth, J.P. Three-carbon annulation. Formation of five-membered rings from olefins via Diels-Alder reactions. J. Am. Chem. Soc. 1977, 99, 5508–5510. [Google Scholar] [CrossRef]
- Marino, J.P.; Linderman, R.J. Chemistry of substituted [.alpha.-(carboethoxy)vinyl]cuprates and their synthetic application to cyclopentenone annulations. J. Org. Chem. 1981, 46, 3696–3702. [Google Scholar] [CrossRef]
- Watanabe, T.; Soma, N. Studies on Seven-membered Ring Compounds. XXXVI. Synthesis of Azulene Derivatives by Ring Closure of β-Cycloheptatrienyl-substituted Carbonyl Compounds. Chem. Pharm. Bull. 1971, 19, 2215–2221. [Google Scholar] [CrossRef][Green Version]
- Mizumoto, K.; Okada, K.; Oda, M. Generation and notable position dependent electrocyclization of cycloheptatrienylphenylcarbonium ions -electrocyclization of a norcaradiene. Tetrahedron Lett. 1984, 25, 2999–3002. [Google Scholar] [CrossRef]
- Oda, M.; Kajioka, T.; Haramoto, K.; Miyatake, R.; Kuroda, S. A Divergent Method for Preparing 1-Aryl- and 1,3-Diarylazulenes from Ethyl 3-(Cyclohepta-1,3,5-trien-1-yl)-3-oxopropionate. Synthesis 1999, 1999, 1349–1353. [Google Scholar] [CrossRef]
- Oda, M.; Kashi, S.; Thanh, N.C.; Kuroda, S. Synthetic Methods for Preparing 1,3-Di(2-pyridyl)azulene. Heterocycles 2007, 71, 1413–1416. [Google Scholar] [CrossRef]
- Kajioka, T.; Oda, M.; Senkura, R.; Kurada, S. Reaction of 1-(ethoxycarbonylacetyl)- cyclohepta-1,3,5-triene with acetic anhydride: A facile synthesis of 1-acetoxy- 2-acetylazulenes. Synth. Commun. 2002, 32, 627–636. [Google Scholar] [CrossRef]
- Yokohama, R.; Ito, S.; Harada, N.; Kabuto, C.; Morita, A. [2+2] Cycloaddition reaction of cycloheptatriene with dichloroketene. A novel and efficient synthetic strategy for the synthesis of 2-hydroxyazulene. J. Chem. Soc. Perkin Trans. 2001, 1, 2257–2261. [Google Scholar] [CrossRef]
- Carret, S.; Blanc, A.; Caquerel, Y.; Berthod, M.; Green, A.E.; Depres, J.-P. Approach to the Blues: A Highly Flexible Route to the Azulenes. Angew. Chem. Ind. Ed. 2005, 44, 5130–5133. [Google Scholar] [CrossRef] [PubMed]
- Carret, S.; Deprés, J.-P. Direct aromatization of chlorohydroazulenones with triflic anhydride: Access to chloroazulenyl triflates. Tetrahedron Lett. 2008, 49, 5642–5644. [Google Scholar] [CrossRef]
- Tsuruta, H.; Sugiyama, T.; Mukai, T. The thermal cyclization of 8-(2,2-disubstituted vinyl) heptafulvenes leading to azulene derivatives. Chem. Lett. 1972, 1, 185–188. [Google Scholar] [CrossRef]
- Prinzbach, H.; Herr, H.-J.; Regel, W. Vinylogous Heptafulvenes from Vinylogous Fulvenes. Angew. Chem. Int. Ed. 1972, 11, 131–133. [Google Scholar] [CrossRef]
- Prinzbach, H.; Herr, H.-J. Neue Wege zum Azulen-System. Angew. Chem. 1972, 84, 117–118. [Google Scholar] [CrossRef]
- Becker, D.A.; Danheiser, R.L. A new synthesis of substituted azulenes. J. Am. Chem. Soc. 1989, 111, 389–391. [Google Scholar] [CrossRef]
- Nozoe, T. Recent advances in the chemistry of troponoids and related compounds in Japan. Pure Appl. Chem. 1971, 28, 239–280. [Google Scholar] [CrossRef]
- Tetsuo Nozoe (1902−1996). Eur. J. Org. Chem. 2004, 2004, 899–928. [CrossRef]
- McDonald, R.N.; Richmond, J.M. Nonbenzenoid aromatic systems. XI. Synthesis and buffered acetolysis of 2-(2-azulyl)ethyl tosylate and nosylate. J. Org. Chem. 1975, 40, 1689–1694. [Google Scholar] [CrossRef]
- Kumar, N.R.; Agrawal, A.R.; Zade, S. Abnormal Nucleophilic Substitution on Methoxytropone Derivatives: Steric Strategy to Synthesize 5-Substituted Azulenes. Chemistry 2019, 25, 14064–14071. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Agrawal, A.R.; Choudhury, A.; Zade, S.S. The Effect of Base and Nucleophile on the Nucleophilic Substitution of Methoxytropone Derivatives: An Easy Access to 4- and 5-Substituted Multifunctional Azulenes. J. Org. Chem. 2020, 85, 9029–9041. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, Y.; Kato, T. The Synthesis of S-Guaiazulene from Hinokitiol. Bull. Chem. Soc. Jpn. 1964, 37, 859–864. [Google Scholar] [CrossRef]
- Nozoe, T.; Takase, K.; Tada, M. The Synthesis of 5- and 6-Aminoazulene Derivatives. Bull. Chem. Soc. Jpn. 1963, 36, 1006–1009. [Google Scholar] [CrossRef]
- Nozoe, T.; Seto, S.; Matsumura, S.; Asano, T. Synthesis of Azulene Derivatives from Troponoids and Cyanoacetic Ester. Proc. Jpn. Acad. 1956, 32, 339–343. [Google Scholar] [CrossRef]
- Nozoe, T.; Seto, S.; Matsumura, S.; Murase, Y. The Synthesis of Azulene Derivatives from Troponoids. Bull. Chem. Soc. Jpn. 1962, 35, 1179–1188. [Google Scholar] [CrossRef]
- Brettle, R.; Dunmur, D.A.; Estdale, S.; Marson, C.M. Synthesis, linear dichroism and mesogenic properties of substituted azulenes. J. Mater. Chem. 1993, 3, 327–331. [Google Scholar] [CrossRef]
- Puodziukynaite, E.; Wang, H.-W.; Lawrence, J.; Wise, A.J.; Russell, T.P.; Barnes, M.D.; Emrick, T. Azulene Methacrylate Polymers: Synthesis, Electronic Properties, and Solar Cell Fabrication. J. Am. Chem. Soc. 2014, 136, 11043–11049. [Google Scholar] [CrossRef]
- Mitsumoto, Y.; Nitta, M. Synthesis, structure, and reactivity of (triphenylarsoranylidene)methylcyclohepta-2,4,6-trienone derivatives: Reactions with heterocumulenes and an activated acetylene. J. Chem. Soc. Perkin Trans. 2002, 1, 2268. [Google Scholar] [CrossRef]
- Doering, W.V.E.; Wiley, D.W. Heptafulvene (methylenecycloheptatriene). Tetrahedron 1960, 11, 183–198. [Google Scholar] [CrossRef]
- Schenk, K.; Kyburz, R.; Neunschwander, M. Synthese und Isolierung von Heptafulven und Sesquifulvalen. Helv. Chim. Acta 1975, 58, 1099–1119. [Google Scholar] [CrossRef]
- Oda, M.; Kitahara, Y. Synthesis of 8-cyanoheptafulvene. Chem. Commun. 1969, 352. [Google Scholar] [CrossRef]
- Hafner, K.; Römer, M.; Der Fünten, W.A.; Komatsu, K.; Tanaka, S.; Okamoto, K. Cyclisch konjugierte 5- und 7-Ringsysteme, IV. Notiz über die Synthese des 8-Acetylheptafulvens. Liebigs Ann. Chem. 1978, 1978, 376–379. [Google Scholar] [CrossRef]
- Hasenhȕndl, A.; Rapp, K.M.; Daub, J. Elektronenreiche heptafulvene als bausteine von azulenen. 8-methoxyheptafulven. Chem. Lett. 1979, 8, 597–600. [Google Scholar] [CrossRef]
- Shono, T.; Okada, T.; Furuse, T.; Kashimura, S.; Nozoe, T.; Maekawa, H. Regioselective synthesis of substituted tropones and azulenes using anodic oxidation of cycloheptatriene systems as the key reaction. Tetrahedron Lett. 1992, 33, 4337–4340. [Google Scholar] [CrossRef]
- Bäumler, A.; Daub, J.; Pickl, W.; Rieger, W. Über die Regiochemie der intermolekularen [8+2]-Cycloaddition elektronenreicher Heptafulvene: Synthese 1,2,3-trisubstituierter Hydroazulene. Chem. Ber. 1985, 118, 1857–1867. [Google Scholar] [CrossRef]
- Oda, M.; Kitahara, Y. Synthesis, Reactions of 8-Cyanoheptafulvene with Enamines. A New Synthesis of Azulene Derivatives. Synthesis 1971, 1971, 367–368. [Google Scholar] [CrossRef]
- Oda, M.; Kitahara, Y. The Reaction of 8-Cyanoheptafulvene with Benzyne. Bull. Chem. Soc. Jpn. 1970, 43, 1920. [Google Scholar] [CrossRef]
- Kuroda, S.; Funamizu, M.; Kitahara, Y.; Asao, T. Syntheses of condensed heptafulvene and 3h-benz[c,d]azulen-3-one derivatives. Tetrahedron Lett. 1975, 37, 3197–3200. [Google Scholar] [CrossRef]
- Rekka, E.; Chrysselis, M.; Siskou, I.; Kourounakis, A. Synthesis of New Azulene Derivatives and Study of Their Effect on Lipid Peroxidation and Lipoxygenase Activity. Chem. Pharm. Bull. 2002, 50, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Bindl, J.; Daub, J.; Hasenhiindl, A.; Meinert, M.; Rapp, K.M. Acenazulendione. Chem. Ber. 1983, 116, 2408–2417. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S.; Yasunami, M. Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties. Int. J. Mol. Sci. 2021, 22, 10686. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Takase, K.; Shimazaki, N. The Synthesis of Diethyl 2-Hydroxyazulene-1, 3-dicarboxylate from Troponoids and Some Reactions of 2-Hydroxyazulene Derivatives. Bull. Chem. Soc. Jpn. 1964, 37, 1644–1648. [Google Scholar] [CrossRef]
- Nozoe, T.; Takase, K.; Nakazawa, T.; Fukuda, S. The formation of azulene derivatives from 2H-cyclohepta[b]furan-2-one derivatives. Tetrahedron 1971, 27, 3357–3368. [Google Scholar] [CrossRef]
- Nozoe, T.; Takase, K.; Kato, M.; Nogi, T. The reaction of 2-arylsulfonyloxytropones and active methylene compounds. Tetrahedron 1971, 27, 6023–6025. [Google Scholar] [CrossRef]
- Nozoe, T.; Takase, K.; Fukuda, S. The Synthesis of 1-Phenylazulene and 2-Phenylazulene from the Troponoid Compound. Bull. Chem. Soc. Jpn. 1971, 44, 2210–2214. [Google Scholar] [CrossRef]
- Iwama, N.; Kashimoto, M.; Othaki, H.; Kato, T.; Sugano, T. A simple and efficient synthesis of 2-alkylazulenes. Tetrahedron Lett. 2004, 45, 9211–9213. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lee, O.; Yao, C.-N.; Chuang, M.-Y.; Chang, Y.-L.; Chang, M.-H.; Chang, M.H.; Wen, Y.F.; Yang, W.H.; Ko, C.H.; et al. Novel azulene-based derivatives as potent multi-receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6129–6132. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Yang, P.-W.; Wu, C.-P.; Huang, T.-S.; Lee, T.-H.; Okai, H.; Wakabayashi, H.; Ishikawa, S.A. Convenient, One-pot Azulene Synthesis from Cyclohepta[b]furan-2-ones and Vinyl Ether and Its Analogues (I). Vinyl Ethyl Ether, Vinyl Acetates, Dihydrofurans, and Dihydropyrans as Reagent. Heterocycles 1989, 29, 1225–1232. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Nozoe, T.; Ishikawa, S.; Wu, C.-P.; Yang, P.-W. A convenient, one-pot azulene synthesis from cycloheptaiblfuran-2-ones and vinyl ether and its analogues. (II): Acetals as reagent. Heterocycles 1990, 31, 17–22. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Nozoe, T.; Shindo, K.; Ishikawa, S.; Wu, C.-P.; Yang, P.-W. A Convenient, One-Pot Azulene Synthesis from 2H-cyclohepta[b]furan-2-ones with Vinyl Ether and Its Analogues III. Orthoesters as a reagent. Heterocycles 1991, 32, 213–220. [Google Scholar] [CrossRef]
- Pham, W.; Weissleder, R.; Tung, C.-H. Intermolecular [8+2] cycloaddition reactions of 2H-3-methoxycarbonylcyclohepta[b]furan-2-one with vinyl ethers: An approach to bicyclo[5.3.0]azulene derivatives. Tetrahedron Lett. 2002, 43, 19–20. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamabe, S.; Minato, T.; Yamazaki, S.; Nozoe, T. Experimental and Theoretical Analyses of Azulene Synthesis from Tropones and Active Methylene Compounds: Reaction of 2-Methoxytropone and Malononitrile. J. Org. Chem. 2012, 77, 5318–5330. [Google Scholar] [CrossRef]
- Kuroda, S.; Obata, Y.; Thanh, N.C.; Miyatake, R.; Horino, Y.; Oda, M. Synthesis and structure of polyunsaturated [10]paracyclophane annulated by two azulene rings. Tetrahedron Lett. 2008, 49, 552–556. [Google Scholar] [CrossRef][Green Version]
- Wu, C.-P.; Devendar, B.; Su, H.-C.; Chang, Y.-H.; Ku, C.-K. Efficient synthesis and applications of 2-substituted azulene derivatives: Towards highly functionalized carbo- and heterocyclic molecules. Tetrahedron Lett. 2012, 53, 5019–5022. [Google Scholar] [CrossRef]
- Yang, P.-W.; Yasunami, M.; Takase, K. The formation of azulene derivatives by the reaction of 2H- cyclohepta[b]furan-2-ones with enamines. Tetrahedron Lett. 1971, 12, 4275–4278. [Google Scholar] [CrossRef]
- Yasunami, M.; Chen, A.; Noro, Y.; Takase, K. The reaction of 3-cyano-2h-cyclohepta[b]furan-2-one with β, β-disubstituted enamines. Chem. Lett. 1981, 10, 555–558. [Google Scholar] [CrossRef]
- Yasunami, M.; Hioki, T.; Kitamori, Y.; Kikuchi, I.; Takase, K. Synthesis of Cyclopent[a]azulenes and 1(3)-Methylenecyclopent[a]azulene Derivatives Having an Electron Withdrawing Group at the 9-Position. Bull. Chem. Soc. Jpn. 1993, 66, 2273–2282. [Google Scholar] [CrossRef]
- Yasunami, M.; Miyoshi, S.; Kanegae, N.; Takase, K. A Versatile Synthetic Method of 1-Alkylazulenes and Azulene by the Reactions of 3-Methoxycarbonyl-2H-cyclohepta[b]furan-2-one with in situ Generated Enamines. Bull. Chem. Soc. Jpn. 1993, 66, 892–899. [Google Scholar] [CrossRef]
- Ueno, T.; Toda, H.; Yasunami, M.; Yoshifuji, M. Synthesis and Properties of Fluoroazulenes. II. Electrophilic Fluorination of Azulenes with N-Fluoro Reagents. Bull. Chem. Soc. Jpn. 1996, 69, 1645–1656. [Google Scholar] [CrossRef]
- Nagel, M.; Hansen, H.-J. Synthesis of Polyalkylphenyl Prop-2-ynoates and Their Flash Vacuum Pyrolysis to Polyalkylcyclohepta[b]furan-2(2H)-ones. Helv. Chim. Acta 2000, 83, 1022–1048. [Google Scholar] [CrossRef]
- Nagel, M.; Hansen, H.-J. From Phenols to Azulenes: An Extended and Versatile Route to Polyalkylated Azulenes with Variable Substitution Patterns at the Seven- and Five-membered Ring. Synlett 2002, 2002, 692–696. [Google Scholar] [CrossRef]
- Nitta, M.; Nishimura, K.; Iino, Y. Synthesis and structural studies of novel azuleno[1,2-a]azulene and its derivatives. Tetrahedron Lett. 1993, 34, 2151–2160. [Google Scholar] [CrossRef]
- Yasunami, M.; Kitamori, Y.; Kikuchi, I.; Ohmi, H.; Takase, K. Peri-Selective Cycloaddition Reactions of 3-Methoxycarbonyl-2H- cyclohepta[b]furan-2-one with 6,6-Dimethylfulvene. Bull. Chem. Soc. Jpn. 1992, 65, 2127–2130. [Google Scholar] [CrossRef]
- Lellek, V.; Hansen, H.-J. Surprising Formation of Highly Substituted Azulenes on Thermolysis of 4,5,6,7,8-Pentamethyl-2H-cyclohepta[b]furan-2-one and Heptalene Formation with the New Azulenes. Helv. Chim. Acta 2001, 84, 1712–1736. [Google Scholar] [CrossRef]
- Plattner, P.A.; Wyss, J. Zur Kenntnis der Sesquiterpene;. (47. Mitteilung). Synthese einiger Mono- und Dimethyl-azulene. Helv. Chim. Acta 1941, 24, 483–492. [Google Scholar] [CrossRef]
- Plattner, P.A.; Fürst, A.; Müller, A.; Somerville, A.R. Zur Kenntnis der Sesquiterpene und Azulene. 96. Mitteilung. Über einige Azulen-carbonsäuren. Helv. Chim. Acta 1951, 34, 971–988. [Google Scholar] [CrossRef]
- Doering, W.V.E.; Mayer, J.R.; DePuy, C.H. Two-Step Synthesis of Azulene. J. Am. Chem. Soc. 1953, 75, 2386. [Google Scholar] [CrossRef]
- Alder, K.; Schmitz, P. Ein Beitrag zur Darstellung der Azulene aus Hydrindenen und über die Dien-Synthese des 3.4-Trimethylen-norcara-diens-(2.4). Chem. Ber. 1953, 86, 1539–1544. [Google Scholar] [CrossRef]
- Zindel, J.; Maitra, S.; Lightner, D.A. Synthesis and Properties of 2,6-Substituted Push-Pull Azulenes. Synthesis 1996, 1996, 1217–1222. [Google Scholar] [CrossRef]
- Luhowy, R.; Keehn, P.M. Cyclophanes. 9. anti-[2.2](2,6)Azulenophane. Synthesis and charge-transfer interaction. J. Am. Chem. Soc. 1977, 99, 3797–3805. [Google Scholar] [CrossRef]
- Sakai, L.T.; Minton, M.A.; Kirms, M.A. A short new azulene synthesis. J. Am. Chem. Soc. 1980, 110, 6311–6314. [Google Scholar] [CrossRef]
- Kane, J.L.; Shea, K.M.; Crombie, A.L.; Danheiser, R.L. A Ring Expansion−Annulation Strategy for the Synthesis of Substituted Azulenes. Preparation and Suzuki Coupling Reactions of 1-Azulenyl Triflates. Org. Lett. 2001, 3, 1081–1084. [Google Scholar] [CrossRef]
- Crombie, A.L.; Kane, J.L.; Shea, K.M.; Danheiser, R.L. Ring Expansion-Annulation Strategy for the Synthesis of Substituted Azulenes and Oligoazulenes. 2. Synthesis of Azulenyl Halides, Sulfonates, and Azulenylmetal Compounds and Their Application in Transition-Metal-Mediated Coupling Reactions. J. Org. Chem. 2004, 69, 8652–8667. [Google Scholar] [CrossRef] [PubMed]
- Leino, T.O.; Johansson, N.G.; Devisscher, L.; Sipari, N.; Yli-Kauhaluoma, J.; Wallen, E.A.A. Synthesis of 1,3,6-Trisubstituted Azulenes Based on the 1-Acyloxyazulene Scaffold. Eur. J. Org. Chem. 2016, 5539–5544. [Google Scholar] [CrossRef]
- Sakai, A.; Aoyama, T.; Shioiri, T. A new preparation of methylenecyclopropanes utilizing trimethylsilyldiazomethane. Tetrahedron 1999, 55, 3687–3694. [Google Scholar] [CrossRef]
- Hari, Y.; Tanaka, S.; Takuma, Y.; Aoyama, T. New Two-Step Synthesis of Azulene-1-carboxylic Esters Using Lithium Trimethylsilyldiazomethane. Synlett 2003, 2003, 2151–2154. [Google Scholar] [CrossRef]
- Aoyama, T.; Sonoda, N.; Yamauchi, M.; Toriyama, K.; Anzai, M.; Ando, A.; Shioiri, T. Chemical Manganese Dioxide (CMD), an Efficient Activated Manganese Dioxide. Application to Oxidation of Benzylic and Allylic Alcohols. Synlett 1998, 1998, 35–36. [Google Scholar] [CrossRef]
- Reppe, W.; Schlichting, O.; Meister, H. Cyclisierende Polymerisation von Acetylen II. Über die Kohlenwasserstoffe C10H10, C12H12 und Azulen. Justus Ann. Chem. 1948, 560, 93. [Google Scholar] [CrossRef]
- Clauß, G.; Ried, W. Photochemische Dimerisierung von 1,4-Dialkinylbenzolen. Chem. Ber. 1975, 108, 528–537. [Google Scholar] [CrossRef]
- Reppe, S.J.; Kharasch, N. Derivatives of Sulfenic Acids. XXXII. The Synthesis of Azulenes via the Interactions of Arylacetylenes with Sulfenyl Halides. Part 1. 1,2,3-Triphenylazulene1,a,b,c. J. Am. Chem. Soc. 1958, 80, 5978–5982. [Google Scholar] [CrossRef]
- De Liefde Meijer, H.J.; Pauzenga, U.; Jellinek, F. Catalytic dimerization of diphenylacetylene to 1,2,3-triphenylazulene: Short communication. Rec. Trav. Chim. Pays-Bas 1966, 85, 634–636. [Google Scholar] [CrossRef]
- Fan, R.L.; Dickstein, J.I.; Miller, S.I. Benzo[b]thiophene S-oxides and related compounds from the reactions of arylalkynes and antimony pentafluoride in sulfur dioxide. J. Org. Chem. 1982, 47, 2466–2469. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Courtneidge, J.L.; Davies, A.G.; Gregory, P.S.; Evans, J.C.; Rowlands, C.C. Generation of azulene radical cations from arylalkynes. J. Chem. Soc. Perkin Trans. 1988, 2, 807–813. [Google Scholar] [CrossRef]
- Lambert, C.; Nöll, G.; Zabel, M.; Hampel, F.; Schmälzlin, E.; Bräuchle, C.; Meerholz, K. Highly Substituted Azulene Dyes as Multifunctional NLO and Electron-Transfer Compounds. Chem. Eur. J. 2003, 9, 4232–4239. [Google Scholar] [CrossRef]
- Brown, D.G.; Hoye, T.R.; Brisbois, R.G. Synthesis of Azulenone Skeletons by Reaction of 2-Phenyl-2-acylketenes [RCO(Ph)CCO] with Alkynyl Ethers: Mechanistic Aspects and Further Transformations. J. Org. Chem. 1998, 63, 1630–1636. [Google Scholar] [CrossRef]
- Usui, K.; Tanoue, K.; Yamamoto, K.; Shimizu, T.; Suemune, H. Synthesis of Substituted Azulenes via Pt(II)-Catalyzed Ring-Expanding Cycloisomerization. Org. Lett. 2014, 16, 4662–4665. [Google Scholar] [CrossRef]
- Staab, H.A.; Ipaktschi, J. Intramolekulare Wechselwirkungen zwischen Dreifachbindungen, V. Parallele Dreifachbindungen: 1.8-Bis-aryläthinyl-naphthaline. Chem. Ber. 1971, 104, 1170–1181. [Google Scholar] [CrossRef]
- Hafner, K.; Häring, J.; Jäkel, W. Cycloadditionen am Azulensystem. Angew. Chem. 1970, 82, 135–136. [Google Scholar] [CrossRef]
- Cresp, T.M.; Wege, D. The addition of benzyne to azulene. Tetrahedron 1986, 42, 6713–6718. [Google Scholar] [CrossRef]
- Le Goff, E.J. The Synthesis of Hexaphenylpentalene. J. Am. Chem. Soc. 1962, 84, 3975–3976. [Google Scholar] [CrossRef]
- Hafner, K.; Diehl, H.; Ulrich Süss, H. Cycloadditionsreaktionen des Pentalens und Azulens—Eine einfache Heptalen-Synthese. Angev. Chem. 1976, 88, 121. [Google Scholar] [CrossRef]
- Dehmlow, E.V.; Slopianka, M. Vakuumpyrolyse von 5,5,10,10-Tetrachlortricyclo[7.1.0.04,6]deca-2,7-dien: 1,5- und 2,6-Dichlorazulene. Angew. Chem. 2006, 94, 461. [Google Scholar] [CrossRef]
- Vogel, E. Südwestdeutsche Chemie-Dozenten-Tagung. Angew. Chem. 1973, 61, 548. [Google Scholar] [CrossRef]
- Sugihara, Y.; Sugimura, T.; Murata, I. Valence isomer of 4-methoxyazulene. Synthesis of 6-methoxytetracyclo[5.3.0.02,4.03,5]deca-6,8,10-triene. J. Am. Chem. Soc. 1981, 103, 6738–6739. [Google Scholar] [CrossRef]
- Sugihara, Y.; Sugimura, T.; Murata, I. Synthesis and thermal behavior of 6-methoxytricyclo[5.3.0.02,5]deca-3,6,8,10-tetraene (methoxy-Dewar azulene). Similarity between valence isomers of azulene and benzene. J. Am. Chem. Soc. 1982, 104, 4295–4298. [Google Scholar] [CrossRef]
- Gassman, P.G.; Nakai, T. Chemistry of bent bonds. XXVI. Transition metal complex induced rearrangement of 1-methyl-2,2-diphenylbicyclo[1.1.0]butane. New route to azulenes. J. Am. Chem. Soc. 1971, 93, 5897–5899. [Google Scholar] [CrossRef]
- Diehl, H. Doctoral thesis, 1976. Technische Hochschule Darmstadt, Germany. In Methoden der Organischen Chemie-Houben-Weil; Thieme Verlag Stuttgart: New York, NY, USA, 1985; Volume V/2c, p. 242. [Google Scholar]
- Nakazawa, T.; Ishihara, M.; Jinguji, M.; Yamaguchi, M.; Sugihara, Y.; Murata, I. Acid-catalyzed rearrangement of 2-substituted and 2,4-disubstituted 8H-3-oxaheptalen-8-ones to 1-Acyl-6-hydroxyazulenes. Tetrahedron Lett. 1992, 33, 6487–6490. [Google Scholar] [CrossRef]
- Dimroth, K.; Wolf, K.H.; Wache, H. Azulenes from Pyrylium Salts and Wittig Reagent. Angew. Chem. Int. Ed. 1963, 2, 621. [Google Scholar] [CrossRef]
- Kende, A.S.; Hebeisen, P. Condensation of a p-diazooxide with dimethyl acetylenedicarboxylate: Formation of a spiro [4.5]decatetraenone and a nonenolizable naphthalenone in a single reaction. Tetrahedron Lett. 1985, 26, 3769–3772. [Google Scholar] [CrossRef]
- Sasabe, M.; Houda, Y.; Takagi, H.; Sugane, T.; Bo, X.; Yamamura, K. A versatile and efficient method for the synthesis of benz[a]azulenic enones starting from readily available o-(2-furyl)cycloheptatrienylbenzenes. J. Chem. Soc. Perkin Trans. 2000, 1, 3786–3790. [Google Scholar] [CrossRef]






























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razus, A.C. A Century of Azulene Chemistry; A Brief Look at Azulenes Building. Symmetry 2025, 17, 335. https://doi.org/10.3390/sym17030335
Razus AC. A Century of Azulene Chemistry; A Brief Look at Azulenes Building. Symmetry. 2025; 17(3):335. https://doi.org/10.3390/sym17030335
Chicago/Turabian StyleRazus, Alexandru C. 2025. "A Century of Azulene Chemistry; A Brief Look at Azulenes Building" Symmetry 17, no. 3: 335. https://doi.org/10.3390/sym17030335
APA StyleRazus, A. C. (2025). A Century of Azulene Chemistry; A Brief Look at Azulenes Building. Symmetry, 17(3), 335. https://doi.org/10.3390/sym17030335



























